Abstract
In mammals, the master circadian clock synchronizes daily rhythms of physiology and behavior with the day–night cycle. Failure of synchrony, which increases the risk for numerous chronic diseases, can be treated by phase adjustment of the circadian clock pharmacologically, for example, with melatonin, or a CK1δ/ε inhibitor. Here, using in silico experiments with a systems pharmacology model describing molecular interactions, and pharmacokinetic and behavioral experiments in cynomolgus monkeys, we find that the circadian phase delay caused by CK1δ/ε inhibition is more strongly attenuated by light in diurnal monkeys and humans than in nocturnal mice, which are common preclinical models. Furthermore, the effect of CK1δ/ε inhibition strongly depends on endogenous PER2 protein levels, which differs depending on both the molecular cause of the circadian disruption and the patient's lighting environment. To circumvent such large interindividual variations, we developed an adaptive chronotherapeutics to identify precise dosing regimens that could restore normal circadian phase under different conditions. Our results reveal the importance of photosensitivity in the clinical efficacy of clock‐modulating drugs, and enable precision medicine for circadian disruption.
Keywords: circadian rhythms, CK1δ/ε inhibitor, personalized chronotherapy, systems pharmacology model
Subject Categories: Computational Biology, Quantitative Biology & Dynamical Systems
Introduction
Circadian (~24 h) rhythms of diverse behavioral and physiological processes such as sleep and hormone secretion are coordinated by an endogenous timer, the circadian clock (Dibner et al, 2010). The key oscillatory molecular mechanism of the mammalian circadian clock centers around the PER1/2 proteins that mediate an autoregulatory negative feedback loop of their own transcription (Ukai & Ueda, 2010; Aryal et al, 2017; Takahashi, 2017). This generates circadian expression of PER1/2 at both the mRNA and protein levels. The period and phase of PER1/2 oscillations are further regulated by post‐translational control of their stability and subcellular localization via CK1δ/ε phosphorylation (Vanselow et al, 2006; Gallego & Virshup, 2007; Kaasik et al, 2013; Qin et al, 2015; Zhou et al, 2015; Shinohara et al, 2017; Narasimamurthy et al, 2018; Ode & Ueda, 2018). Thus, CK1δ/ε is being considered as an effective pharmacologic target for the manipulation of circadian rhythms (Badura et al, 2007; Sprouse et al, 2009, 2010; Meng et al, 2010; Kim et al, 2013; Pilorz et al, 2014). The phase of circadian rhythms can also be adjusted by Per1/2 gene transcription induced by external light, which is transmitted from the retina to the hypothalamic suprachiasmatic nucleus (SCN) via the retinohypothalamic tract (Hattar et al, 2002; Gallego & Virshup, 2007). This leads to the entrainment of the endogenous circadian system to the external day–night cycle (Wright et al, 2013).
The failure of synchrony between the clock and external cycles can occur due to dysfunction of the circadian clock system or alteration of the external environment. Notably, recent epidemiological data suggest that more than 80% of the population appears to live a shift work lifestyle (Sulli et al, 2018). This increases the risk for various chronic diseases such as sleep disorders, cancer, diabetes, and mood disorders (Zhu & Zee, 2012; Sulli et al, 2018). To restore normal circadian phase, several approaches are being used. The most common is timed light exposure, because light can advance and delay circadian phase depending on exposure time (Khalsa et al, 1997). However, as this therapy often requires bright exposure for up to 2 h daily at specific times, patients show poor compliance with it, highlighting the need for different approaches. Pharmacological phase adjustment of circadian rhythms is considered as an attractive alternative to timed light exposure (Skelton et al, 2015). For instance, administration of melatonin in the evening can advance circadian phase of patients with delayed sleep phase disorder (DSPD) (Zhu & Zee, 2012). However, although melatonin given in the morning can delay circadian rhythms in experimental condition (Burgess et al, 2010), its clinical efficacy for advanced sleep phase disorder (ASPD) has not been reported (Sack et al, 2007; Zhu & Zee, 2012). Furthermore, other clock‐modulating drugs including dopamine partial agonists and GABA receptor modulators have only shown efficacy in treating DSPD but not ASPD (Ozaki et al, 1989; Regestein & Monk, 1995; Takaki & Ujike, 2014; Omori et al, 2018; Takeshima et al, 2018). Given the absence of drugs effectively delaying circadian phase, PF‐670462 (PF‐670), a potent and selective CK1δ/ε inhibitor currently in preclinical development, is the most promising candidate compound for treating ASPD as it induces a large phase delay regardless of dosing timing (Badura et al, 2007; Kim et al, 2013).
The effect of clock‐modulating drugs dramatically changes upon external light exposure because light also alters the circadian phase. For instance, the phase shifts induced by PF‐670 or melatonin are attenuated by light exposure (Sprouse et al, 2010; Kim et al, 2013; Nesbitt, 2018). The effect of clock‐modulating drugs is also proposed to be affected by individual variations in the molecular cause of circadian disruption and photosensitivity (Holst et al, 2016; Keijzer et al, 2017). However, the interindividual variability in pharmacological circadian phase shift caused by differences in individual genes and environmental lighting conditions has not been systematically investigated. Thus, whether precision medicine could be applied as successfully to circadian disruption as it has been to other areas of medicine such as oncology is currently unclear (Skelton et al, 2015; Holst et al, 2016; Keijzer et al, 2017). Indeed, current therapeutics are mainly tailored around the patient's clinical features (i.e., phase of sleep–wake cycle) (Zhu & Zee, 2012).
The SCN of diurnal and nocturnal mammals have similar structures and temporal patterns of the core clock gene expression and neuronal firing rate (Inouye & Kawamura, 1982; Cohen et al, 2010; Kumar, 2017; Millius & Ueda, 2018; Mure et al, 2018). Thus, pharmacomanipulation of the circadian clock has been expected to be similar in nocturnal and diurnal mammals. However, they display nearly antiphase rhythms in physiology and behavior (Refinetti, 2006). This suggests that differences may still exist in their responses to clock‐modulating drugs, complicating the translation of preclinical studies into effective therapies.
We used the response to the selective CK1δ/ε inhibitor, PF‐670, to dissect any differences in circadian modulation between diurnal and nocturnal mammals that may be clinically relevant. We found that nocturnal mice and a diurnal non‐human primate (NHP), the cynomolgus monkey (Macaca facicularis), show a quantitatively different response: PF‐670 induces a significantly smaller phase delay in NHPs than in mice. This interspecies variation was analyzed by developing a comprehensive systems chronopharmacology model based on experimental data from the NHPs and humans. The model simulations for the intracellular interactions of PF‐670 with clock components and follow‐up experiments revealed that the weak drug response of NHPs is mainly due to the strong attenuation of PF‐670‐induced phase delay by light exposure, which appears to exist across many diurnal mammals including humans. This explains the absence of effective pharmacotherapy for delaying circadian phase of sleep disorder patients in a daylight cycle (Sack et al, 2007; Zhu & Zee, 2012). Our work reveals a previously unrecognized biological variable in translating the efficacy of clock‐modulating drugs from nocturnal mice to diurnal humans: their different photosensitivity. Besides photosensitivity, we found that the effect of PF‐670 on the circadian phase dramatically decreases as endogenous levels of PER2 decrease. Together with this finding, our in silico experiments revealed that depending on the molecular cause of circadian disruption and the patient's light exposure, which alters PER2 levels, the effect of the CK1δ/ε inhibitor (CK1i) changes. To overcome the inter‐ and intraindividual variations, we developed an adaptive chronotherapeutics that identifies a precise dosing time for CK1i to restore the normal circadian phase. Our study illustrates how a systematic approach can both identify sources of variations in drug response and generate a strategy providing a precise dosing regimen for circadian disruption.
Results
The effect of PF‐670 is more strongly attenuated by light in diurnal NHPs than in nocturnal mice
PER1/2 phosphorylation by CK1δ/ε regulates their degradation, binding to CRY1/2, and nuclear translocation, which are the key processes of the transcriptional–translational negative feedback loop of the mammalian circadian clock (Fig 1Ai; Gallego & Virshup, 2007). When CK1δ/ε is inhibited by PF‐670, these processes are slowed down (Fig 1Ai) and circadian phase is delayed, which is attenuated by light, the strongest zeitgeber (Fig 1Aii) (Badura et al, 2007; Walton et al, 2009; Meng et al, 2010; Sprouse et al, 2010; Kim et al, 2013). To analyze the effect of PF‐670 on the circadian phase in diurnal NHPs, and compare it with nocturnal mice, we first compared the free PF‐670 brain concentrations across species (see Materials and Methods for details). Despite the lower dose level in NHPs (10 mg/kg (mpk)) than used in our previous study in mice (32 mpk), the drug exposure in NHPs (AUC = 3.6 μM·h) was much higher than in mice (AUC = 0.5 μM·h) (Fig 1B; Kim et al, 2013). Due to the higher drug exposure in NHPs, we hypothesized that PF‐670 induces a larger phase delay of activity onset in NHPs than in mice. To investigate this, we compared the phase delays of NHPs induced by 3‐day 10 mpk dosing in a dark–dark cycle (DD) (Fig 1C) with the phase delays of mice dosed with 32 mpk PF‐670 for 3 days in DD (Kim et al, 2013). Indeed, NHPs showed a significantly larger phase delay (5.2 h) compared with mice (3.8 h) (P = 0.03; Fig 1E) (see Materials and Methods for details of phase delay measurement). This larger phase delay in NHPs than in mice might be due to the different dosing times for NHPs (e.g., CT14) than for mice (CT11) as the effect of PF‐670 changes upon dosing time (Badura et al, 2007). However, the phase delay of NHP induced by dosing at CT11 is also likely to be larger than that of mice because the dosing at CT11 is expected to yield a nearly maximal phase delay (Badura et al, 2007) and the drug exposure is much higher in NHPs than in mice (Fig 1B) (see Fig EV3 after reading below two sections for details).
Figure 1. Light attenuates the effect of PF‐670 more strongly in diurnal NHPs than in nocturnal mice.
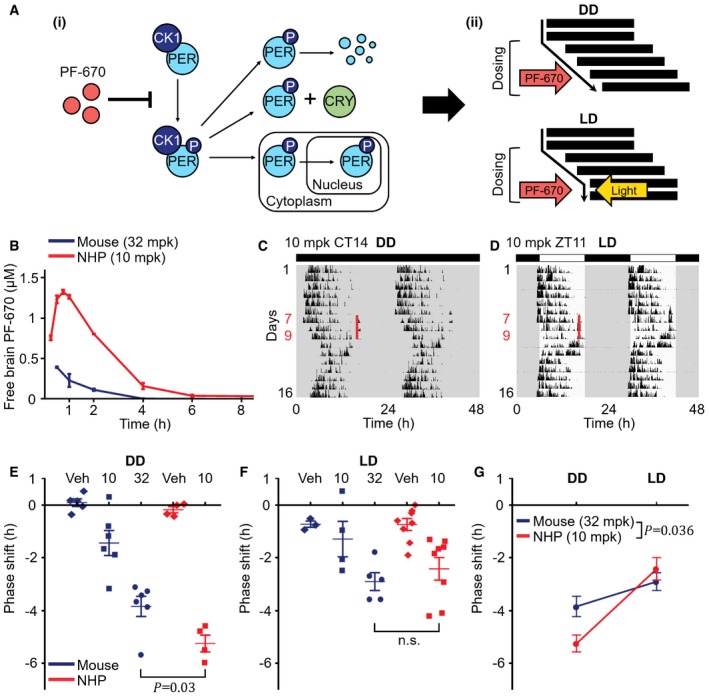
-
APF‐670 inhibits the phosphorylation of PER by CK1δ/ε (i) and delays the circadian phase, which is counterbalanced by light (ii). Thus, daily dosing leads to continually accumulating phase delay in DD and constant stable phase delay in LD.
-
BMeasured free PF‐670 exposure in the brain tissue of mice and NHPs. Drug exposure in NHPs after administration of 10 mpk PF‐670 (AUC = 3.6 μM·h) is ˜7‐fold higher than that in mice given 32 mpk PF‐670 (AUC = 0.5 μM·h) (n = 2–3; mean ± SEM). The mouse data are adopted from (Kim et al, 2013).
-
C, DDouble‐plotted actograms of NHPs’ activity for 16 days. NHPs were treated with 10 mpk PF‐670 for 3 consecutive days at the same solar time of day: 4 pm for the DD experiment (C) and 4:30 pm (ZT11) for the LD experiment (D), respectively, which are highlighted as red lines. White and black rectangles indicate the times of light going on and off (LD 12:12).
-
E, FPhase delay induced by the 3‐day dosing under DD (E) or LD (F). The phase of activity onset in NHPs (10 mpk) is more delayed than that in mice (32 mpk) under DD (E), but not under LD (F) (n = 3–8; P = 0.03, one‐way analysis of variance (ANOVA); n.s., no significant difference). Veh denotes vehicle. The error bars represent mean ± SEM. The mouse data are adopted from (Kim et al, 2013).
-
GThe quantification of (E) and (F) indicates that light has a stronger attenuating effect on the PF‐670‐induced phase delay in NHPs than in mice (n = 5–8; mean ± SEM; P = 0.036, two‐way ANOVA).
Next, we investigated whether there is also an interspecies difference in the alteration of PF‐670‐induced phase delay by light. Specifically, we compared the phase delays of NHPs induced by 10 mpk dosing for 3 days at zeitgeber time (ZT) 11 in a light–dark cycle (LD) (Fig 1D) with the previously reported ones of mice dosed with 32 mpk PF‐670 under the same conditions (Kim et al, 2013). Despite the stronger effect of PF‐670 in NHPs than in mice under DD (Fig 1E), NHPs did not show a larger phase delay than mice under LD (n.s.; Fig 1F). Thus, light attenuates the PF‐670‐induced phase delay more strongly in NHPs than in mice (P = 0.036; Fig 1G).
A systems chronopharmacology model is developed to simulate the effects of PF‐670 and light on circadian rhythms of NHPs
NHPs and mice show large differences in the pharmacokinetics of PF‐670 (Fig 1B), and the effect of PF‐670 on circadian phase, and how this is influenced by light (Fig 1E–G). To analyze such multiple differences systematically, we developed the first systems chronopharmacology model for NHPs by modifying our previous model (Kim et al, 2013), which successfully simulates the effects of PF‐670 and light on the intracellular circadian clock of mice. The modified parts of the model including the newly estimated pharmacokinetic parameters and new equations for the light module are described in the Materials and Methods. See Appendix Equation S1, Tables EV1 and EV2, and Datasets EV1 and EV2 for the detailed description for the equations, variables, and parameters of the NHP model. In the NHP model, inhibition of CK1δ/ε for PER1/2 phosphorylation by PF‐670 (Fig 2Ai) and light‐induced Per1/2 gene transcription via CREB (Fig 2Aii) were incorporated to simulate the resulting phase shift of the circadian clock at the molecular level (orange arrow; Fig 2Aiii).
Figure 2. Systems chronopharmacology model accurately simulates the phase shift of NHPs induced by PF‐670 and light.
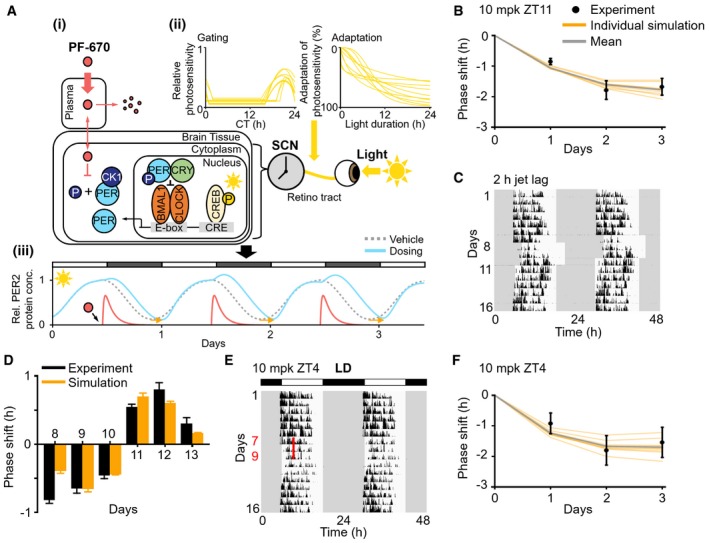
- Description of the systems chronopharmacology model for NHP. (i) The translocation of PF‐670 from plasma to the SCN and its inhibition of PER phosphorylation by CK1δ/ε in NHP (Fig EV1A) were accurately modeled to reproduce the PF‐670 exposure (Figs 1B and EV1B) and its effect in NHPs (Figs 1E and EV1C). (ii) The dependence of light‐induced Per1/2 gene transcription via CREB on circadian time (gating) and light duration (adaptation) is included in the model (Fig EV1F–H). The estimated 10 pairs of gating and adaptation allow for the model to successfully reproduce the light‐induced phase shift in NHPs and humans (Fig EV2). (iii) The model simulates the combined effect of PF‐670 (i) and light (ii) on PER2 rhythms in the SCN of NHPs.
- The model accurately reproduces the phase delay of NHPs induced by 3‐day LD dosing at ZT11. Here, the PF‐670‐induced phase delay from which the vehicle‐induced phase shift is subtracted was used (n = 8; mean ± SEM; Fig 1F). Each orange line is an individual simulation result obtained with each pair of gating and adaptation in (Aii), and their average is represented by the gray line.
- Double‐plotted actogram of NHPs’ activity for 16 days. The light is on at 6 am and off at 6 pm except for days 8 to 10 when the light is on at 8 am and off at 8 pm (i.e., LD is delayed by 2 h).
- The model successfully predicts the phase shift induced by 2‐h jet lag (days 8–10) and its recovery after jet lag (days 11–13) (n = 7; mean ± SEM; C).
- NHPs were treated with 10 mpk PF‐670 for 3 consecutive days at ZT4 in LD 12:12, which are highlighted as the red line.
- The phase delay induced by 3‐day LD dosing at ZT4 (n = 8; mean ± SEM; E) is accurately predicted by the model.
To match the simulated phase shift with the experimentally observed one in NHPs, firstly in the absence of light, we adjusted the model parameters describing the pharmacokinetics of PF‐670 in NHPs (Fig 2Ai) (see Fig EV1A–C, Dataset EV1 and Materials and Methods for details). However, unlike DD dosing, the effect of LD dosing in NHPs (Fig 1F) was not captured by the model (Fig EV1D and E), necessitating revision of the light module. In the previous mouse model, light was assumed to equally promote Per1/2 gene transcription across the entire circadian phase for simplicity (Kim et al, 2013). However, the light‐induced Per1/2 gene transcription is known to be suppressed more strongly during the subjective day than during the subjective night (Albrecht et al, 1997; Shigeyoshi et al, 1997; Miyake et al, 2000; Kiessling et al, 2014). Therefore, this gating for light, which reduces photosensitivity of the circadian clock depending on the circadian time (CT) (Geier et al, 2005), was incorporated into the light module of the new model (Fig 2Aii). Furthermore, adaptation for light, which reduces photosensitivity depending on light duration (Ding et al, 1997; Jagannath et al, 2013; Cao et al, 2015), was also added to further improve the accuracy of the light module (Fig 2Aii).
Figure EV1. The original model (Kim et al, 2013) with modified pharmacokinetic parameters accurately captures DD dosing but not LD dosing in NHPs.
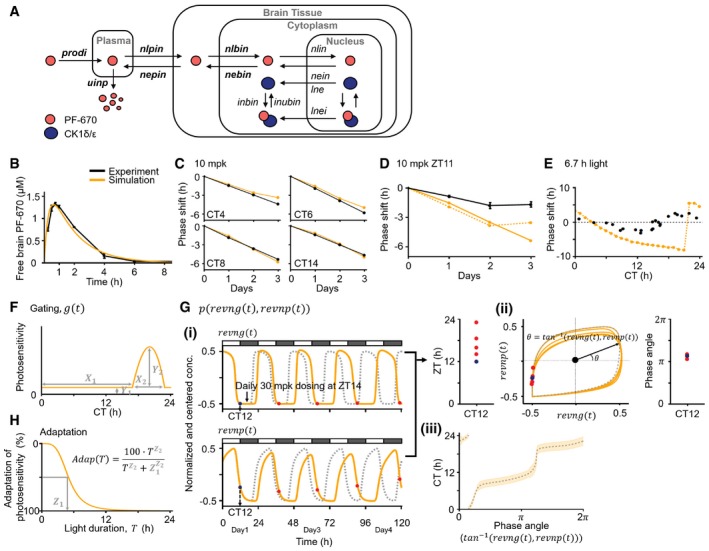
-
AModel diagram describing the dynamics of PF‐670, which is modified from (Kim et al, 2013). As was done in the original model (Kim et al, 2013), due to the fast absorption of PF‐670 into the brain tissue of NHPs (t max < 0.75 h; Fig 1B), we assume that PF‐670 directly comes into the plasma compartment with the initial concentration, prodi, which is cleared with the rate of uinp. Furthermore, the transfer rate of PF‐670 from plasma to brain tissue (nlpin) is assumed to be equal to that from brain tissue to plasma (nepin) as free brain and plasma PF‐670 concentrations are predicted to be equal by physiologically based PK modeling (see Materials and Methods for details). After entering the brain tissue compartment, PF‐670 transfers between brain tissue and cytoplasm with the rates of nlbin and nebin, respectively. PF‐670 then traffics between the cytoplasm and nucleus with the rates of nlin and nein, respectively. In a cell, PF‐670 and CK1δ/ε form a reversible complex with the binding rate, inbin, and unbinding rate, inubin. The rate of nuclear export of CK1δ/ε bound to PF‐670 (lnei) is allowed to be different from the rate of nuclear export of CK1δ/ε unbound to PF‐670 (lne) following the original model (Kim et al, 2013).
-
B, CWhile the six pharmacodynamic parameters were taken from the original model (Kim et al, 2013), the six PK parameters, highlighted as bold in (A), were modified to reproduce the free PF‐670 exposure in the brain tissue of NHPs (n = 2; mean ± SEM; B) and its DD dosing effects in NHPs at various initial dosing times (CT 4–14 h) (C). See Dataset EV1 for the values of the modified PK parameters. Here, the PF‐670‐induced phase delay from which the vehicle‐induced phase shift is subtracted was used (Fig 1E).
-
D, EUnlike DD dosing (C), the model overestimates the phase delay induced by 3‐day 10 mpk LD dosing (n = 8; mean ± SEM; Fig 1F) (solid line; D). On the other hand, when the light‐induced Per1/2 gene transcription is increased to reduce the simulated phase delay (dashed line; D), a 6.7‐h light pulse leads to an unrealistically large phase shift in the model (˜10 h) compared with the experimentally measured one in humans (black dots; E) adopted from (Khalsa et al, 2003). Here, to get the high photosensitivity (dashed line; D and E), the values of parameters describing light‐induced Per1/2 transcription rates (lono/lont; Dataset EV1) are increased threefold from the values used in the original model (Kim et al, 2013).
-
FTo accurately capture the effect of LD dosing in NHPs, gating for light, which is denoted by function g in Materials and Methods and Appendix Equation S1, is incorporated into the model (Fig 2Aii). The shape of the gating is determined by four parameters: X 1 determines the circadian time when the gating becomes weaker and thus the photosensitivity increases. X 2 describes the range of high photosensitivity zones. Y 1 describes the photosensitivity of the circadian clock when it is fully inhibited, which is assumed to be constant for simplicity. Y 2 describes the maximum photosensitivity of the circadian clock. To connect these photo‐insensitive and photosensitive zones continuously, a piecewise polynomial interpolation is used (see Code EV1). Note that the gating depends on the CT.
-
GTo estimate the input CT for gating (F) even when the circadian phase is altered by a stimulus (i.e., light and PF‐670), we constructed the function p, which estimates the CT from the phase angle of limit cycle of two clock variables, revng and revnp (Table EV1). (i) When the circadian clock is entrained by external light (i.e., LD 12:12), the CT can be simply approximated by ZT (e.g., CT12≈ZT12; blue circle). However, if the circadian phase is delayed by PF‐670, ZT, which corresponds to the same CT (e.g., CT12), changes dramatically (e.g., ZT14, 16, 19, and 23; red circles). (ii) On the other hand, the phase angles of limit cycle of revng and revnp, which corresponds to the same CT, change little (e.g., blue and red circles corresponding to CT12 in (i)). The concentration of revng and revnp is normalized by their maximum concentration in LD 12:12 and is centered by the average of their maximum and minimum concentration. (iii) Based on this feature, we constructed the function p, which is the function of the phase angle of the limit cycle (i.e., tan−1 (revng(t), revnp(t)) for CT when the model is entrained to LD 12:12 (gray dashed line). This allows the model to accurately predict the CT from the phase angle even when the circadian phase is altered by PF‐670 (orange range). The orange range represents the mean ± SD of the predicted CTs using the phase angle when a single daily 30 mpk dosing is given at ZT14 for 20 days. Note that p is accurate up to a considerably high dose (˜80 mpk) as the limit cycle is stable.
-
HThe adaptation for light is incorporated into the model (Fig 2Aii). The shape of adaptation is described with a Hill function with two parameters: Z 1 and Z 2 determine the light duration which reduces the photosensitivity by 50% and how sharply the photosensitivity decreases, respectively.
The gating and adaptation, which have not been experimentally quantified in NHPs, were estimated by fitting the model to the LD dosing effect that we observed in NHPs (Fig 2B) and human light phase response curves (PRCs) (Khalsa et al, 1997, 2003) (see Materials and Methods for details). The shapes of the gating and adaptation (Fig EV1F and H) were randomly chosen during the estimation process (Fig EV2A–E), However, despite that, we found that all the identified pairs of gating and adaptation had a similar shape (Figs 2Aii and EV2F). Specifically, the identified gatings commonly yielded high photosensitivity during the subjective night, which is consistent with experimental data from other mammals (Albrecht et al, 1997; Shigeyoshi et al, 1997; Miyake et al, 2000; Kiessling et al, 2014). With the identified 10 adaptations, light‐induced Per1/2 gene transcription gradually decreased and reached a 50% reduction after ~8 h.
Figure EV2. Filtering procedure to identify accurate gating and adaptation for light in the model.
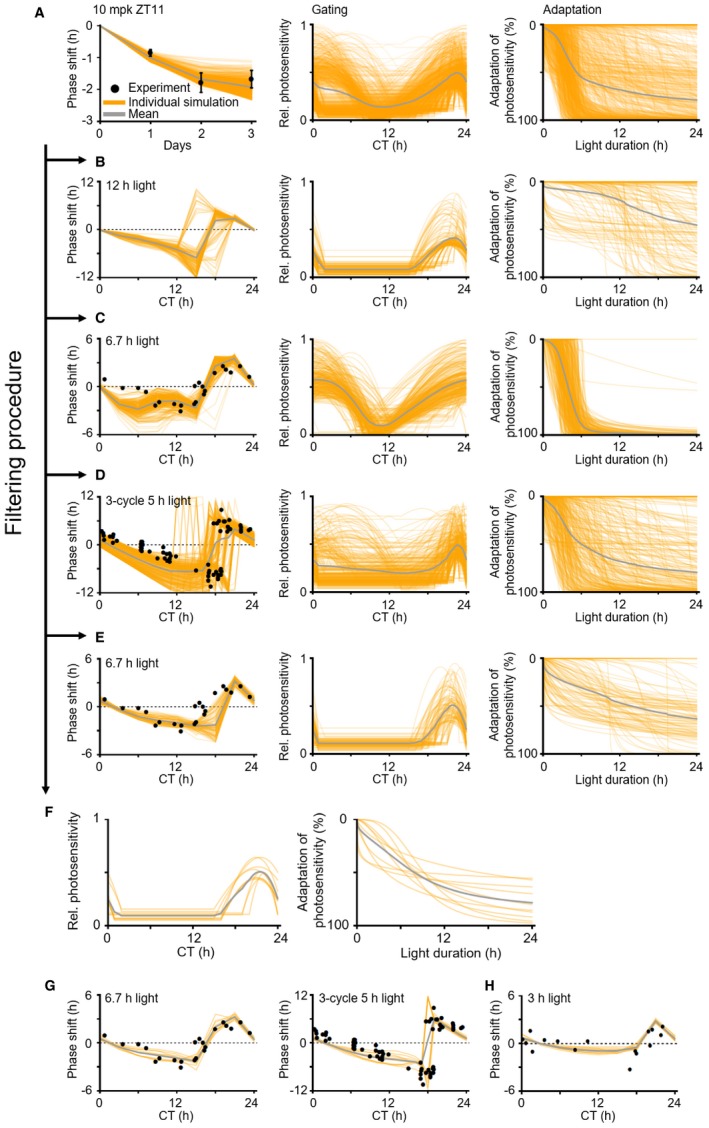
-
A911 pairs of gating and adaptation, with which the model accurately simulates the 3‐day LD dosing effect in NHPs (n = 8; mean ± SEM; Fig 1F) (left) and the magnitude of human PRC to a 6.7‐h light pulse (Khalsa et al, 2003), were found using the simulated annealing method (see Materials and Methods for details). The photosensitivity is normalized so that the maximum photosensitivity of initially estimated gatings becomes unified (center). 100% adaptation in photosensitivity means that Per1/2 gene transcription is no longer induced by light (right).
-
B152 pairs of gating and adaptation with which simulated PRCs to a 12‐h light pulse are discontinuous were filtered out (left), which is caused by the slow adaptation exaggerating the light effect (right).
-
C323 pairs of gating and adaptation were filtered out with which simulated light PRCs to a 6.7‐h light pulse have a too large phase shift between CT0 and CT6 (left) due to overestimated photosensitivity (center). Here, the human PRC to a 6.7‐h light pulse is adopted from (Khalsa et al, 2003).
-
D327 pairs of gating and adaptation were filtered out with which the simulated PRCs to 3‐cycle 5‐h light pulses (left) have either a too large phase shift due to overestimated photosensitivity between CT6 and CT12 (center) or an abrupt jump between CT16 and CT20 due to an underestimated range of high photosensitivity zone (center). Here, the human PRC to 3‐cycle 5‐h light pulses is adopted from (Khalsa et al, 1997).
-
E99 pairs of gating and adaptation with which the model fails to simulate the human PRC to a 6.7‐h light pulse (Khalsa et al, 2003) between CT15 and CT18 were filtered out (left).
-
F10 pairs of gating and adaptation passed the filtering procedure. See Dataset EV2 for the values of the parameters describing the 10 pairs of gating and adaptation.
-
G, HThe model with these 10 pairs of gating and adaptation (F) accurately simulates the human PRC to a 6.7‐h light pulse (left) and 3‐cycle 5‐h light pulses (right) (G). The model accurately predicts the human PRC to a 3‐h light pulse adopted from (Minors et al, 1991), which is not used in the estimation process (H).
The new light module accurately predicts the response of NHPs to light exposure
As the new light module of the model was estimated mainly based on the response of humans to light (Fig EV2), we next investigated whether it could accurately predict the potent light‐induced attenuation of the PF‐670 effect in NHPs. To solely focus on the effect of light, we introduced a 3‐day 2‐h delayed LD on days 8–10, which mimics the ~2‐h phase delay of NHPs induced by the 3‐day LD dosing (Fig 2C). After this 3‐day “jet lag”, the delayed phase was advanced to the original circadian phase for days 11–13. We found that such light‐induced phase shifts that occur during and after jet lag were accurately predicted by the model with the new light module (Fig 2D). This indicates that the new light module precisely captures the light‐induced phase advance in NHPs against the PF‐670‐induced phase delay in LD dosing.
We next investigated whether the model can predict the combined effect of PF‐670 and light even when the dosing time changes. We chose dosing at ZT4 as it leads to a much weaker phase delay in mice than dosing at ZT11 (Badura et al, 2007; Kim et al, 2013). However, in NHPs, dosing at ZT4 led to a similar phase delay as dosing at ZT11 (1.5; ZT4 and 1.7 h; ZT11; Fig 2B, E and F). This unexpected drug effect in NHPs was accurately predicted by the model with the new light module (Fig 2F), which is mainly due to the strong attenuation of the drug effect by light in NHPs (Fig EV3). Taken together, the difference in light response between mice and NHPs is a critical factor leading to their heterogeneous response to a clock‐modulating drug.
Figure EV3. The phase delays induced by 3‐day PF‐670 dosing in NHPs and mice under DD and LD.
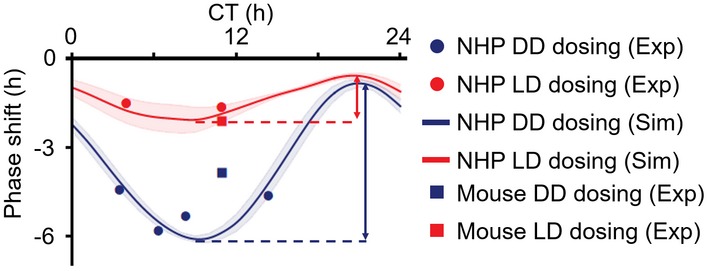
NHPs and mice were treated with PF‐670 for 3 days (Figs 1E and F, and 2F, and EV1C). Due to the higher drug exposure in NHPs than in mice (Fig 1B), all NHPs, which were treated with 10 mpk PF‐670 at various dosing times (CT4, 6, 8, 14) in DD, show a larger phase delay (filled blue circles) than mice dosed with 32 mpk PF‐670 at CT11 in DD (empty blue circle). Thus, the phase delay of NHPs induced by 10 mpk PF‐670 dosing at CT11 is expected to be larger than that of mice dosed with 32 mpk PF‐670 at CT11 under DD, which is supported by the model simulation (blue line). Due to the strong attenuation of the PF‐670 effect by light in NHPs, the change in drug effect upon dosing time is much smaller in LD (from 0.6 h to 2.1 h; red arrow) than in DD (from 0.8 h to 6.1 h; blue arrow). Dosing time is denoted by the x‐axis. The line and colored range represent the mean ± SD of the simulated phase delays of NHP models with the 10 pairs of gating and adaptation (Figs 2Aii and EV2F).
The strong attenuating effect of light is expected in humans
Although the new light module was developed mainly based on the human response to light (Fig EV2), the model accurately captured the effect of light in NHPs in both the presence and absence of dosing (Fig 2). Thus, the strong light‐induced attenuation of the PF‐670 effect in NHPs compared with mice (Figs 1G and 3A) is expected to also exist in humans. To investigate this, we used our previous finding that the magnitude of the advance zone of the 12‐h light PRC can determine how strongly a 12‐h light pulse attenuates the effect of PF‐670 in LD 12:12 (Kim et al, 2013). Indeed, reflecting the stronger light‐induced attenuation in NHPs than in mice (Figs 1G and 3A), the simulated PRC of NHPs had a larger magnitude of the advance zone than the experimentally measured one of mice (Fig 3B) (Comas et al, 2006). This leads to a higher ratio of maximum magnitudes between the advance (A) zone and the delay (D) zone (A/D ratio) of light PRC in NHPs (A/D = 1.25) than in mice (A/D = 0.41). This is consistent with experimental studies in which diurnal animals generally have PRCs with higher A/D ratios than nocturnal animals (Johnson, 1992). In particular, the human PRC has a higher A/D ratio than the mouse PRC for both short and long light pulses (Fig 3C) (Comas et al, 2006; St Hilaire et al, 2012; Ruger et al, 2013). This supports our proposal that the PF‐670‐induced phase delay is also strongly attenuated by external light in humans, similar to NHPs.
Figure 3. Inter‐ and intraspecies variability in photosensitivity causes variation in the effect of PF‐670.
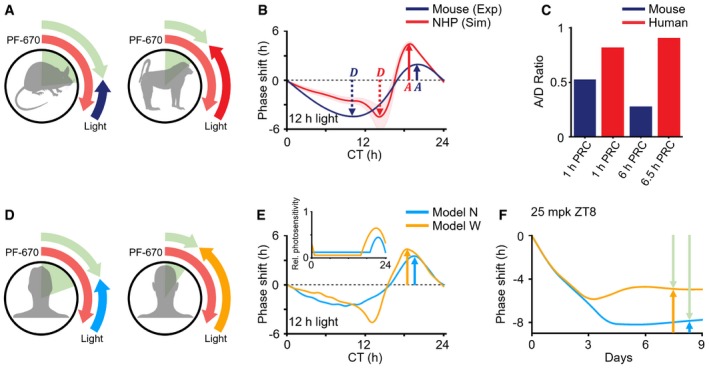
- The stronger attenuating effect of light in diurnal NHPs than in nocturnal rodents leads to the interspecies variation observed in the response to PF‐670.
- The simulated PRCs of diurnal NHPs have a higher ratio of maximal magnitudes between advance and delay zones (A/D = 1.25) than that of the experimentally measured nocturnal mouse PRC (A/D = 0.41), which is adopted from (Comas et al, 2006). Here, the red line and red range represent the mean ± SD of the simulated 12‐h light PRCs of the NHP model with different gating and adaptation (Fig 2Aii).
- The effect of PF‐670 can be heterogeneous due to interindividual variability in photosensitivity.
- Model N with a narrow high photosensitivity zone (inset) simulates a smaller magnitude of the advance zone of 12‐h light PRC than model W with a wide high photosensitivity zone.
- Thus, model N simulates a larger constant stable phase delay than model W when a single daily 25 mpk dose is given at ZT8 under LD 12:12.
The effect of CK1i depends on individual photosensitivity
Large interindividual variability of photosensitivity up to ~3‐fold has been observed in humans (van der Meijden et al, 2016; Stone et al, 2018; Watson et al, 2018). Given the strong attenuating effect of light on CK1δ/ε inhibition in humans (Fig 3C), we would expect a potentially large variation in CK1i response in individuals with different levels of photosensitivity (Fig 3D). To investigate this, among the 10 pairs of gating and adaptation (Fig 2Aii), two pairs were chosen: One has a narrower high photosensitivity zone than the other (Fig 3E inset) (see Fig EV4A and Dataset EV2 for details). Thus, the model with the narrow high photosensitivity zone (model N; Fig 3E) simulates the smaller magnitude of the advance zone of light PRC than the model with the wide high photosensitivity zone (model W; Fig 3E). Due to the weaker attenuating effect of light in model N than in model W (Fig 3E), PF‐670 induced a larger phase delay in model N (Fig EV4B and C). For instance, when a single daily 25 mpk dose is given at ZT8, the PF‐670‐induced phase delay is balanced with the light‐induced phase advance, and reaches the equilibrium point after 1 week of dosing (Fig 3F) (Kim et al, 2013). At this point, with a weaker attenuating effect of light in model N, the constant stable phase delay induced by PF‐670 is larger than in model W. Furthermore, when a higher dose is used, the effect of CK1i under LD differs qualitatively depending on individual photosensitivity: The PF‐670‐induced phase delay overcomes the light‐induced phase advance, and thus, it accumulates in model N while the phase shift unstably alternates between delay and advance in model W due to amplitude suppression (Fig EV4D–G) (Diekman & Bose, 2018). Thus, the degree of photosensitivity of the individual likely has dramatic consequences for the effect of clock‐modulating drugs.
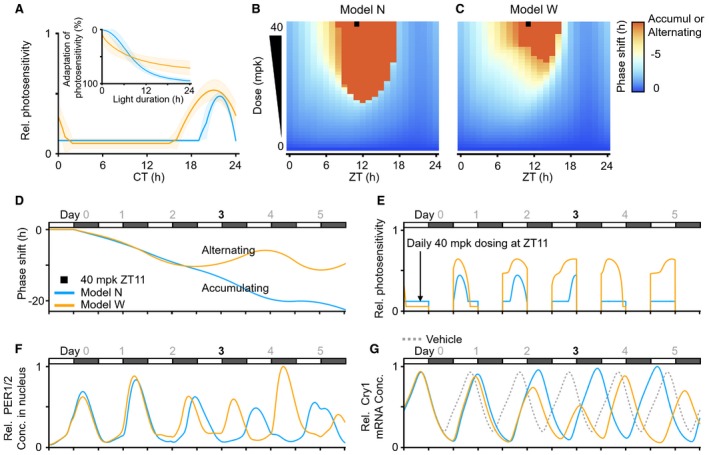
Figure EV4. The effect of PF‐670 highly depends on the individual photosensitivity.
-
AThe models with 10 pairs of gating and adaptation for light in Fig EV2F are divided into two classes: the models with gatings having a narrow (blue) or wide (orange) range of high photosensitivity. Among each class, we chose a representative model: The representative model with the gating having a narrow (wide) range of high photosensitivity zone is referred as model N (W) (see Dataset EV2 for details). The line and colored range represent the mean ± SD of the gatings and adaptations of each class, respectively.
-
B, CThe phase delay induced by LD single daily dosing for 27 days with various dose levels and dosing times was simulated. Overall, model N (B) simulates a larger phase delay than model W (C) due to the weaker attenuating effect of light (Fig 3E). Here, LD 12:12 is used.
-
DA single daily 40 mpk dosing at ZT11 (black square at B) leads to a continually accumulating phase delay in model N as the dosing overcomes the attenuating effect of light, unlike a low dose (Fig 3F). On the other hand, despite the same regimen (black square at C), model W simulates an unstable alternating phase shift between delay and advance from day 3.
-
E–GSuch an alternating phase shift in model W is due to a high dose‐induced amplitude suppression. When the single daily dosing delays the circadian phase by ˜10 h, the circadian time during the daytime corresponds to the time when photosensitivity is high in model W (CT14‐CT2) (A and day 3; E). This prevents the decrease in the levels of PER1/2 proteins during the falling phase (daytime at day 3; F). Furthermore, the increase in the level of PER1/2 proteins is enhanced when PF‐670 is dosed at ZT11 as it stabilizes PER1/2 protein. Due to this synergistic effect between light and PF‐670, the level of PER1/2 protein in model W greatly increases (nighttime at day 3; F) compared with model N, which leads to a strong repression of the transcription for clock genes such as Cry1 mRNA and thus the amplitude suppression at day 3 unlike in model N (day 3; G). This amplitude suppression causes the circadian rhythms to reach a singular point (Diekman & Bose, 2018) and thus leads to an alternating phase shift (D). The vehicle in (G) represents a mean of relative Cry1 mRNA abundances simulated with models N and W.
The effect of CK1i becomes stronger as PER2 levels increase
Due to the larger phase delay induced by PF‐670 compared to other clock‐modulating drugs (Subramanian & Subbaraj, 1993; Badura et al, 2007; Burgess et al, 2010), a daily dosing of PF‐670 can induce a stable phase delay even in the presence of daylight cycle (Fig 3F). This suggests that daily dosing of CK1i could be a promising first pharmacological candidate for ASPD. However, as the effect of PF‐670 depends on the dose level and dosing time, those values need to be carefully selected. This raises the question of what constitutes a precise dosing regimen to treat ASPD. For instance, to treat ASPD patients with a 4 h more advanced circadian phase and sleep schedule than healthy people, one candidate regimen would be a single daily dosing, which is known to induce the ~4‐h stable phase delay in healthy people. Alternatively, dosing time may need to be advanced by 4 h to reflect the advanced circadian phase of ASPD patients compared with healthy people, as is done in timed phototherapy (Bjorvatn & Pallesen, 2009; Zhu & Zee, 2012). Furthermore, dosing regimen could differ depending on the genetics and environmental lighting conditions of ASPD patients.
To investigate this, we performed a series of in silico experiments using the systems chronopharmacology model. We first identified four dosing regimens that would induce the ~4‐h stable phase delay in the WT model having normal circadian phase, which mimics healthy people: single daily 26 mpk, 24 mpk, 22 mpk, or 19 mpk dosing at ZT4, 5, 6, or 7, respectively (blue circles in WT; Fig 4B) (see Dataset EV2 for details of the WT model). Then, these dosing regimens are applied to eight different ASPD models, which we developed by incorporating various mutations including PER2 FASPS (Toh et al, 2001; Vanselow et al, 2006) into the WT model (see Dataset EV3 and Materials and Methods for details). These ASPD models simulate a shorter period than the WT model in DD and thus have a ~4‐h more advanced circadian phase in LD (Fig 4A). Interestingly, the dosing regimens inducing the ~4‐h phase delay in the WT model led to much larger phase delays in all of the ASPD models, with large variations (blue circles; Fig 4B). On the other hand, when the dosing times were advanced by 4 h reflecting the advanced circadian phase of the ASPD models, as is done in timed phototherapy (Bjorvatn & Pallesen, 2009; Zhu & Zee, 2012), the model simulated phase delays closer to the desired ~4‐h phase delay (red squares; Fig 4B). Nevertheless, they were still larger. We found that this is mainly due to the weaker attenuating effect of light in the ASPD models than in the WT model (Fig EV5A). Importantly, despite the same clinical feature of the ASPD models (4‐h advance) (Fig 4A), the PF‐670‐induced phase delay is considerably different (e.g., Per2t and Cry1t; Fig 4B).
Figure 4. CK1i induces a larger phase delay as PER2 levels increase depending on the molecular cause of ASPD and external lighting conditions.
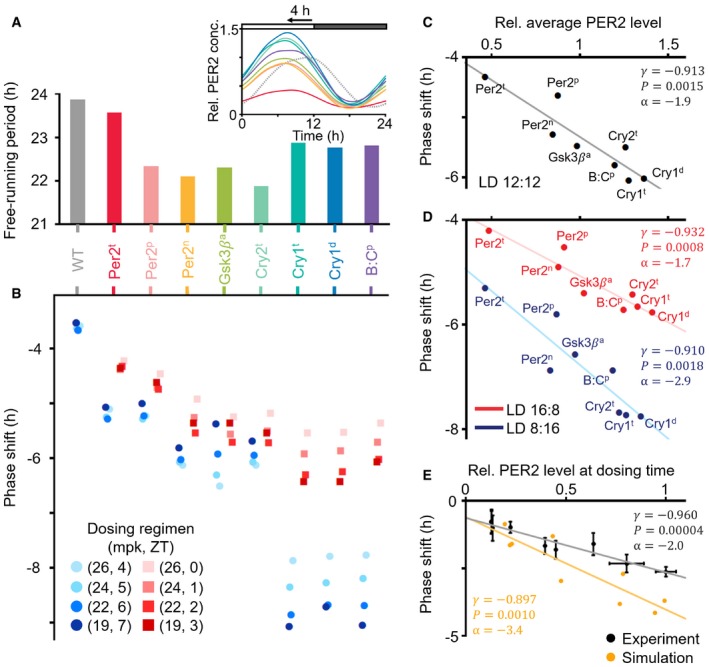
- The simulated period of ASPD models is shorter than that of the WT model in DD. Thus, they have a ˜4 h more advanced phase than the WT model in LD 12:12 (inset). PER2 protein concentrations in each mutant are normalized to the maximum PER2 concentration of the WT model (dashed gray line; inset).
- The phase delays induced by a single daily dosing of PF‐670 were simulated. The dosing regimens inducing ˜4‐h phase delays in the WT model led to much larger phase delays in all ASPD models with large inter‐ and intravariations (blue circles). When the dosing times are advanced by 4 h reflecting the advanced phase of the ASPD models, the phase delays become closer to but still larger than the ˜4‐h phase delays (red squares).
- As the average PER2 level is higher in the ASPD models (A), PF‐670 induces a larger phase delay. Here, the phase shift is the average of the phase delays represented as the red squares in (B). The line represents the least‐square fitting line. r and P denote the Pearson's correlation coefficient and P‐value of Pearson's correlation test, respectively. α denotes the slope of the least‐square fitting line.
- The ASPD models simulate the larger PF‐670‐induced phase delay in LD 8:16 than in LD 16:8 due to the higher PER2 level in LD 8:16 than in LD 16:8 when dosing occurs (Fig EV5G).
Figure EV5. The response of the ASPD models to light and PF‐670: PER2 level is strongly correlated with the effect of CK1i.
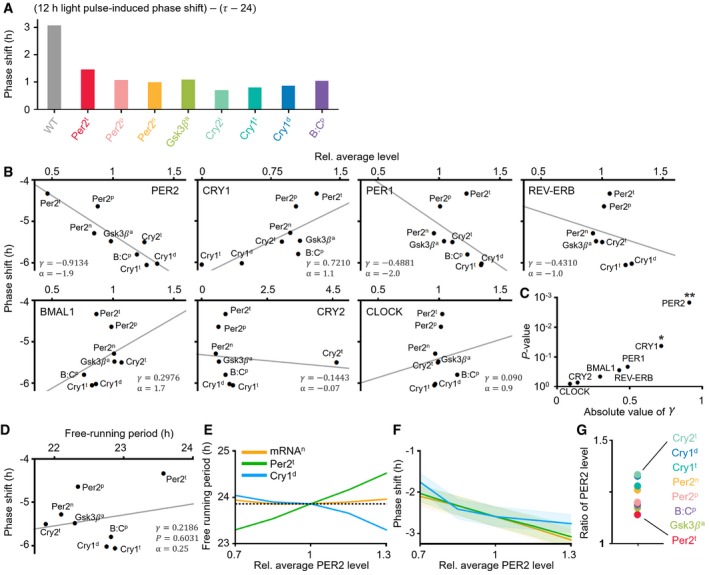
-
AAll ASPD models simulate the weaker light‐induced attenuation of the effect of PF‐670 than the WT model when PF‐670 induces the 4‐h phase delay under LD 12:12. As the free‐running period (τ) of the ASPD models is shorter than 24 h (Fig 4A), the light needs to induce the phase shift (24‐τ) to equalize the period of ASPD models to 24 h. Thus, by subtracting 24‐τ from a light‐induced phase shift to remove the entraining effect of light, the light‐induced attenuation of the effect of PF‐670 is estimated. The light‐induced phase shift is simulated using a 12‐h light pulse given at CT20 since it corresponds to the light pulse in LD 12:12 when PF‐670 induces a 4‐h phase delay. See Datasets EV2 and EV3 and Materials and Methods for details of the WT model and ASPD models.
-
BThe correlation between the effect of PF‐670 (red squares; Fig 4B) and the average level of various core clock proteins of the ASPD models (Dataset EV3). The line represents the least‐square fitting line. r and α denote the Pearson's correlation coefficient and the slope of the least‐square fitting line.
-
CThe average protein level of PER2 is significantly more strongly correlated with the effect of PF‐670 than that of other clock proteins. * and ** indicates P < 0.05 and P < 0.001, respectively. Here, P‐value is estimated by Pearson's correlation test.
- D
-
E, FAs PER2 level increases in the model, regardless of the change in the free‐running period (E), the effect of CK1i becomes stronger (F). The mRNAn (orange line), Per2t (green line), and Cry1d (blue line) were simulated by perturbing the parameters tmc, trPt, and uro, which describe the nuclear export rate of mRNA, the transcription rate for Per2, and the degradation rate for CRY1, respectively (see Dataset EV1 for details). The line and colored range in F represent the mean ± SEM of the phase delays induced by a single 20 mpk DD dosing at CT1, 2, 3, …, 24.
-
GThe ratio of average PER2 levels in LD 8:16 and LD 16:8 when dosing occurs (red squares; Fig 4B) is higher than 1 in all ASPD models: PER2 level is higher in LD 8:16 than in LD16:8 when dosing occurs.
To identify the source of the heterogeneous drug response among the ASPD models, we estimated the relationship between the effect of PF‐670 (red squares; Fig 4B) and the average level of various core clock molecules of the ASPD models (Fig EV5B). Interestingly, we found that the average protein level of PER2 is significantly more strongly correlated with the effect of PF‐670 than that of the other clock proteins (Fig EV5C). Specifically, as the average PER2 protein levels (Fig 4A inset) increase in the ASPD models, the effect of PF‐670 (red squares; Fig 4B) becomes stronger (Fig 4C). This correlation is not due to the different free‐running period of the ASPD models (Fig EV5D) and appears to stem from the fact that phosphorylation of PER2 by CK1δ/ε is the target of PF‐670. Indeed, when we increase PER2 level in the model by tuning model parameters, the effect of CK1i becomes stronger regardless of its effect on the free‐running period (Fig EV5E and F). Furthermore, PER2 abundance also explains the different effect of PF‐670 depending on day length: Due to the higher PER2 abundance at the dosing times in LD 8:16 than in LD 16:8 (Fig EV5G), a larger PF‐670‐induced phase delay is simulated in LD 8:16 than in LD 16:8 (Fig 4D). To support these in silico predictions (Fig 4C and D), we estimated the relationship between the effect of PF‐670 and PER2 levels from experimentally measured PRC to PF‐670 (Badura et al, 2007) and time series of PER2 levels in the SCN (Amir et al, 2004). Indeed, we found a strong positive correlation between them, which is also recapitulated by our model (Fig 4E).
Adaptive chronotherapeutic approach identifies precise dosing times
We found that the molecular cause of ASPD and the patient's environmental lighting conditions dramatically influence PER2 levels and thus the effect of CK1i (Fig 4C and D). This dependence of the drug effect on PER2 level could be used to identify a precise dosing regimen. That is, due to the accumulation of PER2 during the daytime (Fig 4A), the drug effect becomes stronger or weaker when the dosing time of day is delayed or advanced, respectively (Fig EV4B and C) (Badura et al, 2007; Kim et al, 2013). By using this feature, we developed an adaptive chronotherapeutic approach: If the current dosing regimen leads to a weaker or stronger drug effect than the desired one, the dosing time is delayed or advanced by 1 h, respectively, until the desired phase delay is achieved (Figs 5A and EV6A).
Figure 5. An adaptive chronotherapeutic approach identifies the precise dosing regimen.
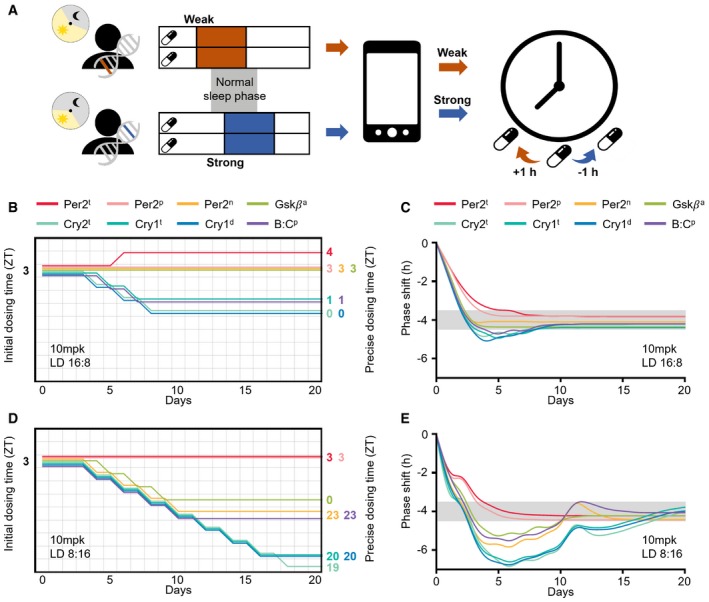
-
ABy tracking the patients’ heterogeneous drug response caused by different genetics and environments, adaptive chronotherapeutics adjusts the dosing time: If the current dosing regimen leads to a weaker or stronger drug effect than the desired one, the dosing time is delayed or advanced by 1 h, respectively (see Fig EV6A for details).
-
B, CBy adjusting the initial dosing time (ZT3) according to the adaptive chronotherapeutics, the precise dosing time can be obtained for each ASPD model (colored number; B), with which a single daily 10 mpk dosing leads to the desired ˜4‐h phase delay in LD 16:8 (gray range; C).
-
D, EWhen day length decreases to 8 h (LD 8:16), the precise dosing times are considerably advanced.
Figure EV6. Two types of adaptive chronotherapeutics.
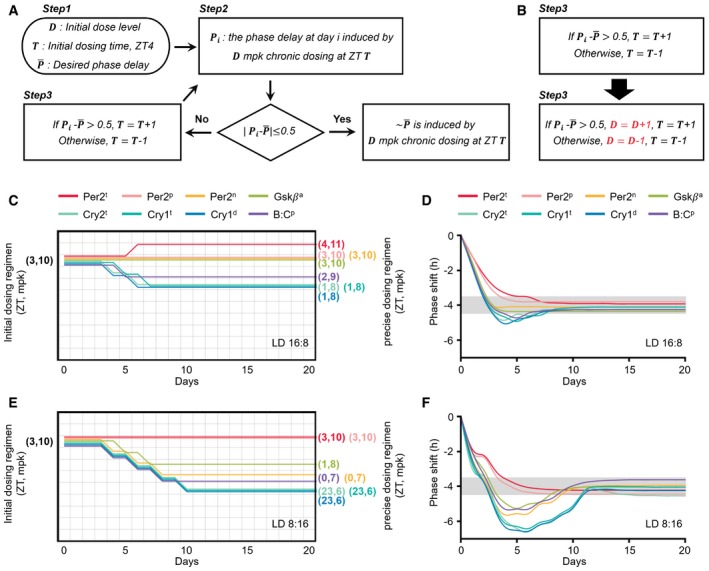
-
ADetailed diagram describing an adaptive chronotherapeutics which identifies a precise dosing regimen by solely adjusting dosing time. Step 1: 10 mpk at ZT3 is recommended so that the following dosing time can be flexibly advanced or delayed depending on current drug effect with a low probability of leading to an accumulating or an alternating phase shift (Fig EV4B–D). Step 2: Single daily dosing is performed until a stable phase delay is observed (i.e., the phase delays at two consecutive days, i−1 and i, are similar, < 0.2 h). However, if a larger phase delay than the desired one is achieved before the stable phase delay is obtained (i.e., > 0.5 h), the dosing is immediately stopped. Step 3: Depending on the drug effect observed in Step 2, the dosing regimen is modified (Fig 5A). If the induced phase delay is smaller than the desired phase delay (i.e., > 0.5 h), the dosing time is delayed by 1 h. Otherwise ( > 0.5 h), the dosing time is advanced by 1 h. Step 2 and Step 3 are repeated until a constant stable phase delay similar to the desired one ( 0.5 h) is achieved.
-
BModification of the adaptive chronotherapeutics (A) to adjust dose level as well as dosing time simultaneously: If the induced phase delay is smaller than the desired phase delay (i.e., > 0.5 h), the dose level is increased by 1 mpk and the dosing time is delayed by 1 h. Otherwise ( > 0.5 h), the dose level is decreased by 1 mpk and the dosing time is advanced by 1 h.
-
C–FDue to the simultaneous change in dose level and dosing time (B), the precise dosing regimen can be more rapidly achieved with a lower dose level than the adaptive chronotherapeutics solely adjusting the dosing time (Fig 5B–E).
To test whether this approach works as expected despite the large perturbation of the circadian clock (e.g., PER2 abundance and phase) by genetic variation or environmental lighting conditions, we applied the adaptive chronotherapeutic approach to all ASPD models with varying day lengths. Specifically, a single daily 10 mpk dose was given at ZT3 to the ASPD models in LD 16:8 (day 1; Fig 5B). Then, depending on the induced phase delay, the initial dosing time (ZT3) was adjusted according to the chronotherapeutics (Figs 5A and EV6A). This identified the precise dosing time (colored number; Fig 5B) inducing the desired phase delay for all ASPD models regardless of their molecular cause (gray range; Fig 5C). This chronotherapeutic approach also successfully identified the precise dosing time when day length changes to LD 8:16 (Fig 5D and E). Due to the stronger drug effect in LD 8:16 than in LD 16:8 (Fig 4D), the identified precise dosing time was more advanced in LD 8:16 (Fig 5D) than in LD 16:8 (Fig 5B). Interestingly, even if ASPD models have the same precise dosing time in LD 16:8 (e.g., ZT3 for Per2P and Per2n; Fig 5B), their precise dosing times in LD 8:16 can be different (e.g., ZT3 for Per2P and ZT23 for Per2n; Fig 5D). This further emphasizes the need for an adaptive chronotherapeutic approach to reflect the patient's environmental lighting condition.
Although solely adjusting the dosing time was successful (Fig 5B–E), it took longer to achieve the desired phase delay (e.g., 18 days for Cry2t in LD 8:16; Fig 5D). Furthermore, the identified precise dosing time could be one at which it is inconvenient to take a drug (e.g., ZT19 for Cry2t; Fig 5D). This problem can be resolved if dose level and dosing time are adjusted simultaneously (Fig EV6B–F).
Discussion
We unexpectedly found that the effect of CK1i on circadian phase differs between nocturnal mice and diurnal NHPs due to their different levels of photosensitivity: Light attenuates the effect of CK1i more strongly in NHPs than in mice. Such a strong attenuating effect of light on a drug‐induced phase delay is expected to exist in humans as their light PRC has a large magnitude of the advance zone (St Hilaire et al, 2012; Ruger et al, 2013). Thus, the phase delay induced by melatonin would also be expected to be strongly attenuated by light. This would explain its weak effectiveness in treating ASPD and jetlag after westward travel in a daylight cycle (Sack et al, 2007; Zhu & Zee, 2012; Spiegelhalder et al, 2017). These results indicate that such interspecies difference in photosensitivity should be considered when translating the efficacy of clock‐modulating drugs from nocturnal mice to diurnal humans.
Our systems chronopharmacology model based on experimental data from NHPs and humans predicts that a regimen reflecting a patient's individual circadian phase can achieve a more precise therapeutic effect than a non‐circadian‐based regimen. This may explain why the current circadian‐based treatment can alleviate sleep disorders (Zhu & Zee, 2012; Sletten et al, 2018). However, our model predicts that even if the circadian phase of patients is similar, their response to CK1i can be considerably different depending on the molecular cause and the environmental lighting condition (Fig 4A–D). We found that such variability in the CK1i effect is mainly due to altered PER2 abundances (Figs 4C–E and EV5B–F). While the interspecies difference in PER2 abundance has not been investigated, it may contribute to the interspecies variability in the CK1i effect (Figs 1E–G and 3A). Furthermore, the interspecies difference in the phase of PER2 rhythms (Vosko et al, 2009; Zhang et al, 2014; Millius & Ueda, 2018; Mure et al, 2018), which leads to the interspecies difference in PER2 abundance when dosing occurs, could also be the source of the interspecies variability in the CK1i effect. It would be interesting to investigate such a relationship between the effect of CK1i and PER2 abundance, and extend this to other clock‐modulating drugs and their target molecules (e.g., KL001 targeting CRY; Hirota et al, 2012). Furthermore, as PER2 regulates the stability of p53, a key tumor suppressor (Gotoh et al, 2016), altered PER2 levels depending on genetics and environmental lighting conditions could vary the level of p53 as well. This may also explain the large inter‐ and intrapatient variability in the chronotherapy of antitumor drugs (Altinok et al, 2009; Levi et al, 2010).
Our work indicates that patient‐specific molecular and lifestyle information should be integrated to achieve a desired therapeutic effect of clock‐modulating drugs (Fig 4B–D). However, because obtaining such information can be challenging, we developed an adaptive chronotherapeutics, which identifies the precise dosing time to achieve normal circadian phase by tracking the patient's drug response (Figs 5A and EV6A and B). This adaptive chronotherapeutics requires a precise quantification of the drug‐induced circadian phase shift in daily life. Recently, to overcome the insufficient accuracy of actigraphy and labor‐intensive measurement of dim light melatonin onset (Duffy & Dijk, 2002; Ancoli‐Israel et al, 2003), various methods have been developed, which are based on the metabolite timetable (Kasukawa et al, 2012), one‐time phase estimation (Wittenbrink et al, 2018), or sleep‐tracking using mobile phone (Walch et al, 2016). With these advances, the adaptive chronotherapeutics could play a critical role in incorporating biomarker data and providing real‐time patient‐tailored chronotherapy via smart devices (i.e., digital medicine) (Elenko et al, 2015).
Materials and Methods
Reagents and Tools table
| Reagent/Resource | Reference or Source | Identifier or catalog number |
|---|---|---|
| Experimental models | ||
| Mauritian Cynomolgus Monkey (Macaca fascicularis) | Charles River BRF, Inc. | |
| PF‐670462 | Pfizer Medicinal Chemistry, Groton, Connecticut USA | |
| Plasma protein binding | Biopharma Drug Disp 23: 327–338 | |
| Brain tissue binding | J Pharm Sci 105: 965–971 | |
| Chemicals, enzymes, and other reagents | ||
| Sulfobutyl ether β‐cyclodextrin | Sigma‐Aldrich | CAT 1065550 |
| Control monkey plasma | Bioreclamation www.bioivt.com | |
| Acetonitrile | Fisher Chemical | CAT A998 |
| Ammonium formate | Sigma‐Aldrich | CAT 78314 |
| Acetic acid | Fisher Chemical | A38‐S |
| Ammonium acetate | Sigma‐Aldrich | A7262 |
| Isopropyl alcohol | Fisher Chemical | A461‐1 |
| Software | ||
| Analyst Software v 1.4.2 | AB Sciex, Inc. https://sciex.com/products/software/analyst-software | |
| Actical Software | Philips Respironics, Bend, Oregon | |
| ClockLab v6.0.52 | Actimetrics, Inc. Wilmette, IL | |
| Wolfram mathematica | Wolfram Research, Inc. https://wolfram.com | |
| GraphPad Quickcalcs software | GraphPad Software, Inc. http://www.graphpad.com/quickcalcs/index.cfm | |
| Other | ||
| Actical activity monitor | Philips Respironics, Bend, Oregon | Model IPX7 |
| ActiReader | Philips Respironics, Bend, Oregon | Model 1063543 |
| Actical animal armored primate collar | Philips Respironics, Bend, Oregon | CAT 198‐0032‐00M |
| Digital light meter | Amprobe, Inc. | Model LM631A |
| Purina Lab Diet | W.F. Fisher and Son | CAT 5K91 |
| Vacutainer spray‐coated K2EDTA | Becton Dickinson Company | CAT 367835 |
| AB Sciex API4000 tandem quadrupole mass spectrometer | AB Sciex, Inc. https://sciex.com/products/mass-spectrometers/triple-quad-systems/api-4000-system | |
| Shimadzu LC20AD pumps | Shimadzu Scientific Instruments https://www.ssi.shimadzu.com/products/liquid-chromatography/lc-20ad.html | |
| CTC PAL autosampler | Leap Technologies https://www.leaptec.com/collections/instruments/products/pal3 | |
| Synergi Max RP UPLC analytical liquid chromatography column 4 μ, 80 Å, 2 × 30 mm | Phenomenex | Part 00A‐4337‐B0 |
Methods and Protocols
Behavioral studies
Animals
All procedures involving animals were conducted with the approval of the Pfizer IACUC and were compliant with the Guide for the Care and Use of Laboratory Animals and the regulations and standards of the Animal Welfare Act (9CFR2, 9CFR3). Eight 5‐ to 6‐year‐old male Mauritian Cynomolgus macaques (Charles River BRF, Inc.) SPF for CHV‐1, SRV 1, SRV 2, and SRV 5, STLV1, and SIV were used for behavioral studies. Six animals were pair‐housed and two singly housed. All monkeys were chair‐trained and fitted with collar‐mounted Actical® activity monitors (Philips Respironics, Bend, Oregon, USA). The Actical® Software (Respironics ActiReader) was set to record activity counts in 5‐min bins. ClockLab 6.0.52 (Actimetrics Wilmette, IL) was used to generate actograms and to determine activity onset and free‐running periods. Animals were acclimated and housed in a room under 12:45/11:15 LD with a 45‐min ramping period to full light on/off as simulated dawn/dusk (i.e., at 5:15 am lights started ramping up to full lights on at 6:00 am; at 5:15 pm lights started ramping down to completely off at 6:00 pm). The direct light intensity in the room was 1,300 lux at 1 meter from the floor in the middle of the room, which is measured using a digital light meter (Model LM631A, Amprobe, Everett, WA). For constant dim light conditions, the light intensity was ~14 lux measured at the cage face. Animals were placed under dim light conditions for 2 weeks prior to study onset and were under dim light conditions for 4 months during the study. Animals were fed Purina LabDiet 5K91 (W.F. Fisher and Son) twice daily, and reverse osmosis purified municipal water was provided ad libitum. Daily environmental enrichment was provided in the form of fruit treats and manipulada. Animals were chair‐restrained for drug administration and data download from the Actical® monitors.
The sample size for behavioral studies was determined to detect the desired magnitude of difference (≥ 20 min), and the variance of the estimates was based on previous data where available and setting α = 0.05 and 1‐β = 0.9. One animal was identified as an outlier in a 2‐h jet lag experiment (Fig 2C) by Grubbs’ test (α = 0.01) and was thus excluded from the analysis.
Study design
A cross‐over design was used for each treatment limb, and animals were randomized into 2 groups of 4 prior to each dosing limb. PF‐670462 was dissolved in 20% w/v sulfobutyl ether β‐cyclodextrin, and filter sterilized. Formulations were prepared 1–2 h prior to administration and given subcutaneously for 3 consecutive days at solar time: 4:00 pm in DD and 4:30 pm in LD. Prior to DD dosing (Fig 1C), 4 NHPs were placed in DD for 2 weeks to estimate their free‐running period. Their different intrinsic periods (23.8, 24, 24.1, and 24.3 h) caused them to be initially dosed at different CTs (CT4, 6, 8, and 14 h) since the CT when the initial dosing occurs was calculated by considering the activity onset at the pre‐dosing day as CT0. Prior to LD dosing (Figs 1D and 2E), NHPs were placed in LD for at least 10 days for a stable base line (i.e., entrainment). This allowed for them to be initially dosed at the same ZT11. For the light‐induced phase shift experiment (Fig 2C), following 7 days of stable activity, the time for lights on/off was delayed 2 h for 3 days and then returned to colony normal.
Pharmacokinetic studies
Distribution profiles
PF‐670462 was synthesized and characterized by Medicinal Chemistry at Pfizer Worldwide Research and Development (WRD). Animal studies were performed in accordance with the Guide for the Care and Use of Laboratory Animals (Clark et al, 1996) using protocols reviewed and approved by the WRD IACUC.
-
1
A single dose (10 mpk, SC) of PF‐670462 was prepared in 20% sulfobutyl ether β‐cyclodextrin in water (10 mg/ml).
-
2
The dose was administered (1 ml/kg) to male cynomolgus macaque monkeys by subcutaneous route (N = 2).
-
3
Serial blood samples were collected from the femoral vein at 0, 0.25, 0.5, 0.75, 1, 2, 4, 6, and 24 h post‐dose into Vacutainer (Becton Dickinson Company, Franklin Lakes, NJ) blood collection tubes pretreated with EDTA and placed immediately on ice.
-
4
The whole blood samples were centrifuged for 10 min at 2,300 × g and plasma transferred to clean tubes for storage at −20°C.
Monkey pharmacokinetic samples were prepared and analyzed using a non‐validated bioanalytical assay. For standard curve preparation, the plasma was purchased from Bioreclamation Inc., Hicksville, NY.
-
5
The standard curves were prepared in control cynomolgus monkey plasma via serial dilution at a concentration range of 0.5–1,000 ng/ml.
-
6
An aliquot of plasma (50 μl) was precipitated with acetonitrile (MeCN) (300 μl) containing an internal standard.
-
7
Samples were vortex‐mixed (1 min) and centrifuged (180 rcf for 10 min) to afford supernatant (250 μl), which was transferred to a 96‐well plate.
-
8
Supernatants were evaporated under N2 at 37°C and reconstituted in 25% MeCN in H2O (100 μl).
Samples were analyzed by an LC‐MS/MS comprising an AB Sciex API 4000 tandem quadrupole mass spectrometer (AB Sciex Inc., Ontario, Canada) with a TurboIonSpray probe (AB Sciex Inc., Ontario, Canada), tertiary Shimadzu LC20AD pumps (Shimadzu Scientific Instruments, Columbia, MD), and a CTC PAL autosampler (Leap Technologies, Carrboro, NC). Instrument settings and potentials were adjusted to provide optimal data. All raw data were processed using Analyst Software version 1.4.2 (AB Sciex Inc., Ontario, Canada).
PF‐670462 and its internal standard within sample aliquots (5 μl) were eluted at 0.5 ml/min on a Synergi Max RP analytical column (4 μ, 80 Å, 2 × 30 mm; Phenomenex, Torrance, CA) using 10 mM ammonium formate in 0.1% HCO2H (solvent A) and MeCN (solvent B) via the following gradient: 0 to 0.75 min, 5% solvent B; 0.75 to 1.25 min, 5 to 90% B; 1.25 to 2.00 min, hold at 90% B; and 2.00 to 2.5 return to 5% B and hold until 3.00 min. Mass spectral data were collected in positive electrospray ionization mode using multiple‐reaction monitoring (MRM) following the m/z 338.2→256.2 fragmentation for PF‐670462.
Plasma protein binding
The plasma‐free fraction of PF‐670462 was determined in monkey plasma by equilibrium dialysis using a previously reported method (Kalvass & Maurer, 2002).
-
1
Aliquots (200 μl, n = 6) of freshly harvested monkey plasma containing PF‐670462 (1 μM) were loaded into a 96‐well equilibrium dialysis apparatus (HTDialysis, Gales Ferry, CT) containing preconditioned Spectra‐Por2 membranes (Spectrum Laboratories Inc., Rancho Dominguez, CA; molecular weight cutoff 12,000–14,000).
-
2
Plasma was dialyzed against an equal volume of phosphate‐buffered saline for 6 h at 37°C.
-
3
After incubation, 150 μl of plasma sample and 150 μl of buffer sample were removed from the apparatus and matrix‐matched for bioanalysis by addition of an equal volume of opposing matrix to yield identical sample composition between buffer and non‐buffer samples.
Protein binding samples were analyzed by HPLC/MS/MS using a non‐validated bioanalytical assay.
-
4
An aliquot of matrix‐adjusted sample (50 μl) was precipitated with acetonitrile (MeCN) (200 μl) containing an internal standard.
-
5
Samples were vortex‐mixed (2 min) and centrifuged (1,811 rcf for 7 min) to afford supernatant (150 μl), which was transferred to a 96‐well plate.
Samples were analyzed by an LC‐MS/MS comprising an AB Sciex API 4000 tandem quadrupole mass spectrometer (AB Sciex Inc., Ontario, Canada) with a TurboIonSpray probe (AB Sciex Inc., Ontario, Canada), tertiary Shimadzu LC20AD pumps (Shimadzu Scientific Instruments, Columbia, MD), and a CTC PAL autosampler (Leap Technologies, Carrboro, NC). Instrument settings and potentials were adjusted to provide optimal data. All raw data were processed using Analyst Software version 1.4.1 (AB Sciex Inc., Ontario, Canada).
PF‐670462 and its internal standard within sample aliquots (10 μl) were eluted at 0.4 ml/min on a Luna C18 analytical column (5 μ, 80 Å, 2.1 × 30 mm; Phenomenex, Torrance, CA) using 50% 20 mM ammonium acetate in 0.1% isopropyl alcohol, and 50% acetonitrile (solvent A) and MeCN (solvent B) via the following gradient: 0–0.5 min, 10% solvent B; 0.5–1.5 min, 10–90% B; 1.5–2.20 min, hold at 90% B; and 2.21 return to 10% B and hold until 3.00 min. Mass spectral data were collected in positive electrospray ionization mode using MRM following the m/z 338.2→256.2 fragmentation for PF‐670462.
The plasma‐free fraction was calculated from the ratio of concentrations determined from the plasma and buffer samples.
Estimation of free brain concentration
Free PF‐670462 brain concentration was estimated from plasma by first correcting for the fraction of unbound PF‐670462 in plasma (fu = 0.15) and subsequently correcting for the free brain and free plasma concentration ratio (Cbu/Cpu) of 1, an approach commensurate with unrestricted equilibrium between the central and plasma compartments as predicted by physiologically based PK modeling (Trapa et al, 2016).
Modeling studies
Mathematical model description
We adopted our previous systems chronopharmacology model (274 variables and 86 parameters), which successfully investigated the effect of PF‐670462 in mice (Kim et al, 2013). This model was developed by extending the Kim–Forger model (Kim & Forger, 2012), a detailed mathematical model of the intracellular mammalian circadian clock, which describes the reactions among core clock molecules (e.g., binding, phosphorylation, subcellular translocation, transcription, and translation) using ordinary differential equations based on mass action kinetics (181 variables and 75 parameters). To develop the systems chronopharmacology model for NHP (Fig 2A), the original mouse model (Kim et al, 2013) was modified by newly estimating the pharmacokinetic (PK) parameters (Fig 2Ai) and incorporating the gating and adaptation into the light module of the model (Fig 2Aii).
Modification of the PK parameters
The six parameters describing the PK properties of PF‐670462 (e.g., transfer rate between plasma and brain tissue) were modified due to the difference in free PF‐670462 exposure in brain tissues (Fig 1B) and its effect in DD (Fig 1D) between NHPs and mice. Specifically, the parameters were fitted to the disposition profiles of PF‐670 and its DD dosing effect in NHPs (see Fig EV1A–C and Dataset EV1 for details).
Incorporation of gating and adaptation for light into the model
To incorporate the gating into the model, we used the function g, which determines the photosensitivity of the circadian clock at each CT (Fig EV1F). To connect the photo‐insensitive and photosensitive zones of g, a piecewise polynomial interpolation was used (see Code EV1). To incorporate the adaptation into the model, we used the Hill function, which expresses light duration‐dependent reduction in photosensitivity (Fig EV1H).
The values of six parameters determining the functions of gating and adaptation (Dataset EV2) were estimated with the simulated annealing (SA) method (Gonzalez et al, 2007) and post‐filtering in two steps. In the first round, we found 991 parameter sets with which the model accurately simulates the phase delay of NHPs induced by the 3‐day LD dosing and the magnitude of human PRC to a 6.7‐h light pulse (Fig EV2A) using SA method with the cost function:
where
m and M are the min and max of the simulated PRC to a 6.7‐h light pulse, respectively. = −3.26 and = 2.63 h are the min and max of the human PRC to a 6.7‐h light pulse, respectively (Khalsa et al, 2003). x j and are the simulated phase delay and experimentally measured phase delay (Fig 1D) on day j of 3‐day LD dosing, respectively.
In the second round, using post‐filtering, we filtered in the final 10 parameter sets (Dataset EV2) with which the model accurately simulates the type 1 PRC to a 12‐h light pulse and the human PRC to a 6.7‐h light pulse and to 3‐cycle 5‐h light pulses (Fig EV2).
To estimate the input CT for g even when the circadian phase is perturbed, we constructed the function p which determines the CT from an internal pace marker: the phase angle of the limit cycle of two clock variables, revng and revnp in the model (Fig EV1G). We first interpolated the phase angles of the limit cycle of revng and revnp to the CTs. Then, we composed the interpolation function with the function, tan−1 (revng(t), revnp(t)), which estimates the phase angle of the limit cycle from the concentrations of revng and revnp at t. As g·p is the composite of the interpolation functions, which do not have explicit form, including g·p into the model increases the computational cost of the simulation. Thus, the approximated g·p using Fourier series was used, which accurately determines the phase‐dependent photosensitivity even when the circadian phase is altered by PF‐670462 or light.
Development of the ASPD models
To develop the ASPD models (Dataset EV3), we investigated the modification of which parameter allows for advancing the phase by ~4 h from the WT model (Fig 4A), reflecting the advanced circadian phase of ASPD patients (Jones et al, 1999). In this process, to reflect the advanced circadian phase, the phase of gating function g is also advanced by 4 h.
Model assumptions
The model of the intracellular mammalian circadian clock, Kim–Forger model, which was developed to accurately simulate the mouse SCN (Kim & Forger, 2012), was used for the new NHP model as clock gene expression profiles in the SCN of cynomolgus monkeys have not been measured. Nevertheless, due to the new light module, the NHP model simulates more advanced clock gene expression compared with the original mouse model under LD, which is consistent with the advanced clock gene expression seen in the SCN of baboons compared with that of mice under LD (Mure et al, 2018).
The pharmacodynamic parameters, describing the intracellular action of PF‐670462 (e.g., binding of PF670 with CK1δ/ε), of the original systems chronopharmacology model (Kim et al, 2013), were kept as they are expected to be similar between NHPs and mice.
The reduced photosensitivity due to adaptation for light during daytime is assumed to be completely recovered after nighttime if it is long enough (≥ 8 h).
To simulate the phase shift of activity onsets with the model, we used the phase shift of the simulated BMAL1 gene expression profile as the phase of BMAL1 gene expression profiles and activity onset is highly correlated (Kiessling et al, 2010). However, as the phases of all clock gene expression profiles are tightly interlocked in the model, the result changes little even if the phase of other clock gene expression profiles is used.
Simulation
All the simulations and parameter searches were performed using MATHEMATICA 11.0 (Wolfram Research, Champaign, IL) with a computer cluster composed of seven machines where each machine is equipped with two Intel Xeon SP‐6148 CPUs (2.4 GHz, 20C), 192GB RAM, and the operating system CentOS 7.4 64bit.
Statistical analysis
For this study, one‐way ANOVA, two‐way ANOVA, and Pearson's correlation test were used to determine statistical significance. Outliers were detected by Grubbs’ test. They are each detailed along with the relevant parameters in the main text, figures, and figure legends. A P‐value of < 0.05 was considered statistically significant. One‐way ANOVA, two‐way ANOVA, and Pearson's correlation test were performed using MATHEMATICA 11.0. Grubbs’ test was performed using GraphPad Quickcalcs software (GraphPad Software Inc, La Jolla, CA, USA, available at http://www.graphpad.com/quickcalcs/index.cfm).
Author contributions
CC, GJD, TW, and JKK designed the study. GJD and ACD performed the experiments. DWK and JKK performed the computational modeling and mathematical analysis. DWK, GJD, XC, ACD, and JKK analyzed the experimental data. CC, FG, and JKK designed the translation strategy. DWK and JKK wrote the manuscript, and all authors contributed to reviewing the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Expanded View Figures PDF
Table EV1
Table EV2
Dataset EV1
Dataset EV2
Dataset EV3
Code EV1
Review Process File
Acknowledgements
We thank Boseung Choi, Daniel B. Forger, and David M. Virshup for valuable comments and Life Science Editors for editorial assistance. This work was supported by a Pfizer grant to Korea Advanced Institute of Science and Technology (G01160179), the Human Frontiers Science Program Organization (RGY0063/2017), and a National Research Foundation of Korea Grant funded by the Korean Government (NRF‐2016 RICIB 3008468 and NRF‐2017‐Fostering Core Leaders of the Future Basic Science Program/Global Ph.D. Fellowship Program).
Mol Syst Biol. (2019) 15: e8838
Contributor Information
Cheng Chang, Email: cheng.chang@pfizer.com.
Jae Kyoung Kim, Email: jaekkim@kaist.ac.kr.
Data availability
The MATHEMATICA codes used in this study are available in Code EV1 and the following database: https://github.com/daewookkim/Non-human-primate-circadian-clock-model-including-CK1-inhibitor.
References
- Albrecht U, Sun ZS, Eichele G, Lee CC (1997) A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light. Cell 91: 1055–1064 [DOI] [PubMed] [Google Scholar]
- Altinok A, Levi F, Goldbeter A (2009) Identifying mechanisms of chronotolerance and chronoefficacy for the anticancer drugs 5‐fluorouracil and oxaliplatin by computational modeling. Eur J Pharm Sci 36: 20–38 [DOI] [PubMed] [Google Scholar]
- Amir S, Lamont EW, Robinson B, Stewart J (2004) A circadian rhythm in the expression of PERIOD2 protein reveals a novel SCN‐controlled oscillator in the oval nucleus of the bed nucleus of the stria terminalis. J Neurosci 24: 781–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancoli‐Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP (2003) The role of actigraphy in the study of sleep and circadian rhythms. Sleep 26: 342–392 [DOI] [PubMed] [Google Scholar]
- Aryal RP, Kwak PB, Tamayo AG, Gebert M, Chiu PL, Walz T, Weitz CJ (2017) Macromolecular assemblies of the mammalian circadian clock. Mol Cell 67: 770–782 e776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badura L, Swanson T, Adamowicz W, Adams J, Cianfrogna J, Fisher K, Holland J, Kleiman R, Nelson F, Reynolds L et al (2007) An inhibitor of casein kinase I epsilon induces phase delays in circadian rhythms under free‐running and entrained conditions. J Pharmacol Exp Ther 322: 730–738 [DOI] [PubMed] [Google Scholar]
- Bjorvatn B, Pallesen S (2009) A practical approach to circadian rhythm sleep disorders. Sleep Med Rev 13: 47–60 [DOI] [PubMed] [Google Scholar]
- Burgess HJ, Revell VL, Molina TA, Eastman CI (2010) Human phase response curves to three days of daily melatonin: 0.5 mg versus 3.0 mg. J Clin Endocrinol Metab 95: 3325–3331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R, Gkogkas CG, de Zavalia N, Blum ID, Yanagiya A, Tsukumo Y, Xu H, Lee C, Storch KF, Liu AC et al (2015) Light‐regulated translational control of circadian behavior by eIF4E phosphorylation. Nat Neurosci 18: 855–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JD, Gebhart GF, Gonder JC, Keeling ME, Kohn DF (1997) Special report: the 1996 guide for the care and use of laboratory animals. ILAR J 38: 41–48 [DOI] [PubMed] [Google Scholar]
- Cohen R, Kronfeld‐Schor N, Ramanathan C, Baumgras A, Smale L (2010) The substructure of the suprachiasmatic nucleus: similarities between nocturnal and diurnal spiny mice. Brain Behav Evol 75: 9–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comas M, Beersma DG, Spoelstra K, Daan S (2006) Phase and period responses of the circadian system of mice (Mus musculus) to light stimuli of different duration. J Biol Rhythms 21: 362–372 [DOI] [PubMed] [Google Scholar]
- Dibner C, Schibler U, Albrecht U (2010) The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol 72: 517–549 [DOI] [PubMed] [Google Scholar]
- Diekman CO, Bose A (2018) Reentrainment of the circadian pacemaker during jet lag: east‐west asymmetry and the effects of north‐south travel. J Theor Biol 437: 261–285 [DOI] [PubMed] [Google Scholar]
- Ding JM, Faiman LE, Hurst WJ, Kuriashkina LR, Gillette MU (1997) Resetting the biological clock: mediation of nocturnal CREB phosphorylation via light, glutamate, and nitric oxide. J Neurosci 17: 667–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JE, Dijk DJ (2002) Getting through to circadian oscillators: why use constant routines? J Biol Rhythms 17: 4–13 [DOI] [PubMed] [Google Scholar]
- Elenko E, Underwood L, Zohar D (2015) Defining digital medicine. Nat Biotechnol 33: 456–461 [DOI] [PubMed] [Google Scholar]
- Gallego M, Virshup DM (2007) Post‐translational modifications regulate the ticking of the circadian clock. Nat Rev Mol Cell Biol 8: 139–148 [DOI] [PubMed] [Google Scholar]
- Geier F, Becker‐Weimann S, Kramer A, Herzel H (2005) Entrainment in a model of the mammalian circadian oscillator. J Biol Rhythms 20: 83–93 [DOI] [PubMed] [Google Scholar]
- Gonzalez OR, Kuper C, Jung K, Naval PC, Mendoza E (2007) Parameter estimation using simulated annealing for S‐system models of biochemical networks. Bioinformatics 23: 480–486 [DOI] [PubMed] [Google Scholar]
- Gotoh T, Kim JK, Liu J, Vila‐Caballer M, Stauffer PE, Tyson JJ, Finkielstein CV (2016) Model‐driven experimental approach reveals the complex regulatory distribution of p53 by the circadian factor Period 2, pp 13516–13521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Liao HW, Takao M, Berson DM, Yau KW (2002) Melanopsin‐containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 295: 1065–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota T, Lee JW, St John PC, Sawa M, Iwaisako K, Noguchi T, Pongsawakul PY, Sonntag T, Welsh DK, Brenner DA et al (2012) Identification of small molecule activators of cryptochrome. Science 337: 1094–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst SC, Valomon A, Landolt HP (2016) Sleep pharmacogenetics: personalized sleep‐wake therapy. Annu Rev Pharmacol Toxicol 56: 577–603 [DOI] [PubMed] [Google Scholar]
- Inouye ST, Kawamura H (1982) Characteristics of a circadian pacemaker in the suprachiasmatic nucleus. J Comp Physiol 146: 153–160 [Google Scholar]
- Jagannath A, Butler R, Godinho SIH, Couch Y, Brown LA, Vasudevan SR, Flanagan KC, Anthony D, Churchill GC, Wood MJA et al (2013) The CRTC1‐SIK1 pathway regulates entrainment of the circadian clock. Cell 154: 1100–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CH (1992) Phase response curves: what can they tell us about circadian clocks? In Circadian clocks from cell to human, Hiroshige T, Honma K. (eds), pp 209–249. Sapporo: Hokkaido University Press; [Google Scholar]
- Jones CR, Campbell SS, Zone SE, Cooper F, DeSano A, Murphy PJ, Jones B, Czajkowski L, Ptacek LJ (1999) Familial advanced sleep‐phase syndrome: a short‐period circadian rhythm variant in humans. Nat Med 5: 1062–1065 [DOI] [PubMed] [Google Scholar]
- Kaasik K, Kivimae S, Allen JJ, Chalkley RJ, Huang Y, Baer K, Kissel H, Burlingame AL, Shokat KM, Ptacek LJ et al (2013) Glucose sensor O‐GlcNAcylation coordinates with phosphorylation to regulate circadian clock. Cell Metab 17: 291–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalvass JC, Maurer TS (2002) Influence of nonspecific brain and plasma binding on CNS exposure: implications for rational drug discovery. Biopharm Drug Dispos 23: 327–338 [DOI] [PubMed] [Google Scholar]
- Kasukawa T, Sugimoto M, Hida A, Minami Y, Mori M, Honma S, Honma K, Mishima K, Soga T, Ueda HR (2012) Human blood metabolite timetable indicates internal body time. Proc Natl Acad Sci USA 109: 15036–15041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keijzer H, Snitselaar MA, Smits MG, Spruyt K, Zee PC, Ehrhart F, Curfs LMG (2017) Precision medicine in circadian rhythm sleep‐wake disorders: current state and future perspectives. Pers Med 14: 171–182 [DOI] [PubMed] [Google Scholar]
- Khalsa SB, Jewett ME, Klerman EB, Duffy JE, Rimmer DW, Kronauer RE, Czeisler CA (1997) Type 0 resetting of the human circadian pacemaker to consecutive bright light pulses against a background of very dim light. Sleep Res 26: 722 [Google Scholar]
- Khalsa SB, Jewett ME, Cajochen C, Czeisler CA (2003) A phase response curve to single bright light pulses in human subjects. J Physiol 549: 945–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiessling S, Eichele G, Oster H (2010) Adrenal glucocorticoids have a key role in circadian resynchronization in a mouse model of jet lag. J Clin Invest 120: 2600–2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiessling S, Sollars PJ, Pickard GE (2014) Light stimulates the mouse adrenal through a retinohypothalamic pathway independent of an effect on the clock in the suprachiasmatic nucleus. PLoS ONE 9: e92959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JK, Forger DB (2012) A mechanism for robust circadian timekeeping via stoichiometric balance. Mol Syst Biol 8: 630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JK, Forger DB, Marconi M, Wood D, Doran A, Wager T, Chang C, Walton KM (2013) Modeling and validating chronic pharmacological manipulation of circadian rhythms. CPT Pharmacometrics Syst Pharmacol 2: e57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V (2017) Biological timekeeping: clocks rhythms and behaviour. New Delhi: Springer; [Google Scholar]
- Levi F, Okyar A, Dulong S, Innominato PF, Clairambault J (2010) Circadian timing in cancer treatments. Annu Rev Pharmacol Toxicol 50: 377–421 [DOI] [PubMed] [Google Scholar]
- van der Meijden WP, Van Someren JL, Te Lindert BH, Bruijel J, van Oosterhout F, Coppens JE, Kalsbeek A, Cajochen C, Bourgin P, Van Someren EJ (2016) Individual differences in sleep timing relate to melanopsin‐based phototransduction in healthy adolescents and young adults. Sleep 39: 1305–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng QJ, Maywood ES, Bechtold DA, Lu WQ, Li J, Gibbs JE, Dupre SM, Chesham JE, Rajamohan F, Knafels J et al (2010) Entrainment of disrupted circadian behavior through inhibition of casein kinase 1 (CK1) enzymes. Proc Natl Acad Sci USA 107: 15240–15245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millius A, Ueda H (2018) Rhythms: the dark side meets the light. Science 359: 1210–1211 [DOI] [PubMed] [Google Scholar]
- Minors DS, Waterhouse JM, Wirz‐Justice A (1991) A human phase‐response curve to light. Neurosci Lett 133: 36–40 [DOI] [PubMed] [Google Scholar]
- Miyake S, Sumi Y, Yan L, Takekida S, Fukuyama T, Ishida Y, Yamaguchi S, Yagita K, Okamura H (2000) Phase‐dependent responses of Per1 and Per2 genes to a light‐stimulus in the suprachiasmatic nucleus of the rat. Neurosci Lett 294: 41–44 [DOI] [PubMed] [Google Scholar]
- Mure LS, Le HD, Benegiamo G, Chang MW, Rios L, Jillani N, Ngotho M, Kariuki T, Dkhissi‐Benyahya O, Cooper HM et al (2018) Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science 359: eaao0318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimamurthy R, Hunt SR, Lu Y, Fustin JM, Okamura H, Partch CL, Forger DB, Kim JK, Virshup DM (2018) CK1delta/epsilon protein kinase primes the PER2 circadian phosphoswitch. Proc Natl Acad Sci USA 115: 5986–5991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesbitt AD (2018) Delayed sleep‐wake phase disorder. J Thorac Dis 10: S103–S111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ode KL, Ueda HR (2018) Design principles of phosphorylation‐dependent timekeeping in eukaryotic circadian clocks. Cold Spring Harb Perspect Biol 10: a028357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori Y, Kanbayashi T, Sagawa Y, Imanishi A, Tsutsui K, Takahashi Y, Takeshima M, Takaki M, Nishino S, Shimizu T (2018) Low dose of aripiprazole advanced sleep rhythm and reduced nocturnal sleep time in the patients with delayed sleep phase syndrome: an open‐labeled clinical observation. Neuropsychiatr Dis Treat 14: 1281–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki N, Iwata T, Itoh A, Ohta T, Okada T, Kasahara Y (1989) A treatment trial of delayed sleep phase syndrome with triazolam. Jpn J Psychiatry Neurol 43: 51–55 [DOI] [PubMed] [Google Scholar]
- Pilorz V, Cunningham PS, Jackson A, West AC, Wager TT, Loudon AS, Bechtold DA (2014) A novel mechanism controlling resetting speed of the circadian clock to environmental stimuli. Curr Biol 24: 766–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Mori T, Zhang Y, Johnson CH (2015) PER2 differentially regulates clock phosphorylation versus transcription by reciprocal switching of CK1epsilon activity. J Biol Rhythms 30: 206–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refinetti R (2006) Variability of diurnality in laboratory rodents. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 192: 701–714 [DOI] [PubMed] [Google Scholar]
- Regestein QR, Monk TH (1995) Delayed sleep phase syndrome: a review of its clinical aspects. Am J Psychiatry 152: 602–608 [DOI] [PubMed] [Google Scholar]
- Ruger M, St Hilaire MA, Brainard GC, Khalsa SB, Kronauer RE, Czeisler CA, Lockley SW (2013) Human phase response curve to a single 6.5 h pulse of short‐wavelength light. J Physiol 591: 353–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack RL, Auckley D, Auger RR, Carskadon MA, Wright KP Jr, Vitiello MV, Zhdanova IV, American Academy of Sleep M (2007) Circadian rhythm sleep disorders: part II, advanced sleep phase disorder, delayed sleep phase disorder, free‐running disorder, and irregular sleep‐wake rhythm. An American Academy of Sleep Medicine review. Sleep 30: 1484–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeyoshi Y, Taguchi K, Yamamoto S, Takekida S, Yan L, Tei H, Moriya T, Shibata S, Loros JJ, Dunlap JC et al (1997) Light‐induced resetting of a mammalian circadian clock is associated with rapid induction of the mPer1 transcript. Cell 91: 1043–1053 [DOI] [PubMed] [Google Scholar]
- Shinohara Y, Koyama YM, Ukai‐Tadenuma M, Hirokawa T, Kikuchi M, Yamada RG, Ukai H, Fujishima H, Umehara T, Tainaka K et al (2017) Temperature‐sensitive substrate and product binding underlie temperature‐compensated phosphorylation in the clock. Mol Cell 67: 783–798 e720 [DOI] [PubMed] [Google Scholar]
- Skelton RL, Kornhauser JM, Tate BA (2015) Personalized medicine for pathological circadian dysfunctions. Front Pharmacol 6: 125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sletten TL, Magee M, Murray JM, Gordon CJ, Lovato N, Kennaway DJ, Gwini SM, Bartlett DJ, Lockley SW, Lack LC et al (2018) Efficacy of melatonin with behavioural sleep‐wake scheduling for delayed sleep‐wake phase disorder: a double‐blind, randomised clinical trial. PLoS Med 15: e1002587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelhalder K, Nissen C, Riemann D (2017) Clinical sleep–wake disorders II: focus on insomnia and circadian rhythm sleep disorders In Handbook of experimental pharmacology, pp 1–16. Berlin, Heidelberg: Springer; [DOI] [PubMed] [Google Scholar]
- Sprouse J, Reynolds L, Swanson TA, Engwall M (2009) Inhibition of casein kinase I epsilon/delta produces phase shifts in the circadian rhythms of Cynomolgus monkeys. Psychopharmacology 204: 735–742 [DOI] [PubMed] [Google Scholar]
- Sprouse J, Reynolds L, Kleiman R, Tate B, Swanson TA, Pickard GE (2010) Chronic treatment with a selective inhibitor of casein kinase I delta/epsilon yields cumulative phase delays in circadian rhythms. Psychopharmacology 210: 569–576 [DOI] [PubMed] [Google Scholar]
- St Hilaire MA, Gooley JJ, Khalsa SB, Kronauer RE, Czeisler CA, Lockley SW (2012) Human phase response curve to a 1 h pulse of bright white light. J Physiol 590: 3035–3045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JE, Sletten TL, Magee M, Ganesan S, Mulhall MD, Collins A, Howard M, Lockley SW, Rajaratnam SMW (2018) Temporal dynamics of circadian phase shifting response to consecutive night shifts in healthcare workers: role of light‐dark exposure. J Physiol 596: 2381–2395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian P, Subbaraj P (1993) Diazepam phase shifts the circadian clock of the field mouse Mus booduga . J Biosci 18: 103–110 [Google Scholar]
- Sulli G, Manoogian ENC, Taub PR, Panda S (2018) Training the circadian clock, clocking the drugs, and drugging the clock to prevent, manage, and treat chronic diseases. Trends Pharmacol Sci 39: 812–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi JS (2017) Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet 18: 164–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaki M, Ujike H (2014) Aripiprazole is effective for treatment of delayed sleep phase syndrome. Clin Neuropharmacol 37: 123–124 [DOI] [PubMed] [Google Scholar]
- Takeshima M, Shimizu T, Echizenya M, Ishikawa H, Kanbayashi T (2018) Inpatient phase‐advance therapy for delayed sleep‐wake phase disorder: a retrospective study. Nat Sci Sleep 10: 327–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh KL, Jones CR, He Y, Eide EJ, Hinz WA, Virshup DM, Ptacek LJ, Fu YH (2001) An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science 291: 1040–1043 [DOI] [PubMed] [Google Scholar]
- Trapa PE, Belova E, Liras JL, Scott DO, Steyn SJ (2016) Insights from an integrated physiologically based pharmacokinetic model for brain penetration. J Pharm Sci 105: 965–971 [DOI] [PubMed] [Google Scholar]
- Ukai H, Ueda HR (2010) Systems biology of mammalian circadian clocks. Annu Rev Physiol 72: 579–603 [DOI] [PubMed] [Google Scholar]
- Vanselow K, Vanselow JT, Westermark PO, Reischl S, Maier B, Korte T, Herrmann A, Herzel H, Schlosser A, Kramer A (2006) Differential effects of PER2 phosphorylation: molecular basis for the human familial advanced sleep phase syndrome (FASPS). Genes Dev 20: 2660–2672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosko AM, Hagenauer MH, Hummer DL, Lee TM (2009) Period gene expression in the diurnal degu (Octodon degus) differs from the nocturnal laboratory rat (Rattus norvegicus). Am J Physiol Regul Integr Comp Physiol 296: R353–R361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch OJ, Cochran A, Forger DB (2016) A global quantification of “normal” sleep schedules using smartphone data. Sci Adv 2: e1501705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton KM, Fisher K, Rubitski D, Marconi M, Meng QJ, Sladek M, Adams J, Bass M, Chandrasekaran R, Butler T et al (2009) Selective inhibition of casein kinase 1 epsilon minimally alters circadian clock period. J Pharmacol Exp Ther 330: 430–439 [DOI] [PubMed] [Google Scholar]
- Watson LA, Phillips AJK, Hosken IT, McGlashan EM, Anderson C, Lack LC, Lockley SW, Rajaratnam SMW, Cain SW (2018) Increased sensitivity of the circadian system to light in delayed sleep‐wake phase disorder. J Physiol 596: 6249–6261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbrink N, Ananthasubramaniam B, Munch M, Koller B, Maier B, Weschke C, Bes F, de Zeeuw J, Nowozin C, Wahnschaffe A et al (2018) High‐accuracy determination of internal circadian time from a single blood sample. J Clin Invest 128: 3826–3839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KP Jr, McHill AW, Birks BR, Griffin BR, Rusterholz T, Chinoy ED (2013) Entrainment of the human circadian clock to the natural light‐dark cycle. Curr Biol 23: 1554–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB (2014) A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci USA 111: 16219–16224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Kim JK, Eng GW, Forger DB, Virshup DM (2015) A Period2 phosphoswitch regulates and temperature compensates circadian period. Mol Cell 60: 77–88 [DOI] [PubMed] [Google Scholar]
- Zhu L, Zee PC (2012) Circadian rhythm sleep disorders. Neurol Clin 30: 1167–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Table EV1
Table EV2
Dataset EV1
Dataset EV2
Dataset EV3
Code EV1
Review Process File
Data Availability Statement
The MATHEMATICA codes used in this study are available in Code EV1 and the following database: https://github.com/daewookkim/Non-human-primate-circadian-clock-model-including-CK1-inhibitor.


