Abstract
Background
Germ cells represent one of the typical cell types that moves over a long period of time and large distance within the animal body. To continue its life cycle, germ cells must migrate to spatially distinct locations for proper development. Defects in such migration processes can result in infertility. Thus, for more than a century, the principles of germ cell migration have been a focus of interest in the field of reproductive biology.
Methods
Based on published reports (mainly from rodents), investigations of germ cell migration before releasing from the body, including primordial germ cells (PGCs), gonocytes, spermatogonia, and immature spermatozoon, were summarized.
Main findings
Germ cells migrate with various patterns, with each migration step regulated by distinct mechanisms. During development, PGCs actively and passively migrate from the extraembryonic region toward genital ridges through the hindgut epithelium. After sex determination, male germline cells migrate heterogeneously in a developmental stage‐dependent manner within the testis.
Conclusion
During migration, there are multiple gates that disallow germ cells from re‐entering the proper developmental pathway after wandering off the original migration path. The presence of gates may ensure the robustness of germ cell development during development, growth, and homeostasis.
Keywords: germ cell migration, gonocyte, gut endoderm, primordial germ cell, spermatogonia
1. INTRODUCTION
Gametes are formed through multiple steps of germ cell migration during development, growth, and adulthood.1 Germ cell migration is of undoubted importance as germ cells must translocate from an original location to spatially distinct locations for survival, fate maintenance, and differentiation. Accordingly, defects in germ cell migration can result in infertility. For more than a century, patterns and mechanisms controlling successful migration of germ cells have been a focus of interest in the field of reproductive biology.1, 2, 3, 4, 5 During migration, there are multiple migration steps that disallow cells from re‐entering the proper developmental pathway after wandering off the original migration path. (In this review, we refer such a migration step as a “gate” for germ cell development.) Herein, we overview the current understanding of mammalian germ cell migration during development, growth, and homeostasis.
2. TECHNOLOGICAL DEVELOPMENT FOR THE ANALYSIS OF GERM CELL MIGRATION
Historically, the development of analytical technologies has enabled the investigation of new aspects of germ cell behavior. In the 1960s, details of germ cell morphologies were described extensively through the use of transmission electron microscopy (TEM), which can provide profound insights for the regulation of cell behavior. Although data provide a static snapshot of a fixed sample, TEM studies revealed pseudopod formation, intracellular organelle distribution, cell polarity, and cell adhesion, all of which are essential components of germ cell migration.6, 7, 8, 9 In most developmental stages, germ cells cannot be reliably distinguished within the embryo because of a lack of prospective germ cell markers. For primordial germ cells (PGCs), TNAP staining can be combined with TEM analysis to enable PGCs to be distinguished from surrounding somatic cells with high reliability.4, 8 Observation of an electron‐dense organelle, nuage, by TEM observation, was found to be a morphological characteristic of germ cells during some steps of spermatogenesis and oogenesis. Nuage is currently proposed to be a center of RNA metabolism, retrotransposon regulation, and interplay with mitochondria.10, 11 To obtain topological information of migrating germ cells, three‐dimensional (3D) morphological analysis has been applied. For germline cells, 3D reconstruction of confocal images revealed the translocation of spermatogenic syncytia through the blood‐testis barrier (BTB).12 Emerging technologies, such as serial block‐face scanning electron microscopy for the analysis of 3D ultrastructure,13, 14 may also be useful to investigate important links between germ cell structure and behavior.
Because cell migration involves temporal components, direct observation of live cell behavior is also key to understanding germ cell movement patterns. First, studies using in vitro cell/organ culture systems provide the knowledge about regulatory mechanisms of germ cell migration such as cell‐autonomous motility, affinity to extracellular matrices, cell‐cell interaction, and requirement of extracellular growth factors.15, 16, 17, 18, 19 Since 2000, live‐imaging analyses combined with fluorescent protein expression driven by the promoter of a specific marker gene have been reported for PGCs and spermatogonia.20, 21, 22, 23 Because of difficulties associated with intrauterine observation of PGCs, an embryonic slice culture method (mainly developed in the field of brain science) was applied for short‐term observations of PGCs.20, 21 For spermatogonia, an intravital testis live‐imaging method to directly observe spermatogonial behaviors was established.22, 23, 24 Recent single‐cell transcriptome analyses have suggested that germ cell states are far more heterogeneous than originally thought.25, 26, 27, 28 Thus, determining germ cell morphologies and behaviors must be combined with information about cell states to fully understand the dynamics of germ cell migration.
3. GERM CELL MIGRATION DURING DEVELOPMENT
Mouse PGCs have provided abundant knowledge about mammalian germ cell migration in terms of patterns and regulatory mechanisms. Germ cells initially form outside of the gonad.4, 5 In mice, a small number of cells begin to express Blimp1/Prdm1, Prdm14, and Tnap (tissue nonspecific alkaline phosphatase) within the proximal epiblast by extraembryonic bone morphogenic proteins at around 7.25 days post coitum (dpc), and thereby, these cells are recognized as PGCs29, 30 From the timing and the location of PGC specification, a pair of genital ridges are formed 3 days after 10.0 dpc at the distant location (coelomic epithelial region) within the developing embryo.20, 31 Therefore, the PGCs are needed to migrate from original location where they specified toward genital ridges over several days. Because improper PGC movement results in infertility and poses a risk of future tumorigenesis, extra‐gonadal PGC migration should be controlled by a robust mechanism.
3.1. Active migration toward endoderm epithelium after specification (Gate 1)
From 7.5 to 8.25 dpc in mice, PGCs form pseudopods and migrate from the primitive streak toward the outermost endodermal epithelium (Figure 1A).8, 20 Thereafter, PGCs sequentially enter the definitive endoderm layer at the central area of the most posterior embryonic endoderm.32 This first migration event has been suggested to be regulated by cellular repulsion between mesodermal cells and PGCs through molecularly repulsive interactions between different members of interferon‐induced transmembrane (IFITM) proteins.33 However, deletion of Ifitm family member genes reportedly did not result in any PGC migration defects, suggesting that IFITMs are not essential for PGC migration, and therefore, other molecules may be redundantly involved in the onset of PGC migration.34 Even if this repulsive mechanism is involved in part, how PGCs recognize the direction of embryonic endoderm remains unclear. Notably, as differentiating endodermal progenitor cells also migrate into the outermost endodermal layer through the primitive streak,35, 36 a close molecular/cellular interaction between endoderm progenitor cells and PGCs may facilitate their concerted movement. In mice, PGCs that fail to enter the embryonic endoderm move to extraembryonic base of allantois, whereby they are unable to contribute to germline development.20 Thus, the initial PGC migration to enter the embryonic endoderm may act as the first gate of proper germ cell development (Figures 1A and 2).
Figure 1.
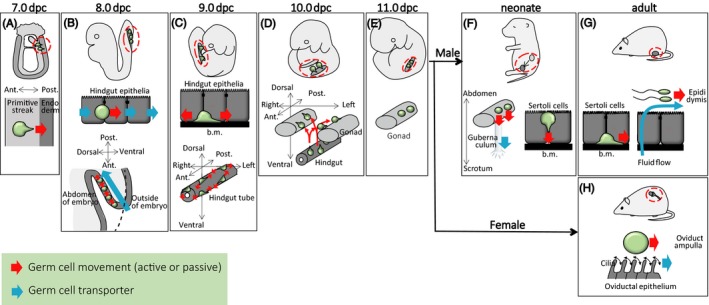
Patterns and mechanisms of germ cell migration during development, growth, and homeostasis. (A‐H) Various germ cell migration steps during embryonic (A‐E), growing (F), and adult (G, H) stages. Green circles indicate germ cells, with or without pseudopod to distinguish active and passive migration processes, respectively. Red dotted circles indicate germ cell location within the body. Red arrows indicate germ cell movement, while blue arrows indicate the mechanism of germ cell transporter. ant, anterior; b.m., basement membrane; dpc, days post coitum; post, posterior
Figure 2.
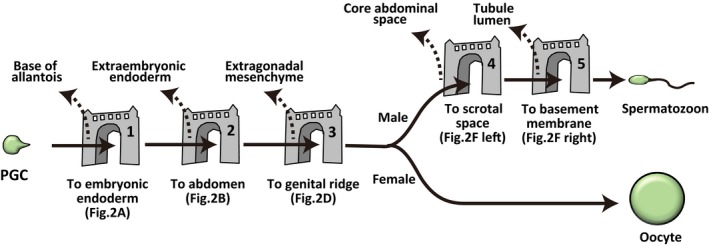
Gates for germ cell development. A cartoon shows possible major gates during germ cell development in mice. Germ cells need to pass through all gates for proper germ cell development (of course, female PGCs do not need to migrate through male‐specific gates). Solid arrows indicate proper germ cell migration, while dotted arrows indicate ectopic germ cells which usually undergo cell death or arrest the differentiation process. Note that scrotal space of rodents is not anatomically separated cavity (see text)
Primate embryo exhibits a planar structure during the perigastrulation period, which is largely different from mouse embryo with an elongated cup‐shaped structure.37 Recent study suggested that cynomolgus monkey PGCs are specified in the nascent amnion around the time of gastrulation of epiblast.38 Thereafter, cynomolgus PGCs become to localize within the endodermal layer.38 Thus, although the original locations of PGCs are different, the initial PGC migration to endoderm might be conserved between mouse and monkey.
3.2. Passive movement with hindgut morphogenesis (Gate 2)
How do PGCs migrate from the embryonic outermost endodermal layer to the abdominal space? Upon entering the embryonic endodermal layer, PGCs adopt a round shape with occasional small pseudopodial projections.7, 8, 21, 32 PGCs at this stage adhere to the basolateral region of endodermal epithelial cells, implying a close cellular interaction between endodermal epithelia and PGCs.7, 8, 32 Thereafter, PGCs are incorporated into the ventral wall of the hindgut tube through gastrulation.1, 31 To the best of our knowledge, there is no report of successful live imaging of PGC behavior from 8.0 to 8.5 dpc because dynamic morphogenetic changes occur in the embryonic body.21 However, considering the absence of obvious pseudopods on PGCs and dynamic morphogenesis of hindgut endoderm, PGCs might be transported by morphogenetic collective movement of the endoderm layer, probably through conveyer‐belt–like transportation (Figure 1B).1 Reports indicating that the emergence of a large number of ectopic PGCs in the extraembryonic visceral endoderm of Sox17 (SRY‐related HMG‐box 17)‐deficient mouse embryos, which exhibits defective differentiation of definitive endoderm, suggest that proper endodermal differentiation and morphogenesis are required for proper PGC movement at an early stage of gastrulation.32, 39 Thus, the PGC movement to enter the abdominal space from the outermost endoderm layer associated with hindgut morphogenesis may act as a second gate for proper germ cell development (Gate 2; Figures 1B and 2).
Conveyer‐belt–like PGC transportation results in dispersed distribution of PGCs along the anterior‐posterior (AP) axis of ventral side of hindgut (Figure 1B lower). The AP length of this PGCs distribution is consistent to the AP length of genital ridge.31 These findings suggest the importance of passive PGC migration by gut morphogenetic movement in allowing PGCs to colonize the entire length of genital ridges.
3.3. Active movement within the gut endoderm
At around 9.0 dpc, PGCs start to actively migrate within the epithelium of the gut endodermal tube in a seemingly random manner (Figure 1C), although the underlying molecular mechanism remains unclear.21 At this stage, PGCs attach to the basement membrane by forming pseudopodia, implying the importance of PGC attachment to the basement membrane for the onset of active PGC migration.8 This may be associated with endodermal epithelial differentiation to form the basement membrane.8, 40 At this stage, randomly migrating PGCs diffuse from the ventral side to the entire (right‐left and dorsal‐ventral) circumference of the hindgut tube (Figure 1C lower).21 Taken together, the combination of passive transportation (Figure 1B) and subsequent active random migration (Figure 1C) may cooperatively support widespread distribution of PGCs within the hindgut tube, which is a prerequisite for proper germ cell migration toward the entire region of left/right gonads.
During migration within the gut endoderm, epigenetic changes such as upregulation of H3K9diMe, downregulation of H3K27triMe, cell cycle arrest at G2 stage, and RNA polymerase II‐dependent transcriptional repression occur in PGCs.41, 42, 43 Reportedly, ectopic PGCs in the extraembryonic visceral endoderm arising from the failure of proper PGC movement undergo changes in H3K27triMe and H3K9diMe with normal manner at a bulk level, suggesting that epigenetic changes in PGCs proceed in a cell‐autonomous manner.32 In the future, it will also be interesting to analyze causal relationships between cell motion patterns, cell cycle progression, and transcriptional activity.
Primordial germ cell migration through hindgut appears to be widely conserved among mammals. For example, cynomolgus monkey PGCs localize within the ventral side of epithelial wall of hindgut in embryo at early somite stage.38 Thereafter, cynomolgus PGCs become to distribute entire circumference of hindgut tube.38 On the other hand, in avian embryos, the PGCs use the vascular system as a vehicle to transport them to the region near the genital ridge.44, 45
3.4. Active movement toward genital ridges (Gate 3)
At around 10.0 dpc in mice, PGCs leave the gut tube and migrate toward bilateral genital ridges through the dorsal mesentery (Figure 1D).21 Morphologically, most PGCs at this stage exhibit a polarized shape with pseudopodia.8 Live‐imaging observations suggest that directional PGC movement occurs at this stage.21 Moreover, after leaving the hindgut, PGCs at this stage particularly spread and actively move in vitro.15 These data support the possibility that PGC locomotion toward genital ridges through the dorsal mesentery is an active process with organized directionality, rather than passive transportation or random migration. Dynamic adhesion to the extracellular matrix is important for PGC migration. Notably, the dorsal mesentery (the region through which PGCs migrate) is rich in fibronectin.46 In addition, laminin, type IV collagen, perlecan, and heparin sulfate are observed on the basement membrane underlying the coelomic epithelium.47, 48 All or a part of these extracellular matrices may provide a scaffold for long‐range PGC migration.
Mechanisms regulating the directionality of PGC active migration continue to be a focus of the field. One of the important chemokine‐receptor combinations controlling PGC migration toward the genital ridge involves stromal‐derived factor‐1 (SDF1; also known as CXCL12) and its G protein‐coupled receptor chemokine (CXC motif) receptor 4 (CXCR4).49, 50 SDF1 is expressed by cells in the genital ridges and surrounding somatic cells, while CXCR4 is expressed by PGCs. Deletion of either SDF1 or CXCR4 causes defects in normal colonization of PGCs within the genital ridges, suggesting that SDF1/CXCR4 interaction is required for the normal colonization of PGCs to genital ridge.49, 50 However, it remains to be clarified whether SDF1/CXCR4 interaction on PGCs plays a role in cues of directionality or motility activation.
Gene knockout experiments have demonstrated the involvement of c‐Kit and its ligand steel in PGC migration, whereby they play a role in PGC survival and proliferation.51, 52, 53 In addition, Wnt5a‐mediated Ror2 activation enhanced the polarity and motility of migrating PGCs as a cell‐autonomous motility regulator.54, 55 During PGC migration in dorsal mesentery, increased expression of E‐cadherin in PGCs might be involved in PGC migration and survival through cadherin‐mediated adhesions of PGCs to one another.18, 56 These molecules may cooperatively support the robust PGC migration toward the genital ridge.
After arriving at the genital ridge, PGCs become immobile and lose their pseudopodia (Figure 1E), although the mechanism underlying suppression of motility remains largely unclear. One possible mechanism was suggested to be downregulation of CXCR4 expression by PGCs.49 Importantly, PGCs that fail to enter the genital ridge remain in the midline region and are mostly eliminated by cell death.21 Thus, the directional PGC migration from the gut to genital ridges may act as a third gate for the proper germ cell development (Gate 3; Figures 1D and 2).
4. MIGRATION OF MALE GERMLINE CELLS AFTER BIRTH
4.1. Testis descent (Gate 4)
Movement of a pair of testes within the body is called the descent of testis. This process passively transports all germ cells from a core abdominal space to scrotal cavity (in rodents, scrotal cavity is not separated from abdominal space anatomically) by shortening of the gubernaculum in mammals with minor exceptions such as elephant, platypus, and dolphin (Figure 1F).57, 58, 59, 60 Hormones such as insulin 3, testosterone, Müllerian‐inhibiting substance, and relaxin are involved in this gubernaculum development.60, 61 In most mammals, proper spermatogenesis requires testicular location within a scrotal cavity (in particular, a temperature slightly lower than body core temperature in scrotal cavity is thought to be an important), because the failure of testis decent causes hypospermatogenesis (cryptorchidism).60, 62, 63, 64 Thus, the germ cell passive movement from core abdominal to scrotal environments may act as a forth gate for proper germ cell development (Gate 4; Figures 1F and 2).
4.2. Directional migration of gonocytes toward the basement membrane (Gate 5)
After sex determination, the testis cord becomes recognizable by the formation of a basal membrane, which provides the structural basis for a future seminiferous tubule.65 In mice, the cell cycle progression of PGCs is arrested at a later stage of development.66 Around the time of birth, PGCs become to be called “gonocytes” (also known as prespermatogonia or prospermatogonia).65, 67, 68, 69 The non‐proliferating phase of gonocytes continues until the neonatal period. At birth, they exhibit a round shape and are separated from the basement membrane by immature Sertoli cells.16, 68 However, after a couple of days, some gonocytes begin to form cytoplasmic processes toward the periphery of the tubule, whereby they make contact with the basement membrane (Figure 1F).6, 70 Yet, how gonocytes sense the peripheral region of seminiferous tubules and acquire motility remain open questions.
At the time when germ cells attach to basement membrane, cells become “spermatogonia.” It is proposed that gonocytes follow two distinct fates: direct differentiation that contributes to first‐round spermatogenesis or maintenance of a stem cell fate supporting spermatogenesis after the second round.66, 71 This fate selection has been suggested to be closely related to the seminiferous epithelial cycle.71 Thus, the arrival location of migrating gonocyte might be related to gonocyte fate selection.
Centrally remaining cells are thought to eventually degenerate.70, 72, 73 Moreover, resumption of cell division mostly occurs after attachment to the basement membrane, suggesting the importance of the environment on the basement membrane for the progression of male germ cell development.74, 75 Therefore, the germ cell active migration to the periphery of tubules can be considered as a fifth gate for proper germ cell development (Gate 5; Figures 1F and 2).
4.3. Spermatogonial migration
During the reproductive period, mammalian sperm production is supported by spermatogenic stem cells that achieve a balance between self‐maintenance and differentiation into spermatozoa.76 A transplantation assay suggested that spermatogenic stem cell function resides within the undifferentiated spermatogonia, which contain singly isolated cells (called Asingle) and syncytia.77 While undifferentiated spermatogonia were shown to have elongated structure on the basement membrane,9 their behaviors had not been observed directly until a decade ago (Figure 3). Undifferentiated spermatogonia are sparsely distributed across the basement membrane with a small bias near vasculature and interstitium.22, 78 Intravital live‐imaging analyses showed that GFRα1‐EGFP+ and Ngn3‐EGFP+ spermatogonia, a distinct subpopulation of undifferentiated spermatogonia, migrate on the basement membrane (Figure 1G).22, 23 Dovere et al19 suggested that glial cell line‐derived neurotrophic factor induces directional movement of spermatogonia in culture. Thus, it is important to understand how this molecule controls spermatogonial migration in vivo. As cell migration can alter the surrounding microenvironment, spermatogonial migration may be related to cell fate behavior through alteration of external regulation. A recent study suggested that migrating spermatogenic stem cells tune their self‐renewal and differentiation in response to fibroblast growth factor consumption on the basement membrane,79 highlighting the importance of spermatogonial migration to support spermatogenesis. However, at present, patterns, regulatory mechanisms, and biological significance of spermatogonial migration are still largely unknown. Considering the long‐term continuity of spermatogenesis, spermatogenic stem cell migration is the longest migration process of germline development. This active migration of undifferentiated spermatogonia in mouse testis is in stark contrast to anchored (immobile) germline stem cells in Drosophila testis.80 Thus, from an evolutionary point of view, the mouse spermatogonial migration can be an intriguing model in the field of germline stem cell biology.
Figure 3.
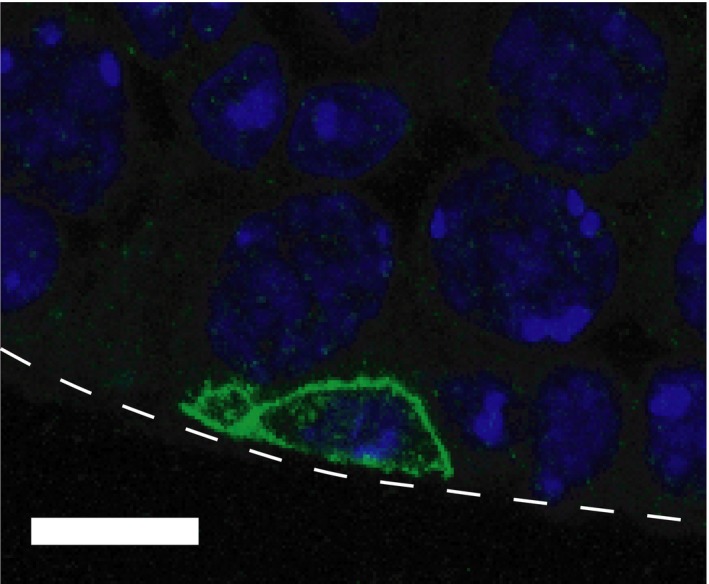
Undifferentiated spermatogonium on the basement membrane in adult mouse testis. A confocal image of GFRα1+ spermatogonium (green) and nuclear (blue) in C57BL6 adult (3‐mo‐old) mouse testis. GFRα1+ cell was visualized by immunohistochemistry by using anti‐GFRα1 antibody and Alexa488‐conjugated anti‐goat IgG antibody with a nuclear staining by Hoechst 33342, following the procedures described in a previous papers.23, 32 White dotted line indicates a periphery of the seminiferous tubule. Bar indicates 10 μm
4.4. Sperm transportation from testis to epididymis
As spermatogenesis proceeds, spermatogenic cell syncytia slowly translocate from basal to adluminal compartments of the seminiferous epithelium through the BTB.12 After the completion of spermatogenesis, spermatozoa are released from the epithelium to the tubular luminal space. Importantly, at this time, spermatozoa have not acquired motility yet, although a sperm tail has already formed. Instead, spermatozoa are thought to be passively transported by luminal fluid flow, which is generated by “water uptake” of Sertoli cells into the tubule lumen and “water outflow” by epididymal epithelial cells (Figure 1G).81 In addition, the contraction of peritubular myoid cells surrounding the seminiferous tubule is also suggested to support sperm transportation by pushing the tubule in a peristalsis‐like manner.82, 83 However, how these two mechanisms work to correctly transport spermatozoa to the epididymis remains an open question. Importantly, spermatogenesis is disrupted soon after the blockage of luminal flow by artificial efferent duct ligation or obstruction of flow by introducing a latex plug into the tubule, suggesting that the intratubular microenvironment formed by luminal flow is important not only for sperm transportation, but also for spermatogenesis.81, 84 Beyond the proposed role of tubular flow for spermatogenesis, molecular regulation and/or physical stress control mechanisms (such as osmolality and shear stress) remain largely unknown. Further study is needed to understand causal relationships between specific molecules and spermatogenesis.
During sperm transportation through epididymis, spermatozoa gradually acquire the ability to move progressively and be able to capacitate in the female reproductive tract.85, 86, 87, 88, 89, 90 Thus, sperm passive movement within the male reproductive tract is essential for successful fertilization. Taken together, the role of sperm movement from testis to epididymis involves functional modification in addition to cell transportation.
5. FEMALE GERMLINE
After sex determination and proliferation, female PGCs initiate meiosis and form primordial follicles in the cortex of the ovary.91 Primordial follicles become active and undergo folliculogenesis at regular intervals initiating at around birth and continuing throughout adulthood. Once activated, follicles and oocytes in a cohort either grow to maturation and ovulation or undergo atresia. In general, mammalian ovaries can release an oocyte from wide area of ovarian surface. As a unique system, horse ovary can undergo ovulation only at a specific narrow region on the ovarian surface (called ovulation fossa).92 While female PGCs and oocytes appear to lack the prominent motion in the cortex of ovary, emerging live‐imaging techniques to observe both germ cells and surrounding somatic cells (such as vasculature) will be useful to reveal the mechanisms of female germ cell development.93
6. CELL MOVEMENTS WITHIN THE FEMALE REPRODUCTIVE TRACT
Finally, because the main focus of this paper is germ cell development, we briefly introduce the key migration events of ejaculated/ovulated gametes and preimplantation embryos. Of course, these movements are essential for the success of fertilization and implantation.
Oocytes cannot actively move because they are coated by a hard structure consisting of a glycoprotein called zona pellucida. It was suggested that ovulated oocytes are passively transported by oviductal luminal flow, which is generated by ciliary movements of epithelia (Figure 1H).94, 95, 96 The key mechanism involves organized directionality of cilia movements of the oviductal epithelium, which is regulated by planar cell polarity.97
Spermatozoa actively and passively move in uterus, uterotubal junction, and oviduct in response to several extracellular signals that control sperm migration and fertilization capacity. For detailed patterns and mechanisms of sperm migration for successful fertilization in the female reproductive tract of mouse and domestic animals, see previous excellent reviews.98, 99
After fertilization, embryos move to implant on the uterine wall. Embryos of multiple‐conception animals move dynamically within the uterus horn to achieve an even distribution of embryos. Notably, embryos of angulates can move extensively throughout both the left and right sides of uterine horns, regardless of their elongated morphologies.100, 101, 102 Because intrauterine movement and distribution are highly related to the efficiency of nutrition and oxygen supplies from mother to embryos, this phenomenon is important for the production efficiency of experimental and industrial animals. However, early embryonic motion dynamics and related regulatory mechanisms remain largely unknown. While intra‐oviductural and intrauterine events are currently difficult to analyze in vivo, continued development of analytical technology will progress this research in the future.
7. CONCLUSION
As summarized, germ cells actively and passively migrate to pass through multiple gates for proper germ cell development. At some developmental stages, germ cells adopt common active migration mechanisms, similar to a typical non‐epithelial cell.103, 104 However, in other developmental stages, germ cell migration follows other mechanisms, such as passive transportation by gut endodermal morphogenesis and luminal fluid flow. Thus, the phenomena of different germ cell migration paths provide unique models for understanding the principles of cell migration in the field of biology.
We would emphasize that mammalian germ cell development is accompanied with their migration through multiple gates (Figures 1 and 2). As we overviewed, there are some other migration steps (such as spermatogonial motion) during germ cell development. In these migration steps, there may also be unidentified gates. Importantly, germ cells are needed to migrate through all gates properly during development, growth, and homeostasis. If germ cells fail to enter the gate, ectopic germ cells cannot return to the original road of their migration, and therefore, they cannot differentiate properly. Following these findings, during migration, multiple gates may ensure the robustness of germ cell development. Elucidation of molecular/cellular identity of gates during germ cell migration will provide the new insight for the mechanisms of germ cell development.
In conclusion, although mechanisms underlying the regulation of germ cell migration appear to be highly variable and complex, the existence of multiple gates during germ cell development may ensure the robustness of germ cell migration.
DISCLOSURES
Conflict of interest: Mizuho Kanamori, Kenta Oikawa, Kentaro Tanemura, and Kenshiro Hara declare that they have no conflicts of interest. Human rights statement and informed consent: In this review, the authors did not conduct any experiments using human‐derived materials. Animal studies: Animal experiment in this review is approved by the institutional animal care and use committee of the Tohoku University (2017AgA‐018).
ACKNOWLEDGMENTS
The authors express the deepest and sincere gratitude to Dr. Yoshiakira Kanai and Dr. Shosei Yoshida for the support and encouragement for this work. A part of this work was funded by JSPS KAKENHI (26450453, 15H01523, 19H05237).
Kanamori M, Oikawa K, Tanemura K, Hara K. Mammalian germ cell migration during development, growth, and homeostasis. Reprod Med Biol. 2019;18:247–255. 10.1002/rmb2.12283
REFERENCES
- 1. Molyneaux K, Wylie C. Primordial germ cell migration. Int J Dev Biol. 2004;48:537‐544. [DOI] [PubMed] [Google Scholar]
- 2. Ruvaschkin W. Zur Frage von der Entstehung der Keimzellen bei Saugetierembryonen. Anat Anz. 1908;32:222‐224. [Google Scholar]
- 3. Simkins CS. Origin and migration of the so‐called primordial germ cells in the mouse and rat. Acta Zool. 1923;4:241‐278. [Google Scholar]
- 4. Chiquoine AD. The identification, origin, and migration of the primordial germ cells in the mouse embryo. Anat Rec. 1954;118(2):135‐146. [DOI] [PubMed] [Google Scholar]
- 5. Barton LJ, LeBlanc MG, Rehmann R. Finding their way: themes in germ cell migration. Curr Opin Cell Biol. 2016;42:128‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Novi AM, Sava P. An electron microscopic study of the development of the rat testis in the first 10 postnatal days. Z Zellforsch Mikrosk Anat. 1968;86:313‐326. [DOI] [PubMed] [Google Scholar]
- 7. Spiegelman M, Bennett D. A light‐ and electron‐microscopic study of primordial germ cells in the early mouse embryo. J Embryol Exp Morphol. 1973;30:97‐118. [PubMed] [Google Scholar]
- 8. Clark JM, Eddy EM. Fine structural observations on the origin and associations of primordial germ cells of the mouse. Dev Biol. 1975;47:136‐155. [DOI] [PubMed] [Google Scholar]
- 9. Chiarini‐Garcia H, Russell LD. Characterization of mouse spermatogonia by transmission electron microscopy. Reproduction. 2002;123:567‐577. [DOI] [PubMed] [Google Scholar]
- 10. Eddy EM. Fine structural observations on the form and distribution of nuage in germ cells of the rat. Anat Rec. 1974;178:731‐757. [DOI] [PubMed] [Google Scholar]
- 11. Chuma S, Hosokawa M, Tanaka T, Nakatsuji N. Ultrastructural characterization of spermatogenesis and its evolutionary conservation in the germline: germinal granules in mammals. Mol Cell Endocrinol. 2009;306:17‐23. [DOI] [PubMed] [Google Scholar]
- 12. Smith BE, Braun RE. Germ cell migration across sertoli cell tight junctions. Science. 2012;338:798‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kremer A, Lippens S, Bartunkova S, et al. Developing 3D SEM in a broad biological context. J Microsc. 2015;259:80‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ohno N, Katoh M, Saitoh Y, Saitoh S. Recent advancement in the challenges to connectomics. Microscopy. 2016;65:97‐107. [DOI] [PubMed] [Google Scholar]
- 15. Donovan PJ, Stott D, Cairns LA, Heasman J, Wylie CC. Migratory and postmigratory mouse primordial germ cells behave differently in culture. Cell. 1986;44:831‐838. [DOI] [PubMed] [Google Scholar]
- 16. McGuinness MP, Orth JM. Reinitiation of gonocyte mitosis and movement of gonocytes to the basement membrane in testes of newborn rats in vivo and in vitro. Anat Rec. 1992;233:527‐537. [DOI] [PubMed] [Google Scholar]
- 17. Gomperts M, Garcia‐Castro M, Wylie C, Heasman J. Interactions between primordial germ cells play a role in their migration in mouse embryos. Development. 1994;120:135‐141. [DOI] [PubMed] [Google Scholar]
- 18. Bendel‐Stenzel MR, Gomperts M, Anderson R, Heasman J, Wylie C. The role of cadherins during primordial germ cell migration and early gonad formation in the mouse. Mech Dev. 2000;91:143‐152. [DOI] [PubMed] [Google Scholar]
- 19. Dovere L, Fera S, Grasso M, et al. The niche‐derived glial cell line‐derived neurotrophic factor (GDNF) induces migration of mouse spermatogonial stem/progenitor cells. PLoS ONE. 2013;8:e59431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anderson R, Copeland TK, Schöler H, Heasman J, Wylie C. The onset of germ cell migration in the mouse embryo. Mech Dev. 2000;91:61‐68. [DOI] [PubMed] [Google Scholar]
- 21. Molyneaux KA, Stallock J, Schaible K, Wylie C. Time‐lapse analysis of living mouse germ cell migration. Dev Biol. 2001;240:488‐498. [DOI] [PubMed] [Google Scholar]
- 22. Yoshida S, Sukeno M, Nabeshima Y. A vasculature‐associated niche for undifferentiated spermatogonia in the mouse testis. Science. 2007;317:1722‐1726. [DOI] [PubMed] [Google Scholar]
- 23. Hara K, Nakagawa T, Enomoto H, et al. Mouse spermatogenic stem cells continually interconvert between equipotent singly isolated and syncytial states. Cell Stem Cell. 2014;14:658‐672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nakagawa T, Sharma M, Nabeshima Y, Braun RE, Yoshida S. Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science. 2010;328:62‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Neuhaus N, Yoon J, Terwort N, et al. Single‐cell gene expression analysis reveals diversity among human spermatogonia. Mol Hum Reprod. 2017;23:79‐90. [DOI] [PubMed] [Google Scholar]
- 26. Guo J, Grow EJ, Yi C, et al. Chromatin and single‐cell RNA‐seq profiling reveal dynamic signaling and metabolic transitions during human spermatogonial stem cell development. Cell Stem Cell. 2017;21:533‐546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li L, Dong J, Yan L, et al. Single‐cell RNA‐seq analysis maps development of human germline cells and gonadal niche interactions. Cell Stem Cell. 2017;20:858‐873. [DOI] [PubMed] [Google Scholar]
- 28. Green CD, Ma Q, Manske GL, et al. A comprehensive roadmap of murine spermatogenesis defined by single‐cell RNA‐seq. Dev Cell. 2018;46:651‐667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lawson KA, Dunn NR, Roelen BA, et al. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev. 1999;13:424‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ohinata Y, Ohta H, Shigeta M, Yamanaka K, Wakayama T, Saitou M. A signaling principle for the specification of the germ cell lineage in mice. Cell. 2009;137:571‐584. [DOI] [PubMed] [Google Scholar]
- 31. Harikae K, Miura K, Kanai Y. Early gonadogenesis in mammals: significance of long and narrow gonadal structure. Dev Dyn. 2013;242:330‐338. [DOI] [PubMed] [Google Scholar]
- 32. Hara K, Kanai‐Azuma M, Uemura M, et al. Evidence for crucial role of hindgut expansion in directing proper migration of primordial germ cells in mouse early embryogenesis. Dev Biol. 2009;330:427‐439. [DOI] [PubMed] [Google Scholar]
- 33. Tanaka SS, Yamaguchi YL, Tsoi B, Lickert H, Tam PP. IFITM/Mil/fragilis family proteins IFITM1 and IFITM3 play distinct roles in mouse primordial germ cell homing and repulsion. Dev Cell. 2005;9(6):745‐756. [DOI] [PubMed] [Google Scholar]
- 34. Lange UC, Adams DJ, Lee C, et al. Normal germ line establishment in mice carrying a deletion of the Ifitm/Fragilis gene family cluster. Mol Cell Biol. 2008;28:4688‐4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kwon GS, Viotti M, Hadjantonakis AK. The endoderm of the mouse embryo arises by dynamic widespread intercalation of embryonic and extraembryonic lineages. Dev Cell. 2008;15:509‐520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lewis SL, Tam PP. Definitive endoderm of the mouse embryo: formation, cell fates, and morphogenetic function. Dev Dyn. 2006;235(9):2315‐2329. [DOI] [PubMed] [Google Scholar]
- 37. Tang WW, Kobayashi T, Irie N, Dietmann S, Surani MA. Specification and epigenetic programming of the human germ line. Nat Rev Genet. 2016;17:585‐600. [DOI] [PubMed] [Google Scholar]
- 38. Sasaki K, Nakamura T, Okamoto I, et al. The germ cell fate of cynomolgus monkeys is specified in the nascent amnion. Dev Cell. 2016;39:169‐185. [DOI] [PubMed] [Google Scholar]
- 39. Kanai‐Azuma M, Kanai Y, Gad JM, et al. Depletion of definitive gut endoderm in Sox17‐null mutant mice. Development. 2002;129:2367‐2379. [DOI] [PubMed] [Google Scholar]
- 40. Spence JR, Lauf R, Shroyer NF. Vertebrate intestinal endoderm development. Dev Dyn. 2011;240:501‐520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Seki Y, Hayashi K, Itoh K, Mizugaki M, Saitou M, Matsui Y. Extensive and orderly reprogramming of genome‐wide chromatin modifications associated with specification and early development of germ cells in mice. Dev Biol. 2005;278:440‐458. [DOI] [PubMed] [Google Scholar]
- 42. Ancelin K, Lange UC, Hajkova P, et al. Blimp1 associates with Prmt5 and directs histone arginine methylation in mouse germ cells. Nat Cell Biol. 2006;8:623‐630. [DOI] [PubMed] [Google Scholar]
- 43. Seki Y, Yamaji M, Yabuta Y, et al. Cellular dynamics associated with the genome‐wide epigenetic reprogramming in migrating primordial germ cells in mice. Development. 2007;134:2627‐2638. [DOI] [PubMed] [Google Scholar]
- 44. Tsunekawa N, Naito M, Sakai Y, Nishida T, Noce T. Isolation of chicken vasa homolog gene and tracing the origin of primordial germ cells. Development. 2000;127:2741‐2750. [DOI] [PubMed] [Google Scholar]
- 45. Nakamura Y, Yamamoto Y, Usui F, et al. Migration and proliferation of primordial germ cells in the early chicken embryo. Poult Sci. 2007;86:2182‐2193. [DOI] [PubMed] [Google Scholar]
- 46. Fujimoto T, Yoshinaga K, Kono I. Distribution of fibronectin on the migratory pathway of primordial germ cells in mice. Anat Rec. 1985;211:271‐278. [DOI] [PubMed] [Google Scholar]
- 47. García‐Castro MI, Anderson R, Heasman J, Wylie C. Interactions between germ cells and extracellular matrix glycoproteins during migration and gonad assembly in the mouse embryo. J Cell Biol. 1997;138:471‐480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Soto‐Suazo M, Abrahamsohn PA, Pereda J, et al. Modulation of hyaluronan in the migratory pathway of mouse primordial germ cells. Histochem Cell Biol. 2002;117:265‐273. [DOI] [PubMed] [Google Scholar]
- 49. Ara T, Nakamura Y, Egawa T, et al. Impaired colonization of the gonads by primordial germ cells in mice lacking a chemokine, stromal cell‐derived factor‐1 (SDF‐1). Proc Natl Acad Sci USA. 2003;100:5319‐5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Molyneaux KA, Zinszner H, Kunwar PS, et al. The chemokine SDF1/CXCL12 and its receptor CXCR50 regulate mouse germ cell migration and survival. Development. 2003;130:4279‐4286. [DOI] [PubMed] [Google Scholar]
- 51. Runyan C, Schaible K, Molyneaux K, Wang Z, Levin L, Wylie C. Steel factor controls midline cell death of primordial germ cells and is essential for their normal proliferation and migration. Development. 2006;133:4861‐4869. [DOI] [PubMed] [Google Scholar]
- 52. Gu Y, Runyan C, Shoemaker A, Surani A, Wylie C. Steel factor controls primordial germ cell survival and motility from the time of their specification in the allantois, and provides a continuous niche throughout their migration. Development. 2009;136:1295‐1303. [DOI] [PubMed] [Google Scholar]
- 53. Gu Y, Runyan C, Shoemaker A, Surani MA, Wylie C. Membrane‐bound steel factor maintains a high local concentration for mouse primordial germ cell motility, and defines the region of their migration. PLoS ONE. 2011;6:e25984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Laird DJ, Altshuler‐Keylin S, Kissner MD, Zhou X, Anderson KV. Ror2 enhances polarity and directional migration of primordial germ cells. PLoS Genet. 2011;7:e1002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chawengsaksophak K, Svingen T, Ng ET, et al. Loss of Wnt5a disrupts primordial germ cell migration and male sexual development in mice. Biol Reprod. 2012;86:1‐12. [DOI] [PubMed] [Google Scholar]
- 56. Di Carlo A, De Felici M. A role for E‐cadherin in mouse primordial germ cell development. Dev Biol. 2000;226:209‐219. [DOI] [PubMed] [Google Scholar]
- 57. Short RV, Mann T, Hay MF. Male reproductive organs of the African elephant, Loxodonta africana . J Reprod Fertil. 1967;13:517‐536. [DOI] [PubMed] [Google Scholar]
- 58. Rommel SA, Pabst DA, McLellan WA, Mead JG, Potter CW. Anatomical evidence for a countercurrent heat exchanger associated with dolphin testes. Anat Rec. 1992;232:150‐156. [DOI] [PubMed] [Google Scholar]
- 59. Lin M, Jones RC. Spermiogenesis and spermiation in a monotreme mammal, the platypus, Ornithorhynchus anatinus . J Anat. 2000;196:217‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Amann RP, Veeramachaneni DN. Cryptorchidism in common eutherian mammals. Reproduction. 2007;133:541‐561. [DOI] [PubMed] [Google Scholar]
- 61. Kubota Y, Temelcos C, Bathgate RA, et al. The role of insulin 3, testosterone, Müllerian inhibiting substance and relaxin in rat gubernacular growth. Mol Hum Reprod. 2002;8:900‐905. [DOI] [PubMed] [Google Scholar]
- 62. Robinson JN, Eengle ET. Some observations on the cryptorchid testis. J Urol. 1954;71:726‐734. [DOI] [PubMed] [Google Scholar]
- 63. Chowdhury AK, Steinberger E. A quantitative study of the effect of heat on germinal epithelium of rat testes. Am J Anat. 1964;115:509‐524. [DOI] [PubMed] [Google Scholar]
- 64. de Rooij DG, Okabe M, Nishimune Y. Arrest of spermatogonial differentiation in jsd/jsd, Sl17H/Sl17H, and cryptorchid mice. Biol Reprod. 1999;61:842‐847. [DOI] [PubMed] [Google Scholar]
- 65. Clermont Y, Perey B. Quantitative study of the cell population of the seminiferous tubules in immature rats. Am J Anat. 1957;100:241‐267. [DOI] [PubMed] [Google Scholar]
- 66. Kluin PM, de Rooij DG. A comparison between the morphology and cell kinetics of gonocytes and adult type undifferentiated spermatogonia in the mouse. Int J Androl. 1981;4:475‐493. [DOI] [PubMed] [Google Scholar]
- 67. Huckins C, Clermont Y. Evolution of gonocytes in the rat testis during late embryonic and early post‐natal life. Arch Anat Histol Embryol. 1968;51:341‐354. [PubMed] [Google Scholar]
- 68. Bellvé AR, Cavicchia JC, Millette CF, O'Brien DA, Bhatnagar YM, Dym M. Spermatogenic cells of the prepuberal mouse. Isolation and morphological characterization. J Cell Biol. 1977;74:68‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Culty M. Gonocytes, from the fifties to the present: is there a reason to change the name? Biol Reprod. 2013;89:46. [DOI] [PubMed] [Google Scholar]
- 70. Beaumont HM, Mandl AM. A quantitative study of primordial germ cells in the male rat. J Embryol Exp Morphol. 1963;11:715‐740. [PubMed] [Google Scholar]
- 71. Yoshida S, Sukeno M, Nakagawa T, et al. The first round of mouse spermatogenesis is a distinctive program that lacks the self‐renewing spermatogonia stage. Development. 2006;133:1495‐1505. [DOI] [PubMed] [Google Scholar]
- 72. Roosen‐Runge EC, Leik J. Gonocyte degeneration in the postnatal male rat. Am J Anat. 1968;122:275‐299. [DOI] [PubMed] [Google Scholar]
- 73. Wang RA, Nakane PK, Koji T. Autonomous cell death of mouse male germ cells during fetal and postnatal period. Biol Reprod. 1998;58:1250‐1256. [DOI] [PubMed] [Google Scholar]
- 74. Vergouwen RP, Jacobs SG, Huiskamp R, Davids JA, de Rooij DG. Proliferative activity of gonocytes, sertoli cells and interstitial cells during testicular development in mice. J Reprod Fertil. 1991;93:233‐243. [DOI] [PubMed] [Google Scholar]
- 75. Nagano R, Tabata S, Nakanishi Y, Ohsako S, Kurohmaru M, Hayashi Y. Reproliferation and relocation of mouse male germ cells (gonocytes) during prespermatogenesis. Anat Rec. 2000;258:210‐220. [DOI] [PubMed] [Google Scholar]
- 76. Yoshida S. Open niche regulation of mouse spermatogenic stem cells. Dev Growth Differ. 2018;60:542‐552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Shinohara T, Orwig KE, Avarbock MR, Brinster RL. Spermatogonial stem cell enrichment by multiparameter selection of mouse testis cells. Proc Natl Acad Sci USA. 2000;97:8346‐8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chiarini‐Garcia H, Hornick JR, Griswold MD, Russell LD. Distribution of type A spermatogonia in the mouse is not random. Biol Reprod. 2001;65:1179‐1185. [DOI] [PubMed] [Google Scholar]
- 79. Kitadate Y, Jörg DJ, Tokue M, et al. Competition for mitogens regulates spermatogenic stem cell homeostasis in an open niche. Cell Stem Cell. 2019;24:79‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Fuller MT, Spradling AC. Male and female Drosophila germline stem cells: two versions of immortality. Science. 2007;316:402‐404. [DOI] [PubMed] [Google Scholar]
- 81. Setchell BP. The secretion of fluid by the testes of rats, rams and goats with some observations on the effect of age, cryptorchidism and hypophysectomy. J Reprod Fertil. 1970;23:79‐85. [DOI] [PubMed] [Google Scholar]
- 82. Roosen‐runge EC. Quantitative studies on spermatogenesis in the albino rat. II. The duration of spermatogenesis and some effects of colchicine. Am J Anat. 1951;88:163‐176. [DOI] [PubMed] [Google Scholar]
- 83. Clermont YC. Contractile elements in the limiting membrane of the seminiferous tubules of the rat. Exp Cell Res. 1958;15:438‐440. [DOI] [PubMed] [Google Scholar]
- 84. Pilsworth LM, Hinton BT, Setchell BP. Effects of obstruction of the flow of seminiferous tubule fluid on the germinal epithelium in the rat. J Reprod Fertil. 1981;63:347‐353. [DOI] [PubMed] [Google Scholar]
- 85. Austin CR. Observations on the penetration of the sperm in the mammalian egg. Aust J Sci Res B. 1951;4:581‐596. [DOI] [PubMed] [Google Scholar]
- 86. Chang MC. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature. 1951;168:697‐698. [DOI] [PubMed] [Google Scholar]
- 87. Young WC. A study of the function of the epididymis. III. Functional changes undergone by spermatozoa during their passage through the epididymis and vas deferens in the guinea‐pig. J Exp Biol. 1931;8:151‐162. [Google Scholar]
- 88. Bedford JM. Changes in the electrophoretic properties of rabbit spermatozoa during passage through the epididymis. Nature. 1963;200:1178‐1180. [DOI] [PubMed] [Google Scholar]
- 89. Bedford JM. Morphological changes in rabbit spermatozoa during passage through the epididymis. J Reprod Fertil. 1963;5:169‐177. [DOI] [PubMed] [Google Scholar]
- 90. Orgebin‐Crist MC. Sperm maturation in rabbit epididymis. Nature. 1967;216:816‐818. [DOI] [PubMed] [Google Scholar]
- 91. Smith P, Wilhelm D, Rodgers RJ. Development of mammalian ovary. J Endocrinol. 2014;221:R145‐R161. [DOI] [PubMed] [Google Scholar]
- 92. Aurich C. Reproductive cycles of horses. Anim Reprod Sci. 2011;124:220‐228. [DOI] [PubMed] [Google Scholar]
- 93. Feng Y, Tamadon A, Hsueh A. Imaging the ovary. Reprod Biomed Online. 2018;36:584‐593. [DOI] [PubMed] [Google Scholar]
- 94. Halbert SA, Tam PY, Blandau RJ. Egg transport in the rabbit oviduct: the roles of cilia and muscle. Science. 1976;191:1052‐1053. [DOI] [PubMed] [Google Scholar]
- 95. Halbert SA, Becker DR, Szal SE. Ovum transport in the rat oviductal ampulla in the absence of muscle contractility. Biol Reprod. 1989;40:1131‐1136. [DOI] [PubMed] [Google Scholar]
- 96. Shi D, Komatsu K, Uemura T, Fujimori T. Analysis of ciliary beat frequency and ovum transport ability in the mouse oviduct. Genes Cells. 2011;16:282‐290. [DOI] [PubMed] [Google Scholar]
- 97. Shi D, Komatsu K, Hirao M, et al. Celsr1 is required for the generation of polarity at multiple levels of the mouse oviduct. Development. 2014;141:4558‐4568. [DOI] [PubMed] [Google Scholar]
- 98. Okabe M. Sperm‐egg interaction and fertilization: past, present, and future. Biol Reprod. 2018;99:134‐146. [DOI] [PubMed] [Google Scholar]
- 99. Harayama H. Flagellar hyperactivation of bull and boar spermatozoa. Reprod Med Biol. 2018;17:442‐448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Geisert RD, Renegar RH, Thatcher WW, Roberts RM, Bazer FW. Establishment of pregnancy in the pig: I. Interrelationships between preimplantation development of the pig blastocyst and uterine endometrial secretions. Biol Reprod. 1982;27:925‐939. [DOI] [PubMed] [Google Scholar]
- 101. Geisert RD, Brookbank JW, Roberts RM, Bazer FW. Establishment of pregnancy in the pig: II. Cellular remodeling of the porcine blastocyst during elongation on day 12 of pregnancy. Biol Reprod. 1982;27:941‐955. [DOI] [PubMed] [Google Scholar]
- 102. Geisert RD, Johnson GA, Burghardt RC. Implantation and establishment of pregnancy in the pig. Adv Anat Embryol Cell Biol. 2015;216:137‐163. [DOI] [PubMed] [Google Scholar]
- 103. Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359‐369. [DOI] [PubMed] [Google Scholar]
- 104. Mattila PK, Lappalainen P. Filopodia: molecular architecture and cellular functions. Nat Rev Mol Cell Biol. 2008;9:446‐454. [DOI] [PubMed] [Google Scholar]


