Abstract
Background:
The aim of this study was to review available evidence for physical therapy treatment (PTT) after single-event multilevel surgery (SEMLS), and to realize a first step towards an accurate and clinical guideline for developing effective PTT for children with cerebral palsy (CP) after SEMLS.
Methods:
A qualitative systematic review (PubMed, Medline, Embase, CINAHL, and the Cochrane Library) investigating a program of PTT after SEMLS in children aged 4–18 years with CP classified by Gross Motor Function Classification System level I–III.
Results:
Six articles meeting the inclusion criteria were selected. The selected studies provide only incomplete descriptions of interventions, and show no consensus regarding PTT after SEMLS. Neither do they show any consensus on the outcome measures or measuring instruments.
Conclusions:
Based on the results of this literature review in combination with our best practice, we propose a preliminary protocol of PTT after SEMLS.
Keywords: cerebral palsy, children, multilevel surgery, physical therapy treatment, qualitative systematic review
Clinical Messages
There is no evidence to support consensus on a PTT in children with CP after SEMLS.
Clear description of the PTT (goals, frequency, duration, intensity, and method), co-intervention, devices and follow-up are necessary to reach consensus on the therapy that children with CP need after SEMLS.
Introduction
The term cerebral palsy (CP) covers a heterogeneous set of neurodevelopmental disorders caused by brain malformation or damage during early development. The defining characteristics are motor and posture impairment, spasticity, and reduced control, and muscle weakness that limits activities of daily live and self-care.1 Affecting 2.11 per 1000 children worldwide, CP is the most common cause of physical disability in infancy.2 CP is usually considered to be caused by a nonprogressive lesion of the developing brain.3 Yet, the musculoskeletal deformities in the growing child with CP are often progressive. Indeed, as the child grows, the proportions of the musculoskeletal system may change with consequent disproportionate strength and lever arms. These secondary deformities, and a different positioning of the body’s center of gravity, mean that children run the risk of further deterioration in their daily functioning; this significantly reduces the quality of life of those affected children.3,4
Over the last 20 years, single-event multilevel surgery (SEMLS) has become an important treatment option for these secondary deformities in children with CP.4 SEMLS is defined as two or more soft-tissue or bony surgical procedures at two or more anatomical levels during one operative procedure that requires only one hospital admission and one period of rehabilitation.4 This medical intervention is used particularly for children walking independently, with or without walking aids, who are progressively threatened in their abilities to stand or walk [Gross Motor Function Classification System (GMFCS II, III)].5,6 The main goal of surgery is to improve stance and gait, both quantitatively and qualitatively, by optimizing the alignment and positioning of the joints in the lower extremities.3,7,8 The most important reasons for children and their parents to choose this extensive surgery are experiences with increasing pain, movement problems, including gait patterns, and fatigue, which interferes with their social relationships in school and everyday life.3,7–9 Dreher and colleagues performed a multicenter cohort study of 231 children after SEMLS, with a follow-up period of 6–12 years. They reported a significant long-term improvement in gait function on the gait profile score.10 Rehabilitation after SEMLS is very complex and needs to be organized efficiently, and must be part of standard care after SEMLS. The Dutch guideline ‘Diagnosis and treatment of children with spastic cerebral palsy, 2014’ stresses the importance of intensive PTT in the healthcare for children with CP.3 However, no information about what an effective rehabilitation strategy after a SEMLS procedure entails is presented in the guideline. As stated by McGinley and colleagues, we concluded that ‘there is little information in the available literature to guide clinicians and scientists as to the optimum post SEMLS rehabilitation.’4
In their systematic review of the efficacy of SEMLS for children with CP, McGinley and colleagues concluded that meticulous reporting of postoperative rehabilitation after SEMLS is often lacking or inadequate (50% of studies), despite widespread acknowledgment of its importance.4 Unfortunately, McGinley and colleagues confine themselves to the rather general conclusion that the information these studies provide about duration and frequency of the rehabilitation program is often sparse, without substantiating this conclusion. This is probably because the focus of their review is on the effectiveness of SEMLS in general, not specifically on subsequent physiotherapeutic treatment. After analysis of the studies included in their review, it was concluded that they could have provided more detailed information to guide clinicians towards more substantive physical therapy treatment (PTT) programs. Based on this lack of results, we would like to identify and assess current evidence for PTT after SEMLS.
Our objective is to contribute to the production of an accurate and clinical guideline for developing an effective PTT for children with CP after SEMLS, based on evidence in the scientific literature. The research questions guiding our systematic search are:
(1) What PTT for children with CP after SEMLS has been described in scientific literature?
(2) What outcome measures are used?
(3) What instruments are used to measure the outcome measures?
In order to address our first research question, we utilize the framework of adequate reporting that McGinley and colleagues suggested in terms of which PTT for children needs to be described as a postoperative rehabilitation protocol after SEMLS.4 This includes duration of the program, type, frequency and duration of physical therapy sessions, and orthotic intervention. McGinley and colleagues advocated the provision of more intervention details in online-only appendices or supplements in cases where results were published.4
For our second and third research questions, we will not address the effects measured by the different measuring instruments, but will describe what outcome measures have been used by different authors, and what measurement tools were used. We expected the measured outcomes and the associated measuring instruments to match the outcome measures and measuring instruments used for daily, evidence-based practice within the CP population. McGinley and colleagues stated that many studies were underpowered to detect convincing effects of SEMLS intervention.4
Methods
We applied a systematic review approach to summarize current literature on PTT interventions for CP after SEMLS in children, and to present a protocol for clinical practice based on the existing evidence of the elements of PTT. Prior to commencement of this systematic review, a protocol outline was registered via the PROSPERO register of systematic reviews (CRD 42015020599), and a detailed protocol was submitted for peer review. There were no deviations from the published protocol.
Identification and selection of trials
A search was performed and updated (up to April 2018) in the PubMed, Medline, EMBASE, CINAHL, and Cochrane libraries. The search strategy was developed by one of the authors (EvB). The detailed search string for PubMed is presented in Appendix 1. The terms are divided into four clusters: ‘Cerebral Palsy’, ‘physical therapy’, ‘child,’ and ‘surgery.’ MeSH terms related to the four clusters were used and these terms were connected by Boolean operators. The reference lists of suitable articles were screened manually for missing items. Eligible studies were randomized controlled trials (RCTs), clinically controlled trails, and cohort studies that reported on at least one PTT after SEMLS. Data were extracted for all outcome measures. Only literature in English from the period between 1990 to 1 April 2018 was eligible for inclusion. The directives of the ‘Preferred Reporting Items for Systematic Reviews and Meta-analysis’ (PRISMA) were used.11
The literature search was carried out by a medical librarian specialist. Records retrieved by the search were assessed for eligibility by two reviewers (EvB, MA) working independently, initially based on titles and abstracts, with potentially eligible articles being assessed in full-text to confirm eligibility. Discrepancies were reviewed and consensus was achieved by discussion, or the decision was completed by consulting a third expert (PJ) in accordance with the inclusion and exclusion criteria of this study.
Inclusion criteria
Articles were included if they met the following criteria: children aged 4–18 years; CP as the main diagnosis; a spastic movement disorder classified GMFCS level I–III; participant having undergone SEMLS; a post-surgery PTT program has been described. Articles that only describe single-level surgery, surgical techniques, and use of specific measuring instruments instead of intervention were excluded.
Data extraction and analysis: assessment of literature
The following data were extracted from all eligible studies and tabulated: first author, year of publication, number of participants, GMFCS level, age, gender, unilateral or bilateral impairment, type of surgery, the description of the PTT, and outcome measures.
The included RCTs and cohort studies were assessed for methodological quality using the Cochrane Centre assessment forms.12 Sum scores for the assessment forms are discouraged by The Cochrane Centre and for this reason were not established.13
Results
Search yield
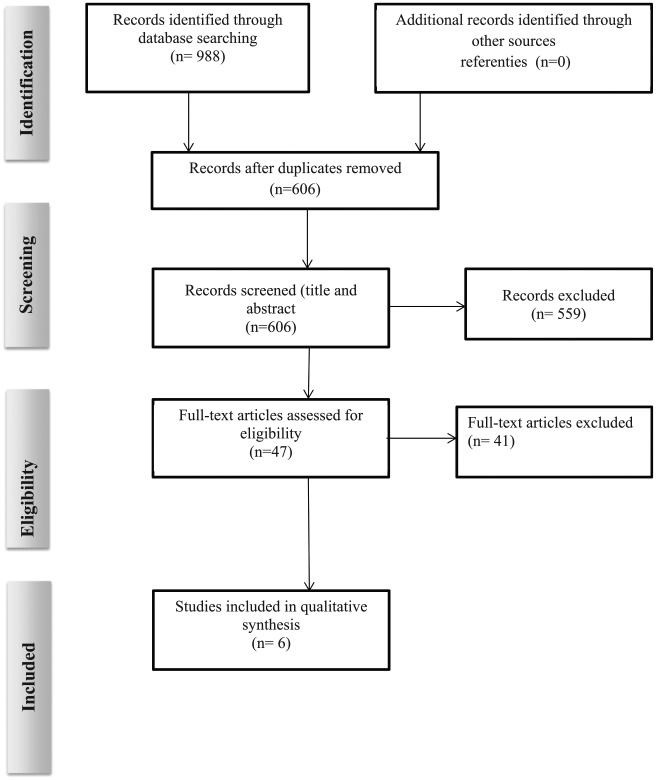
The electronic and reference search yielded 988 articles published in the period from 2006 to 2011. After removing duplicates and reviewing the titles and abstracts, 47 articles were selected. Following the application of the predefined inclusion and exclusion criteria, six articles were included in the process of reviewing (Figure1).
Figure 1.
Flowchart detailing the literature search and selection process according to Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines.
Study design
The included studies represented two cohort studies,14,15 and four RCTs.16–19 Three RCTs described the period after SEMLS and evaluated various physiotherapy approaches.17–19 One RCT described evaluation retrospectively after 2 years.16 Two papers written by Patikas and colleagues appeared to be based on the same population. 16,17 We tried to contact the author for clarification, but did not receive any reply from the author’s correspondence address. We interpreted the articles as two different studies with similar designs. The study population ranged from 11 to 43 participants, with an exception of one cohort study (N = 85).14–19
Quality of the studies
In all studies, the subjects, therapists, and testers were not blinded; participants were distributed randomly; only the study of Seniorou and colleagues had no blind randomization.18 The studies by Patikas et al. and Seniorou et al. did not mention ‘intention to treat.’17,18 The interventions (physical therapy programs) were insufficiently described in all articles. Although the results were described in cohort studies by Åkerstedt and Khan, no statistical testing took place.14,15 Khan15 and Thomason et al.19 mentioned the use of co-interventions (plaster, orthotics, devices), whereas in the other articles nothing was mentioned about co-intervention. Khan was not only the author but also the surgeon of all participants in his study, and additionally evaluated the outcomes.15 This may cause a potential bias (see supplement Table 1).
Physiotherapeutic treatment options
Aims and conclusion are described in more detail per study. Supplement Table 2 shows the details of the study, population, surgery procedures, and follow-up. In Table 1 the treatment in each study is presented in detail, based on goal intervention, frequency, duration, intensity, and method of treatment; Table 2 presents outcome measures and results after SEMLS.
Table 1.
Physical therapy treatment in detail.
| Authors | RPT | Goal | Frequency | Duration | Intensity | Method of treatment |
|---|---|---|---|---|---|---|
| Åkerstedt14 | Mobilization to standing and walking. | 2–4 sessions weekly in the first year after surgery. At 2-year follow-up frequency varied between 0.25 and 1 time/week. | 30 min to 2 h per session in the 1st year after surgery followed by shorter sessions (45–90 min) weekly in the 2nd year after surgery. | Not mentioned. | Every child had a training program with exercises for daily training. The training was based on individual needs and included different quantity of the components; active and passive movement, muscle strength, stretching, balance and gait towards more complex functional tasks. However, the exact treatment and specific exercises were not described. Instruction and guidance to the child, parents, assistant and teachers were very important and integrated with training sessions. | |
| Khan15 | Standing and walking not further specified categorized by the modified functional walking scale of Hoffer et al.* | Two to four times a week. By giving the parents a home programme, which was reinforced each visit, the need of excessive visit became less and the visits gradually became less frequent. | 1–3 h per session without mentioning the length of rehabilitation period. | Not mentioned. | All the children were taught exercises to improve their range of movement as outpatients and were provided with post-operative plasters, ankle foot orthoses (AFO ) and ‘long leg Gutter type’ night splints. Nothing was described about the exercise program. | |
| Patikas ext torque16 | The article lacked a precise exercise description, duration, frequency and intensity of the RPT. Only the EG is described. | An exercise protocol targeted to muscle strengthening could be assistive for maintaining muscle strength in children with CP after surgery. | At least three times a week, with an optimal target of four times a week. | The parents completed a logbook providing information about the frequency of the training session at home. (duration 8.70 ± 0.95 months). Each training session was 30–45 min long depending on the child. | Each training session consisted of 7 exercises for both sides, Two sets of 5 repetitions each exercise; 1 min rest between each set and drill. Movement velocity was 4–5 sec per repetition progressive resistance exercise method, with and without manual assistance and with elastic bands if needed. Additional rubber-band layers were applied if the child could repeat a whole set without compensatory movements from other muscle groups. | The training for the EG started 3–4 weeks after the surgery when it was no longer painful to perform the exercise and there was no danger of recurring injury. Two physiotherapists taught and supervised the training protocol, which consisted of 7 exercises involving the hip and knee extensors and flexors, the abdominals, in supine, prone, sit and high knee position. For exercises 1 and 7, the tights were fastened together distally with rubber bands prohibit excessive hip abduction. They gave instructions to the children’s parents about executing the exercises after hospital discharge and written instruction about the performance were given. RM method was used. |
| Patikas and walking17 | The content of the exercise program (RPT) remained unexplained. | The effect of RPT [control group (CG)] versus RPT combined with muscle strength training [strength training group (EG) program for home]. | Two to four times a week (average 3.2±0.3). | This training program started 3–4 weeks after surgery with a median duration of 40 weeks (40.3 ± 0.4) with a duration of 30–45 min per session until 1 year after operation. | Two sets of five repetitions were performed for 7 exercise with a low velocity to permit movement control and eccentric activation of the muscles. If children succeeded in overcoming the resistance against gravity, elastics bands were used to increase resistance. | The training protocol consisted of 7 exercises involving the hip, knee and ankle extensors and flexors. |
| Seniorou18 | RPT treatment continued uninterrupted during this additional training for both the AE and PRT groups and remained unexplained. | Compared the efficacy of progressive resistance strengthening (PRT) versus active exercises (AE) in children with CP following SEMLS. | Three times a week. | Each of the separate training period lasted 6 weeks. Duration of the training sessions were not mentioned. Frequency and duration of these sessions of RFT were also not mentioned. | Three sets of 10 repetitions for the muscle groups: hip flexors, hip extensors, hip abductors, knee extensors and knee flexors bilaterally. | Weight bearing was delayed 4–6 weeks when derotation osteotomies were performed. No weight-training exercises were included in this initial routine rehabilitation regime in any subject. The PRT group performed progressive resistance training using free weights. The weight was determined using a 10 RM for each muscle group and re-assessment and incremental weight increase were dictated by the child’s progress. The AE group exercised against gravity only. |
| Thomason19 | For the SEMLS group started 3 months after surgery (SEMLS). | Improving the ROM, strength, balance and function. | Three times a week. | 12 weeks. | Not mentioned. | Not mentioned. |
| PRT group (both groups used custom fitted ankle foot ortheses.) | Strengthening the hip abductors and extensors, knee extensors and ankle plantar flexors. | Three times a week. | 12 weeks. | The 3 exercises, involved ankle plantarflexor, knee extensor, and hip extensor with 8–10 repetitions. The training load was adjusted by adding free weights to a backpack worn by the participant. | A progressive resistance strength training mentioned elsewhere.39 RM method was used. |
Hoffer MM, Feiwell E, Perry R, Perry J, Bonnett C. Functional ambulation in patients with myelomeningocele. J Bone Joint Surg Am.1973; 5: 137–148.
AE, active exercise group; CG, control group; CP, cerebral palsy; EG, exercise group; PRT, progressive resistance training; RPT, regular physical therapy; RM, repetion maximum; ROM, range of motion; SEMLS, single event multilevel surgery.
Table 2.
Outcome measures and results after SEMLS.
| Outcome measures | Authors | Results | |
|---|---|---|---|
| Spasticity | MAS | Patikas ext torque16 | Both EG and CG decreased significantly at 6 months, and this decrease remained unchanged until 1 year. Resistance training did not increases spasticity, as was expected and it is likely that the decline in MAS score in both groups was a consequence of the surgery. |
| MAS | Patikas walking17 | The significant difference on the MAS a year after surgery for the group in total showed a decrease in spasticity level. No significant difference was found between the CG and the EG. Resistance training did not increases spasticity, as was expected and it is likely that the decline in MAS score in both groups was a consequence of the surgery. | |
| Range of motion | ROM | Khan15 | Positive ROM changes 2–5 years after surgery (mean 3.5 years). At all levels, the static contractures in all the children (N = 85) were almost completely resolved except for a few degrees of static flexion at the knees in 15 children (17.6%). An estimated 10° of dynamic flexion was retained at the knees in 23 children (27%) when walking unaided, but this did not compromise function. Static hip flexion contractures resolved in all children. None had residual adductor or ankle contracture. |
| ROM of the knee in a standardized fashion with the subject supine and the hip extended or flexed at 90° for the knee extension or flexion was measured, no additional information was given. | Patikas ext torque16 | A significant increase in the passive knee extension in CG was described 6 months after surgery. A significant decrease of the knee flexion in CG was described 1.5 years after surgery. For the knee flexion in EG, there was a significant decrease as well 1 year after surgery. No significant change in ROM of the knee was mentioned for both groups at 1.5 years after surgery. | |
| ROM. | Thomason19 | ROM was measured but no further analyses were conducted. | |
| Muscle strength | RM was used, measured with a isokinetic and isometric dynamo- meter. There were isometric (fixed at 90° flexion) and isokinetic test (assessed concentrically at 60° and 180° with movement ranging from 100° knee flexion to full knee flexion) for the knee extensors and flexors bilaterally. | Patikas ext torque16 | A significant decrease in the isometric and isokinetic
strength of the knee extensors and knee flexors was seen
6 months after surgery in the CG. Followed by a significant
increase a year after surgery. There was a decrease, although not significant in both isometric and isokinetic muscle strength 6 months after surgery in EG. In addition, there was an increase for the EG, but not significant for all. |
| A combination of a fixed and a hand-held dynamo meter. (MIE digital dynamo meter Nm) | Seniorou18 | CP children were generally weaker in all muscle groups compared with the healthy group. Strength decreased significantly at 6 months in both groups. The greatest percentage reduction in strength was seen in the knee-flexors (57.6%) followed by the hip flexors (45.1%). At 7.5 months there was a significant increase in muscle strength in four of the six muscle groups for active exercises group compared with 6 months and in five of the six muscle groups for the progressive resistance training group. At 1 year the strength decreased significantly in four of the six muscle groups compared with Pre-op, for both groups. | |
| The Lafayette handheld dynamometer using 1RM. | Thomason19 | An actual positive change of strength of the plantar flexors was measured, no significant improvement in muscle strength measured 1 year after surgery. | |
| Energy Cost | PCI | Åkerstedt14 | At 1 year, four children had a lower energy level during the test, three had a higher consumption and four were unchanged. At 2 years, six children showed a lower energy consumption. Five children did not benefit from the surgery and were unchanged or showed a higher energy consumption before surgery. |
| V02
EEI |
Patikas walking17 | V02 showed no significant change 1 year after
surgery. A significant increase in energy consumption was calculated after 1 year. |
|
| Gait Paramters | Walking speed, Spatiotemporal and Kinematic parameters are measured with Vicon. | Patikas walking17 | The walking speed did not change throughout the study
period. Spatiotemporal and kinematic parameters were measured before, 1, and 2 years after surgery. Both groups deviated more from the healthy children as compared with 1 year after surgery. |
| Walking speed and Kinematic parameters are measured with Vicon. | Seniorou18 | A significant increase for kinematics and a significant decrease in walking speed at 6 months for both groups. At 7.5 months, there was no significant change for kinematics. However, a significant increase in walking speed occurred for both groups. At 1 year there was a significant improvement of the kinematics, but no significant change of walking speed compared with baseline scores. | |
| GPS and GGI | Thomason19 | 1 year after surgery, a significant improvement for the SEMLS group was described for both the GPS and the GGI. 2 years after surgery only the SEMLS- group was measured and a significant improvement for both outcome measures was present. | |
| Gross Motor Function | GMFM | Åkerstedt14 | Eleven children remained unchanged at 1 year using the GMFM-66. At 2 years, 10 children remained unchanged and 1 child experienced a positive change (clinical important chance). |
| Patikas ext torque16 | A significant improvement on dimension D of the GMFM (and no significant change at dimension E or the total score of the GMFM 1 year after surgery. | ||
| Patikas walking17 | 1 year after surgery there was a significant improvement on dimension D and no significant change at dimension E or the total score. | ||
| Seniorou18 | A significant decrease in the total GMFM and dimension E at 6 months, no difference between the groups was observed. At 7.5 months there was a significant increase in the total GMFM and dimension E for both groups, compared with 6 months. At 1 year, a significant decrease existed in both groups on the GMFM domain E. The total GMFM scores showed no significant differences for both groups 1 year after surgery. | ||
| Thomason19 | No significant improvement on the GMFM was measured one year post surgery. There seems to be an improvement in the SEMLS group and deterioration in PRT group. 2 years after surgery there is a significant improvement on the GMFM for which only the SEMLS group has been measured. | ||
| Walking scale | A self-report measurement was used; The domain ‘mobility’ and category ‘walking’ ‘moving around’ (ICF related) were used and were graded in five steps from 1= no difficulty till 5= total difficulty. Maximum gait distance. | Åkerstedt14 | At 1 year, 10 children showed an improvement in walking
ability and 1 child was unchanged. At 2 years, there are
seven improved and four unchanged. One of those children did
not change at all in comparison with before
surgery. Of the 11 children, 8 have improved their maximum gait distance, 2 deteriorated and 1 was unchanged at 1 year. The maximum gait distance varied between 150 and 7000 m. At 2 years, 10 improved and 1 was unchanged. The maximum gait distance varied between 600 and >10,000 m. |
| MFWS | Khan15 | A significant improvement of MFWS (when the child went up one category) was seen. An average of 3.5 years after surgery all children become walkers according to the categories of Hoffer et al.* [exercise (21%), household (45.9 %), community-walker (32.8 %)]. | |
| FMS | Thomason19 | 2 years after surgery children showed an improvement or no change on the FSM but both were not statistically significant. | |
| Quality of life | CHQ QSC formulated on the basis of predefined expectations (according to the ICF). |
Åkerstedt14 | On the CHQ; psychosocial aspects, three children were
improved and eight unchanged at 1 year. At 2 years, three
improved and six were unchanged, and from two children data
were missing. Three of those children did not change at all
in comparison with before surgery, and six children
improved. Of the 11 children, 8 showed improvement at the CHQ; physical aspects, and three unchanged at 1 year. At 2 years, seven children remain unchanged, one improved, one deteriorated. Two of those children did not change at all in comparison with before surgery, six children improved and one is unclear. At 1 year, five children and parents were satisfied, five partially satisfied and one dissatisfied measured with the questionnaire for satisfaction of care and the question is put to parents and children whether their expectations have been realized. At 2 years, seven children and parents were satisfied and four were partially satisfied. Children were asked 1 and 2 years after surgery, whether the surgery and the rehabilitation was worth taking. Ten children answered yes and one answered no after 2-years follow-up. |
| CHQ | Thomason19 | 1 year after surgery on the CHQ; the physical aspects improved in both groups, but there was no significant difference between the groups. 2 years after surgery, there is a significant improvement on the CHQ; physical aspects. The social/emotional aspects improved significantly for the PRT group and there was a slight decline in the SEMLS group after one year. The CHQ; family/cohesion aspects showed a small but not significant improvement in the SEMLS group. |
Hoffer MM, Feiwell E, Perry R, Perry J, Bonnett C. Functional ambulation in patients with myelomeningocele. J Bone Joint Surg Am.1973; 5:137–148.
CG, control group; CHQ, child health questionnaire; EEI, energy expenditure index; EG, exercise group; FMS, functional mobility scale; GGI, Gillette gait index; GMFM, gross motor function measure; GPS, gait profile score; ICF, international classification of functioning disability and health; MAS, modified Ashworth scale; MFWS, modified functional walking scale; N, number; Nm, newton meter; PCI, physical cost index; PRT, progressive resistance training; QSC, questionnaire for satisfaction of care; RM, repetition maximum; ROM, range of motion; SEMLS, single event multilevel surgery; VO2, oxygen consumption.
Åkerstedt.14
Aim of the study. This study evaluated SEMLS and rehabilitation from a broad perspective, that included clinical and self-reported functional measures at different levels of functioning according to the WHO ‘Classification of Functioning, Disability and Health’ (ICF) and a health-related quality of life (HRQOL). In addition, parents evaluated their satisfaction with the care given.
Conclusion. Self-reported walking ability improved after SEMLS and intensive rehabilitation. This result was partly supported by lower energy cost and improved Child Health Questionnaire (CHQ). Expectations and satisfaction were fulfilled for the majority of children.
Khan.15
Aim of the study. By performing a SEML followed by a structural, supervised rehabilitation program (physiotherapy and orthotic support), walking is initiated in an untreated group. All participants presented as untreated nonwalkers, and had achieved sitting balance by the age of 5–6 years.
Conclusion. Children with CP who cannot walk and have not been treated can be helped by SEMLS, provided that inclusion criteria are followed and a structural, supervised rehabilitation program is in place.
Patikas.17
Aim of the study. To investigate the effects of resistive exercise on the knee extension and flexion torque production during the rehabilitation period after multilevel orthopaedic surgery. The question was raised whether a home-based exercise program of 9 months duration, focused on muscle strength and which started 3–4 weeks after surgery (T1), would be better to maintain muscle power in comparison with regular physiotherapy (RPT). The former program is named the Exercise Group, or EG; the latter is the Control Group, or CG. It was advocated that RPT was executed in a standardized way.
Conclusion. The additional resistive exercise program had only marginal effects on preventing muscle weakness during the postoperative rehabilitation period. Additional long-term, home-based, low-cost, resistive exercise that starts soon after the operation of patients with CP was not more beneficial than conventional PTT only, in terms of strength and gross motor function measure (GMFM).
Patikas and walking.16
Aim of the study. The purpose of this study was to examine the effect of a postoperative long-term strength-training program (EG) in addition to physiotherapy (CG) in children with CP after SEMLS, with minimal requirements in equipment and costs, and with exercises and movements that are important in everyday life.
Conclusion. The examined parameters may be more substantially influenced by factors such as the surgery outcome and the variability of pathologic characteristics than by the strength-training program per se. However, a more significant effect of strength-training may appear if more intense and short-term protocols are used, considering factors such as patients’ motivations, age and postoperative status.
Seniorou.18
Aims of the study:
(1) The reliability of the isometric strength-testing protocol in a pilot study of healthy children and spastic diplegic cerebral palsy (SDCP).
(2) Quantified changes in lower-limb muscle strength, gait and motor function in children with SDCP following SEMLS.
(3) Evaluating two different methods of postoperative physiotherapy (resistance training/active exercise, both focused on muscle strengthening but based on different principles.
At 6 months postoperatively, the participants were divided in two groups: a progressive resistance training group (PRT) versus thane active exercises group (AE). Both groups started 6 months after surgery. The program duration was 6 weeks. Until the start of these specific programs, the children received RPT for 6 months.
Conclusion. The protocol for isometric muscle strength measurement is reliable. Despite kinematic improvements, there was significant reduction of muscle strength in all muscle groups at 6 and 12 months postoperatively. Following 6 weeks of intensive physiotherapy, both groups showed significant improvement in muscle strength, GMFM scores, and gait parameters.
Thomason.19
Aim of the study. The aim was to evaluate the magnitude of change between groups and over time, based on gait indices, physical measures, function, activity, mobility, and health-related quality of life following SEMLS. The PRT group participated in PRT training. The randomized phase of the trial concluded at 12 months. The PRT group then exited the study and progressed to surgery, whereas the surgical group (SEMLS) continued to be followed in a prospective cohort study. The PRT group continued RPT the first 3 months, in the second 3 months they received PRT to match the intensive PTT received by SEMLS.
Conclusion. This article provides level-II evidence that SEMLS improves the gait of children with spastic diplegic cerebral palsy 12 months after surgery. Improvements in other domains, including gross motor function and quality of life, were not observed until 24 months after surgery.
Conclusion
This qualitative systematic review included six articles to answer the following questions: What physical therapy treatment for children with CP after SEMLS has been described in scientific literature? What outcome measures are used? What instruments are used to measure the outcome measures?
Related to the first research question, the review resulted in both an incomplete description of the PTT and a variety of treatment in all studies, with a small level of agreement (see Table 1). All studies described different goals. The goal of the physiotherapy treatment described entailed mainly improving muscle strength training, balance, range of motion (ROM), function, standing, and walking. RPT is mentioned without any description of methods.
All studies mentioned the frequency of the therapy, and the range was from less than one time per week to four times a week. The period for the training programs varied from 6 weeks to 2 years, or was not mentioned at all. The training session varied from 30 to 120 min. Intensity varied from two sets of five repetitions up to three sets of seven repetitions, and from five muscle groups to seven muscle groups per session.
The treatment descriptions varied from progressive resistance training to active exercise and passive movement, muscle strength training, stretching, balance, and gait training, to more complex functional tasks. No specific information was presented related to specific training methods. Because of the variation and the lack of description of the five domains of the PTT, it is difficult to draw straightforward conclusions related to the PTT.
Related to the second and third research questions, a wide range of outcome measures was described. The outcome measures and measurements are categorized into eight domains based on frequently used parameters in RPT in children with CP: spasticity, ROM, muscle strength, energy cost, gait parameters, gross motor function, walking scales, and quality of life, modified Ashworth scale (MAS), and ROM of the knee, hip, ankle using goniometers (see Table 2).20,21 Strength was measured both for different muscle groups in all studies, using different instruments, namely isokinetic and isometric dynamometer, the repetition maximum (RM) method, and a combination of a fixed and a hand-held dynamometer (MIE digital dynamometer Nm, and the Lafayette hand-held dynamometer). All authors measured knee-extensors with different results: a decrease, an increase, and no differences in muscle strength. All these studies indicated that the muscle strength is weaker post SEMLS than before surgery, up to 6 and 12 months after surgery. Different walking scales were used to measure effects on walking: a self-report measurement – the domain ‘mobility’ and category ‘walking’ ‘moving around’ (ICF-related), maximum walking distance, the Modified Functional Walking Scale (MFWS), and the Functional Mobility Scale (FMS). Quality of life was reported using the Child Health Questionnaire (CHQ) and the CHQ-Parent Form 50 (CHQ-PF50). Based on the PTT, a positive effect on walking, energy costs, improvement of the quality of walking after 1 or 2 years, and increase of walking speed. Furthermore, an improvement in GMFM 2 year post SEMSL has been shown, and variable results after 1 year. Harvey and colleagues support that gross motor function deteriorates post SEMLS, and starts to be more efficient 2 years after SEMLS.22 The large variation in outcome measures, the different types of measurements and the noncomparable follow-up period (see Table 2) was expected due to the variety in the individualized treatment goals, such as performing transfers, standing on two legs, walking with heel strike, etc.
To conclude: the studies showed agreement only on muscle strength training, the use of RPT, use of GMFM, and duration of recovery time and intensive rehabilitation of more than 1 year after SEMLS. Because the studies do not provide clear suggestions for PTT after SEMLS, or measuring instruments and outcome measures, we developed a preliminary protocol of PTT after SEMLS (see appendix: supplement preliminary protocol). This protocol includes goals, frequency, duration, intensity, method, and the selection of instruments. We described the most important elements that should be incorporated in PTT based on evidence in SEMLS and CP, and we developed a protocol for PTT after SEMLS.
Supporting evidence for elements in PTT
It is not just the complexity of the surgery but also the period of immobilization following SEMLS that has a great physical impact on function, activity, and participation level (ICF-CY) of the child.23 Any period of immobilization will reduce strength, coordination, and cardiovascular condition,14,16,17 and there is a reduced amount of mobility, which has great impact on the relevant standing and walking ability of the children.24–26 The period of immobilization will vary according to the type of surgery protocol, and will differ by center. On body and function level, strength training, coordination, ROM changes, balance during standing and walking, and fitness training are all essential elements in the treatment for the child to relearn to move within the new alignment. Motor skill and functional gait training are crucial to relearn a new motor pattern on standing and walking at the level of activity and participation.3,16–20,25,27–33 Muscle strength will be trained by using the progressive resistance exercise method according to FITT-factors (Frequency, Intensity, Time, and Type of training).27,34 Strength training deserves extra attention in the post-surgery PTT, as advocated by Dodd et al. and Heyrman et al.35,36 This is in line with conclusions in Dutch and international guidelines about strength training in children with CP.3,27 In addition, the decrease in muscle strength, even a considerable period after surgery, is an important outcome for managing the expectations of parents and children, and thus has a significant influence on the effect of treatment.24,25 Motor skill and functional gait training, especially quality of gait, will be supported with technology such as a partial body weight-supported treadmill and overground training and Gait Real-time Analysis Interactive Lab (GRAIL) training, and has a motivational and valuable influence in the treatment of these children.29–31,33,37 Coordination, ROM changes, balance during standing and walking, and fitness training will be integrated in this treatment.20,32 Because the prospect of improved quality of gait is decisive for the choice of SEMLS, it is important that possible future results related to quality of gait are transparent. The desired effects to achieve after SEMLS also require a long-term effort by parents and children. In order to make adequate use of the new alignment and gait opportunities (possibilities), co-interventions such as plaster, orthotics, and devices are needed in the post-surgery intervention plan.19 It is therefore important for parents, child, and practitioners that the PTT program is very clear in advance; including goal, frequency, duration, intensity, and method of treatment, along with the logistics and the efforts needed pre, peri and post SEMLS. In order to monitor the treatment process, we suggest the importance of managing expectations. Furthermore, we firmly believe that the more active children and parents are engaged in the treatment process, the more effective PTT after SEMLS will be.9,25,26,28
Crucial factors to take into consideration after SEMLS are pain and fatigue due to a period of immobilization. These can be restrictive factors during PTT.25,26,28 It is important for the motivation of the child and parents to keep in mind the aim of performing SEMLS, and to link that aim to participation goals, which affect overall well being.25 The newly developed gait outcome assessment list (GOAL questionnaire will be a good questionnaire to add.38
Based on the description of these elements, we provide a protocol for PTT after SEMLS as a first set-up, based on best practice and available evidence in SEMLS and CP. We hope that the protocol provides directions on all ICF-CY levels. This protocol is described in an accompanying file (see appendix: supplement preliminary protocol).
Supplemental Material
Supplemental material, Supplement_Combined_tables_and_appendices for Physical therapy treatment in children with cerebral palsy after single-event multilevel surgery: a qualitative systematic review. A first step towards a clinical guideline for physical therapy after single-event multilevel surgery by Esther E.H. van Bommel, Marieke M.E. Arts, Peter H. Jongerius, Julia Ratter and Eugene A.A. Rameckers in Therapeutic Advances in Chronic Disease
Acknowledgments
Special thanks goes to Koen Dortmans and Arnt Schellekens for editing the paper, and Tracey Keij-Denton for assistance with English language editing.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplemental material: Supplemental material for this article is available online.
ORCID iD: Eugene AA Rameckers  https://orcid.org/0000-0001-6661-6500
https://orcid.org/0000-0001-6661-6500
Contributor Information
Esther E.H. van Bommel, Department of Pediatric Rehabilitation, Sint Maartenskliniek, Hengstdal 3, 6574 NA Ubbergen, The Netherlands.
Marieke M.E. Arts, Rehabilitation Specialists, Klimmendaal, Arnhem, The Netherlands
Peter H. Jongerius, Department of Pediatric Rehabilitation, Sint Maartenskliniek, Nijmegen, The Netherlands
Julia Ratter, Northwest Clinics, The Netherlands.
Eugene A.A. Rameckers, Adelante Rehabilitation, Maastricht University, Valkenburg, The Netherlands Department of Functioning and Rehabilitation, Maastricht University, The Netherlands; Rehabilitation Science – Pediatric Physical Therapy, Hasselt University, Belgium.
References
- 1. Rosenbaum P, Paneth N, Leviton A, et al. - A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl 2007; 109: 8–14. [PubMed] [Google Scholar]
- 2. Oskoui M, Coutinho F, Dykeman J, et al. An update on the prevalence of cerebral palsy: a systematic review and. Dev Med Child Neurol 2013; 55: 509–519. [DOI] [PubMed] [Google Scholar]
- 3. Kwaliteitsinstituut Voor De Gezondheidszorg (CBO). Richtlijn diagnostiek en behandeling van kinderen met spastische cerebrale parese, https://www.nvpc.nl/uploads/stand/62CP%20Richtlijn%20CBO%20(2).pdf (2014, accessed 01 November).
- 4. McGinley JL, Dobson F, Ganeshalingam R, et al. Single-event multilevel surgery for children with cerebral palsy: a systematic. Dev Med Child Neurol 2012; 54: 117–128. [DOI] [PubMed] [Google Scholar]
- 5. Palisano R, Rosenbaum P, Walter S, et al. Development and reliability of a system to classify gross motor function in. Dev Med Child Neurol 1997; 39: 214–223. [DOI] [PubMed] [Google Scholar]
- 6. Gorter JW, Boonacker CWB, Rubriek KM. ‘Meten in de praktijk’ - gross motor function classification system (GMFCS). Nerderlands tijdschrift voor fysiotherapie 2005; 115. [Google Scholar]
- 7. Damiano DL, Alter KE, Chambers H. New clinical and research trends in lower extremity management for ambulatory. Phys Med Rehabil Clin N Am 2009; 20: 469–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kondratek M, McCollum H, Garland A. Long-term physical therapy management following a single-event multiple level. Pediatr Phys Ther 2010; 22: 427–438. [DOI] [PubMed] [Google Scholar]
- 9. Hilde C, Ida Torunn B. Ambulant children with spastic cerebral palsy and their parents’ perceptions and expectations prior to multilevel surgery. Dev Neurorehabil 2010; 13: 80–87. [DOI] [PubMed] [Google Scholar]
- 10. Dreher T, Thomason P, Svehlik M, et al. Long-term development of gait after multilevel surgery in children with cerebral palsy: a multicentre cohort study. Dev Med Child Neurol 2018; 60: 88–93. [DOI] [PubMed] [Google Scholar]
- 11. David M, Larissa S, Mike C, et al. Preferred reporting items for systematic review and meta-analysis protocols. Syst Rev 2015; 4: 2046–4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scholten RJPM, Offringa M, Assendelft WJJ. Inleiidng in evidence based medicine: Klinisch handelen gebaseerd op bewijsmateriaal. Uitgever: Bohn Stafleu van Loghum, 2013. [Google Scholar]
- 13. The Cochrane Collaboration. Section 8.3.3. Quality scales and Cochrane reviews. In: Higgins JPT, Green S. (eds) Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0. (updated March 2011): Cochrane, 2011. http://handbook.cochrane.org [Google Scholar]
- 14. Akerstedt A, Risto O, Odman P, et al. Evaluation of single event multilevel surgery and rehabilitation in children and. Disabil Rehabil 2010; 32: 530–539. [DOI] [PubMed] [Google Scholar]
- 15. Khan MA. Outcome of single-event multilevel surgery in untreated cerebral palsy in a. J Bone Joint Surg Br 2007; 89: 1088–1091. [DOI] [PubMed] [Google Scholar]
- 16. Patikas D, Wolf SI, Mund K, et al. Effects of a postoperative strength-training program on the walking ability of. Arch Phys Med Rehabil 2006; 87: 619–626. [DOI] [PubMed] [Google Scholar]
- 17. Patikas D, Wolf SI, Armbrust P, et al. - Effects of a postoperative resistive exercise program on the knee extension and. Arch Phys Med Rehabil 2006; 87: 1161–1169. [DOI] [PubMed] [Google Scholar]
- 18. Seniorou M, Thompson N, Harrington M, et al. Recovery of muscle strength following multi-level orthopaedic surgery in diplegic. Gait Posture 2007; 26: 475–481. [DOI] [PubMed] [Google Scholar]
- 19. Thomason P, Baker R, Dodd K, et al. Single-event multilevel surgery in children with spastic diplegia: a pilot. J Bone Joint Surg Am 2011; 93: 451–460. [DOI] [PubMed] [Google Scholar]
- 20. Franki I, Desloovere K, De Cat J, et al. The evidence-base for basic physical therapy techniques targeting lower limb. J Rehabil Med 2012; 44: 385–395. [DOI] [PubMed] [Google Scholar]
- 21. Novak I, Mcintyre S, Morgan C, et al. A systematic review of interventions for children with cerebral palsy: state of the evidence. Dev Med Child Neurol 2013; 55: 885–910. [DOI] [PubMed] [Google Scholar]
- 22. Harvey A, Rosenbaum P, Hanna S, et al. Longitudinal changes in mobility following single-event multilevel surgery in. J Rehabil Med 2012; 44: 137–143. [DOI] [PubMed] [Google Scholar]
- 23. International classification of functioning, disability and health: children and youth version (ICF-CY). http://www.who-fic.nl/Familie_van_Internationale_Classificaties/Afgeleide_classificaties/ICF_CY_International_Classification_of_Functioning_Disability_and_Health_for_Children_and_Youth (2017, accessed 05 October).
- 24. Park MS, Chung CY, Lee KM, et al. Issues of concern before single event multilevel surgery in patients with cerebral palsy. J Pediatr Orthop 2010; 30: 489–495. [DOI] [PubMed] [Google Scholar]
- 25. Park MS, Chung CY, Lee KM, et al. Issues of concern after a single-event multilevel surgery in ambulatory children. J Pediatr Orthop 2009; 29: 765–770. [DOI] [PubMed] [Google Scholar]
- 26. Capjon H, Bjørk IT. Rehabilitation after multilevel surgery in ambulant spastic children with. Dev Neurorehabil 2010; 13: 182–191. [DOI] [PubMed] [Google Scholar]
- 27. Verschuren O, Peterson MD, Balemans AC, et al. Exercise and physical activity recommendations for people with cerebral palsy. Dev Med Child Neurol 2016; 58: 798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thomason P, Selber P, Graham HK. Single event multilevel surgery in children with bilateral spastic cerebral. Gait Posture 2013; 37: 23–28. [DOI] [PubMed] [Google Scholar]
- 29. Booth ATC, Buizer AI, Meyns P, et al. The efficacy of functional gait training in children and young adults with cerebral palsy: a systematic review and meta-analysis. Dev Med Child Neurol 2018; 60: 866–883. [DOI] [PubMed] [Google Scholar]
- 30. Grecco LA, de Freitas TB, Satie J, et al. Treadmill training following orthopedic surgery in lower limbs of children with cerebral palsy. Pediatr Phys Ther 2013; 25: 187–192; discussion 193. [DOI] [PubMed] [Google Scholar]
- 31. Grecco LA, de Freitas TB, Satie J, et al. Treadmill training following orthopedic surgery in lower limbs of children with cerebral palsy. Pediatr Phys Ther 2013; 25: 187–192. [DOI] [PubMed] [Google Scholar]
- 32. Ketelaar M, Vermeer A, Hart HT, et al. Effects of a functional therapy program on motor abilities of children with cerebral palsy. Phys Ther 2001; 81: 1534–1545. [DOI] [PubMed] [Google Scholar]
- 33. Smania N, Bonetti P, Gandolfi M, et al. Improved gait after repetitive locomotor training in children with cerebral. Am J Phys Med Rehabil 2011; 90: 137–149. [DOI] [PubMed] [Google Scholar]
- 34. Rameckers E, Nijhuis-van der Sanden R, Takken T, Engelbert R. 7 Behandelstrategieën in methodisch en didactisch perspectief; A Functieniveau. In: van Empelen R, Nijhuis-van der Sanden R, Hartman A, editors, Kinderfysiotherapie. Amsterdam: Reed Business Education; 2013. p. 203–214. [Google Scholar]
- 35. Heyrman L, DeCat J, Molenaers G, et al. Strength outcome and progression over time following multilevel orthopaedic surgery in children with cerebral palsy. Gait Posture 2008; 28: doi: 10.1016/S0966-6362(08)70106-1. [DOI] [Google Scholar]
- 36. Dodd KJ, Taylor NF, Graham HK. A randomized clinical trial of strength training in young people with cerebral. Dev Med Child Neurol 2003; 45: 652–657. [DOI] [PubMed] [Google Scholar]
- 37. van Gelder L, Booth ATC, van de Port I, et al. Real-time feedback to improve gait in children with cerebral palsy. Gait Posture 2017; 52: 76–82. [DOI] [PubMed] [Google Scholar]
- 38. Thomason P, Tan A, Donnan A, et al. The gait outcomes assessment list (GOAL): validation of a new assessment of gait. Dev Med Child Neurol 2018; 60: 618–623. [DOI] [PubMed] [Google Scholar]
- 39. Dodd KJ, Taylor NF, Graham HK. A randomized clinical trial of strength training in young people with cerebral palsy. Dev Med Child Neurol 2003; 45: 652–657. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplement_Combined_tables_and_appendices for Physical therapy treatment in children with cerebral palsy after single-event multilevel surgery: a qualitative systematic review. A first step towards a clinical guideline for physical therapy after single-event multilevel surgery by Esther E.H. van Bommel, Marieke M.E. Arts, Peter H. Jongerius, Julia Ratter and Eugene A.A. Rameckers in Therapeutic Advances in Chronic Disease