Significance
The last decade has seen an exponential increase in opioid use in the United States, resulting in a subset of the population developing opioid tolerance. In this paper, we unequivocally demonstrate that the gut microbiota plays a significant role in morphine analgesic tolerance. Our studies show that morphine-tolerant mice have a distinct gut microbial composition. Morphine tolerance is dramatically reduced in two microbiota depletion models: germfree mice and pan-antibiotics−treated mice. Reconstitution of the gut microbiota in germfree mice restores morphine tolerance. Furthermore, probiotics, which restore depleted microbial communities, attenuate morphine analgesic tolerance. These studies have significant clinical relevance and support manipulating gut microbiota as a potential therapeutic target for the prevention, treatment, and management of opioid analgesic tolerance.
Keywords: gut−immune−brain axis, morphine tolerance, gut dysbiosis, germfree mice
Abstract
Prolonged exposure to opioids results in analgesic tolerance, drug overdose, and death. The mechanism underlying morphine analgesic tolerance still remains unresolved. We show that morphine analgesic tolerance was significantly attenuated in germfree (GF) and in pan-antibiotic−treated mice. Reconstitution of GF mice with naïve fecal microbiota reinstated morphine analgesic tolerance. We further demonstrated that tolerance was associated with microbial dysbiosis with selective depletion in Bifidobacteria and Lactobacillaeae. Probiotics, enriched with these bacterial communities, attenuated analgesic tolerance in morphine-treated mice. These results suggest that probiotic therapy during morphine administration may be a promising, safe, and inexpensive treatment to prolong morphine’s efficacy and attenuate analgesic tolerance. We hypothesize a vicious cycle of chronic morphine tolerance: morphine-induced gut dysbiosis leads to gut barrier disruption and bacterial translocation, initiating local gut inflammation through TLR2/4 activation, resulting in the activation of proinflammatory cytokines, which drives morphine tolerance.
Opioids are the gold standard for the management of moderate to severe pain (1). Despite their high efficacy, the clinical use of opioids is limited because of comorbidities associated with their prolonged use (2). The last decade has seen a significant rise in opioid use in the United States, resulting in a subset of the population developing opioid tolerance (3, 4). Chronic and repeated opioid use leads to the rapid onset of analgesic tolerance; however, the pharmacokinetics of tolerance to peripheral receptors develops slowly, leading to respiratory depression, immune modulation, nausea, and decreased gastrointestinal motility with escalating doses of opioids (5). Thus, chronic opioid use is associated with poorer outcomes, longer lengths of hospital stay, higher readmission rates, and higher health care costs (6, 7). Therefore, it is imperative that we understand the mechanisms underlying the comorbidities associated with chronic opioid use and delineate specific protocols for the care of chronic pain patients who are on opioids. Studies on opioid analgesic tolerance have revealed several potential mechanisms: receptor desensitization and down-regulation (8), up-regulation of cAMP-protein kinase A systems, release of glutamate, calcitonin gene-related peptide, substance P, noradrenaline, and acetylcholine to enhance synaptic transmission (9) and neuroimmune activation and neuroinflammation (10–16). However, the precise mechanisms underlying morphine tolerance remain unresolved. Recent studies have shown that the gut microbiota plays a crucial and dynamic role in immune response and neuronal function (17). Gut homeostasis confers health benefits, and any disruption resulting in alteration in beneficial bacteria can negatively influence the health and well-being of an individual (18, 19). Toll-like receptors (TLRs) recognize a variety of microbial components and allow the innate immune system to sense and react to the altered microbiota, hence playing a central role in the interaction between host and microbiota (20).
Our recent studies have shown that chronic opioid use in animal models is associated with altered gut microbiota (21, 22). These findings are supported by studies in patients with substance use disorder who demonstrate altered composition and diversity of gut microbiome distinct from healthy controls, thus implicating the gut−brain axis in morphine analgesic tolerance (23–25). In the present studies, we used multiple murine models to demonstrate the essential role of the gut microbiota in morphine analgesic tolerance. These studies suggest that strategies can be developed to alter the gut microbiota to prolong the effectiveness of morphine and prevent analgesic tolerance.
Results
Morphine Tolerance Was Attenuated in Germfree and Pan-Antibiotics Mice.
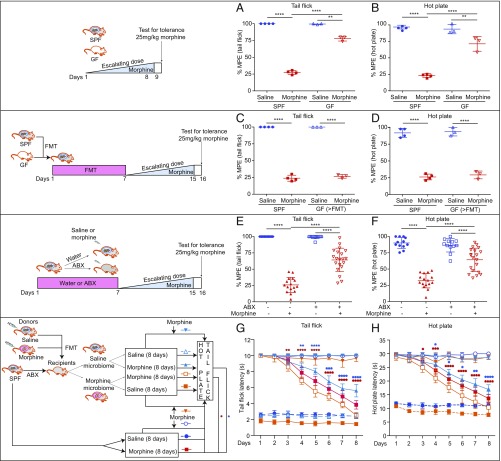
We have previously shown that chronic morphine treatment results in microbial dysbiosis, disruption in gut barrier function, and bacterial translocation, primarily through the μ-opioid receptor (OPRM1) (21). To initially establish the role of the gut microbiota in morphine tolerance, germfree (GF) mice and conventionally raised, specific pathogen-free (SPF) mice were subjected to a well-established tolerance regimen wherein mice were treated with either saline or repeated escalating doses of morphine for 8 d. Interestingly, morphine-treated GF mice displayed less analgesic tolerance in both tail flick and hot plate tests than SPF mice, 75% and 25% maximum possible effect (%MPE), respectively (Fig. 1 A and B). We next repeated this experiment in GF mice that had undergone fecal microbiota transplantation (FMT) with samples obtained from SPF mice. These mice showed significant analgesic tolerance similar to SPF animals treated with morphine (Fig. 1 C and D). These results clearly establish the role of gut microbiota in morphine analgesic tolerance.
Fig. 1.
Gut microbiota are essential for morphine analgesic tolerance. (A and B) Antinociceptive tolerance was attenuated in GF mice. Ftail flick (3, 10) = 869.4, and Fhot plate (3, 10) = 110.9. (C and D) GF mice recapitulated morphine tolerance after FMT with naïve mouse microbiota. Ftail flick (3, 10) = 882.8, and Fhot plate (3, 10) = 147.8; nSPF = 4; nGF = 3. (E and F) Mice exhibited attenuated analgesic tolerance after gut microbiota depletion; n = 12 to 20. Ftail flick (3, 56) = 118.8, and Fhot plate (3, 56) = 58.19. (A–F) Data were analyzed by one-way ANOVA followed by Bonferroni correction. **P < 0.01; ****P < 0.0001. (G and H) Time course of the effects of different microbiota on morphine tolerance; n = 6 to 8. FTreatment x time (56, 343) = 73.15 for tail flick. FTreatment x time (56, 343) = 59.66 for hot plate. Two-way ANOVA followed by Tukey’s multiple comparison was used. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001 for Morphine vs. Saline microbiome + Morphine. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001 for Morphine vs. Morphine microbiome + Morphine. Each dot represents one mouse. Mean ± SD.
To further investigate whether gut microbial dysbiosis contributed to morphine tolerance, SPF mice were administered a mixture of antibiotics in their drinking water for a week to deplete the gut microbiota and then treated with escalating doses of morphine for 8 d. Pan-antibiotics (ABX) treatment was maintained during the whole duration of morphine treatment. Results from these studies show that ABX markedly reduced gut bacteria (SI Appendix, Fig. S1 A and B). ABX and SPF mice were treated with repeated escalating doses of morphine. The morphine-treated ABX mice showed significantly attenuated analgesic tolerance, with 64 %MPE at day 8 in both tail flick and hot plate assays (Fig. 1 E and F). In an additional study, a fixed morphine dose was administered to ABX and SPF control mice twice daily for 8 d. The ABX mice maintained a significantly higher antinociceptive efficacy from day 4 by tail flick (SI Appendix, Fig. S1C) and from day 5 by hot plate (SI Appendix, Fig. S1D) compared with non−antibiotic-treated mice.
To further establish the role of gut microbiota in analgesic tolerance, SPF mice treated with ABX were gavaged with microbiota harvested from either saline- or morphine-treated mice. Mice reconstituted with morphine-tolerant mouse microbiota and then treated with morphine showed exacerbated analgesic tolerance following the same daily injection doses. In contrast, mice that were reconstituted with saline-treated mouse microbiota displayed less analgesic tolerance (Fig. 1 G and H). To validate effective reconstitution, stool samples were collected from recipient mice and subjected to 16S ribosomal RNA (rRNA) sequencing. The microbiome of the recipient animals was similar to their donor profile in principal coordinates analysis clustering (SI Appendix, Fig. S1E). In summary, the data from these studies clearly support a role for gut microbiota in morphine-induced analgesic tolerance.
Morphine-Induced Dysbiosis Disrupted Gut Epithelial Barrier and Promoted Systemic Bacterial Translocation.
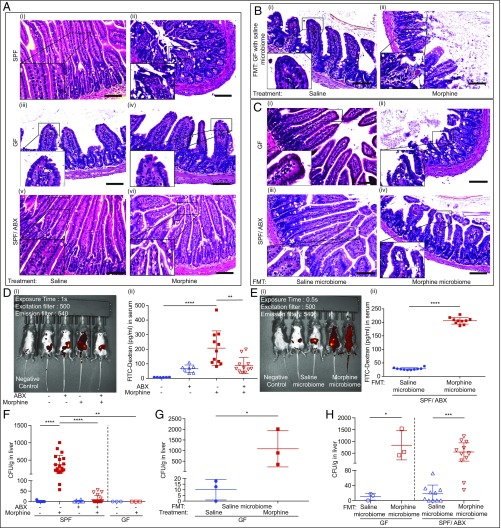
We evaluated gut barrier permeability and bacterial translocation in the mice to determine how morphine-induced microbial dysbiosis contributes to analgesic tolerance. Ileum samples were examined for histopathological changes. In SPF morphine-tolerant mice, we observed impaired epithelia and increased inflammatory infiltrates in small intestinal villi (Fig. 2 A, ii). In contrast, in morphine-treated GF and ABX mice, no morphological damage was observed (Fig. 2 A, iv and vi). Morphine-induced intestinal disruption was also seen in GF mice that received naïve microbiota from SPF mice (Fig. 2 B, ii). In the GF or ABX mice undergoing FMT from saline- or morphine-tolerant mice, we observed that FMT from morphine-tolerant mice alone is sufficient to induce histopathological change in the gut, without requiring direct exposure to morphine (Fig. 2 C, ii and iv). To evaluate the disruption in gut permeability, SPF and ABX mice with or without FMT were gavaged with fluorescein isothiocyanate (FITC)-dextran. Significant increase in gut permeability was observed in SPF morphine-tolerant mice (Fig. 2D). Morphine’s effects on gut permeability of SPF mice were abolished in ABX-treated mice (Fig. 2D). In ABX mice that underwent FMT, a significant increase in FITC-dextran in the serum was observed in the mice receiving gut microbiota from morphine-tolerant mice (Fig. 2E), but not in those receiving gut microbiota from saline-treated mice. We next evaluated gut bacterial translocation to the liver and mesenteric lymph nodes (MLN) in the mice. Significant bacterial translocation was observed in SPF morphine-tolerant mice (Fig. 2F and SI Appendix, Fig. S2A). As expected, morphine treatment failed to induce bacterial translocation in either GF or ABX mice (Fig. 2F and SI Appendix, Fig. S2A). However, when GF mice were reconstituted with the microbiota of naïve SPF mice, and then treated with morphine, bacterial translocation was observed (Fig. 2G and SI Appendix, Fig. S2B). In addition, when GF mice or ABX mice underwent FMT, the mice receiving morphine-tolerant microbiota showed significantly greater bacterial translocation than the mice receiving microbiota of saline-treated animals (Fig. 2H and SI Appendix, Fig. S2C). These studies suggest that morphine-induced gut integrity disruption and subsequent bacterial translocation is mediated by alterations in the gut microbiota. These results provide further evidence for the crucial role of morphine-induced dysbiosis in analgesic tolerance.
Fig. 2.
Gut microbiome is responsible for gut permeability and bacterial translocation induced by morphine. (A–C) Representative H&E stained section of mouse ileum. (Scale bar: 100 μm.) WT and ABX mice: nSPF = 10, nGF = 3. (D and E) Representative images and summary of FITC-dextran fluorescent signal distribution in mice; n = 6 to 11. (F) Bacterial colony-forming unit (CFU) in liver homogenates of GF and SPF mice; nSPF = 10 to 20; nGF = 3. Data were analyzed by one-way ANOVA followed by Bonferroni’s multiple comparisons test. (G) CFU from GF mice reconstituted with normal gut microbiota and treated with either morphine or saline; nGF = 3. (H) CFU from GF mice and ABX-treated SPF mice received gut microbiota either from saline or morphine-tolerant donors; nGF = 3; nSPF/ABX = 10 to 12. Data were analyzed by Student’s t test. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Mean ± SD.
Morphine-Tolerant Animals Displayed Higher Expression of TLR2 and TLR4.
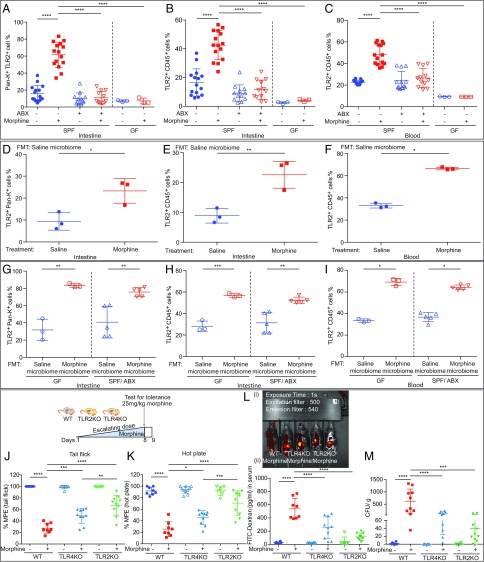
To further explore the characteristics of the translocated bacteria in the liver and MLN, we analyzed DNA isolated from the liver by gel-based PCR using the primers specific for Gram-positive and Gram-negative bacteria. We found both Gram-positive and Gram-negative bacteria translocating to the liver in the morphine-tolerant animals (SI Appendix, Fig. S3 A and B). TLR2 and TLR4 are the major receptors that mediate the host’s response to Gram-positive and Gram-negative bacteria, respectively. To determine the roles of TLR2 and TLR4 in morphine analgesic tolerance, we evaluated expression of these receptors on gut epithelial and immune cells. In morphine-tolerant animals, we found a significant increase in TLR2 and TLR4 expression in both gut epithelial cells (Fig. 3A and SI Appendix, Figs. S3C and S4A) and intraepithelial immune cells (Fig. 3B and SI Appendix, Figs. S3D and S4B). Furthermore, the expression of TLR2 and TLR4 was also significantly increased in circulating immune cells isolated from the blood of morphine-tolerant animals (Fig. 3C and SI Appendix, Figs. S3E and S4C). The expression levels of TLR2 and TLR4 were not up-regulated by morphine treatment in ABX mice (Fig. 3 A and B and SI Appendix, Fig. S3 C and D). Surprisingly, in circulating immune cells, morphine increased TLR4 expression in GF mice (SI Appendix, Fig. S4C). However, in ABX mice, TLR4 expression on circulating immune cells was not elevated by morphine (SI Appendix, Fig. S4C). After reconstituting the GF mice with the naïve microbiome of SPF mice, morphine treatment induced tolerance in these mice, and elevated TLR2 and TLR4 expression on gut epithelial and immune cells (Fig. 3 D–F and SI Appendix, Figs. S3 F–H and S4 D–F). Moreover, transplantation of microbiota from morphine-tolerant mice alone was sufficient to enhance TLR2 and TLR4 protein expression in GF and ABX mice (Fig. 3 G–I and SI Appendix, Figs. S3 I–K and S4 G–I). These data suggest that TLR2 and TLR4 activation, as a consequence of dysbiosis and bacterial translocation, and the resulting induction of proinflammatory cytokines may be mediators of morphine analgesic tolerance. To further delineate the roles of TLR2 and TLR4 in morphine analgesic tolerance, TLR2 and TLR4 Knockout (TLR2KO and TLR4KO) mice were treated with repeated injections of morphine [either with escalated (Fig. 3 J and K) or fixed doses (SI Appendix, Fig. S3 L and M)]. We found that morphine-induced analgesic tolerance was partially attenuated in both TLR2KO and TLR4KO mice using either tail flick (Fig. 3J) or hot plate (Fig. 3K) assays. Although morphine-induced analgesic tolerance was modulated by both TLR2 and TLR4, it was more significantly attenuated in TLR2KO mice (Fig. 3 J and K), with reduced gut barrier disruption (Fig. 3L) and lower bacterial translocation (Fig. 3M). This suggests a more important role for TLR2 in morphine analgesic tolerance.
Fig. 3.
Morphine regulates gut and systemic TLR2 expression through gut microbiome. (A−C) The up-regulation of TLR2 expression by morphine was suppressed in GF and ABX mice; nGF = 3; nSPF = 12 to 15. (D−F) Morphine induced TLR2 expression in GF mice following gavaging with naïve mouse microbiota; nGF = 3. (A–F) Data were analyzed by one-way ANOVA followed by Bonferroni’s multiple comparisons test. (G−I) The microbiota FMT from morphine-tolerant mice alone increased TLR2 expression in GF and ABX mice; nGF = 3; nABX = 5. Two-tailed Student’s t test was used for statistical analysis. (J and K) Morphine analgesic tolerance was determined in WT, TLR2KO, and TLR4KO mice. Ftail flick (5, 46) = 75.71; Fhot plate (5, 46) = 42.04; n = 8 to 10. (L) (i) Representative images and (ii) summary of fluorescent signal distribution in morphine-treated WT, TLR2KO, and TLR4KO mice; n = 6 to 10. (M) Visualization of bacterial colonies in blood agar plates from liver homogenates. (J–M) One-way ANOVA followed by Bonferroni’s multiple comparisons test was used for statistical analysis. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Mean ± SD.
Morphine-Tolerant Animals Displayed Sustained Chronic Systemic Inflammation.
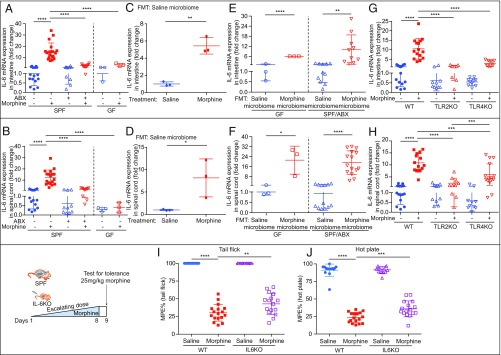
Accumulating evidence implicates inflammation as a contributing factor in morphine-induced analgesic tolerance. To further investigate the role of inflammatory cytokines in this process, animals were subjected to repeated morphine injections. In morphine-tolerant animals, the protein levels of proinflammatory cytokines IL-6, IL-1β, and TNF-α were measured in the liver, MLN, and brain by enzyme-linked immunosorbent assay (ELISA). Additionally, messenger RNA levels of these cytokines were also determined in the spinal cords and intestines. Morphine-tolerant animals displayed significantly elevated levels of these cytokines in all tissues examined. However, morphine administration to GF and ABX mice showed less inflammatory response (Fig. 4 A and B and SI Appendix, Table S1 and Fig. S5 A, B, I, and J). GF mice receiving naïve SPF microbiota developed analgesic tolerance and displayed higher expressions of these cytokines following morphine treatment (Fig. 4 C and D and SI Appendix, Table S2 and Fig. S5 C, D, K, and L). FMT of the gut microbiota from morphine-tolerant mice into GF and ABX recipients resulted in increased expression of these cytokines (Fig. 4 E and F and SI Appendix, Table S3 and Fig. S5 E, F, M, and N). Repeated morphine injections into TLR2KO and TLR4KO mice, as previously mentioned, induced less inflammation than equivalent injections into WT mice (Fig. 4 G and H and SI Appendix, Table S4 and Figs. S4 G and H and S5 G, H, O, and P). Together, these studies demonstrate that alteration in microbial compositions disrupted gut integrity, facilitated bacterial translocation, regulated TLR expressions and activation, and exacerbated inflammation, all contributing to morphine tolerance. Since morphine-tolerant animals showed a significant increase in IL-6 protein levels, we hypothesized that IL-6 may be one of the mediators of morphine tolerance. To test this hypothesis, IL-6 Knockout (IL-6KO) mice were subjected to repeated morphine injections to induce analgesic tolerance. Analgesic tolerance for both tail flick (30.23 %MPE vs. 42.82 %MPE) and hot plate assays (23.44 %MPE vs. 35.89 %MPE) (Fig. 4 I and J and SI Appendix, Fig. S5 Q and R) were only partially attenuated in these mice, indicating that other proinflammatory cytokines may also contribute to modulating analgesic tolerance. Gut histological damage (SI Appendix, Fig. S5U) and bacterial translocation (SI Appendix, Fig. S5 S and T) were also attenuated in IL-6KO mice.
Fig. 4.
Gut microbiome is essential for morphine-induced chronic systemic inflammation. (A and B) IL-6 expression in morphine-treated GF and ABX mice was reduced compared with WT controls; nGF = 3; nSPF = 10 to 17. (C and D) Morphine effect on IL-6 expression was restored after gut microbiota was reconstituted in GF mice; nGF = 3. (A–D) Data were analyzed using one-way ANOVA followed by Bonferroni’s multiple comparisons test. (E and F) GF and ABX mice were gavaged with microbiota from either saline or morphine-tolerant mice; nGF = 3; nSPF/ABX = 13 to 15. Significance was tested by two-tailed Student’s t test. (G and H) IL-6 expression was detected in WT, TLR2KO, and TLR4KO mice; n = 8 to 16. (I and J) WT and IL-6KO mice were treated with escalating morphine dosing for 8 d; n = 13 to 17. Ftail flick (3, 56) = 206.1. Fhot plate (3, 56) = 268.9. Data were analyzed by one-way ANOVA followed by Bonferroni’s multiple comparisons test. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Mean ± SD.
Morphine Analgesic Tolerance Resulted in Significant Alterations in Gut Microbiome.
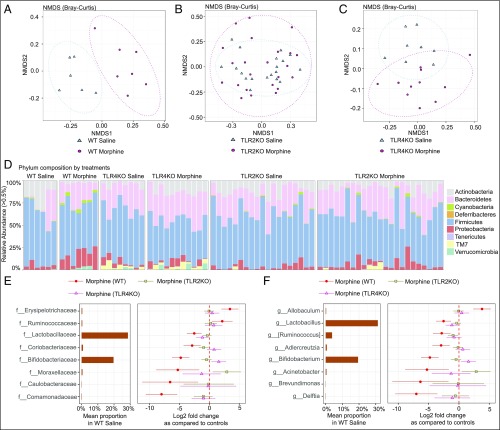
To determine whether microbial dysbiosis contributed to morphine analgesic tolerance, mice were treated with repeated morphine injections to induce analgesic tolerance. On day 8, small intestinal fecal contents were collected from morphine-tolerant mice (WT, TLR2KO, and TLR4KO) and their corresponding saline-treated controls. Fecal DNA was extracted and subjected to 16S rRNA sequencing. β-diversity analysis revealed a distinct clustering of the bacterial communities in the morphine-tolerant animals compared with saline-treated mice (P = 0.00256, permutation ANOVA (PERMANOVA) test with Bonferroni correction) (Fig. 5A). However, in TLR2KO and TLR4KO mice, using the same β-diversity matrix, we found no difference in the bacterial profiles between saline- and morphine-treated groups (P = 0.217, Fig. 5B; P = 0.183, Fig. 5C). Further analysis revealed a reduction in the relative abundance of Actinobacteria and Firmicutes at the phylum level in morphine-tolerant animals (Fig. 5D and Dataset S1). Furthermore, morphine-tolerant animals also displayed a reduction in Bifidobacteriaceae and Lactobacillaceae at the family level and Bifidobacterium and Lactobacillus at the genus level. The changes observed with WT morphine-tolerant animals were not observed in TLR2KO or TLR4KO morphine-treated mice (Fig. 5 E and F). These data imply that the microbiota of TLR2KO and TLR4KO are very stable and resist any change as a consequence of morphine treatment.
Fig. 5.
Morphine analgesic tolerance induces gut dysbiosis. (A−C) Multidimensional scaling analysis of gut microbiota to visualize the Bray−Curtis distance of WT, TLR2KO, and TLR4KO morphine-tolerant mice and their controls. Red circles depict samples from morphine-tolerant mice; blue triangles represent WT saline-treated mice. β-diversity was found to be significantly different between the WT morphine-tolerant and saline-treated groups (P = 0.00256). (D) Taxonomic distribution of WT, TLR2KO, and TLR4KO morphine-tolerant mice and their controls at phylum level. Each column represents a fecal sample from a treatment group. (E and F) Dot plots show changes in abundance of bacteria with morphine treatment in WT, TLR2KO, and TLR4KO at family and genus level using WT saline mean proportion as reference. Microbial taxa with significant difference in WT mice were selected at false discovery rate < 0.1 and average relative abundance of WT control > 0.1%; nWT = 6 to 7; nTLR2KO = 19 to 24; nTLR4KO = 8 to 11. NMDS, nonmetric multidimensional scaling.
Morphine Analgesic Tolerance Was Attenuated by Treatment with Probiotics.
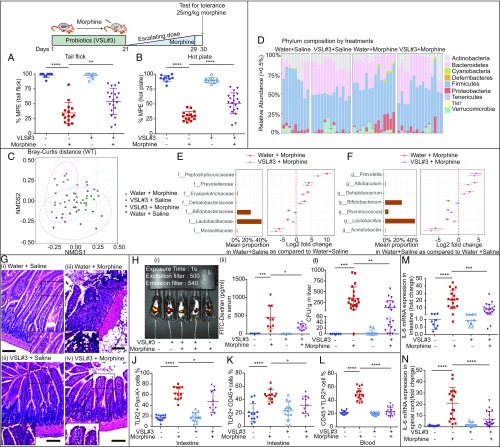
Our microbial analyses showed that the relative abundance in the Operational Taxonomic Unit representing Lactobacillaceae and Bifidobacteriaceae was significantly reduced in morphine-tolerant animals. To investigate whether supplementation with these beneficial bacteria attenuates morphine tolerance, the mice were gavaged with probiotics VSL#3. We found a dramatic decrease in morphine antinociceptive tolerance with VSL#3 pretreatment compared with sham mice following escalated morphine treatment in both tail flick (34.74 %MPE vs. 54.23 %MPE) and hot plate (30.04 %MPE vs. 51.09 %MPE) (Fig. 6 A and B) tests. Similarly, probiotics pretreatment alleviated morphine tolerance after constant doses of morphine treatment (SI Appendix, Fig. S6 A and B). Measured by β-diversity, probiotics pretreatment decreased morphine-induced microbial alterations, indicating that VSL#3 probiotics restored partial gut microbial components (Fig. 6C) (P = 0.00096 for Water+Morphine and Water+Saline; P = 0.00012 for Water+Morphine and VSL#3+Saline; P = 0.0024 for VSL#3+Morphine and Water+Saline; P = 0.02592 for VSL#3+Morphine and VSL#3+Saline). Notably, bacterial communities that were significantly reduced in relative abundance in morphine-tolerant animals were substantially restored compared with saline control sample (Fig. 6 D–F and Dataset S1). A close observation of gut histology showed less immune cell infiltration and gut epithelial damage in probiotics-treated mice (Fig. 6G). Notably, morphine-induced gut permeability (Fig. 6H) and systemic bacterial translocation into liver and MLN (Fig. 6I and SI Appendix, Fig. S6C) were rescued using probiotics treatment. Furthermore, morphine tolerance induced increases in TLR2 and TLR4 expression on the gut epithelia, gut, and systemic immune cells (Fig. 6 J–L and SI Appendix, Fig. S6 D–F and G−I), and elevated proinflammatory cytokines IL-6 (Fig. 6 M and N and SI Appendix, Table S5), IL-1β (SI Appendix, Fig. S6 J and K), and TNF-α (SI Appendix, Fig. S6 L and M), which were also ameliorated by probiotics pretreatment. These data clearly support the use of probiotics as an adjunct therapy in patients using opioids for pain management.
Fig. 6.
Probiotics VSL#3 pretreatment attenuates morphine tolerance and prevents morphine-induced gut microbiota alterations. (A and B) Morphine analgesic effect was determined after escalating doses of morphine; n = 10 to 20. Ftail flick (3, 51) = 46.78. Fhot plate (3, 51) = 82.50. One-way ANOVA followed by Bonferroni’s correction was used to analyze data. (C) Multidimensional scaling was used to visualize the Bray−Curtis distance of different groups; n = 9 to 15. Data were subjected to PERMANOVA test along with Bonferroni correction. (D) Taxonomic distribution of different groups at phylum level. (E and F) Dot plots show changes in abundance of bacteria in Water+Morphine-treated and VSL#3+Morphine-treated mice at family and genus level using Water+Saline mean proportion as reference; n = 9 to 15. (G) Representative H&E stained intestinal sections from different treatment groups; n = 6 to 10. (H) (i) Representative figure of florescent signal distribution and (ii) summary of serum FITC-dextran concentration from different treatment groups; n = 6 to 10. (I) CFU from liver homogenate of each mouse in different treatment groups; n = 6 to 20. (J−L) Probiotics pretreatment inhibits TLR2 up-regulation by morphine on epithelial and immune cells; n = 11 to 14. (M and N) RT-PCR of the IL-6 gene expression; n = 9 to 19. (E–N) The results were analyzed by one-way ANOVA followed by Bonferroni’s multiple comparisons test. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Mean ± SD.
Discussion
In the current study, we found a vicious cycle in the development and maintenance of morphine tolerance. Chronic morphine-induced dysbiosis initiates local gut inflammation through TLR2 and TLR4 activation, resulting in the induction of proinflammatory cytokines, including IL-6, which drives morphine tolerance. Proinflammatory cytokines, in turn, aggravate dysbiosis, resulting in impaired gut integrity and induction of bacterial translocation, thus exacerbating inflammation and sustaining morphine tolerance. However, when gut dysbiosis was restored with probiotics, morphine tolerance was attenuated, with fewer pathological symptoms. These studies indicate that gut bacterial imbalance plays an important role in analgesic tolerance through activation of the gut−immune−brain axis (SI Appendix, Fig. S7). Our studies have clear clinical implications and suggest that probiotic pretreatment can prolong the efficacy of morphine as an analgesic agent.
The gut microbiome is a diverse community that maintains a close relationship with the host. Depletion of the gut microbiome with broad-spectrum antibiotics and/or absence of the gut microbiome as in GF mice have revealed the critical role of the gut microbiome in the behavior, stress- and pain-modulation systems, and central neurotransmitter systems (26–28). The gut microbiome, as a potential contributor to morphine analgesic tolerance, was previously implicated in studies by Kang et al. (29) wherein they demonstrated that antibiotics prevented morphine-induced hypoexcitability of dorsal root ganglion (DRG) nociceptors following chronic morphine exposure. Additional studies demonstrated that gut-derived mediators produced by Gram-positive bacteria were responsible for the development of morphine tolerance by regulating the DRG neuron through tetrodotoxin-resistant Na+ channels (30). In addition, the gut microbiota was also implicated in microglia activation, resulting in alteration in morphine tolerance and impairment in cocaine reward behavior (31). To determine whether morphine tolerance results in dysbiosis, we initially detected distinct microbial changes and the functional characteristics of these changes in morphine-tolerant mice. β-diversity analysis revealed a distinct clustering of the microbiome in the morphine analgesic group with a significant decrease in Bifidobacterium and Lactobacillus at the genus level. The dramatic depletion of Bifidobacterium and Lactobacillus, two essential and common inhabitants of the human intestine, were of particular interest because of their roles in maintaining gut homeostasis and gut epithelial integrity (32). Bifidobacterium and Lactobacillus with antiinflammatory and antioxidative properties are also shown to be significantly decreased in aging and in Alzheimer’s patients with concomitant increase in intestinal permeability and inflammation (33). Together, our studies imply the reduction in these two bacterial communities plays a crucial role in gut inflammation and is thus implicated in morphine tolerance. Taken together, these studies provide strong support for the role of the gut microbiome in the development of morphine analgesic tolerance, and support targeting the microbiome as a druggable site for prolonging the analgesic efficacy of morphine for pain management.
We also observed that depletion of these gut microbial communities in morphine-tolerant animals is associated with an expansion of Allobaculum, Peptostreptococcacea, and Prevotellaceae. We posit that the dominance of pathogenic taxa and depletion of antiinflammatory genus contribute to sequential increase in intestinal permeability, increased microbial translocation, and sustained systemic inflammation in morphine-tolerant animals. In addition, studies have examined the role of gut dysbiosis in abuse of other substances. Pathobionts Thauera, Paracoccus, and Prevotella were significantly increased in patients abusing heroin, crystal methamphetamine (ice), and ephedrine. The functional metagenomics analysis (PiCRUST) of the microbiome in substance abusers indicated that pathways including DNA replication and repair, and cell growth and death, were up-regulated in gut microbiota of substance abusers. The up-regulated pathways might explain the mechanisms through which substance-induced dysbiosis exerts pathogenic effects on cell communication, cardiovascular diseases, and the circulatory system (25). Similarly, chronic opioid use is associated with altered relative abundances of Prevotella and Bacteroides, which, in turn, may affect systemic opioid receptor function and cognition (23). The role of dysbiosis in addiction and cognitive changes following chronic prescription opioid use requires further investigation.
Our studies of morphine tolerance in relation to gut dysbiosis focused exclusively on immune signaling mechanisms. Studies have shown that morphine-induced gut dysbiosis was causally correlated with impaired cocaine-conditioned place preference and morphine hyperalgesia by modulating microglial activation (31). Moreover, our studies showed that chronic morphine-induced gut dysbiosis created an inflammatory environment with compromised gut epithelial barrier function, bacterial translocation, and proinflammatory cytokines. Other studies have shown that this inflammatory environment enhanced ATP release through Connexin-43 hemichannel in enteric glia, resulting in deficits in regulating gastrointestinal motility (34). However, the gut microbes also communicate to the brain through vagal nerves, gastrointestinal hormones, and microbial metabolites (19). It is also plausible that morphine-induced microbial dysbiosis may result in altered morphine metabolism, thus contributing to morphine tolerance. This is consistent with our published data, which showed that depletion in bacterial communities that are associated with deglucuronidation of the major morphine metabolite morphine-3-glucuronide results in decreased enterohepatic recirculation of morphine, resulting in decreased systemic morphine levels over time (22). In the future, studies using stable morphine isotopes (Morphine-D3) to investigate the role of gut dysbiosis in morphine metabolism kinetics, which can influence morphine analgesic tolerance, are warranted.
Recent studies have shown the benefits of probiotics as a means to restore and maintain health in diseased states such as allergic disease, diarrhea, irritable bowel disease, Alzheimer’s disease, anxiety, and depression (35). A recent report showed an increase in OPRM1 expression in human HT-29 epithelial cells following treatment with Lactobacillus (especially Lactobacillus acidophilus NCFM) (36). These studies further suggested that commensal bacteria can control the transmission of nociceptive information of the intestinal nervous system through opioid receptors. It is likely that loss of these commensals, along with reduced expression of opioid receptors on epithelial cells, may contribute to reduced antinociceptive signals and heightened pain. In addition, VSL#3 probiotics have been shown to inhibit disease-associated cytokines IFN-γ, IL-17, and IL-6 while increasing antiinflammatory cytokines TGF-β, IL-10, and IL-4 in experimental autoimmune encephalomyelitis (37). Furthermore, VSL#3 probiotics were reported to ameliorate age-related deficits and reverse the effects of genes associated with aging (38). Together, our studies found that decreases in Lactobacillus and Bifidobacterium play a crucial role in gut inflammation and are thus implicated in morphine tolerance. Our studies support the protective effect of probiotics on reinstating morphine effectiveness through the gut−brain axis in a morphine-tolerant model.
In this study, we focused on the contribution of peripheral mechanisms to morphine-induced tolerance. We implicated microbial dysbiosis, bacterial translocation, and intestinal and systemic TLR2 and TLR4 activation as mediators of morphine analgesic tolerance. We suggested that TLR2 and TLR4 activation are components of the gut−brain axis that contribute to morphine tolerance, which is consistent with findings from Song and Zhao (39) and Eidson and Murphy (40). We propose IL-6 as a downstream factor of TLR2 and TLR4 activation that can contribute to morphine tolerance. However, our results show only partial attenuation of morphine tolerance in the IL-6KO mice. This suggests that other factors may be involved in morphine tolerance, including cytokines/chemokines such as IL-1β, TNFα, CXCL1, IL-10, CXCL12, and other peripheral factors such as complement factor 5 receptor, tissue plasminogen activator, neuronal matrix metalloproteinase 9, and neuronal nitric oxide synthase (41–46). Studies have confirmed the presence of additional peripheral mechanisms affecting morphine tolerance. For example, morphine tolerance could be abolished by blockade of peripheral OPRM1 on DRG by methylnaltrexone bromide, or blockade of JAK2/STAT3 pathway by regulating miR-375, or activation of Mrg3C receptor in DRG (47–49). Taken together, these studies have indicated that peripheral OPRM1 activation plays a crucial role in morphine tolerance, and therapeutics targeted at inhibiting peripheral OPRM1 may prolong morphine’s efficacy at central analgesic sites.
Our studies, however, suggest that probiotic therapy at the time of morphine administration is a promising, safe, and inexpensive treatment for attenuating morphine analgesic tolerance and prolonging its efficacy as an analgesic agent.
Methods
All procedures were approved by Institutional Animal Care and Use Committee at the University of Miami. All mice (C57BL/6, TLR2KO, TLR4KO, and IL-6KO) were housed three to five per cage in SPF conditions and maintained on a 12-h light/dark cycle in a constant temperature (20 °C to 22 °C) and humidity (45 to 55%) with ad libitum access to food and water. GF mice were maintained in CBC (Class Biologically Clean Ltd.) flexible film isolators with sterile food and water. SI Appendix provides complete experimental methods. It includes animal treatment, FMT, behavior study, intestinal permeability and bacterial translocation, Real-Time PCR and PCR, intestinal cell isolation and fluorescence-activated cell sorting, ELISA, histology, statistical analysis, 16S rRNA sequencing, and metagenomic data analysis.
Supplementary Material
Acknowledgments
We thank Dr. April Dawn Mann and Dr. Valerie Gramling at the writing center of University of Miami for assistance with grammar, and Dr. Oliver Umland for providing help and support with flow cytometry. The authors and their work were supported by National Institutes of Health Grants R01 DA044582, R01 DA043252, R01 DA037843, R01 DA047089, and R01 DA034582.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The raw sequencing data that support the findings of this study have been submitted to Sequence Read Archive (SRA) of the National Center for Biotechnology Information under accession number PRJNA531200.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1901182116/-/DCSupplemental.
References
- 1.Wickham R. J., Cancer pain management: Opioid analgesics, Part 2. J. Adv. Pract. Oncol. 8, 588–607 (2017). [PMC free article] [PubMed] [Google Scholar]
- 2.Cherny N., et al. ; Expert Working Group of the European Association of Palliative Care Network , Strategies to manage the adverse effects of oral morphine: An evidence-based report. J. Clin. Oncol. 19, 2542–2554 (2001). [DOI] [PubMed] [Google Scholar]
- 3.Volkow N. D., McLellan A. T., Opioid abuse in chronic pain–Misconceptions and mitigation strategies. N. Engl. J. Med. 374, 1253–1263 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Jalal H., et al. , Dynamics of the drug overdose epidemic in the United States, 1979-2016. Science 361, eaau1184 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dumas E. O., Pollack G. M., Opioid tolerance development: A pharmacokinetic/pharmacodynamic perspective. AAPS J. 10, 537–551 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gulur P., Williams L., Chaudhary S., Koury K., Jaff M., Opioid tolerance—A predictor of increased length of stay and higher readmission rates. Pain Physician 17, E503–E507 (2014). [PubMed] [Google Scholar]
- 7.Cron D. C., et al. , Preoperative opioid use is independently associated with increased costs and worse outcomes after major abdominal surgery. Ann. Surg. 265, 695–701 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Bailey C. P., Connor M., Opioids: Cellular mechanisms of tolerance and physical dependence. Curr. Opin. Pharmacol. 5, 60–68 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Ma W., Zheng W.-H., Powell K., Jhamandas K., Quirion R., Chronic morphine exposure increases the phosphorylation of MAP kinases and the transcription factor CREB in dorsal root ganglion neurons: An in vitro and in vivo study. Eur. J. Neurosci. 14, 1091–1104 (2001). [DOI] [PubMed] [Google Scholar]
- 10.Shavit Y., Wolf G., Goshen I., Livshits D., Yirmiya R., Interleukin-1 antagonizes morphine analgesia and underlies morphine tolerance. Pain 115, 50–59 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Shen C. H., Tsai R. Y., Wong C. S., Role of neuroinflammation in morphine tolerance: Effect of tumor necrosis factor-α. Acta Anaesthesiol. Taiwan. 50, 178–182 (2012). [DOI] [PubMed] [Google Scholar]
- 12.DeLeo J. A., Tanga F. Y., Tawfik V. L., Neuroimmune activation and neuroinflammation in chronic pain and opioid tolerance/hyperalgesia. Neuroscientist 10, 40–52 (2004). [DOI] [PubMed] [Google Scholar]
- 13.Hutchinson M. R., et al. , Exploring the neuroimmunopharmacology of opioids: An integrative review of mechanisms of central immune signaling and their implications for opioid analgesia. Pharmacol. Rev. 63, 772–810 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutchinson M. R., et al. , Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia. Brain Behav. Immun. 22, 1178–1189 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X., et al. , Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc. Natl. Acad. Sci. U.S.A. 109, 6325–6330 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnston I. N., et al. , A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J. Neurosci. 24, 7353–7365 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilbert J. A., et al. , Current understanding of the human microbiome. Nat. Med. 24, 392–400 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schroeder B. O., Bäckhed F., Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 22, 1079–1089 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Fung T. C., Olson C. A., Hsiao E. Y., Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 20, 145–155 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carvalho F. A., Aitken J. D., Vijay-Kumar M., Gewirtz A. T., Toll-like receptor-gut microbiota interactions: Perturb at your own risk! Annu. Rev. Physiol. 74, 177–198 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Banerjee S., et al. , Opioid-induced gut microbial disruption and bile dysregulation leads to gut barrier compromise and sustained systemic inflammation. Mucosal Immunol. 9, 1418–1428 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang F., et al. , Morphine induces changes in the gut microbiome and metabolome in a morphine dependence model. Sci. Rep. 8, 3596 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barengolts E., et al. , Gut microbiota varies by opioid use, circulating leptin and oxytocin in African American men with diabetes and high burden of chronic disease. PLoS One 13, e0194171 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Acharya C., et al. , Chronic opioid use is associated with altered gut microbiota and predicts readmissions in patients with cirrhosis. Aliment. Pharmacol. Ther. 45, 319–331 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Xu Y., et al. , Bacterial diversity of intestinal microbiota in patients with substance use disorders revealed by 16S rRNA gene deep sequencing. Sci. Rep. 7, 3628 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Asmakh M., Zadjali F., Use of germ-free animal models in microbiota-related research. J. Microbiol. Biotechnol. 25, 1583–1588 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Mayer EA, Tillisch K, Gupta A, Gut/brain axis and the microbiota Emeran. Nutr. Cancer 125, 926–938 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amaral F. A., et al. , Commensal microbiota is fundamental for the development of inflammatory pain. Proc. Natl. Acad. Sci. U.S.A. 105, 2193–2197 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang M., et al. , The effect of gut microbiome on tolerance to morphine mediated antinociception in mice. Sci. Rep. 7, 42658 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mischel R. A., et al. , Tolerance to morphine-induced inhibition of TTX-R sodium channels in dorsal root ganglia neurons is modulated by gut-derived mediators. iScience 2, 1–17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee K., et al. , The gut microbiota mediates reward and sensory responses associated with regimen-selective morphine dependence. Neuropsychopharmacology 43, 2606–2614 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang B. G., Xu H. B., Wei H., Zeng Z. L., Xu F., Oral administration of Bifidobacterim bifidum for modulating microflora, acid and bile resistance, and physiological indices in mice. Can. J. Microbiol. 61, 155–163 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi Y., et al. , Therapeutic potential of Bifidobacterium breve strain A1 for preventing cognitive impairment in Alzheimer’s disease. Sci. Rep. 7, 13510 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhave S., et al. , Connexin-purinergic signaling in enteric glia mediates the prolonged effect of morphine on constipation. FASEB J. 31, 2649–2660 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossi G., et al. , Comparison of microbiological, histological, and immunomodulatory parameters in response to treatment with either combination therapy with prednisone and metronidazole or probiotic VSL#3 strains in dogs with idiopathic inflammatory bowel disease. PLoS One 9, e94699 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rousseaux C., et al. , Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat. Med. 13, 35–37 (2007). [DOI] [PubMed] [Google Scholar]
- 37.Salehipour Z., et al. , Bifidobacterium animalis in combination with human origin of Lactobacillus plantarum ameliorate neuroinflammation in experimental model of multiple sclerosis by altering CD4+ T cell subset balance. Biomed. Pharmacother. 95, 1535–1548 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Distrutti E., et al. , Modulation of intestinal microbiota by the probiotic VSL#3 resets brain gene expression and ameliorates the age-related deficit in LTP. PLoS One 9, e106503 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song P., Zhao Z. Q., The involvement of glial cells in the development of morphine tolerance. Neurosci. Res. 39, 281–286 (2001). [DOI] [PubMed] [Google Scholar]
- 40.Eidson L. N., Murphy A. Z., Blockade of Toll-like receptor 4 attenuates morphine tolerance and facilitates the pain relieving properties of morphine. J. Neurosci. 33, 15952–15963 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raghavendra V., Rutkowski M. D., DeLeo J. A., The role of spinal neuroimmune activation in morphine tolerance/hyperalgesia in neuropathic and sham-operated rats. J. Neurosci. 22, 9980–9989 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin C. P., et al. , CXCL12/CXCR4 signaling contributes to the pathogenesis of opioid tolerance: A translational study. Anesth. Analg. 124, 972–979 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Li Y. H., et al. , Complement factor C5a and C5a receptor contribute to morphine tolerance and withdrawal-induced hyperalgesia in rats. Exp. Ther. Med. 4, 723–727 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berta T., Liu Y. C., Xu Z. Z., Ji R. R., Tissue plasminogen activator contributes to morphine tolerance and induces mechanical allodynia via astrocytic IL-1β and ERK signaling in the spinal cord of mice. Neuroscience 247, 376–385 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakamoto K., et al. , Involvement of matrix metalloproteinase-9 in the development of morphine tolerance. Eur. J. Pharmacol. 683, 86–92 (2012). [DOI] [PubMed] [Google Scholar]
- 46.Liu W., et al. , Inhibition of neuronal nitric oxide synthase antagonizes morphine antinociceptive tolerance by decreasing activation of p38 MAPK in the spinal microglia. Neurosci. Lett. 410, 174–177 (2006). [DOI] [PubMed] [Google Scholar]
- 47.Corder G., et al. , Loss of μ opioid receptor signaling in nociceptors, but not microglia, abrogates morphine tolerance without disrupting analgesia. Nat. Med. 23, 164–173 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li H., Tao R., Wang J., Xia L., Upregulation of miR-375 level ameliorates morphine analgesic tolerance in mouse dorsal root ganglia by inhibiting the JAK2/STAT3 pathway. J. Pain Res. 10, 1279–1287 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y., Wang K., Lin M., Li Q., Hong Y., Inhibition of morphine tolerance by MrgC receptor via modulation of interleukin-1β and matrix metalloproteinase 9 in dorsal root ganglia in rats. Eur. J. Pharmacol. 815, 10–17 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.