Abstract
Ovarian cancer is the seventh most common gynaecologic malignancy seen in women. Majority of the patients with ovarian cancer are diagnosed at the advanced stage making prognosis poor. The standard management of advanced ovarian cancer includes tumour debulking surgery followed by chemotherapy. Various types of chemotherapeutic regimens have been used to treat advanced ovarian cancer, but the most promising and the currently used standard first-line treatment is carboplatin and paclitaxel. Despite improved clinical response and survival to this combination of chemotherapy, numerous patients either undergo relapse or succumb to the disease as a result of chemotherapy resistance. To understand this phenomenon at a cellular level, various macromolecules such as DNA, messenger RNA and proteins have been developed as biomarkers for chemotherapy response. This review comprehensively summarizes the problem that pertains to chemotherapy resistance in advanced ovarian cancer and provides a good overview of the various biomarkers that have been developed in this field.
Keywords: Chemo-therapy, advanced ovarian cancer, biomarkers, proteomics
Introduction
Malignant transformation of the ovarian surface epithelium causes epithelial ovarian cancer.1 Ovarian cancer is 1 of the seventh most common cancer affecting women worldwide and accounting for 295 414 new cases and 184 799 deaths annually.2 The lifetime risk of developing ovarian cancer in a woman is 1 in 75, with her chance of mortality due to the disease being 1 in 100.3 It occurs in peri-menopausal and post-menopausal women, with 80% to 90% of cases occurring after the age of 40 with the peak incidence of occurring at age of 60.4 Other risk factors for cancer include family history, BRCA1 and BRCA2 mutations, Lynch II syndrome, infertility, nulliparity, early menarche and late menopause.5 The familial cases account for only 10% to 15% of the patients while most cases are sporadic.6,7 Studies have consistently reported the use of oral contraceptives as being inversely associated with the risk of ovarian cancer, with a protective effect increasing with longer duration of use.8,9
Ovarian cancer is staged according to the FIGO system (Fédération Internationale de Gynécologie et d’Obstétrique) that considers the extent of tissue involvement, lymph node status and the magnitude of metastasis.10 Accordingly, stage I and stage II cancers limited to the pelvic cavity are called early stage cancer and the stage III and stage IV cancers that spread beyond the pelvic cavity are called advanced stage cancer.11 Early detection of ovarian cancer provides an opportunity for successful treatment; however, the disease is rarely diagnosed at an early stage due to lack of symptoms during the early stage. Only one-fourth of the patients present with the disease localized to the ovaries when the 5-year survival rate is 92%, while in contrast more than 75% patients present with the advanced stage disease, with a 5-year survival ranging from 15% to 25%.12,13 As most patients are diagnosed with advanced stage of the disease, it leads to a high fatality-to-case ratio among all gynaecologic malignancies.5
The standard treatment for advanced ovarian cancer is primary cytoreductive surgery followed by platinum-based chemotherapy.14 The cytoreductive surgery is done to accurately establish a diagnosis, to remove poorly perfused tissue that may harbour the disease and to decrease the tumour bulk to enhance adjuvant chemotherapy.15,16 The amount of residual disease after surgery is inversely related to overall survival (OS); patients with optimum cytoreduction (defined as <1 cm residual disease) having a more superior outcome compared with those with sub-optimal cytoreduction (>1 cm residual disease).17 The chemotherapeutic strategy for treating advanced ovarian cancer comprises different chemotherapeutic drugs, and different combinations that have been tried to improve clinical response (CR) and OS and decrease toxicity.18 The chemotherapeutic regimens used to treat advanced ovarian cancer are comprehensively summarized in Table 1 and the important and most pertinent aspects of various combinations have been discussed below.
Table 1.
First-line chemotherapeutic regimens in advanced ovarian cancer.
| Chemotherapy combinations | Clinical response | Median PFS (in months) | Overall survival (in months) | Reference |
|---|---|---|---|---|
| Melphalan | 20.0% | 7.7 | 12.3 | 19,20 |
| Melphalan hexamethylmelamine | 28.0% | 6.0 | 13.5 | 19 |
| Cyclophosphamide doxorubicin | 32.0% | 9.5 | 14.2 | 19 |
| Cyclophosphamide doxorubicin | 26.0% | 7.7 | 15.7 | 21 |
| Carboplatin Etoposide | 43.0% | 8.5 | 19.5 | 22 |
| Carboplatin Hexamethylmelamine Etoposide | 92.0% | – | – | 23 |
| Cisplatin Cyclophosphamide | 60.0% | 17.9 | 24.4 | 24 |
| Cisplatin Cyclophosphamide | 60.0% | 13.0 | 24.0 | 24 |
| Cisplatin Cyclophosphamide | 16.0% | 19.0 | 35.0 | 25 |
| Cisplatin Cyclophosphamide Doxorubicin | 51.0% | 13.1 | 19.7 | 21 |
| Cisplatin Paclitaxel | 73.0% | 18.0 | 38.0 | 24 |
| Cisplatin Paclitaxel | 81.4% | 19.1 | 44.1 | 26 |
| Cisplatin Ifosfamide | 69.0% | 14.0 | 25.0 | 27 |
| Cisplatin Ifosfamide | 67.5% | – | – | 28 |
| Paclitaxel | 55.0% | 6.1 | – | 29 |
| Carboplatin Cyclophosphamide | 14.0% | 26.0 | 37.0 | 25 |
| Carboplatin Ifosfamide | 67.0% | 24.9 | 30 | |
| Carboplatin Paclitaxel Hexamethylmelamine | 76.0% | – | – | 31 |
| Cisplatin Docetaxel | 69.0% | – | – | 32 |
| Cisplatin Docetaxel | 58.0% | 14.4 | 43.0 | 33 |
| Cisplatin thio-TEPA | 80.0% | 12.0 | 18.0 | 34 |
| Cisplatin Paclitaxel Topotecan | 60.0% | – | – | 35 |
| Carboplatin Paclitaxil Epirubicin | 86.0% | 18.7 | – | 36 |
| Carboplatin Paclitaxil Epirubicin | 90.0% | – | 65.0 | 37 |
| Carboplatin Paclitaxel Etoposide Cyclophosphamide with G-CSF | 92.0% | 4.0 | – | 38 |
| Cisplatin Paclitaxel Ifosfamide | 85.0% | 22.2 | 52.8 | 39 |
| Cisplatin Paclitaxel Ifosfamide | 85.0% | – | 51.0 | 37 |
| Carboplatin Paclitaxel Gemcitabine | 94.0% | 16.0 | 28.0 | 40 |
| Carboplatin Paclitaxel Gemcitabine | 97.5% | 19.5 | 31.2 | 41 |
| Cisplatin Gemcitabine | 70.7% | 10.4 | 23.4 | 42 |
| Cisplatin Gemcitabine | 64.9% | 13.4 | 24.0 | 43 |
| Cisplatin Irinotecan | 76.0% | – | 30.9 | 44 |
| Carboplatin Paclitaxel Epidoxorubicin | 86.0% | 19.5 | 36.0 | 45 |
| Carboplatin Docetaxel | 73.0% | 18.0 | 24.4 | 46 |
| Carboplatin Docetaxel | 78.8% | 12.0 | 35.3 | 47 |
| Carboplatin Paclitaxel Topotecan | 77.0% | 10.6 | 22.2 | 48 |
| Carboplatin Paclitaxel Amifostine | 38.0% | 22.0 | – | 49 |
| Carboplatin Paclitaxel Etoposide | 75.0% | 12.0 | 24.0 | 50 |
| Carboplatin Gemcitabine | 83.3% | 11.6 | 29.2 | 51 |
| Carboplatin Paclitaxel Gemcitabine Oxaliplatin | 85.0% | 14.5 | 31.5 | 52 |
| Carboplatin Paclitaxel Epirubicin | 60.1% | 18.4 | 45.8 | 53 |
| Carboplatin Paclitaxel Epirubicin | 65.7% | 16.4 | 42.4 | 54 |
| Carboplatin Paclitaxel Bevacizumab | 80.0% | – | – | 55 |
| Carboplatin Paclitaxel Bevacizumab | 48.0% | 16.9 | 29.9 | 56 |
| Carboplatin Topotecan | 71.0% | – | 47.0 | 57 |
| Cisplatin Paclitaxel Doxorubicin | 64.0% | 18.1 | 44.3 | 58 |
| Carboplatin Doxorubicin | 57.0% | 19.0 | 61.6 | 59 |
| Carboplatin Paclitaxel lonafarnib | – | 11.5 | 20.6 | 60 |
| Oxaliplatin Docetaxel Bevacizumab | 58.6% | 16.3 | 47.3 | 61 |
| Carboplatin Paclitaxel Sorafenib | 69.0% | 15.4 | – | 62 |
Abbreviations: G-CSF, granulocyte colony stimulating factor; PFS, progression-free survival.
Melphalan as a Single Agent and Its Combinations
During the 1950s, the main therapeutic strategy for treating advanced ovarian cancer was cytoreductive surgery and radiotherapy. An improvement in the treatment was achieved with the use of alkylating agents like melphalan which causes cytotoxicity against tumour cells by alkylating DNA at N7 position of guanine and induces DNA inter-strand cross-linkages, leading to inhibition of replication and transcription.63 The use of single-agent melphalan benefitted the patients with advanced ovarian cancer.64 However, the CR was 20%, median progression-free survival (median PFS) was 7.7 months and median OS was 12.3 months, along with toxicity manifestations such as myelosuppression with neutropenia.19,20 The combination of melphalan and hexamethylmelamine produced a CR of 28%, median PFS of 6 months and median OS of 13.5 months as compared with the combination of adriamycin and cyclophosphamide, which produced a slightly improved CR of 32%, median PFS of 9.5 months and median OS of 14.2 months; however, it produced significant hematologic and gastrointestinal toxicity.19 The use of melphalan is limited as it causes severe myelosuppression.65
Cyclophosphamide as a Single Agent and Its Combinations
Previous studies have demonstrated the efficacy of other alkylating agents like cyclophosphamide and anthracycline doxorubicin. The GOG (Gynecologic Oncology Group) trial comparing cyclophosphamide, melphalan and doxorubicin demonstrated an improvement in response rate; however, there was no OS advantage.19 Clinical trials studying the effect of this combination have shown a CR of 26% and median OS of 15.7 months with side effects such as nausea, vomiting and leukocyte toxicity.21
Cisplatin as a Single Agent and Its Combinations
The inclusion of cisplatin in the chemotherapeutic regimen for advanced ovarian cancer proved to be a major landmark. Cisplatin binds to nuclear DNA leading to interference with transcription and/or DNA replication and eventually cell death induced by cell repair machinery.66 A Cochrane review and meta-analysis confirmed a modest 2- and 5-year survival advantage in women with advanced stage epithelial ovarian cancer who were given platinum-based combination chemotherapy compared with those given combination therapy lacking platinum.67 The use of cisplatin in combination with thio-TEPA produced an improved CR; however, OS was not good.34 The combination of cisplatin, cyclophosphamide and doxorubicin showed an increased CR of 51% and median OS of 19.7 months.21 Chemotherapy combinations containing an alkylating agent and a platinum coordination complex produced a high response rate in women with advanced ovarian cancer. Cisplatin-based combination chemotherapy showed improved CR and progression-free interval (PFS) as compared with alkylating agents alone or combinations without cisplatin.24 The CR in the cisplatin-cyclophosphamide group was 60% and median OS was of 24.4 months.24 As long-term disease control in patients with advanced ovarian cancer was not significant, new drug combinations were investigated. The efficacy of cisplatin-ifosfamide combination showed improved CR and OS.27,28 Cisplatin-docetaxel combination produced an improved CR of 69%; however, increasing the docetaxel dose caused significant hematologic toxicity to the patient.32,33 Cisplatin with gemcitabine, a nucleoside antimetabolite, is an active agent in ovarian cancer and produced significantly increased CR.42,43 A similar study reported a 71% CR; however, 63% of patients developed high-grade neutropenia and 28% developed high-grade thrombocytopenia.68 The combination of cisplatin with irinotecan, an inhibitor of DNA topoisomerase I, showed improved activity in chemotherapy-naive patients with advanced ovarian cancer; however, neutropenia was the dose-limiting adverse effect.44,69
Carboplatin as a Single Agent and Its Combinations
Carboplatin which has good efficacy and less toxicity than cisplatin was introduced in the 1980s as first-line chemotherapeutic agent.66,70 Carboplatin crosses the cell membrane where it is hydrolysed to 1,1-cyclobutanedicarboxylate and therefore gains a positive charge.71,72 The positively charged intermediate interacts with nucleophilic molecules such as DNA or RNA by covalent bond formation with the N7 site of purine bases leading to formation of platinum adducts (Figure 1).73,74 Carboplatin is less toxic than cisplatin as the former forms an intermediate 1,1-cyclobutanedicarboxylate which is a poorer leaving group as compared with chloride, which leads to low reactivity rate and there is therefore less adduct formation.75 The clearance of cisplatin occurs majorly by host tissues; however, for carboplatin, it occurs by renal function, therefore targeted area-under-the-curve (AUC) dosing based on estimated renal clearance improved safety and tolerability of carboplatin.76 Carboplatin and etoposide, which showed significant synergistic activity in animal models of ovarian cancer, had a relatively low CR of only 43% along with increased toxicity rate.22 Combination of carboplatin, hexamethylmelamine and etoposide produced a very high CR of 92%; however, the study was on a very small sample size.23 Carboplatin and cyclophosphamide combination proved to be effective in optimally debulked advanced ovarian cancer patients; however, the combination did not significantly prevent tumour progression in a majority of patients.25 A combination of carboplatin and ifosfamide too has showed improved CR.30 Carboplatin and docetaxel produced an improved CR of 73%; however, it has substantial myelotoxicity.46 Gemcitabine, a nucleoside analogue, and carboplatin also showed significant CR.51 Carboplatin and topotecan, which is a specific topoisomerase I inhibitor that causes single-stranded breaks in DNA during replication, showed a CR of 71%.57
Figure 1.
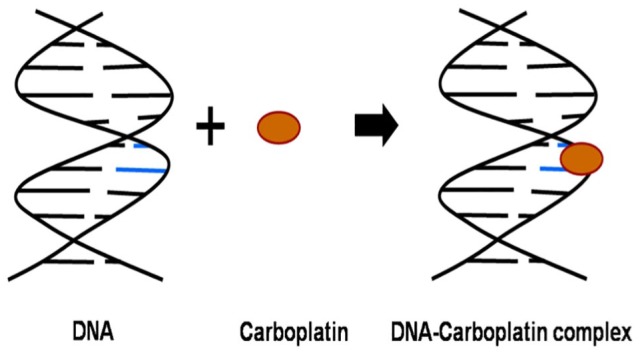
Mechanism of action of cisplatin. Double stranded DNA is shown in black; Cisplatin is shown as a brown oval; and purine bases are shown as blue coloured lines.
Paclitaxel as a Single Agent and Its Combinations
In the 1990s, paclitaxel was found to be the most effective agent in patients with relapsed platinum-refractory disease.77 It acts by binding to intracellular β-tubulin, which leads to microtubule stabilization, G2-M arrest and apoptosis, via both p53-dependent and p53-independent pathways.78 Earlier the most commonly used combination was cyclophosphamide and cisplatin; however, the OS was not sufficient. At this stage, paclitaxel was included in first-line chemotherapy for patients with sub-optimally debulked advanced ovarian cancer which led to increase in the duration of the PFS and OS.24 Paclitaxel used alone emerged as an effective and safe drug for first-line treatment of advanced ovarian cancer.29 Paclitaxel-cisplatin produces an overall higher CR although it has low tolerability than the conventional combination of carboplatin-paclitaxel.24,26 A GOG study along with other randomized studies concluded that the inclusion of paclitaxel with a platinum analogue produced significant improvement in response and survival.24,79 At this stage, combination of paclitaxel, cisplatin and ifosfamide was investigated which produced a CR of 85%.39 Paclitaxel, cisplatin and doxorubicin combination produced a CR of 64%, a marginal improvement in PFS; however, there was improved survival benefit when compared with the standard carboplatin-paclitaxel combination.58 It may be noted here that combinations of paclitaxel and carboplatin along with a third agent have also been tested across various clinical trials (Table 1). However, these combinations have had their own limitations like no improvement in CR as compared with carboplatin and paclitaxel, increased haematological toxicities, neutropenia, alopecia and thrombocytopenia.53,80,81
Carboplatin and Paclitaxel as a Combination
Carboplatin and paclitaxel have been a standard chemotherapy combination used and the related clinical studies summarizing its efficacy are provided in Table 2. It is seen that the combination therapy of carboplatin-paclitaxel achieves a CR ranging from 50% to 81% and median PFS range of 13.6 to 19.3 months. Numerous trials have established that a combination of paclitaxel and carboplatin is well tolerated in advanced ovarian cancer.90 A few earlier randomized trials too demonstrated that the combination of cisplatin and paclitaxel was superior to cisplatin and cyclophosphamide in advanced ovarian cancer.91 Also, trials have showed that carboplatin and paclitaxel was a less toxic and highly effective combination regimen.92 A study conducted by GOG in patients with optimally debulked advanced ovarian cancer revealed that the median PFS and OS were 19.4 and 48.7 months, respectively, for the cisplatin-paclitaxel as compared with 20.7 and 57.4 months, respectively, for carboplatin-paclitaxel. In addition, gastrointestinal, renal, metabolic toxicity and leukopenia were significantly more in cisplatin-paclitaxel group as compared with carboplatin-paclitaxel.92 Another clinical trial compared carboplatin-paclitaxel and carboplatin, paclitaxel and cisplatin combination. The median PFS and OS were not statistically different, although carboplatin-paclitaxel combination was associated with better tolerability and quality of life (median PFS: 17.2 vs 19.1 months; median OS: 43.3 vs 44.1 months). The mean global quality-of-life scores at the end of treatment were statistically significantly better with the use of carboplatin-paclitaxel (65.25 vs 51.97).26 In the HeCOG (Hellenic Cooperative Oncology Group) study comparing carboplatin-paclitaxel and cisplatin, paclitaxel and doxorubicin, the latter showed a slight increase in PFS; however, there was no additional survival benefit (CR: 69% vs 64%; median PFS: 13.25 vs 18.13 months; median OS: 37.97 vs 44.33 months).58 Previous trials comparing cisplatin and paclitaxel against carboplatin and paclitaxel, 1 using different paclitaxel schedules on the 2 arms suggested that carboplatin and paclitaxel had more favourable toxicity profile and convenience of a shorter schedule.93,94 Other studies have demonstrated that quality of life was better during treatment with carboplatin and paclitaxel as compared with cisplatin and paclitaxel.95,96 Such landmark studies established the combination of carboplatin-paclitaxel as the standard of care in advanced ovarian cancer.
Table 2.
First-line chemotherapeutic treatment with carboplatin-paclitaxel in advanced ovarian cancer.
| Clinical response | Median PFS (in months) | Overall survival (in months) | Reference |
|---|---|---|---|
| 57.0% | – | – | 82 |
| 75.0% | – | – | 83 |
| 70.0% | – | – | 83 |
| 57.0% | – | – | 25 |
| 74.0% | – | – | 84 |
| 81.0% | – | 20.0 | 85 |
| 50.0% | – | – | 86 |
| 67.7%, | 17.2 | 43.3 | 26 |
| 60.0% | 17.9 | 41.0 | 53 |
| 69.0% | 18.1 | 38.0 | 58 |
| 77.5% | 19.3 | 51.5 | 87 |
| 59% | 16.8 | 53.2 | 88 |
| 80.0% | 16.0 | 40.2 | 54 |
| 56.2% | 18·3 | – | 89 |
| 74.0% | 16.3 | – | 62 |
Abbreviation: PFS, progression-free survival.
Chemotherapy Resistance in Advanced Ovarian Cancer
First-line chemotherapy with carboplatin and paclitaxel achieves an improved CR; however, recurrence occurs in 25% of patients with early stage disease and more than 80% of patients with advanced disease.97 A majority of advanced ovarian cancer patients experience disease relapse within 2 years of the initial treatment of combination chemotherapy.98 The heterogeneity of tumour cells leads to molecular variations in signalling pathways including oncogene activation, tumour suppressor inactivation and various pro-survival genetic mutations.99 Therefore, chemo-resistance to standard chemotherapy regimen has emerged as a major challenge.100 Whereas the current therapeutic regimens are fixed linear protocols, cancer biology is a highly dynamic system. Adapting a therapeutic strategy using systems biology approach based on temporal and spatial variations in tumour is a futuristic goal in oncology.101 Other studies including poly (ADP-ribose) polymerase inhibitors and anti-angiogenic agents have shown that trial design with restricted eligibility criteria rather than testing chemotherapeutic agents in unselected populations can lead to improved clinical outcomes in the targeted populations.102–104 Drug resistance is 1 of the most important factors for failure of chemotherapy in advanced ovarian cancer. Chemotherapy resistance is of 2 types: (a) intrinsic chemo-resistance, where the cancer cells are inherently resistant to drug treatment, and (b) acquired chemo-resistance, which can be acquired during the course of treatment.105 Intrinsic chemo-resistance is caused due to cancer cells possessing several biological modifications including inhibited drugs uptake, increased drug efflux, increased detoxification of chemotherapeutic drugs, inhibition of apoptosis and so on.106 While acquired chemo-resistance can arise due to genetic and epigenetic alternations that assist the cancer cells to adapt to chemotherapy induced effects such as stress, DNA damage and apoptosis.106 Therefore, chemo-resistance, which is a multifactorial phenomenon, is being investigated with the view to decipher chemo-resistance mechanisms and develop drugs to overcome it.107,108 However, the major problem is that identification of patients pre-disposed to chemo-resistance is challenging as there are no available tests to guide clinicians to make an informed decision to alter treatment course before chemotherapy.
Biomarkers and Chemotherapy Resistance in Advanced Ovarian Cancer
Despite initial responsiveness to combination chemotherapy of carboplatin and paclitaxel, the occurrence of chemo-resistant tumours is a major hurdle and therefore demands elucidation of its pathogenesis. The delineation of molecular signatures from these tissues has paved the way for biomarker discovery. Biomarkers are biological macromolecules that can be objectively measured and evaluated and indicate the functioning of biological processes and pharmacologic response in the human body.109 Biomarker discovery has made many strides in the field of medicine and health.110–112 Over the past decade, clinical proteomics has helped in biomarker discovery in the field of advanced ovarian cancer.113,114 An understanding of biomarkers in chemotherapy resistance in advanced ovarian cancer will have the following benefits: (a) to elucidate the molecular mechanisms at a cellular level that dictate drug resistance, (b) design new therapeutic strategies to overcome drug resistance, (c) plan the best chemotherapeutic strategy and improve patient management, (d) help to improve patient compliance and reduce financial expenditure and (e) predict the sensitivity of tumour to chemotherapeutic regimen allowing chemotherapy administration to the patient who would benefit and prevent the toxic effects of chemotherapy to non-responder patients.
Various biomarkers and their mechanism of action that help to understand chemotherapy resistance are summarized in Table 3. Even though chemo-resistance has plagued the CR and survival in advanced ovarian cancer since the beginning of chemotherapy administration, the research investigating predictive biomarkers began much later. It can be observed that much of the research in chemo-resistance biomarker discovery has focussed on transcriptomics and proteomics with very few genomic studies investigating the same.
Table 3.
Biomarkers for chemo-resistance to first-line chemotherapy in advanced ovarian cancer.
| Chemotherapy | Macromolecule | Biomarker | Source | Cellular mechanism | Clinical phenotype | Chemo-resistance | References |
|---|---|---|---|---|---|---|---|
| Platinum-based drug/Alkylating agent | Protein | Lung resistance protein (LRP) ↑ | Tissue | Increases drug efflux | Non-responder | Intrinsic | 115 |
| Doxorubicin, cisplatin and paclitaxel | mRNA | Human epidermal growth factor receptor 2 (HER-2) ↑ | Cell line | Increases drug efflux and increases drug metabolism | Responder | N/A | 116,117 |
| Paclitaxel | RNA | Inactive X chromosome-specific Transcripts (XIST) ↓ | Cell line and tissue | Reactivates resistance-specific genes on inactive X chromosome in the absence of XIST
RNA. |
Non-responder | Acquired | 118 |
| Cisplatin | Protein | Cycloxegenase-2 (COX-2) ↑ | Tissue | Increases proliferation and inhibits apoptosis | Non-responder | Intrinsic | 119 |
| Cisplatin | Protein | P-glycoprotein (Pgp) ↑ | Tissue | Increases drug efflux | Non-responder | Intrinsic | 119 |
| Cisplatin | mRNA | Trophinin (TRO) ↑ | Cell line | Homophilic adhesion molecule involved in blastocyst implantation | Responder | N/A | 120 |
| Carboplatin and cyclophosphamide | Protein | Excision repair cross-complementation group 1 (ERCC-1) ↑ | Tissue | Increases DNA repair | Non-responder | Intrinsic | 121 |
| Platinum-based drug and paclitaxel | Protein | Vascular endothelial growth factor (VEGF) ↑ | Ascites | Increases tumour angiogenesis | Non-responder | Intrinsic | 122 |
| Platinum-based drug and paclitaxel | Protein | Tumour necrosis factor alpha (TNFα) ↑ | Ascites | Increases inflammation | Non-responder | Intrinsic | 122 |
| Paclitaxel | Protein | Endoplasmic reticulum resident oxidoreductase 57 (ERp57) ↑ | Cell line | Increases protein folding under stress and inhibits apoptosis | Non-responder | Acquired | 123 |
| Platinum-based drug and cyclophosphamide or paclitaxel | mRNA | Mesothelin (MSLN) ↑ | Tissue | Alters the time spent by cytotoxic drugs in the peritoneal cavity or changes the tumour microenvironment of ovarian cancer patients; therefore inhibiting the effects of cytotoxic drugs | Non-responder | Intrinsic | 124 |
| Cisplatin | DNA | Insulin-like growth factor I receptor (IGF1R) ↑ | Cell line | Inhibits apoptosis | Non-responder | Acquired | 125–127 |
| Cisplatin | DNA | Phosphatidylinositol-3-OH kinase (PIK) ↑ | Cell line | Inhibits apoptosis | Non-responder | Acquired | 125–127 |
| Platinum-based-drug | DNA | Cyclin E (CCNE1) ↑ | Tissue | Increases cell proliferation | Non-responder | Intrinsic | 128 |
| Paclitaxel | Protein | Heterogeneous nuclear riboprotein A2 (hnRNPA2) ↓ | Cell line | Increased cell stability | Non-responder | Acquired | 129,130 |
| Paclitaxel | Protein | Rho GDP dissociation inhibitor (GDI 2) ↓ | Cell line | Increases invasion | Non-responder | Acquired | 129,131 |
| Cisplatin | Protein | Pyruvate kinase-M2 (PKM2) ↓ | Cell line | Increases drug inactivation | Non-responder | Acquired | 132,133 |
| Cisplatin | Protein | Heat shock protein D1 (HSP-D1) ↑ | Cell line | Inhibits apoptosis | Non-responder | Acquired | 132,134 |
| Cisplatin | Protein | Hypoxia up-regulated protein 1 precursor (HYOU1) ↑ | Cell line | Inhibits apoptosis | Non-responder | Acquired | 132,135 |
| Cisplatin | Protein | Isoform 1 of collagen XII alpha-1 chain (COL12A1) ↓ | Cell line | Increases tumour angiogenesis and invasion | Non-responder | Acquired | 132,136,137 |
| Cisplatin | Protein | Calnexin (CNX) ↑ | Cell line | Inhibits apoptosis | Non-responder | Acquired | 132,138 |
| Cisplatin | Protein | High mobility group protein B1 (HMGB1) ↑ | Cell line | Reduces DNA damage | Non-responder | Acquired | 132,139 |
| Cisplatin | Protein | Lung resistance protein (LRP) ↑ | Cell line | Increases drug efflux | Non-responder | Acquired | 104,121 |
| Cisplatin | Protein | Nestin (NES)↑ | Tissue | Increases tumour angiogenesis | Non-responder | Intrinsic | 130,131 |
| Cisplatin | Protein | Activated leukocyte cell adhesion molecule (ALCAM)↑ | Cell line | Increased interaction with pro-growth NFκB pathway | Non-responder | Acquired | 132 |
| Cisplatin | Protein | A-kinase anchoring protein 12 (AKAP12) ↑ | Cell line | Inhibits apoptosis | Non-responder | Acquired | 132 |
| Carboplatin | Protein | Steroid receptor coactivator 3 (SRC-3) ↑ | Tissue | Increases cell survival | Non-responder | Intrinsic | 114,133 |
| Cisplatin | Protein | Pro-alpha1(XI) chain (COL11A1) ↑ | Cell line | Increases invasion | Non-responder | Acquired | 134,135 |
| Paclitaxel | mRNA | Chitinase 3-like 1 (CHI3L1) ↑ | Tissue | Inhibits apoptosis | Non-responder | Intrinsic | 136 |
| Platinum-based drug and paclitaxel | mRNA | Insulin-like growth factor 2 mRNA-binding protein (IGF2BP) ↑ | Tissue | Increases tumour proliferation | Non-responder | Intrinsic | 137 |
| Platinum-based and paclitaxel | mRNA | Lin-28 Homolog B (Lin28B) ↑ | Tissue | Acts as oncogene by blocking let-7 miRNA biogenesis and subsequent depression of let-7 miRNA target genes | Non-responder | Intrinsic | 139 |
| Platinum-based drug | Protein | Leptin (LEP) ↑ | Ascites | Inhibits apoptosis | Non-responder | Intrinsic | 138,139 |
| Cisplatin paclitaxel | Protein | Midkine (MK) ↑ | Tissue | Increases cytotoxicity | Responder | N/A | 140 |
| Paclitaxel | Protein | Musashi-2 (MSI2) ↑ | Cell line | Increases drug efflux | Non-responder | Acquired | 141 |
| Platinum-based | Protein | RAN Binding Protein 1 (RANBP1) ↓ | Tissue | Increases tumour proliferation, decreases drug binding to DNA and inhibits apoptosis | Non-responder | Intrinsic | 142 |
| Platinum-based drug | Protein | Actinin Alpha 4 (ACTN4) ↑ |
Tissue | Increases invasion | Non-responder | Intrinsic | 142 |
| Platinum-based drug | Protein | Keratin 19 (KRT19) ↑ | Tissue | Increases invasion | Non-responder | Intrinsic | 142,143 |
| Platinum-based drug | Protein | Lactate dehydrogenase A-like 6A (LDHAL6A) ↑ | Tissue | Increases anaerobic metabolism | Non-responder | Intrinsic | 142,144 |
| Platinum-based drug | Protein |
Thiopurine S-methyltransferase
(TPMT) ↓ |
Tissue | Decreases metabolism of drug | Responder | N/A | 142,145 |
| Platinum-based and paclitaxel | Protein | Mesothelium vascular cell adhesion molecule-1 (VCAM-1) ↑ | Serum | Increases invasion | Non-responder | Acquired | 146,147 |
| Platinum-based drug | Protein | Epithelial cell adhesion molecule (EpCam) ↑ | Tissue | Inhibits apoptosis | Non-responder | Acquired | 148 |
| Platinum-based drug | mRNA and protein | Forkhead box M1 (FOXM1) ↑ | Tissue | Increases cell-cycle progression, response to DNA damage | Non-responder | Intrinsic | 149,150 |
| Carboplatin | mRNA and protein | Keratin 5 (KRT5) ↑ | Cell line, tissue and ascites | Increases cytoskeletal stability | Non-responder | Acquired | 151 |
| Cisplatin | mRNA | Colony-stimulating-factor-1 receptor (CSF-1R) ↑ |
Cell line | Inhibits apoptosis | Non-responder | Acquired | 152 |
| Cisplatin | mRNA | 3-Oxoacid CoA transferase 1 (OXCT1) ↓ | Cell line | Inhibits apoptosis | Non-responder | Acquired | 153,154 |
The field of genomics encompasses the systematic study of the genome of an organism. In advanced ovarian cancer, genomics has been primarily used to reveal chromosomal abnormalities or mutations such as insertions and deletions or abnormal chromosomal numbers in a process.125,128,155,156 Other studies have focused on the study of single nucleotide polymorphisms (SNPs) in deciphering individual response to the chemotherapeutic drugs.157,158 In the past, very few studies have explored the frontier of genomics in the field of biomarker discovery for chemo-resistance in advanced ovarian cancer. The major limitation of genomics approach is that the investigation of mutation and SNPs does not correlate with the level of proteins. For instance, in a previous study 12 genes were identified using DNA microarray technology; however only HSP-10 could be validated using immunohistochemistry.159
Transcriptomics is the study of the complete set of mRNA (messenger RNA) transcripts produced by a tissue or an organism under specific conditions at a particular point in time. Messenger RNA detection and estimation have been widely used in the study of chemo-resistance in advanced ovarian cancer (Table 3). However, many studies have suffered from a major limitation of inaccurate correlation between gene expression and protein expression. Correlation between mRNA and protein level is insufficient to predict protein expression levels from quantitative mRNA data.159 For some genes with similar mRNA levels, the protein levels may vary by more than 20-fold and conversely, for proteins with similar levels, corresponding mRNA levels may vary by as much as 30-folds.160 Such discrepancy can be explained by taking into account post-translational events such as alternative splicing, translational regulation and differences in protein in vivo half-lives.161,162
Proteomics is the study of the set of all expressed proteins in a cell, tissue or organism at a specific time under specific conditions. It can be used to characterize the flow of information in biological pathways and their networks to establish functional relevance of proteins. The proteome of a biological entity represents the dynamic relationship between the genes, environment and pathological states. Proteins are the macromolecules that are majorly affected in diseases and participate in the subsequent disease response. It is evident that proteins have the advantage to be used as biomarkers in various clinical states and to assess associated therapeutic response due to the following advantages: (a) genome is largely similar in individuals of the same species, whereas protein expression is specific to a cell type under specific conditions; (b) effect of environment is reflected in proteome more easily than genome which remains stable; (c) protein expression level is result of transcriptional activation, transcript degradation and translation efficiency and (d) proteins are the key downstream effectors and affecters in various cellular functions. Therefore, a majority of the studies have used proteomics to study the phenomenon of chemo-resistance in advanced ovarian cancer.
Post-translational modifications are important determinants of protein functionality and are an important mechanism to increase the diversity of proteome along with regulatory interactions with the various cellular functions.163 Several studies have investigated the role of post-translational modifications as potential biomarkers in cancer.164 Some post-translational modifications also play a role in chemo-response mechanisms in ovarian cancer and is summarized in Table 4. These modifications have an effect on cytoskeletal integrity, protein folding, metabolic function and apoptotic activity of tumour cells that in turn determines the response of ovarian cancer cells to the chemotherapeutic drug.
Table 4.
Effect of post-translational modifications on chemo-response carboplatin and paclitaxel in ovarian cancer.
| Protein | Post-translational modification | Effect of post-translational modification on cellular function | Phenotype | References |
|---|---|---|---|---|
| Tubulin | De-tyrosination | Microtubule stabilization that is essential for apoptosis | Chemo-sensitive | 165,166 |
| p53 | Phosphorylation | Leads to apoptosis | Chemo-sensitive | 167,168 |
| Tumour rejection antigen | Glycosylation | Tumour proliferation, anti-apoptotic activity, metastasis | Chemo-resistance | 169,170 |
| Triose phosphate Isomerase | Glycosylation | Facilitates glycolysis that helps to keep up with the increased energy demand in rapidly growing tumour | Chemo-resistance | 170,171 |
| Palmitoyl-protein thioesterase 1 precursor | Glycosylation | Anti-apoptotic activity | Chemo-resistance | 170,172 |
| ER-associated DNAJ | Glycosylation | Protein folding, transport, translational initiation and gene expression | Chemo-resistance | 170,173–175 |
| Fas-associated death domain-like interleukin-1b-converting enzyme (FLICE)-like inhibitory protein | Ubiquitination | Suppressor of apoptosis | Chemo-resistance | 176,177 |
| Peptidyl-prolyl cis-trans isomerase A | N-terminal acetylation | Conformational maintenance of oncogenes, cell proliferation, anti-apoptotic activity | Chemo-resistance |
Biomarkers and Their Biological Functions
The identified genes, mRNA or proteins are involved in various biological functions such as cell cycle and checkpoint proteins, protein folding, chaperones, DNA repair proteins, cytoskeletal proteins, metabolic enzymes, transcriptional activators, drug-efflux pumps, and cellular redox protein and regulators of the apoptotic pathway as can be seen in Table 3.
The different mechanisms that cause chemo-resistance are explained. (a) Apoptosis is 1 of the main mechanisms employed by the cells to evade drug-induced cytotoxicity and subsequently manifest as chemo-resistance. PI3K/AKT and ERK1/2 pathway are at the centre stage of mediating anti-apoptotic activity in chemo-resistance. Various proteins interact with this pathway to prevent cell death such as increased expression of insulin-like growth factor I receptor (IGF1R), phosphatidylinositol-3-OH kinase (PIK), A-kinase anchoring protein 12 (AKAP12), chitinase 3-like 1 (CHI3L1), leptin (LEP), epithelial cell adhesion molecule (EpCam) and colony-stimulating-factor-1 receptor (CSF1R) that causes evasion of apoptosis as a downstream effect of PI3K/AKT and ERK1/2 pathway activation.125,126,127,143,147 Other proteins like cyclooxygenase-2 are known to increase production of prostaglandin E2 which is involved in resistance to apoptosis.119 Proteins like endoplasmic reticulum resident oxidoreductase 57 (ERp57) show class III β-tubulin (TUBB3) mediated anti-apoptotic activity.123 (b) Drug efflux from the cells plays an important role in the response of tumour cells to chemotherapy. Some of the interesting mechanisms include HER2 mediated increase in activity of drug-efflux pumps including the adenosine triphosphate (ATP)–binding cassette, sub-family B, member 1 (ABCB1) and ABCC3.14 Other mechanisms include increased activity of drug-transporters like lung resistance protein and ATP-driven P-glycoprotein (Pgp).115,119,132 (c) Cell adhesion and tumour invasion pose a challenge to the efficacy of chemotherapeutic drugs. For example, down-regulation of isoform 1 of collagen XII alpha-1 chain (COL12A1) causes extracellular matrix remoulding and tumour migration.132 Other cytoskeletal modulations include actinin alpha-4 (ACTN4) up-regulation that enhances cell motility by bundling the actin cytoskeleton causing metastasis.153 Other proteins like mesothelium vascular cell adhesion molecule-1 (VCAM1) is up-regulated in non-responders and is known to mediate tumour invasion by the epithelial and mesenchymal transition. (d) Drug metabolism is common in chemo-resistant tumour cells as an important pathway to eliminate active drug molecules and evade cytotoxic effect. Human epidermal growth factor receptor-2 (HER2) up-regulation increases expression of drug metabolism proteins including glutathione S-transferase P1 (GSTP1) and cytochrome P450 3A4 (CYP3A4).117 In contrast, down-regulation of thiopurine S-methyltransferase reduces the metabolism of thiopurine chemotherapeutic agents, such as 6-mercaptopurine. (e) Increase in pathways involved in tumour angiogenesis contributes to increased vascularity and nutrient supply to cells under stress condition induced by chemotherapy. For instance, up-regulation of vascular endothelial growth factor and nestin provide the tumour with a replenished microenvironment to enhance survival.122,141 (f) Increased cell proliferation and survival pathways are an important marker for chemotherapy resistance. An interesting observation is the up-regulation of HER2 mediated activation of pro-survival proteins such as survivin, p21 and p53.117
Research has been done to understand these aspects using an array of human tissue samples. Some of the sources include ascitic fluid, serum, ovarian cancer cell lines and ovarian cancer tissue samples. A comprehensive review by Kaur and group revealed that pharmacological studies using cell lines suffer from some major drawbacks191: (a) Cell lines get genetically altered, and this sometimes alters their phenotype, native functions and their response to stimuli; (b) genotypic and phenotypic variation may occur due to serial passage of cell lines over an extended period of time and (c) unsuspected heterogeneity may occur due to genetic drift. Therefore, cell lines do not provide the actual reflection of molecular events as compared with tissue-based experiments. Also, most of the ovarian cancer cell line studies have been done for individual drugs such as cisplatin and paclitaxel, which may not correspond with the actual use of drug combinations in patients. It is of interest to note that the standard protocol for advanced ovarian cancer is a debulking surgery followed by chemotherapy. The procurement of the ovarian cancer tissue offers a window of opportunity for discovering biomarkers for innate resistance.
Studies have looked into the role of biomarkers to evaluate patient response to carboplatin and paclitaxel. This has been summarized in Table 5. Some of the important observations are as follows: (a) Genomic and transcriptomic studies that have been done to understand chemo-resistance to carboplatin and paclitaxel in ovarian cancer do not correspond to protein expression; (b) the chemotherapy response results from cell-cycle pathways that include apoptosis, drug-efflux mechanisms, regulation of innate immunity and cell survival; (c) observed chemo-response outcomes were mostly intrinsic by nature, indicating pre-mediated cellular mechanisms that determine clinical phenotypes and (d) metabolic proteins, chaperones, transporters, transcription regulators and cytoskeletal proteins are up-regulated in ovarian cancer tissues of patients who had chemo-resistance. Although proteomics was used to study the problem of chemo-resistance in advanced ovarian cancer, substantial progress was not made due to the absence of whole cell proteome comparison between chemo-resistant and chemo-sensitive patient tissue samples. To address this, our group has sought to delineate distinct protein signatures that could red flag an innate chemotherapy resistance in advanced ovarian cancer. In this endeavour, we employed fluorescence-based differential in-gel expression coupled with mass spectrometric analysis to identify differentially expressed proteins in the advanced ovarian cancer tissue of patients resistant and sensitive to carboplatin and paclitaxel combinations.114 Aldehyde reductase, hnRNP, cyclophilin A, heat shock protein-27 and actin that were expressed in the chemo-sensitive state are proteins intricately involved in apoptosis, and prohibitin, enoyl-coA hydratase, peroxiredoxin, fibrin-β and fibrin-γ that were expressed in the chemo-resistant state are proteins that dictate cell survival.225,226 This clearly establishes the importance and relevance of biomarker discovery for chemo-response in ovarian cancer. This is a positive step that can pave the way for better patient management and compliance.
Table 5.
Biomarkers in chemo-resistance to carboplatin and paclitaxel in advanced ovarian cancer.
| Chemotherapy | Macromolecule | Biomarker | Source | Cellular mechanism | Clinical phenotype | Chemo-resistance | Reference |
|---|---|---|---|---|---|---|---|
| Carboplatin and paclitaxel | Protein | Heat shock protein 10 (HSP-10) ↓ | Tissue | Inhibits apoptosis | Non-responder | Intrinsic | 159 |
| Carboplatin and paclitaxel | Protein | Matrix metalloproteinase 1 (MMP-1) ↑ | Tissue | Promotes tumour invasion | Non-responder | Intrinsic | 159,178 |
| Carboplatin and paclitaxel | DNA | Ecotropic virus integration site 1 protein homolog (EVI1) ↑ | Tissue | Inhibits apoptosis | Non-responder | Intrinsic | 156,179 |
| Carboplatin and paclitaxel | DNA | SH3 domain-containing kinase-binding protein 1 (CIN85) ↓ |
Tissue | Inhibits apoptosis | Non-responder | Intrinsic | 156,180 |
| Carboplatin and paclitaxel | DNA | Endophilin A1 (SH3GL2) ↓ | Tissue | Inhibits apoptosis | Non-responder | Intrinsic | 156,181 |
| Carboplatin and paclitaxel | Protein | Ki-67 ↑ | Tissue | Marker for increased cell proliferation | Responder | N/A | 182 |
| Carboplatin and paclitaxel | Protein | Adenosine triphosphate–binding cassette sub-family C member 2 (ABCC2) ↓ | Increases apoptosis | Responder | N/A | 182,183 | |
| Carboplatin and paclitaxel | mRNA | Insulin-like growth factor-1 (IGF1) ↑ | Tissue | Enhances ovarian cancer cell proliferation through PI3K/Akt/mTOR signalling | Non-responder | Intrinsic | 184,185 |
| Carboplatin and paclitaxel | mRNA | Telomerase reverse transcriptase (TERT) ↑ | Tissue | Inhibits apoptosis | Non-responder | Intrinsic | 186,187 |
| Carboplatin and paclitaxel | mRNA | E2F transcription factor 1 (E2F) ↑ | Tissue | Inhibits apoptosis | Non-responder | Intrinsic | 186,188 |
| Carboplatin and paclitaxel | mRNA | Cyclin-dependent kinase inhibitor 1A (CDKN1A) ↓ | Tissue | Inhibits apoptosis | Non-responder | Intrinsic | 186,189 |
| Carboplatin and paclitaxel | mRNA | FBJ murine osteosarcoma viral oncogene homolog (FOS) ↑ | Inhibits apoptosis | Non-responder | Intrinsic | 186,190 | |
| Carboplatin and paclitaxel | mRNA | Tumor necrosis factor (TNF) receptor superfamily, member 10A (TNFRSF10A) ↓ | Tissue | Inhibits apoptosis | Non-responder | Intrinsic | 186 |
| Carboplatin and paclitaxel | mRNA | TNF receptor superfamily, member 10C (TNFRSF10C) ↓ | Tissue | Decreased activity as p53-regulated DNA damage-inducible gene | Non-responder | Intrinsic | 186 |
| Carboplatin and paclitaxel | mRNA | TNF receptor superfamily, member 10D (TNFRSF10D) ↑ | Tissue | Inhibits apoptosis | Non-responder | Intrinsic | 186 |
| Carboplatin and paclitaxel | mRNA | TNF receptor-associated factor 1 (TRAF-1) ↓ | Tissue | Increases apoptosis | Non-responder | Intrinsic | 186,191 |
| Carboplatin and paclitaxel | mRNA | Goosecoid homeobox (GSC) ↑ | Tissue | Increases metastasis | Non-responder | Intrinsic | 186,192 |
| Carboplatin and paclitaxel | mRNA | Snail homolog 1 (SNAI 1) ↑ | Tissue | Inhibits apoptosis | Non-responder | Intrinsic | 186,193 |
| Carboplatin and paclitaxel | Protein | Class III β-tubulin (TUBB3) ↑ | Cell line | Cytoskeletal modulation and overcomes stress conditions | Non-responder | Acquired | 194 |
| Carboplatin and paclitaxel | Protein | Lewis y ↑ | Tissue | Increases cell adhesion and invasion | Non-responder | Intrinsic | 195,196 |
| Carboplatin and paclitaxel | Protein | CD44 ↑ | Tissue | Increases cell adhesion and invasion, increases drug efflux | Non-responder | Intrinsic | 195–197 |
| Carboplatin and paclitaxel | Protein | CD147 ↑ | Tissue | Interacts with drug resistance proteins | Non-responder | Intrinsic | 195,198 |
| Carboplatin and paclitaxel | Protein | Human epididymis protein 4 (HE4) ↑ | Tissue | Increases tumour cell proliferation | Non-responder | Intrinsic | 195,199 |
| Carboplatin and paclitaxel | Protein | Integrin α5β1 ↑ | Tissue | Increases cell adhesion and invasion | Non-responder | Intrinsic | 195,200 |
| Carboplatin and paclitaxel | Protein | Integrin αvβ3 ↑ | Tissue | Increases cell adhesion and invasion | Non-responder | Intrinsic | 195,201 |
| Carboplatin and paclitaxel | Protein | CD44 ↑ | Tissue | Increases cell adhesion and invasion, increases drug efflux | Non-responder | Intrinsic | 196,197,202 |
| Carboplatin and paclitaxel | Protein | IL-8 ↑ | Tissue | Inhibits apoptosis | Non-responder | Intrinsic | 202,203 |
| Carboplatin and paclitaxel | Protein | α-Enolase (ENO1) ↑ | Tissue | Cytoskeleton modulation and inhibition of apoptosis | Non-responder | Intrinsic | 114,204–206 |
| Carboplatin and paclitaxel | Protein | Enoyl CoA hydratase (ECH) ↑ | Tissue | Decreases apoptosis | Non-responder | Intrinsic | 114,207 |
| Carboplatin and paclitaxel | Protein | Prohibitin (PHB) ↑ | Tissue | Inhibits apoptosis | Non-responder | Intrinsic | 114,208 |
| Carboplatin and paclitaxel | Protein | Peroxiredoxin-4 (PRDX4) ↑ | Tissue | Overcomes stress condition and inhibits apoptosis | Non-responder | Intrinsic | 114,209,210 |
| Carboplatin and paclitaxel | Protein | Fibrin β ↑ | Tissue | Increases tumour angiogenesis and inhibits apoptosis | Non-responder | Intrinsic | 114,211–213 |
| Carboplatin and paclitaxel | Protein | Fibrin γ ↑ | Tissue | Increases tumour angiogenesis and inhibits apoptosis | Non-responder | Intrinsic | 114,211–213 |
| Carboplatin and paclitaxel | Protein | Heat shock protein-27 (HSP27) ↑ | Tissue | Increases apoptosis and reduces drug efflux | Responder | Intrinsic | 114,214,215 |
| Carboplatin and paclitaxel | Protein | Actin (ACT) ↑ | Tissue | Increases apoptosis | Responder | Intrinsic | 114,216,217 |
| Carboplatin and paclitaxel | Protein | Heterogeneous ribonucleoprotein particle (hnRNP) ↑ | Tissue | Increases apoptosis | Responder | Intrinsic | 114,218,219 |
| Carboplatin and paclitaxel | Protein | Aldose reductase (ALDR) ↑ | Tissue | Increases apoptosis | Responder | Intrinsic | 114,220,221 |
| Carboplatin and paclitaxel | Protein | α-enolase (ENO1) ↑ | Tissue | Cytoskeleton modulation and inhibition of apoptosis | Non-responder | Intrinsic | 204–206,222 |
| Carboplatin and paclitaxel | Protein | Elongation factor Tu mitochondrial (TUFM) ↑ |
Tissue | Regulation of autophagy and innate immunity | Non-responder | Intrinsic | 222 |
| Carboplatin and paclitaxel | Protein | Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) ↑ | Tissue | Increases glycolysis enhancing cell survival | Non-responder | Intrinsic | 222,223 |
| Carboplatin and paclitaxel | Protein | Stress-70 protein mitochondrial (GRP75) ↑ |
Tissue | Inhibits apoptosis | Non-responder | Intrinsic | 222,224 |
| Carboplatin and paclitaxel | Protein | Annexin A 1 (ANXA1) ↑ | Tissue | Overcomes stress and inhibits apoptosis | Non-responder | Intrinsic | 222 |
Conclusions
Clinical research for chemotherapy combinations in advanced ovarian cancer has progressed over the years with a view to achieve better CR and tolerability. Currently, carboplatin and paclitaxel combination is widely used to treat advanced ovarian cancer globally. Even though there has been significant improvement in the CR and OS, a large proportion of the patients relapse due to chemo-resistance. A lot of progress has been made in the fields of genomics, transcriptomics and proteomics to understand the molecular processes that determine and dictate chemotherapy response. Clinical proteomics holds a lot of promise for biomarker discovery that can pave the way for development of diagnostics that can help monitor chemotherapeutics in patients with advanced ovarian cancer.
Footnotes
Funding:The author(s) received no financial support for the research, authorship and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Author Contributions: Concept was concieved by GH; Drafted by RP, RH and GH; Proof read by LK, RH and GH.
References
- 1. Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–2529. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer, 2018. [Google Scholar]
- 3. Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2008 (Based on November 2010 SEER Data Submission, Posted to the SEER Web Site, 2011). Bethesda, MD: National Cancer Institute; http://seer.cancer.gov/csr/1975_2008/ [Google Scholar]
- 4. Yancik R. Ovarian cancer: age contrasts in incidence, histology, disease stage at diagnosis, and mortality. Cancer. 1993;71:517–523. [DOI] [PubMed] [Google Scholar]
- 5. Holschneider CH, Berek JS. Ovarian cancer: epidemiology, biology, and prognostic factors. Semin Surg Oncol. 2000;19:3–10. [DOI] [PubMed] [Google Scholar]
- 6. Risch HA, McLaughlin JR, Cole DE, et al. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin-cohort study in Ontario, Canada. J Natl Cancer Inst. 2006;98:1694–1706. doi: 10.1093/jnci/djj465. [DOI] [PubMed] [Google Scholar]
- 7. Chen S, Iversen ES, Friebel T, et al. Characterization of BRCA1 and BRCA2 mutations in a large United States sample. J Clin Oncol. 2006;24:863–871. doi: 10.1200/JCO.2005.03.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Whittemore AS, Harris R, Itnyre J. Characteristics relating to ovarian cancer risk: collaborative analysis of 12 US case-control studies. II. Invasive epithelial ovarian cancers in white women. Collaborative ovarian cancer group. Am J Epidemiol. 1992;136:1184–1203. [DOI] [PubMed] [Google Scholar]
- 9. Risch HA, Marrett LD, Howe GR. Parity, contraception, infertility, and the risk of epithelial ovarian cancer. Am J Epidemiol. 1994;140:585–597. doi: 10.1093/oxfordjournals.aje.a117296. [DOI] [PubMed] [Google Scholar]
- 10. Schumer ST, Cannistra SA. Granulosa cell tumour of the ovary. J Clin Oncol. 2003;21:1180–1189. [DOI] [PubMed] [Google Scholar]
- 11. Prat J; FIGO Committee on Gynecologic Oncology. FIGO’s staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet. 2015;26:87–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Raja FA, Chopra N, Ledermann JA. Optimal first-line treatment in ovarian cancer. Ann Oncol. 2012;23:x118–127. doi: 10.1093/annonc/mds315. [DOI] [PubMed] [Google Scholar]
- 13. Salani R, Backes FJ, Fung MF, et al. Posttreatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of Gynecologic Oncologists recommendations. Am J Obstet Gynecol. 2011;204:466–478. doi: 10.1016/j.ajog.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 14. Angioli R, Palaia I, Zullo MA, et al. Diagnostic open laparoscopy in the management of advanced ovarian cancer. Gynecol Oncol. 2006;100:455–461. [DOI] [PubMed] [Google Scholar]
- 15. Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20:1248–1259. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 16. Le T, Faught W, Hopkins L, Fung Kee Fung M. Primary chemotherapy and adjuvant tumour debulking in the management of advanced-stage epithelial ovarian cancer. Int J Gynecol Cancer. 2005;15:770–775. [DOI] [PubMed] [Google Scholar]
- 17. Griffiths CT, Fuller AF. Intensive surgical and chemotherapeutic management of advanced ovarian cancer. Surg Clin North Am. 1978;58:131–142. [DOI] [PubMed] [Google Scholar]
- 18. Piver MS. Treatment of ovarian cancer at the crossroads: 50 years after single-agent melphalan chemotherapy. Oncology (Williston Park). 2006;20 :11561158. [PubMed] [Google Scholar]
- 19. Omura GA, Morrow CP, Blessing JA, et al. A randomized comparison of melphalan versus melphalan plus hexamethylmelamin versus adriamycin plus cyclophosphamide in ovarian carcinoma. Cancer. 1983;51:783–789. [DOI] [PubMed] [Google Scholar]
- 20. Lawton FG, Blackledge G. Melphalan in epithelial ovarian cancer: speed of clinical response as a predictor of activity. J Obstet Gynaecol. 1987;7:135–138. [Google Scholar]
- 21. Omura G, Blessing JA, Ehrlich CE, et al. A randomized trial of cyclophosphamide and doxorubicin with or without cisplatin in advanced ovarian carcinoma: a gynecologic oncology group study. Cancer. 1986;57:1725–1730. [DOI] [PubMed] [Google Scholar]
- 22. Edelmann DZ, Peretz T, Barak V, Anteby SO. Carboplatin and etoposide as first-line chemotherapy in advanced epithelial ovarian cancer. Int J Gynecol Cancer. 1995;5:443–448. [DOI] [PubMed] [Google Scholar]
- 23. Frasci G, Comella G, Comella P, et al. Carboplatin (CBDCA)-hexamethylmelamine (HMM)-oral etoposide (VP-16) first-line treatment of ovarian cancer patients with bulky disease: a phase II study. Gynecol Oncol. 1995;58:68–73. [DOI] [PubMed] [Google Scholar]
- 24. McGuire WP, Hoskins WJ, Brady MF, et al. Cyclophosphamide and cisplatin versus paclitaxel and cisplatin: a phase III randomized trial in patients with suboptimal stage III/IV ovarian cancer (from the Gynecologic Oncology Group). Semin Oncol. 1996;23:40–47. [PubMed] [Google Scholar]
- 25. Meerpohl HG, Sauerbrei W, Kuhnle H, Schumacher M, Pfleiderer A. Randomized study comparing carboplatin/cyclophosphamide and cisplatin/cyclophosphamide as first-line treatment in patients with stage III/IV epithelial ovarian cancer and small volume disease. Gynecol Oncol. 1997;66:75–84. [DOI] [PubMed] [Google Scholar]
- 26. du Bois A, Lück HJ, Meier W, et al. ; and Arbeitsgemeinschaft Gynäkologische Onkologie Ovarian Cancer Study Group. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst. 2003;95:1320–1329.12953086 [Google Scholar]
- 27. Vallejos C, Solidoro A, Gómez H, et al. Ifosfamide plus cisplatin as primary chemotherapy of advanced ovarian cancer. Gynecol Oncol 1997;672:168–171. [DOI] [PubMed] [Google Scholar]
- 28. Aziz Z, Zahid M, Ud Din Ahmed Z, Arshad T. Phase II trial of ifosfamide and cisplatinum in advanced ovarian cancer. Aust N Z J Med. 1998;28:403–409. [DOI] [PubMed] [Google Scholar]
- 29. Trope C, Kaern J, Kristensen G, Rosenberg P, Sorbe B. Paclitaxel in untreated FIGO stage III suboptimally resected ovarian cancer. Ann Oncol. 1997;8:803–806. doi: 10.1023/a. [DOI] [PubMed] [Google Scholar]
- 30. Brandi M, Demitrio A, Lorusso V, et al. Carboplatin (CBDCA) plus ifosfamide (IFO) as first-line chemotherapy in advanced (FIGO III, IV), ovarian cancer. Int J Oncol. 1997;10:509–514. [DOI] [PubMed] [Google Scholar]
- 31. Hartenbach EM, Harris LS, Bailey HH, et al. Paclitaxel, carboplatin, and hexamethyl melamine (taxchex) as first-line therapy for ovarian cancer. Cancer J Sci Am. 1999;5:348–355. [PubMed] [Google Scholar]
- 32. Vasey PA, Paul J, Birt A, et al. Docetaxel and cisplatin in combination as first-line chemotherapy for advanced epithelial ovarian cancer. J Clin Oncol. 1999;17:2069–2080. doi: 10.1200/JCO.1999.17.7.2069. [DOI] [PubMed] [Google Scholar]
- 33. Dieras V, Guastalla JP, Ferrero JM, et al. A multicenter phase II study of cisplatin and docetaxel (Taxotere) in the first-line treatment of advanced ovarian cancer: a GINECO study. Cancer Chemother Pharmacol. 2004;53:489–495. doi: 10.1007/s00280-004-0762-9. [DOI] [PubMed] [Google Scholar]
- 34. Hagen B, Onsrud M, Dale O. A pharmacokinetic and clinical evaluation of thio-TEPA in combination with cisplatin as first-line chemotherapy for advanced epithelial ovarian carcinoma. Int J Gynecol Cancer. 1999;9:110–116. [DOI] [PubMed] [Google Scholar]
- 35. Herben VM, Panday VR, Richel DJ, et al. Phase I and pharmacologic study of the combination of paclitaxel, cisplatin, and topotecan administered intravenously every 21 days as first-line therapy in patients with advanced ovarian cancer. J Clin Oncol. 1999;17:747–755. doi: 10.1200/JCO.1999.17.3.747. [DOI] [PubMed] [Google Scholar]
- 36. Papadimitriou CA, Moulopoulos LA, Vlahos G, et al. Paclitaxel, cisplatin, and epirubicin first-line chemotherapy in stage III and IV ovarian carcinoma: long-term results of a phase II study. Cancer. 2000;89:1547–1554. [DOI] [PubMed] [Google Scholar]
- 37. Fruscio R, Colombo N, Lissoni AA, et al. A phase II randomised clinical trial comparing cisplatin, paclitaxel and ifosfamide with cisplatin, paclitaxel and epirubicin in newly diagnosed advanced epithelial ovarian cancer: long-term survival analysis. Br J Cancer. 2008;98:720–727. doi: 10.1038/sj.bjc.6604231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tung N, Berkowitz R, Matulonis U, et al. Phase I trial of carboplatin, paclitaxel, etoposide, and cyclophosphamide with granulocyte colony stimulating factor as first-line therapy for patients with advanced epithelial ovarian cancer. Gynecol Oncol. 2000;77:271–277. doi: 10.1006/gyno.2000.5778. [DOI] [PubMed] [Google Scholar]
- 39. Papadimitriou CA, Kouroussis C, Moulopoulos LA, et al. Ifosfamide, paclitaxel and cisplatin first-line chemotherapy in advanced, suboptimally debulked epithelial ovarian cancer. Cancer. 2001;92:1856–1863. [DOI] [PubMed] [Google Scholar]
- 40. Fuso L, Amant F, Neven P, Berteloot P, Vergote I. Gemcitabine-carboplatin-paclitaxel combination as first-line therapy in advanced ovarian carcinoma: a single institution phase II study in 24 patients. Int J Gynecol Cancer. 2006;16:60–67. doi: 10.1111/j.1525-1438.2006.00315.x. [DOI] [PubMed] [Google Scholar]
- 41. Friedlander M, Buck M, Wyld D, et al. Phase II study of carboplatin followed by sequential gemcitabine and paclitaxel as first-line treatment for advanced ovarian cancer. Int J Gynecol Cancer. 2007;17:350–358. [DOI] [PubMed] [Google Scholar]
- 42. Nogue M, Cirera L, Arcusa A, et al. Phase II study of gemcitabine and cisplatin in chemonaive patients with advanced epithelial ovarian cancer. Anticancer Drugs. 2002;13:839–845. [DOI] [PubMed] [Google Scholar]
- 43. Belpomme D, Krakowski I, Beauduin M, et al. Gemcitabine combined with cisplatin as first-line treatment in patients with epithelial ovarian cancer: a phase II study. Gynecol Oncol. 2003;91:32–38. [DOI] [PubMed] [Google Scholar]
- 44. Sugiyama T, Yakushiji M, Kamura T, et al. ; and Japan CPT-11 Study Group. Irinotecan (CPT-11) and cisplatin as first-line chemotherapy for advanced ovarian cancer. Oncology. 2002;63:16–22. [DOI] [PubMed] [Google Scholar]
- 45. Romanini A, Tanganelli L, Carnino F, et al. First-line chemotherapy with epidoxorubicin, paclitaxel, and carboplatin for the treatment of advanced epithelial ovarian cancer patients. Gynecol Oncol. 2003;89:354–359. [DOI] [PubMed] [Google Scholar]
- 46. Pfisterer J, du Bois A, Wagner U, et al. ; and Arbeitsgemeinschaft Gynäkologische Onkologie (AGO OVAR) Ovarian Cancer Study Group. Docetaxel and carboplatin as first-line chemotherapy in patients with advanced gynecological tumors. A phase I/II trial of the Arbeitsgemeinschaft Gynäkologische Onkologie (AGO-OVAR) ovarian cancer study group. Gynecol Oncol. 2004;92:949–956. [DOI] [PubMed] [Google Scholar]
- 47. Sorbe B, Graflund M, Nygren L, et al. Phase II study of docetaxel weekly in combination with carboplatin every three weeks as first line chemotherapy in stage IIB-IV epithelial ovarian cancer: neurological toxicity and quality-of-life evaluation. Int J Oncol. 2012;40:773–781. doi: 10.3892/ijo.2011.1286. [DOI] [PubMed] [Google Scholar]
- 48. Guppy AE, Nelstrop AE, Foster T, Agarwal R, Seckl MJ, Rustin GJ. A phase II study of sequential carboplatin, paclitaxel and topotecan in patients with previously untreated advanced ovarian cancer. Br J Cancer. 2004;90:810–814. doi: 10.1038/sj.bjc.6601618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. De Vos FY, Bos AM, Schaapveld M, et al. A randomized phase II study of paclitaxel with carboplatin +/- amifostine as first line treatment in advanced ovarian carcinoma. Gynecol Oncol. 2005;97:60–67. doi: 10.1016/j.ygyno.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 50. Hainsworth JD, Kalman L, Castine M, Sylvester L, Greco FA. Paclitaxel, carboplatin, and oral etoposide as initial treatment for advanced ovarian carcinoma: a Minnie Pearl Cancer Research Network phase II trial. Gynecol Oncol. 2005;97:200–205. doi: 10.1016/j.ygyno.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 51. Tay SK, Ilanchadran A, Tan TY. First-line gemcitabine and carboplatin in advanced ovarian carcinoma: a phase II study. BJOG. 2006;113:1388–1392. [DOI] [PubMed] [Google Scholar]
- 52. Steer CB, Chrystal K, Cheong KA, et al. Gemcitabine and oxaliplatin followed by paclitaxel and carboplatin as first line therapy for patients with suboptimally debulked, advanced epithelial ovarian cancer. Gynecol Oncol. 2006;103:439–445. doi: 10.1016/j.ygyno.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 53. du Bois A, Weber B, Rochon J, et al. ; and Arbeitsgemeinschaft Gynaekologische Onkologie; Ovarian Cancer Study Group; Groupe d’Investigateurs Nationaux pour l’Etude des Cancers Ovariens. Addition of epirubicin as a third drug to carboplatin-paclitaxel in first-line treatment of advanced ovarian cancer: a prospectively randomized gynecologic cancer intergroup trial by the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group and the Groupe d’Investigateurs Nationaux pour l’Etude des Cancers Ovariens. J Clin Oncol. 2006;24:1127–1135. [DOI] [PubMed] [Google Scholar]
- 54. Lindemann K, Christensen RD, Vergote I, et al. First-line treatment of advanced ovarian cancer with paclitaxel/carboplatin with or without epirubicin (TEC versus TC) – a gynecologic cancer intergroup study of the NSGO, EORTC GCG and NCIC CTG. Ann Oncol. 2012;23:2613–2619. doi: 10.1093/annonc/mds060. [DOI] [PubMed] [Google Scholar]
- 55. Micha JP, Goldstein BH, Rettenmaier MA, et al. A phase II study of outpatient first-line paclitaxel, carboplatin, and bevacizumab for advanced-stage epithelial ovarian, peritoneal, and fallopian tube cancer. Int J Gynecol Cancer. 2007;17:771–776. doi: 10.1111/j.1525-1438.2007.00886.x. [DOI] [PubMed] [Google Scholar]
- 56. Fleming ND, Coleman RL, Tung C, et al. Phase II trial of bevacizumab with dose-dense paclitaxel as first-line treatment in patients with advanced ovarian cancer. Gynecol Oncol. 2017;147:41–46. doi: 10.1016/j.ygyno.2017.07.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vecchione F, Fruscio R, Dell’Anna T, et al. A phase II clinical trial of topotecan and carboplatin in patients with newly diagnosed advanced epithelial ovarian cancer. Int J Gynecol Cancer. 2007;17:367–372. doi: 10.1111/j.1525-1438.2007.00797.x. [DOI] [PubMed] [Google Scholar]
- 58. Aravantinos G, Fountzilas G, Bamias A, et al. ; and Hellenic Cooperative Oncology Group study. Carboplatin and paclitaxel versus cisplatin, paclitaxel and doxorubicin for first-line chemotherapy of advanced ovarian cancer: a Hellenic Cooperative Oncology Group (HeCOG) study. Eur J Cancer. 2008;44: 2169–2177. [DOI] [PubMed] [Google Scholar]
- 59. Pignata S, Breda E, Scambia G, et al. A phase II study of weekly carboplatin and paclitaxel as first-line treatment of elderly patients with advanced ovarian cancer. A multicentre Italian trial in ovarian cancer (MITO-5) study. Crit Rev Oncol Hematol. 2008;66:229–236. doi: 10.1016/j.critrevonc.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 60. Meier W, du Bois A, Rau J, et al. Randomized phase II trial of carboplatin and paclitaxel with or without lonafarnib in first-line treatment of epithelial ovarian cancer stage IIB-IV. Gynecol Oncol. 2012;126:236–240. [DOI] [PubMed] [Google Scholar]
- 61. Herzog TJ, Monk BJ, Rose PG, et al. A phase II trial of oxaliplatin, docetaxel, and bevacizumab as first-line therapy of advanced cancer of the ovary, peritoneum, and fallopian tube. Gynecol Oncol. 2014;132:517–525. doi: 10.1016/j.ygyno.2014.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hainsworth JD, Thompson DS, Bismayer JA, et al. Paclitaxel/carboplatin with or without sorafenib in the first-line treatment of patients with stage III/IV epithelial ovarian cancer: a randomized phase II study of the Sarah Cannon research institute. Cancer Med. 2015;4:673–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. https://pubchem.ncbi.nlm.nih.gov/compound/melphalan
- 64. Rutledge F, Burns BC. Chemotherapy for advanced ovarian cancer. Am J Obstet Gynecol. 1966;96:761–772. [DOI] [PubMed] [Google Scholar]
- 65. Young RC, Walton LA, Ellenberg SS, et al. Adjuvant therapy in stage I and stage II epithelial ovarian cancer. Results of two prospective randomized trials. N Engl J Med. 1990;322:1021–1027. doi: 10.1056/NEJM199004123221501. [DOI] [PubMed] [Google Scholar]
- 66. Fuertes MA, Castilla J, Alonso C, Perez JM. Cisplatin biochemical mechanism of action: from cytotoxicity to induction of cell death through interconnections between apoptotic and necrotic pathways. Curr Med Chem. 2003;10:257–266. [DOI] [PubMed] [Google Scholar]
- 67. Advanced Ovarian Cancer Trialists Group. Chemotherapy for advanced ovarian cancer. Cochrane Database of Systematic Reviews. 1999;1:CD001418. [DOI] [PubMed] [Google Scholar]
- 68. Krakowski I, Petit T, Kayitalire L, et al. Gemcitabine (Gemzar [trade]) in combination with cisplatin (CP) in advanced ovarian cancers (AOC): a phase II study. Paper presented at: Program/proceedings of the 34th Annual Meeting of the American Society of Clinical Oncology; October May 16-19 1998; Los Angeles, CA. [Google Scholar]
- 69. Hsiang YH, Hertzberg R, Hecht S, Liu LE. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem. 1985;260:14873–14878. [PubMed] [Google Scholar]
- 70. Aabo K, Adams M, Adnitt P, et al. Chemotherapy in advanced ovarian cancer: four systematic meta-analyses of individual patient data from 37 randomized trials. Br J Cancer. 1998;78:1479–1487. doi: 10.1038/bjc.1998.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. de Sousa GF, Wlodarczyk SR, Monteiro G. Carboplatin: molecular mechanisms of action associated with chemoresistance. Braz J Pharm Sci. 2014;50:693–701. [Google Scholar]
- 72. Adams C, McCarthy HO, Coulter JA, et al. Nitric oxide synthase gene therapy enhances the toxicity of cisplatin in cancer cells. J Gene Med. 2009;11:160–168. doi: 10.1002/jgm.1280. [DOI] [PubMed] [Google Scholar]
- 73. Amptoulach S, Tsavaris N. Neurotoxicity caused by the treatment with platinum analogues. Chemother Res Pract. 2011;2011:843019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. McWhinney SR, Goldberg RM, McLeod HL. Platinum neurotoxicity pharmacogenetics. Mol Cancer Ther. 2009;8:10–16. doi: 10.1158/1535-7163.MCT-08-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hah SS, Stivers KM, de Vere White RW, Henderson PT. Kinetics of carboplatin-DNA binding in genomic DNA and bladder cancer cells as determined by accelerator mass spectrometry. Chem Res Toxicol. 2006;19:622–626. [DOI] [PubMed] [Google Scholar]
- 76. Bookman MA. First-line chemotherapy in epithelial ovarian cancer. Clin Obstet Gynecol. 2012;55:96–113. [DOI] [PubMed] [Google Scholar]
- 77. Einzig AI, Wiernik PH, Sasloff J, Runowicz CD, Goldberg GL. Phase II study and long-term follow-up of patients treated with taxol for advanced ovarian adenocarcinoma. J Clin Oncol. 1992;10:1748–1753. doi: 10.1200/JCO.1992.10.11.1748. [DOI] [PubMed] [Google Scholar]
- 78. Wang TH, Popp DM, Wang HS, et al. Microtubule dysfunction induced by paclitaxel initiates apoptosis through both c-Jun N-terminal kinase (JNK)-dependent and independent pathways in ovarian cancer cells. J Biol Chem. 1999;274: 8208–8216. [DOI] [PubMed] [Google Scholar]
- 79. Neijt JP, ten Bokkel Huinink WW, van der Burg ME, et al. Randomised trial comparing two combination chemotherapy regimens (Hexa-CAF vs CHAP-5) in advanced ovarian carcinoma. Lancet. 1984;2:594–600. [DOI] [PubMed] [Google Scholar]
- 80. Bookman MA, Brady MF, McGuire WP, et al. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: a phase III trial of the gynecologic cancer intergroup. J Clin Oncol. 2009;27:1419–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bolis G, Scarfone G, Raspagliesi F, et al. Paclitaxel/carboplatin versus topotecan/paclitaxel/carboplatin in patients with FIGO suboptimally resected stage III-IV epithelial ovarian cancer: a multicenter, randomized study. Eur J Cancer. 2010;46:2905–2912. [DOI] [PubMed] [Google Scholar]
- 82. Meerpohl HG, du Bois A, Kühnle H, et al. Paclitaxel combined with carboplatin in the first-line treatment of advanced ovarian cancer. Semin Oncol. 1995; 22:7–12. [PubMed] [Google Scholar]
- 83. du Bois A, Lück HJ, Meier W, et al. Carboplatin plus paclitaxel as first-line chemotherapy in previously untreated advanced ovarian cancer. German AGO Study Group Ovarian Cancer. Arbeitsgemeinschaft Gynäkologische Onkologie. Semin Oncol. 1997;24:S1128–S1133. [PubMed] [Google Scholar]
- 84. Zamagni C, Martoni A, Cacciari N, Gentile A, Pannuti F. The combination of paclitaxel and carboplatin as first-line chemotherapy in patients with stage III and stage IV ovarian cancer: a phase I-II study. Am J Clin Oncol. 1998;21:491–497. [DOI] [PubMed] [Google Scholar]
- 85. Mayerhofer K, Bodner-Adler B, Bodner K, Leodolter S, Kainz C. Paclitaxel/carboplatin as first-line chemotherapy in advanced ovarian cancer: efficacy and adverse effects with special consideration of peripheral neurotoxicity. Anticancer Res. 2000;20:4047–4050. [PubMed] [Google Scholar]
- 86. Sehouli J, Stengel D, Elling D, et al. Ovarian cancer study group of the Nordostdeutsche Gesellschaftfür Gynäkologische Onkologie (NOGGO). First-line chemotherapy with weekly paclitaxel and carboplatin for advanced ovarian cancer: a phase I study. Gynecol Oncol. 2002;85:321–326. [DOI] [PubMed] [Google Scholar]
- 87. du Bois A, Herrstedt J, Hardy-Bessard AC, et al. Phase III trial of carboplatin plus paclitaxel with or without gemcitabine in first-line treatment of epithelial ovarian cancer. J Clin Oncol. 2010;28:4162–4169. [DOI] [PubMed] [Google Scholar]
- 88. Pignata S, Scambia G, Ferrandina G, et al. Carboplatin plus paclitaxel versus carboplatin plus pegylated liposomal doxorubicin as first-line treatment for patients with ovarian cancer: the MITO-2 randomized phase III trial. J Clin Oncol. 2011;29:3628–3635. doi: 10.1200/JCO.2010.33.8566. [DOI] [PubMed] [Google Scholar]
- 89. Pignata S, Scambia G, Katsaros D, et al. ; and Multicentre Italian Trials in Ovarian cancer (MITO-7); Groupe d’Investigateurs Nationaux pour l’Etude des Cancers Ovariens et du sein (GINECO); Mario Negri Gynecologic Oncology (MaNGO); European Network of Gynaecological Oncological Trial Groups (ENGOT-OV-10); Gynecologic Cancer Inter Group (GCIG) Investigators. Carboplatin plus paclitaxel once a week versus every 3 weeks in patients with advanced ovarian cancer (MITO-7): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2014;15:396–405. [DOI] [PubMed] [Google Scholar]
- 90. Ozols RF. Optimum chemotherapy for ovarian cancer. Int J Gynecol Cancer. 2000;10:33–37. [DOI] [PubMed] [Google Scholar]
- 91. Piccart MJ, Bertelsen K, James K, et al. Randomized intergroup trial of cisplatin-paclitaxel versus cisplatin-cyclophosphamide in women with advanced epithelial ovarian cancer: three-year results. J Natl Cancer Inst. 2000;92:699–708. [DOI] [PubMed] [Google Scholar]
- 92. Ozols RF, Bundy BN, Greer BE, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 93. Markman M, Bundy BN, Alberts DS, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19:1001–1007. doi: 10.1200/JCO.2001.19.4.1001. [DOI] [PubMed] [Google Scholar]
- 94. Neijt JP, Engelholm SA, Tuxen MK, et al. Exploratory phase III study of paclitaxel and cisplatin versus paclitaxel and carboplatin in advanced ovarian cancer. J Clin Oncol. 2000;18:3084–3092. doi: 10.1200/JCO.2000.18.17.3084. [DOI] [PubMed] [Google Scholar]
- 95. Schroder W, du Bois A, Kuhn W, et al. Treatment of patients with advanced ovarian cancer (FIGO IIb–IV) with cisplatin/paclitaxel or carboplatin/paclitaxel – an interim analysis of the AGO study protocol OVAR–3. Eur J Cancer. 1999;35:S231. [Google Scholar]
- 96. Meier W, du Bois S, Olbricht U, et al. Cisplatin/paclitaxel vs carboplatin/paclitaxel in ovarian cancer: results of a prospective randomized phase III study. Proc Int Gynecol Cancer Soc. 1999:48. [Google Scholar]
- 97. O’Toole O’Leary SJ. Ovarian cancer chemoresistance. In: Schwab M, eds. Encyclopedia of Cancer. Berlin: Springer;2011. [Google Scholar]
- 98. Agarwal R, Kaye SB. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat Rev Cancer. 2003;3:502–516. doi: 10.1038/nrc1123. [DOI] [PubMed] [Google Scholar]
- 99. Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–627. [DOI] [PubMed] [Google Scholar]
- 100. Sapiezynski J, Taratula O, Rodriguez-Rodriguez L, Minko T. Precision targeted therapy of ovarian cancer. J Control Release. 2016;243:250–268. doi: 10.1016/j.jconrel.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Gatenby RA, Silva AS, Gillies RJ, et al. Adaptive therapy. Cancer Res. 2009;69:4894–4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bookman MA. Should studies of maintenance therapy be maintained in women with ovarian cancer? J Gynecol Oncol. 2013;24:105–107. doi: 10.3802/jgo.2013.24.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a pre-planned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15:852–861. [DOI] [PubMed] [Google Scholar]
- 104. Della Pepa C, Tonini G, Pisano C, et al. Ovarian cancer standard of care: are there real alternatives? Chin J Cancer. 2015;34:17–27. doi: 10.5732/cjc.014.10274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Rubin SC, Randall TC, Armstrong KA, Chi DS, Hoskins WJ. Ten-year follow-up of ovarian cancer patients after second-look laparotomy with negative findings. Obstet Gynecol. 1999;93:21–24. [DOI] [PubMed] [Google Scholar]
- 106. Armstrong DK. Relapsed ovarian cancer: challenges and management strategies for a chronic disease. Oncologist. 2002;7:20–28. doi: 10.1634/theoncologist.7-suppl_5-20. [DOI] [PubMed] [Google Scholar]
- 107. Lippert TH, Ruoff HJ, Volm M. Current status of methods to assess cancer drug resistance. Int J Med Sci. 2011;8:245–253. doi: 10.7150/ijms.8.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zahreddine H, Borden KL. Mechanisms and insights into drug resistance in cancer. Front Pharmacol. 2013;4:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Rukmangadachar LA, Makharia GK, Mishra A, et al. Proteome analysis of the macroscopically affected colonic mucosa of Crohn’s disease and intestinal tuberculosis. Sci Rep. 2016;6:23162. doi: 10.1038/srep23162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Rukmangadachar LA, Kataria J, Hariprasad G, Samantaray JC, Srinivasan A. Two-dimensional difference gel electrophoresis (DIGE) analysis of sera from visceral leishmaniasis patients. Clin Proteomics. 2011;8:4. doi: 10.1186/1559-0275-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kataria J, Rukmangadachar LA, Hariprasad G O J, Tripathi M, Srinivasan A. Two dimensional difference gel electrophoresis analysis of cerebrospinal fluid in tuberculous meningitis patients. J Proteomics. 2011;74:2194–2203. doi: 10.1016/j.jprot.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 112. Manral P, Sharma P, Hariprasad G, et al. Can apolipoproteins and complement factors be biomarkers of Alzheimer’s disease. Curr Alzheimer Res. 2012;9: 935–943. [DOI] [PubMed] [Google Scholar]
- 113. Hariprasad G, Hariprasad R, Kumar L, Srinivasan A, Kola S, Kaushik A. Apolipoprotein A1 as a potential biomarker in the ascitic fluid for the differentiation of advanced ovarian cancers. Biomarkers. 2013;18:532–541. doi: 10.3109/1354750X.2013.822561. [DOI] [PubMed] [Google Scholar]
- 114. Sehrawat U, Pokhriyal R, Gupta AK, et al. Proteomic analysis of advanced ovarian cancer tissue to identify potential biomarkers of responders and non-responders to first-line chemotherapy of carboplatin and paclitaxel. Biomark Cancer. 2016;16:43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Izquierdo MA, van der Zee AG, Vermorken JB, et al. Drug resistance-associated marker Lrp for prediction of response to chemotherapy and prognoses in advanced ovarian carcinoma. J Natl Cancer Inst. 1995;87:1230–1237. doi: 10.1093/jnci/87.16.1230. [DOI] [PubMed] [Google Scholar]
- 116. Aigner A, Hsieh SS, Malerczyk C, Czubayko F. Reversal of HER-2 over-expression renders human ovarian cancer cells highly resistant to taxol. Toxicology. 2000;144:221–228. [DOI] [PubMed] [Google Scholar]
- 117. de Hoon JP, Veeck J, Vriens BE, Calon TG, van Engeland M, Tjan-Heijnen VC. Taxane resistance in breast cancer: a closed HER2 circuit. Biochim Biophys Acta. 2012;1825:197–206. doi: 10.1016/j.bbcan.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 118. Huang KC, Rao PH, Lau CC, et al. Relationship of XIST expression and responses of ovarian cancer to chemotherapy. Mol Cancer Ther. 2002;1:769–776. [PubMed] [Google Scholar]
- 119. Raspollini MR, Amunni G, Villanucci A, Boddi V, Taddei GL. Increased cyclooxygenase-2 (COX-2) and P-glycoprotein-170 (MDR1) expression is associated with chemotherapy resistance and poor prognosis. Analysis in ovarian carcinoma patients with low and high survival. Int J Gynecol Cancer. 2005;15:255–260. doi: 10.1111/j.1525-1438.2005.15212.x. [DOI] [PubMed] [Google Scholar]
- 120. Baba T, Mori S, Matsumura N, et al. Trophinin is a potent prognostic marker of ovarian cancer involved in platinum sensitivity. Biochem Biophys Res Commun. 2007;360:363–369. doi: 10.1016/j.bbrc.2007.06.070. [DOI] [PubMed] [Google Scholar]
- 121. Steffensen KD, Waldstrom M, Jeppesen U, Brandslund I, Jakobsen A. Prediction of response to chemotherapy by ERCC1 immunohistochemistry and ERCC1 polymorphism in ovarian cancer. Int J Gynecol Cancer. 2008;18:702–710. doi: 10.1111/j.1525-1438.2007.01068.x. [DOI] [PubMed] [Google Scholar]
- 122. Bamias A, Koutsoukou V, Terpos E, et al. Correlation of NK T-like CD3+CD56+ cells and CD4+CD25+(hi) regulatory T cells with VEGF and TNF alpha in ascites from advanced ovarian cancer: association with platinum resistance and prognosis in patients receiving first-line, platinum-based chemotherapy. Gynecol Oncol. 2008;108:421–427. [DOI] [PubMed] [Google Scholar]
- 123. Cicchillitti L, Di Michele M, Urbani A, et al. Comparative proteomic analysis of paclitaxel sensitive A2780 epithelial ovarian cancer cell line and its resistant counterpart A2780TC1 by 2D-DIGE: the role of ERp57. J Proteome Res. 2009;8:1902–1912. [DOI] [PubMed] [Google Scholar]
- 124. Cheng WF, Huang CY, Chang MC, et al. High mesothelin correlates with chemoresistance and poor survival in epithelial ovarian carcinoma. Br J Cancer. 2009;100:1144–1153. doi: 10.1038/sj.bjc.6604964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Eckstein N, Servan K, Hildebrandt B, et al. Hyperactivation of the insulin-like growth factor receptor I signaling pathway is an essential event for cisplatin resistance of ovarian cancer cells. Cancer Res. 2009;69:2996–3003. doi: 10.1158/0008-5472.CAN-08-3153. [DOI] [PubMed] [Google Scholar]
- 126. Grothey A, Voigt W, Schober C, Muller T, Dempke W, Schmoll HJ. The role of insulin-like growth factor I and its receptor in cell growth, transformation, apoptosis, and chemoresistance in solid tumors. J Cancer Res Clin Oncol. 1999;125: 166–173. [DOI] [PubMed] [Google Scholar]
- 127. Carden CP, Stewart A, Thavasu P, et al. The association of PI3 kinase signaling and chemoresistance in advanced ovarian cancer. Mol Cancer Ther. 2012;11:1609–1617. doi: 10.1158/1535-7163.MCT-11-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Etemadmoghadam D, deFazio A, Beroukhim R, et al. ; and AOCS Study Group. Integrated genome-wide DNA copy number and expression analysis identifies distinct mechanisms of primary chemoresistance in ovarian carcinomas. Clin Cancer Res. 2009;15:1417–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lee DH, Chung K, Song JA, et al. Proteomic identification of paclitaxel-resistance associated hnRNP A2 and GDI 2 proteins in human ovarian cancer cells. J Proteome Res. 2010;9:5668–5676. doi: 10.1021/pr100478u. [DOI] [PubMed] [Google Scholar]
- 130. Ford LP, Wright WE, Shay JW. A model for heterogeneous nuclear ribonucleoproteins in telomere and telomerase regulation. Oncogene. 2002;21:580–583. doi: 10.1038/sj.onc.1205086. [DOI] [PubMed] [Google Scholar]
- 131. Chia WJ, Tang BL. Emerging roles for Rab family GTPases in human cancer. Biochim Biophys Acta. 2009;1795:110–116. doi: 10.1016/j.bbcan.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 132. Li SL, Ye F, Cai WJ, et al. Quantitative proteome analysis of multidrug resistance in human ovarian cancer cell line. J Cell Biochem. 2010;109:625–633. doi: 10.1002/jcb.22413. [DOI] [PubMed] [Google Scholar]
- 133. Mazurek S, Eigenbrodt E. The tumor metabolome. Anticancer Res. 2003;23: 1149–1154. [PubMed] [Google Scholar]
- 134. Kirchhoff SR, Gupta S, Knowlton AA. Cytosolic heat shock protein 60, apoptosis, and myocardial injury. Circulation. 2002;105:2899–2904. [DOI] [PubMed] [Google Scholar]
- 135. Miyagi T, Hori O, Koshida K, Egawa M, Kato H, Kitagawa Y. Antitumor effect of reduction of 150-kDa oxygen-regulated protein expression on human prostate cancer cells. Int J Urol. 2002;9:577–585. [DOI] [PubMed] [Google Scholar]
- 136. Januchowski R, Swierczewska M, Sterzynska K, Wojtowicz K, Nowicki M, Zabel M. Increased expression of several collagen genes is associated with drug resistance in ovarian cancer cell lines. J Cancer. 2016;7:1295–1310. doi: 10.7150/jca.15371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Koenig A, Mueller C, Hasel C, Adler G, Menke A. Collagen type I induces disruption of E-cadherin-mediated cell-cell contacts and promotes proliferation of pancreatic carcinoma cells. Cancer Res. 2006;66:4662–4671. doi: 10.1158/0008-5472.CAN-05-2804. [DOI] [PubMed] [Google Scholar]
- 138. Takizawa T, Tatematsu C, Watanabe K, Kato K, Nakanishi Y. Cleavage of calnexin caused by apoptotic stimuli: implication for the regulation of apoptosis. J Biochem. 2004;136:399–405. doi: 10.1093/jb/mvh133. [DOI] [PubMed] [Google Scholar]
- 139. Lange SS, Mitchell DL, Vasquez KM. High mobility group protein B1 enhances DNA repair and chromatin modification after DNA damage. Proc Natl Acad Sci U S A. 2008;105:10320–10325. doi: 10.1073/pnas.0803181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Steiner E, Holzmann K, Elbling L, Micksche M, Berger W. Cellular functions of vaults and their involvement in multidrug resistance. Curr Drug Targets. 2006;7:923–934. [DOI] [PubMed] [Google Scholar]
- 141. Qin Q, Sun Y, Fei M, et al. Expression of putative stem marker nestin and CD133 in advanced serous ovarian cancer. Neoplasma. 2012;59:310–315. doi: 10.4149/neo_2012_040. [DOI] [PubMed] [Google Scholar]
- 142. Matsuda Y, Hagio M, Ishiwata T. Nestin: a novel angiogenesis marker and possible target for tumor angiogenesis. World J Gastroenterol. 2013;19:42–48. doi: 10.3748/wjg.v19.i1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Chappell NP, Teng PN, Hood BL, et al. Mitochondrial proteomic analysis of cisplatin resistance in ovarian cancer. J Proteome Res. 2012;11:4605–4614. doi: 10.1021/pr300403d. [DOI] [PubMed] [Google Scholar]
- 144. Palmieri C, Gojis O, Rudraraju B, et al. Expression of steroid receptor coactivator 3 in ovarian epithelial cancer is a poor prognostic factor and a marker for platinum resistance. Br J Cancer. 2013;108:2039–2044. doi: 10.1038/bjc.2013.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Teng PN, Wang G, Hood BL, et al. Identification of candidate circulating cisplatin-resistant biomarkers from epithelial ovarian carcinoma cell secretomes. Br J Cancer. 2014;110:123–132. doi: 10.1038/bjc.2013.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Wu YH, Chang TH, Huang YF, Huang HD, Chou CY. COL11A1 promotes tumor progression and predicts poor clinical outcome in ovarian cancer. Oncogene. 2014;33:3432–3440. doi: 10.1038/onc.2013.307. [DOI] [PubMed] [Google Scholar]
- 147. Chiang YC, Lin HW, Chang CF, et al. Overexpression of CHI3L1 is associated with chemoresistance and poor outcome of epithelial ovarian carcinoma. Oncotarget. 2015;6:39740–39755. doi: 10.18632/oncotarget.5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Hsu KF, Shen MR, Huang YF, et al. Overexpression of the RNA-binding proteins Lin28B and IGF2BP3 (IMP3) is associated with chemoresistance and poor disease outcome in ovarian cancer. Br J Cancer. 2015;113:414–424. doi: 10.1038/bjc.2015.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Lane D, Matte I, Garde-Granger P, et al. Inflammation-regulating factors in ascites as predictive biomarkers of drug resistance and progression-free survival in serous epithelial ovarian cancers. BMC Cancer. 2015;15:492. doi: 10.1186/s12885-015-1511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Chen C, Chang YC, Lan MS, Breslin M. Leptin stimulates ovarian cancer cell growth and inhibits apoptosis by increasing cyclin D1 and Mcl-1 expression via the activation of the MEK/ERK1/2 and PI3K/Akt signaling pathways. Int J Oncol. 2013;42:1113–1119. [DOI] [PubMed] [Google Scholar]
- 151. Wu X, Zhi X, Ji M, et al. Midkine as a potential diagnostic marker in epithelial ovarian cancer for cisplatin/paclitaxel combination clinical therapy. Am J Cancer Res. 2015;5:629–638. [PMC free article] [PubMed] [Google Scholar]
- 152. Yu R, Jin H, Jin C, et al. Inhibition of the CSF-1 receptor sensitizes ovarian cancer cells to cisplatin. Cell Biochem Funct. 2018;36:80–87. doi: 10.1002/cbf.3319. [DOI] [PubMed] [Google Scholar]
- 153. Yang SD, Ahn SH, Kim JI. 3-Oxoacid CoA transferase 1 as a therapeutic target gene for cisplatin-resistant ovarian cancer. Oncol Lett. 2018;15:2611–2618. doi: 10.3892/ol.2017.7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Sanchez-Alvarez R, Martinez-Outschoorn UE, Lin Z, et al. Ethanol exposure induces the cancer-associated fibroblast phenotype and lethal tumor metabolism: implications for breast cancer prevention. Cell Cycle. 2013;12:289–301. doi: 10.4161/cc.23109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Prasad M, Bernardini M, Tsalenko A, et al. High definition cytogenetics and oligonucleotide aCGH analyses of cisplatin-resistant ovarian cancer cells. Genes Chromosomes Cancer. 2008;47:427–436. doi: 10.1002/gcc.20547. [DOI] [PubMed] [Google Scholar]
- 156. Osterberg L, Levan K, Partheen K, et al. Potential predictive markers of chemotherapy resistance in stage III ovarian serous carcinomas. BMC Cancer. 2009;9:368. doi: 10.1186/1471-2407-9-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Belotte J, Fletcher NM, Saed MG, et al. A single nucleotide polymorphism in catalase is strongly associated with ovarian cancer survival. PLoS ONE. 2015;10:e0135739. doi: 10.1371/journal.pone.0135739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Zhao YN, He DN, Wang YD, Li JJ, Ha MW. Association of single nucleotide polymorphisms in the MVP gene with platinum resistance and survival in patients with epithelial ovarian cancer. Oncol Lett. 2016;11:2925–2933. doi: 10.3892/ol.2016.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Tetu B, Popa I, Bairati I, et al. Immunohistochemical analysis of possible chemoresistance markers identified by micro-arrays on serous ovarian carcinomas. Mod Pathol. 2008;21:1002–1010. doi: 10.1038/modpathol.2008.80. [DOI] [PubMed] [Google Scholar]
- 160. Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between protein and mRNA abundance in yeast. Mol Cell Biol. 1999;19:1720–1730. doi: 10.1128/mcb.19.3.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Greenbaum D, Colangelo C, Williams K, Gerstein M. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol. 2003;4:117. doi: 10.1186/gb-2003-4-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Beyer A, Hollunder J, Nasheuer HP, Wilhelm T. Post-transcriptional expression regulation in the yeast Saccharomyces cerevisiae on a genomic scale. Mol Cell Proteomics. 2004;3:1083–1092. doi: 10.1074/mcp.M400099-MCP200. [DOI] [PubMed] [Google Scholar]
- 163. Duan G, Walther D. The roles of post-translational modifications in the context of protein interaction networks. Plos Comput Biol. 2015;11:e1004049. doi: 10.1371/journal.pcbi.1004049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Mann M, Jensen ON. Proteomic analysis of post-translational modifications. Nature Biotechnol. 2003;21:255. [DOI] [PubMed] [Google Scholar]
- 165. Ahmed AA, Mills AD, Ibrahim AE, et al. The extracellular matrix protein TGFBI induces microtubule stabilization and sensitizes ovarian cancers to paclitaxel. Cancer Cell. 2007;12:514–527. doi: 10.1016/j.ccr.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Parker AL, Kavallaris M, McCarroll JA. Microtubules and their role in cellular stress in cancer. Front Oncol. 2014;4:153. doi: 10.3389/fonc.2014.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Fraser M, Bai T, Tsang BK. Akt promotes cisplatin resistance in human ovarian cancer cells through inhibition of p53 phosphorylation and nuclear function. Int J Cancer. 2008;122:534–546. doi: 10.1002/ijc.23086. [DOI] [PubMed] [Google Scholar]
- 168. Unger T, Mietz JA, Scheffner M, Yee CL, Howley PM. Functional domains of wild-type and mutant p53 proteins involved in transcriptional regulation, transdominant inhibition, and transformation suppression. Mol Cell Biol. 1993;13:5186–5194. doi: 10.1128/mcb.13.9.5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Fu Y, Lee AS. Glucose regulated proteins in cancer progression, drug resistance and immunotherapy. Cancer Biol Ther. 2006;5:741. doi: 10.4161/cbt.5.7.2970. [DOI] [PubMed] [Google Scholar]
- 170. Di Michele M, Marcone S, Cicchillitti L, et al. Glycoproteomics of paclitaxel resistance in human epithelial ovarian cancer cell lines: towards the identification of putative biomarkers. J Proteomics. 2010;73:879–898. doi: 10.1016/j.jprot.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 171. Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008;13:472. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 172. Cho S, Dawson PE, Dawson G. Antisense palmitoyl protein thioesterase 1 PPT1 treatment inhibits PPT1 activity and increases cell death in LA-N-5 neuroblastoma cells. J Neurosci Res. 2000;62:234. doi: 10.1002/1097-4547(20001015)62. [DOI] [PubMed] [Google Scholar]
- 173. Yu M, Haslam RHA, Haslam DB. HEDJ, an Hsp40 co-chaperone localized to the endoplasmic reticulum of human cells. J Biol Chem. 2000;275:24984. doi: 10.1074/jbc.M000739200. [DOI] [PubMed] [Google Scholar]
- 174. Shen Y, Hendershot LM. ERdj3, a stress-inducible endoplasmic reticulum DnaJ homologue, serves as a cofactor for BiP’s interactions with unfolded substrates. Mol Biol Cell. 2005;16:40. doi: 10.1091/mbc.e04-05-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. De Bessa SA, Salaorni S, Patrao DF, Neto MM, Brentani MM, Nagai MA. JDP1 DNAJC12/Hsp40 expression in breast cancer and its association with estrogen receptor status. Int J Mol Med. 2006;17:363. [PubMed] [Google Scholar]
- 176. Abedini MR, Qiu Q, Yan X, Tsang BK. Possible role of FLICE-like inhibitory protein (FLIP) in chemoresistant ovarian cancer cells in vitro. Oncogene. 2004;23:6997–7004. doi: 10.1038/sj.onc.1207925. [DOI] [PubMed] [Google Scholar]
- 177. Yang BF, Xiao C, Li H, et al. Resistance to Fas-mediated apoptosis in malignant tumors is rescued by KN-93 and cisplatin via downregulation of c-FLIP expression and phosphorylation. Clin Exp Pharmacol Physiol. 2007;34:1245–1251. [DOI] [PubMed] [Google Scholar]
- 178. Januchowski R, Zawierucha P, Rucinski M, Nowicki M, Zabel M. Extracellular matrix proteins expression profiling in chemoresistant variants of the A2780 ovarian cancer cell line. Biomed Res Int. 2014:365867. doi: 10.1155/2014/365867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Liu Y, Chen L, Ko TC, Fields AP, Thompson EA. Evi1 is a survival factor which conveys resistance to both TGFbeta- and taxol-mediated cell death via PI3K/AKT. Oncogene. 2006;25:3565–3575. doi: 10.1038/sj.onc.1209403. [DOI] [PubMed] [Google Scholar]
- 180. Cao C, Lu S, Sowa A, et al. Priming with EGFR tyrosine kinase inhibitor and EGF sensitizes ovarian cancer cells to respond to chemotherapeutical drugs. Cancer Lett. 2008;266:249–262. doi: 10.1016/j.canlet.2008.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Soubeyran P, Kowanetz K, Szymkiewicz I, Langdon WY, Dikic I. Cbl-CIN85-endophilin complex mediates ligand-induced down-regulation of EGF receptors. Nature. 2002;416:183–187. [DOI] [PubMed] [Google Scholar]
- 182. Kim H, Kim K, No JH, Jeon YT, Jeon HW, Kim YB. Prognostic value of biomarkers related to drug resistance in patients with advanced epithelial ovarian cancer. Anticancer Res. 2012;32:589–594. [PubMed] [Google Scholar]
- 183. Ma JJ, Chen BL, Xin XY. Inhibition of multi-drug resistance of ovarian carcinoma by small interfering RNA targeting to MRP2 gene. Arch Gynecol Obstet. 2009;279:149–157. doi: 10.1007/s00404-008-0690-8. [DOI] [PubMed] [Google Scholar]
- 184. Koti M, Gooding RJ, Nuin P, et al. Identification of the IGF1/PI3K/NF κB/ERK gene signalling networks associated with chemotherapy resistance and treatment response in high-grade serous epithelial ovarian cancer. BMC Cancer. 2013;13:549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Lau MT, Leung PC. The PI3K/Akt/mTOR signaling pathway mediates insulin-like growth factor 1-induced E-cadherin down-regulation and cell proliferation in ovarian cancer cells. Cancer Lett. 2012;326:191–198. doi: 10.1016/j.canlet.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 186. Kang KW, Lee MJ, Song JA, et al. Overexpression of goosecoid homeobox is associated with chemoresistance and poor prognosis in ovarian carcinoma. Oncol Rep. 2014;32:189–198. doi: 10.3892/or.2014.3203. [DOI] [PubMed] [Google Scholar]
- 187. Zhang Z, Yu L, Dai G, et al. Telomerase reverse transcriptase promotes chemoresistance by suppressing cisplatin-dependent apoptosis in osteosarcoma cells. Sci Rep. 2017;7:7070. doi: 10.1038/s41598-017-07204-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Meng P, Ghosh R. Transcription addiction: can we garner the Yin and Yang functions of E2F1 for cancer therapy. Cell Death Dis. 2014;5:e1360. doi: 10.1038/cddis.2014.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Fraser M, Leung B, Jahani-Asl A, Yan X, Thompson WE, Tsang BK. Chemoresistance in human ovarian cancer: the role of apoptotic regulators. Reprod Biol Endocrinol. 2003;1:66. doi: 10.1186/1477-7827-1-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Brokers N, Le-Huu S, Vogel S, Hagos Y, Katschinski DM, Kleinschmidt M. Increased chemoresistance induced by inhibition of HIF-prolyl-hydroxylase domain enzymes. Cancer Sci. 2010;101:129–136. doi: 10.1111/j.1349-7006.2009.01367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr. NF-kappa antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. [DOI] [PubMed] [Google Scholar]
- 192. Hartwell KA, Muir B, Reinhardt F, Carpenter AE, Sgroi DC, Weinberg RA. The Spemann organizer gene, Goosecoid, promotes tumor metastasis. Proc Natl Acad Sci U S A. 2006;103:18969–18974. doi: 10.1073/pnas.0608636103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Kajita M, McClinic KN, Wade PA. Aberrant expression of the transcription factors snail and slug alters the response to genotoxic stress. Mol Cell Biol. 2004;24:7559–7566. doi: 10.1128/MCB.24.17.7559-7566.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Roque DM, Buza N, Glasgow M, et al. Class III β-tubulin overexpression within the tumor microenvironment is a prognostic biomarker for poor overall survival in ovarian cancer patients treated with neoadjuvant carboplatin/paclitaxel. Clin Exp Metastasis. 2014;31:101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Zhu L-C, Gao J, Hu Z-H, et al. Membranous expressions of Lewis y and CAM-DR-related markers are independent factors of chemotherapy resistance and poor prognosis in epithelial ovarian cancer. Am J Cancer Res. 2015;5:830–843. [PMC free article] [PubMed] [Google Scholar]
- 196. Gao L, Yan L, Lin B, et al. Enhancive effects of Lewis y antigen on CD44-mediated adhesion and spreading of human ovarian cancer cell line RMG-I. J Exp Clin Cancer Res. 2011;30:15. doi: 10.1186/1756-9966-30-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Bourguignon LYW, Peyrollier K, Xia W, Gilad E. Hyaluronan-CD44 interaction activates stem cell marker, Nanog, stat-3-mediated MDR1 gene expression, and Ankyrin-regulated multidrug Efflux in breast and ovarian tumor cells. J Biol Chem. 2008;283:17635–17651. doi: 10.1074/jbc.M800109200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Hao J, Madigan MC, Khatri A, et al. In vitro and in vivo prostate cancer metastasis and chemoresistance can be modulated by expression of either CD44 or CD147. PLoS ONE. 2012;7:e40716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Moore RG, Hill EK, Horan T, et al. HE4 (WFDC2) gene overexpression promotes ovarian tumor growth. Sci Rep. 2014;4:3574. doi: 10.1038/srep03574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Yan LM, Lin B, Zhu LC, et al. Enhancement of the adhesive and spreading potentials of ovarian carcinoma RMG-1 cells due to increased expression of integrin alpha5beta1 with the Lewis Y-structure on transfection of the alpha1,2-fucosyltransferase gene. Biochimie. 2010;92:852–857. doi: 10.1016/j.biochi.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 201. Wang Y, Liu J, Lin B, et al. Study on the expression and clinical significances of Lewis y antigen and integrin alphav, beta3 in epithelial ovarian tumors. Int J Mol Sci. 2011;12:3409–3421. doi: 10.3390/ijms12063409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Bonneau C, Rouzier R, Geyl C, et al. Predictive markers of chemoresistance in advanced stages epithelial ovarian carcinoma. Gynecol Oncol. 2015;136:112–120. doi: 10.1016/j.ygyno.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 203. Wang Y, Xu RC, Zhang XL, et al. Interleukin-8 secretion by ovarian cancer cells increases anchorage-independent growth, proliferation, angiogenic potential, adhesion and invasion. Cytokine. 2012;59:145–155. doi: 10.1016/j.cyto.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 204. Georges E, Bonneau AM, Prinos P. RNAi-mediated knockdown of alphaenolase increases the sensitivity of tumor cells to antitubulin chemotherapeutics. Int J Biochem Mol Biol. 2011;2:303–308. [PMC free article] [PubMed] [Google Scholar]
- 205. Diaz-Ramos A, Roig-Borrellas A, Garcia-Melero A, Lopez-Alemany R. α-enolase, a multifunctional protein: its role on pathophysiological situations. J Biomed Biotechnol. 2012;2012:156795. doi: 10.1155/2012/156795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Capello M, Ferri-Borgogno S, Cappello P, Novelli F. α-Enolase: a promising therapeutic and diagnostic tumor target. FEBS J. 2011;278:1064–1074. doi: 10.1111/j.1742-4658.2011.08025.x. [DOI] [PubMed] [Google Scholar]
- 207. Zhu XS, Dai YC, Chen ZX, et al. Knockdown of ECHS1 protein expression inhibits hepatocellular carcinoma cell proliferation via suppression of Akt activity. Crit Rev Eukaryot Gene Expr. 2013;23:275–282. [DOI] [PubMed] [Google Scholar]
- 208. Chowdhury I, Thompson WE, Welch C, Thomas K, Matthews R. Prohibitin (PHB) inhibits apoptosis in rat granulosa cells (GCs) through the extracellular signal-regulated kinase 1/2 (ERK1/2) and the Bcl family of proteins. Apoptosis. 2013;18:1513–1525. doi: 10.1007/s10495-013-0901-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Yang S, Luo A, Hao X, et al. Peroxiredoxin 2 inhibits granulosa cell apoptosis during follicle atresia through the NFKB pathway in mice. Biol Reprod. 2011;84:1182–1189. doi: 10.1095/biolreprod.110.087569. [DOI] [PubMed] [Google Scholar]
- 210. Kim TH, Song J, Alcantara Llaguno SR, et al. Suppression of peroxiredoxin 4 in glioblastoma cells increases apoptosis and reduces tumor growth. PLoS ONE. 2012;7:e42818. doi: 10.1371/journal.pone.0042818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. van Hinsbergh VW, Collen A, Koolwijk P. Role of fibrin matrix in angiogenesis. Ann N Y Acad Sci. 2001;936:426–437. doi: 10.1111/j.1749-6632.2001.tb03526.x. [DOI] [PubMed] [Google Scholar]
- 212. Kuehn C, Lakey JR, Lamb MW, Vermette P. Young porcine endocrine pancreatic islets cultured in fibrin show improved resistance toward hydrogen peroxide. Islets. 2013;5:207–215. doi: 10.4161/isl.26989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Guo YH, Hernandez I, Isermann B, et al. Caveolin-1-dependent apoptosis induced by fibrin degradation products. Blood. 2009;113:4431–4439. doi: 10.1182/blood-2008-07-169433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Arany I, Clark JS, Reed DK, Ember I, Juncos LA. Cisplatin enhances interaction between p66Shc and HSP27: its role in reorganization of the actin cytoskeleton in renal proximal tubule cells. Anticancer Res. 2012;32:4759–4763. [PubMed] [Google Scholar]
- 215. Kanagasabai R, Krishnamurthy K, Druhan LJ, Ilangovan G. Forced expression of heat shock protein 27 (Hsp27) reverses P-glycoprotein (ABCB1)-mediated drug efflux and MDR1 gene expression in adriamycin-resistant human breast cancer cells. J Biol Chem. 2011;286:33289–33300. doi: 10.1074/jbc.M111.249102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Cotter TG, Lennon SV, Glynn JM, Green DR. Microfilament-disrupting agents prevent the formation of apoptotic bodies in tumor cells undergoing apoptosis. Cancer Res. 1992;52:997–1005. [PubMed] [Google Scholar]
- 217. Martin SS, Leder P. Human MCF10A mammary epithelial cells undergo apoptosis following actin depolymerization that is independent of attachment and rescued by Bcl–2. Mol Cell Biol. 2001;21:6529–6536. doi: 10.1128/mcb.21.19.6529-6536.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Schwerk C, Schulze-Osthoff K. Regulation of apoptosis by alternative pre-mRNA splicing. Mol Cell. 2005;19:1–13. [DOI] [PubMed] [Google Scholar]
- 219. Garneau D, Revil T, Fisette JF, Chabot B. Heterogeneous nuclear ribonucleoprotein F/H proteins modulate the alternative splicing of the apoptotic mediator Bcl-x. J Biol Chem. 2005;280:22641–22650. doi: 10.1074/jbc.M501070200. [DOI] [PubMed] [Google Scholar]
- 220. Hamaoka R, Fujii J, Miyagawa J, et al. Overexpression of the aldose reductase gene induces apoptosis in pancreatic beta-cells by causing a redox imbalance. J Biochem. 1999;126:41–47. doi: 10.1093/oxfordjournals.jbchem.a022434. [DOI] [PubMed] [Google Scholar]
- 221. Ramana KV, Bhatnagar A, Srivastava SK. Aldose reductase regulates TNF-alpha-induced cell signaling and apoptosis in vascular endothelial cells. FEBS Lett. 2004;570:189–194. doi: 10.1016/j.febslet.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 222. Cruz I, Coley HM, Kramer HB, et al. Proteomics analysis of ovarian cancer cell lines and tissues reveals drug resistance-associated proteins. Cancer Genom Proteom. 2017;14:35–51. doi: 10.21873/cgp.20017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Lincet H, Icard P. How do glycolytic enzymes favour cancer cell proliferation by non-metabolic functions? Oncogene. 2015;34:3751. [DOI] [PubMed] [Google Scholar]
- 224. Wadhwa R, Yaguchi T, Hasan MK, Mitsui Y, Reddel RR, Kaul SC. Hsp70 family member, mot-2/mthsp70/GRP75, binds to the cytoplasmic sequestration domain of the p53 protein. Exp Cell Res. 2002;274:246–253. doi: 10.1006/excr.2002.5468. [DOI] [PubMed] [Google Scholar]
- 225. Zheng HC. The molecular mechanisms of chemoresistance in cancers. Oncotarget. 2017;8:59950–59964. doi: 10.18632/oncotarget.19048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Pandey MK, Prasad S, Tyagi AK, et al. Targeting cell survival proteins for cancer cell death. Pharmaceuticals (Basel). 2016;9:11. doi: 10.3390/ph9010011. [DOI] [PMC free article] [PubMed] [Google Scholar]


