Significance
Darwin argued that differential natural selection across environments leads to the evolution of reproductive isolation and speciation. More recently, this process has been dubbed “ecological speciation.” Although simple in principle, mechanisms that link adaptation and reproductive isolation have seldom been demonstrated. We triggered diversification in the descendants of a single parasite population confined to different host “islands.” We used feather-feeding lice that pass their entire life cycle on the host, making it possible to evolve lice under natural conditions. Lineages of lice transferred to different sized pigeons rapidly evolved differences in size. These size differences reduced the ability of lice from different hosts to mate and reproduce. Our results effectively demonstrate how natural selection can cause species to form.
Keywords: adaptation, ecological speciation, magic trait, ectoparasite, natural selection
Abstract
Ecological speciation occurs when local adaptation generates reproductive isolation as a by-product of natural selection. Although ecological speciation is a fundamental source of diversification, the mechanistic link between natural selection and reproductive isolation remains poorly understood, especially in natural populations. Here, we show that experimental evolution of parasite body size over 4 y (approximately 60 generations) leads to reproductive isolation in natural populations of feather lice on birds. When lice are transferred to pigeons of different sizes, they rapidly evolve differences in body size that are correlated with host size. These differences in size trigger mechanical mating isolation between lice that are locally adapted to the different sized hosts. Size differences among lice also influence the outcome of competition between males for access to females. Thus, body size directly mediates reproductive isolation through its influence on both intersexual compatibility and intrasexual competition. Our results confirm that divergent natural selection acting on a single phenotypic trait can cause reproductive isolation to emerge from a single natural population in real time.
Understanding mechanisms responsible for the origin of new species is a fundamental goal of evolutionary biology (1, 2). Ecological speciation is a process in which local adaptation to different environments leads to reproductive isolation (3–9). However, direct demonstrations of the mechanisms that drive this process have been elusive, especially in natural populations. Most empirical studies of ecological speciation retrospectively investigate closely related species or ecotypes that have already evolved some degree of divergence (9–11). Thus, most studies rely on inferences about past selective events that shaped contemporary patterns of diversification. Examining speciation in this way makes it challenging to directly link natural selection to the evolution of reproductive isolation (12). We took a complementary approach and evolved reproductive isolation from a single natural population adapting to divergent selective environments in real time. In the past, this approach has been largely restricted to studies involving Drosophila (13, 14). We used a host–parasite system to identify the selective agents that trigger divergence of isolated lines from a single natural population of parasites. We further characterized the prezygotic mechanism that causes reproductive isolation.
Theory predicts that ecological speciation happens most readily when natural selection directly acts on traits that govern both survival and reproduction (9, 15, 16). The effectiveness of selection on these “multipurpose” traits lies in the pleiotropy or tight linkage of the underlying genes, which are not broken down by recombination. However, pleiotropy is much more effective than linkage disequilibrium in connecting natural selection to nonrandom mating (16–18). Therefore, selection acting on a single phenotypic trait could conceivably cause automatic reproductive isolation as a by-product of adapting to novel environments (9, 15, 16).
The idea that speciation can be driven by a single trait under divergent selection has a long history and varied nomenclature, including the “pleiotropy model” (19); “by-product mechanism” (5); “magic trait” model (20); and “multiple-effect trait” model (16). However, surprisingly few studies have demonstrated how selection on a single trait can actually generate assortative mating (reviewed in ref. 21). Thus, the importance of such traits in driving speciation remain largely unknown, even in the best studied systems (16, 22).
Host–parasite interactions provide tractable systems to experimentally test how natural selection can trigger reproductive isolation (18, 23–25). Populations of parasites are subject to the same factors thought to lead to adaptive diversification in free-living groups: namely ecological opportunity, fragmented habitats, and local adaptation (12, 26, 27). For parasites, host species are analogous to islands, and host switching is analogous to dispersal among islands. Hosts are patchily distributed and provide barriers to gene flow between parasite populations (28–30). Ecological speciation via host switching makes two clear predictions: (i) following a host switch, parasites may evolve adaptations in response to novel host defenses, (ii) if these adaptations directly influence assortative mating, then reproductive isolation will evolve between conspecifics exploiting different host species (31, 32). Indeed, the role of host switching in parasite diversification has been the subject of more than a century of evolutionary research (28, 31, 33–35). However, despite widespread acceptance that parasites frequently adapt to novel hosts, it remains unclear how these adaptations alone lead to speciation (31, 32).
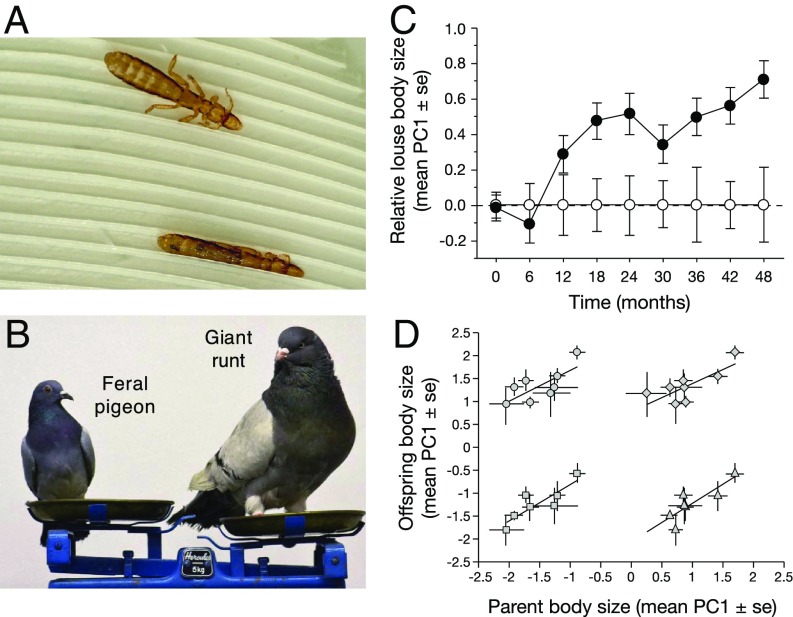
We conducted a test of “host-switching” speciation using a host–parasite system consisting of rock pigeons (Columba livia) and their parasitic feather lice (Insecta: Phthiraptera: Ischnocera). Feather lice are host-specific parasites of birds that feed and pass their entire life cycle on the body of the host. Members of the genus Columbicola are parasites of pigeons and doves (Columbiformes) that feed on the downy regions of feathers, causing energetic stress and a reduction in host fitness through reduced survival and mating success (36). Pigeons combat feather lice by removing them with their beaks during regular bouts of preening. Columbicola columbae, a parasite of feral pigeons, avoids preening by hiding in spaces between adjacent feather barbs (Fig. 1A); preening selects for C. columbae small enough to fit between the barbs (36, 37). Preening also exerts selection on traits critical for locomotion on the host. When lice are placed on feathers from larger hosts, they show decreased running speed, presumably because of the greater distance between the barbs of larger feathers (36, 38). Thus, when lice find themselves on larger hosts, preening will favor larger individuals that are better able to run on larger feathers. In the absence of preening, large bodied lice are also favored because of a size-fecundity correlation: Large lice lay more eggs than small-bodied lice (39). Thus, selection to remain small enough to fit between feather barbs of small bodied hosts is countered by selection for rapid locomotion and higher fecundity on large hosts. These opposing selective forces explain the high correlation between the body sizes of different species of pigeons and their host-specific species of Columbicola, a macroevolutionary pattern known as Harrison’s Rule (36, 40).
Fig. 1.
Experimental evolution of C. columbae body size. (A) Live C. columbae walking on the surface of a feral pigeon wing feather (Upper) and inserted between adjacent feather barbs (Lower) to escape host preening. (B) Relative sizes of a feral pigeon (∼340 g) and a domesticated giant runt pigeon (∼1,100 g), both Columba livia. Image courtesy of Andrew Bartlow (photographer). (C) Increase in the relative size of C. columbae on giant runts (filled circles) over 4 y (ca. 60 louse generations), compared with the size of lice on feral pigeons (open circles, set to zero) (LMM, n = 3,096, t = 3.15, P = 0.002). (D) Common garden experiment showing that C. columbae body size is heritable. Each point compares the mean (±SE) body size of parental and offspring cohorts on a single common garden feral pigeon. Parent and offspring size are highly correlated in all cases. Points are as follows: daughters vs. fathers (circles, Upper Left; linear regression, r = 0.73, df = 7, F = 7.13, P = 0.037), daughters vs. mothers (diamonds, Upper Right; r = 0.77, df = 7, F = 8.65, P = 0.026), sons vs. fathers (squares, Lower Left r = 0.86, df = 6, F = 13.90, P = 0.014), and sons vs. mothers (triangles, Lower Right r = 0.84, df = 6, F = 12.61, P = 0.016).
Because feather lice are “permanent” parasites that pass their entire life cycle on the body of the host, they can be maintained under natural conditions on captive birds (36). This fact, coupled with their relatively short (24-d) generation time, makes feather lice tractable for experimental evolution studies of reproductive isolation. Feather lice essentially provide an ecological intermediate between conventional laboratory models, such as Drosophila (13) and yeast (41), and field-based models, such as Darwin's finches (42), Heliconius butterflies (43), Mimulus flowers (44), Rhagoletis flies (45), and threespine sticklebacks (46).
Results and Discussion
To test for adaptation in response to host body size, we conducted a 4-y experiment [approximately (ca.) 60 louse generations] using C. columbae placed on captive pigeons of different sizes. We transferred lice from wild caught feral pigeons to giant runts, a domesticated breed of pigeon that is threefold larger than feral pigeons (Fig. 1B). We also transferred C. columbae to feral pigeon controls. We quantified the size of lice by measuring louse body length, metathorax width, and head width. These measures are highly correlated, so we used the first principal component (PC1) as an index of overall louse size (SI Appendix, Table S1). Over the course of 4 y, lice on giant runts increased in size, relative to lice on feral pigeon controls (Fig. 1C and SI Appendix, Tables S2–S5). Midway through the study (2 y), we performed a common garden experiment and confirmed that louse body size is heritable (Fig. 1D). These data are consistent with Harrison’s Rule, the observation that larger bodied hosts tend to have larger bodied parasites (40). In the bird-louse system, this macroevolutionary pattern is driven by selection for a match between parasite body size and morphological features of the host that correlate with overall host size. Feather lice routinely seek refuge from preening in the interbarb spaces of flight feathers, and the size of these spaces is highly correlated with host body size (Fig. 1A) (36). This relationship is not only consistent across host species (40), but within species as well; the interbarb space of giant runts is typically 20% larger than the interbarb space of feral pigeons (47). In summary, our 4-y experimental evolution study shows that host-imposed natural selection drives rapid local adaptation of louse body size.
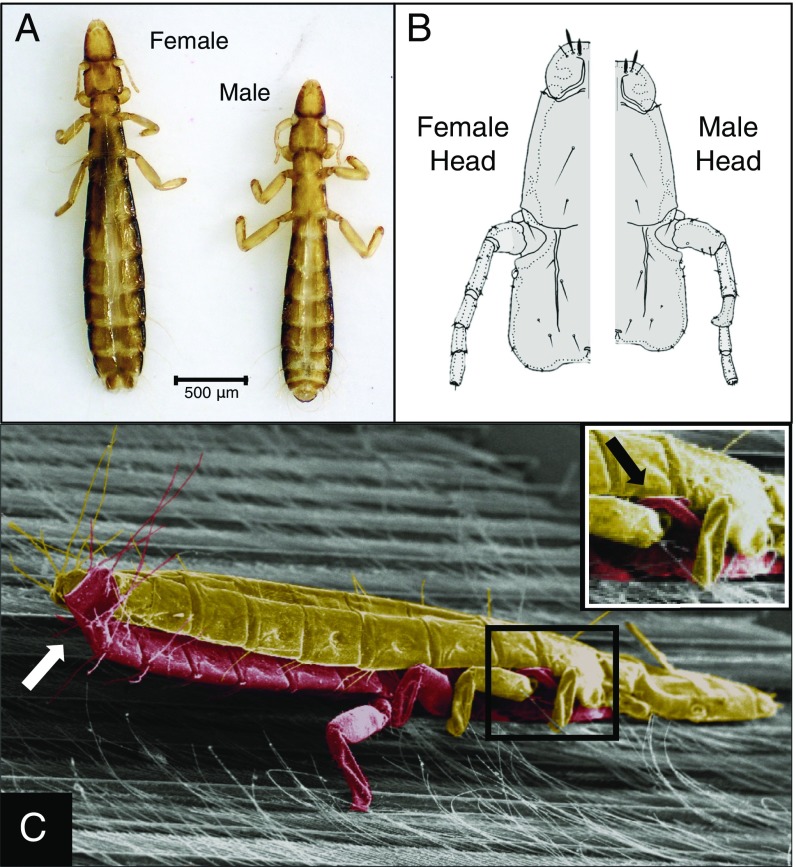
Body size also plays a role in the reproductive biology of C. columbae. Male and female C. columbae are sexually dimorphic in overall body size (Fig. 2A), and in the structure of the male antennae (Fig. 2B), which are used to grasp the female during copulation (Fig. 2C). Behavioral observations of live C. columbae suggest that the extent of body size dimorphism may influence copulation success. During copulation between individuals showing “typical” dimorphism (SI Appendix, Table S6), males use their antennae to grasp the female’s metathorax while aligning the tip of their abdomen with the female’s abdomen (Figs. 2C and 3 A and B and Movie S1). In contrast, when males are either too large or too small, relative to the female, they have difficulty copulating (Fig. 3 C–F and Movies S2 and S3). These observations suggest that increases in the body size of C. columbae on giant runts may reduce their ability to copulate with smaller lice from feral pigeons. Thus, reproductive isolation may evolve as a direct result of adapting to a new environment.
Fig. 2.
Sexual size dimorphism of C. columbae. (A) Females are typically 13% larger than males. (B) Sexually dimorphic heads showing male antenna with larger scape (first segment) and inward pointing spur on the third segment. (C) Colorized SEM of C. columbae copulating on a pigeon feather: male (red) grabbing the female (gold) with his antennae (black arrow; Inset), while curling the tip of his abdomen dorsally to contact the tip of her abdomen (white arrow).
Fig. 3.
Influence of body size on mating position in C. columbae (female on top, male on bottom in all photos). Still photographs are from videos (SI Appendix). (A and B) Abdomens are parallel during copulation in pairs of lice with typical dimorphism (Movie S1). (C and D) Relatively large males seldom succeed in copulating, but when they do, they are S-shaped (Movie S2). (E and F) Females copulating with relatively small males, which is also rare, are arched during copulation (Movie S3). Dimorphism scores (male length − female length): A and B = −346 μm; C and D = −197 μm; E and F = −561 μm.
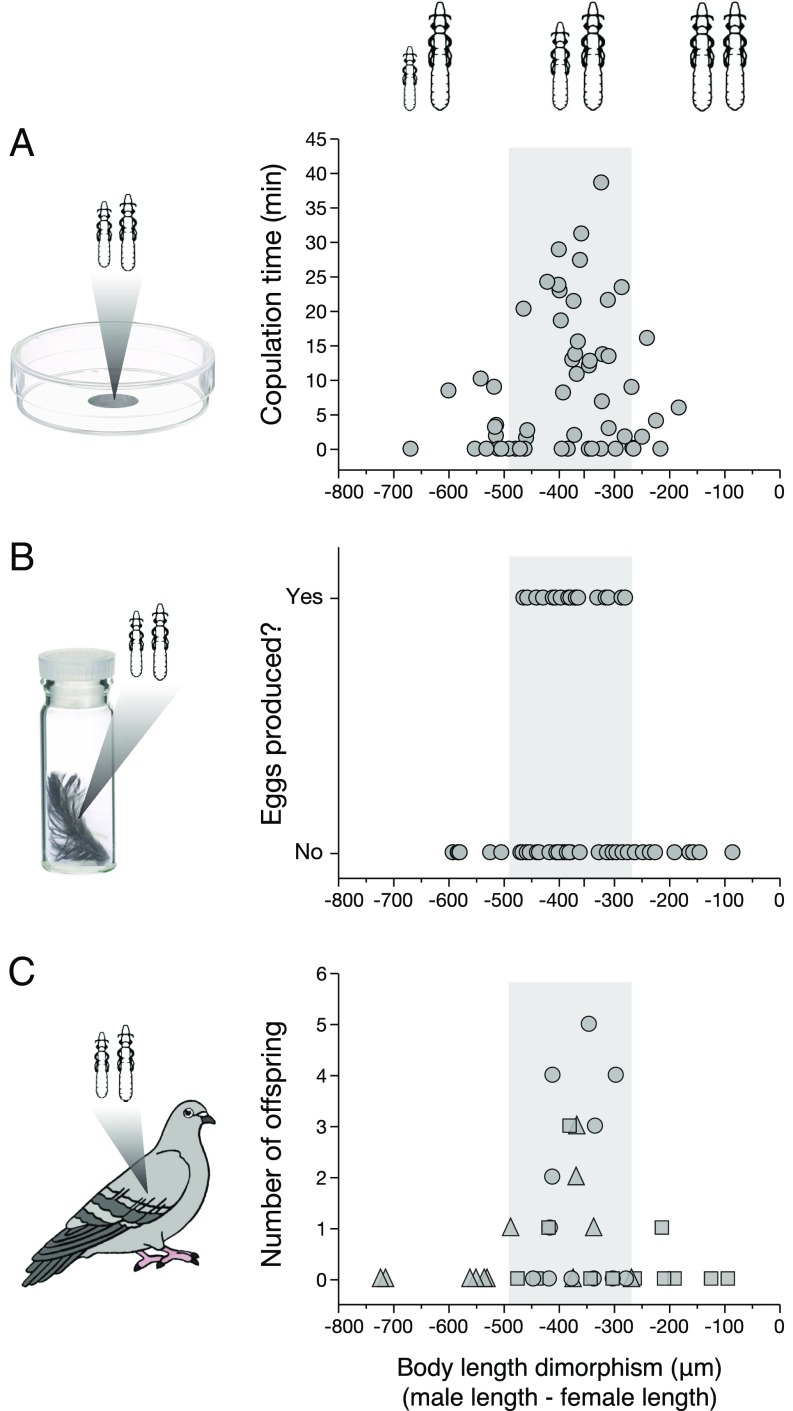
We conducted a series of experiments to test the effect of body size on louse reproductive success (Fig. 4). First, we quantified time spent copulating by pairs of lice that vary in degree of dimorphism. The lice used in this experiment came from feral pigeons (Materials and Methods). Lice were filmed in mating arenas on detached feathers. Pairs of lice with typical dimorphism (SI Appendix, Table S6) copulated for significantly longer than “mismatched” pairs with more or less dimorphism (Fig. 4A). Observations of lice in mating arenas showed that, although virtually all males attempted to copulate, individuals that were too small or too large, relative to females, had difficulty. On average, mismatched pairs spent 70% less time copulating than typical pairs. Thus, copulation time is a function of the relative dimorphism of male and female lice (Fig. 4A).
Fig. 4.
Reproductive performance of C. columbae differing in size dimorphism. Values on the x axis indicate the difference in body length of the male relative to that of the female (e.g., “−400 μm” represents a trial in which the male was 400 μm shorter than the female). Gray shaded region in each plot represents the typical range of dimorphism of lice (SI Appendix, Table S6); relatively small males at left, large males at right (illustrations not to scale). (A) Copulation time of lice in mating arenas was correlated with size dimorphism (NLM, n = 56, t = −2.30, P = 0.03; SI Appendix, Table S7). (B) Pairs of lice with typical degrees of dimorphism were more likely to produce eggs (GNLM, n = 58, z = −1.96, P < 0.05; SI Appendix, Table S8); 17 of 42 typical pairs (40.5%) produced eggs, but none of 16 pairs (0.0%) with relatively small or large males produced eggs. (C) Reproductive success of 36 pairs of lice from feral or giant runt pigeons transferred to 36 louse-free feral pigeons: 12 pairs included a male giant runt louse and a female feral pigeon louse (squares); 12 pairs included a male feral pigeon louse and a female giant runt louse (triangles); 12 (control) pairs included a male feral pigeon louse and a female feral pigeon louse (circles) (SI Appendix, Table S9). Pairs with intermediate levels of dimorphism produced significantly more offspring than pairs with extreme dimorphism (GLNMM, n = 36, z = −2.21, P = 0.03; SI Appendix, Table S10). Reproductive success was governed by the relative size of the male and female lice, independent of the type of host on which they evolved (compare circles, triangles, and squares).
We also tested the effect of size dimorphism on the production of eggs by lice. As before, lice used in this experiment were from feral pigeons (Materials and Methods). Pairs of virgin lice were placed in vials of feathers kept in an incubator at optimal temperature and humidity (48). The vials were checked daily for several weeks until the female in each vial died. The presence or absence of eggs laid by the female was then tallied. Pairs of lice with typical dimorphism (SI Appendix, Table S6) were significantly more likely to produce eggs than mismatched pairs. Nearly all of the eggs (92%) had developing embryos. Thus, the production of viable eggs is also a function of relative dimorphism (Fig. 4B).
Next, we tested the effect of variable dimorphism on the reproductive success of lice on live pigeons. Single pairs of lice were placed on individual feral pigeons for 2 wk to quantify the number of F1 offspring produced by each pair of lice. Lice for this experiment came from populations maintained on either giant runts or feral pigeons (Materials and Methods). Typical pairs of lice had significantly more offspring than mismatched pairs (Fig. 4C). Relative size dimorphism thus dictates the number of offspring produced by lice under natural conditions on live birds. The results of these three experiments (Fig. 4 A–C) show that local adaptation triggers reproductive isolation in cases where male and female lice are either too different, or too similar, in body size.
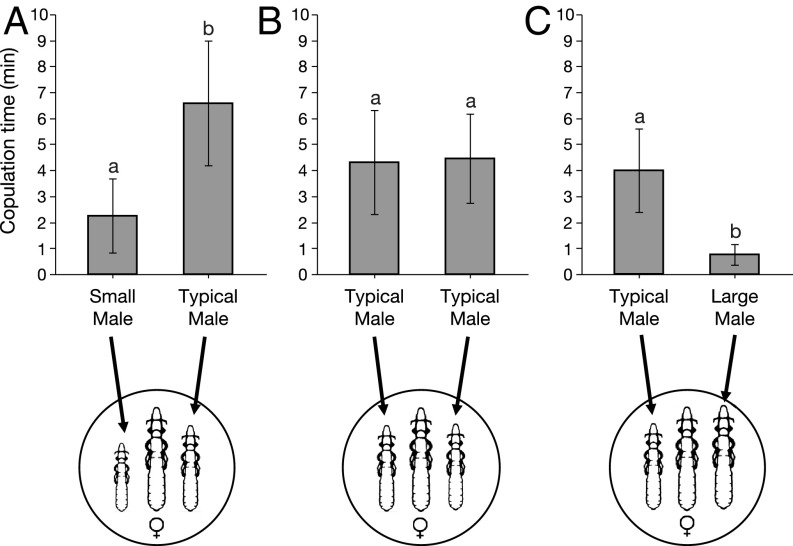
Sexual size dimorphism also influences intrasexual competition for mates (49–51). We tested if the degree of size dimorphism influences male-male competition in C. columbae by allowing two males to compete for single females in mating arenas. Typical males spent significantly more time copulating than either small or large males (Fig. 5). During these trials, we observed males trying to displace other males already in copula by wedging themselves between the first male and the female (Movie S4). We also observed behavior consistent with mate guarding by males following copulation. Thus, sexual selection magnifies the consequences of local adaptation for the evolution of reproductive isolation in C. columbae lice.
Fig. 5.
Relationship of size dimorphism to male-male competition in C. columbae (lice illustrations not to scale). When males of different sizes were combined with a single female in mating arenas, typical sized males spent more time copulating than relatively small (A) or large (C) males; Paired Wilcoxon signed-rank tests: n = 10, S = 24.50, P = 0.02 for A, and n = 10, S = 22.50, P = 0.03 for C. When males of the same (typical) size were combined with a single female (B), the males did not differ significantly in copulation time; n = 10, S = 13.00, P = 0.25. Different letters indicate significant differences for P < 0.05.
In summary, body size governs the survival of lice on different sized hosts (Fig. 1), as well as the reproductive success of lice locally adapted to those hosts (Fig. 4). Thus, body size in lice is consistent with a “magic trait” model of ecological speciation (9, 15, 21). Experimental divergence in size over 4 y (ca. 60 generations) led to partial reproductive isolation between populations adapted to large and small-bodied hosts. Our study is consistent with the theoretical and empirical work suggesting that ecological speciation evolves rapidly when divergent selection acts on a single trait that governs both survival and mating success. These data add to the growing evidence that the speciation process can take place on the same time scale as adaptive divergence (13, 15, 16, 22, 52–56).
The rapid evolution of louse body size and the emergence of reproductive isolation between populations presumably resembles the consequences of host switches by lice in nature. Feather lice are relatively host specific and have phylogenies that are often congruent with those of the host, owing to repeated bouts of host–parasite cospeciation (57). Nevertheless, lice do sometimes switch host species (36). If lice switch to a new host that differs in body size from the original host, then local adaptation should lead to rapid evolution of louse body size. This, in turn, should lead to the automatic emergence of reproductive isolation between populations of lice adapted to different hosts. Alternatively, lice may switch to a new host species that does not differ in body size from the original host. In these cases, lice may not evolve reproductive isolation. Clayton et al. (57) documented several cases of conspecific Columbicola that occur on different host species that are similar in size. Thus, simply infesting more than one host species is not sufficient for ecological speciation in lice. Our study demonstrates how the divergent natural selection following a host switch can trigger reproductive isolation, a scenario often inferred in the diversification of many groups of parasites and phytophagous insects (26, 29, 31, 32, 35).
The partial reproductive isolation that evolved between lice on giant runts and feral pigeons likely represents the first, and arguably most critical, stage of speciation. If lice continue to increase in size over time, the difference in body size between populations on giant runts and feral pigeons may be large enough for complete prezygotic reproductive isolation, resulting in the formation of a new species. However, even if the difference in body size alone does not yield complete reproductive isolation, the partial mechanical isolation could facilitate the evolution of additional reproductive barriers over longer periods of time (1, 2, 9). For example, reduced gene flow could allow a build-up of additional host-specific adaptions through linkage disequilibrium (16, 35). These additional adaptations may lead to further reproductive isolation via pleiotropy, and still further reductions in gene flow (35).
Our study emphasizes the benefit of examining ecological speciation in systems during the earliest stages of divergence. Most empirical studies of ecological speciation retrospectively investigate closely related species or ecotypes that have already evolved some degree of divergence (42–45, 55, 58). We took a complementary approach and experimentally triggered diversification in the descendants of a single population living under natural conditions in real time. This approach allowed us to identify a specific trait that links adaptation to reproductive isolation. By showing that local adaptation leads directly to the rapid emergence of reproductive isolation, our results confirm fundamental predictions of ecological speciation theory (1–5).
Materials and Methods
Elimination of “Background” Lice.
Before using pigeons in experiments, all of their naturally occurring background lice were eradicated by housing birds in low humidity conditions (<25% relative ambient humidity) for ≥10 wk. This method kills lice and their eggs, while avoiding residues from insecticides (59). During experiments, relative humidity in the animal rooms was increased to 35–60%, which provides sufficient humidity for feather lice to extract the moisture they need from the air (60).
Measuring Louse Body Size.
To measure louse body size, lice were first removed from hosts by anesthetizing them with CO2, then ruffling the feathers of the host over a collection tray (61). Each live louse was photographed by placing it dorsal side up on a glass slide. The lice were harmlessly immobilized by placing a 22 × 22 mm micro coverslip (VWR) directly on the body. Digital photographs were taken at high resolution (uncompressed TIFF 2,560 × 1,920 pixels) using an Olympus DP25 digital camera mounted on an Olympus SZ-CTV stereoscope linked to a computer running CellSens image acquisition and analysis software. Once photographed, the live lice were returned to their respective host. All of the photos were measured digitally using the open source imaging software ImageJ 1.3. From each image, we measured three aspects of body size: total body length, metathorax width, and head width.
Experimental Evolution of Louse Body Size.
To test the influence of host size on louse body size, we infested giant runt pigeons with C. columbae. We transferred 800 lice from wild caught feral pigeons to 16 giant runt pigeons and 16 feral pigeon controls (25 lice per bird). At this time (Time 0), we also randomly sampled 800 lice from the source population on wild caught feral pigeons and measured their body size. Pigeons were housed in groups of four in 1.8 × 1.5 × 1.0 m aviaries. Thus, the 32 pigeons used in the experiment were housed in eight aviaries, each containing four birds of the same type. Housing birds in groups reduced the risk of extinction of experimental lineages of lice on individually housed birds. The experiment ran for 48 mo. C. columbae has a mean generation time of 24.4 d (59); hence, the experiment represents ca. 60 generations of lice.
During the experiment, all pigeons were maintained on a 12-h light/dark photoperiod and provided ad libitum grain, grit, and water. When a bird died during the course of the experiment, lice from the dead bird were transferred to a new parasite-free pigeon of the same type. C. columbae can survive for several days on a dead bird yet cannot leave the bird’s feathers under their own power. Thus, few lice were lost (62).
Every 6 mo, random samples of lice were removed from pigeons and digitally photographed. To calculate an index of overall body size, we combined measures of total body length, metathorax width, and head width in a principal component analysis in JMP v13 (63) (SI Appendix, Table S1). We used linear mixed effects models (LMMs) to explore the relationship between host size and louse size over the course of the experiment. We first quantified experimental changes in overall louse body size (PC1) using an LMM that combined male and female lice from feral and giant runt pigeons. We predicted louse size by modeling the fixed effects of host type, time (months), louse sex, and all respective interactions, while individual bird (lineage) and aviary were included as random effects with lineage “nested” within aviary (SI Appendix, Table S2). The random effects were included to account for both repeated measures and the structured nature of the data. Three additional LMMs were used to quantify changes in body length, metathorax width, and head width over the course of the experiment (SI Appendix, Tables S3–S5). The intercept of each model was set to the value of female lice on feral pigeons at the end of the experiment (48 mo). All mixed models were fit in R using the “lme4” library package (64, 65). Degrees of freedom and resulting P values were calculated with a Satterthwaite approximation using the “lmerTest” library package (66).
Heritability of Louse Size.
Halfway through the 48-mo study, lice were randomly sampled using the CO2 procedure described above. A subsample of adult lice was marked by clipping setae with retinal scissors. Setal clipping is a reliable method that has been used to indelibly mark individuals of other species of lice, even under field conditions (67). Removal of setae does not influence survival, and the setae do not grow back. After clipping, lice from each aviary were combined on a single louse-free common garden feral pigeon. The eight common garden birds were isolated in eight wire mesh cages (30 × 30 × 56 cm). Their preening was impaired to prevent them from removing lice. Preening was impaired using harmless poultry bits, which are C-shaped pieces of plastic inserted between the upper and lower beak. Bits spring shut in the nostrils to prevent dislodging, but without damaging the tissue. They create a 1- to 3-mm gap that prevents the forceps-like action of the beak required for efficient preening (68). Bits have no apparent side effects and they do not impair the ability of birds to feed (69).
After a period of 48 d, all lice were removed from each of the eight pigeons using CO2. At this point in time, most F1 offspring had developed to the adult stage, and could be distinguished from members of the parental cohort, which had clipped setae. In contrast, F2 lice had not yet developed to the adult stage. Thus, we were able to compare the morphology of parental and F1 cohorts of lice from each of the eight common garden birds. Parental and F1 lice were removed from each bird and digitally photographed and their size measured.
Since C. columbae are sexually dimorphic, the body size of F1 and parental cohorts were compared in a 2 × 2 matrix: daughters vs. fathers, daughters vs. mothers, sons vs. fathers, and sons vs. mothers. We recovered just one son (F1 male) from one of the common garden birds; this common garden bird was excluded from analyses. Hence, son vs. father and son vs. mother comparisons were restricted to lice from seven of the eight common garden birds.
The body size (PC1) of the F1 cohort from each common garden pigeon (n = 8–30 lice per common garden bird for a total of 139 lice) was then compared with the body size (PC1) of the parental cohort (n = 11–50 lice per common garden bird for a total of 275 lice). The distribution of body sizes within both parents and offspring were normally distributed. Thus, we used four linear regressions, one for each combination of parental and offspring sex, to assess the relationship between the mean parental body sizes and the mean offspring body sizes.
Typical Variation in Sexual Size Dimorphism of C. columbae.
We used walk-in traps to capture 22 feral pigeons in Salt Lake City, Utah. We used the CO2 method to recover 262 adult C. columbae (roughly equal sex ratio). The 22 pigeons had a mean (±SE) of 11.9 (±1.5) adult lice, reflecting typical adult population sizes at this location (70). For each population, we subtracted the mean C. columbae male length from the mean C. columbae female length to generate a range (minimum and maximum) of typical sexual dimorphism scores (SI Appendix, Table S6). “Typical” dimorphism ranged from −492 to −269 μm and had a mean (±SD) of −354 ± 65 μm.
Influence of Sexual Size Dimorphism on Copulation Time.
We filmed the behavior of 56 arranged pairs of lice with a range of size disparities. We used lice from a group of bitted feral pigeons established for this purpose (but different from the bitted birds used in the common garden experiment). Because bits relax preening-mediated selection for small size, the largest lice on bitted pigeons are similar to, but do not exceed, the size of lice on giant runt pigeons. Each pair of lice was placed in a 9 × 12 mm arena on a detached underwing covert feather. The lice were filmed with an Apple iPod Touch (fifth generation) mounted on an Olympus SZ-25 stereoscope at 2× magnification for 60 min. The videos were watched by two of us (J.C.A. or L.I.M.), and copulation time was recorded. Dimorphism among the pairs of lice was normally distributed. Thus, we used a quadratic nonlinear model (NLM) to examine if an intermediate level of dimorphism maximized the time spent copulating. This model was directly compared with a linear model (LM) to evaluate a quadratic vs. linear relationship (SI Appendix, Table S7). Corrected Akaike information criterion (AIC) scores were calculated using AICcmodavg package to aid in model discrimination (71).
Influence of Sexual Size Dimorphism on Egg Production.
We tracked the egg laying success of 58 arranged pairs of lice that varied in size. Again, we used lice from bitted feral pigeons established for this purpose. Female lice are capable of storing sperm (72). We therefore created virgin adult lice by taking immature lice from bitted feral pigeons and rearing them in glass vials in an incubator (48). Single virgin female and male lice were then paired in new glass vials containing feathers. Once the female died, each vial was thoroughly examined for eggs by an observer who was blind to the dimorphism score (H.E.C. or E.J.P.). Because we scored eggs as present or absent, we used a generalized nonlinear model (GNLM) assuming a binomial distribution with a logit link to explore the relationship between dimorphism and the likelihood of producing eggs. This model was directly compared with a generalized linear model (GLM) to evaluate a quadratic vs. linear relationship (SI Appendix, Table S8).
Influence of Sexual Size Dimorphism on Reproductive Success on Live Birds.
We measured the reproductive success of 36 arranged pairs of lice from giant runt and feral pigeons. To avoid removing lice from our experimental evolution lines, we obtained the lice from populations cultured for this purpose on additional giant runt and feral pigeons in our facility. Lice from these birds showed the same adaptive divergence in body size as the lice in our experimental evolution lines. We tested whether local adaptation to different sized hosts also leads to reproductive isolation. We removed immature lice from giant runt and feral pigeons and reared them individually to the adult stage on feathers in glass vials in an incubator (48). We began with 100 immature lice from each type of host, only about half of which typically survived to adulthood.
We infested each of 36 louse-free feral pigeons with a single pair of lice: 12 birds had a male louse from a giant runt and a female louse from a feral pigeon; 12 birds had a female louse from a giant runt and a male louse from a feral pigeon; 12 birds had a male and female louse from a feral pigeon (SI Appendix, Table S9). The 36 birds were isolated in 36 cages for 14 d, which is sufficient time for the lice to breed, but not enough time for offspring to reach the adult stage. After 14 d, the pigeons were killed and the offspring produced by each pair were removed by “body washing” and counted (73). All procedures followed guidelines of the Institutional Animal Care and Use Committee of the University of Utah. To assess whether pairs of lice with intermediate degrees of sexual dimorphism produce more nymphs than those with relatively small or large degrees of dimorphism, we used a generalized nonlinear mixed model (GNLMM) assuming a Poisson distribution with host origin modeled as a random effect. This model was directly compared with a generalized linear mixed model (GLMM) to evaluate a quadratic vs. linear relationship (SI Appendix, Table S10).
Influence of Sexual Size Dimorphism on Male-Male Competition.
We filmed the behavior of different sized male lice in the presence of a single female. Lice in this experiment came from bitted feral pigeons. We arranged 30 trios of lice, each consisting of two males and a single female. In each trio, one of the two males showed typical dimorphism relative to the female. The second male was smaller than the typical male, larger than the typical male, or, in the case of controls, was a second typical male (n = 10 for each combination).
Each trio of lice was placed in a 9 × 12 mm arena on a detached underwing covert feather. Behavior of the lice was filmed with an Apple iPod Touch (fifth generation) mounted on an Olympus SZ-25 stereoscope at 2× magnification for 60 min. The videos were watched by two of us (J.C.A. or L.I.M.) and copulation time was recorded. We explored the relationship between size of males relative to females and how much time they spent copulating. This relationship was assessed with Paired Wilcoxon Signed-Rank Tests, as the copulation times were not normally distributed, and each trial involved two males vying for a female.
Supplementary Material
Acknowledgments
We thank F. Adler, D. Benevides, M. Evans, G. Goodman, B. Levin, S. McNew, N. Phadnis, J. Seger, and E. Waight for discussion and other assistance and the two anonymous reviewers for comments that helped to improve the manuscript. Funding for this work was provided by National Science Foundation Grant DEB-1342600.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper are available on the Dryad Digital Repository (https://doi.org/10.5061/dryad.hr6k12v).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1901247116/-/DCSupplemental.
References
- 1.Dobzhansky T., Genetics and the Origin of Species (Columbia University Press, 1940). [Google Scholar]
- 2.Coyne J. A., Orr H. A., Speciation (Sinauer Associates, Inc., 2004). [Google Scholar]
- 3.Muller H. J., Isolating mechanisms, evolution, and temperature. Biol. Symp. 6, 71–125 (1942). [Google Scholar]
- 4.Mayr E., Ecological factors in speciation. Evolution 1, 263–288 (1947). [Google Scholar]
- 5.Schluter D., Ecology and the origin of species. Trends Ecol. Evol. 16, 372–380 (2001). [DOI] [PubMed] [Google Scholar]
- 6.McKinnon J. S., et al. , Evidence for ecology’s role in speciation. Nature 429, 294–298 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Schluter D., Evidence for ecological speciation and its alternative. Science 323, 737–741 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Schemske D. W., Adaptation and the origin of species. Am. Nat. 176 (suppl. 1), S4–S25 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Nosil P., Ecological Speciation (Oxford University Press, 2012). [Google Scholar]
- 10.Butlin R., et al. ; Marie Curie SPECIATION Network , What do we need to know about speciation? Trends Ecol. Evol. 27, 27–39 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Safran R. J., Scordato E. S., Symes L. B., Rodríguez R. L., Mendelson T. C., Contributions of natural and sexual selection to the evolution of premating reproductive isolation: A research agenda. Trends Ecol. Evol. 28, 643–650 (2013). [DOI] [PubMed] [Google Scholar]
- 12.Schluter D., The Ecology of Adaptive Radiation (Oxford University Press, 2000). [Google Scholar]
- 13.Rice W. R., Hostert E. E., Laboratory experiments on speciation: What have we learned in 40 years? Evolution 47, 1637–1653 (1993). [DOI] [PubMed] [Google Scholar]
- 14.Fry J. D., “Laboratory experiments on speciation” in Experimental Evolution: Concepts, Methods, and Applications of Selection Experiments, Garland T. Jr., Rose M. R., Eds. (University of California Press, 2009), pp. 631–656. [Google Scholar]
- 15.Gavrilets S., Perspective: Models of speciation: What have we learned in 40 years? Evolution 57, 2197–2215 (2003). [DOI] [PubMed] [Google Scholar]
- 16.Smadja C. M., Butlin R. K., A framework for comparing processes of speciation in the presence of gene flow. Mol. Ecol. 20, 5123–5140 (2011). [DOI] [PubMed] [Google Scholar]
- 17.Dieckmann U., Doebeli M., On the origin of species by sympatric speciation. Nature 400, 354–357 (1999). [DOI] [PubMed] [Google Scholar]
- 18.Kirkpatrick M., Ravigné V., Speciation by natural and sexual selection: Models and experiments. Am. Nat. 159 (Suppl. 3), S22–S35 (2002). [DOI] [PubMed] [Google Scholar]
- 19.Maynard Smith J., Sympatric speciation. Am. Nat. 100, 637–650 (1966). [Google Scholar]
- 20.Gavrilets S., Fitness Landscapes and the Origin of Species (Princeton University Press, 2004). [Google Scholar]
- 21.Servedio M. R., Van Doorn G. S., Kopp M., Frame A. M., Nosil P., Magic traits in speciation: ‘Magic’ but not rare? Trends Ecol. Evol. 26, 389–397 (2011). [DOI] [PubMed] [Google Scholar]
- 22.Hendry A. P., Eco-evolutionary Dynamics (Princeton University Press, 2017). [Google Scholar]
- 23.Bush G. L., Sympatric host race formation and speciation in frugivorous flies of the genus Rhagoletis (Diptera, Tephritidae). Evolution 23, 237–251 (1969). [DOI] [PubMed] [Google Scholar]
- 24.Bush G. L., Modes of animal speciation. Annu. Rev. Ecol. Syst. 6, 339–364 (1975). [Google Scholar]
- 25.Via S., Sympatric speciation in animals: The ugly duckling grows up. Trends Ecol. Evol. 16, 381–390 (2001). [DOI] [PubMed] [Google Scholar]
- 26.Price P. W., Evolutionary Biology of Parasites (Princeton University Press, 1980). [Google Scholar]
- 27.Via S., Reproductive isolation between sympatric races of pea aphids. I. Gene flow restriction and habitat choice. Evolution 53, 1446–1457 (1999). [DOI] [PubMed] [Google Scholar]
- 28.Diehl S. R., Bush G. L., An evolutionary and applied perspective of insect biotypes. Annu. Rev. Entomol. 29, 471–504 (1984). [Google Scholar]
- 29.Bush G. L., Howard D. J., “Allopatric and non-allopatric speciation: Assumptions and evidence” in Evolutionary Processes and Theory, Karlin S., Nevo E., Eds. (Academic Press, 1986), pp. 411–438. [Google Scholar]
- 30.Poulin R., Evolutionary Ecology of Parasites (Princeton University Press, ed. 2, 2007). [Google Scholar]
- 31.Matsubayashi K. W., Ohshima I., Nosil P., Ecological speciation in phytophagous insects. Entomol. Exp. Appl. 134, 1–27 (2010). [Google Scholar]
- 32.Forbes A. A., et al. , Revisiting the particular role of host shifts in initiating insect speciation. Evolution 71, 1126–1137 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Walsh B. J., On phytophagic varieties and phytophagic species. Proc. Entomol. Soc. Phil. 3, 403–430 (1864). [Google Scholar]
- 34.Ross H. H., A Synthesis of Evolutionary Theory (Prentice-Hall, 1962). [Google Scholar]
- 35.Drès M., Mallet J., Host races in plant-feeding insects and their importance in sympatric speciation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 357, 471–492 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clayton D. H., Bush S. E., Johnson K. P., Coevolution of Life on Hosts: Integrating Ecology and History (University of Chicago Press, 2015). [Google Scholar]
- 37.Clayton D. H., Lee P. L. M., Tompkins D. M., Brodie Iii E. D., Reciprocal natural selection on host-parasite phenotypes. Am. Nat. 154, 261–270 (1999). [DOI] [PubMed] [Google Scholar]
- 38.Bush S. E., “Evolutionary ecology of host specificity in Columbiform feather lice”, PhD dissertation, University of Utah, (2004).
- 39.Villa S. M., et al. , Body size and fecundity are correlated in feather lice (Phthiraptera: Ischnocera): Implications for Harrison’s Rule. Ecol. Entomol. 43, 394–396 (2018). [Google Scholar]
- 40.Johnson K. P., Bush S. E., Clayton D. H., Correlated evolution of host and parasite body size: Tests of Harrison’s rule using birds and lice. Evolution 59, 1744–1753 (2005). [PubMed] [Google Scholar]
- 41.Dettman J. R., Sirjusingh C., Kohn L. M., Anderson J. B., Incipient speciation by divergent adaptation and antagonistic epistasis in yeast. Nature 447, 585–588 (2007). [DOI] [PubMed] [Google Scholar]
- 42.Lamichhaney S., et al. , Rapid hybrid speciation in Darwin’s finches. Science 359, 224–228 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Jiggins C. D., Naisbit R. E., Coe R. L., Mallet J., Reproductive isolation caused by colour pattern mimicry. Nature 411, 302–305 (2001). [DOI] [PubMed] [Google Scholar]
- 44.Wu C. A., et al. , Mimulus is an emerging model system for the integration of ecological and genomic studies. Heredity 100, 220–230 (2008). [DOI] [PubMed] [Google Scholar]
- 45.Feder J. L., et al. , Mayr, Dobzhansky, and Bush and the complexities of sympatric speciation in Rhagoletis. Proc. Natl. Acad. Sci. U.S.A. 102 (suppl. 1), 6573–6580 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McKinnon J. S., Rundle H. D., Speciation in nature: The threespine stickleback model systems. Trends Ecol. Evol. 17, 480–488 (2002). [Google Scholar]
- 47.Villa S. M., “Evolutionary ecology of parasite diversification: Experiments with pigeons and their ectoparasites”, PhD dissertation, University of Utah, (2016).
- 48.Malenke J. R., Newbold N., Clayton D. H., Condition-specific competition governs the geographic distribution and diversity of ectoparasites. Am. Nat. 177, 522–534 (2011). [DOI] [PubMed] [Google Scholar]
- 49.Crespi B. J., Causes of assortative mating in arthropods. Anim. Behav. 38, 980–1000 (1989). [Google Scholar]
- 50.Andersson M., Sexual Selection (Princeton University Press, 1994). [Google Scholar]
- 51.Bolnick D. I., Doebeli M., Sexual dimorphism and adaptive speciation: Two sides of the same ecological coin. Evolution 57, 2433–2449 (2003). [DOI] [PubMed] [Google Scholar]
- 52.Rice W. R., Disruptive selection on habitat preference and the evolution of reproductive isolation: A simulation study. Evolution 38, 1251–1260 (1984). [DOI] [PubMed] [Google Scholar]
- 53.Rice W. R., Salt G. W., The evolution of reproductive isolation as a correlated character under sympatric conditions: Experimental evidence. Evolution 44, 1140–1152 (1990). [DOI] [PubMed] [Google Scholar]
- 54.Rundle H. D., Chenoweth S. F., Doughty P., Blows M. W., Divergent selection and the evolution of signal traits and mating preferences. PLoS Biol. 3, e368 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chung H., et al. , A single gene affects both ecological divergence and mate choice in Drosophila. Science 343, 1148–1151 (2014). [DOI] [PubMed] [Google Scholar]
- 56.Meyer J. R., et al. , Ecological speciation of bacteriophage lambda in allopatry and sympatry. Science 354, 1301–1304 (2016). [DOI] [PubMed] [Google Scholar]
- 57.Clayton D. H., Bush S. E., Goates B. M., Johnson K. P., Host defense reinforces host-parasite cospeciation. Proc. Natl. Acad. Sci. U.S.A. 100, 15694–15699 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Via S., Natural selection in action during speciation. Proc. Natl. Acad. Sci. U.S.A. 106 (suppl. 1), 9939–9946 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harbison C. W., Bush S. E., Malenke J. R., Clayton D. H., Comparative transmission dynamics of competing parasite species. Ecology 89, 3186–3194 (2008). [DOI] [PubMed] [Google Scholar]
- 60.Nelson B. C., Murray M. D., The distribution of Mallophaga on the domestic pigeon (Columba livia). Int. J. Parasitol. 1, 21–29 (1971). [DOI] [PubMed] [Google Scholar]
- 61.Moyer B. R., Gardiner D. W., Clayton D. H., Impact of feather molt on ectoparasites: Looks can be deceiving. Oecologia 131, 203–210 (2002). [DOI] [PubMed] [Google Scholar]
- 62.Bush S. E., et al. , Host defense triggers rapid adaptive radiation in experimentally evolving parasites. Evol. Lett. 3, 120–128 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.SAS Institute , JMP User’s Guide (SAS Institute, 1985). [Google Scholar]
- 64.Bates D., Maechler M., Bolker B., Walker S., Fitting linear mixed- effects models using lme4. J. Stat. Softw. 67, 1–48 (2015). [Google Scholar]
- 65.R Core Team , R: A Language and Environment for Statistical Computing (R Package Version 3.3.3, R Foundation for Statistical Computing, 2016).
- 66.Kuznetsova A., Brockhoff P. B., Haubo R., Christensen B., lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw., 10.18637/jss.v082.i13 (2016). [DOI] [Google Scholar]
- 67.Durden L. A., Sucking louse (Hoplopleura erratica: Insecta, Anoplura) exchange between individuals of a wild population of eastern chipmunks, Tamias striatus, in central Tennessee, USA. J. Zool. 201, 117–123 (1983). [Google Scholar]
- 68.Clayton D. H., et al. , Adaptive significance of avian beak morphology for ectoparasite control. Proc. Biol. Sci. 272, 811–817 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Clayton D. H., Tompkins D. M., Comparative effects of mites and lice on the reproductive success of rock doves (Columba livia). Parasitology 110, 195–206 (1995). [DOI] [PubMed] [Google Scholar]
- 70.Moyer B. R., Drown D. M., Clayton D. H., Low humidity reduces ectoparasite pressure: Implications for host life history evolution. Oikos 97, 223–228 (2003). [Google Scholar]
- 71.Mazerolle M. J., AICcmodavg: Model Selection and Multimodel Inference Based on (Q)AIC(c). R Package Version 2.1-0. https://CRAN.Rproject.org/package=AICcmodavg. Accessed 14 April 2019.
- 72.Johnson K. P., Clayton D. H., “The biology, ecology, and evolution of chewing lice” in The Chewing Lice: World Checklist and Biological Overview, Price R.D., Hellenthal R. A., Palma R. L., Johnson K. P., Clayton D. H., Eds. (Illinois Natural History Survey Special Publication 24, 2003), p. 501. [Google Scholar]
- 73.Clayton D. H., Drown D. M., Critical evaluation of five methods for quantifying chewing lice (Insecta: Phthiraptera). J. Parasitol. 87, 1291–1300 (2001). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.