Significance
Lyme disease, caused by the spirochete Borrelia burgdorferi, is the most common vector-borne disease in North America. If early infection is untreated, it can result in late-stage manifestations, including arthritis. Although antibiotics are generally effective at all stages of the disease, arthritis may persist in some patients for months to several years despite oral and intravenous antibiotic treatment. Excessive, dysregulated host immune responses are thought to play an important role in this outcome, but the underlying mechanisms are not completely understood. This study identifies the B. burgdorferi peptidoglycan, a major component of the cell wall, as an immunogen likely to contribute to inflammation during infection and in cases of postinfectious Lyme arthritis.
Keywords: Lyme disease, arthritis, peptidoglycan, Borrelia burgdorferi, inflammation
Abstract
Lyme disease is a multisystem disorder caused by the spirochete Borrelia burgdorferi. A common late-stage complication of this disease is oligoarticular arthritis, often involving the knee. In ∼10% of cases, arthritis persists after appropriate antibiotic treatment, leading to a proliferative synovitis typical of chronic inflammatory arthritides. Here, we provide evidence that peptidoglycan (PG), a major component of the B. burgdorferi cell envelope, may contribute to the development and persistence of Lyme arthritis (LA). We show that B. burgdorferi has a chemically atypical PG (PGBb) that is not recycled during cell-wall turnover. Instead, this pathogen sheds PGBb fragments into its environment during growth. Patients with LA mount a specific immunoglobulin G response against PGBb, which is significantly higher in the synovial fluid than in the serum of the same patient. We also detect PGBb in 94% of synovial fluid samples (32 of 34) from patients with LA, many of whom had undergone oral and intravenous antibiotic treatment. These same synovial fluid samples contain proinflammatory cytokines, similar to those produced by human peripheral blood mononuclear cells stimulated with PGBb. In addition, systemic administration of PGBb in BALB/c mice elicits acute arthritis. Altogether, our study identifies PGBb as a likely contributor to inflammatory responses in LA. Persistence of this antigen in the joint may contribute to synovitis after antibiotics eradicate the pathogen. Furthermore, our finding that B. burgdorferi sheds immunogenic PGBb fragments during growth suggests a potential role for PGBb in the immunopathogenesis of other Lyme disease manifestations.
Lyme disease, caused by the spirochete Borrelia burgdorferi, is the most prevalent tick-borne human disease in temperate regions of the Northern hemisphere (1). Clinical manifestations of this disease are highly variable and can involve multiple organ systems at different times (2). Infection in humans is often heralded by a skin lesion (known as erythema migrans) at the site of the tick bite. If left untreated, the infection can disseminate to other tissues (e.g., skin, heart, central nervous system, joints) and give rise to additional skin lesions, carditis, neurological disorders, or arthritis (3–5). These clinical outcomes are thought to result from host immune responses to B. burgdorferi or B. burgdorferi-derived components (6).
Arthritis is the most common late-stage clinical manifestation of Lyme disease in the United States and is often characterized by inflammation of one or more large joints (typically the knee), which are one of the sites the spirochetes frequently infiltrate (6). In ∼10% of cases, an inflammatory proliferative synovitis persists despite 2–3 mo of oral and intravenous (IV) antibiotic therapy and apparent absence of viable organisms in the synovial fluid and adjacent tissues (5, 7, 8). Development of autoimmunity is thought to contribute to the persistence of Lyme arthritis (LA), and recent studies have identified four autoantigens as targets of autoreactive T and B cell responses in patients with postinfectious LA (9–13). It has also been proposed that B. burgdorferi-derived components may persist after initial infection and serve as immunogens, contributing to inappropriate inflammation long after the spirochetes have been killed (14). However, such persistent immunogens have yet to be identified.
B. burgdorferi does not produce lipopolysaccharides (endotoxin), and its genome does not appear to encode effectors that might act as toxins (15, 16). Therefore, most studies to date have focused on surface-exposed lipoproteins anchored in the outer membrane of B. burgdorferi. These lipoproteins play important roles in various aspects of tick colonization, mammalian infection, and host immune evasion and response (17–19). Comparatively, the peptidoglycan (PG), an essential component of bacterial cell envelopes, has received very little attention. The PG, which is made of glycan strands cross-linked by short peptides, forms a polymeric meshwork around the cytoplasmic membrane and provides resistance against intracellular osmotic pressure (20, 21). PG is also a microbe-associated molecular pattern that can stimulate innate immune pathways in animals, resulting in inflammation (22). PG from Gram-positive bacteria administered intraarticularly or systemically can induce acute arthritis in mice and rats (23–29). NOD2, an innate immunity protein recognizing a PG moiety, has been implicated in proinflammatory cytokine production and immune tolerance during B. burgdorferi infection in mice (30, 31). Furthermore, a 1990 report has shown that B. burgdorferi PG (PGBb) stimulates interleukin 1 (IL-1) production in macrophages in vitro and that intradermal injection of PGBb in human volunteers results in skin reactions characteristic of inflammation (32). Despite these observations, a potential role for PGBb in B. burgdorferi pathogenesis has not been directly examined.
In diderm bacteria, including B. burgdorferi, the outer membrane shields the PG meshwork from the external environment. Exposure of PGBb to the host immune system may, however, still be significant for two reasons. First, spirochete death, which occurs during early stages of transmission and dissemination (33), may result in PGBb exposure to host immune cells. Second, sequence homology analyses predict that B. burgdorferi lacks a PG recycling pathway (34). Absence of PG recycling suggests that large amounts of PG fragments (known as muropeptides) may be released into the host environment during spirochetal growth. Bacteria degrade ∼40–50% of their PG per generation, as part of the normal PG remodeling process required for cell wall expansion (34–36). In Gram-negative/diderm bacteria, the vast majority of muropeptides produced during normal PG turnover are typically recycled. During this process, muropeptides are transported into the cytoplasm by an inner membrane permease (AmpG), processed by PG recycling proteins (e.g., AmpD and LdcA), and reincorporated into the PG biosynthetic pathway for reuse (SI Appendix, Fig. S1A) (34). Bacterial mutants that lack AmpG shed a large amount of muropeptides into their environment during growth (SI Appendix, Fig. S1B) (36–39). The apparent absence of a canonical muropeptide recycling pathway in B. burgdorferi suggests the possibility that muropeptides produced during normal PG turnover may be released into the extracellular milieu where the host immune system would be able to detect them. These considerations motivated us to test the hypothesis that PGBb is an antigen contributing to proinflammatory responses during the infectious and postinfectious phases of LA.
Results
B. burgdorferi PG Has an Unusual Chemical Composition.
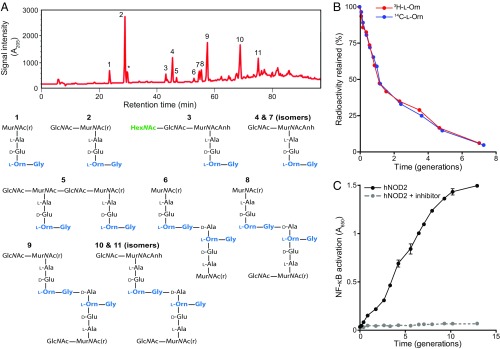
We first characterized the chemical composition and architecture of purified PGBb. Liquid chromatography and mass spectrometry (LC-MS) analysis of cellosyl-digested PG revealed several unusual features (Fig. 1A and SI Appendix, Table S1). For instance, whereas the sugar backbone of the PGBb is made up of alternating N-acetylglucosamine (GlcNAc) and N-acetylmuramic acid (MurNAc), similar to other bacterial PGs, we also observed the occasional presence of an N-acetylhexosamine (HexNAc) linked to GlcNAc (Fig. 1A). To our knowledge, such a modification has not been reported in any other PGs characterized to date. Another feature of the PGBb was the presence of l-ornithine (l-Orn) linked to a single glycine (Fig. 1A and SI Appendix, Table S1), which is congruent with an earlier chemical amino acid analysis (32). The presence of l-Orn has been reported in other spirochetes (40). It is, otherwise, a rare deviation from the typical PG dichotomy in the bacterial domain (41), which generally features a diaminopimelic acid (DAP) or lysine (Lys) at the third amino acid position of the stem peptide. We confirmed the presence of l-Orn in PGBb by using two methods: (i) gas chromatography coupled to mass spectrometry (GC-MS; SI Appendix, Fig. S2A) and (ii) 3H-l-Orn radiolabeling followed by high-performance liquid chromatography (HPLC) analysis and liquid scintillation counting (SI Appendix, Fig. S2B).
Fig. 1.
B. burgdorferi sheds muropeptides into its extracellular environment. (A, Top) Chromatogram of cellosyl-digested and reduced PGBb isolated from B. burgdorferi B31. Numbers correspond to the identified chemical species shown below. The asterisk indicates an unidentified species (SI Appendix, Table S1). Analysis performed on three separate preparations produced highly similar chromatograms. (A, Bottom) Chemical composition of muropeptides in peaks shown in the chromatogram. Muropeptide identification was accomplished by MS. MurNAc(r) and Anh indicate N-acetylmuramitol and 1,6-anhydro group, respectively. (B) Plot showing PG turnover over multiple generations in B. burgdorferi grown in vitro. PGBb was pulse-radiolabeled by incubating cells in medium containing 7.5 µCi/mL of 3H- or 14C-l-Orn for 48 h. Cells were then washed to remove unincorporated isotope, and outgrowth was tracked in complete BSK II medium lacking radioactive l-Orn. At each time point, the same volume of batch culture was removed, bacterial density was determined, and PGBb was purified for quantification of its radioactivity per volume equivalent. The retained radioactivity was then plotted as a percentage of total radioactivity in the PG at time 0 (i.e., start of outgrowth). (C) Muropeptide accumulation in the culture medium. Cultures of B. burgdorferi (5 × 107 cells per milliliter) were diluted to a starting density of 104 cells per milliliter and monitored for muropeptide release during growth in complete BSK II medium (lacking phenol red) using an hNOD2 reporter cell line in the presence or absence of the RIP2 inhibitor gefitinib. NF-κB activity (absorbance at 650 nm) provides a measure of NOD2-specific muropeptide levels present in the culture medium samples collected at the indicated time points. Shown are the mean and SD of NF-κB activation for two biological replicates at each time point.
B. burgdorferi Sheds Muropeptides into Its Environment during Growth.
Because the B. burgdorferi genome appears to lack the requisite proteins (AmpG, AmpD, and LdcA) for muropeptide recycling (SI Appendix, Fig. S1C), we hypothesized that muropeptides produced during normal PGBb turnover are recycled via an unknown pathway or are released into the extracellular milieu. To determine whether PG recycling occurs, we pulse-labeled B. burgdorferi cells with l-Orn containing 3H or 14C isotopes, followed by cell outgrowth in radiolabel-free liquid culture medium. At various time points during outgrowth, we collected cells, purified PGBb, and analyzed these PG preparations by liquid scintillation counting. On average, the PGBb lost 40 ± 2% of radiolabeled l-Orn per generation (Fig. 1B), consistent with the lack of a muropeptide recycling pathway (34, 36). Moreover, we found that PGBb turnover during B. burgdorferi growth resulted in time-dependent muropeptide accumulation in the culture supernatant (Fig. 1C and SI Appendix, Fig. S3), similar to what is observed with mutant strains of other bacteria that lack the PG recycling permease AmpG required for cytoplasmic import of muropeptides (36–39). We showed this muropeptide release by exposing human NOD2 (hNOD2) reporter cells to B. burgdorferi culture supernatant samples. In these cells, binding of PG material containing MurNAc-l-Ala-d-Glu to the hNOD2 receptor drives downstream activation of NF-κB (42). Treatment with gefitinib, an inhibitor of the adaptor protein RIP2 downstream of NOD2 (43), prevented NF-κB activation (Fig. 1C). In addition, NF-κB signaling was not activated when we exposed human NOD1 reporter cells to B. burgdorferi culture supernatants (SI Appendix, Fig. S3). NOD1 specifically recognizes PG containing DAP in the third amino acid position of the stem peptide (44). Collectively, these results demonstrate that B. burgdorferi sheds muropeptides into its local environment, likely because it is unable to recycle them.
Patients with LA Develop an Adaptive Immune Response against B. burgdorferi PG.
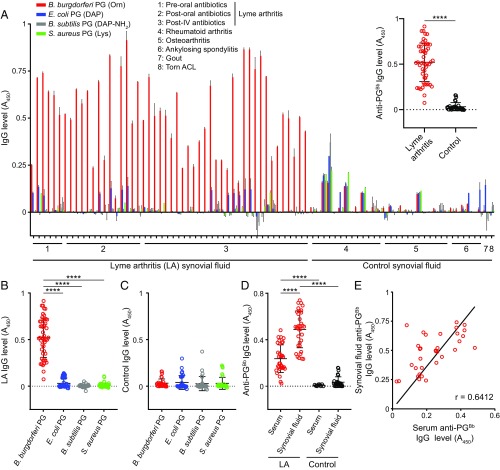
Animals, including humans, produce a humoral response that can discriminate different types of PG chemistry (45, 46). As the chemical composition of PGBb is unusual (Fig. 1A and SI Appendix, Table S1) (32), we postulated that it may contain epitopes that induce a specific immunoglobulin G (IgG) response capable of discriminating between PGBb and other bacterial PGs. To test this idea, we used purified PG from B. burgdorferi (Orn-type PG), Escherichia coli (DAP-type PG), Bacillus subtilis (amidated DAP-type PG), and Staphylococcus aureus (Lys-type PG) in an ELISA to probe for an anti-PG IgG response in 82 blinded synovial fluid samples from patients with different forms of arthritis. Some samples originated from patients with LA and included single and longitudinal samples. These samples were collected before treatment with oral antibiotics, after oral antibiotic treatment, or after oral antibiotic treatment and additional IV antibiotic therapy (Methods). Control synovial fluid samples from patients with rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, or gouty arthropathy were randomly scattered among the coded samples. Another control synovial fluid sample was from a patient with a torn anterior cruciate ligament (ACL), which was the only nonblinded patient sample in our study.
We found that most LA synovial fluid samples contained significant levels of IgG antibodies against B. burgdorferi PG (anti-PGBb), whereas control samples from patients with other forms of arthritis or a torn ACL did not (Fig. 2 A, Inset). This IgG response was largely specific to PGBb, as LA samples displayed little to no IgG reactivity to PGs from other bacteria (Fig. 2B). In contrast, control samples did not exhibit a PG-specific IgG response (Fig. 2C). The levels of anti-PGBb IgG in preoral, postoral, and postoral/IV antibiotic LA patients did not significantly differ based on a Kruskal–Wallis test followed by a Dunn’s post hoc pairwise test (SI Appendix, Fig. S4A). Several control samples contained anti-PG IgG levels above background (Fig. 2A), especially those from patients with rheumatoid arthritis (38%). However, such anti-PG responses, which have been previously reported in patients with rheumatoid arthritis (47, 48), were not specific for a particular type of PG tested (Fig. 2A).
Fig. 2.
Patients with LA develop an adaptive immune response specifically to PGBb. Purified PG from B. burgdorferi B31, E. coli K-12, S. aureus SA113, or B. subtilis 168 was immobilized, and synovial fluid from patients with different types of arthritis (groups 1–6 and 7) were blindly assayed for the presence of IgG by ELISA (****P < 0.0001, Kruskal–Wallis test followed by Dunn’s post hoc pairwise test for B and Mann–Whitney U test for A and D). Horizontal black lines indicate means and SDs. (A) Levels of IgG against different PG types. After the results were obtained, the patient sample type was decoded and organized based on the arthritis type and treatment stage. Results for a control joint fluid sample obtained from a patient with a torn ACL (group 8) were also included. Values were background-subtracted based on the IgG level measured for each individual sample in the absence of PG ligand. (Inset) All anti-PGBb IgG values are shown as a bee-swarm plot for LA samples and controls (all non-LA samples). (B) Specificity of IgGs from LA synovial fluid samples for PGs from different bacteria. (C) Specificity of IgGs from control synovial fluid samples for different PG types. (D) Comparison of IgG responses to PGBb for serum and synovial fluid samples from patients with LA relative to those for serum from healthy humans and synovial fluid samples from control (non-LA) patients. (E) Correlation analysis of anti-PGBb IgG responses between the serum and synovial fluid of patients with LA. The linear fit and Pearson correlation coefficient (r) for the LA synovial fluid samples are also shown.
From the original panel of synovial fluid samples, we had matching serum samples for 34 patients with LA (Methods), which we used as a subset for further analysis. We found that sera from LA patients contained significantly more anti-PGBb IgG than control sera from healthy people (Fig. 2D). Whereas the synovium represents a local environment, the synovial cavity communicates freely with systemic circulation, which likely explains why anti-PGBb IgG levels in paired serum and synovial fluid samples correlate (Fig. 2E). In all LA cases, the synovial fluid had a higher anti-PGBb IgG level than the corresponding serum sample from the same patient (Fig. 2E). Our data indicate that patients with LA produce specific antibodies against PGBb and that these responses are primarily localized to the joint, the site of inflammation.
B. burgdorferi PG Material Is Detected in Synovial Fluid Samples from Patients with LA after Antibiotic Treatment.
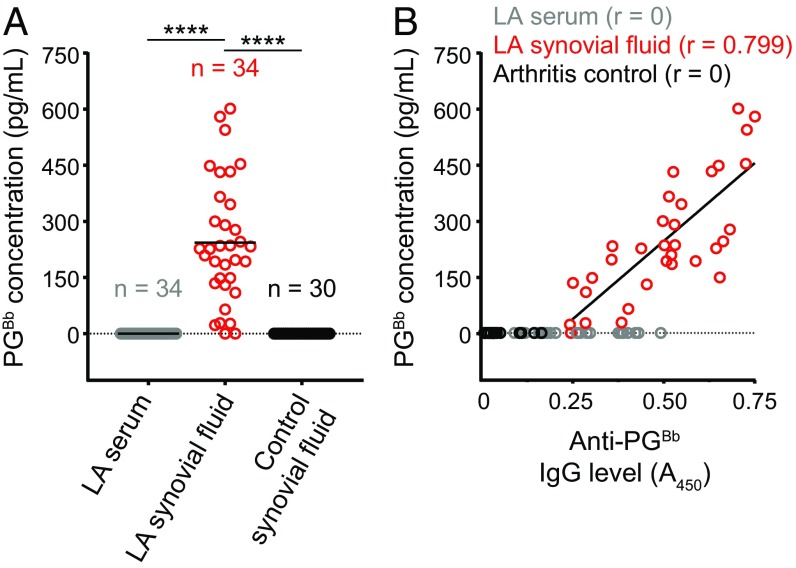
As patients with LA produce a specific anti-PGBb IgG response, we next sought to determine whether we could detect antigenic PGBb material in the synovial fluid of patients with LA. To this end, we generated a polyclonal anti-PGBb antiserum through immunization of New Zealand White rabbits with PGBb. The polyclonal antiserum was specific for PGBb, as it did not react with other common PG types in a competitive ELISA (SI Appendix, Fig. S5). By using this same competitive ELISA, we did not detect PGBb in control synovial fluid samples (Fig. 3A). We also failed to detect PGBb in the sera of patients with LA (Fig. 3A). However, 92% of the tested LA synovial fluid samples contained tens to hundreds of picograms of PG material per milliliter (Fig. 3A). The amount of PG detected strongly correlated with the anti-PGBb IgG level found in the same synovial fluid sample (Fig. 3B), indicating that the antigenic material detected by our rabbit polyclonal antiserum is likely PGBb. Our results show that PGBb is present in LA synovial fluid samples before and after oral and IV antibiotic treatment (SI Appendix, Fig. S4B).
Fig. 3.
Detection of PGBb in synovial fluid samples of patients with LA. (A) Competitive ELISA using rabbit antiserum raised against PGBb to quantify the concentration of PG (in picograms per milliliter) present in each sample. Horizontal black lines indicate means (****P < 0.0001, Kruskal–Wallis test followed by Dunn’s post hoc pairwise test). (B) Plot showing the PGBb concentration of each sample as a function of its anti-PGBb IgG level. The linear fit and the Pearson correlation coefficient (r) for the LA synovial fluid samples are also shown.
PCR analysis showed that serum and synovial fluid samples were often positive for B. burgdorferi DNA before antibiotic treatment (SI Appendix, Fig. S4C). In contrast, all but one serum and synovial fluid sample were negative after oral antibiotic treatment and all samples were negative after IV antibiotic therapy (SI Appendix, Fig. S4C). Thus, our results suggest that PGBb material persists in LA patients long after B. burgdorferi eradication.
B. burgdorferi PG Elicits Proinflammatory Cytokine Responses in Human Peripheral Blood Mononuclear Cells.
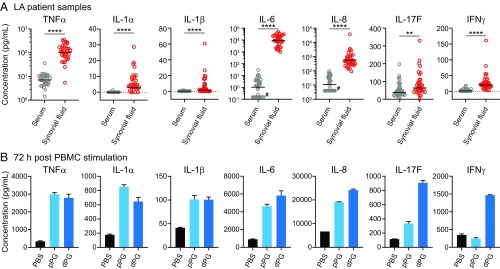
LA is characterized by marked synovial hypertrophy and inflammation. As in other forms of inflammatory arthritis, proinflammatory cytokines such as IL-1, TNFα, IL-6, and IL-8 are found in the synovial fluid of patients with LA (12, 49, 50). Consistent with these previous observations, we found that virtually all major proinflammatory markers were significantly up-regulated in the synovial fluid of patients with LA relative to their serum (SI Appendix, Fig. S6), ranging from 4- to 2,000-fold increases in TNFα, IL-1α, IL-1β, IL-6, IL-8, IL-17F, and INFγ production (Fig. 4A). Inflammation of this magnitude often coincided with a secondary response involving production of antiinflammatory cytokines, including IL-10, the level of which was also significantly increased in the synovial fluid of LA patients (SI Appendix, Fig. S6).
Fig. 4.
Cytokine profile in serum and synovial fluid samples from patients with LA or after in vitro stimulation of human PBMCs with PGBb. (A) Bee-swarm plots showing levels of indicated cytokines in LA patient samples. Horizontal black lines indicate geometric means (****P < 0.0001 and **0.001 < P < 0.01, Mann-Whitney U test). Pound signs indicate samples that yielded no signal but were included for completeness, as zero values cannot be displayed on log-scale axes. (B) Cytokine levels produced by control human PBMCs stimulated by PBS or 100 μg/mL polymeric PG (pPG) or mutanolysin-digested PG (dPG) for 72 h. The 18-h results are shown in SI Appendix, Fig. S7A. All stimulatory studies were performed on pooled, mixed donor samples assayed in duplicate (mean ± SD).
To determine if PGBb alone can elicit an inflammatory response, we stimulated human peripheral blood mononuclear cells (PBMCs) from healthy control subjects with polymeric (whole) or mutanolysin-digested PGBb for 18 or 72 h. The synthesis of virtually all analytes highly represented in synovial fluid samples (Fig. 4A) and previously implicated in LA (12, 50) was induced by polymeric and/or digested PGBb (Fig. 4B and SI Appendix, Fig. S7A). Note that, under these stimulatory conditions, PGBb behaves similarly to other PG types (SI Appendix, Fig. S7). However, stimulation with PGBb resulted in only a two- to threefold increase in the level of antiinflammatory cytokine IL-10 after 72 h relative to the 10-fold increase seen with other PG types (Fig. 4B vs. SI Appendix, Fig. S7). These findings suggest that PGBb may have the ability to cause inflammation without eliciting a compensatory antiinflammatory response of the magnitude normally seen with infectious agents and associated immunogens (51).
Systemic Administration of B. burgdorferi PG Triggers Acute Arthritis in Mice.
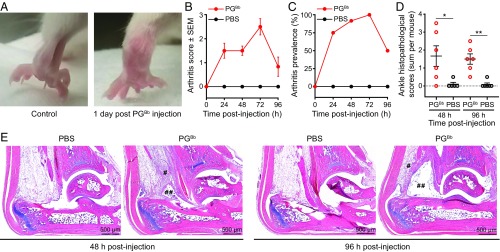
Systemic injection of PG isolated from Gram-positive bacteria is known to induce arthritis in mice and rats (23–26). To test the arthritogenic potential of the chemically unusual PGBb, we injected a sonicated preparation of PGBb into the tail veins of 12 BALB/c mice. In parallel, a control group of 12 mice received the diluent (PBS). All 24 mice were evaluated clinically and scored daily for evidence of swelling and erythema in their paws and tibiotarsal joints. Half of the mice from each group were randomly selected and euthanized on day 2 or 4 postinjection. Both hind limbs from each euthanized animal were immersion-fixed, decalcified, and stained with hematoxylin-eosin for blinded histopathological evaluation (52).
We found that PGBb alone was sufficient to induce acute arthritis, as evidenced by ankle swelling by 24–96 h postinjection (Fig. 5 A–C). In contrast, the control mice injected with PBS alone, as well as additional unmanipulated mice housed under similar conditions, showed no visual evidence of swelling (Fig. 5 A–C). Histopathologic analysis of the hind limbs of mice injected with PGBb confirmed the presence of inflammatory infiltrates in the peritendinous adventitia (Fig. 5 E, single pound symbols) and edema in the synovial space (Fig. 5 E, double pound symbols) at 48-h and 96-h time points (Fig. 5 D and E). Such infiltrates were absent in control mice injected with PBS (Fig. 5 D and E). Our data indicate that systemic exposure to PGBb is sufficient to trigger an acute tenosynovitis, consistent with what is observed in the established mouse model of LA (53).
Fig. 5.
Systemic administration of PGBb induces acute arthritis in mice. (A) A BALB/c mouse 24 h after IV injection of 200 μg PGBb exhibits bilateral ankle edema not present in an uninjected control mouse. (B) Average composite arthritis score (i.e., average sum of individual scores for left and right hind limbs) within each mouse group 24, 48, 72, and 96 h after IV administration of PGBb or PBS. Error bars indicate SEMs; n = 12 mice per group at 24 and 48 h postinjection and n = 6 mice per group at all subsequent time points. (C) Arthritis prevalence as a function of time after injection with PGBb or PBS. Only mice with a composite arthritis score ≥1 were considered as having arthritis. (D) Sum of left and right ankle histopathological scores for individual mice at 48 or 96 h after IV injection of PGBb or PBS. Horizontal black lines indicate means and SEM (**P < 0.01 and *0.01 < P < 0.05, respectively, Mann–Whitney U test). (E) Representative light micrographs of hematoxylin-eosin–stained sections of mouse ankles collected 48 or 96 h after IV administration of PGBb show peritendon inflammation (single pound symbols) and synovial space edema (double pound symbols). PBS-injected control mice lack both histopathological features when examined at the same time points.
Discussion
Our study provides supporting evidence of an important role for PGBb in the pathogenesis of LA. Clinical manifestations of Lyme disease are largely driven by the host immune response rather than toxin-mediated damage (6). PG is recognized by several types of pattern recognition receptors including Toll-like receptors (TLRs), PG recognition proteins (PGRPs), and cytoplasmic NOD proteins (22, 54). Although their downstream effectors may vary (55), the result is often a strong proinflammatory response (56). Similar inflammatory responses are apparent in the synovial fluid of patients with LA based on cytokine profiling (Fig. 4 and SI Appendix, Fig. S6). For instance, TNFα was, on average, up-regulated 16-fold in synovial fluid samples from patients with LA and induced in human PBMCs exposed to PGBb in vitro (Fig. 4 and SI Appendix, Fig. S7A). This is noteworthy, as TNFα is a key effector protein in chronic inflammatory arthritides, and biologic agents targeting TNFα have been used successfully in cases of postinfectious LA (5).
Although autoimmunity has been implicated in the pathogenesis of LA (9–13, 57), genetic and transcriptomic evidence suggests that variability in innate immune responses during and after B. burgdorferi infection is also an important disease determinant. Notably, transcripts encoding PG-cleaving protein lysozyme and PG-sensing protein NOD2 are elevated in synovial tissues of postinfectious LA patients months to several years after antibiotic therapy (58, 59). Therefore, immune responses to PGBb and autoantigens may contribute to pathology, even after the infection itself has been cleared. The role of PGBb and autoantigens may be independent or PGBb may act as an adjuvant, exacerbating immunoreactivity to autoantigens in the synovium. Differences in PGBb-specific immune responses among patients with LA may contribute to variability in disease severity.
How can PGBb material remain in the synovial environment for an extended period (weeks to months) after appropriate antibiotic treatment (Fig. 3)? There are several possibilities.
First, PG material may be left behind after bacterial killing. B. burgdorferi cells that disseminate to the joint may shed muropeptides as they undergo replication. These muropeptides may then diffuse into the synovial cavity over time. Alternatively (or in addition), PG exposure may occur in the absence of spirochete replication; PG material may simply be released following bacterial lysis (through natural death or killing by the immune system or antibiotic treatment). Both possibilities would be consistent with the hypothesis that retained bacterial antigens are a source of inflammatory stimuli in LA (14). In rats, bacterial cell-wall fragments are detected weeks to months after their systemic administration (60, 61), supporting the notion that PG material can persist for an extended period in animals.
Second, tissue-resident synovial macrophages may act as an antigen sink (62). Although our synovial samples were free of cells (Methods), they contained extracellular vesicles, likely derived from immune and stromal cells (63). Vesicles from antigen-presenting cells containing PGBb material may be released into the surrounding environment. Interestingly, antigen-presenting cells containing PG from gut bacteria have been proposed to contribute to inflammation in patients with rheumatoid arthritis (64, 65).
Third, PG-containing immune complexes may accumulate in the synovial fluid. We show that patients with LA develop a specific anti-PGBb antibody response that is higher in the synovial fluid than in the serum (Fig. 2). This, together with the presence of PGBb in the synovial fluid (Fig. 3), may result in accumulation of PGBb immune complexes in the inflamed joints. Previous work on Bacillus anthracis PG has established that PG can form immune complexes with anti-PG antibodies, which can activate human platelets and promote vascular damage (66). Inflammation and damage in and around the microvasculature is a hallmark of the synovial lesions seen in postinfectious LA (67, 68), and immune complexes are known to localize to joints in patients with LA (69). Future studies will be required to discriminate between these three nonexclusive possibilities.
The finding that B. burgdorferi releases PGBb fragments during growth (Fig. 1C) suggests that PGBb may play a broad role in the multifaceted pathogenesis of Lyme disease beyond LA. Released muropeptides have previously been implicated in diseases caused by other bacteria. For example, Neisseria gonorrhoeae recycles most of its PG breakdown products (39, 70), as Gram-negative bacteria generally do. However, the small amount of PG monomers that N. gonorrhoeae releases (39, 71) is thought to induce inflammatory cytokine production and cause ciliated cell death in human fallopian tubes (72). In contrast to N. gonorrhoeae, B. burgdorferi lacks a PG recycling pathway (Fig. 1B and SI Appendix, Fig. S1C), suggesting that significant quantities of muropeptides may be released into the environment during B. burgdorferi proliferation, presumably through the outer-membrane porins. We confirmed that hNOD2-binding muropeptides are shed into the culture supernatant during B. burgdorferi growth (Fig. 1C). Given PGBb immunogenicity (Figs. 2, 4, and 5 and SI Appendix, Fig. S7) (32), muropeptide shedding during active B. burgdorferi infection may, together with surface-exposed lipoproteins (73–75) and glycolipids (76, 77), contribute to early inflammatory manifestations, such as skin lesions, carditis, and meningitis.
After antibiotic treatment of the infection, therapy for postinfectious LA is currently directed at dampening immune responses with disease-modifying antirheumatic drugs, primarily hydroxychloroquine or methotrexate (5). The persistence of immunogenic PGBb material in inflamed joints provides a stronger rationale for targeting innate immune responses with medications, such as TNF or NF-κB inhibitors, for the treatment of such patients. A potential role for bacterial PG in triggering inflammation in rheumatoid arthritis patients has been considered for several decades (61, 62, 64, 65). Our work supports further consideration of this idea.
Methods
Bacterial Strains, Cell Lines, and Growth Conditions.
A clone of the B. burgdorferi type strain B31 (MI) (16) was used in all experiments involving this bacterium. Other bacteria used in this study include S. aureus SA113, B. subtilis 168, and E. coli K-12 MG1655. Unless otherwise noted, B. burgdorferi was cultured at 34 °C in complete BSK II medium containing 6% rabbit serum (78). All other bacteria were grown at 37 °C in LB medium. HEK 293-derived human NOD1 and NOD2 reporter cell lines (InvivoGen) were cultured at 37 °C under 5% CO2 in RPMI medium containing 10% (vol/vol) FBS and blasticidin S (30 µg/mL), Zeocin (100 µg/mL), and Normocin (100 µg/mL). Fresh PBMCs from healthy human subjects were obtained from mixed donor samples (Zen-Bio) and used in assays in the recommended PBMC culture medium (Zen-Bio).
PG Purification.
PGBb was purified as described previously (79), which is an adaptation of the Glauner protocol (80). For immunological and mouse studies, a few modifications were made to increase yield and ensure purity. PGBb was purified from 2–3 L of B. burgdorferi culture. Before protease treatment with 300 µg/mL α-chymotrypsin (Sigma-Aldrich), insoluble PGBb was treated with 50 U of DNase (Zymogen) and 10 U of RNase A (Promega) for 2 h, followed by a 2-h treatment with 10 µg/mL amylase (Sigma-Aldrich). After protease digestion, PGBb sacculi were harvested and washed three times with 10 mL endotoxin-free water, once with 10 mL 0.5 M EDTA, and three more times with water. A similar procedure was performed to purify PG from E. coli. For PG preparations from Gram-positive bacteria, the cell walls were broken using a kit (Precellys Microorganism Lysing Kit) that includes 7-mL tubes containing glass beads before sodium dodecyl sulfate (SDS) solubilization and enzymatic treatment. The Precellys Evolution homogenizer was set to 10 cycles of 30 s at 8,500 rpm with a 60-s rest period between each cycle. Afterward, samples were treated with 48% hydrofluoric acid for 48 h at 4 °C to hydrolyze PG-bound teichoic acids as previously described (81). Post hydrolysis, PG sacculi were harvested and washed as described here earlier.
The concentration of all purified PG preparations was determined by dry weight and confirmed by SLP assay as previously described (82).
PG Structural and Chemical Analysis.
Purified PGBb (∼100 µg) was digested with cellosyl (25 µg/mL) for 14–16 h at 37 °C, and the resulting muropeptides were analyzed by LC-MS as reported previously (83).
For the chemical analysis, purified PGBb (0.7 mg) was hydrolyzed (200 µL 4 N HCl, 100 °C, 16 h) in a sealed ampoule. The hydrolysate was evaporated to dryness in a gentle stream of air at 60 °C. The residue was dissolved in 200 µL water and dried down again to remove residual HCl. The amino acids of the hydrolysate were transformed into N-pentafluoropropionyl amino acid isopropylesters according to protocol 11 described in a previous review (84). These amino acid derivatives were analyzed by GC (GC-14A; Shimadzu) with a CP-ChiraSil-l-Val column (Agilent Technologies, CP495) following protocol 11 and by GC-MS using a 320 Single Quad instrument (Varian) equipped with a VF-5ms column (CP8944; Agilent Technologies) using protocol 10 (84).
To verify the incorporation of l-Orn into the PGBb (SI Appendix, Fig. S2B), B. burgdorferi was cultured in 500 mL complete BSK II medium to a density of 106 cells per milliliter. Cells were harvested by centrifugation (3,500 × g for 20 min) and resuspended in 50 mL of prewarmed, modified medium (25% BSK II in PBS plus 1.2% rabbit serum) (85) containing 7.5 µCi/mL of 3H l-Orn (Perkin-Elmer). After 48 h of incubation, unincorporated radiolabeled l-Orn was removed by centrifugation (3,500 × g for 20 min) and three washes with 40 mL of PBS. After each wash, cells were harvested by centrifugation at 3,500 × g for 10 min. After the washes, the cells were gently resuspended in 5 mL of PBS and PG was purified as described earlier.
PG Turnover Studies.
To track the turnover of PGBb over time (Fig. 1B), we used two different protocols. In the first one, 500 mL culture of B. burgdorferi at a cell density of 106 cells per milliliter was pulse-labeled with 7.5 µCi/mL of 14C l-Orn as described earlier. After three washes and centrifugation, cells were gently resuspended to a final concentration of 5 × 104 cells per milliliter in 250 mL prewarmed BSK II complete medium [which includes rabbit serum that contains Orn (86)]. Retention of radiolabel into the PGBb was tracked by removing a 25-mL culture volume at various time points, harvesting the cells by centrifugation (3,500 × g for 20 min), washing cells once with 25 mL of PBS, and harvesting cells at 3,500 × g for 10 min. Pelleted cells were resuspended, and cells were solubilized in a boiling solution of 4% SDS for 30 min. SDS-insoluble PGBb was pelleted at 145,000 × g for ∼30 min and analyzed by liquid scintillation. In the second protocol, a 250-mL culture of B. burgdorferi at 106 cells per milliliter was centrifuged (4,000 × g for 20 min) and cells were resuspended in 50 mL of prewarmed, modified medium (as described earlier) containing 7.5 µCi/mL of 3H l-Orn. After 48 h of incubation, unincorporated radiolabeled l-Orn was removed by centrifugation (4,000 × g for 20 min) and three washes with 40 mL of PBS. After each wash, cells were harvested by centrifugation at 3,000 × g for 10 min. After washes, cells were resuspended in 125 mL of BSK II complete medium at a density of 5 × 104 cells per milliliter. The remaining steps were the same as the first protocol except that 10 mL of culture was removed at each time point and cells were harvested at 4,000 × g for 20 min. Both protocols gave highly similar results (Fig. 1B).
NOD Activation Assay.
Time-course experiments to monitor the release of muropeptides were performed to ensure that potential stress during the radiolabeling procedure (as detailed earlier) or washes did not significantly alter our findings. In these experiments, 10 mL of culture was removed from a 250-mL batch culture, cells were enumerated, and 8 mL of culture was filtered by using a 0.1-µm filter under a vacuum. From the filtered flow-through, 5 mL was processed through a YM-3 Amicon filter to selectively exclude biomolecules greater than 3,000 Da. Column flow-through (4 mL) was lyophilized and resuspended in 1 mL of endotoxin-free Dulbecco’s PBS (DPBS), resulting in a 4× solution of the culture supernatant. Sterile BSK II complete medium (without phenol red) was processed similarly to serve as control medium to which each signal was background-subtracted.
HEK-Blue hNOD1 and hNOD2 cells were cultured to 60–70% confluence, washed with PBS, enumerated, and resuspended in QUANTI-Blue detection medium (InvivoGen) at a final concentration of 2.5 × 105 cells per milliliter. HEK-Blue hNOD1 or hNOD2 cells (180 µL per well) were incubated in 96-well plate in triplicate with 20 µL of a three-time dilution (in DPBS) of the 4× culture-supernatant solution. Cells were incubated at 37 °C in 5% CO2 for 18 h. Colorimetric quantification of NF-κB activity through NOD1 or NOD2 activation was measured at 650 nm. Gefitinib (Sigma), an inhibitor that interferes with adaptor protein RIP2 signaling (43), was used at a final concentration of 20 µM.
Human Subject Samples.
All work with human samples was approved by the human investigations committee at Massachusetts General Hospital granted to A. Steere. Patients with Lyme disease satisfied the criteria put forth by the Centers for Disease Control and Prevention (87). Patients with LA were treated with 1–2 mo of oral antibiotic therapy (usually doxycycline), followed by an additional 1 mo of IV antibiotic therapy (ceftriaxone) if needed, as described by the Infectious Diseases Society of America (88). Control synovial fluid samples were acquired from patients with rheumatoid arthritis, psoriatic arthritis, and osteoarthritis who met the criteria associated with each disease (89–91).
Serum and synovial fluid samples were collected and then centrifuged at 300 × g for 10 min, followed by another centrifugation at 3,000 × g for another 10 min to remove cells and cell debris as previously described (92). All samples were stored at −80 °C and did not undergo more than two freeze–thaw cycles.
PCR Analysis.
Serum and synovial fluid samples were screened by PCR for amplification of the B. burgdorferi flaB gene by using the fla-3 (5′-GGGTCTCAAGCGTCTTGG-3′) and fla-4 (5′-GAACCGGTGCAGCCTGAG-3′) oligonucleotides and Phusion Polymerase (New England Biolabs). The cycling conditions were as follows: 1 cycle at 98 °C for 30 s and 45 cycles of 98 °C for 12 s, 58 °C for 20 s, and 70 °C for 15 s, followed by a final extension at 70 °C for 5 min. All reactions were subjected to DNA agarose electrophoresis and visualized by ethidium bromide staining. Visible products were apparent for serum samples 9, 13, 17, 20, and 33 and for synovial fluid samples 9, 13, 17, and 20 (SI Appendix, Fig. S4C).
ELISA.
To quantify the level of anti-PG IgG in patient samples, purified PG sacculi (100 µg/mL) in PBS with 0.01% SDS were immobilized on poly-lysine–coated microtiter plates overnight at 4 °C. Unbound material was removed through three washes with PBS-T (PBS plus 0.05% Tween 20). The wells were then “blocked” for 2 h at 37 °C using SEA-BLOCK (Thermo Fisher Scientific). Serum and synovial fluid samples were diluted 1:25 in PBS and incubated with substrates (or diluent control) for 2 h at room temperature with gentle rocking. Unbound material was washed with PBS-T. After washing, plates were incubated with anti-human IgG-HRP (1:25,000; Sigma-Aldrich), and bound IgG was detected by using 1-step Turbo TMB substrate (Thermo Fisher Scientific). Reported IgG response was determined by measuring absorbance at 450 nm, following background subtraction for each patient sample signal attained in the absence of PG ligand.
Detection of PGBb in patient samples was performed by using a competitive ELISA and rabbit anti-PGBb polyclonal antibodies produced as a fee-for-service by Cocalico Biologicals (Thermo Fisher Scientific). Briefly, purified PGBb (0.5 mg/mL) was used to immunize two New Zealand White rabbits according to protocols approved by the animal care and use committee of Cocalico Biologicals. After one dose, two boosters of 0.5 mg/mL were administered 1 wk apart. Serum from blood samples collected on days 53 and 54 was assayed for PGBb specificity by competitive ELISA. Competitive ELISA involved coating plates with 100 µg/mL of PGBb as described earlier. Rabbit serum containing anti-PGBb IgG was diluted 1:350 in PBS and preincubated for 2 h with titrating amounts (10 ng/mL to 10 pg/mL) of different bacterial PG preparations with gentle mixing at room temperature before 1 h incubation with PGBb-coated plates. All patient samples were diluted 1:5 in PBS and otherwise treated exactly as the PGBb standards of known concentration. Rabbit anti-IgG-HRP (Bio-Rad) diluted 1:3,000 was used to detect anti-PGBb. Standard curves were created by using 1/absorbance values (at 450 nm) produced with known concentrations of each PG preparation. Data were fitted by a third-order polynomial equation. Standard curve experiments were performed on the same day as the serum and synovial fluid analyses and used to back-calculate the amount of PGBb in each patient sample.
PBMC Stimulation and Cytokine Analysis.
Muropeptides were generated by digesting 1 mL of purified PG (120 µg/mL) with Streptomyces globisporus mutanolysin (1,000 U/mL; Sigma-Aldrich) for 4 h at 37 °C in buffer (50 mM MES, 1 mM MgCl2, pH 6), followed by another incubation of mutanolysin (∼500 U) overnight at 37 °C. Undigested material was harvested by centrifugation at 150,000 × g for 30 min at 12 °C. The soluble muropeptides were lyophilized, and their amount was determined by weight.
Upon arrival, fresh PBMCs (Zen-Bio) were seeded in 12-well plates at 106 cells per milliliter and allowed to rest at 37 °C under 5% CO2 atmosphere for 24 h before further manipulation. After stimulation with 100 µg/mL of digested or polymeric PG, cells were harvested by centrifugation at 600 × g for 8 min and supernatants were collected and stored at −80 °C for further analysis. All cytokines were assayed using Luminex bead arrays (Agilent) following the manufacturer’s recommendations. All supernatants were diluted 1:5 in PBS and analyzed in duplicate.
Serum and synovial fluid samples from patients with LA, diluted 1:3 in PBS, were similarly analyzed in duplicate and run on the same day as the PBMC supernatants. The concentration of cytokines (in picograms per milliliter) from patient samples were log2-transformed to create the heat map (SI Appendix, Fig. S6).
PG Injection in Mice and Histopathology.
Purified PGBb was lyophilized, weighed, and resuspended to a final concentration of 2 μg/μL in DPBS. To achieve even dispersal PGBb in DPBS, the suspension was subjected to four rounds of sonication (15 s each) on ice using a Branson Digital Sonifier set to 45% amplitude. Fragmented PGBb (100 µL, i.e., 200 μg PGBb) was administered IV to each of 12 female BALB/c mice (5–6 wk old) by tail vein injection. In parallel, 12 BALB/c mice (age- and sex-matched) were injected IV with 100 μL DPBS. All mice were then examined daily for foot and ankle swelling and assigned a clinical arthritis score as previously described (29). Briefly, arthritis scores were computed by summing the individual scores for both hind paws, each graded as follows: 0, normal paw, no redness or swelling; 1, some swelling of ankle; 2, moderate swelling and redness of ankle; 3, moderate swelling and redness of ankle and some swelling of foot pad and/or digits; and 4, pronounced swelling and redness of the whole paw. Each group of mice was also evaluated for the prevalence of arthritis (defined as the percentage of mice with an arthritis score of at least 1). Half of the mice in each group (n = 6) were euthanized by CO2 asphyxiation on days 2 and 4 postinjection, and both hind limbs from each animal were immediately fixed in 10% formalin and subsequently decalcified, embedded in paraffin, sectioned, and stained with hematoxylin-eosin by routine methods. For each mouse, one stained section per hind limb midlevel (to include the stifle and tibiotarsal joints) was analyzed. Sections were analyzed, and stifle and tibiotarsal inflammation was scored blindly by a veterinarian (C.J.B.) formally trained in pathology with years of experience in scoring mice for inflammation using previously published criteria (93). All procedures involving mice were approved by the Yale University Institutional Animal Care and Use Committee.
Supplementary Material
Acknowledgments
We thank Alexia Belperron, Jialing Mao, and Nicole D’Angelo for assistance with the mouse experiments; Roman Dziarski for advice in planning the PG injection studies; and Dr. Patricia Rosa and the laboratories of C.J.‐W. and B.L.J. for valuable discussions and critical reading of the manuscript. This study was supported in part by Wellcome Trust grant 101824/Z/13/Z (to W.V.) and National Institutes of Health grants AI101175 and AI144365. C.J.-W. is an investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1904170116/-/DCSupplemental.
References
- 1.Mead P. S., Epidemiology of Lyme disease. Infect. Dis. Clin. North Am. 29, 187–210 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Stanek G., Strle F., Lyme borreliosis-from tick bite to diagnosis and treatment. FEMS Microbiol. Rev. 42, 233–258 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Koedel U., Fingerle V., Pfister H. W., Lyme neuroborreliosis-epidemiology, diagnosis and management. Nat. Rev. Neurol. 11, 446–456 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Robinson M. L., Kobayashi T., Higgins Y., Calkins H., Melia M. T., Lyme carditis. Infect. Dis. Clin. North Am. 29, 255–268 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Arvikar S. L., Steere A. C., Diagnosis and treatment of Lyme arthritis. Infect. Dis. Clin. North Am. 29, 269–280 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bockenstedt L. K., Wormser G. P., Review: Unraveling Lyme disease. Arthritis Rheumatol. 66, 2313–2323 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlson D., et al. , Lack of Borrelia burgdorferi DNA in synovial samples from patients with antibiotic treatment-resistant Lyme arthritis. Arthritis Rheum. 42, 2705–2709 (1999). [DOI] [PubMed] [Google Scholar]
- 8.Li X., et al. , Burden and viability of Borrelia burgdorferi in skin and joints of patients with erythema migrans or Lyme arthritis. Arthritis Rheum. 63, 2238–2247 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crowley J. T., et al. , A highly expressed human protein, apolipoprotein B-100, serves as an autoantigen in a subgroup of patients with Lyme disease. J. Infect. Dis. 212, 1841–1850 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crowley J. T., et al. , Matrix metalloproteinase-10 is a target of T and B cell responses that correlate with synovial pathology in patients with antibiotic-refractory Lyme arthritis. J. Autoimmun. 69, 24–37 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pianta A., et al. , Annexin A2 is a target of autoimmune T and B cell responses associated with synovial fibroblast proliferation in patients with antibiotic-refractory Lyme arthritis. Clin. Immunol. 160, 336–341 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strle K., et al. , T-helper 17 cell cytokine responses in Lyme disease correlate with Borrelia burgdorferi antibodies during early infection and with autoantibodies late in the illness in patients with antibiotic-refractory Lyme arthritis. Clin. Infect. Dis. 64, 930–938 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drouin E. E., et al. , A novel human autoantigen, endothelial cell growth factor, is a target of T and B cell responses in patients with Lyme disease. Arthritis Rheum. 65, 186–196 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bockenstedt L. K., Gonzalez D. G., Haberman A. M., Belperron A. A., Spirochete antigens persist near cartilage after murine Lyme borreliosis therapy. J. Clin. Invest. 122, 2652–2660 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takayama K., Rothenberg R. J., Barbour A. G., Absence of lipopolysaccharide in the Lyme disease spirochete, Borrelia burgdorferi. Infect. Immun. 55, 2311–2313 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fraser C. M., et al. , Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390, 580–586 (1997). [DOI] [PubMed] [Google Scholar]
- 17.Hirschfeld M., et al. , Cutting edge: Inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. J. Immunol. 163, 2382–2386 (1999). [PubMed] [Google Scholar]
- 18.Salazar J. C., et al. , Lipoprotein-dependent and -independent immune responses to spirochetal infection. Clin. Diagn. Lab. Immunol. 12, 949–958 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kenedy M. R., Lenhart T. R., Akins D. R., The role of Borrelia burgdorferi outer surface proteins. FEMS Immunol. Med. Microbiol. 66, 1–19 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vollmer W., Blanot D., de Pedro M. A., Peptidoglycan structure and architecture. FEMS Microbiol. Rev. 32, 149–167 (2008). [DOI] [PubMed] [Google Scholar]
- 21.Typas A., Banzhaf M., Gross C. A., Vollmer W., From the regulation of peptidoglycan synthesis to bacterial growth and morphology. Nat. Rev. Microbiol. 10, 123–136 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolf A. J., Underhill D. M., Peptidoglycan recognition by the innate immune system. Nat. Rev. Immunol. 18, 243–254 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Stimpson S. A., et al. , Effect of acetylation on arthropathic activity of group A streptococcal peptidoglycan-polysaccharide fragments. Infect. Immun. 55, 16–23 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fox A., et al. , Arthropathic properties related to the molecular weight of peptidoglycan-polysaccharide polymers of streptococcal cell walls. Infect. Immun. 35, 1003–1010 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koga T., et al. , Acute joint inflammation in mice after systemic injection of the cell wall, its peptidoglycan, and chemically defined peptidoglycan subunits from various bacteria. Infect. Immun. 50, 27–34 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onta T., et al. , Induction of acute arthritis in mice by peptidoglycan derived from Gram-positive bacteria and its possible role in cytokine production. Microbiol. Immunol. 37, 573–582 (1993). [DOI] [PubMed] [Google Scholar]
- 27.Liu Z. Q., Deng G. M., Foster S., Tarkowski A., Staphylococcal peptidoglycans induce arthritis. Arthritis Res. 3, 375–380 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X., et al. , CD14 mediates the innate immune responses to arthritopathogenic peptidoglycan-polysaccharide complexes of Gram-positive bacterial cell walls. Arthritis Res. Ther. 6, R273–R281 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saha S., et al. , PGLYRP-2 and Nod2 are both required for peptidoglycan-induced arthritis and local inflammation. Cell Host Microbe 5, 137–150 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oosting M., et al. , Recognition of Borrelia burgdorferi by NOD2 is central for the induction of an inflammatory reaction. J. Infect. Dis. 201, 1849–1858 (2010). [DOI] [PubMed] [Google Scholar]
- 31.Petnicki-Ocwieja T., et al. , Nod2 suppresses Borrelia burgdorferi mediated murine Lyme arthritis and carditis through the induction of tolerance. PLoS One 6, e17414 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beck G., Benach J. L., Habicht G. S., Isolation, preliminary chemical characterization, and biological activity of Borrelia burgdorferi peptidoglycan. Biochem. Biophys. Res. Commun. 167, 89–95 (1990). [DOI] [PubMed] [Google Scholar]
- 33.Montgomery R. R., Lusitani D., de Boisfleury Chevance A., Malawista S. E., Human phagocytic cells in the early innate immune response to Borrelia burgdorferi. J. Infect. Dis. 185, 1773–1779 (2002). [DOI] [PubMed] [Google Scholar]
- 34.Park J. T., Uehara T., How bacteria consume their own exoskeletons (turnover and recycling of cell wall peptidoglycan). Microbiol. Mol. Biol. Rev. 72, 211–227 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodell E. W., Schwarz U., Release of cell wall peptides into culture medium by exponentially growing Escherichia coli. J. Bacteriol. 162, 391–397 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacobs C., Huang L. J., Bartowsky E., Normark S., Park J. T., Bacterial cell wall recycling provides cytosolic muropeptides as effectors for beta-lactamase induction. EMBO J. 13, 4684–4694 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adin D. M., Engle J. T., Goldman W. E., McFall-Ngai M. J., Stabb E. V., Mutations in ampG and lytic transglycosylase genes affect the net release of peptidoglycan monomers from Vibrio fischeri. J. Bacteriol. 191, 2012–2022 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nigro G., et al. , Muramylpeptide shedding modulates cell sensing of Shigella flexneri. Cell. Microbiol. 10, 682–695 (2008). [DOI] [PubMed] [Google Scholar]
- 39.Garcia D. L., Dillard J. P., Mutations in ampG or ampD affect peptidoglycan fragment release from Neisseria gonorrhoeae. J. Bacteriol. 190, 3799–3807 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holt S. C., Anatomy and chemistry of spirochetes. Microbiol. Rev. 42, 114–160 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schleifer K. H., Joseph R., A directly cross-linked L-ornithine-containing peptidoglycan in cell walls of Spirochaeta stenostrepta. FEBS Lett. 36, 83–86 (1973). [DOI] [PubMed] [Google Scholar]
- 42.Girardin S. E., et al. , Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 278, 8869–8872 (2003). [DOI] [PubMed] [Google Scholar]
- 43.Tigno-Aranjuez J. T., Asara J. M., Abbott D. W., Inhibition of RIP2's tyrosine kinase activity limits NOD2-driven cytokine responses. Genes Dev. 24, 2666–2677 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Girardin S. E., et al. , Peptidoglycan molecular requirements allowing detection by Nod1 and Nod2. J. Biol. Chem. 278, 41702–41708 (2003). [DOI] [PubMed] [Google Scholar]
- 45.Verbrugh H. A., Peters R., Rozenberg-Arska M., Peterson P. K., Verhoef J., Antibodies to cell wall peptidoglycan of Staphylococcus aureus in patients with serious staphylococcal infections. J. Infect. Dis. 144, 1–9 (1981). [DOI] [PubMed] [Google Scholar]
- 46.Wergeland H. I., Endresen C., Antibodies to various bacterial cell wall peptidoglycans in human and rabbit sera. J. Clin. Microbiol. 25, 540–545 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson P. M., Phua K. K., Perkins H. R., Hart C. A., Bucknall R. C., Antibody to streptococcal cell wall peptidoglycan-polysaccharide polymers in seropositive and seronegative rheumatic disease. Clin. Exp. Immunol. 55, 115–124 (1984). [PMC free article] [PubMed] [Google Scholar]
- 48.Todome Y., et al. , Detection of antibodies against streptococcal peptidoglycan and the peptide subunit (synthetic tetra-D-alanyl-bovine serum albumin complex) in rheumatic-diseases. Int. Arch. Allergy Immunol. 97, 301–307 (1992). [DOI] [PubMed] [Google Scholar]
- 49.Shin J. J., Glickstein L. J., Steere A. C., High levels of inflammatory chemokines and cytokines in joint fluid and synovial tissue throughout the course of antibiotic-refractory Lyme arthritis. Arthritis Rheum. 56, 1325–1335 (2007). [DOI] [PubMed] [Google Scholar]
- 50.Lochhead R. B., et al. , Robust interferon signature and suppressed tissue repair gene expression in synovial tissue from patients with postinfectious, Borrelia burgdorferi-induced Lyme arthritis. Cell. Microbiol. 21, e12954 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Couper K. N., Blount D. G., Riley E. M., IL-10: The master regulator of immunity to infection. J. Immunol. 180, 5771–5777 (2008). [DOI] [PubMed] [Google Scholar]
- 52.Belperron A. A., Liu N., Booth C. J., Bockenstedt L. K., Dual role for Fcγ receptors in host defense and disease in Borrelia burgdorferi-infected mice. Front. Cell. Infect. Microbiol. 4, 75 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Belperron A. A., Dailey C. M., Booth C. J., Bockenstedt L. K., Marginal zone B-cell depletion impairs murine host defense against Borrelia burgdorferi infection. Infect. Immun. 75, 3354–3360 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Royet J., Dziarski R., Peptidoglycan recognition proteins: Pleiotropic sensors and effectors of antimicrobial defences. Nat. Rev. Microbiol. 5, 264–277 (2007). [DOI] [PubMed] [Google Scholar]
- 55.Kawai T., Akira S., Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34, 637–650 (2011). [DOI] [PubMed] [Google Scholar]
- 56.Boneca I. G., The role of peptidoglycan in pathogenesis. Curr. Opin. Microbiol. 8, 46–53 (2005). [DOI] [PubMed] [Google Scholar]
- 57.Singh S. K., Girschick H. J., Lyme borreliosis: From infection to autoimmunity. Clin. Microbiol. Infect. 10, 598–614 (2004). [DOI] [PubMed] [Google Scholar]
- 58.Strle K., Shin J. J., Glickstein L. J., Steere A. C., Association of a toll-like receptor 1 polymorphism with heightened Th1 inflammatory responses and antibiotic-refractory Lyme arthritis. Arthritis Rheum. 64, 1497–1507 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lochhead R. B., et al. , Interferon-gamma production in Lyme arthritis synovial tissue promotes differentiation of fibroblast-like synoviocytes into immune effector cells. Cell. Microbiol. 21, e12992 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dalldorf F. G., Cromartie W. J., Anderle S. K., Clark R. L., Schwab J. H., The relation of experimental arthritis to the distribution of streptococcal cell wall fragments. Am. J. Pathol. 100, 383–402 (1980). [PMC free article] [PubMed] [Google Scholar]
- 61.Lichtman S. N., et al. , Bacterial cell wall polymers (peptidoglycan-polysaccharide) cause reactivation of arthritis. Infect. Immun. 61, 4645–4653 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Melief M. J., Hoijer M. A., Van Paassen H. C., Hazenberg M. P., Presence of bacterial flora-derived antigen in synovial tissue macrophages and dendritic cells. Br. J. Rheumatol. 34, 1112–1116 (1995). [DOI] [PubMed] [Google Scholar]
- 63.Malda J., Boere J., van de Lest C. H., van Weeren P., Wauben M. H., Extracellular vesicles—New tool for joint repair and regeneration. Nat. Rev. Rheumatol. 12, 243–249 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schrijver I. A., et al. , Peptidoglycan from sterile human spleen induces T-cell proliferation and inflammatory mediators in rheumatoid arthritis patients and healthy subjects. Rheumatology 40, 438–446 (2001). [DOI] [PubMed] [Google Scholar]
- 65.Schrijver I. A., Melief M. J., Tak P. P., Hazenberg M. P., Laman J. D., Antigen-presenting cells containing bacterial peptidoglycan in synovial tissues of rheumatoid arthritis patients coexpress costimulatory molecules and cytokines. Arthritis Rheum. 43, 2160–2168 (2000). [DOI] [PubMed] [Google Scholar]
- 66.Sun D., et al. , Bacillus anthracis peptidoglycan activates human platelets through FcγRII and complement. Blood 122, 571–579 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Johnston Y. E., et al. , Lyme arthritis. Spirochetes found in synovial microangiopathic lesions. Am. J. Pathol. 118, 26–34 (1985). [PMC free article] [PubMed] [Google Scholar]
- 68.Londoño D., et al. , Antibodies to endothelial cell growth factor and obliterative microvascular lesions in the synovium of patients with antibiotic-refractory Lyme arthritis. Arthritis Rheumatol. 66, 2124–2133 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hardin J. A., Steere A. C., Malawista S. E., Immune complexes and the evolution of Lyme arthritis. Dissemination and localization of abnormal C1q binding activity. N. Engl. J. Med. 301, 1358–1363 (1979). [DOI] [PubMed] [Google Scholar]
- 70.Greenway D. L., Perkins H. R., Turnover of the cell wall peptidoglycan during growth of Neisseria gonorrhoeae and Escherichia coli. Relative stability of newly synthesized material. J. Gen. Microbiol. 131, 253–263 (1985). [DOI] [PubMed] [Google Scholar]
- 71.Rosenthal R. S., Release of soluble peptidoglycan from growing gonococci: Hexaminidase and amidase activities. Infect. Immun. 24, 869–878 (1979). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Melly M. A., McGee Z. A., Rosenthal R. S., Ability of monomeric peptidoglycan fragments from Neisseria gonorrhoeae to damage human fallopian-tube mucosa. J. Infect. Dis. 149, 378–386 (1984). [DOI] [PubMed] [Google Scholar]
- 73.Norgard M. V., Riley B. S., Richardson J. A., Radolf J. D., Dermal inflammation elicited by synthetic analogs of Treponema pallidum and Borrelia burgdorferi lipoproteins. Infect. Immun. 63, 1507–1515 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gondolf K. B., Mihatsch M., Curschellas E., Dunn J. J., Batsford S. R., Induction of experimental allergic arthritis with outer surface proteins of Borrelia burgdorferi. Arthritis Rheum. 37, 1070–1077 (1994). [DOI] [PubMed] [Google Scholar]
- 75.Batsford S., Dunn J., Mihatsch M., Outer surface lipoproteins of Borrelia burgdorferi vary in their ability to induce experimental joint injury. Arthritis Rheum. 50, 2360–2369 (2004). [DOI] [PubMed] [Google Scholar]
- 76.Schröder N. W., et al. , Acylated cholesteryl galactoside as a novel immunogenic motif in Borrelia burgdorferi sensu stricto. J. Biol. Chem. 278, 33645–33653 (2003). [DOI] [PubMed] [Google Scholar]
- 77.Pozsgay V., et al. , Synthesis and antigenicity of BBGL-2 glycolipids of Borrelia burgdorferi, the causative agent of Lyme disease. Carbohydr. Res. 346, 1551–1563 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barbour A. G., Isolation and cultivation of Lyme disease spirochetes. Yale J. Biol. Med. 57, 521–525 (1984). [PMC free article] [PubMed] [Google Scholar]
- 79.Jutras B. L., et al. , Lyme disease and relapsing fever Borrelia elongate through zones of peptidoglycan synthesis that mark division sites of daughter cells. Proc. Natl. Acad. Sci. U.S.A. 113, 9162–9170 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Glauner B., Separation and quantification of muropeptides with high-performance liquid chromatography. Anal. Biochem. 172, 451–464 (1988). [DOI] [PubMed] [Google Scholar]
- 81.Gründling A., Schneewind O., Cross-linked peptidoglycan mediates lysostaphin binding to the cell wall envelope of Staphylococcus aureus. J. Bacteriol. 188, 2463–2472 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tsuchiya M., Asahi N., Suzuoki F., Ashida M., Matsuura S., Detection of peptidoglycan and beta-glucan with silkworm larvae plasma test. FEMS Immunol. Med. Microbiol. 15, 129–134 (1996). [DOI] [PubMed] [Google Scholar]
- 83.Bui N. K., et al. , The peptidoglycan sacculus of Myxococcus xanthus has unusual structural features and is degraded during glycerol-induced myxospore development. J. Bacteriol. 191, 494–505 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schumann P., Peptidoglycan structure. Methods Microbiol. 38, 101–129 (2011). [Google Scholar]
- 85.Jutras B. L., Chenail A. M., Stevenson B., Changes in bacterial growth rate govern expression of the Borrelia burgdorferi OspC and Erp infection-associated surface proteins. J. Bacteriol. 195, 757–764 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Molnár D., Soltész G., Mestyán J., The metabolic effects of cold exposure in the newborn rabbit. Biol. Neonate 36, 215–219 (1979). [DOI] [PubMed] [Google Scholar]
- 87.Wharton M., Chorba T. L., Vogt R. L., Morse D. L., Buehler J. W., Case definitions for public health surveillance. MMWR Recomm. Rep. 39, 1–43 (1990). [PubMed] [Google Scholar]
- 88.Wormser G. P., et al. , The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: Clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 43, 1089–1134 (2006). [DOI] [PubMed] [Google Scholar]
- 89.Aletaha D., et al. , 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 62, 2569–2581 (2010). [DOI] [PubMed] [Google Scholar]
- 90.Veale D., Rogers S., Fitzgerald O., Classification of clinical subsets in psoriatic arthritis. Br. J. Rheumatol. 33, 133–138 (1994). [DOI] [PubMed] [Google Scholar]
- 91.Altman R., et al. ; Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Arthritis Rheum. 29, 1039–1049 (1986). [DOI] [PubMed] [Google Scholar]
- 92.Lochhead R. B., et al. , MicroRNA expression shows inflammatory dysregulation and tumor-like proliferative responses in joints of patients with postinfectious Lyme arthritis. Arthritis Rheumatol. 69, 1100–1110 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Montgomery R. R., et al. , Recruitment of macrophages and polymorphonuclear leukocytes in Lyme carditis. Infect. Immun. 75, 613–620 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.