Abstract
Background: Human JAKs are responsible for generating docking sites for human SSTAT phosphorylation. The role of JAKs in psoriasis pathogenesis has not been clearly explained.
Aim: To investigate the role of JAK1 in psoriasis pathogenesis and to assess if this role is mediated through STAT3 or not, through evaluation of their immunohistochemical expression in the skin of psoriatic patients.
Methods: This case–control study was carried out on 26 patients presenting with psoriasis vulgaris versus 26 age- and sex-matched apparently healthy volunteers. Psoriasis Area and Severity Index (PASI) scores were used to evaluate psoriasis severity. From all controls and cases (lesional and perilesional), skin biopsies were taken for histopathological and immunohistochemical JAK1 and STAT3 evaluation.
Results: There was significant stepwise upregulation of JAK1 from controls to perilesional to lesional psoriatic skin of the patient group in both epidermis and dermis (P≤0.001 for both). Dermal JAK1 H-score was significantly associated with psoriasis severity (P=0.01). STAT3 was significantly overexpressed in lesional psoriatic skin over nonlesional skin (P<0.001). There were significant positive correlations between lesional H-scores for STAT3 and Psoriasis Area and Severity Index scores in epidermis (r=0.63, P<0.001), and in dermis (r=0.47, P=0.04). There was a significant positive correlation between JAK1 and STAT3 expression in epidermal lesional psoriatic skin (r=0.44, P=0.03).
Conclusion: JAK1 has a proinflammatory effect in psoriasis pathogenesis, which could be mediated through increasing STAT3 expression in psoriasis. JAK1 and STAT3 tissue expression could be markers of psoriasis severity. JAK1 may be used as a target for immunotherapy in psoriasis-management programs.
Keywords: JAK1, STAT3, psoriasis, immunohistochemistry
Introduction
Psoriasis is a common, chronic, and immunomediated inflammatory skin disorder.1,2 Although its pathogenesis remains unclear, psoriasis is considered as a mixed T helper 1 (Th1)/Th17 cell-mediated autoimmune disease, because the activated T lymphocytes release different cytokines, such as IL17 and interferon IFNγ, to enhance keratinocyte proliferation and recruit neutrophils. Environmental triggers, in addition to skin-barrier disruption and immunodysfunction, activate dendritic cells that migrate to the skin, draining lymphatics, and activate antigen-specific T cells to differentiate into effector T cells. Then these cells reach the skin, releasing proinflammatory cytokines, which in turn stimulate keratinocyte proliferation with cytokine interaction between T lymphocytes and keratinocytes.3,4
JAKs are intracellular, non–receptor tyrosine kinases that first gained attention as signaling mediators of type I and type II cytokine receptors.5 There are four types of JAKs.6 They are responsible for protein–protein interactions, such as adaptor and scaffolding interactions with membrane-associated proteins.7
There are seven STATs.8 It has been shown that STAT3 has an active role in regulating fundamental cellular biological processes such as differentiation, proliferation, malignant transformation, apoptosis, and survival. The JAK–STAT pathway plays an important role in the inflammatory immunoresponse through regulation of the differentiation and proliferation of immune cells.9
Therefore, the aim of this study was to investigate the role of JAK1 and STAT3 in psoriasis pathogenesis through assessment of their immunohistochemical expression in lesional and perilesional skin of psoriasis cases. Also, the relationship between JAK1 and STAT3 was investigated to assess if the role of JAK1 in psoriasis is mediated through STAT3 or not.
Methods
This prospective case–control study was carried out on 52 subjects. They included 26 patients presenting with chronic plaque psoriasis and 26 age- and sex-matched apparently healthy individuals as a control group. Psoriasis patients were selected from the Outpatient Clinic, Dermatology Department, Faculty of Medicine, Menoufia University ibetween January 2017 and December 2017. Diagnosis of psoriasis was based on its clinical and pathological aspects. The control group was collected from the Plastic Surgery Department, Faculty of Medicine, Menoufia University.
Each participant in the study signed a written informed consent approved by the Ethics Committee, Faculty of Medicine, Menoufia University that was in accordance with the Declaration of Helsinki of 1975. No patients investigated had been receiving any systemic/topical immunosuppressant or immunomodulator drugs for a minimum of 15 days before the excision.10 Each selected patient was subjected to complete history taking and general and dermatological examinations, including assessment of psoriasis severity and stability. Assessment of severity of the disease was done using Psoriasis Area and Severity Index (PASI) scores.11 Regarding psoriasis stability, it was noted that, scaling predominated in stable chronic plaque psoriasis, while erythema was the dominant feature of unstable progressing lesions. The transition to more extensive involvement, due to unidentifiable triggering factors, was frequently known by the onset of the inflammatory phase with predominant erythema and limited scaling associated with itching and rapidly progressing lesions. Unstable psoriasis may evolve to whole-body involvement.12
Skin biopsies
Skin biopsies were taken under 2% lignocaine local anesthesia. Two biopsies were taken from each patient — one from lesional skin and the other from perilesional skin (2 cm beyond the plaque border) — and one from matched sites of controls.
All biopsies were fixed in 10% neutral buffered formalin and submitted to the Pathology Department, Faculty of Medicine, Menoufia University for routine processing and sectioning. Three sections of 4 µm thickness were taken from each block. One was stained with H&E for evaluation of histopathological changes. The other two sections were mounted on positively charged slides and stored at room temperature for immunohistochemical staining for JAK1 and STAT3.
Immunohistochemical staining
Immunohistochemical staining was performed using antibodies: JAK1 (mouse monoclonal antibody, 0.1 mL, each vial containing 200 μg IgG2b in 1.0 mL PBS; A9, sc1677; Santa Cruz Biotechnology) and STAT3 (mouse monoclonal antibody, 0.1 mL, each vial containing 200 μg IgG1 in 1.0 mL PBS; F2, sc8019; Santa Cruz Biotechnology). Antibodies used were diluted 1:200 and 1:100 for JAK1 and STAT3, respectively. Deparaffinized sections were stained using Dako immunohistochemistry products (Heverlee, Belgium) in an automatic immunohistochemistry apparatus. Antigens were retrieved using target-retrieval solution (pH 6.1 or 9, 97°C) for 10–30 minutes. Slides were blocked using peroxidase-blocking reagent and incubated with the primary antibody for 1 hour at room temperature. Detection was performed using the detection reagent and DAB. The slides were counterstained using Mayer’s hematoxylin. Finally, sections were mounted and covered using distyrene, a plasticizer, and xylene
Positive-control slides were mouse kidney tissue and human fallopian tube tissue for JAK1 and STAT3, respectively. Negative-control slides were prepared by omitting the primary antibodies from the staining procedure.
Interpretation of JAK1 and STAT3 expression
Positive expression was considered when nuclear, cytoplasmic, and/or membranous staining was seen in any number of cells.5,9
Histoscores (H-scores) were applied in both epidermis and dermis, where both the intensity and percentage of positivity were considered using the formula: H-score = percentage of mildly stained cells + double the percentage of moderately stained cells + triple the percentage of strongly stained cells.13
Statistical analysis
Data were collected, tabulated, and analyzed by SPSS version 20 on an IBM computer. Qualitative data are expressed as numbers and percentages. Quantitative data are expressed as arithmetic mean (X), SD, percentages, and medians. For qualitative variables, we used χ2 and Fisher’s exact tests. Mann–Whitney U tests were used in comparing two nonparametric quantitative variables. ANOVA (F) was used for comparison among three or more quantitative variables. P≤0.05 was considered statistically significant.
Results
Personal data of the studied groups
Age and sex of studied subjects are shown in Table 1. Both psoriatic patients and controls were matched regarding age (P=0.07) and sex (P=0.53; Table 1).
Table 1.
Demographic data of studied groups and clinicopathologic characteristics of psoriatic patients
| Cases | Control | t-test | P-value | |
|---|---|---|---|---|
| Age, years | ||||
| Mean ± SD | 39.3±14.9 | 33.6±4.7 | U=1.8 | 0.07 |
| Median | 37.5 | 34 | ||
| Range | 17–75 | 23–42 | ||
| Sex n, (%) | ||||
| Male | 18 (69.2) | 20 (76.9) | χ2=0.39 | 0.53 |
| Female | 8 (30.8) | 6 (23.1) | ||
| Family history n, (%) | ||||
| Positive | 2 (7.7) | — | — | — |
| Negative | 24 (92.3) | — | — | — |
| Duration, years | ||||
| Mean ± SD | 10.3±3.2 | — | — | — |
| Median | 10 | — | — | — |
| Range | 2–15 | — | — | — |
| Age at onset, years | ||||
| Mean ± SD | 29.1±13.8 | — | — | — |
| Median | 27 | — | — | — |
| Range | 13–61 | — | — | — |
| PASI score | ||||
| Mean ± SD | 23.5±13.7 | — | — | — |
| Median | 21.5 | — | — | — |
| Range | 4–53 | — | — | — |
| Severity n, (%) | ||||
| Mild | 1 (3.8) | — | — | — |
| Moderate | 6 (23.1) | — | — | — |
| Severe | 19 (73.1) | — | — | — |
| Stability: | ||||
| Stable | 21 (80.8) | — | — | — |
| Progressive | 5 (19.2) | — | — | — |
| Histopathological parameters | ||||
| Epidermal changes | ||||
| Acanthosis n, (%) | ||||
| Present | 26 (100) | — | — | — |
| Absent | 0 | — | — | — |
| Parakeratosis n, (%) | ||||
| Confluent | 5 (19.2) | — | — | — |
| Focal | 21 (80.8) | — | — | — |
| Granular cell layer n, (%) | ||||
| Absent | 18 (69.2) | — | — | — |
| Partially absent | 8 (30.8) | — | — | — |
| Munro microabcesses n, (%) | ||||
| Present | 21 (80.8) | — | — | — |
| Absent | 5 (9.2) | — | — | — |
| Suprapaillry epidermis n, (%) | ||||
| Normal | 5 (7.7) | — | — | — |
| Thin | 21 (92.3) | — | — | — |
| Spongyform pustules of Kogoj n, (%) | ||||
| Present | 21 (80.8) | — | — | — |
| Absent | 5 (9.2) | — | — | — |
| Tip of rete ridge n, (%) | ||||
| Normal | 6 (23.1) | — | — | — |
| Coalescent | 20 (76.9) | — | — | — |
| Dermal changes | ||||
| Dilated blood vessels n, (%) | ||||
| Present | 22 (84.6) | — | — | — |
| Absent | 4 (15.4) | — | — | — |
| Perivascular lymphocytic infiltrate n, (%) | ||||
| Present | 21 (80.8) | — | — | — |
| Absent | 5 (9.2) | — | — | — |
Abbreviation: PASI, Psoriasis Area and Severity Index.
Clinical and pathological data of psoriasis patients
Clinical criteria of psoriasis patients and the pathological data of their lesional skin biopsies are given in Table 1.
Comparison between controls and psoriatic skin (lesional and perilesional) regarding JAK1 immunohistochemical expression
JAK1 immunoreactivity in controls (Figure 1) and perilesional (Figure 2) and lesional (Figure 3) psoriatic skin showed that epidermal JAK1 expression in controls (42.3%) and perilesional (80%) and lesional psoriatic skin sections (88.5%) had a significant stepwise increase (P≤0.001). Similarly, dermal expression of JAK1 was expressed in a significant ascending manner from controls (11.5%), to perilesional psoriatic skin sections (30.8%) to lesional ones (73.1%; P≤0.001). Moreover, there was a significant stepwise upregulation of JAK1 H-score in epidermis and dermis in perilesional and lesional psoriatic skin over normal skin of the control group (P≤0.001). Also, there were significant differences between studied groups regarding JAK1 dermal localization (P=0.03; Table 2).
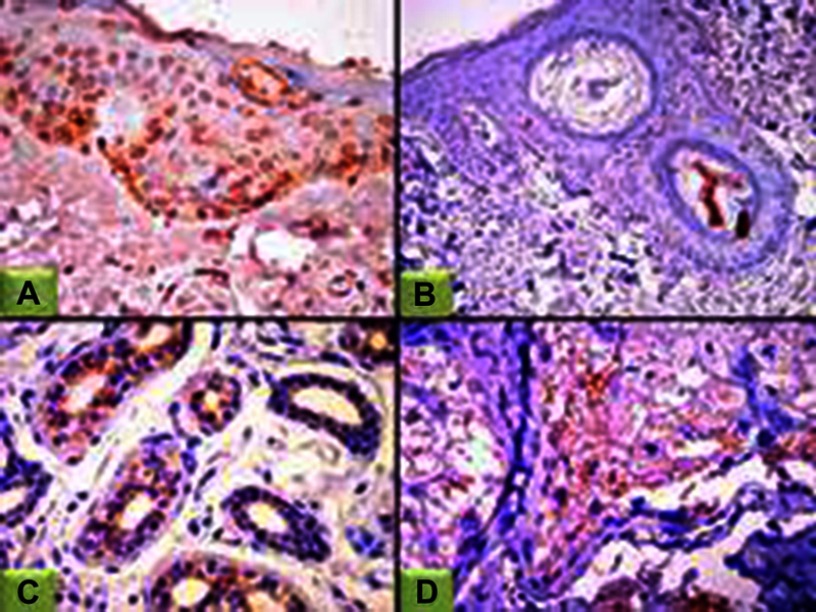
Figure 1.
JAK1 expression in controls.
Notes: (A) Epidermal nucleocytoplasmic JAK1 expression (200×); (B) negative epidermal and dermal JAK1 expression (100×); (C) cytoplasmic JAK1 expression in eccrine sweat-gland ducts (100×); (D) sebaceous gland (200×).
Abbreviation: JAK1, janus kinase 1.
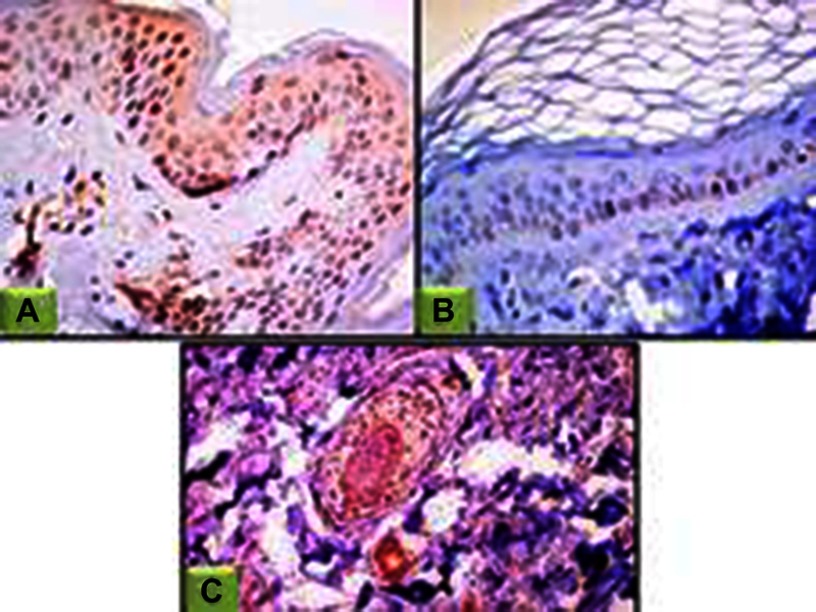
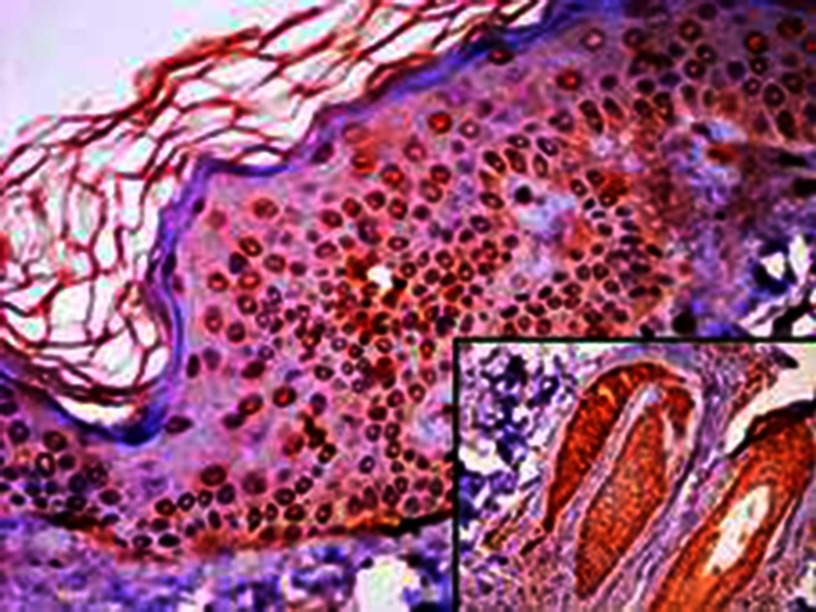
Figure 2.
JAK1 expression in perilesional skin sections.
Notes: (A) Epidermal cytoplasmic JAK1 expression with focal nucleocytoplasmic localization (200×); (B) negative epidermal and dermal JAK1 expression (200×); (C) nucleocytoplasmic JAK1 expression in hair follicles, eccrine sweat-gland ducts and dermal fibroblasts (100×).
Abbreviation: JAK1, janus kinase 1.
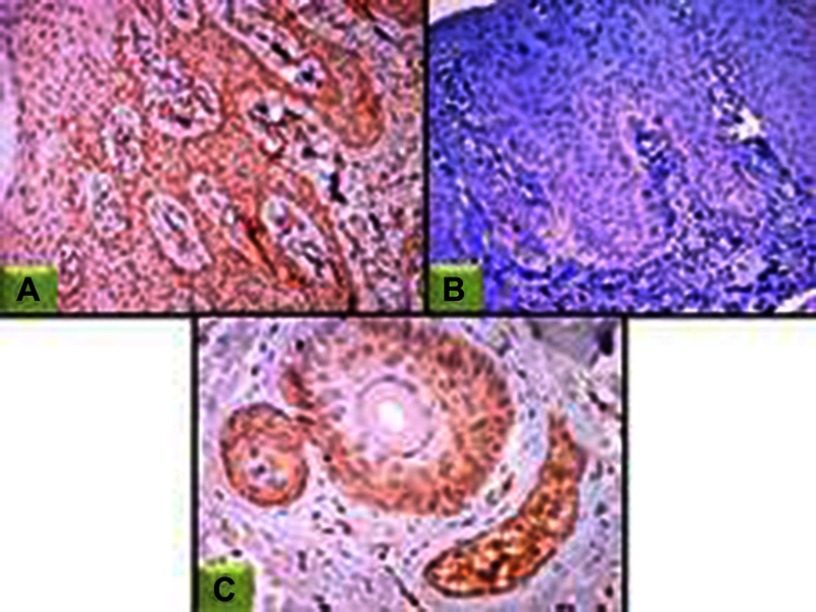
Figure 3.
JAK1 expression in lesional skin sections.
Notes: (A) Epidermal nucleocytoplasmic JAK1 expression (100×); (B) negative epidermal and dermal JAK1 expression (100×); (C) nucleocytoplasmic JAK1 expression in hair follicles and sebaceous gland (200×).
Table 2.
Comparison between controls and psoriatic skin (lesional and perilesional) regarding JAK1 and STAT3 tissue immunoreactivity
| Immunoreactivity parameters | Controls (total 26), n (%) |
Perilesional (total 26), n (%) |
Lesional (total 26), n (%) |
Test of significance |
P-value & post hoc test |
|---|---|---|---|---|---|
| Epidermal JAK1 | |||||
| Expression: | |||||
| Positive | 11 (42.3) | 21 (80.0) | 23 (88.5) | χ2=15.2 | P≤0.001 |
| Negative | 15 (57.7) | 5 (19.2) | 3 (11.5) | ||
| Localization: | |||||
| Nuclear | 0 | 0 | 2 (8.7) | Fisher’s exact = 4.6 | P=0.28 |
| Cytoplasmic | 8 (72.7) | 19 (90.5) | 16 (69.6) | ||
| Nucleocytoplasmic | 3 (27.3) | 2 (9.5) | 5 (21.7) | ||
| H score: | P1≤0.001* | ||||
| Mean ± SD | 90.8±32.3 | 130.9±3.9 | 164.7±20.4 | P2≤0.001* | |
| Median | 130 | 130 | 170 | KW=20.1 | P3≤0.001* |
| Range | 30–130 | 80–180 | 120–200 | P≤0.001* | |
| Dermal JAK1 | |||||
| Expression | |||||
| Positive | 3 (11.5) | 8 (30.8) | 19 (73.1) | χ2=21.7 | P≤0.001* |
| Negative | 23 (88.5) | 18 (69.2) | 7 (26.9) | ||
| Localization | |||||
| Nuclear | 0 | 3 (37.5) | 0 | Fisher’s exact =10.4 | P=0.03* |
| Cytoplasmic | 2 (66.7) | 5 (62.5) | 14 (73.7) | ||
| Nucleocytoplasmic | 1 (33.3) | 0 | 5 (26.3) | ||
| H score | P1=0.02* | ||||
| Mean ± SD | 50±17.32 | 86.2±32.9 | 160±18.8 | KW=32.7 | P2≤0.001* |
| Median | 40 | 85 | 160 | P3≤0.001* | |
| Range | 40–70 | 30–120 | 120–190 | P≤0.001* | |
| Epidermal STAT3 | |||||
| Expression: | |||||
| Positive | 26 (100) | 26 (100) | 26 (100) | NA | — |
| Negative | 0 | 0 | 0 | ||
| Localization: | |||||
| Nuclear | 2 (7.7) | 0 | 0 | ||
| Cytoplasmic | 0 | 0 | 0 | ||
| Nucleocytoplasmic | 24 (92.3) | 26 (100) | 26 (100) | ||
| H score | P1<0.001* | ||||
| Mean ± SD | 180.3±34.2 | 110.0±41.1 | 225.0±43.1 | P2<0.001* | |
| Median | 170 | 115 | 230 | P3<0.001* | |
| Range | 110–250 | 50–180 | 170–300 | P<0.001* | |
| Expression: | |||||
| Positive | 26 (100) | 22 (84.6) | 19 (73.1) | Fisher’s exact=7.8 | P=0.01* |
| Negative | 0 | 4 (15.4) | 7 (26.9) | ||
| Localization: | |||||
| Nuclear | 2 (7.7) | 0 | 0 | ||
| Cytoplasmic | 0 | 0 | 0 | NA | — |
| Nucleocytoplasmic | 24 (92.3) | 22 (100) | 19 (100) | ||
| H score | P1=0.43 | ||||
| Mean ± SD | 185.3±30.6 | 129.1±35.1 | 193.6±40.9 | KW=21.5 | P2<0.001* |
| Median | 170 | 120 | 280 | P3<0.001* | |
| Range | 160–250 | 80–180 | 120–280 | P≤0.001* | |
Notes: *Significant; P1, control vs lesional; P2, control vsperilesional; P3, lesional vs perilesional.
Abbreviations: NA, not applicable; KW, Kruskal–Wallis; JAK1, janus kinase 1; STAT3, signal transducer and activator of transcription.
Relationship of JAK1 lesional H-score with demographic and clinical data of psoriatic patients
There was a significant association between lesional epidermal JAK1 H-score and psoriasis stability (P=0.01). There were significant associations between lesional dermal JAK1 H-score with sex (P=0.007) and psoriasis severity (P=0.01; Table 3).
Table 3.
Relationship between lesional H scores of JAK1 and STAT3 with demographic and clinical parameters
| Parameters | JAK1 H score in epidermis | STAT3 H score in epidermis | Test | P-value |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| Gender | ||||
| Male | 164.3±21.2 | 241.1±40.8 | t1 test=0.14 | P1=0.88 |
| Female | 165.7±19.8 | 188.7±21.01 | t2 test=3.4 | P2=0.002* |
| Stability | ||||
| Stable | 170±`4.5 | 211.9±35.1 | U =2.61 | P1=0.01* |
| Progressive | 146±28.8 | 280±29.1 | t2 test=4.01 | P1=0.01* |
| Disease severity | ||||
| Mild | 160±2.1 | 170 | F1=1.7 | P1=0.27 |
| Moderate | 178±14.8 | 178±14.8 | F2=11.4 | P2=0.001* |
| Severe | 161.1±21.1 | 243.1±35.7 | ||
| JAK1 H score in dermis | STAT 3 H score in dermis | |||
| Mean ± SD | Mean ± SD | |||
| Gender: | ||||
| Male | 168.3±11.9 | 199.2±44.8 | t1 test=3.04 | P1=0.007* |
| Female | 145.7±20.7 | 178±24.9 | t2 test=0.99 | P2=0.33 |
| Stability | ||||
| Stable | 160±1.3 | 182.8±29.7 | t1 test=0.09 | P1=0.11 |
| Progressive | 160.3±1.5 | 253.3±46.1 | t2 test=3.50 | P2=0.003* |
| Disease severity | ||||
| Mild | 120±1.3 | 220 | F1 test=5.8 | P1=0.01* |
| Moderate | 151.6±19.4 | 158±22.8 | F2 test=3.30 | P2=0.06 |
| Severe | 167.5±12.8 | 205.3±40.1 |
Notes: *, significant; P1, p-value in JAK1; P2, p-value in STAT3; t1, student t-test in JAK1; t2, student t-test in STAT3; F1, ANOVA test in JAK1; F2, ANOVA test in STAT3; U, Mann–Whitney test.
Abbreviations: JAK1, janus kinase 1; STAT3, signal transducer and activator of transcription.
Relationship between lesional H score of JAK1 expression and histopathological parameters
There were significant associations between epidermal lesional H-scores of JAK1 and parakeratosis, presence of Munro microabscesses, presence of spongiform pustules of Kogoj (P=0.029 for all), and dilated blood vessels (P=0.05; data not shown).
Comparison between controls and psoriatic skin (lesional and perilesional) regarding STAT3 immunohistochemical expression
Analysis of STAT3 immunoreactivity in controls (Figure 4) and perilesional (Figure 5) and lesional (Figure 6) psoriatic skin showed a significant difference between studied groups regarding STAT3 dermal expression (P=0.01). STAT3 was significantly more expressed in lesional psoriatic skin than perilesional and controls in both epidermis and dermis (P<0.001 for both; Table 2).
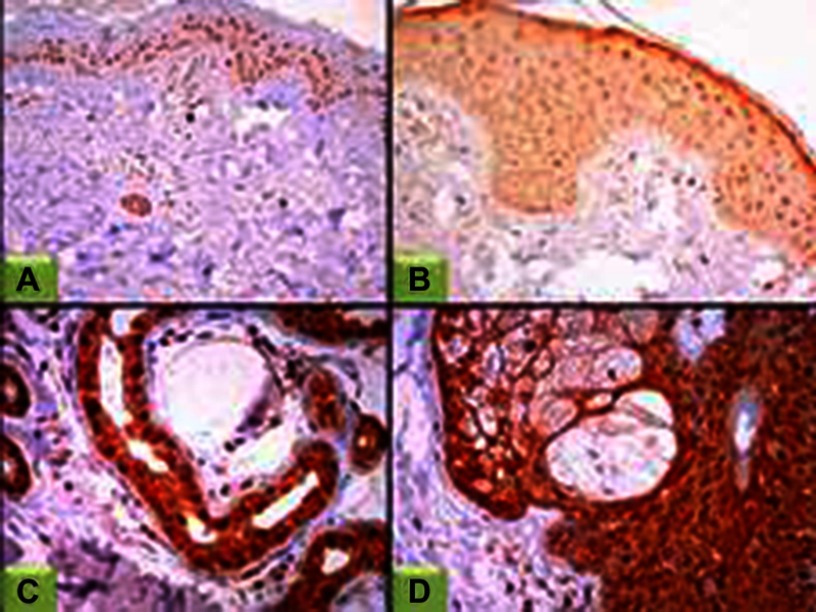
Figure 4.
STAT3 expression in controls.
Notes: (A) Epidermal nuclear STAT3 expression (100×); (B) epidermal nucleocytoplasmic STAT3 expression, (200×); (C) STAT3 nucleocytoplasmic expression in eccrine sweat gland (400×); (D) STAT3 nucleocytoplasmic expression in sebaceous gland (400×).
Abbreviation: STAT3, signal transducer and activator of transcription.
Figure 5.
STAT3 expression in perilesional skin sections.
Notes: Epidermal nucleocytoplasmic STAT3 expression (400×); dermal adnexa showed nucleocytoplasmic STAT3 expression (inset, 100×).
Abbreviation: STAT3, signal transducer and activator of transcription.
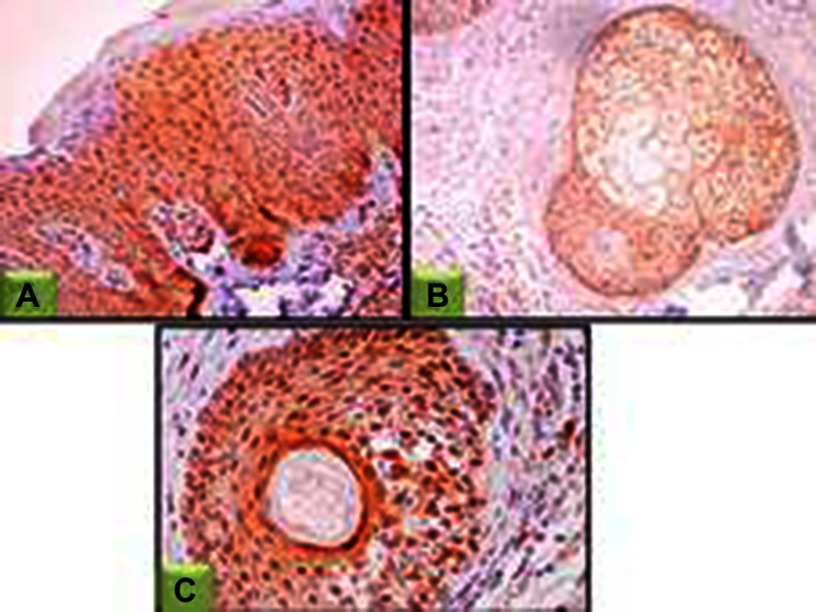
Figure 6.
STAT3 expression in lesional skin sections.
Notes: (A) Epidermal nucleocytoplasmic STAT3 expression (200×); (B) nucleocytoplasmic STAT3 expression in sebaceous gland and dermal fibroblasts (100×); (C) nucleocytoplasmic STAT3 expression in hair follicles and dermal fibroblasts (200×).
Abbreviation: STAT3, signal transducer and activator of transcription.
Relationship of STAT3 lesional H-score of with demographic and clinical data of psoriatic patients
Lesional epidermal STAT3 H-scores were significantly associated with male sex (P=0.002) and progressive (P=0.003) and severe forms of psoriasis (P=0.001). There was a significant association between lesional dermal STAT3 H-scores and progressive forms of psoriasis (P=0.003; Table 3).
Relationship between lesional H-score of STAT3 expression and histopathological parameters
There were significant associations between dermal lesional H-score of STAT3 and parakeratosis, epidermal hyperplasia, presence of spongiform pustules of Kogoj, perivascular lymphocytic infiltration (P=0.04 for all), and dilated blood vessels (P=0.03 for both; data not shown).
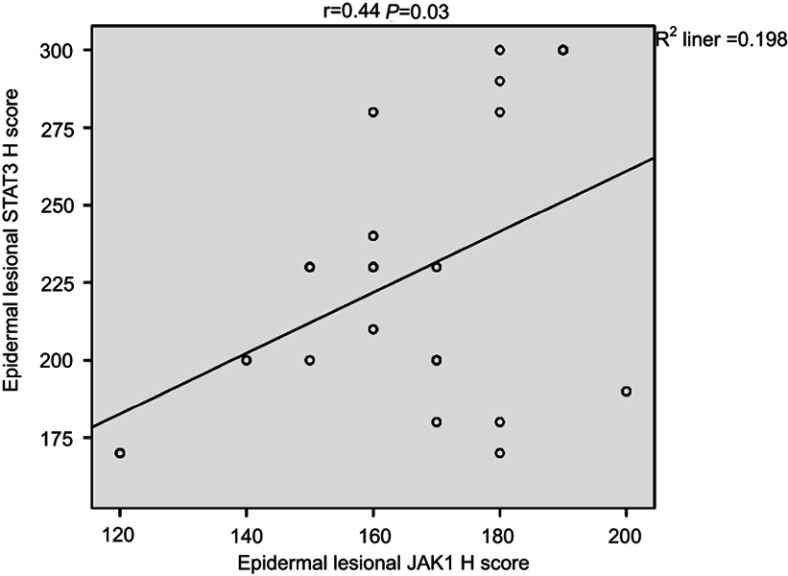
Correlation between JAK1 and STAT3 expression in psoriasis patients
There was a significant positive correlation between epidermal lesional JAK1 and STAT3 H-scores (r=0.44, P=0.03; Figure 7).
Figure 7.
Correlation between epidermal JAK1 and STAT3 H-scores in lesional psoriatic skin sections in psoriatic patients.
Abbreviations: JAK1, janus kinase 1; STAT3, signal transducer and activator of transcription.
Discussion
To best of our knowledge, this is the first study to investigate immunohistochemical expression of both JAK1 and STAT3 in involved and uninvolved skin of the same psoriasis patients versus controls.
The present study showed a significant stepwise increase of JAK1 expression from controls to perilesional to lesional psoriatic skin sections in both the epidermis and the dermis. In agreement with our findings, Nada et al14 reported significant upregulation of tissue JAK1 in psoriatic skin sections over controls. Also, de Medeiros et al10 found that JAK1 expression was significantly enhanced in dermal inflammatory cells of psoriatic lesional sections compared to healthy skin. These results confirm the previously reported active role of JAK1 in many inflammatory disorders, including rheumatoid arthritis, polycythemia vera, myeloproliferative diseases, and psoriasis.15
In the pathogenesis of psoriasis, many of the implicated inflammatory cytokines utilize JAKs for signaling. JAK1 is critical for signal transduction of IL6, IL22, IL23, and IL12, and all are upregulated in psoriasis.16,17
In the current study, different topographic cellular localizations of JAK1 were noted (cytoplasmic, nuclear, and nucleocytoplasmic). Supporting this finding, Zouein et al5 found that JAK1 and JAK2 were present in nuclei of certain cells under conditions related to high rates of growth, and de Medeiros et al10 reported JAK1 expression in the cytoplasm and along the cell membrane of keratinocytes. Nuclear JAKs have been shown to affect gene expression by activating other transcription factors, and may have a role in stem-cell biology.5
In the current study, overexpression of lesional dermal JAK1 was significantly related to male sex, which is explained by activation of the androgen receptor by IL6 being blocked by inhibition of JAKs.18
The current study also showed significant associations of JAK1 overexpression with both psoriasis stability and severity. This finding points to the role of JAK1 in psoriasis pathogenesis, which may be mediated through its proinflammatory effects.5 Supporting this speculation, the current study also showed significant associations of JAK1 overexpression and presence of Munro microabscesses, parakeratosis, and spongiform pustules of Kogoj, all of which are indicators of inflammatory processes in active severe forms of psoriasis.19
Parallel to our results, in cutaneous lupus erythematosus (an immunoinflammatory disorder), JAK1 levels have been found to be significantly higher in patients than controls and were associated with activity of clinical symptoms and disease severity.10 On the other hand, Nada et al14 found that there was no significant association between JAK1 level and severity of psoriasis. This variation may be due to the small sample in their study (n=10).
In the same context, we also investigated STAT3 immunohistochemal expression in the same sections to take a closer look at its role in psoriasis pathogenesis and a more detailed relationship with JAK1 action.
We revealed significant epidermal and dermal STAT3 overexpression in lesional psoriatic skin sections over nonlesional ones (controls and perilesional sections), in addition to significant associations of this overexpression with progressive and severe psoriasis. Moreover, the presence of parakeratosis, epidermal hyperplasia, spongiform pustules of Kogoj, and perivascular lymphocytic infiltration and absence of granular cell layers and dilated blood vessels, all of which are indicators of inflammatory processes in active severe forms of psoriasis,19 showed significant associations with STAT3 overexpression.
Matching these findings, Andres et al9 reported significant upregulation of STAT3 in lesional psoriatic skin compared to nonlesional skin. Additionally, de Medeiros et al10 found stronger epidermal and dermal STAT3 overexpression in psoriatic skin lesions than nonlesional ones.
STAT3 has been implicated in the regulation of fundamental cellular biological processes, such as differentiation, proliferation, apoptosis, and survival.20 Moreover, several cytokines and growth factors upregulated in psoriasis, such as IL6 and IL20, are able to induce STAT3 activation.21 Also, STAT3 has been shown to have a role in the psoriasis-associated IL23-signaling pathway.20
Our sections showed nuclear and nucleocytoplasmic topographic cellular localization of STAT3 in the epidermis and dermis. This clarifies that some STATs go to the nucleus after its activation and phosphorylation to enhance gene transcription or induction,9,22,23 while other STATs that are not phosphorylated remain in the cytoplasm in an inactive form.9
In the current study, overexpression of lesional epidermal STAT3 was significantly noted in male, akin to Li et al24 reporting that STAT3 was significantly higher in male mice than female mice. As activation of the human androgen receptor by IL6, was mediated through STAT3 signal-transduction pathways.18
Moreover, we reported a significant positive correlation between perilesional and lesional STAT3 H-scores in the dermis, confirming that perilesional skin could be considered the next victim and has the same genetic alteration in a subtle manner.
Herein, we demonstrated a significant positive correlation between epidermal JAK1 and STAT3 in psoriatic patients. Supporting this observation, activated JAKs phosphorylate tyrosine residues on the receptor, making docking sites for latent STATs. After the arrival of STATs to the receptor, they are also phosphorylated by JAKs. Activated STATs go to the nucleus and promote gene transcription or induction.22,23 Therefore, we confirmed that JAK1 and STAT3 have orchestrated synergistic inflammatory roles in the pathogenesis of psoriasis, as well as in its progression.
It has been reported that the JAK–STAT axis transduces signals of IL6 and IFNs. Both nonlesional and lesional psoriatic keratinocytes produce IL6, which is involved in the growth and differentiation of dermal and epidermal cells, growth and differentiation of cytotoxic cells, activation of NK cellss, and maturation of hematopoietic stem cells.25,26 Furthermore, IL6 acts as a chemotactic factor for T cells, and thus can directly stimulate T-cell migration to the epidermis.27
Conclusion
JAK1 has a proinflammatory effect in psoriasis pathogenesis, which could be mediated through increasing STAT3 expression in psoriasis. JAK1 and STAT3 tissue expression could be markers of psoriasis severity. JAK1 may be used as a target for immunotherapy in psoriasis-management programs.
Acknowledgments
The authors are grateful to administrative and technical staff at the Dermatology Clinic and Pathology Department, Faculty of Medicine, Menoufia University, Egypt, who kindly helped throughout this study.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Torres T, Raposo I, Selores M. IL-17 blockade in psoriasis: friend or foe in cardiovascular risk? Am J Clin Dermatol. 2016;17:107–112. doi: 10.1007/s40257-015-0166-0 [DOI] [PubMed] [Google Scholar]
- 2.Vassantachart JM, Soleymani T, Wu JJ. Comparison of phototherapy guidelines for psoriasis: a critical appraisal and comprehensive review. J Drugs Dermatol. 2016;15:995–1000. [PubMed] [Google Scholar]
- 3.Raychaudhuri SK, Maverakis E, Raychaudhuri SP. Diagnosis and classification of psoriasis. Autoimmun Rev. 2014;13(4–5):490–495. doi: 10.1016/j.autrev.2014.01.008 [DOI] [PubMed] [Google Scholar]
- 4.Ghoreschi K, Weigert C, Rocken M. Immunopathogenesis of T-cells in psoriasis. Clin Dermatol. 2007;25(6):574–580. doi: 10.1016/j.clindermatol.2007.08.012 [DOI] [PubMed] [Google Scholar]
- 5.Zouein FA, Duhé RJ, Booz GW. JAKs go nuclear: emerging role of nuclear JAK1 and JAK2 in gene expression and cell growth. Growth Factors. 2011;29:245–252. doi: 10.3109/08977194.2011.614949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227 [DOI] [PubMed] [Google Scholar]
- 7.Tepass U. FERM proteins in animal morphogenesis. Curr Opin Genet Dev. 2009;19:357–367. doi: 10.1016/j.gde.2009.05.006 [DOI] [PubMed] [Google Scholar]
- 8.Darnell JE. STATs and gene regulation. Science. 1997;277:1630–1635. [DOI] [PubMed] [Google Scholar]
- 9.Andrés RM, Hald A, Johansen C, Kragballe K, Iversen L. Studies of Jak/STAT3 expression and signalling in psoriasis identifies STAT3-Ser727 phosphorylation as a modulator of transcriptional activity. Exp Dermatol. 2013;22:323–328. doi: 10.1111/exd.12204 [DOI] [PubMed] [Google Scholar]
- 10.de Medeiros AK, Speeckaert R, Desmet E, Gele MV, Schepper SD, Lambert J. JAK3 as an emerging target for topical treatment of inflammatory skin diseases. PLoS One. 2016;16:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van de Kerkhof PC, Schalkwijk J. Psoriasis. J Am Acad Dermatol. 2008;11(5):48–55. [Google Scholar]
- 12.Naldi L, Gambini D. The clinical spectrum of psoriasis. Clin Dermatol. 2007;25:510–518. doi: 10.1016/j.clindermatol.2007.08.003 [DOI] [PubMed] [Google Scholar]
- 13.Bilalovic N, Sandstad B, Torlakovic E. CD10 protein expression in tumor and stromal cells of malignant melanoma is associated with tumor progression. Mod Pathol. 2004;17:1251–1258. doi: 10.1038/modpathol.3800174 [DOI] [PubMed] [Google Scholar]
- 14.Nada HR, El Sharkawy DA, Elmasry MF, Rashed LA. Expression of Janus Kinase 1 in vitiligo & psoriasis before and after narrow band UVB: a case-control study. Arch Dermatol Res. 2018;310:39–46. doi: 10.1007/s00403-017-1792-6 [DOI] [PubMed] [Google Scholar]
- 15.Smyth JF, Gourley C, Walker G, et al. Antiestrogen therapy is active in selected ovarian cancer cases; the use of letrozolin estrogen receptor-posative patients. Clin Cancer Res. 2007;13(12):3617–3622. [DOI] [PubMed] [Google Scholar]
- 16.Ishizaki M, Akimoto T, Muromoto R, et al. Involvement of tyrosine kinase-2 in both the IL-12/Th1 and IL-23/Th17 axes in vivo. J Immunol. 2011;187:181–189. doi: 10.4049/jimmunol.1100967 [DOI] [PubMed] [Google Scholar]
- 17.Sohn SJ, Barrett K, Van Abbema A, et al. A restricted role for TYK2 catalytic activity in human cytokine responses revealed by novel TYK2-selective inhibitors. J Immunol. 2013;191:2205–2216. doi: 10.4049/jimmunol.1202859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ueda T, Bruchovsky N, Sadar M. Activation of the androgen receptor N-terminal domain by interleukin-6 via MAPK and STAT3 signal transduction pathways. J Biol Chem. 2002;277:7076–7085. doi: 10.1074/jbc.M108255200 [DOI] [PubMed] [Google Scholar]
- 19.Mignogna C, Rosa DG. The histopathology of psoriasis. Reumatismo. 2007;46:46–48. [DOI] [PubMed] [Google Scholar]
- 20.Saeki Y, Nagashima T, Kimura S, Okada-Hatakeyama M. An ErbB receptor-mediated AP-1 regulatory network is modulated by STAT3 and c-MYC during calcium-dependent keratinocyte differentiation. Exp Dermatol. 2012;21:293–298. doi: 10.1111/j.1600-0625.2012.01453.x [DOI] [PubMed] [Google Scholar]
- 21.Wolk K, Haugen HS, Xu W, et al. IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-gamma are not. J Mol Med. 2009;87:523–536. doi: 10.1007/s00109-009-0457-0 [DOI] [PubMed] [Google Scholar]
- 22.O’Shea JJ, Schwartz DM, Villarino AV, Gadina M, McInnes IB, Laurence A. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311–328. doi: 10.1146/annurev-med-051113-024537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.A V V, Kanno Y, Ferdinand JR, O’Shea JJ. Mechanisms of Jak/STAT signaling in immunity and disease. J Immunol. 2015;194:21–27. doi: 10.4049/jimmunol.1402705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li SQ, Wang P, Wang DM, et al. Molecular mechanism for the influence of gender dimorphism on alcoholic liver injury in mice. Hum Exp Toxicol. 2018;10:1–2. [DOI] [PubMed] [Google Scholar]
- 25.Saggini A, Sergio C, Andrea C. IL-6 as a druggable target in psoriasis: focus on pustular variants. J Immunol Res. 2014;137:541–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Danziger O, Pupko T, Bacharach E, Ehrlich M. Interleukin-6 and interferon-a signaling via JAK1-STAT differentially regulate oncolytic versus cytoprotective antiviral states. Front Immunol. 2018;9:94–95. doi: 10.3389/fimmu.2018.00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elloso MM, Gomez-Angelats M, Fourie AM. Targeting the Th17 pathway in psoriasis. J Leukoc Biol. 2012;92:1187–1197. doi: 10.1189/jlb.0212101 [DOI] [PubMed] [Google Scholar]