Abstract
Purpose
This study aimed to examine the relationship between granulosa cells (GCs), number of follicles, and the ability of follicular fluid to support in vitro growth of oocytes.
Methods
The culture medium was supplemented with follicular fluid (FF) collected from GC‐rich ovaries and GC‐poor ovaries, and its effect on in vitro growth and quality of oocytes derived from early antral follicles (EAFs) was assessed.
Results
GC‐rich FF treatment enhanced oocyte growth and augmented changes in the chromatin configuration and lipid content of oocytes when compared to oocytes treated with GC‐poor FF. Moreover, oocytes treated with GC‐rich FF had a higher ability to progress to the blastocyst stage, than oocytes derived from large antral follicles (3‐5 mm in diameter). In addition, supplementation of the culture medium with either GC‐rich or GC‐poor FF enhanced histone acetylation in oocytes grown in vitro.
Conclusion
GC‐rich FF contains key factors that support in vitro oocyte growth; hence, oocytes grown in GC‐rich FF medium had high developmental competence, which was comparative to the oocytes grown in vivo.
Keywords: early antral follicles, embryos, follicular fluids, granulosa cells, oocyte growth
1. INTRODUCTION
Ovaries have a myriad of follicles of various sizes, and tens of small early antral follicles (EAFs) are present in the ovarian cortex. However, the developmental ability of oocytes, derived from EAFs, grown in vitro remains low owing to the lack of proper culture conditions. Therefore, only large antral follicles have been used for embryo development in humans and domestic animals. Follicle development depends on granulosa cell proliferation. Granulosa cells (GCs) plays a crucial role in oocyte development by providing energy and small molecules that closely interact with the oocytes.1 During growth, the oocytes accumulate mRNA, proteins, and lipids. Additionally, changes in the chromatin configuration occur in fully, grown oocytes, including condensed chromatin and high levels of acetylated histones.1, 2, 3 High ATP and lipid content, highly condensed chromatin, and enhanced histone acetylation are markers for developmental competence of the oocytes.4, 5, 6 Although factors supporting these oocyte changes have not been fully elucidated, the number of GCs surrounding the oocytes has been reported to be associated with the lipid content and histone acetylation levels. For example, when oocytes derived from EAFs were cultured in vitro, the chromatin condensed7 and the GC number at the end of the culture period was closely related to the lipid and ATP content and the level of acetylation in oocytes.8, 9, 10 It has been previously reported that even in the antral follicles of same size, the average number of GCs differs among donor gilts but is related to the developmental ability of the enclosed oocytes.11 In this study, ovaries were categorized into GC‐rich, GC‐poor, and intermediate ovaries based on the average number of GCs per follicle. It was reported that FF collected from GC‐rich ovaries profoundly supported oocyte maturation and subsequent embryo development.11 Hence, we hypothesized that GC‐rich FF may strongly support to growth of oocyte in vitro (IVG). In the present study, oocyte‐granulosa cell complexes (OGCs) derived from EAFs of gilts were cultured in medium containing either GC‐rich, GC‐poor, or bovine serum albumin (BSA). The viability of oocyte grown in vitro was examined based on developmental markers such as chromatin configuration, granulosa cell number, histone acetylation level, and development capability to the blastocyst stage.
2. MATERIALS AND METHODS
2.1. Chemicals and media
All reagents were purchased from Nacalai Tesque (Kyoto, Japan), unless otherwise stated. For culture of OGCs, we used α‐MEM (Sigma‐Aldrich, SaintLouis, Missouri, USA) supplemented with 10 mmol/L taurine, 0.1 mAU/mL follicle‐stimulating hormone (Kawasaki Mitaka, Tokyo, Japan), 2% (w/v) polyvinylpyrrolidone‐360 (Sigma‐Aldrich), 2 mmol/L hypoxanthine (Sigma‐Aldrich), 1% (w/v) insulin transferrin selenium (Gibco BRL, Paisley, UK), 1 µg/mL 17β‐estradiol, 3 mg/mL BSA, and antibiotics. The in vitro maturation (IVM) medium was Medium 199 (Gibco) supplemented with 10% (v/v) porcine follicular fluid (pFF), 0.5 mmol/L L‐cysteine, 0.9 mmol/L sodium pyruvate, 1 mmol/L l‐glutamine, 10 ng/mL epidermal growth factor, 5% (v/v) fetal calf serum, 10 IU/mL equine chorionic gonadotropin (ASKA Pharma Co. Ltd, Tokyo, Japan), and 10 IU/mL human chorionic gonadotropin (Fuji Pharma Co. Ltd, Tokyo, Japan). In vitro culture (IVC) of embryos and oocyte activation was conducted in porcine zygote medium 3 (PZM3).12 In vitro culture of OGCs and oocyte maturation was performed at 38.5°C in an atmospheric condition of 5% CO2 and 95% air, whereas in vitro embryo culture was performed at 38.5°C in an atmospheric condition of 5% O2, 5% CO2, and 90% N2.
2.2. Collection of OGCs from early antral follicles
Ovaries were collected from prepubertal gilts at a local slaughterhouse and transported within 1 hour to the laboratory (at approximately 35°C, in phosphate buffer saline (PBS) containing antibiotics). The ovarian cortical tissues were excised from the ovarian surface under a stereomicroscope, and OGCs were collected from EAFs (0.5‐0.7 mm in diameter). OGCs containing oocytes with diameters ranging from 90 to 100 µm were then selected using a digital microscope (BZ‐8000; Keyence, Tokyo, Japan).
2.3. IVG, IVM, parthenogenetic activation (PA), and IVC
Oocyte‐granulosa cell complexes collected from EAFs were individually transferred to a well (96‐well plates, Becton Dickinson) containing 200 µL of IVG medium and cultured for 14 days. OGCs were cultured on polyacrylamide gel (PAG) set at the bottom of the culture well. Half of the medium was replaced with fresh medium, and antrum formation was examined at 4 days intervals based on their morphology (Figure 2B,C). After IVG (14 days), OGCs having an antrum cavity were subjected to IVM for 48 hours.
Figure 2.
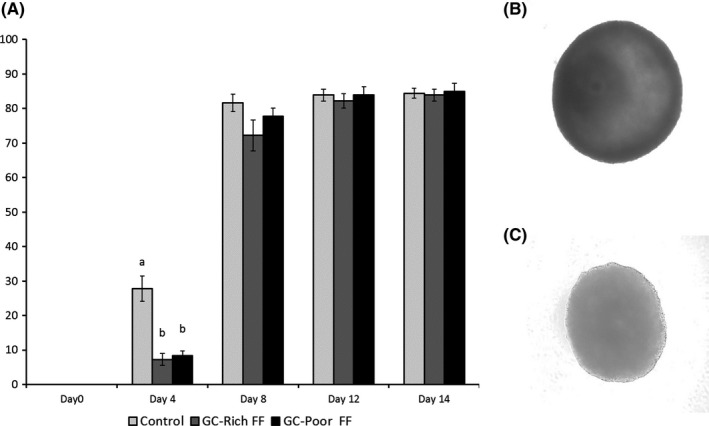
A, Antrum formation rate of oocyte‐granulosa cell complexes (OGCs) grown in vitro. OGCs were cultured in medium containing BSA (Control), 10% GC‐rich FF or GC‐poor FF. Representative picture of OGCs with antrum (Day 14) (B) and without antrum (Day 6) (C). OGCs were cultured in medium containing 10% of GC‐rich FF or GC‐poor FF for 14 days. Data are presented as mean ± standard error of the mean (SEM) of 12 replicates. ab: P < 0.05
In addition, oocyte‐cumulus cells complexes (COCs) were collected from the antral follicles (3‐5 mm in diameter) and were subjected to IVM. After IVM, oocytes were denuded from the surrounding GCs, and parthenogenetically activated in IVC medium containing 10 μg/mL ionomycin for 5 minutes, and incubated for 5 hours in PZM3 containing 10 μg/mL cytochalasin B and 10 μg/mL cycloheximide at 38.5°C. After activation, embryos were cultured for 8 days in culture medium (PZM3) and the rate of blastulation and total blastocyst cell number were determined. Blastocyst was fixed in 4% paraformaldehyde and mounted onto a microscope slide using an antifade reagent containing DAPI (ProLong Gold antifade reagent with DAPI; Invitrogen, OR, USA). The total number of cells in the blastocysts was counted using a fluorescence digital microscope (BZ‐8000; Keyence).
2.4. Preparation of 0.3% polyacrylamide gel sheet
Polyacrylamide gel (N‐methylenebisacrylamide, water, acrylamide, ammonium persulfate, and N,N,N′,N′‐Tetramethylethylenediamine) were prepared according to the general methods for Western blot analysis. Detailed information on PAG preparation for culture of OGCs derived from EAFs is described elsewhere.13
2.5. Preparation of follicular fluid used for oocyte in vitro growth and oocyte nucleic maturation
In vitro maturation medium of oocytes was supplemented with 10% (v/v) FF. The FF was aspirated from antral follicles (AFs; 3‐5 mm in diameter) of hundreds of gilts and centrifuged (10 000 g) for 20 minutes. The resulting supernatants were collected, sterilized, and stored at −20°C until use.
In vitro growth medium of OGCs was supplemented with BSA or FF collected from antral follicles (3‐5 mm in diameter) obtained from GC‐rich ovaries or GC‐poor ovaries. Ovaries were categorized based on the number of GCs present in the AFs as reported previously.11 In brief, follicular contents were carefully collected from 20 randomly selected antral follicles (3‐5 mm in diameter) of the ovaries of each of the 25 gilts, and the average number of GCs per follicle was determined for each gilt using real‐time PCR targeting one copy gene. Based on the average GC numbers, samples with the 5 highest and 5 lowest average GC counts per follicle were selected together with the corresponding FF samples (Figure 1). The categorized 5 GC‐rich and 5 GC‐poor FF samples were divided into equal amounts to create batches of granulosa cell rich‐FF (GC‐rich FF) and granulosa cell‐poor FF (GC‐poor FF). Each batch was produced from a different ovary series, and each experiment was conducted using different batches of FF.
Figure 1.
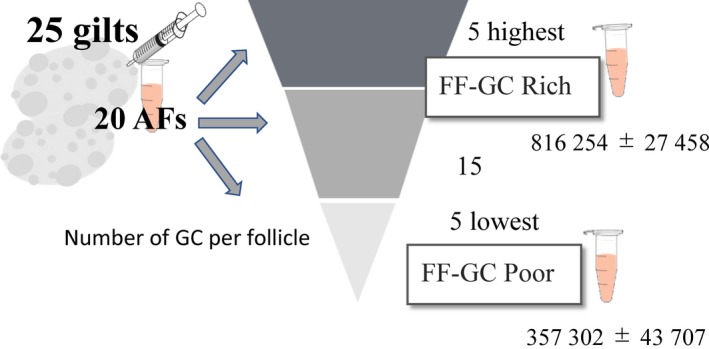
Follicular contents were carefully collected from randomly selected 20 antral follicles (3‐5 mm in diameter) of the ovaries of 25 gilts, and the average granulosa cell (GC) number per follicle was determined by real‐time PCR. Based on the average GC number, follicular fluid (FF) with the 5 highest and 5 lowest average GC number per follicle were selected. These categorized 5 GC‐rich and 5 GC‐poor FF samples were equally mixed to create a batches of GC‐rich FF and GC‐poor FF. We prepared 12 batches of GC‐rich and GC‐poor FF using different ovary, and randomly selected batch was used for each experiment. Average granulosa cell number of GC‐rich FF and GC‐poor FF was 816 254 ± 27 458 and 357 302 ± 43 707, respectively
2.6. Measurement of the number of the GCs surrounding the oocyte grown in vitro
After IVG, GCs were enzymatically dispersed (Accumax; Innovative Cell Technologies, San Diego, CA, USA) and the total number of GCs was calculated based on the volume and concentration of the cell suspensions using a hematocytometer.
2.7. Measurement of oocyte diameter
The diameter of each ooplasm was measured (x‐axis and y‐axis at 90°angle) under a digital microscope (BZ‐8000; Keyence). Averages of the x‐axis and y‐axis values were calculated as the diameter of the oocytes.
2.8. Chromatin configurations
After IVG, oocytes were denuded from OGCs and fixed in PBS containing 4% paraformaldehyde. Oocytes were stained with Hoechst 33342 (1 µg/mL, Sigma) for 15 minutes and observed under a fluorescence microscope (BZ‐8000; Keyence). According to a previous report,3 chromatin configuration was subdivided into five categories, and the prominent features of full‐grown oocytes at germinal vesicle stages were categorized as GV1 (oocytes having a nuclear membrane and intact but condensed chromatin forming a ring or horseshoe around the nucleolus). In the present study, the percentage of oocytes at the stage GV1, considered as full‐grown oocytes, was determined under a digital microscope (BZ‐8000; Keyence).
2.9. Immunostaining
After IVG, oocytes denuded from OGCs were fixed in 4% paraformaldehyde. Immunostaining was conducted as a described previously.14 The primary antibodies used were polyclonal anti‐H4K12 (1:200; Novus International Saint Charles, MO, USA); secondary antibodies were anti‐rabbit IgG Alexa Fluor 555 (1:500; Cell Signaling Technology Inc, Danvers, MA). Oocytes were mounted on a slide with an antifade reagent containing DAPI (Invitrogen, OR, USA). These were observed under a Leica DMI 6000B microscope using Leica Application Suite Advanced Fluorescence (LAS AF) software (Leica, Wetzlar, Germany), and the fluorescence intensities were quantified using ImageJ software (NIH, Bethesda, MD, USA).
2.10. Measurement of lipid in oocytes
Oocytes denuded from OGCs were fixed in 4% paraformaldehyde followed by staining with 10 μg/mL Nile Red (Wako, Osaka, Japan) for 10 minutes. After washing, oocytes were mounted onto a microscope slide with prolong gold antifade reagent with DAPI (Invitrogen). Fluorescence image of lipids was captured using a fluorescence digital microscope (BZ‐8000; Keyence), and fluorescence intensity was measured using ImageJ software (NIH, Bethesda, MD, USA).
2.11. Experimental design
In the present study, 15 OGCs derived from EAFs were allocated to different experimental groups (BSA, GC‐rich FF, and GC‐poor FF) and cultured for 14 days. IVG was repeated 12 times with differential lots of GC‐rich and GC‐poor FF. In the first 4 trials, oocytes grown in vitro were examined for their diameter, chromatin configuration and acetylation level of histone 4 K12. In addition, the number of GCs in the OGCs was counted. In the other 8 trials, OGCs developed in vitro were subjected to IVM, PA, and IVC to determine the rate of development to the blastocyst stage. In addition, IVG was repeated 3 times with differential lots of GC‐rich and GC‐poor FF in which lipid content in in vitro growth oocytes were examined.
2.12. Data analysis
All data were analyzed using ANOVA followed by post hoc Tukey's test. Percentages were arcsine transformed before analysis. The rates of GV1 were analyzed using the chi‐square test. Alpha values less than 0.05 were considered to be significantly different.
3. RESULTS
3.1. Follicular fluid delayed the timing of antrum formation
Oocyte‐granulosa cell complexes derived from EAFs were individually cultured in a medium containing 5mg/mL BSA, 10% GC‐rich FF, or 10% GC‐poor FF. At day 4 of the culture period, 27.8% of OGCs formed antrum in the medium containing BSA; this value was higher than in those cultured with either Rich FF or Poor FF (Figure 2A). At the end of the culture period (day 14), the number of OGCs forming antrum was comparable among the three groups.
3.2. Supplementation with FF increased diameter and acetylation level of histones in oocytes grown in vitro
Oocyte diameter, histone acetylation, and chromatin configuration are markers of oocyte growth.15, 16 Both GC‐rich FF and GC‐poor FF increased the diameter of oocytes grown in vitro, which was significantly greater than the oocyte diameter grown with BSA (Table 1; BSA, 120.4 ± 0.7 μm; GC‐rich FF, 128.5 ± 0.9 μm; GC‐poor FF, 126.9 ± 0.9 μm; P < 0.05). Moreover, both GC‐rich and GC‐poor FF increased the levels of acetylation of histone H4K12 when compared with the histone acetylation levels in oocytes grown in BSA (Figure 3 and Table 1; BSA, 1.0 ± 0.0; GC‐rich FF, 1.4 ± 0.1; GC‐poor FF, 1.3 ± 0.1; P < 0.05). GC‐rich FF significantly helped the progression of chromatin configuration, such that the rate of GV1 was 85.5% ± 1.8% for GC‐rich FF, which was significantly higher than the progression observed in oocytes grown with BSA (Table 1; BSA, 66.5% ± 2.1%; GC‐poor FF, 80.7% ± 3.6%; P < 0.05).
Table 1.
Effect of FF on developmental competence of oocyte grown in vitro
| FF (10%) | No. of replicates | No. of OGCs | No. of oocytes | Oocyte diameter (μm) | Acetylation of H4K12 (Mean ± SEM%) | Rate of GV1 (Mean ± SEM%) |
|---|---|---|---|---|---|---|
| Control | 4 | 60 | 51 | 120.4 ± 0.7a | 1.0 ± 0.0a | 66.5 ± 2.1a |
| GC‐rich FF | 4 | 60 | 49 | 128.5 ± 0.9b | 1.4 ± 0.1b | 85.5 ± 1.8b |
| GC‐poor FF | 4 | 60 | 51 | 126.9 ± 0.9b | 1.3 ± 0.1b | 80.7 ± 3.6ab |
Data are represented as mean ± SEMa,b; P < 0.05.
BSA, bovine serum albumin; FF, follicular fluid; GC, granulosa cell; OGCs, oocyte‐granulosa cell complexes.
Level of acetylation was determined by immunostaining normalized to the acetylation levels in control (BSA). OGCs were cultured in medium containing BSA (Control), 10% GC‐rich FF or GC‐poor FF.
Figure 3.
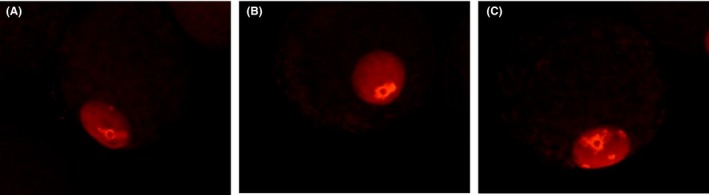
Effect of Rich FF and Poor FF on acetylation level of histone in oocytes grown in vitro. Oocyte‐granulosa cell complexes (OGCs) were cultured for 14 days in medium containing 5 mg/mL BSA (A, Control), 10% GC‐rich FF (B), and GC‐poor FF (C). Acetylation levels of H4K12 were examined by immunostaining
3.3. GC‐rich FF increased lipid content in oocytes in vitro growth oocytes
Lipid content in oocytes is another marker of oocyte competence6; therefore, we examined the effect of FF on the lipid content of oocytes grown in vitro. GC‐rich FF significantly increased the lipid content of oocytes (Figure 4A,B). Furthermore, when average GC number per follicles (3‐5 mm in diameter) and average lipid content in corresponding oocytes (average of 20 cohort oocytes) was determined for 53 gilts, there was significantly positive correlation between them (P < 0.05, Figure 4C).
Figure 4.
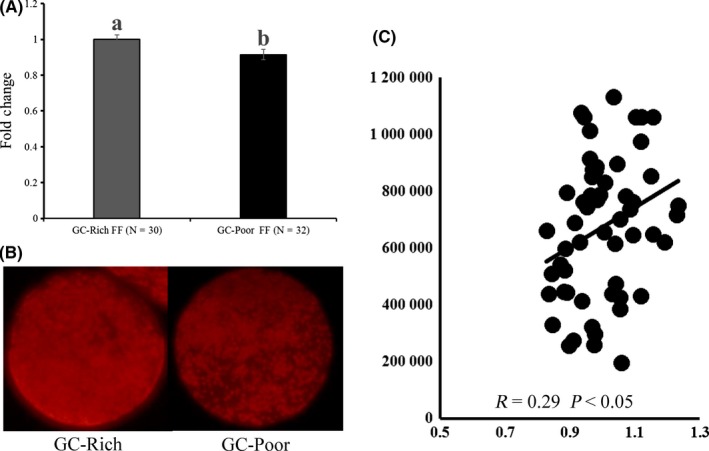
Effect of Rich‐FF and Poor‐FF on lipid content in oocytes grown in vitro (A). Oocyte‐granulosa cell complexes (OGCs) were cultured for 14 days in medium containing 10% GC‐rich FF or GC‐poor FF. Lipid content in oocytes was determined by Nile red staining (B). Average lipid content in oocytes was normalized with the lipid content of GC‐rich FF. Relationship between average granulosa cell number per large antral follicles (3‐5 mm in diameter) and average lipid content in corresponding oocytes (average of 20 cohort oocytes) for 53 gilts (C). Twenty antral follicles were aspirated to determine average granulosa cell number per follicles. All oocytes collected were stained with Nile red to determine average lipid content in oocytes
3.4. GC‐rich FF enhanced granulosa cell proliferation and assisted oocytes to develop to the blastocyst stage
In one study, OGCs collected from EAFs were reported to have 7977 ± 776 GCs.7 In our study, GC‐rich FF stimulated cellular proliferation and the GC numbers increased to 199000.0 ± 6676.4, which were significantly higher than the GC numbers observed in OGCs treated with GC‐poor FF or BSA (Figure 5; BSA, 177208.3 ± 5250.0; GC‐poor FF, 174208.3 ± 5082.4; P < 0.05). In addition, when in vitro grown oocytes were subjected to IVM followed by activation and IVC for 8 days, the highest rate of development to the blastocyst stage was observed for oocytes grown with GC‐rich FF (Table 2; BSA, 4.7 ± 2.8%; GC‐rich FF, 20.9 ± 5.8%; GC‐poor FF, 6.7 ± 3.7%; P < 0.05). When COCs derived from antral follicles were subjected to IVM, PA, and IVC, the rate of development to the blastocyst stage was 14.6 ± 2.1% (15 trials with each 15 COCs).
Figure 5.
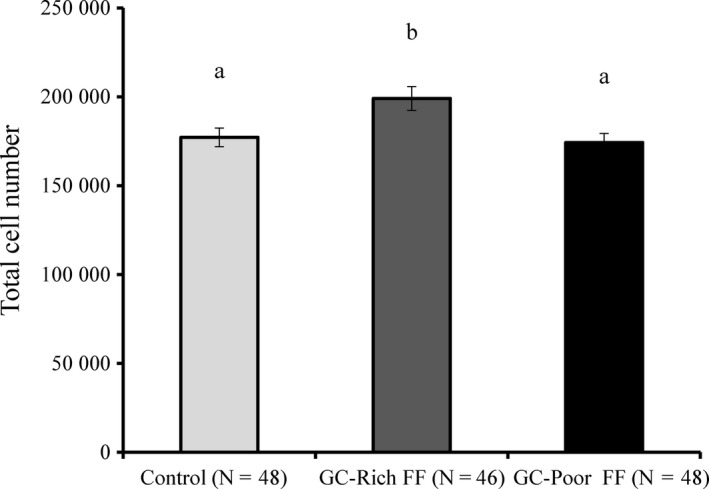
Effect of GC‐rich FF and GC‐poor FF on total cell number of OGCs grown in vitro. OGCs were cultured in medium containing BSA (Control), 10% GC‐rich FF or GC‐poor FF. Data are presented as means ± standard error of the mean (SEM) of four replicates. ab: P < 0.05
Table 2.
Effect of FF on developmental competence of oocyte grown in vitro
| FF(10%) | No. of replicates | No. of oocytes | No. of blastocysts (%) | Total cell number (Mean ± SEM%) |
|---|---|---|---|---|
| Control | 8 | 100 | 5 (5.0)a | 42.7 ± 5.9 |
| GC‐rich FF | 8 | 94 | 21 (22.3)b | 42.1 ± 3.7 |
| GC‐poor FF | 8 | 96 | 6 (6.3)a | 40.6 ± 7.5 |
Data are represented as mean ± SEMa,b; P < 0.05.
BSA, bovine serum albumin; FF, follicular fluid; GC, granulosa cell; OGCs, oocyte‐granulosa cell complexes.
OGCs were cultured in medium containing BSA (Control), 10% GC‐rich FF or GC‐poor FF.
4. DISCUSSION
The results of the present study revealed that supplementation of culture medium with FF delayed antrum formation but increased the diameter and levels of histone H4K12 acetylation of oocytes grown in vitro. Furthermore, GC‐rich FF stimulated GC proliferation and changes in chromatin configuration of oocytes during in vitro culture, and drastically improved the developmental ability to the blastocyst stage.
The diameter, acetylation levels, and chromatin configuration are known to be useful markers of oocytes growth.7, 10 Our study showed that GC‐rich FF enhanced the progression of chromatin configuration which mirrors the high ability of GC‐rich FF to support oocyte growth in vitro. Histone acetylation is established during oocyte growth in mouse,15 and high acetylation level of H4K12 in GV stage oocytes has been reported in cows.17, 18 Acetylation levels of the GV stage of an oocyte have been reported to be linked to the oocyte quality. It is reported that oocytes of aged mice have low acetylation levels of H4K12.5 Moreover, it is suggested that the low acetylation levels in the GV stage oocytes are associated with high acetylation levels at the M2 stage, which may cause some abnormalities in oocytes, such as chromatin abnormalities.5 Taken together, this study shows that supplementation of in vitro growth medium with FF enhanced acetylation levels and the diameter of oocytes. The result indicates that irrespective of the FF categories (GC‐rich or GC‐poor) FF contains certain components that enhance oocyte acetylation and growth. Oocytes accumulate lipid during follicle development, and the lipid content reflects oocyte developmental competence.6 We are the first to show significant positive correlation between the number of GC in antral follicle and lipid content in enclosed oocytes. Furthermore, GC‐rich FF assisted accumulation of lipids in oocytes grown in vitro. The result indicates GC‐rich FF improved the quality of oocytes grown in vitro.
Although Metoki et al18 reported the beneficial effects of FF on oocyte grown in vitro, the study was focused on the origin of FF in large and small antral follicles. Our study is the first of its kind to demonstrate that the ability of FF to support oocyte growth and its association with granulosa cell number in the follicles. Furthermore, supplementation of in vitro growth medium with GC‐rich FF resulted in a 20% increase in the rate of development of oocytes to the blastocyst stage, which was similar to or higher than that of oocytes derived from large follicles (14.6%). In addition, it was observed that when OGCs derived from EAFs were cultured in optimal conditions, the quality of oocytes grown in vitro was far superior to those collected from germinal antral follicles. The results suggest that some key factors, which can stimulate in vitro oocyte growth, are present in GC‐rich FF. Therefore, we conducted RNA‐seq experiments using GCs collected from GC‐rich and GC‐poor ovaries (DDJB database: accession number DRA00632). In this analysis, we obtained thousands of genes differentially expressed in GCs between the GC‐rich and GC‐poor ovaries.11 Subsequent Ingenuity Pathway Analysis showed upstream regulators; for example, IGF, IGF‐R, FSH, cAMP, GATA4, E2, and EGF‐ERK pathways. Thus, these data suggest that certain components present in GC‐rich FF influence the above‐mentioned signaling, but elucidation of these factors is a subject of future studies.
In conclusion, FF selected based on the granulosa cell number improved the growth of oocyte in vitro, and the oocytes had comparable developmental ability to the oocytes grown in vivo. In addition, FF has certain components to help bring about changes in histone acetylation and oocyte growth.
DISCLOSURES
Conflict of interest: All authors declare no conflict of interest. Human rights statements and informed consent: This article does not contain any studies with human subjects. Animal study: In this study, porcine ovaries were collected from a slaughterhouse. The ovaries were discarded and were not used for edible meat; hence, this study was approved by the Ethical Committee for Animal Experiment of Tokyo University of Agriculture.
Shibahara H, Ishiguro A, Shirasuna K, Kuwayama T, Iwata H. Follicular factors determining the developmental competence of porcine oocyte. Reprod Med Biol. 2019;18:256–262. 10.1002/rmb2.12269
Funding information
This work was supported by Grant‐in‐Aid for Scientific Research C (KAKENHI, grant number: 16K07996) from the Japan Society for the Promotion of Science.
REFERENCES
- 1. Bui HT, Van Thuan N, Kishigami S, et al. Regulation of chromatin and chromosome morphology by histone H3 modifications in pig oocytes. Reproduction. 2007;133:371‐382. [DOI] [PubMed] [Google Scholar]
- 2. De La Fuente R. Chromatin modifications in the germinal vesicle (GV) of mammalian oocytes. Dev Biol. 2006;292:1‐12. [DOI] [PubMed] [Google Scholar]
- 3. Sun XS, Liu Y, Yue KZ, Ma SF, Tan JH. Changes in germinal vesicle (GV) chromatin configurations during growth and maturation of porcine oocytes. Mol Reprod Dev. 2004;69:228‐234. [DOI] [PubMed] [Google Scholar]
- 4. Jeong WJ, Cho SJ, Lee HS, et al. Effect of cytoplasmic lipid content on in vitro developmental efficiency of bovine IVP embryos. Theriogenology. 2009;72:584‐589. [DOI] [PubMed] [Google Scholar]
- 5. Manosalva I, González A. Aging alters histone H4 acetylation and CDC2A in mouse germinal vesicle stage oocytes. Biol Reprod. 2009;81(6):1164‐1171. [DOI] [PubMed] [Google Scholar]
- 6. Niu Y, Wang C, Xiong Q, et al. Distribution and content of lipid droplets and mitochondria in pig parthenogenetically activated embryos after delipation. Theriogenology. 2015;83:131‐138. [DOI] [PubMed] [Google Scholar]
- 7. Oi A, Tasaki H, Munakata Y, Shirasuna K, Kuwayama T, Iwata H. Effects of reaggregated granulosa cells and oocytes derived from early antral follicles on the properties of oocytes grown in vitro. J Reprod Dev. 2015;61:191‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Itami N, Munakata Y, Shirasuna K, Kuwayama T, Iwata H. Promotion of glucose utilization by insulin enhances granulosa cell proliferation and developmental competence of porcine oocyte grown in vitro. Zygote. 2017;25:65‐74. [DOI] [PubMed] [Google Scholar]
- 9. Munakata Y, Ichinose T, Ogawa K, et al. Relationship between the number of cells surrounding oocytes and energy states of oocytes. Theriogenology. 2016;86:1789‐1798. [DOI] [PubMed] [Google Scholar]
- 10. Sugiyama M, Sumiya M, Shirasuna K, Kuwayama T, Iwata H. Addition of granulosa cell mass to the culture medium of oocytes derived from early antral follicles increases oocyte growth, ATP content, and acetylation of H4K12. Zygote. 2016;24:848‐856. [DOI] [PubMed] [Google Scholar]
- 11. Munakata Y, Ueda M, Kawahara‐Miki R, et al. Follicular factors determining granulosa cell number and developmental competence of porcine oocytes. J Assist Reprod Genet. 2018;35:1809‐1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yoshioka K, Suzuki C, Onishi A. Defined system for in vitro production of porcine embryos using a single basic medium. J Reprod Dev. 2011;57:9‐16. [DOI] [PubMed] [Google Scholar]
- 13. Munakata Y, Kawahara‐Miki R, Shirasuna K, Kuwayama T, Iwata H. Polyacrylamide gel as a culture substrate im malce proves in vitro oocyte growth from porcine early antral follicles. Mol Reprod Dev. 2017;84:44‐54. [DOI] [PubMed] [Google Scholar]
- 14. Takeo S, Kawahara‐Miki R, Goto H, et al. Age‐associated changes in gene expression and developmental competence of bovine oocytes, and a possible countermeasure against age‐associated events. Mol Reprod Dev. 2013;80:508‐521. [DOI] [PubMed] [Google Scholar]
- 15. Kageyama S, Liu H, Kaneko N, Ooga M, Nagata M, Aoki F. Alterations in epigenetic modifications during oocyte growth in mice. Reproduction. 2007;133:85‐94. [DOI] [PubMed] [Google Scholar]
- 16. Sun MJ, Zhu S, Li YW, et al. An essential role the intra‐oocyte MAPK activity in the NSN‐to‐SN transition of germinal vesicle chromatin configuration in porcine oocyte. Sci Rep. 2016;6:23555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maalouf WE, Alberio R, Campbell KH. Differential acetylation of histone H4 lysine during development of in vitro fertilized, cloned and parthenogenetically activated bovine embryos. Epigenetics. 2008;3. [DOI] [PubMed] [Google Scholar]
- 18. Metoki T, Iwata H, Itoh M, et al. Effects of follicular fluids on the growth of porcine preantral follicle and oocyte. Zygote. 2008;16:239‐247. [DOI] [PubMed] [Google Scholar]


