Significance
Lung endothelial cells express high levels of glucose metabolic enzymes, such as PFKFB3, and consequently produce large amounts of glucose metabolites. These metabolites are able to stabilize the cell signaling molecule HIF2A, similar to that which occurs under hypoxic conditions. This stabilization of HIF2A by glucose metabolites in lung endothelial cells stimulates production of growth and inflammatory factors, thereby enhancing proliferation and inflammation of the pulmonary vessels and exacerbating pulmonary hypertension (PH). In this study, blockade of endothelial PFKFB3 inhibits PH development in rodent models, suggesting that targeting glucose metabolic enzymes is a promising strategy for the treatment of PH.
Keywords: endothelial cells, glycolysis, pulmonary hypertension
Abstract
Increased glycolysis in the lung vasculature has been connected to the development of pulmonary hypertension (PH). We therefore investigated whether glycolytic regulator 6-phosphofructo-2-kinase/fructose-2, 6-bisphosphatase (PFKFB3)-mediated endothelial glycolysis plays a critical role in the development of PH. Heterozygous global deficiency of Pfkfb3 protected mice from developing hypoxia-induced PH, and administration of the PFKFB3 inhibitor 3PO almost completely prevented PH in rats treated with Sugen 5416/hypoxia, indicating a causative role of PFKFB3 in the development of PH. Immunostaining of lung sections and Western blot with isolated lung endothelial cells showed a dramatic increase in PFKFB3 expression and activity in pulmonary endothelial cells of rodents and humans with PH. We generated mice that were constitutively or inducibly deficient in endothelial Pfkfb3 and found that these mice were incapable of developing PH or showed slowed PH progression. Compared with control mice, endothelial Pfkfb3-knockout mice exhibited less severity of vascular smooth muscle cell proliferation, endothelial inflammation, and leukocyte recruitment in the lungs. In the absence of PFKFB3, lung endothelial cells from rodents and humans with PH produced lower levels of growth factors (such as PDGFB and FGF2) and proinflammatory factors (such as CXCL12 and IL1β). This is mechanistically linked to decreased levels of HIF2A in lung ECs following PFKFB3 knockdown. Taken together, these results suggest that targeting PFKFB3 is a promising strategy for the treatment of PH.
Pulmonary hypertension (PH) is a severe lung disease characterized by the remodeling of small pulmonary vessels, leading to a progressive increase in pulmonary vascular resistance and ultimately culminating in right ventricular failure and death. The cardinal pathological changes of PH include increased proliferation and resistance to apoptosis of pulmonary arterial endothelial and smooth muscle cells (PAECs and PASMCs), generation and accumulation of extracellular matrix, and local expression of proinflammatory cytokines and chemokines and the subsequent infiltration of leukocytes to the perivascular areas of the lung (1–3). The mechanisms underlying these pathologies remain poorly understood, and currently available therapeutic agents have limited efficacy against the pathologic remodeling, despite the accumulation of a large body of extensive research over the past decade (1, 2, 4).
Aberrant metabolism, especially aerobic glycolysis or the Warburg effect, has been proposed as an important pathogenic mechanism in the development of PH. Positron emission tomography (PET) scans with [18F]-fluoro-deoxy-d-glucose (FDG) performed in rodents with experimental PH and patients with idiopathic pulmonary arterial hypertension (IPAH) show significantly higher glucose uptake in the lungs (5–8), suggesting increased glycolytic activity in PH lungs. Furthermore, it has been found that many cell types in the PH lung, including endothelial cells from patients with IPAH (5, 7, 9), PASMCs from rodents with experimental PH and humans with IPAH (7, 10), as well as vascular fibroblasts isolated from IPAH patients and calves with severe hypoxia-induced pulmonary hypertension (8), rely heavily on glycolysis for increased growth. Although aerobic glycolysis is an inefficient way to generate adenosine 5′-triphosphate (ATP), it nevertheless provides macromolecules, lipids, and many other molecules that support the rapid growth of proliferating cells (11). The up-regulation of many glycolytic enzymes and glycolysis-related molecules or regulators such as glucose transporter 1 (GLUT1) and 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatases (PFKFBs) has been observed in many of the aforementioned lung cells under hypertensive conditions (5, 7–10). Despite these associations, the functional importance of these glycolytic enzymes or regulators in the development of PH has not yet been determined.
Among the numerous glycolytic regulators, PFKFB enzymes catalyze the synthesis of fructose-2,6-bisphosphate (F-2,6-P2), which is the most potent allosteric activator of 6-phosphofructo-1-kinase (PFK-1), one of three rate-limiting enzymes for glycolysis (12). Among the four isoforms of PFKFBs, expression of the PFKFB3 isoform is dominant in vascular cells, leukocytes, and many transformed cells (12, 13). Compared with the other PFKFB isoforms, PFKFB3 has the highest kinase-to-phosphatase ratio (740:1) and controls the steady-state concentration of F-2,6-P2 in the cells (12). Recent studies indicated that blockade or deletion of endothelial PFKFB3 reduces pathological angiogenesis (14, 15). Moreover, PFKFB3 knockdown or inhibition in cancer cells substantially inhibits cell survival, growth, and invasiveness (16). However, it is unclear whether this critical glycolytic regulator plays an important role in the development of PH.
Pulmonary endothelial cells are directly involved in the development and progression of PH, i.e., in the early and late stages of the disease. As such, pulmonary vasoconstriction during the early stages of PH has been attributed to endothelial dysfunction (1, 2), and plexiform lesions of late-stage PH result from excessive proliferation of endothelial cells (1, 2). Recent studies using genetically modified mice have shown that excessive endothelial inflammation mediated by hypoxia-inducible factor-2α (HIF-2α, HIF2A), prolyl hydroxylase domain-containing protein 2 (PHD2), and 5′ AMP-activated protein kinase (AMPK) is critical for the development of PH (17–19). Given the importance of these molecules to cellular energy production, it is likely that endothelial HIF2A, PHD2, and AMPK promote PH through changes in endothelial metabolism. As glycolysis is the predominant metabolic pathway for energy production in endothelial cells, and increased glycolysis in pulmonary endothelial cells from IPAH patients has been observed (5, 9), we hypothesized that the glycolytic regulator PFKFB3 may have an important role in the development of PH.
In the present study, we used a combination of genetic and pharmacological approaches in rodent PH models to uncover a critical role of the glycolytic regulator PFKFB3 in lung endothelial cells in the development of PH. We found that inhibition of endothelial PFKFB3 reduces the levels of HIF2A, leading to reduced generation of growth factors, proinflammatory cytokines, and chemokines, suppression of adhesion molecules, and attenuation of PH.
Results
Heterozygous Pfkfb3 Deficiency in Mice Inhibits the Development of Hypoxia-Induced PH.
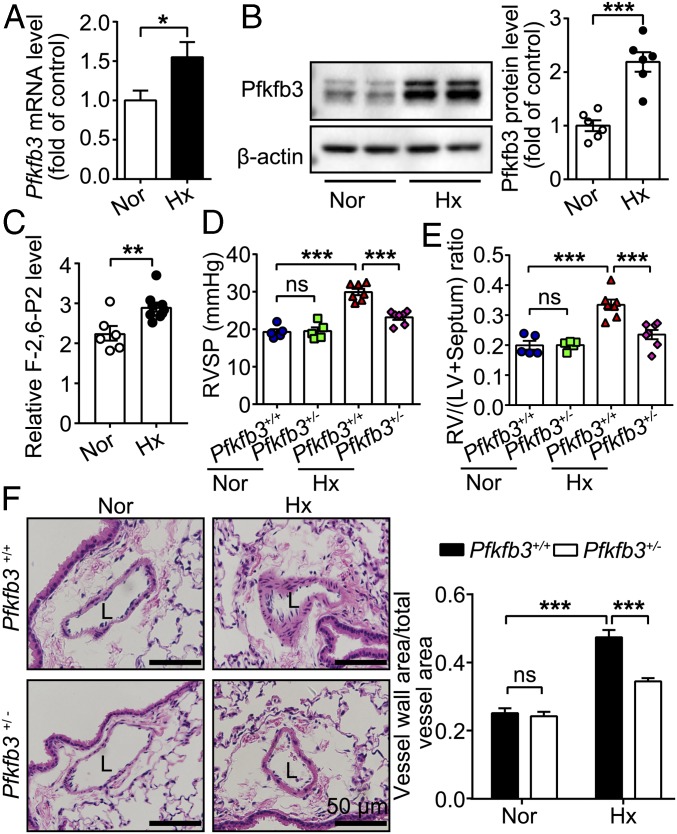
A mouse hypoxia-induced PH model was used to test the effect of genetic Pfkfb3 deletion in the development of PH. These PH mice exhibited higher levels of Pfkfb3 at the mRNA and protein levels and higher activity levels of Pfkfb3 in the lungs compared with control mice (Fig. 1 A–C). As a result of embryonic lethality for homozygous deficiency of Pfkfb3 in mice (20), heterozygous Pfkfb3-deficient mice (Pfkfb3+/−) were used. By using the experimental design indicated in SI Appendix, Fig. S1, Pfkfb3+/− and Pfkfb3+/+ mice were exposed to hypoxia (10% O2) for 4 wk. As shown in Fig. 1 D and E, the right ventricular systolic pressure (RVSP) and right ventricular hypertrophy [assessed by the ratio of right ventricular (RV) weight to left ventricular (LV) plus septum weight (RV/LV+septum)] were 65.4% and 74.2% lower in Pfkfb3+/− mice than in Pfkfb3+/+ mice, respectively. Furthermore, the medial wall thickness of distal pulmonary arteries was 54.1% less in Pfkfb3+/− mice than in Pfkfb3+/+ mice (Fig. 1F). No difference was found in RVSP and RV/LV+septum between the two groups of mice under normoxic conditions.
Fig. 1.
Heterozygous Pfkfb3 deficiency in mice inhibits the development of hypoxia (Hx)-induced PH. (A) Real-time PCR analysis of Pfkfb3 mRNA levels in lung homogenates of mice exposed to hypoxia (10% O2) or ambient oxygen levels (21% O2) for 4 wk (n = 6). (B) Western blot analysis and densitometric quantification of Pfkfb3 protein levels in lung homogenates of mice exposed to hypoxia (10% O2) or ambient oxygen levels (21% O2) for 4 wk (n = 6 mice per group). (C) Relative F-2,6-P2 levels in lung homogenates of mice exposed to normoxia (Nor) or hypoxia (10% O2) for 4 wk (n = 6 mice for normoxia group, n = 9 mice for hypoxia group). (D) RVSP and (E) RV hypertrophy assessed by the ratio of RV/LV+septum. (F) (Left) Representative images of H&E staining of distal pulmonary arteries from control mice and Pfkfb3+/− mice exposed to normoxia or hypoxia (10% O2) for 4 wk. L, lumen. (Scale bars, 50 µm.) (Right) Quantification of pulmonary artery thickness as measured by the ratio of vessel wall area to total vessel area (n = 3 for control mice under normoxic or hypoxic condition, n = 4 for normoxic Pfkfb3+/− mice, and n = 7 for hypoxic Pfkfb3+/− mice). All data are expressed as mean ± SEM. Statistical significance was determined by unpaired Student’s t test (A–C) and one-way ANOVA followed by Bonferroni test (D–F). *P < 0.05 was considered significant, **P < 0.01, ***P < 0.001. ns, no significance.
PFKFB3 Inhibitor 3PO Suppresses Sugen 5416/Hypoxia-Induced PH in Rats.
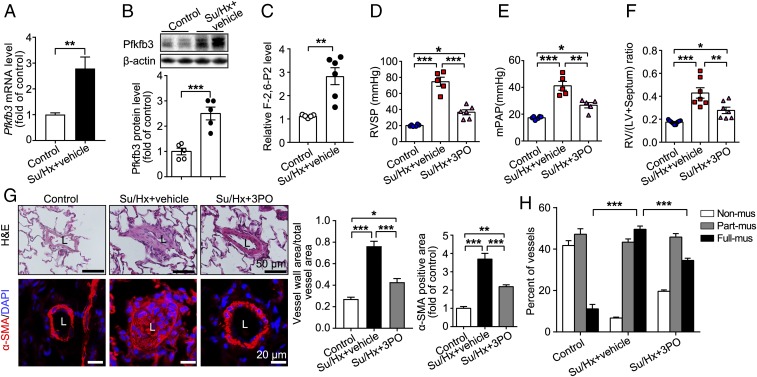
By using the experimental design indicated in SI Appendix, Fig. S2A, we generated the Sugen 5416/hypoxia (Su/Hx)-induced rat PH model. Pfkfb3 expression at mRNA and protein levels and its activity were significantly increased in the lungs of Su/Hx rats compared with control rats (Fig. 2 A–C). To examine the effect of pharmacological inhibition of PFKFB3 in suppression of PH development, 3PO, a specific PFKFB3 inhibitor, was administered to the Su/Hx rats intraperitoneally at a dose of 50 mg/kg/d for 5 wk. No apparent side effects, such as decreased body weight and systolic blood pressure (SI Appendix, Fig. S2 B and C), were observed in 3PO-treated rats compared with vehicle-treated rats. Treatment with 3PO almost completely abrogated the ability of Su/Hx to induce PH in rats. By using echocardiography approaches, 3PO-treated rats had a smaller RV chamber compared with vehicle-treated Su/Hx-PH rats (SI Appendix, Fig. S2D), indicating that the shift of the interventricular septum toward the left ventricle was normalized by 3PO treatment. 3PO-treated Su/Hx-PH rats also showed a higher pulmonary artery (PA) acceleration time (PAAT) and velocity time integral (VTI) compared with vehicle-treated Su/Hx-PH rats (SI Appendix, Fig. S2E), indicating improved PA diastolic function. In addition, RV hypertrophy in 3PO-treated Su/Hx-PH rats was ameliorated, as evidenced by decreased RV wall thickness, which was consistent with improved RV systolic function [tricuspid annulus plane systolic excursion (TAPSE)] compared with vehicle-treated Su/Hx-PH rats (SI Appendix, Fig. S2 F and G). No difference in LV function was noted between groups of 3PO-treated Su/Hx-PH rats and vehicle-treated Su/Hx-PH rats (SI Appendix, Fig. S2H). In line with data from echocardiography examination, 3PO treatment reduced the increased RVSP and mPAP by 69.7% and 60.8%, respectively, and increased RV hypertrophy by 59.8% in Su/Hx rats compared with vehicle-treated Su/Hx rats (Fig. 2 D–F). Additionally, histology of rat lungs with hematoxylin & eosin (H&E) staining and immunostainings showed that increases in pulmonary vascular wall thickness, percentage of muscularized vessels, and proliferative endothelial cells in pulmonary vascular lesions seen in vehicle-treated Su/Hx rats were strikingly blunted in 3PO-treated Su/Hx rats (Fig. 2 G and H and SI Appendix, Fig. S2I).
Fig. 2.
PFKFB3 inhibitor 3PO reduces Sugen 5416/hypoxia (Su/Hx)-induced PH in rats. (A) Real-time PCR analysis of Pfkfb3 mRNA levels in lung homogenates of control rats and Su/Hx-treated rats (n = 6). (B) Western blot analysis and densitometric quantification of Pfkfb3 protein levels in lung homogenates of control rats and Su/Hx-treated rats (n = 5). (C) Relative F-2,6-P2 levels in lung homogenates of control rats and Su/Hx-treated rats (n = 5 for control group, n = 6 for Su/Hx-treated group). (D) Quantification of RVSP, (E) mean pulmonary arterial pressure (mPAP), and (F) RV hypertrophy assessed by the ratio of RV/LV+septum. (G) (Left) Representative images of H&E staining and α-SMA immunostaining of the distal pulmonary arteries of control and treated (Su/Hx + vehicle and Su/Hx + 3PO) rats. (Right) Quantification of the ratio of vessel wall area to total vessel area and α-SMA immunostaining-positive area. (H) Quantitative assessment of nonmuscularized, partially muscularized, and fully muscularized arteries as percentages of total assessed arteries (n = 6–8). All data are expressed as mean ± SEM. Statistical significance was determined by unpaired Student’s t test (A–C) and one-way ANOVA followed by Bonferroni test (D–H). *P < 0.05 was considered significant, **P < 0.01, ***P < 0.001. ns, no significance.
Pfkfb3/PFKFB3 Expression Is Increased in PAECs of Rodents with PH and Patients with IPAH.
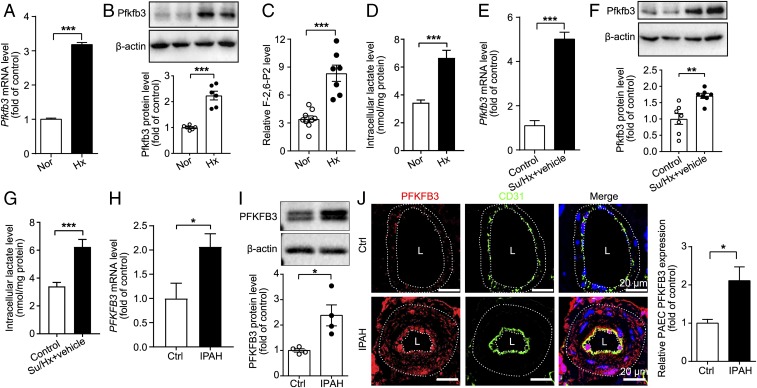
It has been shown that glycolysis is increased in lung endothelial cells from IPAH patients (5). The mechanism for this increased glycolysis has been linked to mitochondrial defects (9, 21). To determine whether increased endothelial glycolysis in PH is caused by up-regulation of PFKFB3, we examined Pfkfb3/PFKFB3 expression in PAECs of rodents with PH and patients with IPAH. The results showed that PAECs of mice and rats with PH showed much higher levels of Pfkfb3 at the mRNA and protein levels as well as higher levels of Pfkfb3 activity compared with control rodents (Fig. 3 A–G). Likewise, much greater Pfkfb3 immunostaining was detected on the endothelial layer of distal pulmonary arteries from PH lungs than on the endothelial layer of lungs from control rodents (SI Appendix, Fig. S3 A and B). PFKFB3 expression and functions were examined in PAECs from patients with IPAH. PFKFB3 expression at the mRNA and protein levels in PAECs from IPAH patients was also substantially increased compared with those from control subjects (Fig. 3 H and I). The PFKFB3 immunostaining showed higher levels of expression of PFKFB3 on the endothelium of distal pulmonary arteries of IPAH lungs compared with control lungs (Fig. 3J). Furthermore, PAECs from IPAH patients and PH rats exhibited increased glycolysis, as evidenced by lactate measurement and Seahorse flux analysis (Fig. 3G and SI Appendix, Fig. S3 C, and E–F). To determine the contribution of PFKFB3 to the increased glycolysis in PAECs of IPAH patients, PAECs were transduced with Ad-shPFKFB3. Knockdown of PFKFB3 with Ad-shPFKFB3 suppressed lactate production (SI Appendix, Fig. S3E). In a Seahorse assay, PFKFB3 knockdown markedly decreased glycolysis, glycolytic capacity, and glycolytic reserve of PAECs from IPAH patients (SI Appendix, Fig. S3F). These data indicate that PFKFB3 contributes to increased glycolysis in PAECs of IPAH patients.
Fig. 3.
Pfkfb3/PFKFB3 expression level is significantly increased in PAECs isolated from mice, rats, and humans with PH. (A) Real-time PCR analysis of Pfkfb3 mRNA levels in PAECs of control and PH mice (n = 6). (B) Western blot analysis and densitometric quantification of Pfkfb3 protein levels in PAECs of control and PH mice (n = 6). (C) Relative F-2,6-P2 levels in PAECs of control and PH mice (n = 9 for normoxia [Nor] group, n = 7 for hypoxia [Hx] group). (D) Intracellular lactate levels of PAECs isolated from control and PH mice (n = 10). (E) Real-time PCR analysis of Pfkfb3 mRNA levels in PAECs of control and Su/Hx-treated rats (n = 5). (F) Western blot analysis and densitometric quantification of Pfkfb3 protein levels in PAECs of control and Su/Hx-treated rats (n = 7). (G) Intracellular lactate levels of PAECs isolated from control and Su/Hx-treated rats (n = 8). (H) Real-time PCR analysis of PFKFB3 mRNA levels in PAECs of control subjects or patients with IPAH (n = 6). Experiments were repeated four times independently with cells from four patients or control subjects. (I) Western blot analysis and densitometric quantification of PFKFB3 protein levels in PAECs of control subjects or patients with IPAH (n = 4). (J) (Left) Representative micrographs of PFKFB3 expression in the pulmonary endothelium of control (Ctrl) or IPAH patients. Sections were costained for PFKFB3 (red) and CD31 (green). (Right) Quantification of the relative PFKFB3 fluorescence intensity in the pulmonary endothelium of Ctrl or IPAH patients. L, lumen. (Scale bar, 20 μm.) All data are expressed as mean ± SEM. Statistical significance was determined by unpaired Student’s t test (A, H, and J) and Mann–Whitney U test (I). *P < 0.05 was considered significant, **P < 0.01, ***P < 0.001.
Endothelial-Specific Pfkfb3 Deficiency in Mice Reduces the Development and Progression of Hypoxia-Induced PH.
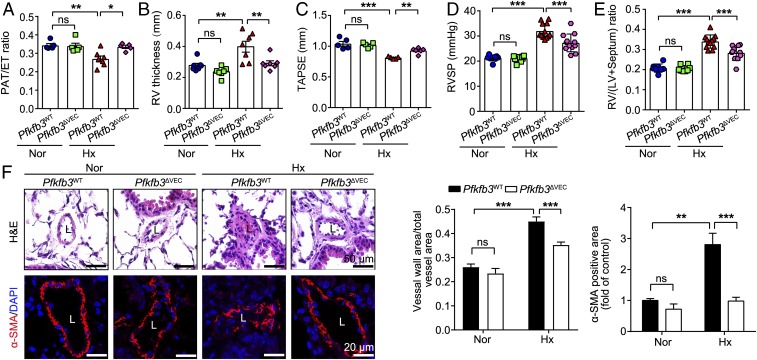
To determine whether endothelial PFKFB3 plays a causal role in PH development, we generated endothelial-specific Pfkfb3-knockout (KO) mice (Pfkfb3ΔVEC) and control mice (Pfkfb3WT) and placed these mice in a hypoxic chamber to promote PH development (SI Appendix, Fig. S4 A–C). Echocardiography analysis showed that Pfkfb3ΔVEC mice exhibited a 94.2% increase in PA acceleration/ejection time (PAT/ET) ratio compared with hypoxic Pfkfb3WT mice (Fig. 4A and SI Appendix, Fig. S4D). Pfkfb3ΔVEC mice also showed a 54.1% reduction in RV wall thickness compared with Pfkfb3WT mice (Fig. 4B). Furthermore, Pfkfb3ΔVEC mice had as much as 60% improvement in RV systolic function (evaluated with TAPSE) compared with Pfkfb3WT mice (Fig. 4C). There was no difference in LV function between the two groups of mice under normoxic or hypoxic conditions (SI Appendix, Fig. S4E).
Fig. 4.
Endothelial-specific Pfkfb3 deficiency in mice ameliorates the development of hypoxia-induced PH. (A–C) Quantification of PAT/ET ratio (A), RV thickness (in millimeters) (B), and TAPSE (in millimeters) (C). (D) Quantification of RVSP and (E) RV hypertrophy as assessed by RV/LV+septum. (F) (Left) Representative images of H&E staining and α-SMA immunostaining of the distal pulmonary arteries of Pfkfb3WT and Pfkfb3ΔVEC mice under normoxic (Nor) or hypoxic (Hx) conditions for 4 wk and (Right) quantification of pulmonary artery thickness as measured by the ratio of vessel wall area to total vessel area and α-SMA immunostaining-positive area. L, lumen (n = 6). All data are expressed as mean ± SEM. Statistical significance was determined by one-way ANOVA followed by Bonferroni test. *P < 0.05 was considered significant, **P < 0.01, ***P < 0.001. ns, no significance.
Consistent with echocardiography analysis, Pfkfb3ΔVEC mice showed a 42.5% decrease in RVSP elevation compared with Pfkfb3WT mice (Fig. 4D). The increase in the Fulton’s index was also reduced by 40.9% in Pfkfb3ΔVEC mice compared with Pfkfb3WT mice (Fig. 4E). Moreover, the robust increase in the thickness of the distal PA walls seen in Pfkfb3WT mice was almost completely abolished in Pfkfb3ΔVEC mice (Fig. 4F). These data demonstrate that Pfkfb3-mediated endothelial glycolysis is critical for PH development.
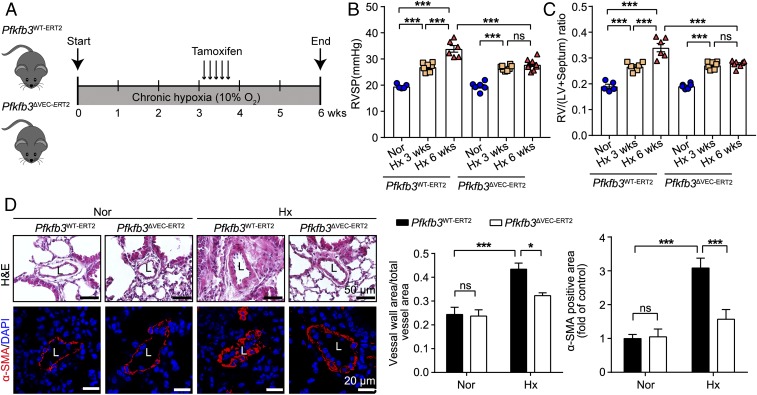
To further study whether endothelial Pfkfb3-mediated glycolysis is also vital for PH progression, we generated inducible endothelial Pfkfb3-KO mice (Pfkfb3ΔVEC-ERT2) and control mice (Pfkfb3WT-ERT2; SI Appendix, Fig. S5 A and B). These mice were placed in a hypoxic chamber for 3 wk to develop PH (Fig. 5 B and C), followed by tamoxifen treatment to knock out the Pfkfb3 gene in endothelial cells of Pfkfb3ΔVEC-ERT2 mice but not Pfkfb3WT-ERT2 mice (Fig. 5A). After continuous hypoxic exposure for another 2 wk, Pfkfb3ΔVEC-ERT2 mice displayed suppressed PH, as evident by decreased RVSP (Fig. 5B), RV/LV+septum ratio (Fig. 5C), vessel wall area, and α-smooth muscle actin (α-SMA)-positive area (Fig. 5D) in Pfkfb3ΔVEC-ERT2 mice compared with Pfkfb3WT-ERT2 mice, indicating that inhibition of Pfkfb3-mediated endothelial glycolysis is able to suppress PH progression.
Fig. 5.
Inducible deficiency of endothelial-specific Pfkfb3 in mice suppresses the progression of hypoxia-induced PH. (A) Schematic diagram of the experimental design. Inducible endothelial-specific Pfkfb3-deficient mice (Pfkfb3ΔVEC-ERT2) and control mice (Pfkfb3WT-ERT2) were exposed to chronic hypoxia (Hx; 10% O2) for 3 wk followed by 75 mg/kg of tamoxifen injection for 5 d under hypoxia condition. Mice continued to be exposed to hypoxia for 2 wk before assessment. (B) Quantification of RVSP and (C) RV hypertrophy as assessed by RV/LV+septum. (D) (Left) Representative images of H&E staining and α-SMA immunostaining of the distal pulmonary arteries of Pfkfb3WT-ERT2 and Pfkfb3ΔVEC-ERT2 mice under normoxic (Nor) or hypoxic conditions for 6 wk and quantification (Right) of pulmonary artery thickness as measured by the ratio of vessel wall area to total vessel area and the α-SMA immunostaining-positive area. L, lumen (n = 6–7). All data are expressed as mean ± SEM. Statistical significance was determined by one-way ANOVA followed by Bonferroni test. *P < 0.05 was considered significant, ***P < 0.001. ns, no significance.
PAEC PFKFB3/Pfkfb3 Knockdown or Deficiency Results in Decreased Proliferation of PASMCs.
A significant decrease in the level of proliferating cell nuclear antigen (PCNA), the marker for cell proliferation in lungs of Pfkfb3ΔVEC mice with PH, was observed compared with Pfkfb3WT mice with PH (SI Appendix, Fig. S6A). Given the fact that PASMC proliferation is critical for the development of PH, we investigated the effect of endothelial PFKFB3 deficiency on PASMC proliferation. The numbers of Ki67- and α-SMA–positive cells on lung sections were significantly decreased in Pfkfb3ΔVEC mice compared with Pfkfb3WT mice (SI Appendix, Fig. S6B). In an in vitro assay, as indicated in SI Appendix, Fig. S6C, conditioned medium (CM) from human PAECs transduced with Ad-shCTRL or Ad-shPFKFB3 under normoxic and hypoxic conditions was incubated with the human PASMCs, after which 5-ethynyl-2′-deoxyuridine (EdU) staining and WST-1 proliferation assay were performed. The EdU staining and WST-1 proliferation assay showed that proliferation of human PASMCs exposed to CM from hypoxic human PAECs with PFKFB3 knockdown was significantly reduced compared with cells cultured with CM from control hypoxic human PAECs (SI Appendix, Fig. S6 E and F). The experiments were also conducted with CM from IPAH PAECs and normal PAECs under normoxic conditions. Interestingly, proliferation of human PASMCs exposed to CM of IPAH PAECs was markedly increased compared with cells cultured with CM of normal PAECs (SI Appendix, Fig. S6G). Moreover, the proliferation of human PASMCs exposed to CM from IPAH PAECs with PFKFB3 knockdown was much lower compared with cells cultured with CM of control IPAH PAECs (SI Appendix, Fig. S6H). Similarly, mouse PASMCs responded to CM of mouse PAECs in the same way as occurred in the aforementioned human cells (SI Appendix, Fig. S6I). These results indicate that PFKFB3 in lung endothelial cells plays an important role in PASMC proliferation in PH.
Endothelial Pfkfb3/PFKFB3 Deficiency/Inhibition or Knockdown Suppresses Expression and Release of Growth Factors from PAECs.
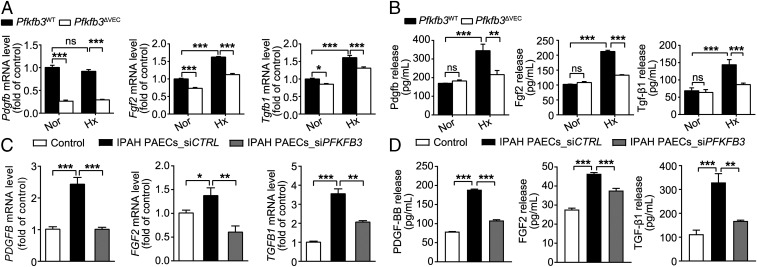
Growth factors have been shown to participate in the pathogenesis of PH (1). The mRNA levels of growth factors including Pdgfb, Fgf2, and Tgfb1 were much lower in the lungs of hypoxic Pfkfb3ΔVEC mice and 3PO-treated Su/Hx rats than in the lungs of hypoxic Pfkfb3WT mice and vehicle-treated Su/Hx rats (SI Appendix, Fig. S7 A and B), suggesting that endothelial PFKFB3 may regulate the expression and release of those growth factors in PH. We further determined the mRNA expression of the aforementioned growth factors and their release by PAECs isolated from Pfkfb3ΔVEC mice, 3PO-treated rats with PH, and their controls. The results showed that PAECs from hypoxic Pfkfb3ΔVEC mice expressed and released much lower levels of Pdgfb, Fgf2, and Tgfb1 than those from hypoxic Pfkfb3WT mice (Fig. 6 A and B). PAECs from 3PO-treated Su/Hx rats also expressed lower mRNA levels of growth factors than cells from vehicle-treated Su/Hx rats (SI Appendix, Fig. S7C). The different patterns of Pdgfb expression in PAECs of mice, rats, and humans were noted and may require further study in the future. The mRNA levels of these growth factors and their release by PAECs from IPAH patients were also much greater than those in PAECs from controls (Fig. 6 C and D). More importantly, PFKFB3 knockdown significantly blunted the mRNA expression and protein release of these growth factors in PAECs from IPAH patients (Fig. 6 C and D). In addition, PAECs from healthy individuals released more growth factors in response to hypoxia, and this increased release from hypoxic PAECs was inhibited by PFKFB3 knockdown (SI Appendix, Fig. S8A). These results indicate that PFKFB3 in lung endothelial cells regulates the expression and release of growth factors that promote PASMC proliferation in PH.
Fig. 6.
Endothelial Pfkfb3/PFKFB3 deficiency or knockdown decreases the hypoxia (Hx)-induced gene expression and protein release of growth factors. (A) Real-time PCR analysis of mRNA levels of Pdgfb, Fgf2, and Tgfb1 in PAECs of Pfkfb3ΔVEC and Pfkfb3WT mice exposed to normoxia (Nor) or hypoxia (10% O2) for 4 wk (n = 6). (B) Levels of released Pdgfb, Fgf2, and Tgf-β1 in the culture supernatants of mouse PAECs exposed to hypoxia (1% O2) for 24 h (n = 4–6). (C) Real-time PCR analysis of mRNA levels of PDGFB, FGF2, and TGFB1 in normal PAECs or siCTRL- and siPFKFB3-transfected PAECs of patients with IPAH (n = 6). Experiments were repeated four times independently with cells from four patients with IPAH or control subjects. (D) Levels of released PDGF-BB, FGF2, and TGF-β1 in the culture supernatants of normal PAECs or siCTRL- and siPFKFB3-transfected PAECs of patients with IPAH (n = 4–6). All data are expressed as mean ± SEM. Statistical significance was determined by one-way ANOVA followed by Bonferroni test. *P < 0.05 was considered significant, **P < 0.01, ***P < 0.001. ns, no significance.
Endothelial Pfkfb3/PFKFB3 Deficiency/Inhibition or Knockdown Reduces Endothelial Inflammation and Macrophage Infiltration in the PH Lungs.
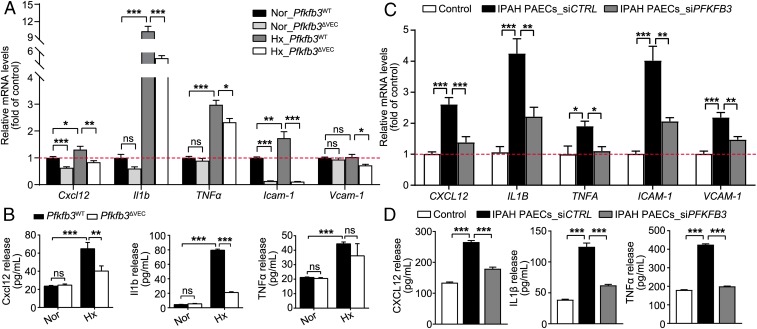
It has been shown that endothelial inflammation plays an important role in the development of PH (3). By using real-time RT-PCR, the mRNA levels of proinflammatory cytokines, including Cxcl12, Il1b, and TNFα, and adhesion molecules Icam-1 and Vcam-1 in lung tissues of Pfkfb3ΔVEC mice or 3PO-treated rats with PH were evaluated. The levels of these proinflammatory molecules in the lungs of hypoxic Pfkfb3ΔVEC mice and 3PO-treated Su/Hx rats were much lower than those in the lungs of hypoxic Pfkfb3WT mice and vehicle-treated Su/Hx rats, respectively (SI Appendix, Figs. S9A and S10A). In an immunostaining assay, the protein levels of Icam-1 and Vcam-1 on the pulmonary endothelium and the numbers of infiltrated macrophages were dramatically decreased in the lungs of hypoxic Pfkfb3ΔVEC mice and 3PO-treated Su/Hx rats compared with their control hypoxic Pfkfb3WT mice and vehicle-treated Su/Hx rats (SI Appendix, Figs. S9 B–D and S10 C and D). These data suggest that endothelial PFKFB3 may play an important role in the vascular inflammation status of PH. We further examined the expression of proinflammatory cytokines in PAECs isolated from Pfkfb3ΔVEC mice, 3PO-treated rats with PH, and IPAH patients. The mRNA levels of Cxcl12, Il1b, TNFα, Icam-1, and Vcam-1 in PAECs from hypoxic mice and Su/Hx rats were much higher than those in PAECs from normoxic mice and control rats. The mRNA levels of these molecules were dramatically decreased in PAECs isolated from hypoxic Pfkfb3ΔVEC mice and 3PO-treated rats compared with those in PAECs from hypoxic Pfkfb3WT mice and Su/Hx rats (Fig. 7A and SI Appendix, Fig. S10B). The aforementioned alteration of these cytokines at the mRNA level in mouse PAECs was also observed at the protein level (Fig. 7B). PAECs from IPAH patients also exhibited higher mRNA and protein levels of CXCL12, IL1B, TNFA, ICAM-1, and VCAM-1, as well as higher adherence of THP-1 monocytic cells, than normal PAECs. In contrast, PFKFB3 knockdown abrogated increases in expression and release of these proinflammatory molecules and in adherence of THP-1 monocytic cells in PAECs from IPAH patients (Fig. 7 C and D and SI Appendix, Fig. S11). Also, PAECs from healthy individuals released more proinflammatory cytokines in response to hypoxia, and this increased release from hypoxic PAECs was suppressed by PFKFB3 knockdown (SI Appendix, Fig. S8B). These results indicate that endothelial PFKFB3 regulates the production and release of proinflammatory molecules that promote endothelial inflammation in PH lungs.
Fig. 7.
Endothelial Pfkfb3/PFKFB3 deficiency or knockdown decreases inflammatory responses. (A) Real-time PCR analysis of mRNA levels of Cxcl12, Il1b, TNFα, Icam-1, and Vcam-1 in PAECs of Pfkfb3ΔVEC and Pfkfb3WT mice exposed to normoxia (Nor) or hypoxia (Hx; 10% O2) for 4 wk (n = 6). (B) Levels of released Cxcl12, Il1b, and TNFα in the culture supernatants of mouse PAECs exposed to hypoxia (1% O2) for 24 h (n = 4–6). (C) Real-time PCR analysis of mRNA levels of CXCL12, IL1B, TNFA, ICAM-1, and VCAM-1 in normal PAECs or siCTRL- and siPFKFB3-transfected PAECs of patients with IPAH (n = 6). Experiments were repeated four times independently with cells from four patients with IPAH or control subjects. (D) Levels of released CXCL12, IL1β, and TNFα in the culture supernatants of normal PAECs or siCTRL- and siPFKFB3-transfected PAECs of patients with IPAH (n = 8–9). All data are expressed as mean ± SEM. Statistical significance was determined by one-way ANOVA followed by Bonferroni test. *P < 0.05 was considered significant, **P < 0.01, ***P < 0.001. ns, no significance.
PFKFB3/Pfkfb3-Mediated Expression of Growth Factors and Proinflammatory Cytokines in PAECs Is through HIFs/Hifs.
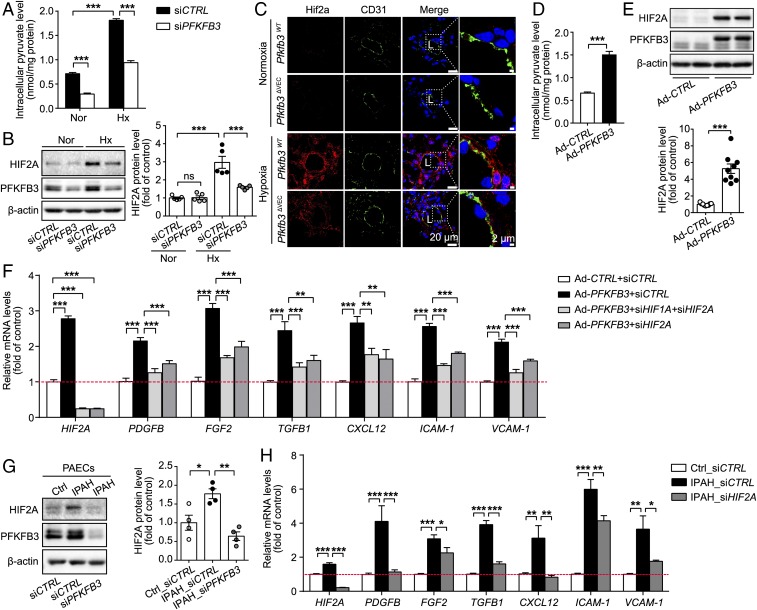
Previous studies have indicated that high glucose and pyruvate levels promoted HIF accumulation and that endothelial Hif2a is highly involved in PH development in rodents (17, 19, 22–24). To explore whether HIFs are the possible molecular mechanism underlying PFKFB3-regulated proinflammatory cytokines and growth factors, the levels of pyruvate, HIF1A, and HIF2A were examined in hypoxic human PAECs transduced with siCTRL or siPFKFB3. The increased levels of pyruvate and protein HIF1A and HIF2A in response to hypoxia were markedly decreased in PFKFB3-knockdown PAECs compared with control PAECs (Fig. 8 A and B and SI Appendix, Fig. S12A). In line with these in vitro results, Hif2a expression on lung vascular endothelium was much lower in hypoxic Pfkfb3ΔVEC mice than hypoxic Pfkfb3WT mice (Fig. 8C). In a gain-of-function assay, the levels of pyruvate, HIF1A, and HIF2A were up-regulated in PFKFB3-overexpressing PAECs compared with control PAECs (Fig. 8 D and E and SI Appendix, Fig. S12B). Also, addition of pyruvate to human PAECs substantially elevated HIF2A levels compared with vehicle treatment (SI Appendix, Fig. S12C). PFKFB3-overexpressing PAECs expressed high levels of growth factors and proinflammatory cytokines (Fig. 8F). To examine whether HIF1A or HIF2A is critical in mediating expression of growth factors and proinflammatory cytokines in PFKFB3-overexpressed PAECs, PFKFB3-overexpressed PAECs were treated with siRNA of HIF1A, HIF2A, or both, and the mRNA levels of proinflammatory cytokines and adhesion molecules were evaluated with real-time RT-PCR. As shown in Fig. 8F, the increased mRNA levels of PDGFB, FGF2, TGF-B1, CXCL12, ICAM-1, and VCAM-1 in PFKFB3-overexpressing PAECs were dramatically reduced by siHIF2A, whereas siHIF1A was able to only marginally decrease the mRNA levels of those molecules (SI Appendix, Fig. S12D). siHIF1A and siHIF2A did not decrease the increased mRNA levels of IL1B and TNFA (SI Appendix, Fig. S12 D and E). These results indicate that HIF2A mediates most of the up-regulated growth and inflammatory factors in high glycolytic PAECs. In addition, PAECs from IPAH patients exhibited higher levels of HIF2A and both growth factors and proinflammatory cytokines compared with PAECs from healthy individuals (Fig. 8 G and H). In these PAECs from IPAH patients, HIF2A knockdown dramatically decreased the increased levels of growth factors and proinflammatory cytokines except IL1B and TNFA (Fig. 8H and SI Appendix, Fig. S12F).
Fig. 8.
Expression of growth factors and inflammatory cytokines induced by PFKFB3/Pfkfb3 in endothelial cells is mediated by HIF2A/Hif2a. (A) Intracellular pyruvate levels in human PAECs transfected with siCTRL and siPFKFB3 under normoxic (Nor) and hypoxic (Hx; 1% O2) conditions for 24 h (n = 5). (B) Western blot analysis and densitometric quantification of HIF2A protein levels in human PAECs transfected with siCTRL and siPFKFB3 under normoxic and hypoxic (1% O2) conditions for 6 h (n = 5). (C) Representative micrographs of Hif2a expression in the distal pulmonary arteries of Pfkfb3WT and Pfkfb3ΔVEC mice under normoxic or hypoxic conditions for 4 wk. Sections were costained for Hif2a (red) and CD31 (green). L, lumen. (D) Intracellular pyruvate levels in human PAECs transfected with Ad-CTRL and Ad-PFKFB3 (n = 9). (E) Western blot analysis and densitometric quantification of HIF2A protein levels in human PAECs transfected with Ad-CTRL and Ad-PFKFB3 (n = 9). (F) Real-time PCR analysis of mRNA levels of HIF2A, PDGFB, FGF2 and TGFB1, CXCL12, ICAM-1, and VCAM-1 in human PAECs transfected with Ad-CTRL-siCTRL, Ad-PFKFB3-siCTRL, Ad-PFKFB3-siHIF1A+siHIF2A, and Ad-PFKFB3-siHIF2A (n = 6). (G) (Left) Representative Western blot images and (Right) densitometric quantification of HIF2A expression in normal or IPAH PAECs with or without PFKFB3 KD (n = 4). (H) Real-time PCR analysis of mRNA levels of HIF2A, PDGFB, FGF2 and TGFB1, CXCL12, ICAM-1, and VCAM-1 in normal or IPAH PAECs transfected with siCTRL or siHIF2A (n = 6). Experiments were repeated four times independently with cells from four patients with IPAH or control subjects. All data are expressed as mean ± SEM. Statistical significance was determined by one-way ANOVA followed by Bonferroni test (A, B, F, and H) and unpaired Student’s t test (D and E). *P < 0.05 was considered significant, **P < 0.01, ***P < 0.001. ns, no significance.
To further define the involvement of Hif2a in the actions of endothelial Pfkfb3, PAECs isolated from PH models of Pfkfb3ΔVEC-ERT2 and Pfkfb3WT-ERT2 mice were further assayed for mRNA expression of Hif2a-targeted genes by using real-time RT-PCR. The expression of Hif2a-targeted genes was increased in wild-type PAECs from PH mice compared with those in PAECs from control normoxic mice (SI Appendix, Fig. S13). The increased mRNA levels of Hif2a-targeted genes were diminished dramatically in PAECs from Pfkfb3ΔVEC-ERT2 mice with PH compared with PAECs from Pfkfb3WT-ERT2 mice with PH (SI Appendix, Fig. S13). These results validate the critical involvement of Hif2a in the action of endothelial Pfkfb3 in PH development.
Discussion
In the present study, we have uncovered a critical role of the glycolytic regulator PFKFB3 in the development of PH. Our data show that endothelial PFKFB3-mediated glycolysis elevates the production of growth factors and proinflammatory cytokines in lung endothelial cells. These factors, through autocrine and paracrine pathways, promote inflammation in lung endothelial cells and proliferation of PASMC in models of PH (SI Appendix, Fig. S14). The ability of PFKFB3 to promote dysfunction of the endothelium was caused by increased expression of HIFs, which are stabilized by higher levels of glycolytic metabolites (SI Appendix, Fig. S14).
Glycolysis plays an important role in the development of PH. PET scans have shown that FDG uptake is increased in the lungs of experimental PH in rodents and human with IPAH (6–8). Vascular cells isolated from PH lungs express high levels of glycolytic enzymes such as PDK1 and HK1 and glycolysis-related molecules such as GLUT1 and PFKFB3 (6–8). To assess the functional role of glycolysis in the development of PH, others have shown that dichloroacetate (DCA), an inhibitor of pyruvate dehydrogenase kinase (PDK), suppresses the development and progression of PAH (7, 8). PDK phosphorylates and inactivates pyruvate dehydrogenase complex (PDC) (25). The latter catalyzes the conversion of pyruvate into acetyl CoA, which enters the Krebs cycle, where it fuels mitochondrial oxidative metabolism (25). Therefore, DCA can restore oxidative phosphorylation in treated cells (26). However, DCA has other actions, and these studies do not provide direct evidence in support of a causal role of glycolysis in the development and progression of PH. PFKFB3 is a critical regulatory enzyme of glycolysis. Its product, F-2,6-P2, is the most potent allosteric activator of PFK-1, the second of three rate-limiting enzymes for glycolysis (12). PFKFB3-promoted glycolysis has been well documented in endothelial cells (14, 16, 27). In the present study, the pharmacological inhibitor of PFKFB3 3PO or haplodeficiency dramatically suppressed the development of PH in two distinct rodent models. The results provide direct evidence that PFKFB3-mediated glycolysis is critical for the development of PH.
PFKFB3-mediated glycolysis in PAECs enhances proinflammatory and proliferative responses in pulmonary arteries. Growth factors such as PDGFB, FGF2, and TGFB1 are highly expressed in the lungs of IPAH patients, and pharmacological inhibition or deletion of these molecules/genes suppresses the development of PH in rodents (28–30). Additionally, increased expression of endothelial adhesion molecules and proinflammatory cytokines/chemokines, as well as leukocyte recruitment, are also significant features of PH (31, 32). Mice deficient in proinflammatory cytokines and endothelial adhesion molecules, as well as those harboring defects in leukocyte recruitment, are not capable of developing severe PH (33–35). Inflammation, as reflected by the expression levels of proinflammatory cytokines and degree of macrophage infiltration, as well as proliferation of PASMCs, were decreased, along with reduced indices of PH in hypoxic Pfkfb3+/− mice or 3PO-treated Su/Hx rats. These antiinflammatory and antiproliferative effects are likely caused at least in part by the decreased ability of PFKFB3 to regulate endothelial glycolysis, as endothelial-specific deficiency of PFKFB3 also suppressed the expression of growth factors and reduced endothelial inflammation in the lung. Cross-talk between PAECs and PASMCs is an important factor in the development of PH (36). Growth factors and proinflammatory cytokines that are released from PAECs can stimulate proliferation of PASMCs and fibroblasts, as well as vascular inflammation in the lungs (24, 36). Additionally, the growth factors and proinflammatory cytokines released from PAECs also further activate ECs (37). Pfkfb3-deficient ECs release low levels of growth factors and proinflammatory cytokines, alleviating inflammation in lung ECs and proliferation of PASMCs in PH lungs through autocrine and paracrine pathways.
Recent studies have shown an association between glycolysis and endothelial inflammation. Endothelial cells are highly glycolytic, and the rate of endothelial glycolysis is equal to or exceeds that of cancer cells (38). PFKFB3-mediated glycolysis in endothelial cells has been shown to be critical in the development of angiogenic and inflammatory diseases such as retinopathy, Crohn’s disease, and metastatic cancer (15, 39). In microvessels of tumors, haplodeficiency of Pfkfb3 lowers the expression of adhesion molecules in endothelial cells by decreasing NF-κB signaling (39). In endothelial cells of arteries in areas prone to develop atherosclerotic lesions and endothelial cells exposed to disturbed flow in vitro, glycolysis is increased and is accompanied by heightened inflammatory signaling. The knockdown of glycolytic enzymes in endothelial cells reduces NF-κB activation and inflammation (40, 41). In the present study, we noted low levels of HIF1A and HIF2A in PFKFB3-knockdown PAECs, but high levels of HIF1A and HIF2A, in PFKFB3-overexpressing PAECs. Knockdown of HIF2A, but not HIF1A, reduced the increased expression of growth factors and proinflammatory cytokines in PFKFB3-overexpressing PAECs, indicating the critical involvement of HIF2A in PFKFB3-mediated expression of growth factors and proinflammatory cytokines in PAECs. Endothelial Hif2a KO lowers increased production of many growth factors and proinflammatory cytokines in lungs of mice deficient in prolyl hydroxylase domain 2 (PHD2) (17, 19, 22, 42). Glycolytic metabolites such as lactate or/and pyruvate are able to inhibit HIF prolyl hydroxylase to reduce HIF degradation (23, 43). Increased glycolytic metabolites in PFKFB3-overexpressing PAECs may stabilize HIF2A via suppressed PHD2. However, in this study, HIF2A knockdown did not affect increased expression of IL1B and TNF1A, indicating that mechanisms other than HIF2A also participate in the signaling events driven by PFKFB3-mediated glycolysis in PAECs.
In addition to endothelial cells, many other lung cells, such as PASMCs, fibroblasts, T cells, and macrophages, participate in the pathology of PH via a multitude of mechanisms (1, 2). Although we have shown a role for PFKFB3 in endothelial cells, glycolytic metabolism and PFKFB3 may also contribute to the development of PH in other cell types through unique pathways. Indeed, we have recently demonstrated a role of Pfkfb3 in VSMCs in the development of PH (44). A more expansive investigation will be required to define the role of PFKFB3 in other lung cell types, such as leukocytes and fibroblasts, in the setting of PH.
PFKFB3 holds significant promise as a therapeutic target for the treatment of PH. PFKFB3 is one of four isoforms of PFKFBs. The structural differences among the four isoforms are large enough that small molecules selectively targeting PFKFB3 should not significantly affect overall PFKFB activity in major metabolic tissues/organs such as the heart, liver, and skeletal muscle. Indeed, several PFKFB3 inhibitors have been developed that exhibit high specificity for PFKFB3 (45–48). Among the published PFKFB3 inhibitors, 3PO is most frequently used in experimental models and has demonstrated its efficacy and safety (15, 46, 49). PFK158 is another small-molecule inhibitor that was developed by using 3PO as a scaffold. It has been tested in a clinical trial with tumor patients and exhibited excellent safety and efficacy (50). In the present study, haploinsufficiency for Pfkfb3 protected mice from hypoxia-induced PH, and 3PO markedly suppressed Su/Hx-induced PH in rats. These promising preclinical findings, along with glycolytic changes in the lungs of IPAH patients, provide a strong rationale for the clinical testing of PFKFB3 inhibitors in patients with IPAH, which may shed new light on the treatment of this disease.
Methods
Animals and Rodent PH Models.
The animal use protocol was approved by the institutional animal care & use committee of Augusta University. The Pfkfb3-floxed (Pfkfb3flox/flox, Pfkfb3WT) mice (14) were generated by Xenogen Biosciences (Cranbury, NJ). Mice with constitutive or inducible Pfkfb3 deficiency in endothelial cells (Pfkfb3ΔVEC or Pfkfb3ΔVEC-ERT2) were generated by cross-breeding Pfkfb3flox/flox (Pfkfb3WT) mice with Cdh5-Cre transgenic mice (stk. no. 006137; The Jackson Laboratory) or Cdh5-CreERT2 (51). Global heterozygous Pfkfb3 (Pfkfb3+/−) KO mice were generated as previously described (20). All mice were on a C57BL/6J background. Mouse PH models were generated by exposing mice to 10% oxygen for the indicated time. Male Sprague–Dawley (SD) adult rats were purchased from Envigo RMS. Rat PH models were generated by s.c. injection of Sugen 5416 (APExBIO) and exposure to 10% hypoxia. At the times indicated, mice and rats were anesthetized for echocardiographic and hemodynamic assay. After euthanasia, lung samples were collected and processed for morphometric analysis.
Measurement of Glycolytic Metabolites, Cytokines, and Growth Factors.
F-2,6-P2 levels were assayed with a previously described method (52). The levels of lactate or pyruvate were measured with the lactate or pyruvate assay kits according to the manufacturer’s instructions. The levels of cytokines and growth factors were measured with R&D ELISA kits according to the manufacturer’s instructions.
Adenovirus Transduction of PAECs.
HPAECs were incubated with 500 µL basal medium containing adenoviral vectors of PFKFB3 knockdown (Ad-shPFKFB3) or overexpression (Ad-PFKFB3) and their negative control adenovirus (Ad-shCTRL or Ad-CTRL) for 2 h. The medium was then replaced with fresh complete growth medium for continuous culture. After 48 h, the cells were treated as indicated and collected for Western blot and quantitative RT-PCR analysis.
RNA Interference.
When HPAECs reached 70% confluence, they were transfected with 25 nM of siRNAs targeting human PFKFB3 (siPFKFB3), HIF1A (siHIF1A), or HIF2A (siHIF2A) or with a nontargeting negative control (siCTRL) by using Lipofectamine RNAiMax reagent according to the manufacturer’s protocol. Four hours later, the medium was changed to complete growth medium for continuous culture. The cells underwent different treatments within 48 h after siRNA transduction and were then collected for various assays.
Statistical Analysis.
Statistical analysis was performed with GraphPad Prism software. The significance of the differences between two groups was assessed by using unpaired Student’s t test for n > 5 and Mann–Whitney U test for n < 5. Multiple comparisons were performed by one-way ANOVA followed by Bonferroni’s post hoc test. All results are presented as mean ± SEM. P < 0.05 was considered significant. All biological experiments were repeated at least three times using independent cell cultures or individual animals (biological replications).
Supplementary Material
Acknowledgments
We thank Ziyi Wang for her effort on culturing IPAH cells. This work is supported by Shenzhen Science and Technology Innovation Committee Grants JCYJ20170412150405310, JCYJ20160525154531263, JCYJ20160506170316776, and JCYJ20170810163238384; Guangdong Natural Science Foundation Grant 2014A030312004; American Heart Association Grant 16GRNT30510010; National Institutes of Health Grants R01HL134934, R01DK095862, R01HL142097, and R01HL138410; and VA Merit Review Grant BX002035.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. M.G.V.H. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1821401116/-/DCSupplemental.
References
- 1.Rabinovitch M., Molecular pathogenesis of pulmonary arterial hypertension. J. Clin. Invest. 118, 2372–2379 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tuder R. M., Pathology of pulmonary arterial hypertension. Semin. Respir. Crit. Care Med. 30, 376–385 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Rabinovitch M., Guignabert C., Humbert M., Nicolls M. R., Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ. Res. 115, 165–175 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elinoff J. M., et al. , Challenges in pulmonary hypertension: Controversies in treating the tip of the iceberg. A joint national institutes of health clinical center and pulmonary hypertension association symposium report. Am. J. Respir. Crit. Care Med. 198, 166–174 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu W., et al. , Alterations of cellular bioenergetics in pulmonary artery endothelial cells. Proc. Natl. Acad. Sci. U.S.A. 104, 1342–1347 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagan G., et al. , (18)FDG PET imaging can quantify increased cellular metabolism in pulmonary arterial hypertension: A proof-of-principle study. Pulm. Circ. 1, 448–455 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marsboom G., et al. , Lung 18F-fluorodeoxyglucose positron emission tomography for diagnosis and monitoring of pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 185, 670–679 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao L., et al. , Heterogeneity in lung (18)FDG uptake in pulmonary arterial hypertension: Potential of dynamic (18)FDG positron emission tomography with kinetic analysis as a bridging biomarker for pulmonary vascular remodeling targeted treatments. Circulation 128, 1214–1224 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Fijalkowska I., et al. , Hypoxia inducible-factor1alpha regulates the metabolic shift of pulmonary hypertensive endothelial cells. Am. J. Pathol. 176, 1130–1138 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonnet S., et al. , An abnormal mitochondrial-hypoxia inducible factor-1alpha-Kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: Similarities to human pulmonary arterial hypertension. Circulation 113, 2630–2641 (2006). [DOI] [PubMed] [Google Scholar]
- 11.Vander Heiden M. G., Cantley L. C., Thompson C. B., Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 324, 1029–1033 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rider M. H., et al. , 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase: Head-to-head with a bifunctional enzyme that controls glycolysis. Biochem. J. 381, 561–579 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okar D. A., Wu C., Lange A. J., Regulation of the regulatory enzyme, 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. Adv. Enzyme Regul. 44, 123–154 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Xu Y., et al. , Endothelial PFKFB3 plays a critical role in angiogenesis. Arterioscler. Thromb. Vasc. Biol. 34, 1231–1239 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schoors S., et al. , Partial and transient reduction of glycolysis by PFKFB3 blockade reduces pathological angiogenesis. Cell Metab. 19, 37–48 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Yalcin A., Telang S., Clem B., Chesney J., Regulation of glucose metabolism by 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatases in cancer. Exp. Mol. Pathol. 86, 174–179 (2009). [DOI] [PubMed] [Google Scholar]
- 17.Tang H., et al. , Endothelial HIF-2α contributes to severe pulmonary hypertension due to endothelial-to-mesenchymal transition. Am. J. Physiol. Lung Cell Mol. Physiol. 314, L256–L275 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Omura J., et al. , Protective roles of endothelial AMP-activated protein kinase against hypoxia-induced pulmonary hypertension in mice. Circ. Res. 119, 197–209 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Dai Z., Li M., Wharton J., Zhu M. M., Zhao Y. Y., Prolyl-4 hydroxylase 2 (PHD2) deficiency in endothelial cells and hematopoietic cells induces obliterative vascular remodeling and severe pulmonary arterial hypertension in mice and humans through hypoxia-inducible factor-2α. Circulation 133, 2447–2458 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chesney J., et al. , Targeted disruption of inducible 6-phosphofructo-2-kinase results in embryonic lethality. Biochem. Biophys. Res. Commun. 331, 139–146 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Haslip M., et al. , Endothelial uncoupling protein 2 regulates mitophagy and pulmonary hypertension during intermittent hypoxia. Arterioscler. Thromb. Vasc. Biol. 35, 1166–1178 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cowburn A. S., et al. , HIF2α-arginase axis is essential for the development of pulmonary hypertension. Proc. Natl. Acad. Sci. U.S.A. 113, 8801–8806 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu H., Forbes R. A., Verma A., Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J. Biol. Chem. 277, 23111–23115 (2002). [DOI] [PubMed] [Google Scholar]
- 24.Armulik A., Abramsson A., Betsholtz C., Endothelial/pericyte interactions. Circ. Res. 97, 512–523 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Michelakis E. D., et al. , Dichloroacetate, a metabolic modulator, prevents and reverses chronic hypoxic pulmonary hypertension in rats: Role of increased expression and activity of voltage-gated potassium channels. Circulation 105, 244–250 (2002). [DOI] [PubMed] [Google Scholar]
- 26.Knoechel T. R., et al. , Regulatory roles of the N-terminal domain based on crystal structures of human pyruvate dehydrogenase kinase 2 containing physiological and synthetic ligands. Biochemistry 45, 402–415 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Tawakol A., et al. , HIF-1α and PFKFB3 mediate a tight relationship between proinflammatory activation and anerobic metabolism in atherosclerotic macrophages. Arterioscler. Thromb. Vasc. Biol. 35, 1463–1471 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perros F., et al. , Platelet-derived growth factor expression and function in idiopathic pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 178, 81–88 (2008). [DOI] [PubMed] [Google Scholar]
- 29.Yung L. M., et al. , A selective transforming growth Factor-β ligand trap attenuates pulmonary hypertension. Am. J. Respir. Crit. Care Med. 194, 1140–1151 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Izikki M., et al. , Endothelial-derived FGF2 contributes to the progression of pulmonary hypertension in humans and rodents. J. Clin. Invest. 119, 512–523 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stacher E., et al. , Modern age pathology of pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 186, 261–272 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huertas A., et al. , Immune dysregulation and endothelial dysfunction in pulmonary arterial hypertension: A complex interplay. Circulation 129, 1332–1340 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Bryant A. J., et al. , Myeloid-derived suppressor cells are necessary for development of pulmonary hypertension. Am. J. Respir. Cell Mol. Biol. 58, 170–180 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Hiress M., et al. , Proinflammatory signature of the dysfunctional endothelium in pulmonary hypertension. Role of the macrophage migration inhibitory factor/CD74 complex. Am. J. Respir. Crit. Care Med. 192, 983–997 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Burton V. J., et al. , Attenuation of leukocyte recruitment via CXCR1/2 inhibition stops the progression of PAH in mice with genetic ablation of endothelial BMPR-II. Blood 118, 4750–4758 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eddahibi S., et al. , Cross talk between endothelial and smooth muscle cells in pulmonary hypertension: Critical role for serotonin-induced smooth muscle hyperplasia. Circulation 113, 1857–1864 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Tu L., et al. , Autocrine fibroblast growth factor-2 signaling contributes to altered endothelial phenotype in pulmonary hypertension. Am. J. Respir. Cell Mol. Biol. 45, 311–322 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Bock K., et al. , Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 154, 651–663 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Cantelmo A. R., et al. , Inhibition of the glycolytic activator PFKFB3 in endothelium induces tumor vessel normalization, impairs metastasis, and improves chemotherapy. Cancer Cell 30, 968–985 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feng S., et al. , Mechanical activation of hypoxia-inducible factor 1α drives endothelial dysfunction at atheroprone sites. Arterioscler. Thromb. Vasc. Biol. 37, 2087–2101 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu D., et al. , HIF-1α is required for disturbed flow-induced metabolic reprogramming in human and porcine vascular endothelium. eLife 6, e25217 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kapitsinou P. P., et al. , The endothelial prolyl-4-hydroxylase domain 2/hypoxia-inducible factor 2 axis regulates pulmonary artery pressure in mice. Mol. Cell. Biol. 36, 1584–1594 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu H., et al. , Reversible inactivation of HIF-1 prolyl hydroxylases allows cell metabolism to control basal HIF-1. J. Biol. Chem. 280, 41928–41939 (2005). [DOI] [PubMed] [Google Scholar]
- 44.Kovacs L., et al. , PFKFB3 in smooth muscle promotes vascular remodeling in pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 10.1164/rccm.201812-2290OC (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boyd S., et al. , Structure-based design of potent and selective inhibitors of the metabolic kinase PFKFB3. J. Med. Chem. 58, 3611–3625 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Clem B., et al. , Small-molecule inhibition of 6-phosphofructo-2-kinase activity suppresses glycolytic flux and tumor growth. Mol. Cancer Ther. 7, 110–120 (2008). [DOI] [PubMed] [Google Scholar]
- 47.Clem B. F., et al. , Targeting 6-phosphofructo-2-kinase (PFKFB3) as a therapeutic strategy against cancer. Mol. Cancer Ther. 12, 1461–1470 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seo M., Kim J. D., Neau D., Sehgal I., Lee Y. H., Structure-based development of small molecule PFKFB3 inhibitors: A framework for potential cancer therapeutic agents targeting the Warburg effect. PLoS One 6, e24179 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xie N., et al. , Glycolytic reprogramming in myofibroblast differentiation and lung fibrosis. Am. J. Respir. Crit. Care Med. 192, 1462–1474 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Redman R., Pohlmann P., Kurman M., Tapolsky G. H., Chesney J., PFK-158, first-in-man and first-in-class inhibitor of PFKFB3/ glycolysis: A phase I, dose escalation, multi-center study in patients with advanced solid malignancies [abstract]. Cancer Res. 75(15 suppl), Abstract CT206 (2015). [Google Scholar]
- 51.Benedito R., et al. , The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell 137, 1124–1135 (2009). [DOI] [PubMed] [Google Scholar]
- 52.Van Schaftingen E., Lederer B., Bartrons R., Hers H. G., A kinetic study of pyrophosphate: fructose-6-phosphate phosphotransferase from potato tubers. Application to a microassay of fructose 2,6-bisphosphate. Eur. J. Biochem. 129, 191–195 (1982). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.