Abstract
Background
A mixture of spermatozoa and accessory gland secretions (from seminal vesicles, prostates, and coagulating glands) is ejaculated into the female reproductive tract at copulation. However, the physiological function of accessory glands on male fecundity remains unclear.
Methods
Publications regarding the physiological functions of male accessory glands were summarized.
Main findings (Results)
The functions of accessory glands have been studied using male rodents surgically removed coagulating glands (CG), prostates (PR), or seminal vesicles (SV). CG‐removed males are fertile or subfertile, while the fecundity of PR‐removed males is controversial. SV‐removed males show copulatory plug defects, leading to fewer sperm in the uterus and severe subfertility. TGM4, SVS2, and PATE4 were identified as essential factors for copulatory plug formation. When the sufficient number of epididymal spermatozoa was artificially injected into a uterus (AI method), they could efficiently fertilize oocytes, implicating that accessory gland secretions are not essential. Seminal vesicle secretions (SVSs) improved fertilization rates only when low numbers of spermatozoa were used for AI. The changes of uterine environment by SVSs could not improve the pregnancy rate.
Conclusion
Accessory gland factors are critical for copulatory plug formation and support sperm fertilizing ability.
Keywords: artificial insemination, copulatory plug, male accessory gland, sperm fertilizing ability, uterine environment
1. INTRODUCTION
Testicular spermatozoa are incapable of fertilizing eggs. Spermatozoa acquire their fertilizing ability (e.g., motility, capacitation, acrosome reaction, and sperm‐egg fusion capabilities) during transition through the epididymis. At copulation, a mixture of cauda epididymal spermatozoa and accessory gland secretions is ejaculated into the female reproductive tract. The accessory gland secretions mainly come from the seminal vesicles (SV), prostates, bulbourethral gland (also known as “Cowper's gland”), and urethral glands.1 Specifically, men ejaculate 3–3.5 mL of semen into the female reproductive tract, which is mainly composed of secretions from SV (1.5–2.0 mL), prostates (0.5 mL), and bulbourethral gland and urethral glands (0.1 mL).2 As summarized in Table 1, the role of accessory glands in male fecundity has been studied using mice and rats by surgically removing accessory glands individually. The prostate is composed of four regions (ventral, lateral, dorsal, and anterior regions), and the fecundity of males having undergone an anterior prostatectomy (also known as “coagulating glands”; CG) was either reduced3, 4 or comparable to control males.5, 6, 7 The remaining regions in the prostate tightly adhere to the SV and urinary bladder,8 complicating surgical removal of each region. In fact, there are discrepancies between previous studies on the fecundity of male mice and rats with ventral and dorsal prostatectomies.3, 5, 9 Thus, the physiological function of these separate regions of the prostates on male fecundity remains unclear. There is no report on male fecundity after surgically removing the bulbourethral gland or urethral glands. When the SV of male mice and rats was surgically removed, the males become severely subfertile.3, 4, 5, 6, 7 Thus, the SV is thought to play a beneficial role in fertilization in vivo. Here, we mainly introduce the physiological function of SV on male fecundity at the molecular level based on recent finding.
Table 1.
Fecundity of male mice and rats with accessory glands surgically removed
| Reference | Treated | Pregnancy rate (%) | Litter size | Plug weight (mg) |
|---|---|---|---|---|
| Mouse | ||||
| Pang et al.5 | Control | 73 | 9.4 ± 0.3 | ND |
| VP and DP (‐) | 38 | 9.8 ± 0.9 | ND | |
| CG (‐) | 73 | 9.2 ± 2.8 | ND | |
| SV (‐) | 7 | 4 | ND | |
| Peitz and Olds‐Clarke28 | Control | 95.2 ± 1.9 | 8.3 ± 0.3 | ND |
| SV (‐) | 77.8 ± 5.1 | 8.0 ± 0.4 | ND | |
| Kawano et al.7 | Control | ND | 13.6 ± 0.5 | ND |
| CG (‐) | ND | 9.6 ± 2.0 | ND | |
| SV (‐) | ND | 0 | 0 | |
| Noda et al.6 | Control | 1.4 ± 0.3# | 8.8 ± 2.0 | 43.5 ± 13.8 |
| CG (‐) | 1.5 ± 0.0# | 9.1 ± 2.4 | 21.6 ± 11.2 | |
| SV (‐) | 0.6 ± 0.5# | 6.1 ± 3.7 | 3.5 ± 3.6 | |
| Rat | ||||
| Gunn and Gould9 | Control | 61.4 ± 1.9 | 9.9 ± 0.4 | ND |
| DP (‐) | 58.4 ± 7.8 | 9.1 ± 0.4 | ND | |
| Queen et al.3 | Control | 100 | 5.5 ± 0.5## | ND |
| VP (‐) | 100 | 5.3 ± 0.4## | ND | |
| DP (‐) | 0 | 0 | ND | |
| CG (‐) | 25 | 5.2 ± 1.6## | ND | |
| SV (‐) | 0 | 0 | ND | |
| Carballada and Esponda4 | Control | N.D | 14.8 ± 0.6 | 58.5 ± 3.7 |
| CG (‐) | 33.3 | 13.0 ± 4.6 | 14.2 ± 18.4 | |
| SV (‐) | N.D | 0 | 0 | |
Sham‐operated males were used as the control.
Abbreviations: #, No. of litters/female/month; ##, Some data from Table 1 of Queen et al. [3] were used; (‐), males with specified organ surgically removed; CG, coagulating gland; DP, dorsal prostate; N.D., not determined; SV, seminal vesicle; VP, ventral prostate.
2. PHYSIOLOGICAL FUNCTIONS OF ACCESSORY GLAND SECRETIONS
2.1. Copulatory plug
As one of the physiological functions of accessory gland secretions, copulatory plug formation is well known in several primates (e.g. chimpanzee) and rodents.10, 11 Proteins from CG and SV are required for copulatory plug formation in vitro.12 In fact, CG‐removed male mice and rats show decreases in the copulatory plug weight, but these males are fertile or subfertile (Table 1).3, 4, 5, 6, 7 SV‐removed males hardly make the copulatory plug (Figure 1 and Table 1), and these males become severely subfertile.3, 4, 5, 6, 7 We revealed that plug formation defects caused semen leakage from the vagina, resulting in a decrease in sperm numbers in the uterus and male fecundity (Figure 1).6 When the females without copulatory plugs after mating were immediately re‐caged with other males, the females had subsequent productive matings.6 Thus, we concluded that the copulatory plug has the dual function of not only inhibiting sequential matings but to maintain spermatozoa in the uterus to ensure male fecundity, as a winner‐take‐all strategy to advance male reproduction.
Figure 1.
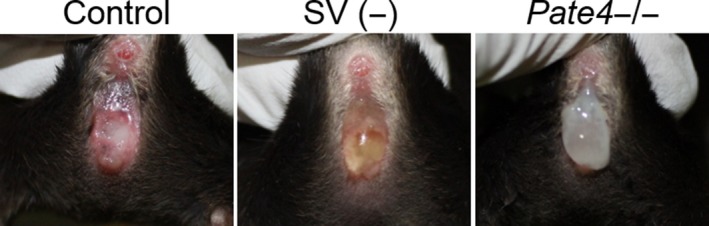
Observation of vaginas immediately after mating. Sham‐operated (control), seminal vesicle removed, and Pate4 KO males were mated with wild‐type females
In copulatory plug formation, it is thought that transglutaminase 4 (TGM4) from prostates and coagulating glands catalyze the formation of ε‐(γ‐glutamyl)lysine cross‐bridges among seminal vesicle secretion 1 (SVS1) to SVS3 (Table 2).6, 11, 13, 14, 15, 16, 17, 18, 19, 20 In fact, previous papers showed that single KO males of Tgm4 or Svs2 are subfertile due to plug formation defects.7, 21 Lin et al. showed that the peptide sequence “QXK(S/T)” within SVS3 acts as the cross‐linking sites by reacting of guinea pig liver transglutaminase with recombinant polypeptides from SVS3.16 SVS2 also contains this peptide sequence.6 Although SVS1 does not contain the sequence “QXK(S/T),” Tseng et al. showed that two glutamine residues in SVS1 (Q232 and Q254) were the major site for TGM4 cross‐linking by mass spectrometry.14 Recently, we reported that prostate and testis expression 4 (PATE4; also known as SVS7) is the essential factor for copulatory plug formation with Pate4 KO mice (Figure 1 and Table 2).6 Though we could not find “QXK(S/T)” in PATE4, our results suggest that PATE4 may be cross‐linked by a TGM4‐dependent/independent manner or have an unknown function to facilitate copulatory plug formation. Other reports suggest that several glutamine and lysine residues (eg, Q86 and K59) in SVS4 are the target sites for TGM4 cross‐linking (Table 2).22, 23, 24 Thus, the mechanism of copulatory plug formation may be more complicated than expected.
Table 2.
Physiological functions of proteins in accessory gland secretions
| Function | Proteins | Summary of results |
|---|---|---|
| Copulatory plug formation | SVS1 | Two glutamines (Q232 and Q254) in SVS1 are the site for TGM4 cross‐linking.14 |
| SVS2 | SVS2 has the TGM4 cross‐linking site and conserves the peptide sequence “QXK(S/T)” for TGM4.6, 11, 13 Svs2 KO males show plug formation defects.7 | |
| SVS3 | The peptide sequence “QXK(S/T)” in SVS3 was identified as the site for TGM4 cross‐linking.16 | |
| SVS4 | Several glutamine and lysine residues (eg, Q86 and K59) in SVS4 were identified as the substrate for TGM4.22, 23, 24 | |
| PATE4 | Pate4 KO males show plug formation defects.6 | |
| TGM4 | TGM4, an enzyme from prostates and coagulating glands, catalyzes the formation of ε‐(γ‐glutamyl)lysine cross‐bridges among SVSs. 6, 11, 13, 14, 15, 16, 17, 18, 19, 20 Tgm4 KO males show plug formation defects.21 | |
| Sperm fertilizing ability | ||
| Motility | SPMI | These proteins from seminal vesicles function as sperm motility inhibitors in vitro (SPMI42, 43 and SVA44). |
| SVA | ||
| PATE4 | PATE4 improved sperm motility in vitro.45 | |
| Capacitation | SVS2 | These proteins were identified as decapacitation factors in vitro (SVS2,48 SPINKL,49, 50 and SERPINE251). |
| SPINKL | ||
| SERPINE2 | ||
| Survival | SVS2 | SVS2 protects the spermatozoa from an immunological response in the uterus using Svs2 KO males.7 |
| Uterine environment | TGFβ | These proteins in seminal plasma are involved in the inflammatory response of the uterus to seminal fluid.55, 56, 58, 59, 60 |
| Prostaglandin E | ||
| TLR4 ligands | ||
Abbreviations: PATE, prostate and testis expression; SERPINE2, serine protease inhibitor, clade E, member 2; SPINKL, serine protease inhibitor Kazal‐type‐like; SPMI, seminal plasma motility inhibitor; SVA, seminal vesicle autoantigen; SVS, seminal vesicle secretion; TGF, transforming growth factor; TGM, transglutaminase; TLR, Toll‐like receptor.
2.2. Sperm fertilizing ability
It is known that the accessory gland secretions aid the sperm fertilizing ability (e.g., sperm motility, capacitation, sperm survival). Seminal plasma components improve the sperm motility in human25, 26 and boar.27 In addition, ejaculated spermatozoa from SV‐removed male mice show decreased motility.28 The ejaculated spermatozoa acquire fertilizing ability after they stay in the female reproductive tract for several hours (known as “sperm capacitation”).29, 30, 31 Spermatozoa from some subfertile bulls display the premature capacitation,32 and it has been shown components of seminal plasma can inhibit sperm capacitation.33 These results suggest that the accessory gland secretions regulate the timing of sperm capacitation to improve male fertility. Accessory gland secretions help the survival and cervical transit of epididymal spermatozoa34 and to prevent an immunological response to spermatozoa in the female reproductive tract.35
Interestingly, the ejaculated spermatozoa of SV‐removed boars36 and bulls37 could efficiently fertilize eggs with artificial insemination (AI). Also, cauda epididymal spermatozoa from mice,6, 38, 39 bulls,40 and boars41 can fertilize oocytes when these spermatozoa were used for AI. From these results, accessory gland secretions appear to be unnecessary for sperm fertilizing ability. Recently, we observed improvement of sperm fertilization rates by SVSs only when the low sperm numbers were used for AI.6 Thus, we concluded that the positive effects of accessory gland secretions on the sperm fertilizing ability only appear when the amount of sperm numbers in the uterus is low referring at least in mice.
There are several functional studies of accessory gland secretions on sperm fertilizing ability at the molecular level (Table 2). Specifically, seminal plasma motility inhibitor,42, 43 seminal vesicle autoantigen,44 and PATE445 were reported as modulators of sperm motility in seminal vesicle secretions. Also, Ca2+ signaling cascades induced by the extracellular vesicles secreted from prostate epithelial cells (known as prostasomes) improved sperm motility.46, 47 SVS2,48 a serine protease inhibitor Kazal‐type‐like (SPINKL),49, 50 and a serine protease inhibitor, clade E, member 2 (SERPINE2)51 from SV were identified as decapacitation factors. SVS2 and SPINKL attached on the plasma membrane of spermatozoa immediately after ejaculation, which then disappear in spermatozoa by the time they reach the oviduct.48, 50 This result suggests that decapacitation factors on the sperm surface are removed while the spermatozoa pass through the uterus. Further, SVS2 acts to protect the spermatozoa against the uterus‐derived cytotoxic factors.7 As more than 700 proteins from accessory glands were identified with proteomics,52 the functional analysis of these proteins will be required to further dissect the physiological function of accessory gland secretions on sperm fertilizing ability at the molecular level.
2.3. Uterine environment
It is known that the seminal plasma is not only involved in sperm fertilizing ability, but also in female reproductive physiology in insects and mammals (e.g., immune tolerance for pregnancy).53, 54 Seminal plasma contains the signaling molecules that interact with estrogen‐primed epithelial cells lining the female reproductive tract to accelerate the expression levels of cytokine and chemokine genes.55, 56 These upregulated genes facilitate leukocyte recruitment and activation of the innate and adaptive immune system that resembles an inflammatory cascade, leading to the preparation of the female reproductive tract for pregnancy.53, 55, 57 The inflammatory‐like response to seminal fluid depends on seminal plasma factors, such as transforming growth factor (TGFβ), E‐series prostaglandins, and Toll‐like receptor 4 (TLR4) ligand (such as bacterial lipopolysaccharide [LPS]; Table 2).55, 56, 58, 59, 60 The lack of accessory gland secretions causes the slower cleavage rates in embryogenesis and placental hypertrophy in vivo, from studies using mouse and hamster males with accessory glands surgically removed,61, 62 leading to changes of postnatal growth and fetal programming.62, 63 Despite these effects, the females artificially injected with cauda epididymal spermatozoa become pregnant.38, 64 Recently, we revealed no differences in the pregnancy rate and the litter size between uterine environments with and without stimulation by SVSs when the cauda epididymal spermatozoa were injected into a uterus by AI.6 Thus, factors in accessory gland secretions may contribute to regulate the uterine environment, but the physiological functions on embryogenesis and pregnancy remain limited. The detailed effects of accessory gland secretions on postnatal growth and fetal programming need further examination.
3. CONCLUSION
In this review, we mainly highlighted positive functions of SV on copulatory plug formation and sperm fertilizing ability. More than 700 proteins were detected in the accessory glands with proteomics,52 but the physiological functions of these proteins remain unknown. The emergence of the clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 system opened a new era in mammalian genome editing.65, 66, 67 Our previous works demonstrated that CRISPR/Cas9‐mediated KO mice generation and phenotypic analysis are a cost‐effective and labor‐effective approach to quickly identify essential gene functions in vivo.68, 69, 70 Thus, utilizing CRISPR/Cas9 genome editing to examine the function of these 700 genes identified as accessory glands will accelerate elucidation of accessory glands on male fecundity at the molecular level.
CONFLICT OF INTEREST
The authors have declared that no conflict of interest exists.
HUMAN AND ANIMAL SUBJECTS
This review article does not contain any studies with human and animal subjects performed by any of the authors.
ACKNOWLEDGEMENT
The authors would like to thank Dr Julio Castaneda for critical reading of the manuscript.
Noda T, Ikawa M. Physiological function of seminal vesicle secretions on male fecundity. Reprod Med Biol. 2019;18:241–246. 10.1002/rmb2.12282
REFERENCES
- 1. Verze P, Cai T, Lorenzetti S. The role of the prostate in male fertility, health and disease. Nat Rev Urol. 2016;13:379‐386. [DOI] [PubMed] [Google Scholar]
- 2. Coffey DS. Androgen action and the sex accessory tissues, in the physiology of reproduction In: Knobil E, Neill DJ, eds. The Physiology of Reproduction. New York, NY: Raven Press; 1988:1081‐1119. [Google Scholar]
- 3. Queen K, Dhabuwala CB, Pierrepoint CG. The effect of the removal of the various accessory sex glands on the fertility of male rats. J Reprod Fertil. 1981;62:423‐426. [DOI] [PubMed] [Google Scholar]
- 4. Carballada R, Esponda P. Role of fluid from seminal vesicles and coagulating glands in sperm transport into the uterus and fertility in rats. J Reprod Fertil. 1992;95:639‐648. [DOI] [PubMed] [Google Scholar]
- 5. Pang SF, Chow PH, Wong TM. The role of the seminal vesicles, coagulating glands and prostate glands on the fertility and fecundity of mice. J Reprod Fertil. 1979;56:129‐132. [DOI] [PubMed] [Google Scholar]
- 6. Noda T, Fujihara Y, Matsumura T, Oura S, Kobayashi S, Ikawa M. Seminal vesicle secretory protein 7, PATE4, is not required for sperm function but for copulatory plug formation to ensure fecundity. Biol Reprod. 2019;100:1035‐1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kawano N, Araki N, Yoshida K, et al. Seminal vesicle protein SVS2 is required for sperm survival in the uterus. Proc Natl Acad Sci USA. 2014;111:4145‐4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Risbridger GP, Taylor RA. Physiology of the male accessory sex structures: the prostate gland, seminal vesicles, and bulbourethral glands In: Neill JD, ed. Knobil and Neill's Physiology of Reproduction. Amsterdam: Elsevier; 2006:1149‐1172. [Google Scholar]
- 9. Gunn SA, Gould TC. Role of zinc in fertility and fecundity in the rat. Am J Physiol. 1958;193:505‐508. [DOI] [PubMed] [Google Scholar]
- 10. Tinklepaugh OL. Occurrence of vaginal plug in a chimpanzee. Anat Rec. 1930;46:329‐332. [Google Scholar]
- 11. Seitz J, Aumuller G. Biochemical properties of secretory proteins from rat seminal vesicles. Andrologia. 1990;22(Suppl 1):25‐32. [DOI] [PubMed] [Google Scholar]
- 12. Gotterer B, Ginsberg D, Schulman T, Banks J, Williams‐Ashman HG. Enzymatic coagulation of semen. Nature. 1955;176:1209‐1211. [DOI] [PubMed] [Google Scholar]
- 13. Tseng H‐C, Tang J‐B, Sudhakar Gandhi PS, et al. Mutual adaptation between mouse transglutaminase 4 and its native substrates in the formation of copulatory plug. Amino Acids. 2012;42:951‐960. [DOI] [PubMed] [Google Scholar]
- 14. Tseng HC, Lin HJ, Tang JB, Gandhi PS, Chang WC, Chen YH. Identification of the major TG4 cross‐linking sites in the androgen‐dependent SVS I exclusively expressed in mouse seminal vesicle. J Cell Biochem. 2009;107:899‐907. [DOI] [PubMed] [Google Scholar]
- 15. Tseng HC, Lin HJ, Sudhakar Gandhi PS, Wang CY, Chen YH. Purification and identification of transglutaminase from mouse coagulating gland and its cross‐linking activity among seminal vesicle secretion proteins. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;876:198‐202. [DOI] [PubMed] [Google Scholar]
- 16. Lin HJ, Luo CW, Chen YH. Localization of the transglutaminase cross‐linking site in SVS III, a novel glycoprotein secreted from mouse seminal vesicle. J Biol Chem. 2002;277:3632‐3639. [DOI] [PubMed] [Google Scholar]
- 17. Williams‐Ashman HG. Transglutaminases and the clotting of mammalian seminal fluids. Mol Cell Biochem. 1984;58:51‐61. [DOI] [PubMed] [Google Scholar]
- 18. Folk JE. Transglutaminases. Annu Rev Biochem. 1980;49:517‐531. [DOI] [PubMed] [Google Scholar]
- 19. Hart RG. Cowper's gland secretion in rat semen coagulation. I. Isolation and amino acid analysis of the seminal vesicle substrate. Biol Reprod. 1970;3:347‐352. [DOI] [PubMed] [Google Scholar]
- 20. Lundwall A, Peter A, Lovgren J, Lilja H, Malm J. Chemical characterization of the predominant proteins secreted by mouse seminal vesicles. Eur J Biochem. 1997;249:39‐44. [DOI] [PubMed] [Google Scholar]
- 21. Dean MD. Genetic disruption of the copulatory plug in mice leads to severely reduced fertility. PLoS Genet. 2013;9:e1003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Porta R, Esposito C, Gentile V, Mariniello L, Peluso G, Metafora S. Transglutaminase‐catalyzed modifications of SV‐IV, a major protein secreted from the rat seminal vesicle epithelium. Int J Pept Protein Res. 1990;35:117‐122. [DOI] [PubMed] [Google Scholar]
- 23. Porta R, Esposito C, Metafora S, et al. Mass spectrometric identification of the amino donor and acceptor sites in a transglutaminase protein substrate secreted from rat seminal vesicles. Biochemistry. 1991;30:3114‐3120. [DOI] [PubMed] [Google Scholar]
- 24. Metafora S, Esposito C, Caputo I, et al. Seminal vesicle protein IV and its derived active peptides: a possible physiological role in seminal clotting. Semin Thromb Hemost. 2007;33:53‐59. [DOI] [PubMed] [Google Scholar]
- 25. Lindholmer C, Carlstrom A, Eliasson R. Occurrence and origin of proteins in human seminal plasma with special reference to albumin. Andrologia. 1974;6:181‐196. [DOI] [PubMed] [Google Scholar]
- 26. Stegmayr B, Ronquist G. Promotive effect on human sperm progressive motility by prostasomes. Urol Res. 1982;10:253‐257. [DOI] [PubMed] [Google Scholar]
- 27. Tso WW, Lee WM. Seminal plasma and progressive motility of boar spermatozoa. Int J Androl. 1980;3:243‐250. [DOI] [PubMed] [Google Scholar]
- 28. Peitz B, Olds‐Clarke P. Effects of seminal vesicle removal on fertility and uterine sperm motility in the house mouse. Biol Reprod. 1986;35:608‐617. [DOI] [PubMed] [Google Scholar]
- 29. Austin CR. Observations on the penetration of the sperm in the mammalian egg. Aust J Sci Res B. 1951;4:581‐596. [DOI] [PubMed] [Google Scholar]
- 30. Chang MC. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature. 1951;168:697‐698. [DOI] [PubMed] [Google Scholar]
- 31. Chang MC. Development of fertilizing capacity of rabbit spermatozoa in the uterus. Nature. 1955;175:1036‐1037. [DOI] [PubMed] [Google Scholar]
- 32. Kuroda K, Fukushima M, Harayama H. Premature capacitation of frozen‐thawed spermatozoa from subfertile Japanese black cattle. J Reprod Dev. 2007;53:1079‐1086. [DOI] [PubMed] [Google Scholar]
- 33. Abney TO, Williams WL. Inhibition of sperm capacitation by intrauterine deposition of seminal plasma decapacitation factor. Biol Reprod. 1970;2:14‐17. [DOI] [PubMed] [Google Scholar]
- 34. Rickard JP, Pini T, Soleilhavoup C, et al. Seminal plasma aids the survival and cervical transit of epididymal ram spermatozoa. Reproduction. 2014;148:469‐478. [DOI] [PubMed] [Google Scholar]
- 35. Bedford JM. The functions–or not–of seminal plasma? Biol Reprod. 2015;92:18. [DOI] [PubMed] [Google Scholar]
- 36. Davies DC, Hall G, Hibbitt G, Moore HD. The removal of the seminal vesicles from the boar and the effects on the semen characteristics. J Reprod Fertil. 1975;43:305‐312. [DOI] [PubMed] [Google Scholar]
- 37. King GJ, Macpherson JW. Influence of seminal vesiculectomy on bovine semen. J Dairy Sci. 1969;52:1837‐1842. [Google Scholar]
- 38. Leckie PA, Watson JG, Chaykin S. An improved method for the artificial insemination of the mouse (Mus musculus). Biol Reprod. 1973;9:420‐425. [DOI] [PubMed] [Google Scholar]
- 39. Watson JG, Wright RW Jr, Chaykin S. Collection and transfer of preimplantation mouse embryos. Biol Reprod. 1977;17:453‐458. [DOI] [PubMed] [Google Scholar]
- 40. Igboeli G, Foote RH. Maturation changes in bull epididymal spermatozoa. J Dairy Sci. 1968;51:1703‐1705. [DOI] [PubMed] [Google Scholar]
- 41. Holtz W, Smidt D. The fertilizing capacity of epididymal spermatozoa in the pig. J Reprod Fertil. 1976;46:227‐229. [DOI] [PubMed] [Google Scholar]
- 42. Iwamoto T, Gagnon C. Purification and characterization of a sperm motility inhibitor in human seminal plasma. J Androl. 1988;9:377‐383. [DOI] [PubMed] [Google Scholar]
- 43. Iwamoto T, Hiroaki H, Furuichi Y, et al. Cloning of boar SPMI gene which is expressed specifically in seminal vesicle and codes for a sperm motility inhibitor protein. FEBS Lett. 1995;368:420‐424. [DOI] [PubMed] [Google Scholar]
- 44. Huang YH, Chu ST, Chen YH. Seminal vesicle autoantigen, a novel phospholipid‐binding protein secreted from luminal epithelium of mouse seminal vesicle, exhibits the ability to suppress mouse sperm motility. Biochem J. 1999;343(Pt 1):241‐248. [PMC free article] [PubMed] [Google Scholar]
- 45. Luo CW, Lin HJ, Chen YH. A novel heat‐labile phospholipid‐binding protein, SVS VII, in mouse seminal vesicle as a sperm motility enhancer. J Biol Chem. 2001;276:6913‐6921. [DOI] [PubMed] [Google Scholar]
- 46. Park K‐H, Kim B‐J, Kang J, et al. Ca2+ signaling tools acquired from prostasomes are required for progesterone‐induced sperm motility. Sci Signal. 2011;4:ra31. [DOI] [PubMed] [Google Scholar]
- 47. Aalberts M, Stout TA, Stoorvogel W. Prostasomes: extracellular vesicles from the prostate. Reproduction. 2014;147:R1‐14. [DOI] [PubMed] [Google Scholar]
- 48. Kawano N, Yoshida M. Semen‐coagulating protein, SVS2, in mouse seminal plasma controls sperm fertility. Biol Reprod. 2007;76:353‐361. [DOI] [PubMed] [Google Scholar]
- 49. Lin M‐H, Lee R‐K, Hwu Y‐M, et al. SPINKL, a Kazal‐type serine protease inhibitor‐like protein purified from mouse seminal vesicle fluid, is able to inhibit sperm capacitation. Reproduction. 2008;136:559‐571. [DOI] [PubMed] [Google Scholar]
- 50. Tseng HC, Lee RK, Hwu YM, Lu CH, Lin MH, Li SH. Mechanisms underlying the inhibition of murine sperm capacitation by the seminal protein, SPINKL. J Cell Biochem. 2013;114:888‐898. [DOI] [PubMed] [Google Scholar]
- 51. Lu CH, Lee RK, Hwu YM, et al. SERPINE2, a serine protease inhibitor extensively expressed in adult male mouse reproductive tissues, may serve as a murine sperm decapacitation factor. Biol Reprod. 2011;84:514‐525. [DOI] [PubMed] [Google Scholar]
- 52. Dean MD, Clark NL, Findlay GD, et al. Proteomics and comparative genomic investigations reveal heterogeneity in evolutionary rate of male reproductive proteins in mice (Mus domesticus). Mol Biol Evol. 2009;26:1733‐1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Robertson SA. Seminal plasma and male factor signalling in the female reproductive tract. Cell Tissue Res. 2005;322:43‐52. [DOI] [PubMed] [Google Scholar]
- 54. Avila FW, Sirot LK, LaFlamme BA, Rubinstein CD, Wolfner MF. Insect seminal fluid proteins: identification and function. Annu Rev Entomol. 2011;56:21‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Robertson SA, Petroff MG, Hunt JS. Immunology of pregnancy In: Plant TM, Zeleznik AJ, eds. Knobil and Neill's Physiology of Reproduction. Amsterdam: Elsevier; 2015:1835‐1874. [Google Scholar]
- 56. Schjenken JE, Glynn DJ, Sharkey DJ, Robertson SA. TLR4 signaling is a major mediator of the female tract response to seminal fluid in mice. Biol Reprod. 2015;93:68. [DOI] [PubMed] [Google Scholar]
- 57. Robertson SA, Guerin LR, Moldenhauer LM, Hayball JD. Activating T regulatory cells for tolerance in early pregnancy ‐ the contribution of seminal fluid. J Reprod Immunol. 2009;83:109‐116. [DOI] [PubMed] [Google Scholar]
- 58. Tremellen KP, Seamark RF, Robertson SA. Seminal transforming growth factor beta1 stimulates granulocyte‐macrophage colony‐stimulating factor production and inflammatory cell recruitment in the murine uterus. Biol Reprod. 1998;58:1217‐1225. [DOI] [PubMed] [Google Scholar]
- 59. Fujita Y, Mihara T, Okazaki T, et al. Toll‐like receptors (TLR) 2 and 4 on human sperm recognize bacterial endotoxins and mediate apoptosis. Hum Reprod. 2011;26:2799‐2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kelly RW. Prostaglandins in primate semen: biasing the immune system to benefit spermatozoa and virus? Prostaglandins Leukot Essent Fatty Acids. 1997;57:113‐118. [DOI] [PubMed] [Google Scholar]
- 61. O. WS , Chen HQ, Chow PH. Effects of male accessory sex gland secretions on early embryonic development in the golden hamster. J Reprod Fertil. 1988;84(1):341–344. [DOI] [PubMed] [Google Scholar]
- 62. Bromfield JJ, Schjenken JE, Chin PY, Care AS, Jasper MJ, Robertson SA. Maternal tract factors contribute to paternal seminal fluid impact on metabolic phenotype in offspring. Proc Natl Acad Sci USA. 2014;111:2200‐2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Burton GJ, Fowden AL. Review: the placenta and developmental programming: balancing fetal nutrient demands with maternal resource allocation. Placenta. 2012;33(Suppl):S23‐27. [DOI] [PubMed] [Google Scholar]
- 64. Stone BJ, Steele KH, Fath‐Goodin A. A rapid and effective nonsurgical artificial insemination protocol using the NSET device for sperm transfer in mice without anesthesia. Transgenic Res. 2015;24:775‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cong L, Ran FA, Cox D, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819‐823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wang H, Yang H, Shivalila CS, et al. One‐step generation of mice carrying mutations in multiple genes by CRISPR/Cas‐mediated genome engineering. Cell. 2013;153:910‐918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yang H, Wang H, Shivalila CS, Cheng AW, Shi L, Jaenisch R. One‐step generation of mice carrying reporter and conditional alleles by CRISPR/Cas‐mediated genome engineering. Cell. 2013;154:1370‐1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Miyata H, Castaneda JM, Fujihara Y, et al. Genome engineering uncovers 54 evolutionarily conserved and testis‐enriched genes that are not required for male fertility in mice. Proc Natl Acad Sci USA. 2016;113:7704‐7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Oji A, Noda T, Fujihara Y, et al. CRISPR/Cas9 mediated genome editing in ES cells and its application for chimeric analysis in mice. Sci Rep. 2016;6:31666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Noda T, Sakurai N, Nozawa K, et al. Nine genes abundantly expressed in the epididymis are not essential for male fecundity in mice. Andrology. 2019. [Epub ahead of print]. 10.1111/andr.12621 [DOI] [PMC free article] [PubMed] [Google Scholar]


