Abstract
Background:
Diagnostic ultrasound has been used to detect human disease especially fetus abnormalities in recent decades. Although the harmful effects of diagnostic ultrasound on human have not been established so far, several researchers showed it has had bioeffects in cell lines and in experimental animals. Three-dimensional (3D), four-dimensional (4D), and color Doppler sonography are new techniques which are widely used in diagnostic fetal ultrasonography.
Objective:
The study aims to evaluate some bioeffects of 3D, 4D, and color Doppler sonography in different exposure times according to the acoustic output which is set as ultrasound scanner’s default for fetal sonography in the second trimester on human dermal fibroblast (HDF) cells.
Material and Methods:
Exposure times selected consist of 10, 40, 70, and 100 seconds for 3D sonography, 10, 20, and 30 minutes for 4D sonography, and 10, 30, and 50 seconds for color Doppler. Cell viability, cell proliferation, and apoptosis induction on HDF cells were assessed using MTT assay, immunocytochemistry of Ki-67, and Terminal Transferase-mediated dUTP End-labeling (TUNEL) assay, respectively.
Results:
Exposure of cells to 3D, 4D, and color Doppler modes led to decreased cell viability and increased proliferation rate of HDF. None of the diagnostic ultrasound modes induced cell apoptosis. .
Conclusion:
The results indicated that 3D, 4D, and color Doppler techniques may affect the cell viability and proliferation of HDF cells, however, have no effects on the induction of apoptosis probability. Further long-term studies with other molecular endpoints are required.
Keywords: Diagnostic Ultrasound , 3-dimentional Ultrasound , 4-dimentional Ultrasound , Color Doppler Ultrasound , Human Dermal Fibroblast Cells
Introduction
Diagnostic (medical) ultrasound has been applied to detect many types of human diseases for more than 6 decades [1]. One of the most favored features of this imaging modality is the non-ionizing nature of ultrasound which has led it to be widely used in pregnant women in order to diagnose abnormalities and defect in the fetus. Over the past decades, ultrasonography has rapidly developed. Conventional B-mode (two-dimensional) sonography has been expanded to three-dimensional (3D) and four-dimensional (4D) imaging [2]. While human studies have failed to indicate the detrimental effects of ultrasound [3], it has potential to cause some bioeffects particularly with increasing utility of ultrasound for monitoring fetuses. Several researchers have investigated adverse effects of ultrasound in vitro (in cell lines) and in vivo (in experimental animals) [4-7]. Cavitation and heating are known as the two likely mechanisms of ultrasound’s biological effects [8]. Wamel et al. explored the effects of various diagnostic ultrasound parameters on CHO cell viability and found that cell viability strongly depends on total exposure time and also other technical parameters [9]. A direct relationship between Doppler exposure time and apoptotic activity was also reported [7]. Cell viability was reduced in longer exposure times. Udroiu et al. studied genotoxicity effects of medical ultrasound in murine fibroblasts (NIH-3T3) at low-intensity exposure in vitro [4]. They indicated that ultrasound can induce significant genotoxicity when compared to control cells. Li et al. studied bioeffects of different exposure times of 4D ultrasound on the ultrastructure of cerebral cells of fetal mice in late pregnancy [5]. Their results showed that 4D ultrasound exposure for more than 10 minutes can lead to abnormal neuronal ultrastructure changes and apoptosis cells in the fetal mouse cerebrum. In recent years, 3D and 4D ultrasound examinations have been widely performed for nonmedical purposes to provide keepsake images of developing fetus without diagnostic indications. To increase the acoustic output of ultrasound instrumentation from 94 mW/cm2 (in 1985) to 720 mW/cm2 (in 1992) so as to examine fetal’ health based on FDA approval, there is some concerns about bioeffects of diagnostic ultrasonography on human beings [10]. The American Institute of Ultrasound in Medicine (AIUM) recommends further research on safety of diagnostic ultrasound [11] and this recommendation has motivated us to conduct this study. In the present study, we investigated the biological effects of diagnostic ultrasound under different exposure times of 3D, 4D, and color Doppler techniques according to the acoustic output that is set as ultrasound scanner’s default for fetal sonography in the second trimester on human dermal fibroblast (HDF) cells using MTT assay, immunocytochemistry of Ki-67 and Terminal Transferase-mediated dUTP End-labeling (TUNEL) assay. Fibroblasts are the major cell type in connective tissue and have some critical roles in tissue restoration [12]. It has been shown that low-intensity pulsed ultrasound can promote proliferation of fibroblast cells [13] via activation of integrin receptors and a Rho/ROCK/Src/ERK signaling pathway [14]. MTT assay is colorimetric to evaluate cell metabolic activity and widely used to assess cell viability [15]. Ki-67 protein is known as an excellent marker for determining the growth fraction of a given cell population [16]. TUNEL is a method to detect apoptotic DNA fragmentation widely used to identify and quantify apoptotic cells in individual cells [17].
Material and Methods
Cell culture
Normal HDF cells were provided from the Cell Bank Center of Royan Institute, (Tehran, Iran). The cells cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (Gibco, Invitrogen, US) were supplemented with 15% fetal bovine serum (FBS, Gibco, Invitrogen, US) and 1% penicillin-streptomycin solution (Gibco, Invitrogen, UK). All cultures were maintained in a humidified incubator at 37 °C and 5% CO2 atmosphere.
Ultrasound irradiation
Ultrasonic exposure was provided by a VOLUSON E8 (GE Medical Systems, Kretz Ultrasound, USA) medical diagnostic ultrasound instrumentation equipped with a RAB4-8D 4D Convex ultrasound transducer. In this study, acoustic parameters in 3D, 4D, and color Doppler were set as instrumentation default for fetal sonography in the second trimester. Exposure time was the only parameter that changed. Exposure times selected consist of 10, 40, 70, and 100 seconds for 3D sonography, 10, 20, and 30 minutes for 4D sonography, 10, 30, and 50 seconds for color Doppler. The selection of these times was based on consultation with medical sonographers as the most common exposure times in 3D, 4D and color Doppler sonography of fetal in the second trimester. Non-exposed cells were set as control. In the all experimental conditions, on-screen thermal and mechanical indices (TI and MI) were kept below 1.0 according to the ALARA principle.
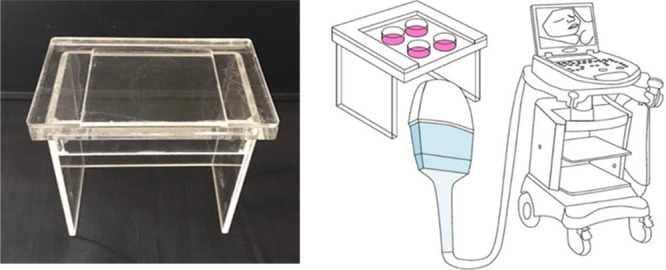
Phantom design
In this study, the cells cultured in 4-well plates were located on an in-house designed phantom and exposed to different modes of ultrasound. This phantom is made of polymethyl methacrylate (PMMA) with a surface area of 10 × 20 cm2 and a thickness of 12.22 mm. Phantom thickness was calculated according to a real sonography condition of the second-trimester embryos. In the second trimester, the distance between the mother’s skin and the fetus’s skin is about 4.5 cm which is considered to be 2.5 cm amniotic fluid and 2 cm soft tissue with an acoustic absorption coefficient of 0.005 dB/cm/MHz and 0.8 dB/cm/MHz, respectively. Attenuation (intensity loss) was calculated according to equation 1:
dB =µfz (1)
Where µ is the intensity attenuation coefficient (expressed in dB/cm), f is the frequency (MHz) and z is the distance traveled in the medium (cm). Considering f =1 MHz (used in this study), attenuation was (0.005 ×2.5 + 0.8 × 2) = 1.6125 dB. Taking into account the 1.1 mm bottom wall thickness of the 4-well plates (with an acoustic absorption coefficient of 0.36 dB/cm/MHz), total attenuation was 1.5729 dB. Equivalent thickness of PMMA (with an acoustic absorption coefficient of 1.288 dB/cm/MHz) which gives this amount of attenuation was L= 1.5729/ 1.288 = 12.22 mm. The ultrasonic gel was used between the transducer and bottom of phantom to allow ultrasound waves to transmit without reflection in the coupling surface.
The phantom designed for cell irradiation and illustration of the irradiation set up is shown in Figure 1.
Figure1.
Roadmap of developed classification scheme
MTT assay
The viability of HDFs was evaluated using MTT assay. HDFs were seeded in 96-well plates at 5 × 104 cells/well in 200 μL of complete media and incubated for 24 hours in the incubator to allow cell adhesion. The cells were exposed to ultrasound beam according to the procedures mentioned above and then returned to the humidified incubator for other 24 hours. Then, supernatant medium was discarded and 100 μL fresh serum-free medium was added. Then, we added a 50 μL MTT solution (0.5 mg/mL) (Bio idea, Tehran, Iran) to each well and incubated plates for an additional 4 hours in dark conditions. After that, we removed the medium and added 200 μL DMSO (Bio idea, Tehran, Iran) to each well in order to dissolve the purple formazan precipitate. Absorbance was measured using an ELISA microplate reader (Anthos 2020, Biochrom Ltd, and UK) at a wavelength of 570 nm. We calculated the percentage of viability according to the following formula: Viability (%) = (average OD (optical density) in the treated sample/ average OD in the control sample) × 100.
Immunocytochemistry staining of Ki-67
The proliferation rate of HDFs was determined using immunocytochemistry staining of Ki-67 as a molecular marker of proliferation. 24 hours after the ultrasound exposure, we fixed the cells with 4% formaldehyde for 20 minutes and then washed twice with PBS. After fixation, we permeabilized the cells with 0.3 % Triton X-100 and 0.1 % sodium citrate in PBS at room temperature for 15 minutes and subsequently blocked with 0.3 % Triton X-100 and 0.5 % BSA for 60 minutes at room temperature. The cells were then labeled with the primary antibody (clone MIB-1, Dako) at 4°C overnight and anti-mouse HRP- secondary antibody (Dako) at 37°C for 90 minutes. The cell nuclei were stained using 3-3′-Diaminobenzidine (DAB) solution (Dako) and imaged on an inverted microscope (Nikon, Eclipse Ti-E, Japan) at 10X magnification. Nuclei were considered as Ki-67 positive if every specific brown stain overlies on them. A total of 4 wells were used for each exposure time group. All Ki-67 positive and negative cells were counted in 10 fields of each well. Then, the fraction of Ki-67 positive cells was considered as the Ki-67 index.
TUNEL assay
Apoptosis induction in HDFs was evaluated using the TUNEL assay. After ultrasound exposure, we incubated the cells in the incubator for an additional 24 hours and then performed TUNEL assay. We fixed the cells with 4 % formaldehyde for 20 minutes and washed them twice with PBS. Subsequently, we permeabilized the cells with 0.1 % Triton X-100 and 0.1 % sodium citrate in PBS at room temperature for 20 minutes. Then, we incubated HDFs with 50 μL of TUNEL reaction mixture containing a 45 μl nucleotide mixture and a 5 μl terminal deoxynucleotidyl transferase (TdT) (Roche Diagnostics GmbH, Germany), for 60 minutes at 37°C. Then, we stained the cells with 4’, 6-diamidino-2-phenylindole (DAPI) (5 μg/mL) (Sigma-Aldrich, USA) for 5 minutes at room temperature and washed them with PBS. The cells were analyzed using an inverted fluorescence microscope (Ceti, Belgium).
Statistical analysis
Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Tukey test for multiple comparisons by the SPSS software version 16.0 (SPSS Inc. USA). P value of < 0.05 was considered statistically significant.
Results
Cellular viability analysis using MTT assay
To determine the possible effects of three different modes of diagnostic ultrasonography on viability of HDF cells, MTT assay was performed 24 hours after exposure. The results were reported as the cell viability % relative to the non-exposed cells and shown in Figure 2. In the case of 3D mode (Figure 2A), cell viability at times of 10 s (P < 0.05) and 100 s (P < 0.01) showed a significant reduction compared to non-exposed cells, while irradiation at times of 40 s and 70 s did not have any significant effects on cell viability (P > 0.05). In the case of 4D mode (Figure 2B), exposure time of 10 minutes did not change the cell viability of HDFs (P > 0.05), while exposure times of 20 minutes (P <0.01) and 30 minutes (P < 0.05) significantly reduced cell viability compared to non-exposed cells. In the case of color Doppler mode (Figure 2C), although the cell viability of exposed cells at times of 10, 30 and 50 s were reduced compared to non-exposed cells, these effects were not significant (P > 0.05). In general, exposure of cells to different modes of diagnostic ultrasound led to a decrease in cell viability and this effect did not show exposure time dependency.
Figure2.
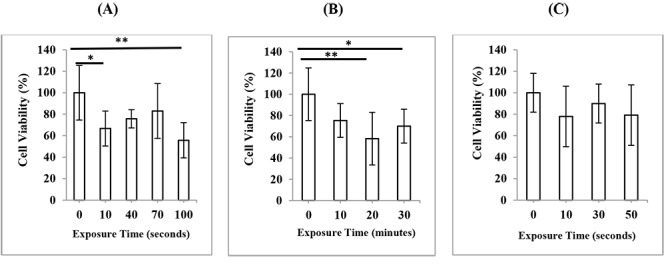
Cell viability of HDF cells, 24 h after exposure to ultrasound in (A) 3D mode (B) 4D mode (C) color Doppler mode of second-trimester fetal sonography condition. Data represent the mean ± SD of three independent experiments.* P < 0.05; ** P < 0.01.
Cell proliferation analysis by immunocytochemistry of Ki-67
To determine the effects of three different modes of diagnostic ultrasonography on the proliferation rate of HDF cells, immunostaining of the proliferation marker Ki-67 was performed 24 hours after exposure. The experiments were repeated three times and the percent of Ki-67 positive cells in total 1000 scored cells was determined. In the case of 3D mode (Figure 3), the exposure time of 40 s significantly increased the proliferation rate of HDFs (P < 0.05), while other exposure times of 10, 70, and 100 s did not change the cell proliferation rate compared to non-exposed cells (P > 0.05). In the case of 4D mode (Figure 4), exposure time of 10 minutes had no significant effects on the proliferation rate of HDFs (P > 0.05) but exposure times of 20 minutes (P < 0.05) and 30 minutes (P < 0.01) significantly increased cell proliferation rate compared to non-exposed cells. In the case of color Doppler mode (Figure 5), exposure time of 10 s had no significant effects on proliferation rate of HDFs (P > 0.05) but exposure times of 20 and 30 s (P < 0.001) significantly increased cell proliferation rate compared to non-exposed cells. In general, the exposure of cells to different modes of diagnostic ultrasound led to an increase in the cell proliferation rate and this effect showed exposure time dependence, in particular in 4D and color Doppler modes.
Figure3.
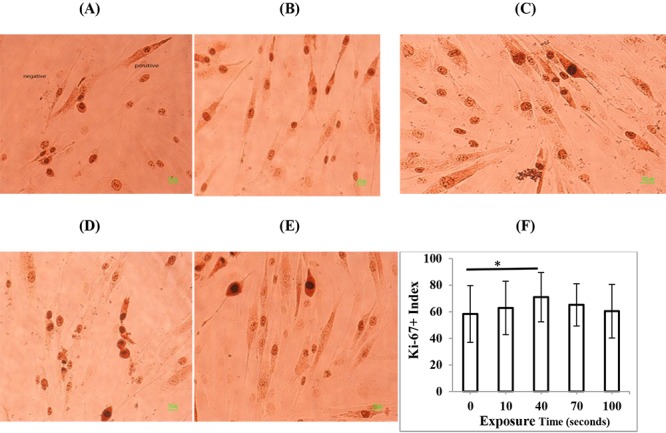
Representative immunostaining images of the proliferation marker Ki-67 in HDF cells, 24 h after exposure to (A) 0 s (control) (B) 10 s (C) 40 s (D) 70 s, and (E) 100 s 3D mode of second-trimester fetal sonography condition. (F) Quantification of Ki-67-positive cells after exposure to different times of 3D mode of diagnostic ultrasonography. Data represent the mean ± SD of three independent experiments.* P < 0.05.
Figure4.
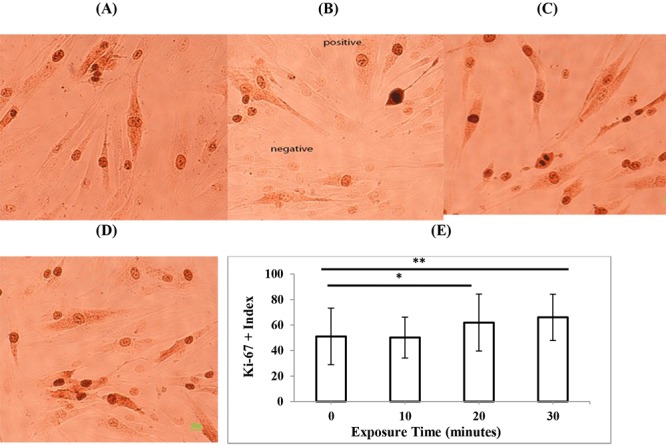
Representative immunostaining images of the proliferation marker Ki-67 in HDF cells, 24 h after exposure to (A) 0 min (control) (B) 10 min (C) 20 min, and (D) 30 min 4D mode of second-trimester fetal sonography condition. (E) Quantification of Ki-67-positive cells after exposure to different times of 4D mode of diagnostic ultrasonography. Data represent the mean ± SD of three independent experiments.* P < 0.05; ** P < 0.01.
Figure5.
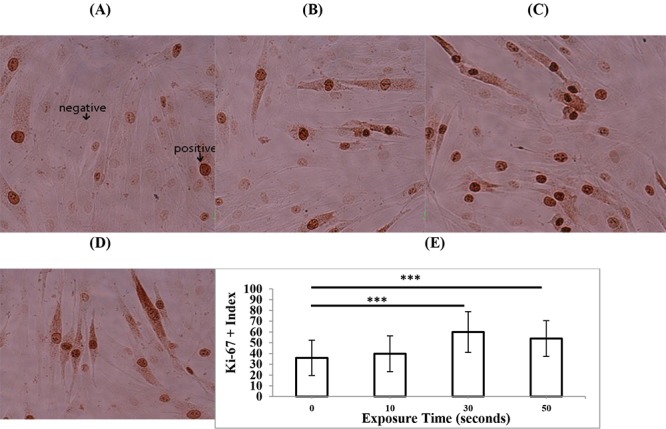
Representative immunostaining images of the proliferation marker Ki-67 in HDF cells, 24 h after exposure to (A) 0 s (control) (B) 10 s (C) 30 s, and (D) 50 s color Doppler mode of second-trimester fetal sonography condition. (E) Quantification of Ki-67-positive cells after exposure to different times of color Doppler mode of diagnostic ultrasonography. Data represent the mean ± SD of three independent experiments. *** P < 0.001.
Apoptosis analysis using TUNEL assay
To determine the effects of three different modes of diagnostic ultrasonography on apoptosis induction in HDF cells, the TUNEL assay was performed 24 hours after exposure. We observed that none of the diagnostic ultrasound modes resulted in induction of apoptosis in HDF cells in any of the exposure times. Representative images of TUNEL staining of HDF cells exposed to maximum exposure times for different modes of diagnostic ultrasound are shown in Figure 6. In any image, TUNEL-positive cells were not observed.
Figure6.
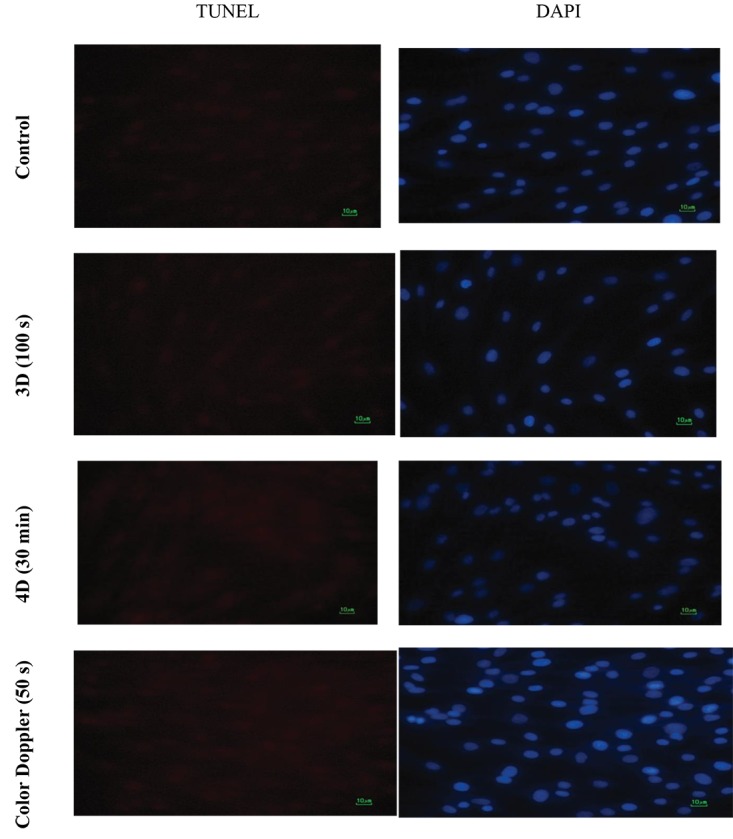
Representative images of TUNEL staining of HDF cells in control, 3D mode exposure (100 s), 4D mode exposure (30 min), and Color Doppler mode (50 s).
Discussion
Ultrasound could result in several bioeffects on cells, including changes in cell division ability, membrane permeability, enzymes activity, calcium influx, cellular functions, and gene expression [18-24]. Ultrasound may also trigger inflammatory cells, resulting in the production of chemical mediators, which activate fibroblasts [22,25]. This stimulation leads to the enhancement of fibroblast proliferation [22]. Furthermore, Lai and Pittelkow indicated that the ultrasound treatment of dermal fibroblasts activates several compensatory signaling pathways mediating cellular repair [12]. The reduced cell viability under the conditions of our study may be linked to compensatory responses mediating subsequent cellular proliferation. Further long-term studies with other endpoints are required to determine the underlying mechanism by ultrasound interacts with cells. No apoptosis was observed in our study representing that unrepairable DNA damage and programmed cell death are unlikely to happen through diagnostic ultrasonography. Previous studies indicated that ultrasound can produce DNA damage which may not necessarily end in cell killing [24]. However, the acoustic output levels at which the ultrasound induces lethal effects appear to be somewhat above the normal range of diagnostic ultrasonography. Despite significant reduction in cell viability of 4 time groups in our study, the increased cell proliferation, measured by ki-67 index, was observed in 5 groups. Oliveira et al. also indicated that the ultrasonic irradiation of the fibroblast cells may accelerate proliferation [26]. As a possible explanation for the reduced viability (assessed by MTT) and increased proliferation (assessed by ki-67) in several groups of the study, we hypothesized the fetal ultrasound can produce some harms and bioeffects and reduce cell viability; however, a number of harms can be compensated via acceleration of cell proliferation. Decreased viability could also be related to the combination of the ultrasound treatment and the technique used in the experimental procedure [20]. At acoustic output levels used in diagnostic ultrasound, there is no proven evidence of any specific harmful effects [27]. In agreement with present experimental evidence, no causal association between fetal ultrasonography during pregnancy period and negative biological effects to the fetus has been shown in human studies [28]. As a limitation, cultured cells could not suitably reflect thermal effects because these effects may be better observed through energy absorption of tissue. This in vitro study has examined the effect of ultrasound irradiation on cultured HDFs. It should be noted that the way ultrasound affects cells in culture environment is different from the way it affects intact tissues [29]. Although we have indicated the potential of ultrasound to cause some bioeffects in cellular level, similar effects occurring in human require further studies. In addition, the obtained results should be interpreted with caution because several uncontrolled parameters in the study could result in cell damage, including the acoustic pressure level, flow properties and, temperature. Therefore, as stated in the ALARA (as Low as Reasonably Achievable) principle, it is still rational to expose patients to the least ultrasound irradiation needed to obtain diagnostic information.
Conclusion
According to the results, we concluded that 3D, 4D, and color Doppler modes of diagnostic ultrasound can affect the cell viability and proliferation of HDF cells; however, they have no effects on the induction of apoptosis probability due to non-fetal damage to the DNA molecule. The ultrasonography should be performed by experts in the shortest possible time according to the ALARA principle and based on medical indications only. We suggest further investigation in terms of different acoustic outputs, post-exposure times, cellular and molecular endpoints.
Acknowledgement
This study was supported by Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Conflict of Interest:None
References
- 1.Troxclair L, Smetherman D, Bluth E I. Shades of gray: a history of the development of diagnostic ultrasound in a large multispecialty clinic. The Ochsner Journal. 2011;11:151–5. [PMC free article] [PubMed] [Google Scholar]
- 2.Wagai T. Studies on the foundation and development of diagnostic ultrasound. Proc Jpn Acad Ser B Phys Biol Sci. 2007;83:256–65. doi: 10.2183/pjab/83.256. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newnham J P, Doherty D A, Kendall G E, Zubrick S R, Landau L L, Stanley F J. Effects of repeated prenatal ultrasound examinations on childhood outcome up to 8 years of age: follow-up of a randomised controlled trial. The Lancet. 2004;364:2038–44. doi: 10.1016/s0140-6736(04)17516-8. [DOI] [PubMed] [Google Scholar]
- 4.Udroiu I, Domenici F, Giliberti C, Bedini A, Palomba R, Luongo F, et al. Potential genotoxic effects of low-intensity ultrasound on fibroblasts, evaluated with the cytokinesis-block micronucleus assay. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2014;772:20–4. doi: 10.1016/j.mrgentox.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Zhang L. Bioeffects of Diagnostic Dynamic 3-Dimensional (4-Dimensional) Ultrasound on Ultrastructure of Cerebral Cells of Fetal Mice in Late Pregnancy. Ultrasound Q. 2016;32:296–301. doi: 10.1097/RUQ.0000000000000216. [DOI] [PubMed] [Google Scholar]
- 6.Schneider-Kolsky M E, Ayobi Z, Lombardo P, Brown D, Kedang B, Gibbs M E. Ultrasound exposure of the foetal chick brain: effects on learning and memory. Int J Dev Neurosci. 2009;27:677–83. doi: 10.1016/j.ijdevneu.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Pellicer B, Herraiz S, Taboas E, Felipo V, Simon C, Pellicer A. Ultrasound bioeffects in rats: quantification of cellular damage in the fetal liver after pulsed Doppler imaging. Ultrasound Obstet Gynecol. 2011;37:643–8. doi: 10.1002/uog.8842. [DOI] [PubMed] [Google Scholar]
- 8.Zeqiri B. Exposure criteria for medical diagnostic ultrasound: II. Criteria based on all known mechanisms:(NCRP Report No. 140) National Council on Radiation Protection and Measurements (NCRP), 2002. Ultrasound Med Biol . 2003;29:1809. [Google Scholar]
- 9.Van Wamel A, Bouakaz A, ten Cate, F de, Jong N, editors . Effects of diagnostic ultrasound parameters on molecular uptake and cell viability. 8-11 Oct. 2002. Munich; IEEE Ultrasonics Symposium, 2002 Proceedings. [Google Scholar]
- 10.Cibull S L, Harris G R, Nell D8M. Trends in diagnostic ultrasound acoustic output from data reported to the US Food and Drug Administration for device indications that include fetal applications. J Ultrasound Med. 2013;32:1921–32. doi: 10.7863/ultra.32.11.1921. [DOI] [PubMed] [Google Scholar]
- 11.Fowlkes J B, Bioeffects Committee of the American Institute of Ultrasound in M. American Institute of Ultrasound in Medicine consensus report on potential bioeffects of diagnostic ultrasound: executive summary. J Ultrasound Med. 2008;27:503–15. doi: 10.7863/jum.2008.27.4.503. [DOI] [PubMed] [Google Scholar]
- 12.Lai J, Pittelkow M R. Physiological effects of ultrasound mist on fibroblasts. Int J Dermatol. 2007;46:587–93. doi: 10.1111/j.1365-4632.2007.02914.x. [DOI] [PubMed] [Google Scholar]
- 13.Franco de Oliveira R, Pires Oliveira D A, Soares C P. Effect of low-intensity pulsed ultrasound on l929 fibroblasts. Arch Med Sci. 2011;7:224–9. doi: 10.5114/aoms.2011.22071. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou S, Schmelz A, Seufferlein T, Li Y, Zhao J, Bachem M G. Molecular mechanisms of low intensity pulsed ultrasound in human skin fibroblasts. J Biol Chem. 2004;279:54463–9. doi: 10.1074/jbc.M404786200. [DOI] [PubMed] [Google Scholar]
- 15.Stockert J C, Horobin R W, Colombo L L, Blazquez-Castro A. Tetrazolium salts and formazan products in Cell Biology: Viability assessment, fluorescence imaging, and labeling perspectives. Acta Histochem. 2018;120:159–67. doi: 10.1016/j.acthis.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–22. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 17.Darzynkiewicz Z, Galkowski D, Zhao H. Analysis of apoptosis by cytometry using TUNEL assay. Methods. 2008;44:250–4. doi: 10.1016/j.ymeth.2007.11.008. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dinno M A, Dyson M, Young S R, Mortimer A J, Hart J, Crum L A. The significance of membrane changes in the safe and effective use of therapeutic and diagnostic ultrasound. Phys Med Biol. 1989;34:1543–52. doi: 10.1088/0031-9155/34/11/003. [DOI] [PubMed] [Google Scholar]
- 19.al-Karmi A M, Dinno M A, Stoltz D A, Crum L A, Matthews J C. Calcium and the effects of ultrasound on frog skin. Ultrasound Med Biol. 1994;20:73–81. doi: 10.1016/0301-5629(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 20.Mortimer A J, Dyson M. The effect of therapeutic ultrasound on calcium uptake in fibroblasts. Ultrasound Med Biol. 1988;14:499–506. doi: 10.1016/0301-5629(88)90111-1. [DOI] [PubMed] [Google Scholar]
- 21.Ma H, Huang L, Jia J, He R, Luo L, Zhu W. Effect of energy-gathered ultrasound on Alcalase. Ultrason Sonochem. 2011;18:419–24. doi: 10.1016/j.ultsonch.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 22.De Deyne P G, Kirsch-Volders M. In vitro effects of therapeutic ultrasound on the nucleus of human fibroblasts. Phys Ther. 1995;75:629–34. doi: 10.1093/ptj/75.7.629. [DOI] [PubMed] [Google Scholar]
- 23.Hanson M A. Health effects of exposure to ultrasound and infrasound: Report of the independent advisory group on non-ionising radiation. Health Protection Agency; 2010. [Google Scholar]
- 24.Furusawa Y, Hassan M A, Zhao Q L, Ogawa R, Tabuchi Y, Kondo T. Effects of therapeutic ultrasound on the nucleus and genomic DNA. Ultrason Sonochem. 2014;21:2061–8. doi: 10.1016/j.ultsonch.2014.02.028. [DOI] [PubMed] [Google Scholar]
- 25.Young S R, Dyson M. Macrophage responsiveness to therapeutic ultrasound. Ultrasound Med Biol. 1990;16:809–16. doi: 10.1016/0301-5629(90)90045-e. [DOI] [PubMed] [Google Scholar]
- 26.De Oliveira R F, Pires Oliveira D A, Machado A H, da Silva N S, Magini M, Pacheco-Soares C. Assessment of fibroblast cells submitted to ultrasonic irradiation. Cell Biol Int. 2008;32:1329–35. doi: 10.1016/j.cellbi.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 27.Izadifar Z, Babyn P, Chapman D. Mechanical and Biological Effects of Ultrasound: A Review of Present Knowledge. Ultrasound Med Biol. 2017;43:1085–104. doi: 10.1016/j.ultrasmedbio.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 28.Abramowicz J S, Barnett SB, Duck FA, Edmonds PD, Hynynen KH, Ziskin MC. Fetal thermal effects of diagnostic ultrasound. J Ultrasound Med. 2008;27:541–59. doi: 10.7863/jum.2008.27.4.541. [DOI] [PubMed] [Google Scholar]
- 29.Ter Haar G. Ultrasound bioeffects and safety. Proc Inst Mech Eng H. 2010;224:363–73. doi: 10.1243/09544119JEIM613. [DOI] [PubMed] [Google Scholar]


