Significance
Long-term fasting for more than 24 h has been reported to ameliorate liver ischemia and reperfusion injury (IRI). However, it is difficult to apply long-term fasting for human preoperative management, and the underlying molecular mechanisms remain unclear. Our present research demonstrates that 12-h fasting remarkably ameliorates liver IRI. The 12-h fasting induces up-regulation of FOXO1 by raising the level of acetylated histone and β-hydroxybutyric acid, followed by up-regulation of antioxidative enzyme and autophagy activity, and improves liver IRI through the reduction of the inflammation and apoptotic cell death. Perioperative administration of β-hydroxybutyric acid or histone deacetylase inhibitor may have beneficial effects by avoiding liver injury at liver surgery. This is an insight on the prevention of liver IRI.
Keywords: fasting, β-hydroxybutyric acid, FOXO1, ischemia and reperfusion injury
Abstract
Liver ischemia and reperfusion injury (IRI) is a major challenge in liver surgery. Diet restriction reduces liver damage by increasing stress resistance; however, the underlying molecular mechanisms remain unclear. We investigated the preventive effect of 12-h fasting on mouse liver IRI. Partial warm hepatic IRI model in wild-type male C57BL/6 mice was used. The control ischemia and reperfusion (IR) group of mice was given food and water ad libitum, while the fasting IR group was given water but not food for 12 h before ischemic insult. In 12-h fasting mice, serum liver-derived enzyme level and tissue damages due to IR were strongly suppressed. Serum β-hydroxybutyric acid (BHB) was significantly raised before ischemia and during reperfusion. Up-regulated BHB induced an increment in the expression of FOXO1 transcription factor by raising the level of acetylated histone. Antioxidative enzyme heme oxigenase 1 (HO-1), a target gene of FOXO1, then increased. Autophagy activity was also enhanced. Serum high-mobility group box 1 was remarkably lowered by the 12-h fasting, and activation of NF-κB and NLRP3 inflammasome was suppressed. Consequently, inflammatory cytokine production and liver injury were reduced. Exogenous BHB administration or histone deacetylase inhibitor administration into the control fed mice ameliorated liver IRI, while FOXO1 inhibitor administration to the 12-h fasting group exacerbated liver IRI. The 12-h fasting exerted beneficial effects on the prevention of liver IRI by increasing BHB, thus up-regulating FOXO1 and HO-1, and by reducing the inflammatory responses and apoptotic cell death via the down-regulation of NF-κB and NLRP3 inflammasome.
Ischemia and reperfusion injury (IRI) is an important issue that requires resolution for reducing the prevalence of organ failures in patients requiring cardiopulmonary resuscitation, and those with cerebral or myocardial infarction and organ transplantation (1). Liver IRI may occur during temporary interruption of blood flow (i.e., Pringle maneuver) for hepatectomy and graft reperfusion for transplantation and cause liver tissue damage (2, 3). In the case of liver transplantation, about 10% of early graft failures are related to IRI, resulting in a higher incidence of acute and chronic rejection (4). Thus, establishment of a method to overcome IRI would help reduce the risk of graft rejection and increase the number of donor organs available for liver transplantation.
In liver IRI, immune cells, such as macrophages, lymphocytes, and neutrophils, infiltrate into the liver tissues after reperfusion and produce inflammatory cytokines and reactive oxygen species (ROS) leading to hepatocytes apoptosis and necrosis (5). NF-κB activation in the inflammatory liver cells is critical for the onset of liver IRI. NF-κB plays a key role in regulating the immune response and inflammation (6), and its activation is regulated by signals from Toll-like receptor 4 (TLR-4) (7). In liver IRI, high-mobility group box 1 (HMGB1), secreted from the inflammatory cells and injured hepatocytes, is an endogenous ligand of TLR-4 and activates inflammatory cells such as macrophages and Kuppfer cells (8).
Many types of clinically available medicines, such as atrial natriuretic peptide (9), inhibitor of neutrophil elastase (10, 11), and recombinant thrombomodulin (12), protect against liver IRI. Immune checkpoints that regulate the immune system also reveal a strong affect against liver IRI (13). Stimulating Programmed Death-1 (14) or T cell Ig and mucin domain-3 (15) ameliorated liver IRI by inhibiting T cell activation and macrophage function. Recently, some types of nutrients have been shown to exert an effect on leukocytes and affect proinflammatory, carcinogenic effects or anticancer immune responses (16). Liver IRI is also influenced quantitatively by various nutrients. Protein restriction ameliorated liver IRI by up-regulating endogenous hydrogen sulfide (17). The enrichment of vitamins C and E in the ordinal diet exhibited preventive effects against liver IRI by up-regulating antioxidant enzymes and down-regulating cell adhesion molecules (18). These findings strongly suggest the importance of dietary control for preventing IRI.
Diet restriction also exerts a protective effect against IRI in several organs (19). Starvation for 48 h to 72 h reduced liver IRI by up-regulating antioxidative enzymes or autophagy (20, 21). In addition, fasting for 1 d, but not 2 d or 3 d, can prevent mouse liver IRI via the Sirtuin1-mediated down-regulation of circulating HMGB1 (22). We report here that 12-h fasting can remarkably suppress mouse liver IRI. This study aimed to clarify the effect of the 12-h fasting against liver IRI and the underlying molecular mechanisms.
Results
Preoperative 12-h Fasting Protects Against Ischemia and Reperfusion-Triggered Hepatocellular Damage.
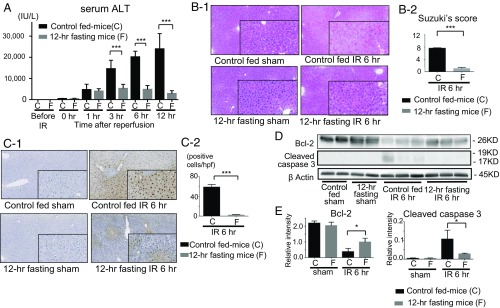
After ischemia and reperfusion (IR), serum liver-derived enzyme, alanine aminotransferase (sALT), levels markedly increased in the control fed mice. However, sALT remained significantly lower in the 12-h fasting group (Fig. 1A and SI Appendix, Fig. S1B), indicating that 12-h fasting can ameliorate IR-induced liver damage. The livers of the 12-h fasted mice exhibited clear reduction in the hepatocellular necrosis caused by IR (Fig. 1 B, 1). Moreover, Suzuki’s score was significantly lowered for the 12-h fasting group than for the control fed mice (Fig. 1 B, 2). During liver IRI, leukocytes infiltrate into the liver tissues and produce inflammatory cytokines, which lead to hepatocyte apoptosis and necrosis. The numbers of T cells (CD3), neutrophils (Ly-6G), and macrophages (CD68) infiltrating into the liver tissue after IR treatment were significantly lesser in the 12-h fasted mice (SI Appendix, Fig. S2). Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL)-positive cells induced by IR were significantly reduced in the liver of the 12-h fasting group compared with those of control fed mice (Fig. 1 C, 1 and 2), indicating that the 12-h fast clearly reduced liver cell injury caused by IR. Moreover, the 12-h fasting suppressed the expression of cleaved caspase-3, while it significantly increased the expression of antiapoptotic protein B cell lymphoma 2 (Bcl-2) in the liver (Fig. 1 D and E).
Fig. 1.
Hepatocellular damage induced by IR in the controls and the 12-h fasting mice. (A) The sALT levels were measured. Means and SD are shown (n = 8 mice per group; two-way ANOVA; P < 0.0001, Bonferroni’s posttest: ***P < 0.001 vs. control mice). (B-1) Liver histology (H & E staining) after IR and sham-operated (magnification 200×; in box, 400×). (B-2) Suzuki’s histological score after IR. Means and SD are shown (n = 8 mice per group; ***P < 0.001). (C-1) TUNEL-assisted detection of hepatic apoptosis after IR (magnification 200×; in box, 400×). (C-2) Quantification of TUNEL positive cells. Means and SD are shown (n = 8 mice per group; ***P < 0.001). (D) Western blot-assisted expression of cleaved caspase-3, Bcl-2 at 6 h of reperfusion. (E) Quantification of Western blot bands shown in D. Means and SD are shown (*P < 0.05).
Preoperative 12-h Fasting Suppressed the Secretion of Proinflammatory Cytokines and Damage-Associated Molecular Patterns Induced by IR.
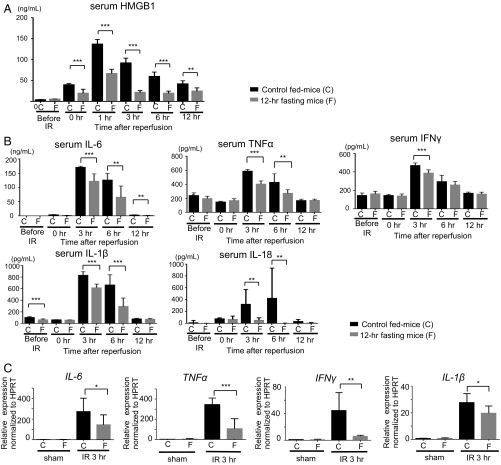
Damage-associated molecular patterns (DAMPs) strongly influence the induction of inflammation (23, 24). HMGB1, a DAMP, is released into the extracellular space from the necrotic cells and stimulates immune cells (8, 23). Serum HMGB1 level increased during ischemia and was rapidly boosted following reperfusion in the controls. In contrast, the serum HMGB1 level was significantly suppressed during ischemia and reperfusion period in the 12-h fasting group (Fig. 2A). HMGB1 activates the inflammatory cells in the liver through TLR-4 and induces the production of proinflammatory cytokines that are crucial for the development of liver IRI (25). In the inflammatory process of hepatic IRI, inflammatory cytokines, such as interleukin-6 (IL-6), tumor necrosis factor α (TNFα), and IFNγ are released from the macrophages and lymphocytes (25). Serum levels of IL-6, IL-18, TNFα, IFNγ, and IL-1β rapidly increased during reperfusion, and returned to the baseline level at 12 h after reperfusion, while the serum levels of all of these proinflammatory cytokines were significantly suppressed in the 12-h fasting group (Fig. 2B). Furthermore, the gene expressions of these proinflammatory cytokines in the liver tissue were also significantly suppressed in the 12-h fasting group compared with the control fed mice (Fig. 2C). These results clearly indicate that pretreatment with 12-h fasting can ameliorate liver IRI via suppression of HMGB1 and inflammatory cytokines induced by IR.
Fig. 2.
Preoperative fasting suppressed the release of HMGB1 and proinflammatory cytokines. (A) Serum HMGB1 levels were measured. Means and SD are shown (n = 7 mice per group; two-way ANOVA: P < 0.0001; Bonferroni’s posttest: **P < 0.01, ***P < 0.001 vs. control mice). (B) Serum levels of proinflammatory cytokines (IL-6, TNFα, IL-1β, IL-18, and IFNγ) were measured. Means and SD are shown (n = 8 mice per group; two-way ANOVA; IL-6, P < 0.0001; TNFα, P < 0.0001; IFNγ, P < 0.001; IL-1β, P < 0.0001; IL-18, P < 0.001; Bonferroni’s posttest: **P < 0.01, ***P < 0.001 vs. control mice). (C) Quantitative PCR detection of proinflammatory cytokines (IL-6, TNFα, IL-1β, and IFNγ) was performed at 3 h of reperfusion after 60 min of ischemia. Data were normalized to HPRT gene expression. Means and SD are shown (sham, n = 4; IR 3 h, n = 8 mice per group; *P < 0.05; **P < 0.01; ***P < 0.001).
Suppression of NF-κB and NLRP3 Induced by 12-h Fasting Before Treatment.
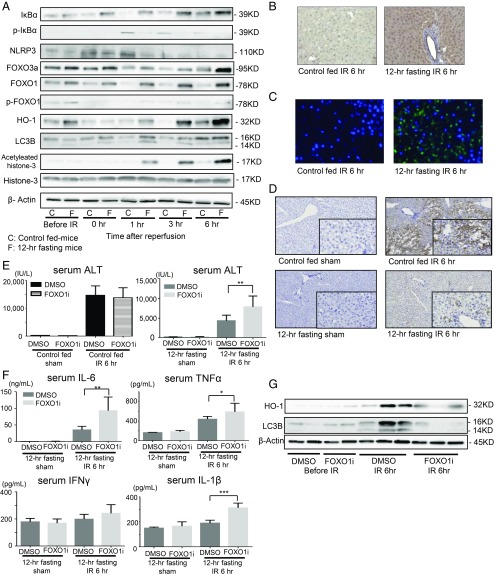
NF-κB is a protein complex that controls cytokine production and cell survival (26), also playing a key role in regulating the immune response against inflammation (27). NF-κB heterodimer comprises p65 and p50 proteins. In an inactivated state, NF-κB locates in the cytosol complexed with the inhibitory protein IκBα as the nonphosphorylated form. The amounts of phosphorylated IκBα (p-IκBα) reflect the activation state of NF-κB (26, 28). The p-IκBα expression at 1 h and more after reperfusion was significantly lower, while the amounts of cytosol NF-κB inversely increased in the 12-h fasting group compared with that in the control fed mice (Fig. 3A), indicating that the 12-h fasting clearly suppressed NF-κB activation in relation to the reduction in serum HMGB1. NLRP3 inflammasome also contributes to the early inflammatory phase and regulates inflammatory response (29). The NLRP3 expression was significantly decreased in the 12-h fasting group at early phase of IR (1 h of reperfusion) compared with that in the control fed mice (Fig. 3A). The reduced expression of nuclear NF-κB and the reduction of NLRP3 from the early phase of reperfusion in the mice pretreated with the 12-h fasting may strongly suppress inflammatory cytokines, resulting in IRI amelioration.
Fig. 3.
Preoperative fasting protected liver from injury through the up-regulation of FOXO1 and suppressed NF-κB and NLRP3 signaling induced by IR. (A) Western blot-assisted analyses of the total and phosphorylated protein levels of FOXO1, IκBα and acetylated histone-3, histone-3, FOXO3a, HO-1, LC3B, and NLRP3 before ischemia and during reperfusion. β-actin was used as the internal control. (B) Representative of immunohistochemistry of FOXO1 (dark brown) at 6 h of reperfusion (magnification 400×). (C) Representative of immunofluorescence staining of FOXO1 (green) and DAPI (blue) at 6 h of reperfusion (magnification 400×). (D) Representative immunohistochemistry of 4-HNE (dark brown) at 6 h of reperfusion (magnification 200×; in box, 400×). (E) The sALT levels were measured for mice pretreated with FOXO1 inhibitor and the 12-h fasting mice pretreated with DMSO only. Means and SD are shown (n = 8 mice per group; **P < 0.01). (F) Serum levels of proinflammatory cytokines (IL-6, TNFα, IL-1β, and IFNγ) were measured at 6 h of reperfusion. Means and SD are shown (sham, n = 4; IR 6 h, n = 8 mice per group; *P < 0.05; **P < 0.01; ***P < 0.001). (G) Western blot-assisted expression of HO-1 and LC3B at 6 h of reperfusion.
Preoperative 12-h Fasting Protects Liver from Oxidative Stress through the Up-Regulation of FOXO1.
FOXO transcription factor is an important target of the glycolytic pathway (30) that regulates gluconeogenesis and β-oxidation of fatty acids in the liver (31). Furthermore, FOXO1 is involved in the transcriptional regulation of antioxidant enzymes and in autophagy regulation (32, 33). FOXO1/3 expression in the liver of the 12-h fasted mice was higher than that in the control fed mice before ischemia, particularly during reperfusion. It was increased from the beginning of reperfusion and peaked at the later phase (3 h to 6 h), while the FOXO1/3 levels in the liver of the controls remained lower during reperfusion. The antioxidative enzymes, heme oxygenase 1 (HO-1), a target gene of FOXO1 (32), also clearly increased in the 12-h fasting group before ischemia and during the later phase (3 h to 6 h) of reperfusion. However, such an increase was not observed in the control fed mice (Fig. 3A). Autophagosome membrane protein LC3B, the marker of autophagy and a target of FOXO1 (33), was up-regulated from the early phase of reperfusion in the liver of the 12-h fasting group but not in the control fed mice (Fig. 3A). Immunohistochemistry (Fig. 3B) and immunofluorescence staining (Fig. 3C) of the liver tissues of the 12-h fasting group revealed that most FOXO1 positive cells were localized in the parenchymal and endothelial cells. These positive cells were significantly higher in the 12-h fasting group than in the controls 6 h after reperfusion (Fig. 3 B and C).
In the development of liver IRI, ROS are generated and induce oxidation of local macromolecules. Lipid peroxidation is essential to assess oxidative stress, and 4-hydroxynonenal (4-HNE) in the liver is an indicator of lipid peroxidation (34). Immunohistochemistry staining of 4-HNE in the liver tissues revealed clear reduction of lipid oxidation in the 12-h fasted mice (Fig. 3D), suggesting that the 12-h fasting could ameliorate oxidative stress in liver caused by IR.
The i.p. administration of FOXO1 inhibitor (AS1842856) significantly exacerbated liver IRI in case of the 12-h fasting group (Fig. 3E and SI Appendix, Fig. S1C). Serum levels of IL-1β, IL-6, and TNFα were also increased upon administration of FOXO1 inhibitor at 6 h after reperfusion (Fig. 3F). The up-regulated expressions of antioxidative enzyme, HO-1, and autophagosome protein, LC3B, which are induced by 12-h fasting, were suppressed by the FOXO1 inhibitor at 6 h after reperfusion (Fig. 3G). Conversely, the effect of FOXO1 inhibitor against liver IRI was not observed in the control fed mice (Fig. 3E). These results indicate that FOXO1 plays a preventive role against liver cell damage caused by IR via the up-regulation of antioxidative responses and autophagy.
β-Hydroxybutyric Acid Controls the Benefits of 12-h Fasting.
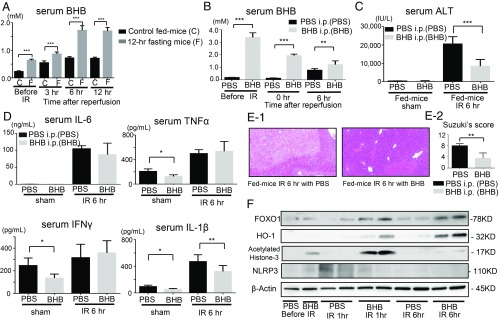
In the animal body, fats are metabolized to acetoacetate and β-hydroxybutyric acid (BHB) as sources of energy when the glycolytic pathway does not function (35). The serum BHB levels were significantly increased even before ischemia in the 12-h fasting group (Fig. 4A). The serum BHB levels slightly increased in the control fed mice 3 h to 12 h after reperfusion. However, the serum BHB level in the 12-h fasting group increased even after ischemia, and at 3 h of reperfusion. It was markedly up-regulated at 6 h of reperfusion. These results strongly suggest that the up-regulation of BHB may be a crucial event for preventing liver IRI in 12-h fasting mice.
Fig. 4.
BHB controls the benefits of 12-h fasting. (A) Serum BHB levels were measured in control fed mice and 12-h fasting mice. Means and SD are shown (n = 8 mice per group; two-way ANOVA: P < 0.0001; Bonferroni’s posttest: ***P < 0.001 vs. control fed mice). (B) After the administration of BHB or PBS into the fed mice, serum BHB levels were measured. Means and SD are shown (n = 8 mice per group; two-way ANOVA: P < 0.0001; Bonferroni’s posttest: **P < 0.01, ***P < 0.001 vs. PBS-administered mice). (C) The sALT levels were measured. Means and SD are shown (n = 8 mice per group; ***P < 0.001). (D) After the administration of BHB or PBS, serum levels of proinflammatory cytokines (IL-6, TNFα, IL-1β, and IFNγ) were measured. Means and SD are shown (sham, n = 4; IR 6 h, n = 8 mice per group; *P < 0.05; **P < 0.01). (E-1) Liver histology (H & E staining) after IR (magnification 200×). (E-2) Suzuki’s histological score after IR. Means and SD are shown (n = 8 mice per group; **P < 0.01). (F) Western blot-assisted analyses of the FOXO1, HO-1 acetylated histone-3, and NLRP3.
To examine the effect of BHB on liver IRI, the control fed mice received i.p. administration of BHB 30 min before ischemia treatment (SI Appendix, Fig. S1E). After BHB administration, the serum BHB levels increased significantly and remained at higher levels during IR than that of the PBS-administered mice (Fig. 4B). The sALT levels and serum IL-1β levels were significantly lowered in the mice administered BHB at 6 h of reperfusion (Fig. 4 C and D). The livers of the BHB-administered mice exhibited histological reduction in hepatocellular necrosis after IR treatment, and Suzuki’s score improved significantly in the BHB-administered mice compared with the PBS-administered mice (Fig. 4 E, 1 and 2).
The expression of FOXO1 is up-regulated through the increment in acetylated histone (36). Thus, the FOXO1 expression is negatively regulated by histone deacetylase (HDAC). BHB has an endogenous HDAC inhibitory activity at a low concentration level (1 mM) (37). BHB induced histone acetylation at the promoter of oxidative stress resistance genes FOXO by inhibiting HDACs classes I and II (37, 38). Western blotting analyses showed that the expressions of FOXO1 and acetylated histone-3 markedly increased in the 12-h fasting group from the beginning of reperfusion (Fig. 3A). These results suggest that the FOXO1 expression in the liver was up-regulated owing to the increased amounts of acetylated histone induced by increased BHB activity; in turn, increased BHB was stimulated by the 12-h fasting. Exogenous BHB also induced acetylated histone-3, which resulted in higher expressions of FOXO1, followed by HO-1 up-regulation in the BHB-administered mice (Fig. 4F). BHB is also known to suppress NLRP3 inflammasome (39). The expression of NLRP3 in the liver and serum levels of IL-1β were suppressed in the BHB-administered mice (Fig. 4 D and F).
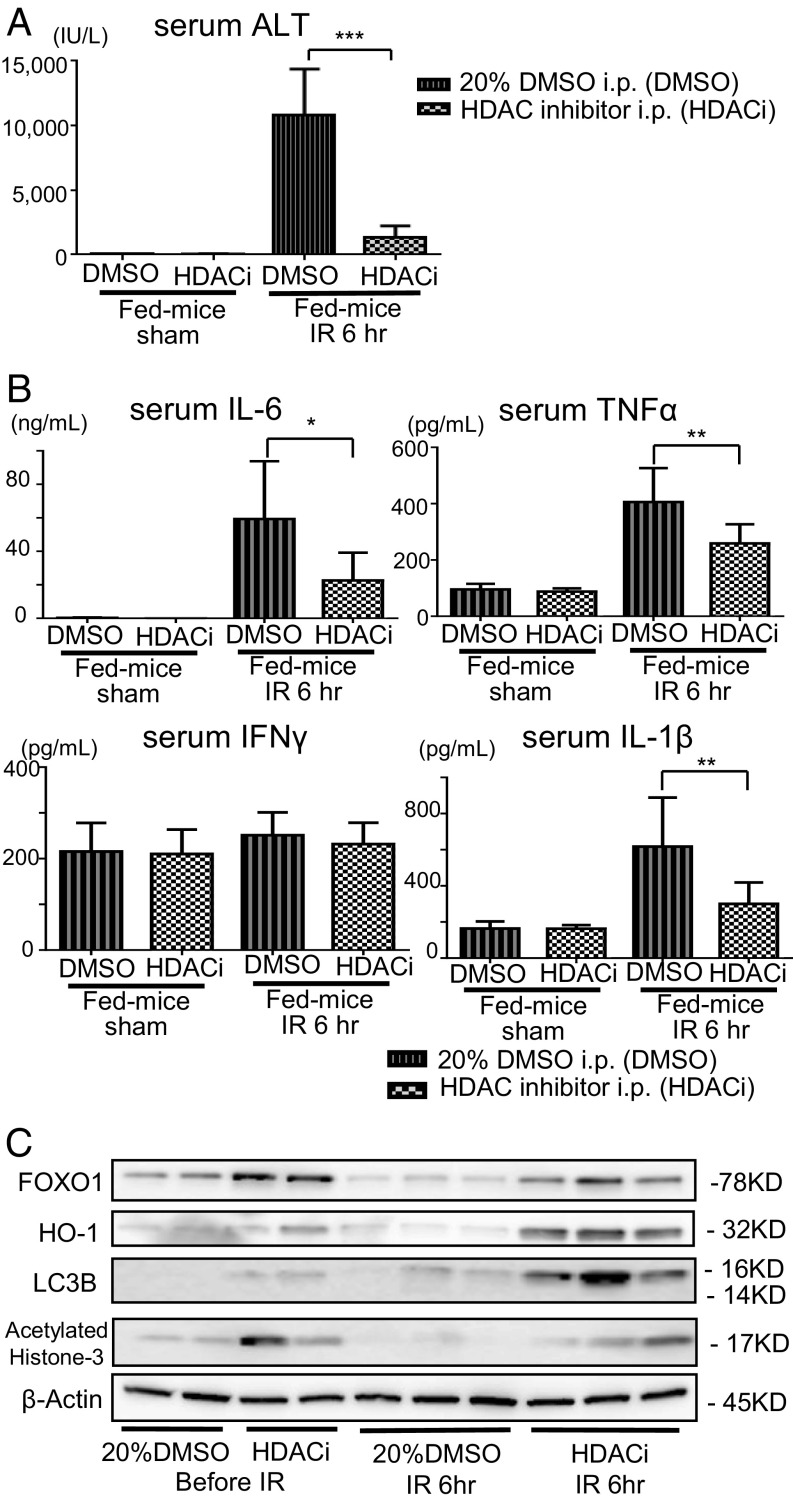
The control fed mice received i.p. administration of Trichostatin A, which is an inhibitor of HDACs classes I and II (HDACi) (40), at 16 h before IR treatment and just before IR treatment (SI Appendix, Fig. S1D). The sALT levels after reperfusion were significantly lowered in the HDACi administered fed mice (Fig. 5A). Serum levels of IL-6, TNFα, and IL-1β were also significantly suppressed in the HDACi administered fed mice (Fig. 5B). The expressions of FOXO1, HO-1, and LC3B were reversely up-regulated by the HDACi administration at 6 h of reperfusion (Fig. 5C).
Fig. 5.
HDAC inhibition reduces liver IRI through the up-regulation of FOXO1. (A) After the administration of DMSO or HDAC inhibitor in DMSO, sALT levels were measured. Means and SD are shown (n = 8 mice per group; ***P < 0.001). (B) Serum levels of proinflammatory cytokines (IL-6, TNFα, IL-1β, and IFNγ) were measured. Means and SD are shown (sham, n = 4; IR 6 h, n = 8 mice per group; *P < 0.05; **P < 0.01). (C) Western blot-assisted analyses of the FOXO1, acetylated histone-3, HO-1, and LC3B.
These results indicate that the improvement in liver IRI induced by the 12-h fasting was partly owing to the enhancement of FOXO1 expression as well as the suppression of NLRP3, both of which were induced by the up-regulation of serum BHB, followed by the suppression of HDAC activity.
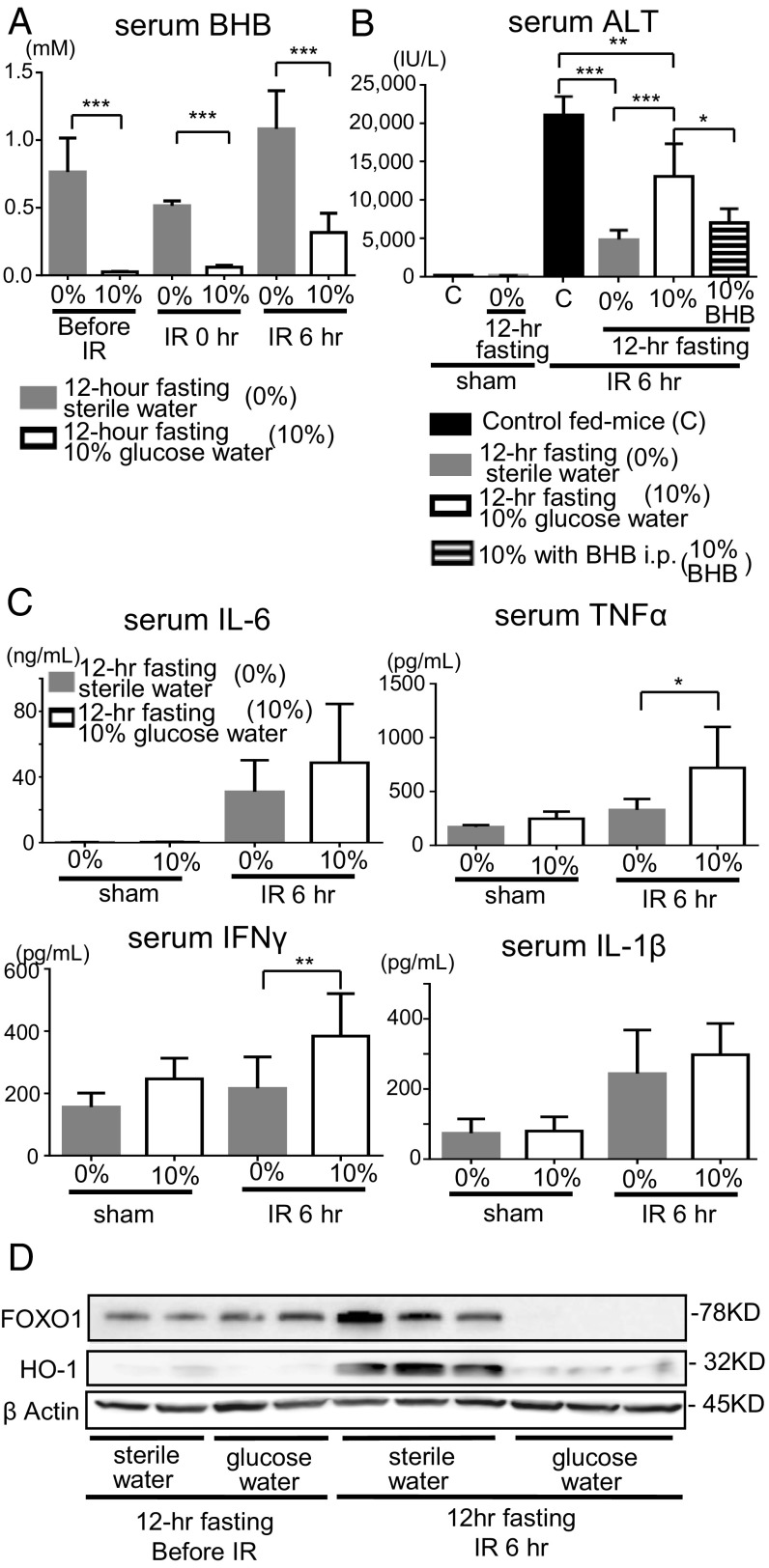
Addition of Glucose to the Drinking Water Reversed the Preventive Effects on Liver IRI of the 12-h Fasting Group.
When glycolysis converts glucose to pyruvate, BHB is not produced in the animal body. We examined the effect of 10% glucose water on liver IRI in the 12-h fasting group (SI Appendix, Fig. S1B). The serum BHB level clearly increased during ischemia and reperfusion in the 12-h fasting group that was given only water but without glucose (Fig. 4A). However, in mice of the 12-h fasting group that was given 10% glucose water, the serum BHB level was strongly lowered (Fig. 6A). Amelioration of liver injury (measured by the elevation of sALT) was cancelled in mice fed with 10% glucose water (Fig. 6B). The i.p. administration of BHB inversely showed the protective effect against liver IRI in the mice of the 12-h fasting given water containing 10% glucose (Fig. 6B). Serum levels of IFNγ and TNFα at 6 h of reperfusion were significantly elevated in 12-h fasting mice given water containing 10% glucose (Fig. 6C). Increased expressions of FOXO1 and HO-1 in the 12-h fasting group at 6 h of reperfusion were markedly suppressed by the feeding with water containing 10% glucose (Fig. 6D).
Fig. 6.
Addition of glucose to drinking water reversed the preventive effects of the 12-h fast on liver IRI. (A) Serum BHB levels were measured in 12-h fasting mice fed with water only or 10% glucose water. Means and SD are shown (n = 8 mice per group; two-way ANOVA; P < 0.0001, Bonferroni’s posttest: ***P < 0.001 vs. 12-h fasting without glucose). (B) The sALT levels were measured in the control fed mice, 12-h fasting mice, and 12-h fasting mice fed with 10% glucose water. Means and SD are shown (n = 8 mice per group; *P < 0.05; **P < 0.01; ***P < 0.001). (C) Serum levels of proinflammatory cytokines (IL-6, TNFα, IL-1β, and IFNγ) were measured. Means and SD are shown (sham, n = 4; IR 6 h, n = 8 mice per group; *P < 0.05; **P < 0.01). (D) Western blot-assisted analyses of FOXO1 and HO-1.
Discussion
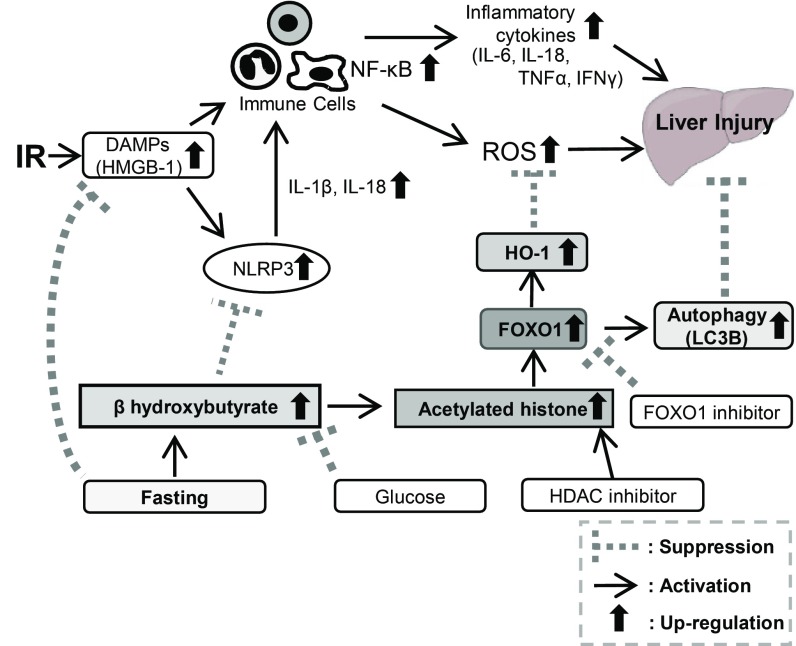
Long-term diet restriction without malnutrition improved stress resistance and extended lifespan (41); however, the underlying nutritional and molecular mechanisms remain unclear. It was shown that fasting for 48 h to 72 h reduced IR-induced liver damage via the up-regulation of antioxidative enzymes (20). Furthermore, 24-h fasting but not 48-h or 72-h fasting prevented liver IRI through Sirtuin1-mediated down-regulation of circulating HMGB1 (22). However, long-term diet restriction of more than 24 h might prove a big burden for the animal and may be difficult to apply for human preoperative management. Therefore, it is important to resolve the underlying mechanisms. The present study showed that 12-h fasting achieves a remarkable amelioration of liver IRI through 1) the marked reduction of serum HMGB1, resulting in the suppression of inflammation and prevention of liver cell injury, and 2) the enhanced expression of BHB, followed by the suppression of inflammasome as well as the increased expressions of acetylated histone, FOXO1, and HO-1, which prevented liver injury by the enhancement of autophagy and antioxidant activity (Fig. 7).
Fig. 7.
Schematic summary of the present study.
In liver IRI, ROS and proinflammatory cytokines such as IL-1β, IL-6, TNFα, and IFNγ are released from the infiltrated immune inflammatory cells, such as macrophages, lymphocytes, and neutrophils, and cause liver injury by inducing hepatocyte apoptosis and necrosis (1, 5). NF-κB is activated following TLR-4 signaling and is crucial for regulating inflammations. HMGB1, an endogenous ligand of TLR-4, thus induces NF-κB activation and liver IRI deterioration (8). The 24-h fasting reportedly suppresses liver IRI via Sirtuin1-mediated down-regulation of HMGB1 translocation (22). In the 12-h fasting group, the serum HMGB1 levels start increasing after ischemia and peak in the early phase of reperfusion. On the other hand, the HMGB1 levels were significantly suppressed immediately following ischemia and further throughout the reperfusion phase (Fig. 2A). NF-κB activation was suppressed from the early phase of reperfusion in conjunction with HMGB1 down-regulation (Fig. 3A). The serum liver-derived enzyme, ALT, was also strongly suppressed from the early stage of reperfusion in the 12-h fasting group (Fig. 1A), suggesting that suppression of HMGB1 secretion from the ischemia phase is crucial for preventing IR insult.
In the previous reports, overexpression of HO-1 has a protective effect against liver IRI (42). HO-1 transcription is regulated by multiple signaling pathways such as MAPK, STAT3, and nuclear factor erythroid 2-related factor 2 (43, 44). FOXO1 transcriptional factor is also known to up-regulate antioxidative enzymes, such as HO-1 (32). Before ischemic insults, FOXO1 and HO-1 were already increased (Fig. 3A). During reperfusion (at 1 h to 6 h), FOXO1 and HO-1 expressions were remarkably increased in the 12-h fasting group, but remained lower in the control fed mice (Fig. 3A).
The NLRP3 inflammasome-mediated cell pyroptosis promotes HMGB1 secretion (45), and HMGB1 release is partly dependent on NLRP3 inflammasome (46). The NLRP3 expression in the 12-h fasting mice was significantly lowered before the ischemic phase (Fig. 3A), suggesting that the down-regulation of the NLRP3 inflammasome may induce suppression of HMGB1 at the early phase of IR in the 12-h fasting group. Thus, the enhanced expression of FOXO1 and HO-1, with reduced expression of NLRP3, may suppress HMGB1 release during ischemia throughout reperfusion in the 12-h fasting group.
It has been shown that the administration of BHB protected various organs from IRI (47, 48); however, the underlying molecular mechanisms are unclear. Recently, BHB has been reported to exert an inhibitory activity against NLRP3 inflammasome and prevent NLRP3-mediated inflammatory diseases (39). Activated NLRP3 inflammasome induces IL-1β and IL-18, and innate immune responses (49, 50), and promotes the maturation and secretion of proinflammatory cytokines from immune cells (28). Serum BHB levels were higher in the 12-h fasting group even before ischemia and during reperfusion (3 h to 12 h) (Fig. 4A). In contrast, the liver expressions of NLRP3 were suppressed during ischemia (before reperfusion) and at 1 h after reperfusion (Fig. 3A). Serum IL-1β and IL-18 levels were significantly lower during reperfusion (3 h to 6 h) in the 12-h fasting group (Fig. 2B). Thus, the enhanced expression of BHB may reduce inflammation by suppressing NLRP3 inflammasome. Exogenous BHB also suppressed the expression of NLRP3 at 1 h of reperfusion (Fig. 4F), and serum IL-1β levels at 6 h after reperfusion were also significantly suppressed (Fig. 4D). These results indicate a crucial role of BHB in suppressing liver inflammation and ameliorating liver IRI. BHB also causes the reduction of serum HMGB1 through the suppression of NLRP3 in the 12-h fasting group.
The serum BHB is known to increase during prolonged exercise or starvation (6 mM to 8 mM) and diabetic ketoacidosis (>25 mM) (51). BHB is known to display an endogenous HDAC inhibitory activity from a low concentration level (1 mM) (37). The expression of FOXO1 is reportedly up-regulated through the increment in acetylated histone (36, 38). The serum BHB concentrations in the 12-h fasting mice were 0.8 mM to 1.7 mM during reperfusion, much higher than in the control fed mice (Fig. 4A). Expressions of acetylated histone-3 were up-regulated in the 12-h fasting group (Fig. 3A). These data indicate that the up-regulation of FOXO1 was induced through the increment of acetylated histone-3, which was accelerated by increased BHB. Exogenously administered BHB or HDACi also induced acetylated histone-3, leading to the up-regulation of FOXO1 and HO-1 (Figs. 4F and 5C). Thus, BHB-mediated enhancement of FOXO1 and then HO-1 are crucial events for the amelioration of IRI in the 12-h fasting group.
Autophagy is an intracellular self-digesting pathway responsible for removing long-lived proteins, damaged organelles, and malformed proteins. Autophagy exerts a protective effect against cell apoptosis (52). The inhibition of autophagy reportedly exacerbates liver IRI (52–54). Autophagy is regulated by FOXO1 and HO-1 (33). The expression of LC3B, an autophagosome membrane marker, was up-regulated in the 12-h fasting group (Fig. 3A). The up-regulation of autophagy induced by 12-h fasting through the increased expression of FOXO1 may also play an important role in suppression of liver IRI.
FOXO1 is regulated not only through acetylation but also through phosphorylation (55). The activation of FOXO1 is reported to be primarily regulated through phosphorylation by the insulin−Phosphoinositide 3-kinase (PI3K)−Akt pathway (55). Activated Akt (p-Akt) inhibits the activity of FOXO1 transcription factors via phosphorylation, resulting in nuclear exclusion and ubiquitination (56). Serum insulin levels were lowered before and during ischemia in the 12-h fasting group, suggesting that the serum insulin level was slightly affected before and during ischemia, due to 12-h fasting. The insulin level clearly increased at 3 h of reperfusion; however, no significant difference was observed between the 12-h fasting group and the control fed group (SI Appendix, Fig. S3A). The expression of p-Akt was significantly increased at 1 h of reperfusion in the control fed mice; however, this was not observed in the 12-h fasting group (SI Appendix, Fig. S3C). Although p-Akt was increased after reperfusion, the expression of phosphorylated FOXO1 (p-FOXO1) remained unchanged (Fig. 3A). In sum, these results suggest that the up-regulation of FOXO1 observed in the 12-h fasted mice may not be directly regulated by the insulin−PI3K−Akt pathway.
The activity of FOXO1 is reportedly regulated by the AMP-activated protein kinase (AMPK) pathway in response to nutrient deprivation (57). Activated AMPKα (p-AMPKα) directly phosphorylates FOXO1 at six regulatory residues and enhances FOXO1 transcriptional activity (57). There was no difference in tissue adenosine triphosphate (ATP) levels in both the control fed group and the 12-h fasting group before ischemia and even during reperfusion. However, at 6 h of reperfusion, the ATP level was significantly increased in the 12-h fasting group (SI Appendix, Fig. S3B). Although tissue ATP levels were sustained, the expressions of p-AMPKα were significantly higher in the 12-h fasting group during the early phase of reperfusion (∼3 h); however, there was no change at 6 h of reperfusion (SI Appendix, Fig. S3C). These results suggest the possibility that the levels of tissue ATP and p-AMPKα may have contributed to the amelioration of IRI in the 12-h fasting group via the up-regulation of FOXO1 activity.
Deacetylation also occurs in FOXO1 proteins. Sirtuin1 deacetylates FOXO1 and enhances FOXO1 transcriptional activity (58). The expression of Sirtuin1 was increased during ischemia in both the control fed group and the 12-h fasting group. Although Sirtuin1 was significantly higher in the 12-h fasted group at 3 h of reperfusion than in the control fed group (SI Appendix, Fig. S3C), the increment in Sirtuin1 occurred later than the up-regulation of FOXO1. Sirtuin1 might affect the increment in FOXO1 as well as HO-1 during the late phase of reperfusion and plays a role in the fasting-induced amelioration of IRI.
The present study showed that the preventive effect of fasting against liver IRI could be induced more rapidly by 12-h fasting. Higher level of serum BHB was quickly and efficiently induced by the 12-h fast, and the enhanced BHB increased FOXO1. Antioxidative enzyme HO-1 and autophagy activity were also enhanced through FOXO1 up-regulation. Furthermore, BHB inhibited the NLRP3 inflammasome activity during early reperfusion. HMGB1 release was suppressed by the 12-h fasting at the early stage of IR insult, leading to NF-κB inactivation.
Taken together, the 12-h fasting helped suppress inflammatory responses by enhancing the BHB expression, followed by the up-regulation of acetylated histone-3 and the activation of FOXO1 and HO-1, together with the consequence of the reduced expression of HMGB1 and the inactivation of NF-κB and NLRP3. These changes resulted in a protective effect against hepatocyte apoptosis and necrosis caused by IRI.
Materials and Methods
Animals.
Male C57BL/6 mice (8 wk, 22 g to 25 g weight) were purchased from Shimizu Laboratory Supplies 7 d before operation. Mice were housed (four mice per cage) in individually ventilated cages (TECNIPLAST S.p.A.), kept under constant environmental conditions with a 12-h light−dark cycle (light 8:00 AM to 8:00 PM), and maintained under specific pathogen-free conditions. All mice were bred with standard rodent breeding chow (CA-1, CLEA Japan, Inc.) and sterile water ad libitum unless otherwise indicated, and received human care as per the Guide for Care and Use of Laboratory Animals (National Institute of Health Publication, eighth edition, 2011).
Reagents.
DL-BHB was purchased from Sigma-Aldrich Co. LLC., AS1842856 (FOXO1 inhibitor) (59) was purchased from Merck Millipore, and Trichostatin A (HDAC inhibitor) (40) was purchased from Tokyo Chemical Industry Co., Ltd.
Liver IRI Model.
An established mouse model of partial warm hepatic IRI was used (10–13, 15, 18, 60). Surgical manipulation was performed from 8:30 AM. The mice were anesthetized under isoflurane (2 to 2.5%) and injected with heparin (100 U/kg). An atraumatic clip was used to interrupt the artery and portal venous supply and bile duct to the left and middle liver lobes. This method prevents mesenteric venous congestion by permitting portal decompression through the right and caudate lobes. After 60 min of ischemia, the clamp was removed, and reperfusion was initiated. The mice were killed at different time points (SI Appendix, Fig. S1A). Liver samples were immediately dissected, mounted in optimal cutting temperature embedding compound, frozen at −80 °C, fixed overnight in 10% formaldehyde or frozen in liquid nitrogen, and reserved at −80 °C until extraction. Sham-operated mice underwent the same procedure without vascular occlusion.
The mice were divided into groups (SI Appendix, Fig. S1B). The control fed group was provided food and sterile water ad libitum, while the fasting group was deprived of food but given free access to water for 12 h before the IR treatment. In the cases where the fasting group was fed 10% glucose water, sterile water was exchanged with 10% glucose water at the start of fasting.
The mice received i.p. administration of AS1842856 (FOXO1 inhibitor, 20 mg/kg) in 40 μL of dimethyl sulfoxide (DMSO) or 40 μL DMSO alone at 36 h and 12 h before ischemia (SI Appendix, Fig. S1C). The dose and usage of AS1842856 were determined based on a previous report (61). The control fed mice received i.p. administration of Trichostatin A (HDAC inhibitor, 1 mg/kg) in 0.2 mL of 20% DMSO in PBS or 0.2 mL of 20% DMSO in PBS alone 16 h before ischemia and just before ischemia (SI Appendix, Fig. S1D). The dose and route of administration of Trichostatin A were determined based on a previous report (62). The control fed mice received i.p. administration of BHB (10 mmoL/kg) in 0.5 mL of PBS or 0.5 mL of PBS alone 30 min before ischemia (SI Appendix, Fig. S1E). The dose and route of administration of BHB were determined based on a previous report (47).
In each experiment, both groups comprised eight mice. Experimental protocols were approved by the Animal Research Committee of The Tazuke Kofukai Medical Research Institute, Kitano Hospital.
Analysis of Blood Samples.
The sALT level, an indicator of hepatocellular injury, was measured using a standard spectrophotometric method with an automated clinical analyzer (JCA-BM9030; JEOL Ltd.). Serum BHB was measured using an enzymatic method (ORIENTAL YEAST Co., Ltd.).
ELISA.
The serum HMGB1 was quantified with a HMGB1 ELISA Kit II (Shino-Test). Serum insulin was measured using Ultra-sensitive Mouse/Rat Insulin ELISA kit (Morinaga Institute of Biological Science, Inc.). Serum IL-18 was measured using the Mouse IL-18 ELISA Kit (Abcam).
Measurement of Serum Cytokine.
Serum levels of IL-6, TNFα, IFNγ, and IL-1β were measured using the Luminex multiplex cytokine analysis kit (Bio-plex Assay Kit; Bio-Rad).
Histology.
After overnight fixation in 10% formaldehyde, the liver was stored in 70% ethanol until paraffin embedding. Liver paraffin sections (5-μm thick) were stained with hematoxylin and eosin (H & E). Severity of liver damage due to IR was blindly graded using modified Suzuki’s criteria (Congestion, Vacuolization, and Necrosis) on a scale from 0 to 4 (63).
Immunohistochemistry for Detecting of CD3, CD68, Ly-6G, FOXO1, and 4-HNE.
To detect CD3, Ly-6G, CD68, and FOXO1, antigen retrieval (Citrate, pH 6) was performed on paraffin-embedded sections of liver. To detect 4-HNE, liver frozen sections were fixed with cold acetone. After blocking, the sections were incubated in primary antibody (SI Appendix, Table S1) overnight at 4 °C. Then, biotinylated rabbit anti-rat IgG or goat anti-rabbit IgG were applied. After incubation, immunoperoxidase (VECTASTAIN ABC Kit; Vector Labs) was applied to the sections and developed using 3,3′-diaminobenzidine (DAB). Negative controls were prepared by incubation with normal rat IgG instead of the first Ab.
Immunofluorescence Staining for Detecting FOXO1.
Liver frozen sections were fixed with cold acetone and incubated in 0.3% (vol/vol) polyethylene glycol monop-isooctylphenyl ether in PBS. Thereafter, Protein Block Serum Free (Dako) was applied. Rabbit mAbs against FOXO1 (Novus Biologicals) were applied on sections and incubated for one night at 4 °C. Thereafter, Alexa Fluor 488-labeled goat anti-rabbit IgG (Abcam) was applied, and incubated for 1 h. After staining with DAPI, the sections were examined using fluorescence microscope (EVOS FL Auto; Invitrogen).
Measurement of Tissue ATP Concentrations.
Liver tissue samples were frozen in liquid nitrogen and preserved at −80 °C until analyses. Liver tissues were homogenized in 10 volumes of 0.25 mol/L sucrose in 10 mmol/L Hepes-NaOH buffer (pH 7.4). The extracts were cleared via centrifugation, and amounts of protein were estimated using the BCA protein assay kit (Pierce Biotechnology, Inc.). Tissue ATP concentrations were determined using the luciferin−luciferase method with Luminescent ATP Detection Assay Kit (Abcam).
Western Blotting Assay.
Proteins extracted from the liver (20 μg per sample) were subjected to sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis and transferred to the polyvinylidene difluoride membrane (Bio-Rad). Thereafter, the membrane was incubated in blocking buffer [5% skim milk in Tris-buffered saline with polyoxyethylene sorbitan monolaurate (TBS-T)] for 1 h at room temperature. After blocking, the membrane was incubated in unconjugated primary antibody (SI Appendix, Table S2) in the dilution buffer (2.5% skim milk in TBS-T) with overnight agitation at 4 °C. Then, the membrane was incubated in horseradish peroxidase-linked anti-rabbit IgG antibody (CST) in the dilution buffer with gentle agitation for 1 h at room temperature. Enhanced Chemi Luminescence (ECL prime; Amersham) and Lumino image analyzer (Image Quant LAS 4000; GE Healthcare) were used to detect each molecule. The intensity of the bands was quantified using Image J software (National Institutes of Health).
Apoptosis Assay.
Apoptosis in 5-µm-thick liver paraffin sections was detected with TUNEL performed with the In Situ Apoptosis Detection Kit (TAKARA BIO) as per the manufacturer’s protocol. Positive cells were counted blindly at 10 high power field (HPF)/section (magnification 400×).
Quantitative Reverse-Transcription PCR.
Total RNA was extracted from liver tissue using the NucleoSpin RNA (Takara Bio). cDNA was prepared using the Prime Script RT reagent Kit (Takara Bio). Quantitative PCR was performed using the StepOnePlusTM Real-Time PCR System (Life Technologies). Primers used to amplify specific gene fragments are listed in SI Appendix, Table S3. Target gene expression was calculated by the ratio to the housekeeping gene hypoxanthine phosphoribosyl transferase (HPRT).
Statistical Analyses.
Data are expressed as mean ± SD values. Differences between the experimental groups were analyzed using the Student’s t tests; two-way repeated-measures analysis of variance (ANOVA) with Bonferroni’s posttest was used to assess time-dependent changes and differences between the groups at each time point. P values of <0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank Marika Hirao for her technical assistance and Koichi Hirano for providing his animal care. This study was supported by Grant-in-Aid for Scientific Research 18K08609 (to H.T.), 15K10177 (to Y.U.), and 15K10041 (to H.T.) from the Ministry of Education, Culture, Science, and Sports, Japan; and by Kitano Research Grant and Translational Medical Research Project from The Tazuke Kofukai Medical Research Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. V.D.D. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1820282116/-/DCSupplemental.
References
- 1.Eltzschig H. K., Eckle T., Ischemia and reperfusion–From mechanism to translation. Nat. Med. 17, 1391–1401 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serracino-Inglott F., Habib N. A., Mathie R. T., Hepatic ischemia-reperfusion injury. Am. J. Surg. 181, 160–166 (2001). [DOI] [PubMed] [Google Scholar]
- 3.Dünschede F., et al. , Reduction of ischemia reperfusion injury after liver resection and hepatic inflow occlusion by α-lipoic acid in humans. World J. Gastroenterol. 12, 6812–6817 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farmer D. G., Amersi F., Kupiec-Weglinski J., Busuttil R. W., Current status of ischemia and reperfusion injury in the liver. Transplant. Rev. 14, 106–126 (2000). [Google Scholar]
- 5.Klune J. R., Tsung A., Molecular biology of liver ischemia/reperfusion injury: Established mechanisms and recent advancements. Surg. Clin. North Am. 90, 665–677 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Suetsugu H., et al. , Nuclear factor κB inactivation in the rat liver ameliorates short term total warm ischaemia/reperfusion injury. Gut 54, 835–842 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akira S., Takeda K., Kaisho T., Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat. Immunol. 2, 675–680 (2001). [DOI] [PubMed] [Google Scholar]
- 8.Tsung A., et al. , The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J. Exp. Med. 201, 1135–1143 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiemer A. K., Vollmar A. M., Bilzer M., Gerwig T., Gerbes A. L., Atrial natriuretic peptide reduces expression of TNF-α mRNA during reperfusion of the rat liver upon decreased activation of NF-kappaB and AP-1. J. Hepatol. 33, 236–246 (2000). [DOI] [PubMed] [Google Scholar]
- 10.Uchida Y., Freitas M. C., Zhao D., Busuttil R. W., Kupiec-Weglinski J. W., The inhibition of neutrophil elastase ameliorates mouse liver damage due to ischemia and reperfusion. Liver Transpl. 15, 939–947 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uchida Y., Freitas M. C., Zhao D., Busuttil R. W., Kupiec-Weglinski J. W., The protective function of neutrophil elastase inhibitor in liver ischemia/reperfusion injury. Transplantation 89, 1050–1056 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kadono K., et al. , Thrombomodulin attenuates inflammatory damage due to liver ischemia and reperfusion injury in mice in toll-like receptor 4-dependent manner. Am. J. Transplant. 17, 69–80 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Uchida Y., et al. , T-cell immunoglobulin mucin-3 determines severity of liver ischemia/reperfusion injury in mice in a TLR4-dependent manner. Gastroenterology 139, 2195–2206 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji H., et al. , Programmed death-1/B7-H1 negative costimulation protects mouse liver against ischemia and reperfusion injury. Hepatology 52, 1380–1389 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirao H., et al. , The protective function of galectin-9 in liver ischemia and reperfusion injury in mice. Liver Transpl. 21, 969–981 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zitvogel L., Pietrocola F., Kroemer G., Nutrition, inflammation and cancer. Nat. Immunol. 18, 843–850 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Hine C., et al. , Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell 160, 132–144 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyauchi T., et al. , Preventive effect of antioxidative nutrient-rich enteral diet against liver ischemia and reperfusion injury. JPEN J. Parenter. Enteral Nutr. 43, 133–144 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Mitchell J. R., et al. , Short-term dietary restriction and fasting precondition against ischemia reperfusion injury in mice. Aging Cell 9, 40–53 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verweij M., et al. , Preoperative fasting protects mice against hepatic ischemia/reperfusion injury: Mechanisms and effects on liver regeneration. Liver Transpl. 17, 695–704 (2011). [DOI] [PubMed] [Google Scholar]
- 21.Qin J., et al. , Short-term starvation attenuates liver ischemia-reperfusion injury (IRI) by Sirt1-autophagy signaling in mice. Am. J. Transl. Res. 8, 3364–3375 (2016). [PMC free article] [PubMed] [Google Scholar]
- 22.Rickenbacher A., et al. , Fasting protects liver from ischemic injury through Sirt1-mediated downregulation of circulating HMGB1 in mice. J. Hepatol. 61, 301–308 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Sternberg E. M., Neural regulation of innate immunity: A coordinated nonspecific host response to pathogens. Nat. Rev. Immunol. 6, 318–328 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Q., et al. , Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464, 104–107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sosa R. A., et al. , Early cytokine signatures of ischemia/reperfusion injury in human orthotopic liver transplantation. JCI Insight 1, e89679 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perkins N. D., Integrating cell-signalling pathways with NF-κB and IKK function. Nat. Rev. Mol. Cell Biol. 8, 49–62 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Hayden M. S., West A. P., Ghosh S., NF-κB and the immune response. Oncogene 25, 6758–6780 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Karin M., Ben-Neriah Y., Phosphorylation meets ubiquitination: The control of NF-κB activity. Annu. Rev. Immunol. 18, 621–663 (2000). [DOI] [PubMed] [Google Scholar]
- 29.Próchnicki T., Latz E., Inflammasomes on the crossroads of innate immune recognition and metabolic control. Cell Metab. 26, 71–93 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Puigserver P., et al. , Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1α interaction. Nature 423, 550–555 (2003). [DOI] [PubMed] [Google Scholar]
- 31.Matsumoto M., Han S., Kitamura T., Accili D., Dual role of transcription factor FoxO1 in controlling hepatic insulin sensitivity and lipid metabolism. J. Clin. Invest. 116, 2464–2472 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu X., et al. , Cobalt protoporphyrin induces HO-1 expression mediated partially by FOXO1 and reduces mitochondria-derived reactive oxygen species production. PLoS One 8, e80521 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sengupta A., Molkentin J. D., Yutzey K. E., FoxO transcription factors promote autophagy in cardiomyocytes. J. Biol. Chem. 284, 28319–28331 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Esterbauer H., Schaur R. J., Zollner H., Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 11, 81–128 (1991). [DOI] [PubMed] [Google Scholar]
- 35.Lin A.-L., Zhang W., Gao X., Watts L., Caloric restriction increases ketone bodies metabolism and preserves blood flow in aging brain. Neurobiol. Aging 36, 2296–2303 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mihaylova M. M., et al. , Class IIa histone deacetylases are hormone-activated regulators of FOXO and mammalian glucose homeostasis. Cell 145, 607–621 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimazu T., et al. , Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 339, 211–214 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J., et al. , Histone deacetylase inhibitors induce autophagy through FOXO1-dependent pathways. Autophagy 11, 629–642 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Youm Y. H., et al. , The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 21, 263–269 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vanhaecke T., Papeleu P., Elaut G., Rogiers V., Trichostatin A-like hydroxamate histone deacetylase inhibitors as therapeutic agents: Toxicological point of view. Curr. Med. Chem. 11, 1629–1643 (2004). [DOI] [PubMed] [Google Scholar]
- 41.Fontana L., Partridge L., Promoting health and longevity through diet: From model organisms to humans. Cell 161, 106–118 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ke B., et al. , Small interfering RNA targeting heme oxygenase-1 (HO-1) reinforces liver apoptosis induced by ischemia-reperfusion injury in mice: HO-1 is necessary for cytoprotection. Hum. Gene Ther. 20, 1133–1142 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang J., et al. , Adoptive transfer of heme oxygenase-1 (HO-1)-modified macrophages rescues the nuclear factor erythroid 2-related factor (Nrf2) antiinflammatory phenotype in liver ischemia/reperfusion injury. Mol. Med. 20, 448–455 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryter S. W., Otterbein L. E., Morse D., Choi A. M., Heme oxygenase/carbon monoxide signaling pathways: Regulation and functional significance. Mol. Cell. Biochem. 234–235, 249–263 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chu J., Alber S., Watkins S., Núñez G., Salter R., NLRP3 inflammasome-dependent HMGB1 release induced by a bacterial pore-forming toxin (136.15). J. Immunol. 184 (suppl. 1), 136.15 (2010). [Google Scholar]
- 46.Hou L., et al. , NLRP3/ASC-mediated alveolar macrophage pyroptosis enhances HMGB1 secretion in acute lung injury induced by cardiopulmonary bypass. Lab. Invest. 98, 1052–1064 (2018). [DOI] [PubMed] [Google Scholar]
- 47.Eiger S. M., Kirsch J. R., D’Alecy L. G., Hypoxic tolerance enhanced by β-hydroxybutyrate-glucagon in the mouse. Stroke 11, 513–517 (1980). [DOI] [PubMed] [Google Scholar]
- 48.Suzuki M., et al. , β-hydroxybutyrate, a cerebral function improving agent, protects rat brain against ischemic damage caused by permanent and transient focal cerebral ischemia. Jpn. J. Pharmacol. 89, 36–43 (2002). [DOI] [PubMed] [Google Scholar]
- 49.Ghiringhelli F., et al. , Activation of the NLRP3 inflammasome in dendritic cells induces IL-1β-dependent adaptive immunity against tumors. Nat. Med. 15, 1170–1178 (2009). [DOI] [PubMed] [Google Scholar]
- 50.Ketelut-Carneiro N., et al. , IL-18 triggered by the Nlrp3 inflammasome induces host innate resistance in a pulmonary model of fungal infection. J. Immunol. 194, 4507–4517 (2015). [DOI] [PubMed] [Google Scholar]
- 51.Stephens J. M., Sulway M. J., Watkins P. J., Relationship of blood acetoacetate and 3-hydroxybutyrate in diabetes. Diabetes 20, 485–489 (1971). [DOI] [PubMed] [Google Scholar]
- 52.Choi A. M., Ryter S. W., Levine B., Autophagy in human health and disease. N. Engl. J. Med. 368, 651–662 (2013). [DOI] [PubMed] [Google Scholar]
- 53.Nakamura K., et al. , Heme oxygenase-1 regulates sirtuin-1-autophagy pathway in liver transplantation: From mouse to human. Am. J. Transplant. 18, 1110–1121 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang J. H., et al. , Autophagy suppresses age-dependent ischemia and reperfusion injury in livers of mice. Gastroenterology 141, 2188–2199.e6 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Daitoku H., Sakamaki J., Fukamizu A., Regulation of FoxO transcription factors by acetylation and protein-protein interactions. Biochim. Biophys. Acta 1813, 1954–1960 (2011). [DOI] [PubMed] [Google Scholar]
- 56.Matsuzaki H., Daitoku H., Hatta M., Tanaka K., Fukamizu A., Insulin-induced phosphorylation of FKHR (Foxo1) targets to proteasomal degradation. Proc. Natl. Acad. Sci. U.S.A. 100, 11285–11290 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Greer E. L., et al. , An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr. Biol. 17, 1646–1656 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hariharan N., et al. , Deacetylation of FoxO by Sirt1 plays an essential role in mediating starvation-induced autophagy in cardiac myocytes. Circ. Res. 107, 1470–1482 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nagashima T., et al. , Discovery of novel forkhead box O1 inhibitors for treating type 2 diabetes: Improvement of fasting glycemia in diabetic db/db mice. Mol. Pharmacol. 78, 961–970 (2010). [DOI] [PubMed] [Google Scholar]
- 60.Uchida Y., et al. , The emerging role of T cell immunoglobulin mucin-1 in the mechanism of liver ischemia and reperfusion injury in the mouse. Hepatology 51, 1363–1372 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chung S., et al. , FoxO1 regulates allergic asthmatic inflammation through regulating polarization of the macrophage inflammatory phenotype. Oncotarget 7, 17532–17546 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Levine M. H., et al. , Class-specific histone/protein deacetylase inhibition protects against renal ischemia reperfusion injury and fibrosis formation. Am. J. Transplant. 15, 965–973 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Suzuki S., Toledo-Pereyra L. H., Rodriguez F. J., Cejalvo D., Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation 55, 1265–1272 (1993). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.