Significance
The ERK signaling pathway is hyperactivated in a majority of cancers. However, because it mediates myriad physiological responses, the clinical efficacy of current ERK pathway inhibitors has been severely limited by toxicity. This study uncovers both SHOC2 phosphatase complex-dependent and -independent mechanisms of RAF and ERK activation that are differentially engaged in a context and spatio-temporal–dependent manner. KRAS oncogenic signaling preferentially depends on SHOC2 dependent-mechanisms, which thus presents a therapeutic opportunity. This study provides a molecular framework for how targeting the SHOC2-holophosphatase regulatory node of the RAF activation process provides a mechanism for selective inhibition of ERK signaling.
Keywords: SHOC2, RAF, MRAS, RAS, ERK
Abstract
Despite the crucial role of RAF kinases in cell signaling and disease, we still lack a complete understanding of their regulation. Heterodimerization of RAF kinases as well as dephosphorylation of a conserved “S259” inhibitory site are important steps for RAF activation but the precise mechanisms and dynamics remain unclear. A ternary complex comprised of SHOC2, MRAS, and PP1 (SHOC2 complex) functions as a RAF S259 holophosphatase and gain-of-function mutations in SHOC2, MRAS, and PP1 that promote complex formation are found in Noonan syndrome. Here we show that SHOC2 complex-mediated S259 RAF dephosphorylation is critically required for growth factor-induced RAF heterodimerization as well as for MEK dissociation from BRAF. We also uncover SHOC2-independent mechanisms of RAF and ERK pathway activation that rely on N-region phosphorylation of CRAF. In DLD-1 cells stimulated with EGF, SHOC2 function is essential for a rapid transient phase of ERK activation, but is not required for a slow, sustained phase that is instead driven by palmitoylated H/N-RAS proteins and CRAF. Whereas redundant SHOC2-dependent and -independent mechanisms of RAF and ERK activation make SHOC2 dispensable for proliferation in 2D, KRAS mutant cells preferentially rely on SHOC2 for ERK signaling under anchorage-independent conditions. Our study highlights a context-dependent contribution of SHOC2 to ERK pathway dynamics that is preferentially engaged by KRAS oncogenic signaling and provides a biochemical framework for selective ERK pathway inhibition by targeting the SHOC2 holophosphatase.
Signaling by the RAF-MEK-ERK (ERK-MAPK) pathway is used by many extracellular signals to mediate a vast array of biological responses in a cell-type–dependent manner. The mechanisms regulating signal specificity remain poorly understood but are known to include modulators, scaffolds, feedbacks, and crosstalk with other signaling pathways that jointly control spatial and temporal dynamics of ERK activation. This in turn regulates phosphorylation of different ERK substrates in a cell-type–, compartment-, and context-dependent manner (1, 2).
Aberrant activation of the ERK pathway is one of the most common defects in human cancer, with oncogenic mutations in RAS and RAF genes found in ∼30% and ∼8% of cancers, respectively. Up-regulated ERK signaling is also responsible in a family of developmental disorders, referred to as RASopathies (3–5).
ERK pathway inhibitors have shown little clinical benefit against RAS mutant tumors because of resistance and toxicity (5). Strikingly, in both RAS and BRAF mutant cells, most resistance mechanisms lead to ERK pathway reactivation, highlighting a strong “oncogene addiction” of these cancers to ERK signaling. However, the potent pathway suppression required for antitumor activity is limited by the inhibitor doses that can be administered safely because of toxicity (6, 7). ERK activity is essential for normal tissue homeostasis and systemic ablation of MEK1/2 or ERK1/2 genes in adult mice leads to death of the animals from multiple organ failure within 2–3 wk, even under conditions of partial inactivation (8), highlighting the difficulties of inhibiting the ERK pathway with a therapeutic index. To effectively harness the addiction of RAS mutant cancers to ERK signaling into viable therapies, new strategies to inhibit the pathway with improved therapeutic margins are needed, for example by inhibiting ERK signaling in a context- or compartment-dependent manner (9, 10).
MEK and ERK kinases are fully activated by phosphorylation in two sites within its kinase domain by RAF and MEK, respectively. On the other hand, RAF activation is a complex multistep process that remains incompletely understood (11). A consensus model stipulates that under resting conditions, the three RAF kinases (ARAF, BRAF, and CRAF/RAF1) are kept in the cytosol in an inactive state by an intramolecular interaction mediated by 14-3-3 dimers binding in a phosphorylation-dependent manner to conserved sites at the N terminus (S214 ARAF, S365 BRAF, S259 CRAF, hereby referred to as the “S259” site) and C-terminal end (S729 in BRAF, S621 in CRAF) (11–13). Upon activation, RAS-GTP binds with high affinity to the RAS binding domain (RBD) of RAF and recruits RAF to the membrane where the cysteine-rich domain (CRD) also plays a role in membrane anchoring. Dephosphorylation of the S259 site is known to provide an additional activating input that releases the 14-3-3 from this site and allows RAF to adopt an open conformation where RAF dimerizes with other RAFs, as well as KSR proteins. Definitive confirmation of this model, however, awaits the crystal structure of full-length RAF with or without bound 14-3-3. Nevertheless, the importance of the S259 dephosphorylation regulatory step is highlighted by RAF1 gain-of-function mutations in Noonan syndrome that cluster around S259 to disrupt the interaction with 14-3-3 (14–17). Furthermore, although RAF1 mutations are rare in cancer, they cluster on residues S257 and S259 (cosmic database).
The precise dynamics and mechanism of S259 dephosphorylation remain unclear (11). We have previously shown that MRAS, a closely related member of the RAS family, upon activation forms a complex with the leucine-rich repeat protein SHOC2 and protein phosphatase 1 (PP1) that functions as a highly specific S259 RAF holophosphatase (18, 19). The importance of the SHOC2-MRAS-PP1 complex (SHOC2 complex) in RAF-ERK regulation is validated by gain-of-function mutations in Noonan syndrome in all three components—SHOC2, MRAS, and PP1—which promote phosphatase complex formation (20–23). On the other hand, the phosphatase PP2A has also been variously implicated in mediating S259 dephosphorylation (24–27), although this was primarily based on the use of okadaic acid and the misconception that it behaves as a specific PP2A inhibitor (28) (in addition to not discriminating between direct or indirect effects). Furthermore, in contrast to its role as a regulatory subunit within a phosphatase complex, other studies have suggested that SHOC2 can function as a scaffold that promotes the RAS–RAF interaction (29–33).
RAF proteins also undergo multiple activating phosphorylation events. Among them, phosphorylation within the negative-charge regulatory region (N-region) plays a key divergent role among RAF paralogues (11). In CRAF, S338 and Y341 phosphorylation within the S338SYY341 motif by PAK and SRC family kinases (SFK) plays a crucial role in regulated activation (34). In contrast, the homologous S446SDD motif in BRAF constitutively provides the negative charges required for activity by virtue of acidic D amino acids and constitutive S446 phosphorylation (11, 34, 35). This difference in N-region regulation is believed to account for BRAF having higher basal activity, being the most frequent RAF target for mutational activation in cancer and for BRAF being the initial activator in asymmetric RAF heterodimers (11, 36).
In this study, we have used RNAi and CRISPR to ablate SHOC2 and RAF function, as well as phosphoproteomics to comprehensively characterize the role of the SHOC2 phosphatase complex in RAF and ERK pathway regulation. We have uncovered a selective role for SHOC2 in ERK pathway dynamics, and show that although SHOC2 phosphatase-mediated dephosphorylation of the S259 site is critically required for growth factor-induced RAF heterodimerization, there also exist SHOC2-independent mechanisms of ERK activation, which are dependent on N-region phosphorylation of CRAF. Importantly, KRAS oncogenic signaling differentially relies on SHOC2-dependent mechanisms, which provides both a therapeutic opportunity and a molecular framework for selective inhibition of ERK signaling in a compartment and context-dependent manner.
Results
MRAS and SHOC2 Expression Promotes S365 BRAF/S259 CRAF Dephosphorylation, BRAF-MEK Dissociation, and BRAF-CRAF Dimerization.
To study the role of the SHOC2 complex in the regulation of RAF kinases, we generated an inducible T-REx-293 cell line (T-17 cells) where addition of the tetracycline analog Doxycycline (Dox) leads to expression of active MRAS-Q71L and SHOC2. In these cells, Dox-induced MRAS/SHOC2 expression led to potent S365 dephosphorylation of ectopic TAP6-BRAF that is inhibited in a dose-dependent manner by the serine/threonine phosphatase inhibitor calyculin A (Fig. 1A). To assess possible RAF regions involved in S259 dephosphorylation, transiently transfected BRAF and CRAF mutants were tested for dephosphorylation upon expression of MRAS/SHOC2. Among the mutants tested, only the RBD mutants R188L BRAF and R89L CRAF were defective for MRAS/SHOC2-induced S365/S259 dephosphorylation (Fig. 1 B and C). Interestingly, when the CRAF R89L RBD mutant was constitutively localized to the membrane by fusion with a RAS membrane-targeting region (CRAF-CAAX R89L), S259 dephosphorylation was efficiently induced by MRAS/SHOC2 expression (Fig. 1C). Taken together, these data suggest that membrane recruitment through interaction with the RBD is required for efficient S259 RAF dephosphorylation.
Fig. 1.
MRAS and SHOC2 expression promotes S365 BRAF/S259 CRAF dephosphorylation, BRAF-MEK dissociation, and BRAF-CRAF dimerization. (A) Calyculin A inhibits BRAF S365 dephosphorylation and ERK activation by MRAS-SHOC2 expression. Expression of MRAS L71 and SHOC2 was induced in T-17 cells stably expressing T6-BRAF by 1 μg/mL Dox treatment for 24 h. Cells were incubated with a Calyculin A dose–response for 20 min and lysates immunoprobed, as indicated. (B) Intact RBD is required for efficient S365 BRAF dephosphorylation by MRAS-SHOC2 expression: T6-BRAF WT and mutants were transiently transfected into T-17 cells and MRAS-SHOC2 expression induced for 24 h. (C) Impaired MRAS-SHOC2 induced S259 dephosphorylation by R89L RBD CRAF mutation is rescued by constitutive membrane localization. As in B but with T6-CRAF mutants. (D) MRAS-SHOC2 expression stimulates BRAF S365 dephosphorylation, MEK dissociation, and CRAF binding to BRAF. T-17 T6-BRAF cells as in A were treated with different Dox concentrations for 24 h. StrepTactin pull-downs of T6-BRAF and lysates were immunoprobed and visualized using a Li-COR Odyssey scanner. (E) Li-Cor quantification of D. (F) MRAS-SHOC2 expression stimulates MEK1 dissociation from BRAF and CRAF but not KSR1. GST-fusion genes were cotransfected into HEK293T cells, together with Myc-MEK1 and either empty vector or MRAS-L71 and SHOC2. GST-S6K was used as a control. GST pull-downs and lysates were probed as indicated.
MRAS/SHOC2 expression levels in T-17 cells did not prove to be tuneable because at the lowest Dox concentration that induced expression, there was a maximum effect on MRAS/SHOC2 protein levels and concomitant S365 dephosphorylation (Fig. 1 D and E). When ectopic T6-BRAF in these cells was purified with streptactin beads, MRAS/SHOC2 expression led to a decrease in the amount of MEK bound to T6-BRAF and a concomitant interaction of T6-BRAF with CRAF (Fig. 1 D and E). To further study the specificity of the role for MRAS/SHOC2 on RAF–MEK interactions, GST-pulldown assays were performed after cotransfection of myc-MEK1 with GST-tagged CRAF, BRAF, and KSR1 in HEK293T cells. Under basal conditions, MEK1 bound most strongly to KSR1 and only weakly to CRAF (KSR1 > BRAF >> CRAF), and Dox-induced MRAS/SHOC2 expression led to strong dissociation of MEK from BRAF and CRAF but not from KSR1 (Fig. 1F). Taken together, the above data suggest that MRAS/SHOC2-induced S365 BRAF dephosphorylation promotes MEK dissociation from BRAF and BRAF heterodimerization with CRAF.
SHOC2 Is Required for EGF-Induced S365/S259 Dephosphorylation, RAF Dimerization, BRAF-MEK Dissociation, and Efficient ERK Pathway Activation.
To assess the role of endogenous SHOC2 within the context of growth factor signaling, T-REx-293 cells where SHOC2 expression was stably inhibited by shRNA expression were used to analyze lysates and immunoprecipitates (IPs) of endogenous RAS and RAF proteins in a time course of EGF treatment. EGF-stimulated S365 BRAF dephosphorylation, MEK, ERK, and RSK phosphorylation, but not AKT and EGFR Y1068 phosphorylation, were severely impaired in SHOC2 knockdown (KD) cells, consistent with a selective role of SHOC2 in RAF-ERK pathway activation (Fig. 2A).
Fig. 2.
SHOC2 is required for EGF-induced S365/S259 dephosphorylation, RAF dimerization, BRAF-MEK dissociation, and efficient ERK pathway activation. (A) SHOC2 is required for EGF-induced BRAF-S365 dephosphorylation and efficient ERK pathway activation in T-REx-293 cells. Serum-starved cells stably expressing control or SHOC2 shRNA were treated with 25 ng/mL EGF for the indicated times and lysates immunoprobed as indicated. (B) SHOC2 is required for EGF-induced BRAF-CRAF heterodimerization and dissociation of BRAF–MEK complexes but not RAS–RAF interaction. Endogenous BRAF, CRAF, or RAS (238) IPs from lysates used in A were probed for as indicated. (C) SHOC2 is required for EGF-induced KSR/BRAF dimerization. Endogenous KSR1 IPs and lysates from T-REx-293 cells were immunoprobed as in B. (D) Impaired S365 dephosphorylation and ERK pathway activation by EGF in SHOC2 KO DLD-1 cells is rescued by reexpression of WT but not SHOC2 mutants defective for complex formation with MRAS and PP1. Lysates from DLD-1 parental (P) and SHOC2 KO cells transduced with lentivirures expressing flag-SHOC2 WT, mutants, or empty vector were stimulated with 25 ng/mL EGF for 5 min after serum starvation. (E) SHOC2 is required for EGF-stimulated MEK and 14-3-3 dissociation from BRAF and BRAF dimerization with ARAF and CRAF. Serum-starved DLD-1 cells were stimulated with EGF for the indicated times and endogenous RAF IPs immunoblotted using the Li-COR Odyssey system. (F) Li-COR quantification of CRAF, MEK, 14-3-3 and P-S365 BRAF from BRAF IPs in E, relative to EGF-untreated parental cells. (G) BRAF S365A does not bind MEK and rescues ERK activation in SHOC2 KO cells. DLD-1 cells, nontransduced or stably expressing T6-BRAF WT or S365A, were treated with EGF for 10 min and Streptactin pull-downs of T6-BRAF and lysates probed as indicated.
When immunoprecipitating RAF, MEK can be readily detected in complex with BRAF but not CRAF under basal conditions (37), and higher levels of P-S365 BRAF in SHOC2 KD cells correlate with higher levels of MEK and 14-3-3 bound to BRAF (Fig. 2 A and B). EGF stimulated MEK and 14-3-3 dissociation from BRAF and BRAF binding to CRAF, and this response is strongly inhibited in SHOC2 KD cells (Fig. 2B). EGF-induced BRAF interaction with KSR is also impaired in the absence of SHOC2 (Fig. 2C and SI Appendix, Fig. S1A). In clear contrast, RAF interaction with RAS, as measured on RAS IPs, was not impaired but enhanced in SHOC2 KD cells (Fig. 2B), likely as a result of loss of inhibitory feedbacks (see Discussion).
To extend these observations to other cell lines, a CRISPR/CAS9 strategy was used to completely ablate SHOC2 function in DLD-1 KRASG13D colon carcinoma cells. EGF-induced dephosphorylation of P-S365/S259 B/CRAF is impaired in SHOC2 knockout (KO) cells (Fig. 2D). Similarly, EGF-stimulated phosphorylation of MEK, ERK, and RSK, but not AKT, is strongly inhibited in SHOC2 KO cells and this response is rescued by reexpression of SHOC2 WT but not SHOC2 mutants defective for interaction with MRAS and PP1, such as D175N or RVxF-SILK (18, 19, 23) (Fig. 2D). SHOC2 E457K disrupts MRAS/PP1 interaction less efficiently (19, 23) and only partially rescues ERK pathway activation by EGF. Therefore, ERK pathway regulation by SHOC2 correlates well with its ability to form a ternary complex with MRAS and PP1.
To analyze RAF interactions in DLD-1 KO cells, endogenous RAF IPs were performed on a time course of EGF stimulation as before. In parental DLD-1 cells, EGF stimulates transient S365 BRAF dephosphorylation with dynamics that mirror MEK and 14-3-3 dissociation from BRAF and BRAF dimerization with ARAF and CRAF (Fig. 2 E and F). As seen in T-REx-293 KD cells, SHOC2 KO DLD-1 have higher basal levels of MEK and 14-3-3–bound BRAF complexes. Moreover, EGF-simulated MEK and 14-3-3 dissociation from BRAF and BRAF heterodimerization with CRAF and ARAF are strongly impaired in SHOC2 KO cells (Fig. 2E).
To further validate that the effect of SHOC2 ablation on ERK pathway activation was dependent on its function within an S259 RAF holophosphatase, T6-BRAF WT and S365A mutant (which cannot be phosphorylated and therefore should be insensitive to the phosphatase function of the SHOC2 complex) were stably expressed in parental and SHOC2 KO DLD-1 cells. Expression of BRAF S365A (unlike BRAF WT) leads to higher basal P-MEK and P-ERK levels in both parental and SHOC2 KO cells, consistent with ERK pathway activation by these RAF mutants being insensitive to regulation by SHOC2 (Fig. 2G). When ectopic T6-BRAF was purified from these cells with streptactin beads, T6-BRAF WT displayed higher basal MEK binding in SHOC2 KO cells, whereas no MEK can be detected in complex with T6-BRAF S365A, consistent with a role for S365 dephosphorylation in the regulation of the BRAF-MEK interaction (Fig. 2G).
Taken together, the above results strongly suggest that SHOC2 complex-mediated S259 RAF dephosphorylation is required for 14-3-3 dissociation from RAFs, MEK dissociation from BRAF, and BRAF heterodimerization with ARAF, CRAF, and KSR, but not for RAF binding to RAS (SI Appendix, Fig. S2).
SHOC2 Is Selectively Required for Early but Not Late ERK Pathway Activation by EGF in DLD-1 cells.
When ERK pathway dynamics were studied in an EGF time course in DLD-1 isogenic cells, MEK, ERK, and RSK phosphorylation was strongly impaired at early time points (2.5–5 min) in SHOC2 KO cells compared with parental cells, whereas little differences were seen between them by 20 min of EGF treatment (Fig. 3 A and B). Similar effects were seen on downstream ERK targets sites, such as BRAF T753, CRAF S289/296/301, EGFR T699, and IRS S363/639 feedback sites, as well as RSK targets, such as YB1 S102 (Fig. 3A). No effect was seen in ERK-independent sites on AF6 or RPS6, whereas AKT S473 phosphorylation is enhanced in the absence of SHOC2, consistent with a negative feedback crosstalk upon ERK pathway inhibition (38, 39). This response is reproducibly seen in multiple DLD-1 SHOC2 KO clones tested, ruling out clonal variation (SI Appendix, Fig. S3 A and B) and is completely rescued by reexpression in KO cells of SHOC2 WT but not the MRAS/PP1 interaction-defective SHOC2 D175N (SI Appendix, Fig. S3 C and D).
Fig. 3.
SHOC2 is selectively required for early, but not delayed ERK pathway activation by EGF in DLD-1 cells. (A) Serum-starved DLD-1 parental or SHOC2 KO cells were stimulated with 25 ng/mL EGF for the indicated times. Lysates were probed and visualized by Li-COR. (B) Quantification of P-S365 BRAF, P-S259 CRAF, P-MEK, P-ERK, P-S380 RSK, P-S473 AKT in A (mean ± SD) (n = 3), relative to EGF-untreated parental condition. (C) Model of biphasic ERK activation by EGF with an early and transient phase that requires SHOC2 and a delayed, sustained phase that is SHOC2-independent. Based on ref. 1.
When other agonists, such as lysophosphatidic acid and FBS were used to stimulate DLD-1 cells, ERK activation was similarly impaired preferentially at early time points in the absence of SHOC2. On the other hand, ERK activation by TNF-α (which is RAS-RAF independent) was completely unaffected (SI Appendix, Fig. S3 E and F). Taken together, these results are consistent with an agonist-dependent biphasic ERK activation response in which a rapid, transient phase requires the SHOC2 complex, whereas a slow, sustained phase is independent of SHOC2 (Fig. 3C).
Phosphoproteomic Analysis of SHOC2’s Contribution to EGF-Regulated Dynamics.
To further study the contribution of SHOC2 to ERK pathway dynamics in an unbiased manner, a label-free phosphoproteomic approach was used to compare global EGF-regulated phosphorylation in parental or SHOC2 KO DLD-1 cells. The MEK inhibitor Trametinib was also used in parental cells to compare global pharmacological pathway inhibition to genetic SHOC2 inhibition (Fig. 4A).
Fig. 4.
Phosphoproteomic analysis of SHOC2’s contribution to EGF-regulated dynamics. (A) Experimental strategy used for quantitative phosphoproteomics. DLD-1 parental or SHOC2 KO cells were serum-starved and left untreated or stimulated with 25 ng/mL EGF for 5 or 20 min. Parental cells were also pretreated with the MEK inhibitor Trametinib (100 nM) for 20 min. (B) Significantly regulated phosphorylated sites (cutoffs: fold-change ± 2, adjusted P < 0.05) at 5 and 20 min of EGF stimulation. Representative of n = 3 experiments. (C) Volcano plots of quantified phosphosites regulated by EGF at 5 and 20 min in parental but not SHOC2 KO cells. Blue up-regulated, red down-regulated upon EGF stimulation; gray no regulation. See SI Appendix, Fig. S4C for representative phosphosite plots.
In total, 7,053 phosphosites were quantified, corresponding to 3,091 inferred proteins. In parental cells that were stimulated with EGF, 89 and 78 phosphosites were found to be significantly regulated at 5 and 20 min, respectively (cutoffs: fold-change ± 2, adjusted P < 0.05) (Fig. 4B and Dataset S1). Functional and phosphorylation motif analysis of the inferred proteins in parental cells are shown in SI Appendix, Fig. S4. Pretreatment with Trametinib dramatically reduced EGF-regulated phosphorylation events with only 5 and 10 phosphosites significantly regulated at 5 and 20 min, respectively (94% and 87% inhibition compared with untreated cells) (Fig. 4B). This highlights the crucial role of the ERK pathway in early signaling by EGF either directly or indirectly by providing priming phoshophorylation for other EGF-regulated kinases (40).
In SHOC2 KO cells, inhibition of EGF-regulated phosphorylation was significantly more pronounced at 5 min than 20 min of EGF treatment (90% vs. 38.5% inhibition, respectively) (Fig. 4B). When the phosphoproteomes of parental and SHOC2 KO cells were compared at either 5 or 20 min of EGF treatment, only 1 phosphosite was significantly changed at 20 min, whereas 26 phosphosites were differentially regulated by EGF in parental but not SHOC2 KO cells at 5 min (21 down-regulated in SHOC2 KO cells, 5 up-regulated) (Fig. 4C). Selected examples of these phosphosites are shown in SI Appendix, Fig. S4C. In conclusion, using phosphoproteomic profiling, we independently determined a selective contribution of SHOC2 to ERK pathway dynamics with a preferential role of SHOC2 at early (5 min) vs. late (20 min) times of EGF treatment.
SHOC2-Independent Late ERK Activation Requires CRAF.
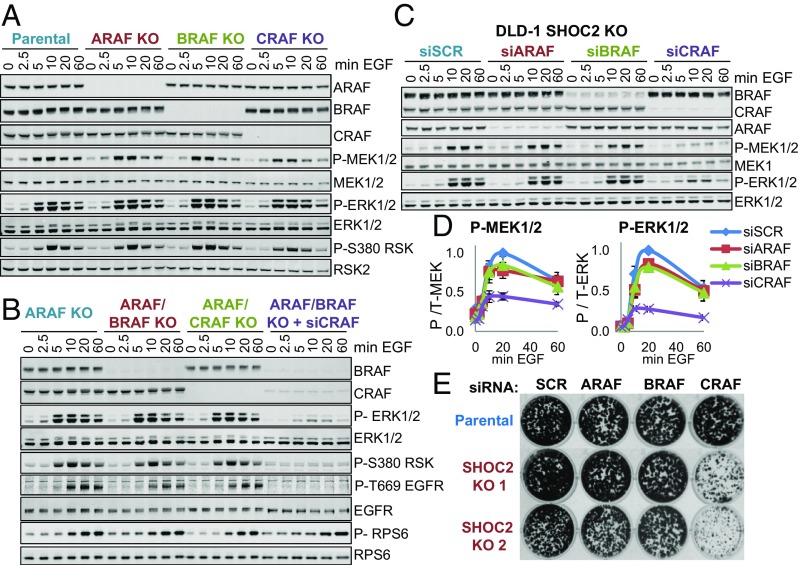
To address the contribution of RAF isoforms to early vs. late SHOC2-dependent and -independent mechanisms of ERK activation, CRISPR was used to knock out the three RAF paralogues in DLD-1 cells. In contrast to SHOC2 deletion, ablation of one or any two combinations of RAF isoforms had no significant effect on EGF-stimulated ERK activation (Fig. 5 A and B and SI Appendix, Fig. S5 A–D). However, KD of the remaining CRAF in dual A/B RAF KO cells potently inhibits EGF-stimulated ERK activation (Fig. 5B) and proliferation in colony formation assays (SI Appendix, Fig. S5E). Thus, as observed in other systems (41, 42), there is redundancy among RAF isoforms but RAF function is essential for ERK activation and proliferation of DLD-1 cells.
Fig. 5.
SHOC2-independent ERK activation requires CRAF. (A) KO of individual RAF isoforms does not affect ERK pathway activation by EGF. Serum-starved DLD-1 parental (P) or ARAF, BRAF, and CRAF KO cells generated by CRISPR were stimulated with 25 ng/mL EGF. (B) ERK pathway activation by EGF in CRAF-only or BRAF-only double KO DLD-1 cells (generated by second round CRISPR of BRAF or CRAF respectively in ARAF KO cells) is normal. However, KD of CRAF in ARAF/BRAF KO (CRAF-only) cells inhibits ERK activation. DLD-1 cells transfected with SCR or CRAF siRNAs were stimulated with EGF as before. (C) CRAF KD (but not ARAF or BRAF) inhibits delayed ERK activation in SHOC2 KO cells. DLD-1 parental and SHOC2 KO cells transfected with ARAF, BRAF, or CRAF siRNAs, stimulated with 25 ng/mL EGF and lysates probed by Li-COR. (D) Li-COR quantification of P-MEK and P-ERK in C (mean ± SD) (n = 2). (E) CRAF KD inhibits growth in SHOC2 KO cells. Parental and two SHOC2 KO clones transfected with SCR or A/B/CRAF siRNAs were used for colony formation assays.
When siRNAs where used to acutely inhibit expression of individual RAF isoforms, transient KD of individual RAF proteins in parental DLD-1 cells had no effect on EGF-stimulated ERK phosphorylation, consistent with the complementation observed in RAF KO cells. In clear contrast, however, CRAF KD (but not ARAF or BRAF) strongly inhibited MEK and ERK phosphorylation in SHOC2 KO cells (Fig. 5 C and D). Similar results were observed in HEK293T cells, although CRAF KD has a modest inhibitory effect in control cells as well (SI Appendix, Fig. S5F). Strong ERK pathway inhibition upon combined SHOC2 and CRAF inhibition correlates with a strong inhibition of proliferation in DLD-1 cells (Fig. 5E). Taken together, these data suggest that, whereas there is redundancy among RAF isoforms in an early phase of SHOC2-dependent ERK pathway activation, CRAF is the primary RAF kinase driving sustained ERK activation by EGF in the absence of SHOC2.
SHOC2-Independent ERK Activation Requires Palmitoylated HRAS/NRAS and CRAF N-Region Phosphorylation.
Previous studies have shown a biphasic HRAS activation response to EGF with a rapid transient phase occurring at the plasma membrane, followed—with a 10- to 20-min delay—by a sustained phase at the Golgi (43, 44), that is strikingly reminiscent of the ERK response observed in this study. Futhermore, HRAS can differentially activate CRAF in some contexts (45, 46). We thus used siRNAs to investigate the contribution of RAS isoforms to ERK activation by EGF.
KD of any RAS protein had no effect on ERK activity in parental DLD-1 cells, consistent with redundancy as observed for RAF isoforms. However, in SHOC2 KO cells, KD of HRAS and NRAS, but not KRAS, significantly impaired EGF-stimulated ERK activation (Fig. 6A). Furthermore, combined KD of HRAS and NRAS inhibited ERK activity more strongly than NRAS/KRAS or HRAS/KRAS combinations in SHOC2 KO cells (SI Appendix, Fig. S6A). Unlike KRAS, NRAS and HRAS are modified by palmitoylation (47) and pretreatment of DLD-1 cells with the palmitoylation inhibitor 2-bromopalmitate (2-BP) selectively reduced ERK activation at 20 min in SHOC2 KO cells (Fig. 6B). These results thus suggest that the SHOC2-independent/CRAF-dependent sustained phase of ERK activity is driven by palmitoylated NRAS/HRAS proteins.
Fig. 6.
SHOC2-independent ERK activation requires palmitoylated HRAS/NRAS and CRAF N-region phosphorylation. (A) NRAS and HRAS KD (but not KRAS) inhibit ERK activation in SHOC2 KO cells. SHOC2 KO DLD-1 cells transfected with siRNAs were stimulated with 25 ng/mL EGF for the indicated times. (B) The palmitoylation inhibitor 2-BP reduces sustained ERK activation in SHOC2 KO cells. DLD-1 cells were pretreated with 2-BP (100 µM) for the indicated times before EGF stimulation. (C) PAK, FAK, and SRC family inhibitors (besides RAF and MEK inhibitors) impair sustained ERK pathway activation by EGF in SHOC2 KO DLD-1 cells. Cells were stimulated with 25 ng/mL EGF for 20 min after 30-min pretreatment with indicated kinase inhibitors (and 2-BP). Lysates were probed and P-ERK quantified by Li-COR (mean ± SD) (n = 3–7). Significance is determined using a two tailed t-test *P < 0.05, **P < 0.01, or ***P < 0.001. See SI Appendix, Fig. S6B for representative experiment. (D) Cells were pretreated with 10 μM PAK (FRAX597), SRC (SU6656), and FAK (PF-562271) inhibitors alone or in combination, 30 min before stimulation with EGF for 20 min. (E) Model of selective contribution of the SHOC2 complex to ERK pathway spatiotemporal dynamics. EGF Receptor activation leads to N/H/K-RAS and MRAS/SHOC2 complex activation at the plasma membrane and an early phase of ERK activation involving A/B/C-RAF isoforms. As a result of intracellular trafficking of palmitoylated proteins (by the constitutive de/reacylation cycle and/or receptor-mediated endocytosis and/or other nonmutually exclusive mechanisms not shown), H/N-RAS travel to endomembrane compartments from where they signal through CRAF to drive sustained ERK pathway activation. Because poly-basic motif-containing KRAS-4B and MRAS (and associated proteins) remain at the plasma membrane, this CRAF is now uncoupled from regulation by the SHOC2 complex, but is instead dependent on N-region phosphorylation by kinases, such as PAK, SFK, and FAK. See Discussion for further details. Membrane anchors represent farnesyl (red) and palmitate (black) groups. S338 and S341 residues in CRAF belong to the N-region. ERK may phosphorylate diverse substrates in different compartments, as shown by different color arrows.
To further investigate additional molecular mechanisms that may be contributing to SHOC2-independent CRAF activation, a panel of kinase inhibitors was tested for their ability to modulate sustained ERK activation. In addition to ERK pathway inhibitors, PAK (FRAX597), FAK (PF-562271), and SRC family (SU6656) kinase inhibitors significantly impaired ERK phosphorylation at 20 min of EGF treatment in SHOC2 KO cells (Fig. 6C and SI Appendix, Fig. S6B). Both PAK and SRC are known to phosphorylate the CRAF N-region at S338 and Y341, respectively, whereas FAK has been linked to both SRC and RAC/PAK signaling. Indeed, FAK inhibitors impaired PAK1 phosphorylation and PAK, FAK, and SFK inhibitors also impaired CRAF S338 phosphorylation (Fig. 6D). Taken together, these results suggest that N-region phosphorylation in CRAF plays an important role in sustained ERK activation by EGF in the absence of SHOC2. A model summarizing all our data is shown in Fig. 6E.
SHOC2 Is Selectively Required for ERK Pathway Activation under Anchorage-Independent Conditions in KRAS Mutant Cells.
We have previously shown that SHOC2 is preferentially required for anchorage-independent proliferation in some RAS mutant cell lines (18). We thus set out to use our isogenic DLD-1 system to elucidate a biochemical mechanism for this observation. SHOC2 KO DLD-1 clones had similar growth rates as parental cells in 2D but were impaired in their ability to grow under anchorage-independent conditions in 3D (Fig. 7 A and B). This effect was partially rescued by reexpression of SHOC2 WT, but not the D175N mutant defective for MRAS/PP1 interaction (Fig. 7B), and is consistent with a selective requirement for the RAF phosphatase function of SHOC2 for tumorigenic properties in some RAS mutant cells.
Fig. 7.
SHOC2 is selectively required for ERK pathway activation under anchorage-independent conditions in KRAS mutant cells. (A) SHOC2 is dispensable for anchorage-dependent/2D growth. Incucyte growth curves of DLD-1 parental and three independent SHOC2 KO clones stably expressing WT and D175N SHOC2 were generated using the IncuCyte Live Cell imaging system. Representative of n = 2 experiments. (B) SHOC2 KO impairs growth in 3D. Cells in A were seeded in low attachment plates and growth at day 5 measured by Alamar blue staining (mean ± SD) (n = 2–4). Significance is determined using a two tailed t test *P < 0.05, **P < 0.01, or ***P < 0.001. (C) SHOC2 is preferentially required for ERK pathway activation in 3D in DLD-1 cells. DLD-1 cells were grown for 24 h on regular or poly-HEMA–coated plates and lysates immunoprobed as indicated. (D) As in C but with HCT116 KRASG13D cells. (E) As in C and D with SW480 KRASG12V cells. (F) SHOC2 is dispensable for ERK phosphorylation in 2D and 3D in the BRAF mutant (V600E) RKO and HT-29 cell lines. (G) PAK (FRAX597), SRC (SU6656), and FAK (PF-562271) family inhibitors inhibit basal ERK signaling more potently in the absence of SHOC2. DLD-1 cells growing in log phase in the presence of 10% FBS were incubated with 10-µM inhibitors for 1 h and lysates immunoblotted as indicated.
To study a molecular mechanism for this selective SHOC2 contribution to 3D growth, lysates of parental and SHOC2 KO DLD-1 cells growing in 2D or suspension (poly-HEMA–coated dishes) were compared. In suspension cells, phosphorylation of AKT and its downstream substrate site S1718 AF6 is strongly impaired [consistent with PI3K/AKT signaling being adhesion-dependent in many cell types (48–50)], but this is unaffected in SHOC2 KO cells (Fig. 7 C–F). Similarly, phosphorylation of FAK and PAK kinases, also known to be regulated by integrin-mediated attachment to the extracellular matrix (48), was similarly down-regulated in suspension in both parental and SHOC2 KO cells, which correlated with decreased phosphorylation of known PAK sites on CRAF (S338) and MEK (S298) (Fig. 7C). In clear contrast, basal ERK signaling, as determined by phosphorylation of ERK and ERK substrate sites on BRAF (T753) and CRAF (S289/290/296), was unaffected in parental DLD-1 cells, but significantly decreased in SHOC2 KO clones only in suspension. A selective inhibition of ERK signaling in cells in suspension upon SHOC2 ablation was also seen in other SHOC2 KO KRAS mutant colorectal cell lines, such as HCT116 (Fig. 7D) and SW480 (Fig. 7E) cells, but not in V600E, dimerization-independent BRAF mutant RKO or HT29 cells (Fig. 7F). Thus, SHOC2 is preferentially required for ERK signaling under anchorage-independent conditions in the context of oncogenic KRAS but not BRAF signaling.
An implication of these observations is that SHOC2-independent mechanisms of ERK activation must predominate under 2D basal growth conditions and that a mechanism similar to that observed in the sustained phase of EGF stimulation involving N-region CRAF phosphorylation by FAK/SRC or PAK kinases (Fig. 6) may also independently operate in the context of anchorage-dependent/2D growth. Consistent with this possibility, treatment of DLD-1 cells growing in 2D with PAK, FAK, and SRC family inhibitors led to decreased CRAF S338 phosphorylation in both parental and SHOC2 KO cells, but more potently inhibited ERK phosphorylation in the absence of SHOC2 (Fig. 7G).
Taken together, our observations suggest that SHOC2-dependent and CRAF/N-region–dependent mechanism of RAF activation differentially contribute to ERK activation in a context-dependent manner: whereas redundancy makes SHOC2 dispensable for ERK activity under anchorage-dependent 2D growth conditions, in the absence of attachment to the extracellular matrix KRAS-mutant cells preferentially rely on SHOC2-dependent mechanism for ERK signaling (Discussion and SI Appendix, Fig. S8).
Discussion
This study highlights a key role for S259 RAF dephosphorylation by the SHOC2 phosphatase complex in regulating the dissociation of 14-3-3 from the N-terminal RAF regulatory region and RAF dimerization. In the absence of SHOC2, EGF-stimulated BRAF-ARAF, BRAF-CRAF, and BRAF-KSR heterodimerization are strongly impaired, whereas RAF interaction with RAS is actually increased (Fig. 2B). This result shows that the RAS–RAF interaction can be uncoupled from RAF dimerization in some contexts and is consistent with a model where coordinate inputs from RAS and the SHOC2 holophosphatase are required for RAF heterodimerization and activation. Increased RAS–RAF interaction in the absence of SHOC2 is incompatible with a role for SHOC2 as a scaffold promoting RAS–RAF interaction as suggested by some overexpression studies (29, 30). Instead, it is consistent with decreased ERK activity in the absence of SHOC2, leading to relief of ERK inhibitory feedbacks, both upstream of RAS and at the level of RAF, such as CRAF S289/296/301 and BRAF T753 that disrupt RAF–RAS interaction (51, 52). Similarly, inhibitory ERK feedback sites on EGFR (T699) and IRS-1 (S636/639) are also inhibited in the absence of SHOC2 and likely contribute to increased AKT phosphorylation upon SHOC2 and ERK pathway inhibition (Fig. 3 A and B) (38, 39, 53).
There is controversy around the precise order of the initial steps in the RAF activation cycle and whether S259 dephosphorylation precedes or follows RAS-GTP binding (11). S259A mutation in CRAF promotes association with RAS (54), which can be interpreted to suggest that S259 dephosphorylation may precede RAS binding, possibly by 14-3-3 dissociation facilitating access of the RAF RBD to RAS. However, our studies support an alternative model (SI Appendix, Fig. S2) where RAS-GTP binding to RAF and recruitment to the membrane is independent of, and precedes S259 dephosphorylation by the SHOC2 complex: in a time course of EGF stimulation, RAF binding to RAS peaks at 2.5 min and precedes S259 dephosphorylation, which peaks at 5–10 min (Fig. 2B). Additionally, in the absence of SHOC2, under conditions where levels of P-S259 RAF and RAF–14-3-3 complexes are high, RAF readily interacts with RAS in response to EGF (in fact there is increased RAS–RAF interaction; see discussion above and Fig. 2B). Furthermore, we have previously shown that S259 phosphorylation can be readily detected on the RAS-bound RAF (19). Taken together, these observations suggest that S259-phosphorylated RAF is able to bind to RAS and that the RAF RBD is likely to be accessible for interaction with RAS within the closed RAF conformation.
Our proposed model also allows for the observations that S259A CRAF promotes RAS binding or that SHOC2 can accelerate the RAS–RAF interaction (31, 54–56) when the tandem arrangement of RBD and CRD and their cooperation in RAF membrane localization is considered: the CRD can interact with RAS as well as phospholipids, and helps anchor RAF at the membrane (57–60). The CRD hydrophobic loops are likely to be buried in the closed/inactive RAF conformation and may only be exposed for membrane interaction in the open/active conformation upon release of 14-3-3 from the regulatory domain in a mechanism analogous to that proposed for KSR (61). According to this possibility, CRD exposure upon S259 dephosphorylation or experimentally in S259A RAF mutants, would increase membrane avidity and stabilize RAS binding to the RBD (59, 60). We also note that our model is consistent with the observation that a CRAF-CAAX mutant that is constitutively localized at the membrane, is independent of RAS but can still be further activated by EGF (62, 63) as well as by S259 dephosphorylation (SI Appendix, Fig. S1B).
Our study suggests a role for SHOC2-mediated BRAF S365 dephosphorylation in the regulation of the BRAF–MEK interaction, which inversely correlates with BRAF dimerization. Because under resting conditions MEK interacts with BRAF much more strongly than ARAF or CRAF, we speculate that the unique N-terminal BRAF-specific (BRS) domain of BRAF may mediate an additional interaction with MEK in the inactive BRAF conformation. The BRAF BRS domain also interacts with KSR1 (64), suggesting a mechanism for competitive displacement upon growth factor stimulated BRAF-KSR dimerization (SI Appendix, Fig. S2). A definitive answer awaits determination of the crystal structure of full-length BRAF in complex with 14-3-3 and MEK.
Our study has uncovered a selective contribution of the SHOC2 phosphatase complex to ERK pathway dynamics. In DLD-1 cells EGF stimulates ERK pathway activation in a pattern consistent with a biphasic response in which SHOC2 is required for a rapid, transient phase, but not a slower, sustained phase that instead depends on palmitoylated HRAS/NRAS and CRAF signaling. SHOC2 complex formation is driven by MRAS-GTP and thus its cellular location is likely to be determined primarily by the membrane localization signals within the carboxyl-terminal hypervariable region (HVR) of MRAS. The HVR of RAS proteins directs their differential spatial segregation, with palmitoylated HRAS and NRAS being able to signal from the plasma membrane as well as endomembrane compartments, whereas the polybasic-motif–containing KRAS-4B is thought to signal exclusively from the plasma membrane (47). The MRAS HVR contains a polybasic motif as a second membrane targeting signal and is thus expected to closely mirror KRAS-4B in its plasma membrane localization, while being refractory to the intracellular trafficking mechanisms of palmitoylated proteins. Indeed, overexpression of YFP/mCherry-fusion proteins in human mammary epithelial cells supports this scenario, as in addition to the plasma membrane, HRAS and NRAS (but not KRAS-4B or MRAS) can be readily detected to colocalize with CRAF at the Golgi and/or other intracellular compartments (SI Appendix, Fig. S7).
We propose a model (Fig. 6E) where upon EGF stimulation, the rapid phase of SHOC2-dependent ERK activation occurs at the plasma membrane, where SHOC2 complex formation upon MRAS activation leads to S259 dephosphorylation on proximal A/B/C-RAF proteins recruited by H/N/K-RAS proteins. In this phase there is redundancy among RAS and RAF isoforms for ERK pathway activation, whereas SHOC2 appears to play an essential, nonredundant role (Fig. 3C). The slow, sustained phase of ERK activation may be driven by internalization of palmitoylated RAS proteins that thereby become spatially segregated from the SHOC2 complex that remains anchored at the plasma membrane by MRAS, alongside KRAS-4B. Internalization may result from intracellular trafficking by the constitutive acylation cycle of palmitoylated proteins and/or receptor-mediated endocytosis and/or other mechanisms operating in a nonmutually exclusive manner (44, 65, 66). From these intracellular compartments, H/N-RAS proteins signal primarily through CRAF, which is now uncoupled from regulation by the SHOC2 complex but dependent on N-region phosphorylation by kinases such as PAK, SRC, and FAK (directly or indirectly).
A biphasic HRAS activation by EGF with a slow sustained phase at the Golgi dependent on the acylation cycle (44, 65, 67), as well as differential CRAF activation by HRAS (but not KRAS) dependent on endocytosis (45), are both consistent with this model. A similar biphasic ERK response upon G protein-coupled receptor internalization has been linked to phosphorylation of different ERK substrates from spatially distinct signaling platforms (1, 68) and it is likely that SHOC2-dependent and -independent phases of ERK activation are also associated with phosphorylation of ERK substrates at distinct spatial compartments. We also note that similar biphasic kinetics linked to compartment-specific RAS-ERK signaling have been observed during the process of thymocyte selection (66, 69) and future studies should address the role of the SHOC2 complex in immune tolerance.
The contribution of CRAF S259 dephosphorylation and/or dimerization to the slow ERK activation phase remains unclear. We were unable to detect significant S259 dephosphorylation or RAF heterodimerization in the absence of SHOC2, but low levels below the sensitivity of our experimental conditions cannot be ruled out. On the other hand, we note that experimental constraints when analyzing endogenous proteins have not allowed us to measure homodimerization, and S259-independent CRAF homodimerization during the slow, sustained phase remains a distinct possibility. Reports of N-region CRAF phosphorylation promoting relief from autoinhibition and dimerization (70, 71) and of high levels of S338 phosphorylation activating CRAF in the presence of high levels of inhibitory phosphorylation at S43 and S259 (72) support this scenario. It is also worth noting that both SFK and PAK activators, such as RAC and CDC42, are palmitoylated and expected to travel with H/N-RAS during both endocytosis and acylation cycle scenarios of intracellular trafficking, which would thus facilitate N-region phosphorylation of the H/N-RAS bound CRAF at these compartments.
The biochemical mechanisms of SHOC2-independent, CRAF N-region–dependent ERK activation observed in the sustained phase of EGF stimulation in DLD-1 cells appear to operate as well in the context of anchorage-dependent proliferation in 2D (SI Appendix, Fig. S8). Integrin signaling regulates both FAK-SRC and PAK activation and cooperates with RTKs to regulate sustained ERK activation in multiple contexts (73–79). Thus, integrins are well poised to mediate, at least in part, SHOC2-independent ERK activation from sites of attachment to the extracellular matrix.
Redundant SHOC2-dependent and SHOC2-independent/CRAF-dependent mechanisms of ERK activation under basal 2D conditions are likely to account for the observation that both SHOC2 and CRAF ablation alone are well tolerated, whereas combined inhibition potently inhibits growth (Fig. 5E), as complete inhibition of the ERK response is incompatible with proliferation (8, 41, 42). In clear contrast however, in the absence of adhesion to the extracellular matrix, a key contribution of SHOC2 to ERK activity in KRAS mutant cells is uncovered in 3D (Fig. 7). Basal PI3K/AKT and FAK/PAK activation is strongly impaired in the absence of matrix-dependent attachment, which is likely to enhance the dependency on SHOC2-dependent ERK signaling for anchorage-independent growth in RAS mutant cells (18). Taken together, our results thus provide a molecular mechanism for a selective RAS oncogene addiction to SHOC2 that has also been observed in other studies (80, 81) (https://depmap.org) and presents a therapeutic opportunity.
Current ERK pathway inhibitors have failed in the clinic against RAS-driven cancers primarily because toxicity precludes a therapeutic index. Our study suggests the SHOC2 phosphatase complex functions as a regulatory node for only a subset of the ERK signaling response. Thus, in contrast to targeting RAF/MEK/ERK core pathway components that inhibit global ERK signaling, targeting the SHOC2 complex may provide a mechanism for selective ERK pathway inhibition that may provide better therapeutic margins against RAS-driven tumors. PP1 holophophosphatases remain underexplored targets of pharmacological inhibition (82–84) and future efforts should drive development of inhibitors of the SHOC2 holophosphatase.
In summary, this study highlights a selective contribution of the SHOC2 phosphatase complex to RAF regulation and ERK pathway spatiotemporal dynamics that is differentially engaged by KRAS oncogenic signaling and that may allow for context and compartment-specific inhibition of ERK signaling.
Materials and Methods
Cell Proliferation in Anchorage-Dependent and Independent Assays.
Growth curves in 24-well plates were generated using the IncuCyte system (Essen BioScience). Pictures were taken every 2 h, with each data point a composite of four different images from the same well. Growth medium was replaced every 2 d.
Anchorage-independent growth (or growth in 3D) was assessed by seeding 1,000 cells in 384-well ultralow attachment plates (Greiner). After 5 d, Alamar blue was added to cells and fluorescence measured using a plate reader.
Colony assays were performed 2 d after the siRNA transfection by seeding 2,000 cells in 24-well plates or 30,000 cells in 6-well plates. Cells were grown for 10 d replacing media every 2 d, stained with 0.5% Crystal violet, and photographed using a digital scanner.
Cell Lysis and IP Assays.
Cells were lysed in PBS with 1% Triton-X-100, protease inhibitor mixture (Roche), phosphatase inhibitor mixture, and either 1 mM EDTA or 5 mM MgCl2. Tagged proteins were immunoprecipitated/pulled down from cleared lysates using either FLAG (M2) agarose (Millipore Sigma), glutathione Sepharose, or Streptactin beads (GE Healthcare). Endogenous proteins were immunoprecipitated using antibodies (SI Appendix, Supplementary Materials and Methods) and protein A/G beads (GE Healthcare). After 2-h rotating incubation at 4 °C, beads were extensively washed with PBS-E or PBS-M lysis buffer, drained, and resuspended in NuPAGE LDS sample buffer (Life Technologies). Samples were analyzed by Western blot with HRP (GE Healthcare) and DyLight (Thermo Scientific) conjugated secondary antibodies. Membranes were visualized using an Odyssey scanner (Li-COR) or Image Quant system (GE Healthcare).
Statistical Analysis.
Data are presented as mean ± SD. Significance was determined with GraphPad Prism 7 software using the Student’s t test, where *P < 0.05, **P < 0.01, or ***P < 0.001.
Supplementary Material
Acknowledgments
We thank Miriam Molina, Benoit Bilanges, Aditya Shroff, and Andrew Wolfe for critical reading of this manuscript. The contribution of G.G.J. and I.B.R. was supported by Cancer Research UK grants. S. Sari is sponsored by The Republic of Turkey Ministry of National Education. Rosetrees Trust and Stoneygate Trust Award M190-F2 supported this work.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1902658116/-/DCSupplemental.
References
- 1.Kholodenko B. N., Hancock J. F., Kolch W., Signalling ballet in space and time. Nat. Rev. Mol. Cell Biol. 11, 414–426 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaul Y. D., Seger R., The MEK/ERK cascade: From signaling specificity to diverse functions. Biochim. Biophys. Acta 1773, 1213–1226 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Rauen K. A., The RASopathies. Annu. Rev. Genomics Hum. Genet. 14, 355–369 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simanshu D. K., Nissley D. V., McCormick F., RAS proteins and their regulators in human disease. Cell 170, 17–33 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samatar A. A., Poulikakos P. I., Targeting RAS-ERK signalling in cancer: Promises and challenges. Nat. Rev. Drug Discov. 13, 928–942 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Caunt C. J., Sale M. J., Smith P. D., Cook S. J., MEK1 and MEK2 inhibitors and cancer therapy: The long and winding road. Nat. Rev. Cancer 15, 577–592 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Bollag G., et al. , Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature 467, 596–599 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blasco R. B., et al. , c-Raf, but not B-Raf, is essential for development of K-Ras oncogene-driven non-small cell lung carcinoma. Cancer Cell 19, 652–663 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jameson K. L., et al. , IQGAP1 scaffold-kinase interaction blockade selectively targets RAS-MAP kinase-driven tumors. Nat. Med. 19, 626–630 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrero A., et al. , Small molecule inhibition of ERK dimerization prevents tumorigenesis by RAS-ERK pathway oncogenes. Cancer Cell 28, 170–182 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Lavoie H., Therrien M., Regulation of RAF protein kinases in ERK signalling. Nat. Rev. Mol. Cell Biol. 16, 281–298 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Tzivion G., Luo Z., Avruch J., A dimeric 14-3-3 protein is an essential cofactor for Raf kinase activity. Nature 394, 88–92 (1998). [DOI] [PubMed] [Google Scholar]
- 13.McKay M. M., Morrison D. K., Integrating signals from RTKs to ERK/MAPK. Oncogene 26, 3113–3121 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Pandit B., et al. , Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nat. Genet. 39, 1007–1012 (2007). [DOI] [PubMed] [Google Scholar]
- 15.Razzaque M. A., et al. , Germline gain-of-function mutations in RAF1 cause Noonan syndrome. Nat. Genet. 39, 1013–1017 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi T., et al. , Molecular and clinical analysis of RAF1 in Noonan syndrome and related disorders: Dephosphorylation of serine 259 as the essential mechanism for mutant activation. Hum. Mutat. 31, 284–294 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Molzan M., et al. , Impaired binding of 14-3-3 to C-RAF in Noonan syndrome suggests new approaches in diseases with increased Ras signaling. Mol. Cell. Biol. 30, 4698–4711 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young L. C., et al. , An MRAS, SHOC2, and SCRIB complex coordinates ERK pathway activation with polarity and tumorigenic growth. Mol. Cell 52, 679–692 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez-Viciana P., Oses-Prieto J., Burlingame A., Fried M., McCormick F., A phosphatase holoenzyme comprised of Shoc2/Sur8 and the catalytic subunit of PP1 functions as an M-Ras effector to modulate Raf activity. Mol. Cell 22, 217–230 (2006). [DOI] [PubMed] [Google Scholar]
- 20.Cordeddu V., et al. , Mutation of SHOC2 promotes aberrant protein N-myristoylation and causes Noonan-like syndrome with loose anagen hair. Nat. Genet. 41, 1022–1026 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins E. M., et al. , Elucidation of MRAS-mediated Noonan syndrome with cardiac hypertrophy. JCI Insight 2, e91225 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zambrano R. M., et al. , Further evidence that variants in PPP1CB cause a rasopathy similar to Noonan syndrome with loose anagen hair. Am. J. Med. Genet. A. 173, 565–567 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Young L. C., et al. , SHOC2-MRAS-PP1 complex positively regulates RAF activity and contributes to Noonan syndrome pathogenesis. Proc. Natl. Acad. Sci. U.S.A. 115, E10576–E10585 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ory S., Zhou M., Conrads T. P., Veenstra T. D., Morrison D. K., Protein phosphatase 2A positively regulates Ras signaling by dephosphorylating KSR1 and Raf-1 on critical 14-3-3 binding sites. Curr. Biol. 13, 1356–1364 (2003). [DOI] [PubMed] [Google Scholar]
- 25.Abraham D., et al. , Raf-1-associated protein phosphatase 2A as a positive regulator of kinase activation. J. Biol. Chem. 275, 22300–22304 (2000). [DOI] [PubMed] [Google Scholar]
- 26.Kubicek M., et al. , Dephosphorylation of Ser-259 regulates Raf-1 membrane association. J. Biol. Chem. 277, 7913–7919 (2002). [DOI] [PubMed] [Google Scholar]
- 27.Jaumot M., Hancock J. F., Protein phosphatases 1 and 2A promote Raf-1 activation by regulating 14-3-3 interactions. Oncogene 20, 3949–3958 (2001). [DOI] [PubMed] [Google Scholar]
- 28.Swingle M., Ni L., Honkanen R. E., Small-molecule inhibitors of ser/thr protein phosphatases: Specificity, use and common forms of abuse. Methods Mol. Biol. 365, 23–38 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li W., Han M., Guan K. L., The leucine-rich repeat protein SUR-8 enhances MAP kinase activation and forms a complex with Ras and Raf. Genes Dev. 14, 895–900 (2000). [PMC free article] [PubMed] [Google Scholar]
- 30.Jang E. R., Galperin E., The function of Shoc2: A scaffold and beyond. Commun. Integr. Biol. 9, e1188241 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsunaga-Udagawa R., et al. , The scaffold protein Shoc2/SUR-8 accelerates the interaction of Ras and Raf. J. Biol. Chem. 285, 7818–7826 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jang E. R., et al. , HUWE1 is a molecular link controlling RAF-1 activity supported by the Shoc2 scaffold. Mol. Cell. Biol. 34, 3579–3593 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dai P., Xiong W. C., Mei L., Erbin inhibits RAF activation by disrupting the sur-8-Ras-Raf complex. J. Biol. Chem. 281, 927–933 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Mason C. S., et al. , Serine and tyrosine phosphorylations cooperate in Raf-1, but not B-Raf activation. EMBO J. 18, 2137–2148 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ritt D. A., et al. , CK2 is a component of the KSR1 scaffold complex that contributes to Raf kinase activation. Curr. Biol. 17, 179–184 (2007). [DOI] [PubMed] [Google Scholar]
- 36.Hu J., et al. , Allosteric activation of functionally asymmetric RAF kinase dimers. Cell 154, 1036–1046 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haling J. R., et al. , Structure of the BRAF-MEK complex reveals a kinase activity independent role for BRAF in MAPK signaling. Cancer Cell 26, 402–413 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Turke A. B., et al. , MEK inhibition leads to PI3K/AKT activation by relieving a negative feedback on ERBB receptors. Cancer Res. 72, 3228–3237 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gan Y., et al. , Differential roles of ERK and Akt pathways in regulation of EGFR-mediated signaling and motility in prostate cancer cells. Oncogene 29, 4947–4958 (2010). [DOI] [PubMed] [Google Scholar]
- 40.Pan C., Olsen J. V., Daub H., Mann M., Global effects of kinase inhibitors on signaling networks revealed by quantitative phosphoproteomics. Mol. Cell. Proteomics 8, 2796–2808 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dorard C., et al. , RAF proteins exert both specific and compensatory functions during tumour progression of NRAS-driven melanoma. Nat. Commun. 8, 15262 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo W., Hao B., Wang Q., Lu Y., Yue J., Requirement of B-Raf, C-Raf, and A-Raf for the growth and survival of mouse embryonic stem cells. Exp. Cell Res. 319, 2801–2811 (2013). [DOI] [PubMed] [Google Scholar]
- 43.Chiu V. K., et al. , Ras signalling on the endoplasmic reticulum and the Golgi. Nat. Cell Biol. 4, 343–350 (2002). [DOI] [PubMed] [Google Scholar]
- 44.Rocks O., et al. , An acylation cycle regulates localization and activity of palmitoylated Ras isoforms. Science 307, 1746–1752 (2005). [DOI] [PubMed] [Google Scholar]
- 45.Roy S., Wyse B., Hancock J. F., H-Ras signaling and K-Ras signaling are differentially dependent on endocytosis. Mol. Cell. Biol. 22, 5128–5140 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roy S., et al. , Dominant-negative caveolin inhibits H-Ras function by disrupting cholesterol-rich plasma membrane domains. Nat. Cell Biol. 1, 98–105 (1999). [DOI] [PubMed] [Google Scholar]
- 47.Rocks O., Peyker A., Bastiaens P. I., Spatio-temporal segregation of Ras signals: One ship, three anchors, many harbors. Curr. Opin. Cell Biol. 18, 351–357 (2006). [DOI] [PubMed] [Google Scholar]
- 48.Moreno-Layseca P., Streuli C. H., Signalling pathways linking integrins with cell cycle progression. Matrix Biol. 34, 144–153 (2014). [DOI] [PubMed] [Google Scholar]
- 49.Riedl A., et al. , Comparison of cancer cells in 2D vs 3D culture reveals differences in AKT-mTOR-S6K signaling and drug responses. J. Cell Sci. 130, 203–218 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Muranen T., et al. , Inhibition of PI3K/mTOR leads to adaptive resistance in matrix-attached cancer cells. Cancer Cell 21, 227–239 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ritt D. A., Monson D. M., Specht S. I., Morrison D. K., Impact of feedback phosphorylation and Raf heterodimerization on normal and mutant B-Raf signaling. Mol. Cell. Biol. 30, 806–819 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dougherty M. K., et al. , Regulation of Raf-1 by direct feedback phosphorylation. Mol. Cell 17, 215–224 (2005). [DOI] [PubMed] [Google Scholar]
- 53.Bouzakri K., et al. , Reduced activation of phosphatidylinositol-3 kinase and increased serine 636 phosphorylation of insulin receptor substrate-1 in primary culture of skeletal muscle cells from patients with type 2 diabetes. Diabetes 52, 1319–1325 (2003). [DOI] [PubMed] [Google Scholar]
- 54.Dhillon A. S., Meikle S., Yazici Z., Eulitz M., Kolch W., Regulation of Raf-1 activation and signalling by dephosphorylation. EMBO J. 21, 64–71 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Y., Takahashi M., Stork P. J., Ras-mutant cancer cells display B-Raf binding to Ras that activates extracellular signal-regulated kinase and is inhibited by protein kinase A phosphorylation. J. Biol. Chem. 288, 27646–27657 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dumaz N., Marais R., Protein kinase A blocks Raf-1 activity by stimulating 14-3-3 binding and blocking Raf-1 interaction with Ras. J. Biol. Chem. 278, 29819–29823 (2003). [DOI] [PubMed] [Google Scholar]
- 57.Brtva T. R., et al. , Two distinct Raf domains mediate interaction with Ras. J. Biol. Chem. 270, 9809–9812 (1995). [DOI] [PubMed] [Google Scholar]
- 58.Hu C. D., et al. , Cysteine-rich region of Raf-1 interacts with activator domain of post-translationally modified Ha-Ras. J. Biol. Chem. 270, 30274–30277 (1995). [DOI] [PubMed] [Google Scholar]
- 59.Li S., Jang H., Zhang J., Nussinov R., Raf-1 cysteine-rich domain increases the affinity of K-Ras/Raf at the membrane, promoting MAPK signaling. Structure 26, 513–525.e2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Travers T., et al. , Molecular recognition of RAS/RAF complex at the membrane: Role of RAF cysteine-rich domain. Sci. Rep. 8, 8461 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kolch W., Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat. Rev. Mol. Cell Biol. 6, 827–837 (2005). [DOI] [PubMed] [Google Scholar]
- 62.Leevers S. J., Paterson H. F., Marshall C. J., Requirement for Ras in Raf activation is overcome by targeting Raf to the plasma membrane. Nature 369, 411–414 (1994). [DOI] [PubMed] [Google Scholar]
- 63.Stokoe D., Macdonald S. G., Cadwallader K., Symons M., Hancock J. F., Activation of Raf as a result of recruitment to the plasma membrane. Science 264, 1463–1467 (1994). [DOI] [PubMed] [Google Scholar]
- 64.Lavoie H., et al. , MEK drives BRAF activation through allosteric control of KSR proteins. Nature 554, 549–553 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Choy E., et al. , Endomembrane trafficking of ras: The CAAX motif targets proteins to the ER and Golgi. Cell 98, 69–80 (1999). [DOI] [PubMed] [Google Scholar]
- 66.Daniels M. A., et al. , Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature 444, 724–729 (2006). [DOI] [PubMed] [Google Scholar]
- 67.Goodwin J. S., et al. , Depalmitoylated Ras traffics to and from the Golgi complex via a nonvesicular pathway. J. Cell Biol. 170, 261–272 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luttrell L. M., Composition and function of g protein-coupled receptor signalsomes controlling mitogen-activated protein kinase activity. J. Mol. Neurosci. 26, 253–264 (2005). [DOI] [PubMed] [Google Scholar]
- 69.Perez de Castro I., Bivona T. G., Philips M. R., Pellicer A., Ras activation in Jurkat T cells following low-grade stimulation of the T-cell receptor is specific to N-Ras and occurs only on the Golgi apparatus. Mol. Cell. Biol. 24, 3485–3496 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takahashi M., Li Y., Dillon T. J., Kariya Y., Stork P. J. S., Phosphorylation of the C-Raf N-region promotes Raf dimerization. Mol. Cell Biol. 37, e00132-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tran N. H., Frost J. A., Phosphorylation of Raf-1 by p21-activated kinase 1 and Src regulates Raf-1 autoinhibition. J. Biol. Chem. 278, 11221–11226 (2003). [DOI] [PubMed] [Google Scholar]
- 72.Edin M. L., Juliano R. L., Raf-1 serine 338 phosphorylation plays a key role in adhesion-dependent activation of extracellular signal-regulated kinase by epidermal growth factor. Mol. Cell. Biol. 25, 4466–4475 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eliceiri B. P., Klemke R., Strömblad S., Cheresh D. A., Integrin alphavbeta3 requirement for sustained mitogen-activated protein kinase activity during angiogenesis. J. Cell Biol. 140, 1255–1263 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Howe A. K., Juliano R. L., Regulation of anchorage-dependent signal transduction by protein kinase A and p21-activated kinase. Nat. Cell Biol. 2, 593–600 (2000). [DOI] [PubMed] [Google Scholar]
- 75.Lin T. H., Chen Q., Howe A., Juliano R. L., Cell anchorage permits efficient signal transduction between ras and its downstream kinases. J. Biol. Chem. 272, 8849–8852 (1997). [PubMed] [Google Scholar]
- 76.Roovers K., Davey G., Zhu X., Bottazzi M. E., Assoian R. K., Alpha5beta1 integrin controls cyclin D1 expression by sustaining mitogen-activated protein kinase activity in growth factor-treated cells. Mol. Biol. Cell 10, 3197–3204 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.del Pozo M. A., Price L. S., Alderson N. B., Ren X. D., Schwartz M. A., Adhesion to the extracellular matrix regulates the coupling of the small GTPase Rac to its effector PAK. EMBO J. 19, 2008–2014 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.King A. J., et al. , The protein kinase Pak3 positively regulates Raf-1 activity through phosphorylation of serine 338. Nature 396, 180–183 (1998). [DOI] [PubMed] [Google Scholar]
- 79.Chaudhary A., et al. , Phosphatidylinositol 3-kinase regulates Raf1 through Pak phosphorylation of serine 338. Curr. Biol. 10, 551–554 (2000). [DOI] [PubMed] [Google Scholar]
- 80.McDonald E. R. 3rd, et al. , Project DRIVE: A compendium of cancer dependencies and synthetic lethal relationships uncovered by large-scale, deep RNAi screening. Cell 170, 577–592.e10 (2017). [DOI] [PubMed] [Google Scholar]
- 81.Wang T., et al. , Gene essentiality profiling reveals gene networks and synthetic lethal interactions with oncogenic Ras. Cell 168, 890–903.e15 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Das I., et al. , Preventing proteostasis diseases by selective inhibition of a phosphatase regulatory subunit. Science 348, 239–242 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peti W., Page R., Strategies to make protein serine/threonine (PP1, calcineurin) and tyrosine phosphatases (PTP1B) druggable: Achieving specificity by targeting substrate and regulatory protein interaction sites. Bioorg. Med. Chem. 23, 2781–2785 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.De Munter S., Köhn M., Bollen M., Challenges and opportunities in the development of protein phosphatase-directed therapeutics. ACS Chem. Biol. 8, 36–45 (2013).23214403 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.