Significance
The specific activation of B lymphocytes via the binding of antigen to their B cell antigen receptor (BCR) is of central importance for the establishment of humoral immunity and a successful vaccination. A better understanding of the antigen sensing process of B cells requires insight into the structure of the BCR comprising the mIg molecule and the Igα/Igβ heterodimer in a 1:1 complex. How a symmetric molecule such as the mIg molecule is asymmetrically associated with only one Igα/Igβ heterodimer has been a puzzle. We suggest that inside the lipid bilayer the BCR forms a symmetric Igα-mHC:mHC-Igβ complex. Our results give insight into the BCR structure and the B cell activation mechanism.
Keywords: B cell antigen receptor, assembly, ER retention, symmetry
Abstract
B lymphocytes have the ability to sense thousands of structurally different antigens and produce cognate antibodies against these molecules. For this they carry on their surface multiple copies of the B cell antigen receptor (BCR) comprising the membrane-bound Ig (mIg) molecule and the Igα/Igβ heterodimer functioning as antigen binding and signal transducing components, respectively. The mIg is a symmetric complex of 2 identical membrane-bound heavy chains (mHC) and 2 identical light chains. How the symmetric mIg molecule is asymmetrically associated with only one Igα/Igβ heterodimer has been a puzzle. Here we describe that Igα and Igβ both carry on one side of their α-helical transmembrane domain a conserved amino acid motif. By a mutational analysis in combination with a BCR rebuilding approach, we show that this motif is required for the retention of unassembled Igα or Igβ molecules inside the endoplasmic reticulum and the binding of the Igα/Igβ heterodimer to the mIg molecule. We suggest that the BCR forms within the lipid bilayer of the membrane a symmetric Igα-mHC:mHC-Igβ complex that is stabilized by an aromatic proline-tyrosine interaction. Outside the membrane this symmetry is broken by the disulfide-bridged dimerization of the extracellular Ig domains of Igα and Igβ. However, symmetry of the receptor can be regained by a dimerization of 2 BCR complexes as suggested by the dissociation activation model.
The B cell antigen receptor (BCR) plays a key role for the clonal selection of B cells. It can bind to self or nonself antigens and translate this binding into cellular signals resulting in B lymphocyte deletion or activation (1). To fulfill its antigen sensing function, the BCR comprises antigen binding and signal transducing components. These are the membrane-bound Ig (mIg) molecule and an Igα/Igβ heterodimer (also known as CD79a/CD79b), respectively (2). The mIg molecule consists of 2 membrane-bound heavy chains (mHC) and 2 light chains (LC). It has a symmetric structure that is stabilized by interchain disulfide bonds. The BCR signaling components, Igα and Igβ, each have a similar structure consisting of an extracellular Ig domain, a short linker, a conserved transmembrane (TM) domain, and a cytoplasmic tail carrying an immunoreceptor tyrosine-based activation motif (ITAM) that connects the BCR to the protein tyrosine kinase Syk (2, 3). Igα and Igβ are covalently linked to each other by a disulfide bond that connects the 2 Ig domains of these proteins (3, 4). In contrast, the Igα/Igβ heterodimer is noncovalently associated with the mIg molecule involving specific contact sites in the membrane-proximal C-domain and TM region of mIg (5). As is the case for most type I transmembrane proteins, the TM regions of Igα, Igβ, and mHC are thought to form an α-helix within the lipid bilayer of the plasma membrane and α-helical TM interactions seem to be important for the stability of the BCR complex as well as for the oligomeric structure that the BCR forms on the surface of resting B lymphocytes (6, 7).
B cells can produce different classes of antibody (IgA, IgD, IgE, IgG, and IgM) and mIg molecules. The different classes of mIg are associated with the same Igα/Igβ heterodimer to express isotype-specific BCRs on the B cell surface (6). A sequence comparison of the α-helical TM region of different mIg isotypes revealed 2 evolutionary conserved sides. One side (TM-S) is specific for each isotype and the other side (TM-C) is conserved between isotypes (7, 8). While the TM-S side is expected to be involved in the formation of an mHC:mHC homodimer as well as the BCR oligomer, the TM-C side is implicated in the binding to the common Igα/Igβ heterodimer (7). Indeed, mutations of the highly conserved tyrosine Y18 and serine S19 (numbered from the start of the TM region) that are both located on the TM-C side prevent the binding of the Igα/Igβ heterodimer to the mIgM molecule (9, 10).
The 4 BCR components are assembled in the endoplasmic reticulum (ER) and then transported together onto the cell surface. Nonassembled BCR components are recognized by a quality control system and retained inside the ER (2, 6, 11). Thus, a deficiency in any BCR component prevents the transport and expression of the remaining components on the cell surface. Molecular requirement for BCR assembly and signaling can be studied by a rebuilding approach using Drosophila S2 cells that display a high cotransfection rate. With this system, we provided evidence for the oligomeric structure of the BCR and studied the role of Syk in BCR opening and amplification of the BCR signal (12–15).
Originally, it was thought that, similar to the T cell antigen receptor, the BCR forms a symmetric complex with an Igα/Igβ heterodimer binding to each site of the mHC:mHC homodimer (7). However, this 1:2 mIg:Igα/Igβ interaction model was abandoned after a biochemical study demonstrated that a digitonin solubilized BCR complex contains only one Igα/Igβ heterodimer per mIg molecule (16). This 1:1 interaction model was further confirmed by a quantification of fluorescence-labeled BCR components (17). How one Igα/Igβ heterodimer is asymmetrically interacting with the symmetric mIg molecule remained a puzzle of the 1:1 interaction model. We here use the S2 rebuilding approach to study the interaction of wild-type (wt) and mutant TM regions of Igα and Igβ with the mIg molecule. We find that the formation of symmetric Igα-mHC:mHC-Igβ complex within the membrane is required for the stable expression of the BCR on the cell surface, thus confirming the 1:1 interaction as well as the oligomeric BCR model.
Results
Retention of Igα in the ER via a Conserved Amino Acid Motif in the TM Region.
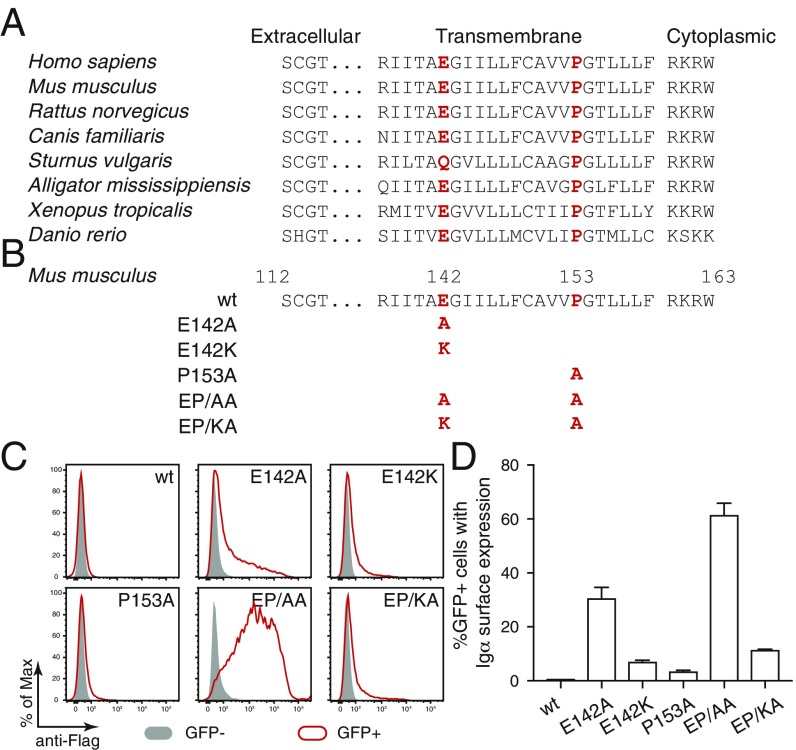
The sequence of the TM region of Igα is highly conserved during evolution (Fig. 1A). Interestingly, this sequence contains 2 amino acids (aa), namely glutamic acid E142 and proline P153, that, in this combination, are rarely found inside the TM region of type I transmembrane proteins (18). A negative charged aa such as glutamic or aspartic acid is, however, also found in the TM region of signaling subunits of other immunoreceptors and plays an important role in the proper assembly of immunoreceptor complexes as well as in the retention of unassembled signaling subunits in the ER (19, 20). To test whether or not the conserved E142 retained unpaired Igα in the ER, we mutated this aa to either alanine (E142A) or lysine (E142K) (Fig. 1B). Furthermore, we generated a proline to alanine (P153A) mutation of Igα. Expression vectors for either wt, single or double mutants of a Flag-tagged murine Igα were transiently transfected together with a GFP vector into Drosophila S2 cells (21). The S2 cells have a high cotransfection rate and most GFP+ S2 cells coexpress Igα. We thus compared Igα expression on the surface of GFP− and GFP+ S2 cell using flow cytometry after anti-Flag antibody staining (Fig. 1C). This analysis showed that Igα-wt failed to be expressed on the S2 cell surface whereas small amounts of the E142A mutant of Igα were transported onto the cell surface. ER retention was not released by the P153A mutation alone but the EP/AA double mutant of Igα was found in large amounts on the cell surface of GFP+ S2 cells. The replacement of the negatively charged glutamic acid E142 with a positively charged lysine again reduced the expression of E142K single or EP/KA double mutant of Igα on the S2 cell surface, indicating that a charged aa at position 142 promotes the ER retention of Igα (Fig. 1C). The quantified analysis of repeated S2 experiments confirmed that the EP/AA double mutant of Igα was most efficiently transported onto the surface of up to 60% of the GFP+ S2 cells (Fig. 1D). This indicates that the conserved E-X10-P motif in the TM region of Igα functions as an ER retention signal.
Fig. 1.
A conserved E-X10-P motif in the transmembrane region of Igα is responsible for its retention in ER. (A) Sequence comparison of Igα of different species. The conserved glutamic acid and proline are highlighted in red. (B) Sequence comparison of wt and mutant forms of Igα. The mutated amino acids are highlighted in red. (C) Flow cytometry analysis of the expression of Flag-tagged Igα on the surface of S2 cells transfected with plasmids encoding the indicated wt and mutant forms of Igα. Gray: GFP− untransfected cells; Red: GFP+ transfected cells. (D) Quantified Igα surface expression results presented as a bar graph. Data represent the mean and SE of a minimum of 3 independent experiments.
A Similar Conserved Amino Acid Motif in the TM Region of Igβ.
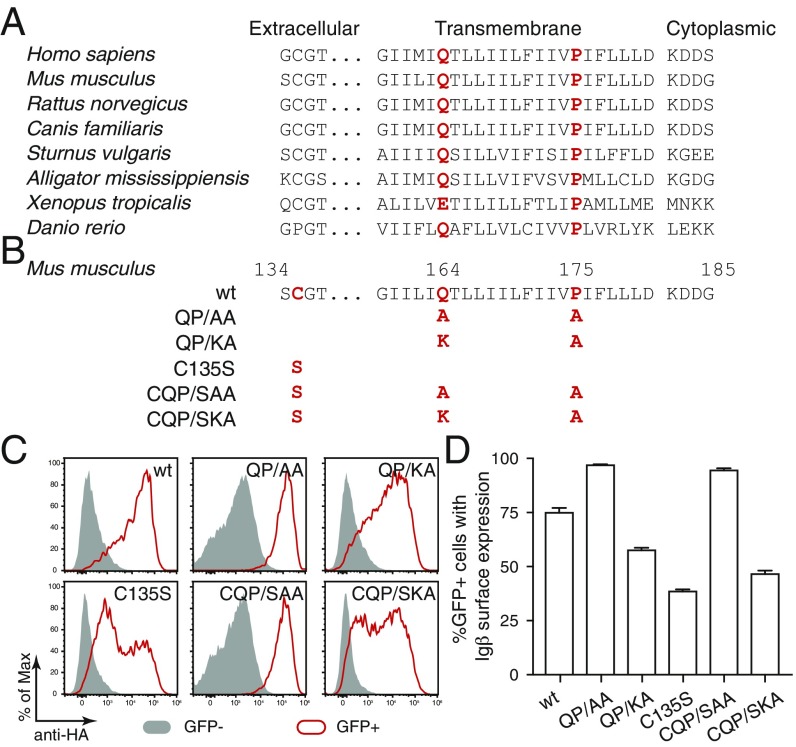
The sequence of the TM region of Igβ is also evolutionary conserved and contains a Q-X10-P motif that is similar to the E-X10-P motif of Igα (Fig. 2A). Furthermore, the aa of both motifs are situated at an identical position in their respective TM region. To test the function of the Q-X10-P motif, we mutated glutamine Q164 of HA-tagged murine Igβ to either alanine (Q164A) or lysine (Q164K) and combined these mutations with a P175A mutation (Fig. 2B). In addition, we mutated the cysteine 135 to serine (C135S) to prevent the formation of covalent Igβ/Igβ homodimers or Igα/Igβ heterodimers (3, 4). Expression vectors for either wt, double, or triple mutant Igβ were transiently transfected together with a GFP vector into Drosophila S2 cells that we tested by flow cytometry for Igβ expression using anti-HA antibody (Fig. 2C). In contrast to Igα-wt, the Igβ-wt protein could be transported as a homodimer onto the S2 cell surface where it was detected by the anti-HA antibody. A covalent Igβ/Igβ homodimer no longer formed after the C135S mutation of Igβ (SI Appendix, Fig. S1) and this mutant was also less well expressed on the S2 cell surface (Fig. 2C). The QP/AA double mutation increased the expression of Igβ on the S2 cell surface. Furthermore, in comparison with the C135S single mutant, the CQP/SAA triple mutant of Igβ was found in larger amounts on the S2 cell surface. The introduction of a positively charged lysine at the 164 aa position again increased ER retention of the double QP/KA as well as the triple CQP/SKA mutated Igβ. The statistical analysis of repeated S2 experiments confirmed that QP/AA and CQP/SAA mutated Igβ was most efficiently transported onto the S2 surface, indicating that the conserved Q-X10-P motif in the TM region of Igβ also functions as an ER retention signal for unpaired Igβ (Fig. 2D).
Fig. 2.
A conserved Q-X10-P motif is responsible for retention of Igβ in the ER. (A) Sequence comparison of Igβ of different species. The conserved glutamine and proline are highlighted in red. (B) Sequence comparison of wt and mutant forms of Igβ. The mutated amino acids are highlighted in red. (C) Flow cytometry analysis of the expression of HA-tagged Igβ on the surface of S2 cells transfected with plasmids encoding the indicated wt and mutant forms of Igβ. Gray: GFP− untransfected cells; Red: GFP+ transfected cells. (D) Quantified Igβ surface expression results presented as a bar graph. Data represent the mean and SE of a minimum of 3 independent experiments.
The Conserved E/Q-X10-P Motif Is Not Required for Igα/Igβ Heterodimerization.
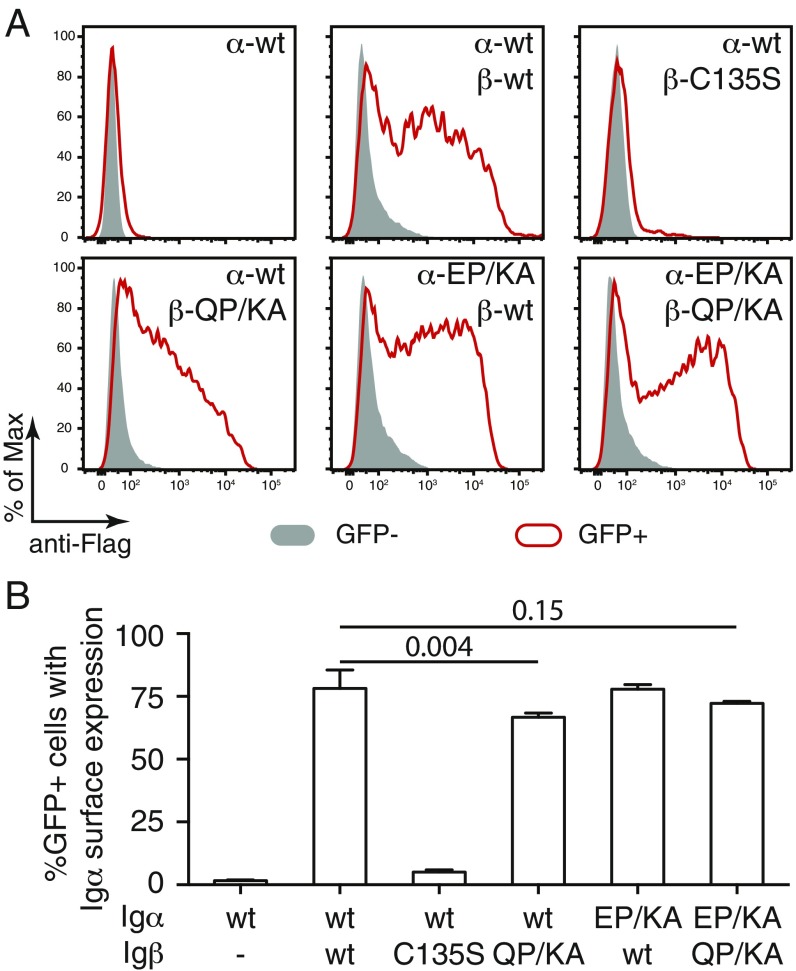
Normal B cells coexpress Igα and Igβ and assemble an Igα/Igβ heterodimer that binds to the mIg molecule thus forming the BCR complex (3). In contrast, the Igβ/Igβ homodimer is not part of a BCR complex and only poorly expressed on the B cell surface (22). We next tested whether or not the Igα/Igβ heterodimerization requires the E/Q-X10-P motif. For this, we transiently expressed different combinations of wt or double-mutated Igα and Igβ as well as C135S mutated Igβ in S2 cells and monitored the presence of Igα on the cell surface by flow cytometry using an anti-Flag antibody (Fig. 3A). While Igα-wt alone was retained inside the ER, the Igα/Igβ heterodimer was transported onto the S2 cell surface efficiently (Fig. 3A). This expression, however, requires the formation of a disulfide bond between the 2 subunits as the Igβ-C135S mutant was not bringing the Igα-wt onto the cell surface (Fig. 3A). The Igα-EP/KA and/or Igβ-QP/KA mutant still could form an Igα/Igβ heterodimer that was transported onto the S2 cell surface. Thus, a double mutation of the E/Q-X10-P motif in either Igα, Igβ, or both components does not prevent the assembly of the Igα/Igβ heterodimer. The statistical analysis of repeated S2 experiments confirmed that cysteine C135 of Igβ but not the E/Q-X10-P motif is required for the formation of the Igα/Igβ heterodimer and its transport onto the S2 cell surface (Fig. 3B).
Fig. 3.
The conserved E/Q-X10-P motif is dispensable for Igα/Igβ heterodimer formation. (A) Flow cytometry analysis of the expression of Flag-tagged Igα on the surface of S2 cells transfected with plasmids encoding the indicated wt and mutant forms of Igα and Igβ. Gray: GFP− untransfected cells; Red: GFP+ transfected cells. (B) Quantified Igα surface expression results presented as a bar graph. Data represent the mean and SE of a minimum of 3 independent experiments.
Both Igα and Igβ Interact with the mIg Molecule via the E/Q-X10-P Motif.
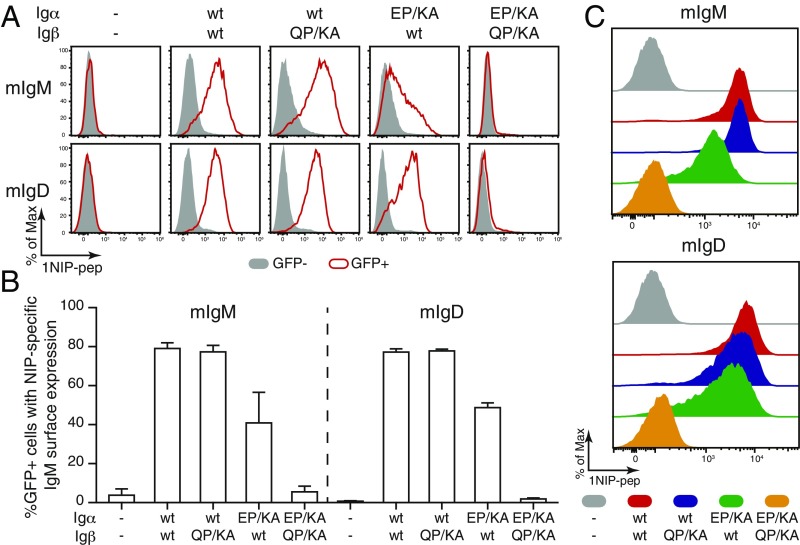
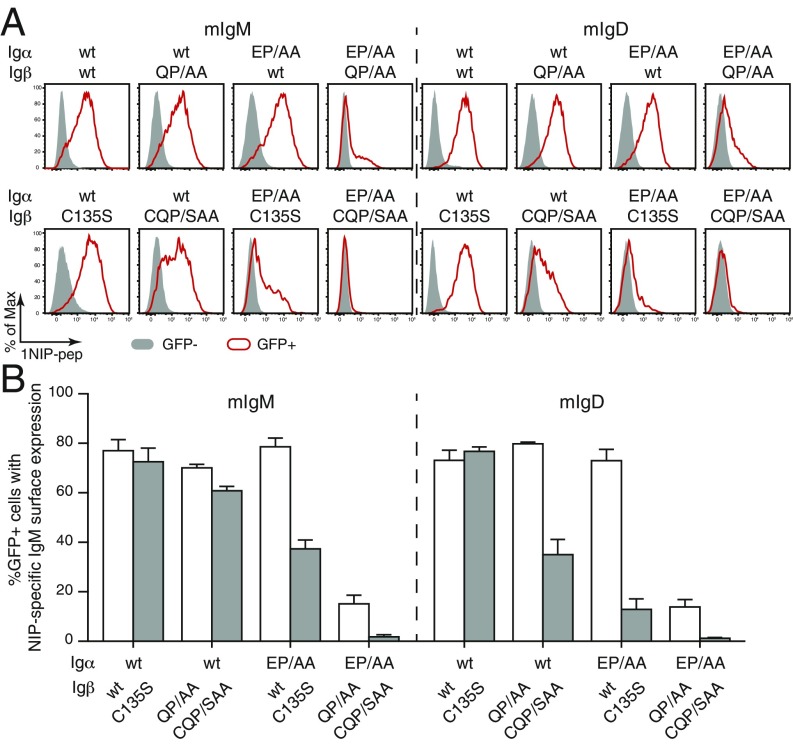
We previously demonstrated that mIg requires assembly with an Igα/Igβ heterodimer for its expression on the S2 cell surface (12). To test the function of the conserved E/Q-X10-P motif in BCR assembly, we expressed wt or the double mutants of Igα and Igβ together with either the mIgM or the mIgD molecule. The expressed mIg molecule comprises the B1-8 mHC and lambda-1 LC and binds to the hapten 4-hydroxy-3-iodo-5-nitrophenylacetyl (NIP). This allowed us to monitor BCR expression by flow cytometry using a NIP- and DyLight 649-coupled fluorescent peptide (1NIP-pep) (12). As expected, the mIgM or mIgD molecules were transported onto the S2 cell surface only in the presence of the Igα/Igβ heterodimer (Fig. 4 A, Upper and Lower). A replacement of Igβ-wt with Igβ-QP/KA did not change the expression of mIg on the S2 surface, whereas the exchange of Igα-wt by Igα-EP/KA reduced the expression of mIgM and to a lesser extent that of mIgD on the S2 surface (Fig. 4A). More strikingly, S2 cells producing an Igα/Igβ heterodimer with double-mutated Igα-EP/KA and Igβ-QP/KA failed to express either class of the mIg at the cell surface (Fig. 4A). The statistical analysis of repeated S2 experiments confirmed that IgD-BCR assembly is less affected by the Igα-EP/KA mutation than IgM-BCR assembly and that both BCR classes were no longer transported onto the S2 cell surface when both Igα and Igβ are double mutated (Fig. 4B).
Fig. 4.
Both Igα and Igβ interact with the mIg molecule via the E/Q-X10-P motif. (A) Flow cytometry analysis of the expression of NIP-specific IgM- or IgD-BCR on the surface of S2 cells transfected with plasmids encoding mIgM or mIgD and the indicated wt and mutant forms of Igα and Igβ. Gray: GFP− untransfected cells; Red: GFP+ transfected cells. (B) Quantified BCR surface expression results presented as a bar graph. Data represent the mean and SE of a minimum of 3 independent experiments. (C) Flow cytometry analysis of the expression of NIP-specific IgM- or IgD-BCR on the surface of 3046β-KO cells transfected with plasmids encoding mIgM or mIgD and the indicated wt and mutant forms of Igα and Igβ. Data are representative of 5 independent experiments.
Our study of the requirement for BCR assembly in the S2 cell system was complemented by a parallel study within a murine pro-B cell line 3046 lacking the expression of mHC, LC, Igα, and SLP65 (12). Using the CRISPR/Cas9 technique (23), we rendered the 2 endogenous Igβ alleles of 3046 inactive and confirmed the defective Igβ production in 12 of 13 tested 3046 cell clones by Western blot (SI Appendix, Fig. S2A). A mixture of 5 different Igβ-KO clones (3046β-KO) were retrovirally transduced with mHC and LC vectors for the expression of NIP-specific mIgM or mIgD molecules. The sorted mIg expressing B cells were further transfected in different combination with vectors encoding Igα-wt, Igβ-wt, Igα-EP/KA, and Igβ-QP/KA and monitored for their BCR expression by flow cytometry (Fig. 4C). The BCR was transported onto the cell surface as long as the Igα/Igβ heterodimer contained one wt form. However, a double-mutated heterodimer consisting of Igα-EP/KA and Igβ-QP/KA failed in BCR assembly and the transport of BCR onto the 3046 pro-B cell surface (Fig. 4C). The 3046 pro-B cell transfectants expressing Igα-EP/KA in combination with Igβ-wt showed in comparison with the vice versa transfectants a reduced BCR expression, in particular of the IgM-BCR, indicating that the TM interaction between Igα and mHC is more important than the one between Igβ and mHC for BCR assembly. We also expressed wt or double-mutant Igα and Igβ alone in the 3046b-KO cells and found that in these cells the ER retention of each component is released by the AA but not the KA double mutations of the E/Q-X10-P motif (SI Appendix, Fig. S3). Our mutational analysis of the Igα/Igβ heterodimer thus showed similar phenotypes in both the Drosophila S2 and the murine 3046 pro-B cells.
The Disulfide Bond Between Igα and Igβ Plays a Supportive Role in BCR Assembly.
As the KA and AA double mutants of the E/Q-X10-P motif display different phenotypes in the retention of isolated Igα and Igβ components, we next tested how the AA mutations of Igα and Igβ affect the assembly and transport of the IgM- or IgD-class BCR onto the S2 cell surface (Fig. 5A). Furthermore, we combined these mutations with a C135S mutation of Igβ preventing the formation of a covalent disulfide bond between Igα and Igβ (3, 12). Unlike the KA mutant, the AA double mutant of Igα is not defective in forming a BCR complex in combination with Igβ-wt (compare Fig. 4A and Fig. 5A). However, the Igα/Igβ heterodimer comprising Igα-EP/AA and Igβ-QP/AA failed to be efficiently expressed together with either the mIgM or mIgD molecule on the cell surface (Fig. 4A). Interestingly, when we replaced in these experiments Igβ-wt with the Igβ-C135S mutant or the double-mutant Igβ-QP/AA with the triple-mutant Igβ-CQP/SAA, the BCR assembly was more strongly affected. In particular, the combination of the Igα-EP/AA mutant with either the Igβ-wt or the Igβ-C135S mutant showed a reduced BCR expression on the S2 cell surface only in the latter case, indicating that the disulfide bond between Igα and Igβ supports BCR assembly. The statistical analysis of repeated S2 experiments confirmed these conclusions and also showed that the Igα/Igβ disulfide bond is more important for the stability of the IgD-BCR than the IgM-BCR (Fig. 5B). This is in agreement with previous finding that mIgD associates with Igα/Igβ heterodimer mainly through its TM region (5).
Fig. 5.
The disulfide bond between Igα and Igβ plays a supportive role in BCR assembly. (A) Flow cytometry analysis of the expression of NIP-specific IgM- or IgD-BCR on the surface of S2 cells transfected with plasmids encoding mIgM or mIgD and the indicated wt and mutant forms of Igα and Igβ. Gray: GFP− untransfected cells; Red: GFP+ transfected cells. (B) Quantified BCR surface expression results presented as a bar graph. Data represent the mean and SE of a minimum of 3 independent experiments.
Discussion
The molecular interactions that stabilize the BCR complex within the membrane are currently poorly understood. We here show that both Igα and Igβ carry in their TM sequences a conserved E/Q-X10-P motif that is required for the retention of isolated Igα and Igβ proteins in the ER and the stable expression of the BCR on the cell surface. Furthermore, we show that this motif is specifically involved in mIg binding but not in the formation of the Igα/Igβ heterodimer or Igβ/Igβ homodimer.
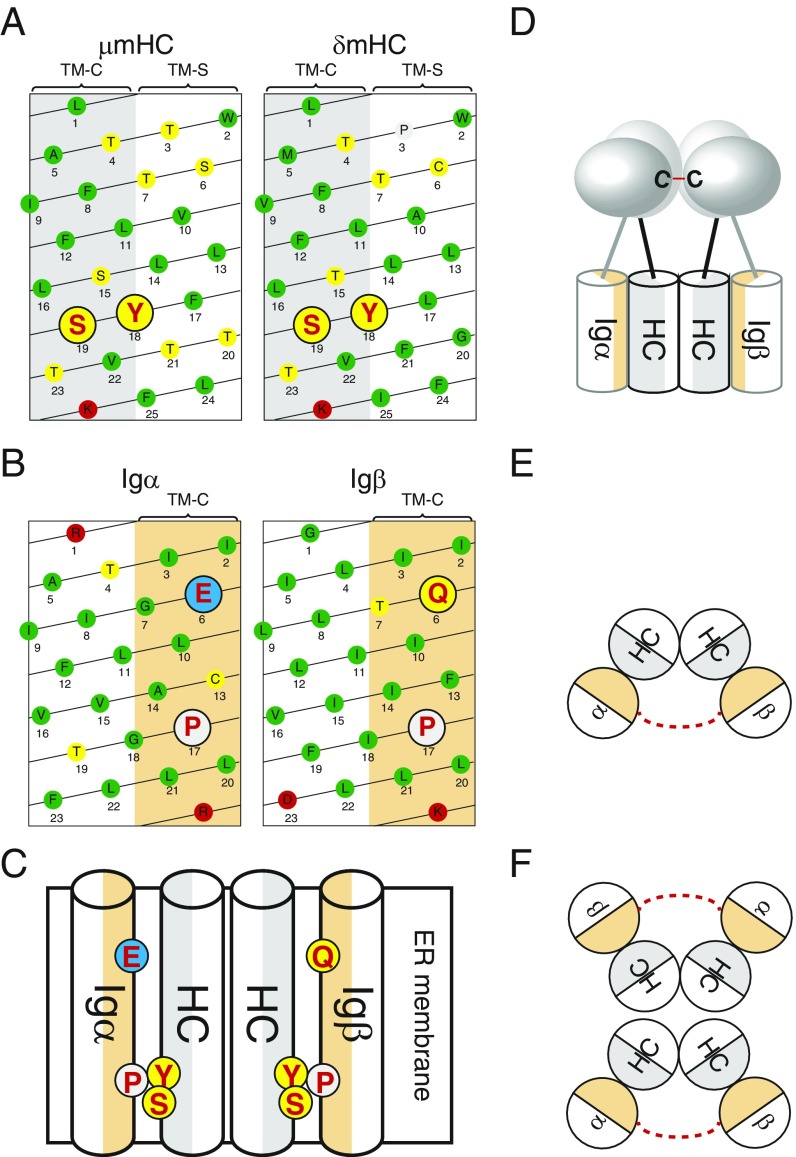
The mIg molecule is a symmetric homodimer containing 2 identical mHC. It thus was previously thought that an Igα/Igβ heterodimer is binding to each side of the mIg molecule. However, this 1:2 model of the BCR complex was discarded after a biochemical study and a fluorescent spectroscopy study both supported a 1:1 interaction between the mIg molecule and the Igα/Igβ heterodimer (16, 17). According to textbook drawings of the 1:1 BCR model, only 1 of the 2 TM-C side of the mHC:mHC homodimer is interacting with the Igα/Igβ heterodimer (24). This asymmetric binding of Igα/Igβ to the symmetric mIg molecule is an unsolved problem of this model. The TM-C side of the μmHC and the δmHC both contain a Y18-S19 aa pair (Fig. 6A) required for the binding of the Igα/Igβ heterodimer (9, 10). One thus should assume that both mHCs of the mIg molecule are involved in Igα/Igβ binding and we suggest here that this is indeed the case. The Igα and Igβ TM sequences most likely cross the lipid bilayer as an α-helix (25). Interestingly, the residues of the conserved E/Q-X10-P motif of Igα and Igβ are situated all on one side of such an α-helix (Fig. 6B). This side contains I2, I3, L20, and L21 (numbered from the start of the TM region) and 4 other conserved aa that are found in both the Igα and Igβ TM sequence. Thus, the TM α-helixes of Igα and Igβ have both a conserved side (here referred to as αTM-C and βTM-C) whose aa composition is nearly identical between these 2 different proteins. It is thus feasible that αTM-C and βTM-C each interacts with one TM-C side of the mIg molecule thus forming inside the membrane a symmetric Igα-mHC:mHC-Igβ complex (Fig. 6C). According to this model, the charged E6 of Igα and the polar Q6 of Igβ (numbered from the start of the TM region) would interact with a polar patch comprising T4 and T7 of mHC whereas P17 of αTM-C and βTM-C would be in close contact with Y18 and S19 of the TM-C sides of the mHC:mHC homodimer. Thus, amino acids whose mutation in their respective protein results in the disruption of the BCR complex are conjugated in this model. It is satisfactory to see that according to this symmetric model most charged or polar aa of the TM domains of the BCR complex are interacting with each other and thus are shielded from the hydrophobic environment of the plasma membrane. Furthermore, the alignment of P17 with Y18 suggest that these 2 conserved aa interact with each other via an aromatic proline interaction (26, 27).
Fig. 6.
Models of BCR complex assembly and BCR dimer formation. (A) Scheme of the TM α-helix of μmHC and δmHC. Amino acids are indicated by single-letter code in circles with different shades indicating their properties. Hydrophobic, polar, positively charged and negatively charged aa are marked with green, yellow, red, and blue, respectively. Proline is marked with light gray. The tyrosine and serine residues known for interacting with Igα and Igβ are highlighted by red color and big size. (B) Scheme of the TM α-helix of Igα and Igβ. Amino acids are indicated by single-letter code in circles with different shades indicating their properties. The residues of the conserved E/Q-X10-P motif of Igα and Igβ are highlighted by red color and big size. (C) Schematic drawing of the symmetric arrangement of the 4 TM regions of one BCR complex. Amino acids involved in the TM interactions are highlighted. (D) Schematic drawing of the BCR complex, showing that the extracellular Ig domains of Igα and Igβ are in tight contact to form the disulfide bond (red line). (E) Schematic drawing showing that the extracellular disulfide bond (red dashed line) linked Igα and Igβ tilt their TM region and resulted in the exposure of the TM-S side of mHC to the lipid environment of the plasma membrane. (F) Schematic drawing showing that BCR forms dimers through the interaction between the exposed TM-S side.
The extracellular Ig domain of Igα and Igβ are covalently bound to each other by a disulfide bridge (3, 4). Based on the crystal structure of the Igβ/Igβ homodimer, a 3-dimensional model of the extracellular part of the Igα/Igβ heterodimer was generated. It shows that the cysteines forming the disulfide bridge are situated in the middle of the respective Ig domain (4). Thus, whereas inside the membrane Igα and Igβ are separated from each other by the mHC:mHC homodimer, extracellularly they form a tight complex (Fig. 6D). We think that this feature implies that the TM regions of the Igα-mHC:mHC-Igβ complex are tilted in a way that allows the extracellular part of Igα and Igβ to move close together (Fig. 6E). In such a tilted structure, the TM-S side of the mHC homodimer would be more exposed to the lipid environment and this could promote a dimerization of the BCR complex that we previously described (12, 16). According to this model, it is the rotation and the shielding of the TM-S side from the lipid bilayer that stabilizes a symmetric, dimeric BCR structure (Fig. 6F). In favor of this model is the phenotype of a mutant IgD-BCR carrying several aa alterations at the TM-S side of the δm TM region and lacking the disulfide bridge between Igα and Igβ. This hyperactive δmTM-S/Igα-S mutant still forms an IgD-BCR complex but no longer a BCR dimer and is not stably expressed on the B cell surface (12). It is thus likely that TM regions of the mutant IgD-BCR are reorganized, preventing a closed autoinhibited BCR dimer conformation. A similar reorganization of the TM regions may occur upon the binding of an antigen to the BCR that according to the DAM hypothesis involves the opening of the BCR dimer (28). The reorganization of the TM regions upon BCR dissociation may induce a conformational change that is transmitted across the membrane to the cytoplasmic tail of Igα and Igβ, thus increasing the accessibility of the ITAM sequences to cytosolic kinases such as Syk. The evolutionary high conservation of the TM regions of the BCR complex may thus be important not only for the stabilization of the dimeric BCR but also for its activation.
According to the symmetric Igα-mHC:mHC-Igβ TM model, the TM-C side of Igα and Igβ are nearly equivalent in their binding to the mHC:mHC homodimer. It thus should be feasible that the Igβ:Igβ homodimer also binds to the mIg molecule and promotes the expression of a BCR complex on the cell surface, but this is not the case (22). This feature suggests that Igα plays a more important role in the binding of the mIg molecule than Igβ. Indeed, our mutational analysis showed that the IgM-BCR expression is more affected by the Igα-EP/KA than the Igβ-QP/KA (Fig. 4A), and the same is true for the AA mutations of the E/Q-X10-P in the absence of an Igα/Igβ stabilizing disulfide bond (Fig. 5A). One explanation for this may be that the E6 at the αTM-C side promotes stronger mHC binding than the Q6 at the βTM-C side. More likely, however, is that the extracellular Ig domain of Igα has a more extended interface and stronger binding to the mIg molecule than that of Igβ. This notion is supported by the finding that N-linked glycosylation sites in the Ig domain of Igα are affected by the binding to different mIg classes (29, 30).
Recently, a molecular dynamics simulation technique was used to generate a structural model of TM region interactions within the IgM-BCR complex (31). This study also supports a 1:1 stoichiometry of the BCR complex but the TM region interaction model suggested by this study is not in line with our mutational analysis. Most importantly, that model failed to identify the conserved αTM-C and βTM-C sides of the Igα/Igβ heterodimer and does not contribute a special role of the E/Q-X10-P motif for the interaction with the TM-C region of the mHC molecule. A resolution of this conflict has to await the generation of a cryo-EM structure of the complete BCR complex.
Materials and Methods
Cells and Cell Culture.
Drosophila Schneider (S2) (a gift from K. Karjalainen, NTU Singapore) were cultured in S2 Drosophila medium (Invitrogen) and transfected using FuGENE HD (Roche) as described (21).
The 3046 (Igα−/−Slp65−/−) pro-B cells were maintained and transfected as described previously (12). Retroviral transfection of the 3046 cells were performed as previously described (32). In brief, Phoenix cells were transfected using PolyJet DNA in vitro transfection reagent following the manufacturer’s protocol (SignaGen Laboratories). Retrovirus-containing supernatants were collected 48 h after transfection and used for transduction.
Generate the 3046β-KO Pro-B Cells by CRISPR/Cas9.
CRISPR/Cas9 KO plasmids for murine Igβ were purchased from Santa Cruz. The KO plasmids were delivered to the 3046 cells using the Neon transfection system (Invitrogen). For 1 reaction, 1 million cells were resuspended with 100 μL transfection medium containing 20 mM Hepes (Gibco) and 1.25% DMSO (Sigma) in RPMI medium (Gibco) and then mixed together with 4 μg of KO plasmid. The cells were then transfected using a single pulse at 1,350 V, with a 30 ms pulse width. The transfected GFP+ cells were single sorted into 96-well plates 24 h to 48 h posttransfection. The single sorted cells were cultured in 96-well plates for 10–14 d and then transferred to larger wells for expansion. Inactivation of the target gene was verified by Western blot and/or genotyping. The resulted KO cells were maintained and transfected as described for 3046 cells.
Plasmids and Mutagenesis.
The vectors for the expression of the BCR components in Drosophila S2 cells and 3046 pro-B cells were described previously (12). Site-directed mutagenesis is achieved using the Quikchange site-directed mutagenesis kit (Stratagene) following the manufacturer’s instructions, and the introduced mutations were verified by sequencing.
Western Blotting.
Cells were collected and lysed as described (14). Cleared lysates were boiled in Laemmli buffer for 5 min and aliquots equivalent to 1 × 106 cells were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and Western blotted using Odyssey (Licor). The following antibodies were used for Western blot: anti-Flag (Mouse, M2, Sigma-Aldrich), anti-HA (Rat, 3F10, Roche), Alexa Fluro 680 Goat anti-Mouse IgG, Alexa Fluor 680 Goat anti-Rat IgG (Invitrogen) and IRdye 800 Goat anti-Mouse IgG, IRdye 800 Goat anti-Rat IgG (Licor).
Flow Cytometry.
For flow cytometry analysis of BCR component surface expression, cells were stained with anti-Flag APC (1:100, BioLegend) for the Flag-tagged Igα, or anti-HA PE (1:100, BioLegend) for the HA-tagged Igβ, or anti-IgM APC (1:100, eBioscience), or 1NIP-pep (200 nM, custom order from IRIS Biotech) (12, 33), and measured with LSRII or LSRFortessa (BD Biosciences) or Attune NxT (Thermo Fisher) flow cytometer. Data were exported in FCS-3 or FCS-3.1 format and analyzed with FlowJo software (TreeStar).
Data Processing and Statistical Analysis.
Means and SEM from a minimum of 3 independent experiments were used for plotting with Prism software (GraphPad). To determine differences between data sets, a 2-tailed unpaired t test was performed. P values for each test are given in the figure.
Supplementary Material
Acknowledgments
This study was supported by the European Research Council Grant 322972, the Deutsche Forschungsgemeinschaft through TRR130-P02. Support came also through the Excellence Initiative of the German Federal and State Governments (EXC 294).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1907481116/-/DCSupplemental.
References
- 1.Niiro H., Clark E. A., Regulation of B-cell fate by antigen-receptor signals. Nat. Rev. Immunol. 2, 945–956 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Hombach J., Tsubata T., Leclercq L., Stappert H., Reth M., Molecular components of the B-cell antigen receptor complex of the IgM class. Nature 343, 760–762 (1990). [DOI] [PubMed] [Google Scholar]
- 3.Siegers G. M., et al. , Identification of disulfide bonds in the Ig-alpha/Ig-beta component of the B cell antigen receptor using the Drosophila S2 cell reconstitution system. Int. Immunol. 18, 1385–1396 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Radaev S., et al. , Structural and functional studies of Igalphabeta and its assembly with the B cell antigen receptor. Structure 18, 934–943 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schamel W. W., Reth M., Stability of the B cell antigen receptor complex. Mol. Immunol. 37, 253–259 (2000). [DOI] [PubMed] [Google Scholar]
- 6.Venkitaraman A. R., Williams G. T., Dariavach P., Neuberger M. S., The B-cell antigen receptor of the five immunoglobulin classes. Nature 352, 777–781 (1991). [DOI] [PubMed] [Google Scholar]
- 7.Reth M., Antigen receptors on B lymphocytes. Annu. Rev. Immunol. 10, 97–121 (1992). [DOI] [PubMed] [Google Scholar]
- 8.Yang J., Reth M., Receptor dissociation and B-cell activation. Curr. Top. Microbiol. Immunol. 393, 27–43 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Shaw A. C., et al. , Mutations of immunoglobulin transmembrane and cytoplasmic domains: Effects on intracellular signaling and antigen presentation. Cell 63, 381–392 (1990). [DOI] [PubMed] [Google Scholar]
- 10.Sanchez M., et al. , Signal transduction by immunoglobulin is mediated through Ig alpha and Ig beta. J. Exp. Med. 178, 1049–1055 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsuuchi L., et al. , The membrane IgM-associated proteins MB-1 and Ig-beta are sufficient to promote surface expression of a partially functional B-cell antigen receptor in a nonlymphoid cell line. Proc. Natl. Acad. Sci. U.S.A. 89, 3404–3408 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J., Reth M., Oligomeric organization of the B-cell antigen receptor on resting cells. Nature 467, 465–469 (2010). [DOI] [PubMed] [Google Scholar]
- 13.Kläsener K., Maity P. C., Hobeika E., Yang J., Reth M., B cell activation involves nanoscale receptor reorganizations and inside-out signaling by Syk. elife 3, e02069 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rolli V., et al. , Amplification of B cell antigen receptor signaling by a Syk/ITAM positive feedback loop. Mol. Cell 10, 1057–1069 (2002). [DOI] [PubMed] [Google Scholar]
- 15.Kulathu Y., Zuern C., Yang J., Reth M., Synthetic biology of B cell activation: Understanding signal amplification at the B cell antigen receptor using a rebuilding approach. Biol. Chem. 400, 555–563 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Schamel W. W., Reth M., Monomeric and oligomeric complexes of the B cell antigen receptor. Immunity 13, 5–14 (2000). [DOI] [PubMed] [Google Scholar]
- 17.Tolar P., Sohn H. W., Pierce S. K., The initiation of antigen-induced B cell antigen receptor signaling viewed in living cells by fluorescence resonance energy transfer. Nat. Immunol. 6, 1168–1176 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Landolt-Marticorena C., Williams K. A., Deber C. M., Reithmeier R. A., Non-random distribution of amino acids in the transmembrane segments of human type I single span membrane proteins. J. Mol. Biol. 229, 602–608 (1993). [DOI] [PubMed] [Google Scholar]
- 19.Zhou F. X., Merianos H. J., Brünger A. T., Engelman D. M., Polar residues drive association of polyleucine transmembrane helices. Proc. Natl. Acad. Sci. U.S.A. 98, 2250–2255 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gratkowski H., Lear J. D., DeGrado W. F., Polar side chains drive the association of model transmembrane peptides. Proc. Natl. Acad. Sci. U.S.A. 98, 880–885 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang J., Reth M., Drosophila S2 Schneider cells: A useful tool for rebuilding and redesigning approaches in synthetic biology. Methods Mol. Biol. 813, 331–341 (2012). [DOI] [PubMed] [Google Scholar]
- 22.He X., et al. , Continuous signaling of CD79b and CD19 is required for the fitness of Burkitt lymphoma B cells. EMBO J. 37, e97980 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ran F. A., et al. , Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281–2308 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy K. M., Janeway’s Immunobiology (Garland Science, 2011). [Google Scholar]
- 25.White S. H., Wimley W. C., Membrane protein folding and stability: Physical principles. Annu. Rev. Biophys. Biomol. Struct. 28, 319–365 (1999). [DOI] [PubMed] [Google Scholar]
- 26.Leitgeb B., Tóth G., Aromatic-aromatic and proline-aromatic interactions in endomorphin-1 and endomorphin-2. Eur. J. Med. Chem. 40, 674–686 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Wu W. J., Raleigh D. P., Local control of peptide conformation: Stabilization of cis proline peptide bonds by aromatic proline interactions. Biopolymers 45, 381–394 (1998). [DOI] [PubMed] [Google Scholar]
- 28.Yang J., Reth M., The dissociation activation model of B cell antigen receptor triggering. FEBS Lett. 584, 4872–4877 (2010). [DOI] [PubMed] [Google Scholar]
- 29.Campbell K. S., Cambier J. C., B lymphocyte antigen receptors (mIg) are non-covalently associated with a disulfide linked, inducibly phosphorylated glycoprotein complex. EMBO J. 9, 441–448 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wienands J., Hombach J., Radbruch A., Riesterer C., Reth M., Molecular components of the B cell antigen receptor complex of class IgD differ partly from those of IgM. EMBO J. 9, 449–455 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friess M. D., Pluhackova K., Böckmann R. A., Structural model of the mIgM B-cell receptor transmembrane domain from self-association molecular dynamics simulations. Front. Immunol. 9, 2947 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Storch B., Meixlsperger S., Jumaa H., The Ig-alpha ITAM is required for efficient differentiation but not proliferation of pre-B cells. Eur. J. Immunol. 37, 252–260 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Volkmann C., et al. , Molecular requirements of the B-cell antigen receptor for sensing monovalent antigens. EMBO J. 35, 2371–2381 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.