Abstract
Oddball studies have shown that sounds unexpectedly deviating from an otherwise repeated sequence capture attention away from the task at hand. While such distraction is typically regarded as potentially important in everyday life, previous work has so far not examined how deviant sounds affect performance on more complex daily tasks. In this study, we developed a new method to examine whether deviant sounds can disrupt reading performance by recording participants’ eye movements. Participants read single sentences in silence and while listening to task-irrelevant sounds. In the latter condition, a 50-ms sound was played contingent on the fixation of five target words in the sentence. On most occasions, the same tone was presented (standard sound), whereas on rare and unexpected occasions it was replaced by white noise (deviant sound). The deviant sound resulted in significantly longer fixation durations on the target words relative to the standard sound. A time-course analysis showed that the deviant sound began to affect fixation durations around 180 ms after fixation onset. Furthermore, deviance distraction was not modulated by the lexical frequency of target words. In summary, fixation durations on the target words were longer immediately after the presentation of the deviant sound, but there was no evidence that it interfered with the lexical processing of these words. The present results are in line with the recent proposition that deviant sounds yield a temporary motor suppression and suggest that deviant sounds likely inhibit the programming of the next saccade.
Keywords: Deviance distraction, reading, eye-movements, auditory distractors
Oddball studies have shown that task-irrelevant sounds that unexpectedly differ from an otherwise structured or repeated sequence of sounds yield specific electrophysiological responses and behavioural distraction in an unrelated task (Berti, 2008; Berti & Schröger, 2001, 2003; Horváth, Roeber, Bendixen, & Schröger, 2008; Schröger, 1996). In the oddball paradigm, an irrelevant sound is presented before the appearance of a target stimulus on the screen. On most trials, the same sound is presented (standard) whereas on rare and unpredictable occasions it is replaced by a different sound (deviant). The typical finding from such studies is that deviant sounds delay responses in categorisation tasks where participants must respond to target stimuli while ignoring task-irrelevant sounds (Ljungberg & Parmentier, 2012; Parmentier, 2014; Parmentier, Elford, Escera, Andrés, & Miguel, 2008; Parmentier, Vasilev & Andrés, 2018; Parmentier, 2008).
Previous research has shown that deviant sounds are distracting because they violate the cognitive system’s predictions (Bubic, von Cramon, Jacobsen, Schröger, & Schubotz, 2009; Parmentier, Elsley, Andrés, & Barceló, 2011). In fact, attentional distraction has been observed at the electrophysiological and behavioural level for both small pitch differences and larger spectral differences between the standard and the deviant sound (Parmentier et al., 2008; Schröger, 1996). In addition, there is abundant evidence showing that deviance distraction does not depend on the specific identity of the sounds: it occurs regardless of whether sound A (e.g., a sinewave tone) is used as the standard and sound B (e.g., white noise) is used as the deviant, or vice versa (Leiva, Parmentier, & Andrés, 2015b). This latter finding has also been shown to generalise to the tactile modality (Parmentier, Ljungberg, Elsley, & Lindkvist, 2011).
Interestingly, deviant sounds that convey meaning can also yield distraction because they undergo some automatic semantic evaluation (Parmentier & Kefauver, 2015; Parmentier, Pacheco-Unguetti, & Valero, 2018; Parmentier, Turner, & Elsley, 2011; Parmentier, Turner, & Perez, 2014; Roye, Jacobsen, & Schröger, 2007; Schröger, Giard, & Wolff, 2000). For example, participants show neural responses to the semantic content of unexpected sounds even when they are passively exposed to them (Czigler, Cox, Gyimesi, & Horváth, 2007; Frangos, Ritter, & Friedman, 2005; Friedman, Cycowicz, & Dziobek, 2003; Roye et al., 2007; Shtyrov, Hauk, & Pulvermuller, 2004; Shtyrov & Pulvermuller, 2003). In addition, the semantics of deviant sounds can be processed even when the words’ meaning bears no connection to the primary task (Escera, Yago, Corral, Corbera, & Nuñez, 2003). Finally, deviance distraction can also be modulated by other factors, such as participants’ age. For example, deviant sounds cause greater behavioural distraction in old age under certain conditions (Leiva, Andrés, & Parmentier, 2015; Leiva, Parmentier, & Andrés, 2015a) although this does not appear to reflect age-related differences in the electrophysiological orienting response (Berti, Vossel, & Gamer, 2017).
Deviance distraction is traditionally viewed as an involuntary switch of attention away from the main task caused by the detection of subtle auditory changes in the human brain (Escera, Alho, Winkler, & Näätänen, 1998; Schröger, 1996). This is essentially an orienting response (see Sokolov, 1963) characterised by a burst of arousal and a reflexive orienting of attention towards the eliciting stimulus (Näätänen, 1992). Deviant sounds are associated with a specific neurophysiogical signature reflected by three distinct event-related potential (ERP) components: (1) mismatch negativity (MMN; Näätänen, Gaillard, & Mäntysalo, 1978; Näätänen, Paavilainen, Rinne, & Alho, 2007), an early ERP component that reflects a pre-attentive mechanism for detecting auditory changes in the brain (Berti & Schröger, 2001); (2) a P3a component reflecting the involuntary shift of attention towards the deviant sound (Berti & Schröger, 2001; Escera, Alho, Schröger, & Winkler, 2000; Schröger & Wolff, 1998a, 1998b); and (3) a reorientation negativity (RON) component reflecting the refocusing of attention back to the main task (Berti, 2008; Schröger et al., 2000; Schröger & Wolff, 1998a).
Interestingly, while deviance distraction has typically been regarded as an example of attentional distraction, recent work suggests that deviant sounds may also temporarily inhibit motor cortical areas (Wessel, 2017; Wessel & Aron, 2013). For example, Wessel and Aron (2013) observed reduced corticospinal excitability following the presentation of novel sounds, leading them to suggest that they activate the same neural circuits that are used to interrupt ongoing actions. The reduction in corticospinal excitability by unexpected sounds has also been found to be positively correlated with action-stopping behaviour in a Go/NoGo task (Dutra, Waller, & Wessel, 2018). This extends the traditional explanation of deviance distraction as an orienting response by suggesting that unexpected sounds may also induce global motor inhibition through the same neural circuits that are used to stop ongoing action plans. Such global inhibition may serve the purpose of temporarily suspending ongoing processes to facilitate the effective and timely processing of unexpected stimuli (Wessel, 2017; Wessel & Aron, 2017). Consequently, the orienting response and motor inhibition accounts are not necessarily mutually exclusive and could both be a consequence of encountering unexpected sounds in the environment.
The potential role of motor inhibition in deviance distraction is exciting as it suggests that deviant sounds may potentially affect a large range of activities, including those relying on relatively automatic motor processes. However, despite this potential impact on everyday life situations, previous studies have used simple laboratory tasks that may have limited ecological validity. We address this issue by exploring for the first time whether deviant sounds affect performance on one important and complex everyday task: reading. To do so, we developed a new method to measure the effect of deviant sounds on eye movements during reading.
Reading is a theoretically interesting task for studying the effect of deviant sounds on human performance because it does not require any specific response from participants upon hearing the task-irrelevant sounds. Unlike categorisation tasks where participants need to make a dichotomous response after the presentation of the sound (e.g., judging whether a number is odd or even), subjects simply have to read the text for comprehension and ignore the task-irrelevant sounds. This makes it possible to investigate how deviant sounds affect performance on a natural, everyday task that does not involve any response preparation or the need to act upon a specific stimulus after the sound is presented. In addition, skilled adult reading is a fairly automatised process (LaBerge & Samuels, 1974), which involves the intricate coordination of oculomotor and cognitive processes that determines when and where to move the eyes next. As a result, it can yield valuable insights into how deviant sounds influence cognitive and oculomotor processes.
While there is a very long history of research into auditory distraction, most studies have only considered the influence of continuous auditory distractors (e.g., irrelevant speech or music) on behavioural measures such as comprehension accuracy (Vasilev, Kirkby, & Angele, 2018). However, recording participants’ eye movements makes it possible to investigate how irrelevant sounds affect the moment-to-moment decision of when and where to move the eyes next. Because eye movements during reading are sensitive to the underlying cognitive processing of the text (Rayner, 1998, 2009), this method has the potential to uncover subtle auditory-distraction effects that may not appear in behavioural measures of comprehension.
It is not known whether eye movements during reading are sensitive to discrete deviant sounds. However, recent work does indicate that they are affected by certain types of continuous sounds. For example, background music and unintelligible speech in a foreign language do not appear to affect fixation durations or fixation probabilities during reading (Cauchard, Cane, & Weger, 2012; Hyönä & Ekholm, 2016; Johansson, Holmqvist, Mossberg, & Lindgren, 2012). However, semantically meaningful irrelevant speech disrupts the reading process by prompting participants to make more re-reading fixations on previously read words (Cauchard et al., 2012; Hyönä & Ekholm, 2016; Yan, Meng, Liu, He, & Paterson, 2018). This latter finding is interesting because it suggests that certain task-irrelevant sounds can have a direct influence on eye movements and interfere with the ongoing cognitive processing of the text.
While discrete deviant sounds could also potentially influence fixation durations during reading, this is not expected to occur through the same mechanism that is responsible for distraction by meaningful speech. The available evidence suggests that meaningful speech causes distraction because readers process its semantic features, which in turn interferes with extracting the meaning of the written text (e.g., Hyönä & Ekholm, 2016; Martin, Wogalter, & Forlano, 1988; see also Marsh, Hughes, & Jones, 2008, 2009). However, we hypothesise that deviant sounds cause distraction not because of semantic interference, but rather because they violate readers’ expectation that another standard sound will be presented (Bubic et al., 2009; Parmentier, Elsley, et al., 2011). Therefore, this study builds upon previous work on distraction by continuous sounds by exploring a different mechanism through which task-irrelevant sounds may influence eye movements during reading.
Present study
We developed a new manipulation in which the presentation of task-irrelevant sounds was contingent on participants’ eye movements. While participants read sentences for comprehension, short sounds were presented upon fixating five target words in each sentence. On most occasions, the sound was a sine-wave tone (i.e., the standard sound) whereas on rare and unpredictable occasions it was replaced by a short burst of white noise (i.e., the deviant sound). Few studies to date have used gaze-contingent auditory presentation in a reading task (Eiter & Inhoff, 2010; Inhoff, Connine, Eiter, Radach, & Heller, 2004; Inhoff, Connine, & Radach, 2002). In these studies, participants heard an irrelevant spoken word once their eyes crossed an invisible boundary (Rayner, 1975) located just before a target word in the sentence. In this study, an invisible boundary was inserted before each of the five target words in the sentence.
If deviant sounds in this task elicit an orienting response (e.g., Escera et al., 1998; Parmentier, 2014), this may happen either overtly or covertly. According to Posner (1980), overt orienting occurs when there is an eye movement directed towards the eliciting stimulus. Conversely, covert orienting occurs when there is a shift of attention without any corresponding eye movements. If deviant sounds trigger an overt shift of attention, this would manifest in shorter fixation durations after the presentation of the sound because the current fixation will be interrupted and the orienting response (i.e., the eye movement) will occur. However, because the deviant stimulus is in the auditory domain and because it is not linked to a specific location on the screen, it is more likely that the orienting response would occur covertly (i.e., without an eye movement). In this case, attention would be redirected away from processing the words in the sentence and towards the deviant sound, resulting in an increase in fixation durations due to a disruption in the lexical processing of the target words. Alternatively, if the orienting response occurs later in time, it may disrupt the allocation of attention to other words in the sentence, or the post-lexical stages of sentence integration. Similarly, if deviant sounds elicit global motor inhibition (Wessel & Aron, 2013), this would also lead to longer fixation durations after the presentation of the sound, likely due to a delay in saccade programming.
On the basis of previous work suggesting that deviant sounds delay the processing of target stimuli (Parmentier et al., 2008) and the notion that this might reflect the temporary suppression of ongoing actions (Wessel & Aron, 2013), we hypothesised that the deviant sounds would lead to increased fixation durations on the target words. In addition, to determine if any potential increase in fixation durations is due to a disruption in the initial stages of lexical processing, we examined the time course of the effect and tested whether it is modulated by the corpus lexical frequency of the target words.
Method
Participants
Forty-eight Bournemouth University students participated for course credit1 (45 female). Their average age was 19.7 years (SD = 2.4 years, range: 18-32 years). All were native speakers of British English, reported normal or corrected-to-normal vision, normal hearing, no prior diagnosis of reading disorders, and were naïve as to the purpose of the experiment. The study was approved by the Bournemouth University Research Ethics Committee (protocol No. 16999). Informed written consent was obtained from all participants who took part in the study.
Materials and design
The standard sound was a 400-Hz sine wave and the deviant sound was a burst of white noise. The sounds were generated in Matlab R2014a (MathWorks, 2014). Both sounds were 50 ms long and had a 10-ms fade-in and fade-out. The amplitude resolution of the sounds was 16 bits and the sampling frequency was 48 kHz.
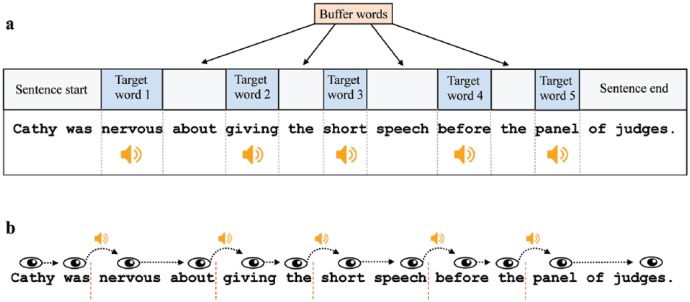
The reading materials consisted of 120 English sentences (see the Supplementary Material). Their average length was 14.3 words (range: 13-18 words). As illustrated in Figure 1a, each sentence contained five target words. The sound stimuli were played once the target words were fixated (we use the term “target” to denote the words on which sounds were played, as opposed to other words in the sentence where no sounds were played). The 5 target words were always the 3rd, 5th, 7th, 9th, and 11th word in the sentence. Their mean length was 6.75 letters (SD = 1.89 letters, range: 3-13 letters). The average lexical frequency of the target words was 180 counts per million in the SUBTLEX-UK database (Van Heuven, Mandera, Keuleers, & Brysbaert, 2014; SD = 786, range: 0.06-15,450 counts per million). Short function words that are likely to be skipped during first-pass reading were not used as targets. Target words were always separated by one non-target word, which served as buffer to increase the sound inter-stimulus interval. In addition, there were always at least two words following the last target word in a sentence to avoid artefacts due to sentence wrap-up effects.
Figure 1.
An illustration of the sound presentation in the experiment. (a) An example sentence and the position of the target words. (b) The gaze-contingent sound presentation. The sound was played once the eye moved to the right of each invisible boundary (illustrated here as red dashed lines).
Because the reading stimuli were specifically developed for this experiment and were not part of a pre-existing corpus, a norming study was carried out to determine their difficulty and naturalness. Twelve participants (nine female, mean age = 29.1 years) who did not take part in the eye-tracking experiment were asked to read each sentence and rate it on a scale from 1 to 10 based on how difficult and how natural they thought it was (1 = very difficult/ very unnatural; 10 = very easy/ very natural). The results showed that the sentences were rated both as very natural (M = 8.84, SD = 1.53) and very easy to understand (M = 9.29, SD = 1.23) by speakers of English.
There were two experimental blocks: one block of 40 sentences that was completed in silence, and another block of 80 sentences that contained the gaze-contingent sound presentation. The silence block was added to determine whether the presentation of five gaze-contingent standard sounds leads to a change in reading behaviour compared to reading in silence. This was important as previous studies have not presented multiple gaze-contingent sounds in a reading task. The gaze-contingent block contained 2 types of trials: (1) 40 trials contained five standard sounds and (2) 40 trials contained four standard sounds and one deviant sound. In trials with a deviant sound, the first sound (on Target Word 1) was also always a standard sound. This was done to re-activate the representation of the standard sound at the beginning of the trial. The deviant sound was then presented on one of the four remaining target words (two through five) with equal probability across the experiment. Overall, deviant sounds represented 10% of all sounds in the experiment. The assignment of conditions to sentences, the position of the deviant sound, and the order of the two experimental blocks were counter-balanced with a full Latin square design. The sentences within each block appeared in random order. The gaze-contingent block always started with three standard-only trials to establish the sine wave as the standard sound.
Apparatus
Participants’ eye movements were recorded with an EyeLink 1000 Tower Mount at a sampling frequency of 1,000 Hz. The resolution noise was <0.01° and the velocity noise was <0.5° on average. Participants rested their chin on a headrest to minimise head-movement artefacts. Viewing was binocular, but only the right eye was recorded. The experiment was programmed in Matlab R2014a (MathWorks, 2014) with the Psychophysics toolbox (Brainard, 1997; Pelli, 1997) and Eyelink (Cornelissen, Peters, & Palmer, 2002) libraries. The sentences were presented on a Cambridge Research Systems LCD ++ monitor (screen resolution: 1920 × 1080 pixels; refresh rate: 100 Hz). The sentences were formatted in a Courier New 18pt. font and appeared as black text over white background on a single line in the middle of the screen. The width of each letter was 14 pixels. The distance between the eye and the monitor was 80 cm. At this distance, each letter subtended a visual angle of approximately 0.34°. The sounds were played through a Creative Labs Sound Blaster X-Fi SB0770 sound card using the low-latency presentation mode of the Psychophysics toolbox. The sound stimuli were presented binaurally at 65 dB(A) through Bose QuietComfort 25 noise-cancelling headphones. The experiment was performed on a personal computer (PC) running Windows 7.
Procedure
Participants were tested individually in a 30- to 40-min session. Participants were instructed to ignore the task-irrelevant sounds and focus on the reading task. Before the experiment, participants were calibrated on a three-point calibration grid. A drift check was presented before each trial and participants were re-calibrated whenever necessary. The calibration error was kept at <0.3° across the experiment. The experiment started with six silent practice trials. Each trial began with a black gaze box that appeared at 50 pixels on the left side of the screen. Upon fixating this box for 100 ms, it disappeared and the sentence was presented on the screen, with the first letter appearing in the middle of the location where the box was.
The gaze-contingent sound manipulation is illustrated in Figure 1b. An invisible boundary (Rayner, 1975) was placed at the first pixel of the empty space before each of the five target words. Once the eye crossed a boundary, the command to play the sound was sent. The delay between sending this command and the actual onset of the sound through the headphones was 14 ms. However, because the boundary was usually crossed during a saccade towards the target word, the sound onset delay relative to fixation onset was normally much less (mean delay = 4.8 ms, SD = 8.9 ms). To avoid sound overlap when participants made a long saccade and crossed more than one boundary, a sound was played only when at least 10 ms had passed since the offset of the previous sound. Each sound was played only once when the target word was first fixated; the sound was not repeated if the target word was subsequently re-fixated during a regression. Participants pressed a button on the mouse to terminate each trial. Comprehension of the sentences was assessed with a “Yes/No” question after 33% of the trials. For example, in the sentence “Cathy was nervous about giving the short speech before the panel of judges.” the comprehension question was “Was Cathy relaxed about giving her speech? Yes/No.”
Data analysis
We analysed fixations on Target Words 2 to 5 as a function of the sound condition (silence, standard, deviant). To ensure that the comparisons were equivalent with respect to the words’ position, one target word was sampled per trial in the silence and standard sound conditions. This word was selected based on the design matrix used to allocate the deviant sounds to word positions in trials where a deviant sound was presented. This made it possible to form a fully balanced dataset with an equal number of observations per sound condition and target word position.
We analysed four standard fixation duration measures: first fixation duration (FFD; the duration of the first fixation on a word), single fixation duration (SFD; the duration when a word is fixated only once), gaze duration (GD; the sum of all fixations on a word before the eyes move on to another word), and total viewing time (TVT; the sum of all fixations on a word, including the ones made during a regression). In addition, a few measures of global reading were also analysed: sentence reading time, fixation duration, number of fixations, and saccade length. The data were analysed with (Generalised) linear mixed models ([G]LMMs) using the lme4 package v.1.1-12 (Bates, Machler, Bolker, & Walker, 2014) in R 3.3.0 (R Core Team, 2016). Sound type was entered as a fixed effect in the model. Random intercepts and random slopes for sound type were added for both participants and items2 (Baayen, Davidson, & Bates, 2008; Barr, Levy, Scheepers, & Tily, 2013). Fixation durations were log transformed in all analyses. Treatment contrast coding was used with the standard sound as the baseline. This contrast coding made it possible to do the two key comparisons in the experiment: (1) silence versus standard sound—to determine whether the presentation of gaze-contingent sounds in general influenced reading behaviour compared to silent reading and (2) deviant versus standard sound—to test for the presence of a sound deviance effect. The results were considered statistically significant if the|t| or|z| values were ⩾1.96. Standardised effect sizes in Cohen’s d (Borenstein, 2009) are also reported for the significant results.
Results
The average comprehension accuracy was 95%, thus indicating that participants understood the sentences (Silence: M = 95.1%, SD = 21.7%; Standard: M = 94.7%, SD = 22.4%; Deviant: M = 95.2%, SD = 21.3%). There were no differences in comprehension accuracy across the sound conditions (all|z|s < 0.49). After the experiment, only two participants (4.1%) reported some awareness that the sounds were played depending on their eye movements.3 Trials with blinks (6.2%) and trials in which the command to play the sound was sent after the fixation onset of the target word (12.2%) were excluded from the data. In addition, trials with boundary “hooks” (8.5%) were also excluded. A hook occurs when the gaze crosses the invisible boundary and triggers the sound, but then returns to the left of the boundary and lands on a previous word. Fixations shorter than 80 ms that occurred within one letter of another fixation were combined with that fixation. Trials with fixation durations longer than 800 ms for FFD, 2,000 ms for GD, and 4,000 ms for TVT (0.44%) were excluded as outliers in all analyses. This left 72.6% of the data for analysis (a total of 4,206 trials).
Global reading
The descriptive statistics and linear mixed model (LMM) results for global reading measures are shown in Tables 1 and 2, respectively. No significant differences were observed between the sound conditions on any of these measures: deviant sounds did not affect performance relative to the standard condition, and performance was comparable in the silence and standard conditions. Therefore, global reading was not disrupted by deviant sounds or the presence of gaze-contingent sounds in general.
Table 1.
Mean descriptive statistics for global reading measures.
| Sound | Sentence reading time (in ms) | Fixation duration (in ms) | Number of fixations | Saccade length (in letters) |
|---|---|---|---|---|
| Silence | 3,670 (1,781) | 237 (114) | 15.4 (6.25) | 9.76 (9.44) |
| Standard | 3,580 (1,715) | 237 (109) | 15.1 (5.81) | 9.59 (9.04) |
| Deviant | 3,560 (1,697) | 236 (110) | 15.1 (5.83) | 9.51 (8.64) |
Standard deviations in parenthesis.
Table 2.
Results from LMMs for global reading measures.
| Effect | Sentence reading timea |
Fixation duration |
Number of fixations |
Saccade lengtha |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| b | SE | t | b | SE | t | b | SE | t | b | SE | t | |
| Intercept | 8.09 | 0.04 | 185 | 5.38 | 0.02 | 282.5 | 15.08 | 0.56 | 26.75 | 2.03 | 0.02 | 95.82 |
| Deviant vs. standard | <–0.01 | 0.01 | −0.57 | <–0.01 | <0.01 | −1.24 | −0.01 | 0.13 | −0.08 | <–0.01 | 0.01 | −0.57 |
| Silence vs. standard | 0.02 | 0.03 | 0.81 | <0.01 | <0.01 | 0.09 | 0.35 | 0.42 | 0.83 | 0.01 | 0.01 | 0.87 |
Statistically significant t-values are formatted in bold.
LMMs = linear mixed models; SE = standard error.
The random intercept for subjects was removed due to convergence failure.
Target words
As Table 3 shows, fixation durations were significantly longer immediately following the deviant sound compared to the standard sound (FFD: b = 0.03, SE = 0.02, t = 2.02, d = 0.20; SFD: b = 0.04, SE = 0.02, t = 2.43, d = 0.29; GD: b = 0.05, SE = 0.02, t = 2.8, d = 0.21; TVT: b = 0.04, SE = 0.02, t = 2.16, d = 0.18). However, fixation durations were comparable in the standard and silent conditions (FFD: b = 0.02, SE = 0.01, t = 1.35; SFD: b = 0.03, SE = 0.02, t = 1.86; GD: b = 0.01, SE = 0.02, t = 0.2; TVT: b = 0.01, SE = 0.02, t = 0.55). When the position of the target word in the sentence was added as a fixed effect in the model, there were no significant interactions with the contrast between the standard and the deviant sound (all|t|s ⩽ 1.75). This shows that the deviance effect was not modulated by the position of the target word in the sentence. Furthermore, there was a 3% greater probability of making a regressive saccade immediately after hearing the deviant sound compared to hearing the standard sound (b = 0.25, SE = 0.13, z = 1.96, d = 0.25; Deviant: M = 21.3%, SD = 40.9%; Standard: M = 17.9%, SD = 38.3%). No such difference was observed between the standard sound and the silence condition (b = 0.14, SE = 0.12, z = 1.23; Standard: M = 17.9%, SD = 38.3%; Silence: M = 19.2%, SD = 39.4%). These results clearly indicate that the deviant sound significantly and selectively affected fixation durations on the target word immediately after presentation. However, this effect did not appear in the global reading measures because they are averages across all words in the sentence whereas the deviant sound manipulation was specific to fixating a single word.
Table 3.
Mean fixation durations on the target words.
| Sound type | FFD |
SFD |
GD |
TVT |
|---|---|---|---|---|
| Silence | 246 (83) | 250 (83) | 296 (137) | 362 (222) |
| Standard | 243 (85) | 243 (83) | 301 (160) | 356 (222) |
| Deviant | 254 (102) | 255 (98) | 317 (166) | 375 (232) |
FFD = first fixation duration; SFD = single fixation duration; GD = gaze duration; TVT = total viewing time.
Standard deviations in parenthesis.
Post-hoc analyses were carried out to test if the disruption by the deviant sound also affected fixation durations on the subsequent word in the sentence (i.e., the post-target word). The results (presented in the Supplementary Material) indicated that the deviant sound had no effect on fixation durations on the next word in the sentence, which suggests that the disruption occurred before the next word was fixated. In addition, the magnitude of the deviance effect on the target words was not modulated by whether participants were slow or fast readers (see the Supplementary Material).
To establish the point in time where the deviant sound first starts to affect fixation durations, we used the confidence interval divergence point analysis (CI-DPA; Reingold & Sheridan, 2014, 2018). This survival analysis technique can determine the earliest point in time where the distributions of two experimental conditions begin to diverge (i.e., significantly differ from one another). The CI-DPA analysis was run with 10,000 bootstrap iterations on the FFD of the target words using the method described in Reingold and Sheridan (2018). The analysis indicated that the deviant sound first started to affect fixation durations at 180 ms (95% CI = [167, 198]). This is illustrated in Figure 2.
Figure 2.
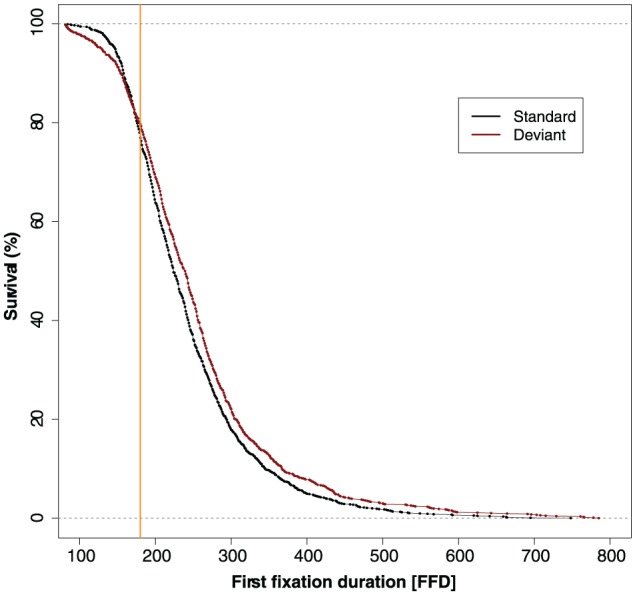
Survival curves of the first fixation during which participants heard the sound. The divergence point (at 180 ms) is shown by the vertical orange line.
Finally, the lexical frequency of target words was entered into a model with the fixation durations in the three sound conditions. If the deviant sound interfered with the lexical processing of the target words, we would expect to see an interaction between lexical frequency and the deviant condition. The results showed no significant interactions between lexical frequency and the deviant sound (see Table 4), suggesting that the deviant sound did not interfere with accessing the lexical representation of words.
Table 4.
Interactions between fixation durations on the target words and corpus lexical frequency.
| Effect | FFD | SFD | GD | ||||||
|---|---|---|---|---|---|---|---|---|---|
| b | SE | t | b | SE | t | b | SE | t | |
| Intercept | 5.44 | 0.02 | 254.29 | 5.45 | 0.02 | 251.45 | 5.60 | 0.03 | 191.26 |
| Frequency | −0.02 | 0.01 | –2.55 | −0.03 | 0.01 | –3.50 | −0.05 | 0.01 | –4.99 |
| Deviant | 0.03 | 0.02 | 2.02 | 0.04 | 0.02 | 2.37 | 0.05 | 0.02 | 2.86 |
| Standard | 0.02 | 0.01 | 1.31 | 0.03 | 0.01 | 1.86 | < 0.01 | 0.02 | 0.18 |
| Frequency: Deviant | < 0.01 | 0.01 | 0.36 | 0.02 | 0.01 | 1.52 | 0.02 | 0.02 | 1.27 |
| Frequency: Standard | −0.01 | 0.01 | −0.83 | −0.01 | 0.01 | −0.39 | −0.01 | 0.02 | −0.35 |
FFD = first fixation duration; SFD = single fixation duration; GD = gaze duration; SE = standard error.
Statistically significant t-values are formatted in bold. Lexical frequency was log transformed.
Discussion
The present results showed clear evidence of deviance distraction in eye movements during reading. Indeed, fixation durations on the target words were longer after hearing the deviant sound compared to hearing the standard sound. Interestingly, global reading measures were unaffected by either sound—the mere presence of gaze-contingent sounds did not influence reading behaviour. However, deviant sounds prolonged fixation durations on the currently read word at the time of the sound’s presentation. As comprehension accuracy was not affected by the presentation of a deviant sound in the sentence, it is unlikely that the observed longer fixation durations on the target words are related to comprehension difficulties. This is in line with previous studies demonstrating that irrelevant speech can disrupt fixation durations during reading without an associated disruption in comprehension (Cauchard et al., 2012; Hyönä & Ekholm, 2016; Yan et al., 2018).
The time-course analysis of deviance distraction and the absence of modulation by lexical frequency enabled us to further localise the source of the effect. For example, in the E-Z Reader model of eye-movement control during reading (Reichle, Pollatsek, Fisher, & Rayner, 1998; Reichle, Rayner, & Pollatsek, 2003; Reichle, Warren, & McConnell, 2009), word processing starts with an early visual processing stage during which the visual features of the word are propagated from the retina to the brain. This stage is then followed by two lexical-processing stages: familiarity check (L1) and lexical access (L2). In this model, completion of L1 initiates the programming of the next saccade because the next stage (L2) is likely to be completed soon (Reichle et al., 1998). The programming of the next saccade also happens in two stages: a labile stage (M1) during which the saccade programme can be cancelled and a non-labile stage (M2) during which the saccade programme can no longer be cancelled.
Our data suggest that the deviant sound did not interfere with the lexical-processing stages for a few reasons. First, the CI-DPA analysis indicated that the earliest discernible effect of the deviant sound occurs somewhat late, at 180 ms. This exceeds the temporal estimates of lexical processing reported in the neurophysiological literature (127-172 ms on average; Reichle & Reingold, 2013). Second, Reingold and Sheridan (2014) used the CI-DPA analysis to estimate that the lexical frequency effect (i.e., the difference in fixation times between high- and low-frequency words) starts at 138 ms after fixation onset, that is, some 42 ms earlier than the sound deviance effect. Finally, deviance distraction was not modulated by target-word lexical frequency. Taken together, these findings suggest that deviant sounds did not increase fixation durations on the target words because of delayed lexical processing.
As the deviance effect appears to occur after the lexical-processing stages of reading, we hypothesise that the interference is likely due to saccadic inhibition during the programming of the next saccade. This conclusion is generally consistent with Wessel and Aron’s (2013) proposition of a general action suppression upon the presentation of a deviant sound. In their study, action inhibition took the form of a reduced corticospinal excitability of the hand following transcranial magnetic stimulation (TMS) of the corresponding motor cortex some 150 ms after the deviant sound’s onset. Because the programming and execution of saccades involves subcortical structures, such as the superior colliculus, cerebellum, and the brainstem (Munoz, 2002), our results in fact extend Wessel and Aron’s (2013) by suggesting that deviant sounds may also inhibit subcortical brain areas. Similar evidence for saccadic inhibition was also found in a study where a sequence of auditory distractors (which also included a deviant) was presented in a picture viewing task (Graupner, Velichkovsky, Pannasch, & Marx, 2007). Graupner et al. reported that the deviant distractor reduced the proportion of terminated fixations, first at around 90 ms and then at around 150 ms, a finding they interpreted as “first” and “second” saccadic inhibition. The secondary inhibition is consistent with both the present findings and Wessel and Aron’s (2013) results.
Further evidence in support of this saccadic inhibition account is the finding that the deviant effect was not modulated by the position of the target word in the sentence. Because inhibition of the oculomotor system should be independent of any underlying word identification or syntactic processes, the effect should not vary with respect to where it occurs in the sentence. Even though deviant sounds had no effect on spatial measures of saccades (e.g., saccade length), this is not inconsistent with the saccadic inhibition explanation because the hypothesised inhibition occurs during the planning stages rather than during the execution of the next saccade. This is in line with previous evidence showing that transient visual changes can result in saccadic inhibition some 60 to 70 ms after the onset of the display change (Reingold & Stampe, 1999, 2002, 2004).
This saccadic inhibition explanation is in line with the notion that deviant sounds capture attention away from ongoing processing (e.g., Escera et al., 1998; Schröger, 1996) and inhibit motor processes (Wessel & Aron, 2013). The present experiment contributes to our understanding of the time course of this effect by suggesting that a covert orienting of attention to the deviant sound does not occur during the lexical-processing stages of the fixated word but at a subsequent stage (namely, the preparation of the next saccade). In oddball tasks, the effect of deviant sounds on electrophysiological measures of the orienting response is traceable from about 150 to 600 ms from the sound’s onset (Berti & Schröger, 2003; Escera et al., 1998). So far, the temporal dynamics of the motor inhibition yielded by deviant sounds remain to be established, but early evidence places it at around 150 ms after the sound’s onset. Our experiment does not allow us to disentangle the potential contributions of the orienting response and the motor inhibition. Exploring this issue could be an objective for future research. For example, the precise time course of the orienting response in our task could be studied by using electroencephalogram (EEG) and eye-tracking co-registration (e.g., Dimigen, Sommer, Hohlfeld, Jacobs, & Kliegl, 2011; Plöchl, Ossandón, & König, 2012), capitalising on the well-known ERP signature of the orienting response (i.e., the P3a component; Berti & Schröger, 2001) to study its potential correlation with the observed disruption in eye movements and to better understand its influence on the reading process.
It should be noted that the size of the deviance-distraction effect in the present experiment was relatively small: the numerical effect size was 14.5 ms on average and the standardised effect size was d = 0.22 on average. While the numerical effect size was generally comparable to that of previous studies using the auditory–visual cross-modal task (mean effect size = 17 ms), the standardised effect size was almost three times smaller (mean d = 0.65 in previous oddball experiments; Andrés, Parmentier, & Escera, 2006; Escera et al., 1998; Ljungberg, Parmentier, Leiva, & Vega, 2012; Parmentier, 2016; Parmentier et al., 2008; Parmentier, Elsley, & Ljungberg, 2010; Parmentier, Vasilev & Andrés, 2018; Wetzel, Schröger, & Widmann, 2013). This discrepancy between the numerical and standardised effect size is most likely due to the greater variability in eye-movement responses compared with behavioural reaction time responses. We speculate that our reading task may introduce more variability into the data because, unlike categorisation tasks, it does not require a specific response from participants upon the presentation of task-irrelevant sounds. Nevertheless, it may be possible to increase the magnitude of the effect in future studies. For example, this could be done by using novel sounds that are not repeated throughout the experiment (Berti, 2012; Escera et al., 1998; Wetzel, Schröger, & Widmann, 2016; cf. Wetzel et al., 2013) or by studying children or elderly populations who can show greater distraction (Andrés et al., 2006; Gumenyuk et al., 2001; Gumenyuk, Korzyukov, Alho, Escera, & Näätänen, 2004; Leiva, Andrés, Servera, Verbruggen, & Parmentier, 2016; Leiva et al., 2015a; Wetzel, Scharf, & Widmann, 2018; Wetzel et al., 2016; cf. Leiva et al., 2016).
Traditionally, the majority of research on deviance distraction has used categorisation tasks, such as judging the parity of numbers presented on the screen (e.g., Parmentier et al., 2008) or judging the duration of the irrelevant sound (short vs. long; e.g., Schröger & Wolff, 1998b). Critically, the present experiment differs from such studies in that it did not require any response from participants after the presentation of task-irrelevant sounds. Rather, participants simply needed to do well overall on the task (i.e., comprehend the sentences) and ignore the sounds. In contrast, participants in categorisation tasks typically need to make a binary response after each sound. As a result, the sound may serve as an unspecific warning signal that alerts them to the imminent presentation of a target stimulus that requires a response (Parmentier, 2014). While deviance distraction in oddball tasks occurs even when the sound does not fulfil this unspecific warning function (Parmentier, 2016), the evidence for this again comes from studies that required a response on at least some of the trials (typically on half of them; see Ljungberg et al., 2012; Parmentier et al., 2010; Wetzel et al., 2013). Therefore, the present experiment contributes to our theoretical understanding of deviance distraction by showing that deviant sounds disrupt ongoing processes even when the sounds are not relevant in any way to the main task and do not constitute cues for goal-directed behaviour.
More broadly, the present results are also relevant for understanding how the external auditory environment can influence readers’ eye-movement behaviour. Recent work has shown that certain sounds, such as meaningful speech, can result in attentional distraction that is detectible at the level of eye fixations whereas other sounds, such as background music or unintelligible speech, generally have no influence on eye-movement behaviour (Cauchard et al., 2012; Hyönä & Ekholm, 2016; Johansson et al., 2012; Yan et al., 2018). These results suggest that certain auditory environments can interfere with readers’ ability to maintain sustained attention on the task and process the text in an efficient way. The present experiment builds upon these findings by demonstrating that deviant sounds that violate readers’ expectations can also result in an immediate disruption of eye-movement behaviour. However, unlike continuous distractors such as meaningful speech that typically result in an increase in re-reading behaviour (e.g., Hyönä & Ekholm, 2016; Yan et al., 2018), deviance distraction may occur due to motor inhibition that interferes with the programming of the next saccade.
Another interesting finding in the present experiment was that the standard sound did not lead to significantly longer fixation durations compared with silence. This is in contrast to some evidence showing that the presentation of a short auditory distractor similar to the standard sound used in this study can lead to an increase in fixation durations (e.g., Pannasch, Dornhoefer, Unema, & Velichkovsky, 2001; Pannasch & Velichkovsky, 2009, Experiment 3; but see Reingold & Stampe, 2004, Experiment 1). The lack of difference may have occurred because the standard sound was not long enough for it to cause any meaningful distraction. In addition, the standard sound was presented very frequently and in quick succession, which may have helped participants habituate to it. This is consistent with previous evidence showing that participants can habituate to distracting sounds, such as irrelevant speech (e.g., Banbury & Berry, 1997; Bell, Röer, Dentale, & Buchner, 2012; Morris & Jones, 1990).
One limitation of this study was that it did not experimentally manipulate the psycholinguistic properties of target words, such as their lexical frequency or predictability given the preceding sentence context. Therefore, future studies should include a lexical frequency or a predictability manipulation on the target words to replicate and extend the present results. If the sound deviance effect is due to inhibition during the programming of the next saccade, it should fail to interact with these variables and it should lead to an increase in fixation durations that is independent of the cognitive processing of the word. The present data also do not allow us to determine with certainty the specific component of saccade programming that may be affected. For example, both the E-Z Reader (Reichle et al., 1998) and SWIFT (Engbert, Nuthmann, Richter, & Kliegl, 2005) models assume that saccade programming occurs in a labile (i.e., cancellable) and a non-labile (i.e., non-cancellable) stage. At present, it is not known which of the two stages may be affected. However, if this effect is due to a general motor inhibition (see Wessel & Aron, 2013), it can be speculated that the resulting inhibition should be generally similar regardless of the exact saccade programming stage in which it occurs.
In summary, the present experiment introduced a new method to study deviance distraction during reading by utilising a gaze-contingent presentation of task-irrelevant sounds (e.g., Inhoff et al., 2002). The results showed that deviant sounds lead to an increase in fixation durations, which is most likely due to saccadic inhibition. This finding contributes to our growing understanding of how task-irrelevant sounds influence eye-movement control during reading by showing that unexpected sounds can have an immediate effect on the programming of the next saccade. More broadly, this study also raises the possibility that unexpected sounds may inhibit ongoing motor processes in everyday tasks similar to reading that rely on relatively automatic motor control.
Supplemental Material
Supplemental material, Supplementary_Materials_rev for Distraction by deviant sounds during reading: An eye-movement study by Martin R Vasilev, Fabrice BR Parmentier, Bernhard Angele and Julie A Kirkby in Quarterly Journal of Experimental Psychology
Acknowledgments
Fabrice Parmentier is also an Adjunct Senior Lecturer at the University of Western Australia and a Visiting Professor at Bournemouth University.
Two more participants were tested, but they were excluded due to tracking problems.
Only the models for gaze duration (GD) and total viewing time (TVT) converged with a random slope for items. In all remaining models, the slope for items was removed. In addition, the random intercept for subjects was removed for sentence reading time and saccade length due to convergence failure. When there was a convergence failure, we first tried to remove the random intercept before removing the random slope. If the model still did not converge, the intercept was retained, but the slope was removed. This was done following Barr, Levy, Scheepers, and Tily’s (2013) recommendation that, when the “maximal” model (i.e., a model with both a random slope and a random intercept for sound type) fails to converge, a model with a missing intercept is preferable over a model with a missing slope. This was because models without random slopes led to greater Type 1 error probability in their analyses.
Their data were retained because they were not completely sure of this and they reported that it did not influence how they read the sentences.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: Martin Vasilev was supported by a PhD studentship from Bournemouth University. Fabrice Parmentier was supported by a research grant (PSI2014-54261-P) from the Spanish Ministry of Science, Innovation and Universities, the Spanish State Agency for Research (AEI) and the European Regional Development Fund (FEDER). Fabrice Parmentier’s contract at the University of the Balearic Islands is co-financed by the Spanish Ministry of Economy, Industry and Competitiveness through their programme for the incentivisation and permanent incorporation of doctors (2016 call, IEDI-2016-00742).
Open practices:


The data and materials from the present experiment are publicly available at the Open Science Framework website: https://osf.io/e5r6f/
Supplemental material: The Supplemental material is available at: qjep.sagepub.com
ORCID iD: Martin R Vasilev  https://orcid.org/0000-0003-1944-8828
https://orcid.org/0000-0003-1944-8828
References
- Andrés P., Parmentier F. B. R., Escera C. (2006). The effect of age on involuntary capture of attention by irrelevant sounds: A test of the frontal hypothesis of aging. Neuropsychologia, 44, 2564–2568. doi: 10.1016/j.neuropsychologia.2006.05.005 [DOI] [PubMed] [Google Scholar]
- Baayen R. H., Davidson D. J., Bates D. M. (2008). Mixed-effects modeling with crossed random effects for subjects and items. Journal of Memory and Language, 59, 390–412. doi: 10.1016/j.jml.2007.12.005 [DOI] [Google Scholar]
- Banbury S., Berry D. C. (1997). Habituation and dishabituation to speech and office noise. Journal of Experimental Psychology: Applied, 3, 181–195. doi: 10.1037//1076-898X.3.3.181 [DOI] [Google Scholar]
- Barr D. J., Levy R., Scheepers C., Tily H. J. (2013). Random effects structure for confirmatory hypothesis testing: Keep it maximal. Journal of Memory and Language, 68, 255–278. doi: 10.1016/j.jml.2012.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D. M., Machler M., Bolker B. M., Walker S. C. (2014). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67(1), 1–48. doi: 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Bell R., Röer J. P., Dentale S., Buchner A. (2012). Habituation of the irrelevant sound effect: Evidence for an attentional theory of short-term memory disruption. Journal of Experimental Psychology: Learning Memory and Cognition, 38, 1542–1557. doi: 10.1037/a0028459 [DOI] [PubMed] [Google Scholar]
- Berti S. (2008). Cognitive control after distraction: Event-related brain potentials (ERPs) dissociate between different processes of attentional allocation. Psychophysiology, 45, 608–620. doi: 10.1111/j.1469-8986.2008.00660.x [DOI] [PubMed] [Google Scholar]
- Berti S. (2012). Automatic processing of rare versus novel auditory stimuli reveal different mechanisms of auditory change detection. NeuroReport, 23, 441–446. doi: 10.1097/WNR.0b013e32835308b5 [DOI] [PubMed] [Google Scholar]
- Berti S., Schröger E. (2001). A comparison of auditory and visual distraction effects: Behavioral and event-related indices. Cognitive Brain Research, 10, 265–273. doi: 10.1016/S0926-6410(00)00044-6 [DOI] [PubMed] [Google Scholar]
- Berti S., Schröger E. (2003). Working memory controls involuntary attention switching: Evidence from an auditory distraction paradigm. European Journal of Neuroscience, 17, 1119–1122. doi: 10.1046/j.1460-9568.2003.02527.x [DOI] [PubMed] [Google Scholar]
- Berti S., Vossel G., Gamer M. (2017). The orienting response in healthy aging: Novelty P3 indicates no general decline but reduced efficacy for fast stimulation rates. Frontiers in Psychology, 8, 1780. doi: 10.3389/fpsyg.2017.01780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein M. (2009). Effect sizes for continuous data. In Cooper H. M., Hedges L. V., Valentine J. C. (Eds.), The handbook of research synthesis and meta-analysis (2nd ed., pp. 221–235). New York, NY: Russel Sage Foundation. [Google Scholar]
- Brainard D. H. (1997). The Psychophysics Toolbox. Spatial Vision, 10, 433–436. doi: 10.1163/156856897X00357 [DOI] [PubMed] [Google Scholar]
- Bubic A., von Cramon D. Y., Jacobsen T., Schröger E., Schubotz R. I. (2009). Violation of expectation: Neural correlates reflect bases of prediction. Journal of Cognitive Neuroscience, 21, 155–168. doi: 10.1162/jocn.2009.21013 [DOI] [PubMed] [Google Scholar]
- Cauchard F., Cane J. E., Weger U. W. (2012). Influence of background speech and music in interrupted reading: An eye-tracking study. Applied Cognitive Psychology, 26, 381–390. doi: 10.1002/acp.1837 [DOI] [Google Scholar]
- Cornelissen F. W., Peters E. M., Palmer J. (2002). The Eyelink Toolbox: Eye tracking with MATLAB and the Psychophysics Toolbox. Behavior Research Methods, Instruments, & Computers, 34, 613–617. doi: 10.3758/BF03195489 [DOI] [PubMed] [Google Scholar]
- Czigler I., Cox T. J., Gyimesi K., Horváth J. (2007). Event-related potential study to aversive auditory stimuli. Neuroscience Letters, 420, 251–256. doi: 10.1016/j.neulet.2007.05.007 [DOI] [PubMed] [Google Scholar]
- Dimigen O., Sommer W., Hohlfeld A., Jacobs A. M., Kliegl R. (2011). Coregistration of eye movements and EEG in natural reading: Analyses and review. Journal of Experimental Psychology: General, 140, 552–572. doi: 10.1037/a0023885 [DOI] [PubMed] [Google Scholar]
- Dutra I., Waller D. A., Wessel J. R. (2018). Perceptual surprise improves action stopping by non-selectively suppressing motor activity via a neural mechanism for motor inhibition. Journal of Neuroscience, 38, 1482–1492. doi: 10.1523/JNEUROSCI.3091-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiter B. M., Inhoff A. W. (2010). Visual word recognition during reading is followed by subvocal articulation. Journal of Experimental Psychology: Learning Memory and Cognition, 36, 457–470. doi: 10.1037/a0018278 [DOI] [PubMed] [Google Scholar]
- Engbert R., Nuthmann A., Richter E. M., Kliegl R. (2005). SWIFT: A dynamical model of saccade generation during reading. Psychological Review, 112, 777–813. doi: 10.1037/0033-295X.112.4.777 [DOI] [PubMed] [Google Scholar]
- Escera C., Alho K., Schröger E., Winkler I. (2000). Involuntary attention and distractibility as evaluated with event-related brain potentials. Audiology and Neurotology, 5, 151–166. doi: 10.1159/000013877 [DOI] [PubMed] [Google Scholar]
- Escera C., Alho K., Winkler I., Näätänen R. (1998). Neural mechanisms of involuntary attention to acoustic novelty and change. Journal of Cognitive Neuroscience, 10, 590–604. doi: 10.1162/089892998562997 [DOI] [PubMed] [Google Scholar]
- Escera C., Yago E., Corral M. J., Corbera S., Nuñez M. I. (2003). Attention capture by auditory significant stimuli: Semantic analysis follows attention switching. European Journal of Neuroscience, 18, 2408–2412. doi: 10.1046/j.1460-9568.2003.02937.x [DOI] [PubMed] [Google Scholar]
- Frangos J., Ritter W., Friedman D. (2005). Brain potentials to sexually suggestive whistles show meaning modulates the mismatch negativity. NeuroReport, 16, 1313–1317. doi: 10.1097/01.wnr.0000175619.23807.b7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D., Cycowicz Y. M., Dziobek I. (2003). Cross-form conceptual relations between sounds and words: Effects on the novelty P3. Cognitive Brain Research, 18, 58–64. doi: 10.1016/j.cogbrainres.2003.09.002 [DOI] [PubMed] [Google Scholar]
- Graupner S. T., Velichkovsky B. M., Pannasch S., Marx J. (2007). Surprise, surprise: Two distinct components in the visually evoked distractor effect. Psychophysiology, 44, 251–261. doi: 10.1111/j.1469-8986.2007.00504.x [DOI] [PubMed] [Google Scholar]
- Gumenyuk V., Korzyukov O., Alho K., Escera C., Näätänen R. (2004). Effects of auditory distraction on electrophysiological brain activity and performance in children aged 8–13 years. Psychophysiology, 41, 30–36. doi: 10.1111/1469-8986.00123 [DOI] [PubMed] [Google Scholar]
- Gumenyuk V., Korzyukov O., Alho K., Escera C., Schröger E., Ilmoniemi R. J., Näätänen R. (2001). Brain activity index of distractibility in normal school-age children. Neuroscience Letters, 314, 147–150. doi: 10.1016/S0304-3940(01)02308-4 [DOI] [PubMed] [Google Scholar]
- Horváth J., Roeber U., Bendixen A., Schröger E. (2008). Specific or general? The nature of attention set changes triggered by distracting auditory events. Brain Research, 1229, 193–203. doi: 10.1016/j.brainres.2008.06.096 [DOI] [PubMed] [Google Scholar]
- Hyönä J., Ekholm M. (2016). Background speech effects on sentence processing during reading: An eye movement study. PLoS ONE, 11(3), e0152133. doi: 10.1371/journal.pone.0152133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inhoff A. W., Connine C., Eiter B., Radach R., Heller D. (2004). Phonological representation of words in working memory during sentence reading. Psychonomic Bulletin & Review, 11, 320–325. doi: 10.3758/BF03196577 [DOI] [PubMed] [Google Scholar]
- Inhoff A. W., Connine C., Radach R. (2002). A contingent speech technique in eye movement research on reading. Behavior Research Methods, Instruments, & Computers, 34, 471–480. doi: 10.3758/BF03195476 [DOI] [PubMed] [Google Scholar]
- Johansson R., Holmqvist K., Mossberg F., Lindgren M. (2012). Eye movements and reading comprehension while listening to preferred and non-preferred study music. Psychology of Music, 40, 339–356. doi: 10.1177/0305735610387777 [DOI] [Google Scholar]
- LaBerge D., Samuels S. J. (1974). Toward a theory of automatic information processing in reading. Cognitive Psychology, 6, 293–323. doi: 10.1016/0010-0285(74)90015-2 [DOI] [Google Scholar]
- Leiva A., Andrés P., Parmentier F. B. R. (2015). When aging does not increase distraction: Evidence from pure auditory and visual oddball tasks. Journal of Experimental Psychology: Human Perception and Performance, 41, 1612–1621. doi: 10.1037/xhp0000112 [DOI] [PubMed] [Google Scholar]
- Leiva A., Andrés P., Servera M., Verbruggen F., Parmentier F. B. R. (2016). The role of age, working memory, and response inhibition in deviance distraction: A cross-sectional study. Developmental Psychology, 52, 1381–1393. doi: 10.1037/dev0000163 [DOI] [PubMed] [Google Scholar]
- Leiva A., Parmentier F. B. R., Andrés P. (2015. a). Aging increases distraction by auditory oddballs in visual, but not auditory tasks. Psychological Research, 79, 401–410. doi: 10.1007/s00426-014-0573-5 [DOI] [PubMed] [Google Scholar]
- Leiva A., Parmentier F. B. R., Andrés P. (2015. b). Distraction by deviance: Comparing the effects of auditory and visual deviant stimuli on auditory and visual target processing. Experimental Psychology, 62, 54–65. doi: 10.1027/1618-3169/a000273 [DOI] [PubMed] [Google Scholar]
- Ljungberg J. K., Parmentier F. B. R. (2012). Cross-modal distraction by deviance: Functional similarities between the auditory and tactile modalities. Experimental Psychology, 59, 355–363. doi: 10.1027/1618-3169/a000164 [DOI] [PubMed] [Google Scholar]
- Ljungberg J. K., Parmentier F. B. R., Leiva A., Vega N. (2012). The informational constraints of behavioral distraction by unexpected sounds: The role of event information. Journal of Experimental Psychology: Learning Memory and Cognition, 38, 1461–1468. doi: 10.1037/a0028149 [DOI] [PubMed] [Google Scholar]
- Marsh J. E., Hughes R. W., Jones D. M. (2008). Auditory distraction in semantic memory: A process-based approach. Journal of Memory and Language, 58, 682–700. doi: 10.1016/j.jml.2007.05.002 [DOI] [Google Scholar]
- Marsh J. E., Hughes R. W., Jones D. M. (2009). Interference by process, not content, determines semantic auditory distraction. Cognition, 110, 23–38. doi: 10.1016/j.cognition.2008.08.003 [DOI] [PubMed] [Google Scholar]
- Martin R. C., Wogalter M. S., Forlano J. G. (1988). Reading comprehension in the presence of unattended speech and music. Journal of Memory and Language, 27, 382–398. doi: 10.1016/0749-596X(88)90063-0 [DOI] [Google Scholar]
- MathWorks. (2014). Matlab R2014a [Computer software]. Natick, MA: Author. [Google Scholar]
- Morris N., Jones D. M. (1990). Habituation to irrelevant speech: Effects on a visual short-term memory task. Perception & Psychophysics, 47, 291–297. doi: 10.3758/BF03205003 [DOI] [PubMed] [Google Scholar]
- Munoz D. P. (2002). Saccadic eye movements: Overview of neural circuitry. Progress in Brain Research, 140, 89–96. [DOI] [PubMed] [Google Scholar]
- Näätänen R. (1992). Attention and brain function. Hillsdale, NJ: Lawrence Erlbaum. [Google Scholar]
- Näätänen R., Gaillard A. W. K., Mäntysalo S. (1978). Early selective-attention effect on evoked potential reinterpreted. Acta Psychologica, 42, 313–329. doi: 10.1016/0001-6918(78)90006-9 [DOI] [PubMed] [Google Scholar]
- Näätänen R., Paavilainen P., Rinne T., Alho K. (2007). The mismatch negativity (MMN) in basic research of central auditory processing: A review. Clinical Neurophysiology, 118, 2544–2590. doi: 10.1016/j.clinph.2007.04.026 [DOI] [PubMed] [Google Scholar]
- Pannasch S., Dornhoefer S. M., Unema P. J. A., Velichkovsky B. M. (2001). The omnipresent prolongation of visual fixations: Saccades are inhibited by changes in situation and in subject’s activity. Vision Research, 41, 3345–3351. doi: 10.1016/S0042-6989(01)00207-3 [DOI] [PubMed] [Google Scholar]
- Pannasch S., Velichkovsky B. M. (2009). Distractor effect and saccade amplitudes: Further evidence on different modes of processing in free exploration of visual images. Visual Cognition, 17, 1109–1131. doi: 10.1080/13506280902764422 [DOI] [Google Scholar]
- Parmentier F. B. R. (2008). Towards a cognitive model of distraction by auditory novelty: The role of involuntary attention capture and semantic processing. Cognition, 109, 345–362. doi: 10.1016/j.cognition.2008.09.005 [DOI] [PubMed] [Google Scholar]
- Parmentier F. B. R. (2014). The cognitive determinants of behavioral distraction by deviant auditory stimuli: A review. Psychological Research, 78, 321–338. doi: 10.1007/s00426-013-0534-4 [DOI] [PubMed] [Google Scholar]
- Parmentier F. B. R. (2016). Deviant sounds yield distraction irrespective of the sounds’ informational value. Journal of Experimental Psychology: Human Perception and Performance, 42, 837–846. doi: 10.1037/xhp0000195 [DOI] [PubMed] [Google Scholar]
- Parmentier F. B. R., Elford G., Escera C., Andrés P., Miguel I. S. (2008). The cognitive locus of distraction by acoustic novelty in the cross-modal oddball task. Cognition, 106, 408–432. doi: 10.1016/j.cognition.2007.03.008 [DOI] [PubMed] [Google Scholar]
- Parmentier F. B. R., Elsley J. V., Andrés P., Barceló F. (2011). Why are auditory novels distracting? Contrasting the roles of novelty, violation of expectation and stimulus change. Cognition, 119, 374–380. doi: 10.1016/j.cognition.2011.02.001 [DOI] [PubMed] [Google Scholar]
- Parmentier F. B. R., Elsley J. V., Ljungberg J. K. (2010). Behavioral distraction by auditory novelty is not only about novelty: The role of the distracter’s informational value. Cognition, 115, 504–511. doi: 10.1016/j.cognition.2010.03.002 [DOI] [PubMed] [Google Scholar]
- Parmentier F. B. R., Kefauver M. (2015). The semantic aftermath of distraction by deviant sounds: Crosstalk interference is mediated by the predictability of semantic congruency. Brain Research, 1626, 247–257. doi: 10.1016/j.brainres.2015.01.034 [DOI] [PubMed] [Google Scholar]
- Parmentier F. B. R., Ljungberg J. K., Elsley J. V., Lindkvist M. (2011). A behavioral study of distraction by vibrotactile novelty. Journal of Experimental Psychology: Human Perception and Performance, 37, 1134–1139. doi: 10.1037/a0021931 [DOI] [PubMed] [Google Scholar]
- Parmentier F. B. R., Pacheco-Unguetti A. P., Valero S. (2018). Food words distract the hungry: Evidence of involuntary semantic processing of task-irrelevant but biologically-relevant unexpected auditory words. PLoS ONE, 13(1), e0190644. doi: 10.1371/journal.pone.0190644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmentier F. B. R., Turner J., Elsley J. V. (2011). Distraction by auditory novelty: The course and aftermath of novelty and semantic effects. Experimental Psychology, 58, 92–101. doi: 10.1027/1618-3169/a000072 [DOI] [PubMed] [Google Scholar]
- Parmentier F. B. R., Turner J., Perez L. (2014). A dual contribution to the involuntary semantic processing of unexpected spoken words. Journal of Experimental Psychology: General, 143, 38–45. doi: 10.1037/a0031550 [DOI] [PubMed] [Google Scholar]
- Parmentier F. B. R., Vasilev M. R., Andrés P. (2018). Surprise as an explanation to auditory novelty distraction and post-error slowing. Journal of Experimental Psychology: General. Advance online publication. doi: 10.1037/xge0000497 [DOI] [PubMed] [Google Scholar]
- Pelli D. G. (1997). The VideoToolbox software for visual psychophysics: \forming numbers into movies. Spatial Vision, 10, 437–442. doi: 10.1163/156856897X00366 [DOI] [PubMed] [Google Scholar]
- Plöchl M., Ossandón J. P., König P. (2012). Combining EEG and eye tracking: Identification, characterization, and correction of eye movement artifacts in electroencephalographic data. Frontiers in Human Neuroscience, 6, 278. doi: 10.3389/fnhum.2012.00278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner M. I. (1980). Orienting of attention. The Quarterly Journal of Experimental Psychology, 32, 3–25. doi: 10.1080/00335558008248231 [DOI] [PubMed] [Google Scholar]
- R Core Team. (2016). R: A language and environment for statistical computing. Vienna, Austria. Available from http://www.r-project.org
- Rayner K. (1975). The perceptual span and peripheral cues in reading. Cognitive Psychology, 7, 65–81. doi: 10.1016/0010-0285(75)90005-5 [DOI] [Google Scholar]
- Rayner K. (1998). Eye movements in reading and information processing: 20 years of research. Psychological Bulletin, 124, 372–422. doi: 10.1037/0033-2909.124.3.372 [DOI] [PubMed] [Google Scholar]
- Rayner K. (2009). Eye movements and attention in reading, scene perception, and visual search. The Quarterly Journal of Experimental Psychology, 62, 1457–1506. doi: 10.1080/17470210902816461 [DOI] [PubMed] [Google Scholar]
- Reichle E. D., Pollatsek A., Fisher D. L., Rayner K. (1998). Toward a model of eye movement control in reading. Psychological Review, 105, 125–157. doi: 10.1037/0033-295X.105.1.125 [DOI] [PubMed] [Google Scholar]
- Reichle E. D., Rayner K., Pollatsek A. (2003). The E-Z reader model of eye-movement control in reading: Comparisons to other models. The Behavioral and Brain Sciences, 26, 445–476. doi: 10.1017/S0140525X03430100 [DOI] [PubMed] [Google Scholar]
- Reichle E. D., Reingold E. M. (2013). Neurophysiological constraints on the eye-mind link. Frontiers in Human Neuroscience, 7, 361. doi: 10.3389/fnhum.2013.00361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichle E. D., Warren T., McConnell K. (2009). Using E-Z Reader to model the effects of higher level language processing on eye movements during reading. Psychonomic Bulletin & Review, 16, 1–21. doi: 10.3758/PBR.16.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reingold E. M., Sheridan H. (2014). Estimating the divergence point: A novel distributional analysis procedure for determining the onset of the influence of experimental variables. Frontiers in Psychology, 5, 1432. doi: 10.3389/fpsyg.2014.01432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reingold E. M., Sheridan H. (2018). On using distributional analysis techniques for determining the onset of the influence of experimental variables. The Quarterly Journal of Experimental Psychology, 71, 260–271. doi: 10.1080/17470218.2017.1310262 [DOI] [PubMed] [Google Scholar]
- Reingold E. M., Stampe D. M. (1999). Saccadic inhibition in complex visual tasks. In Becker W., Deubel H., Mergner T. (Eds.), Current oculomotor research: Physiological and psychological aspects (pp. 249–255). Boston, MA: Springer. doi: 10.1007/978-1-4757-3054-8 [DOI] [Google Scholar]
- Reingold E. M., Stampe D. M. (2002). Saccadic inhibition in voluntary and reflexive saccades. Journal of Cognitive Neuroscience, 14, 371–388. doi: 10.1162/089892902317361903 [DOI] [PubMed] [Google Scholar]
- Reingold E. M., Stampe D. M. (2004). Saccadic inhibition in reading. Journal of Experimental Psychology: Human Perception and Performance, 30, 194–211. doi: 10.1037/0096-1523.30.1.194 [DOI] [PubMed] [Google Scholar]
- Roye A., Jacobsen T., Schröger E. (2007). Personal significance is encoded automatically by the human brain: An event-related potential study with ringtones. European Journal of Neuroscience, 26, 784–790. doi: 10.1111/j.1460-9568.2007.05685.x [DOI] [PubMed] [Google Scholar]
- Schröger E. (1996). A neural mechanism for involuntary attention shifts to changes in auditory stimulation. Journal of Cognitive Neuroscience, 8, 527–539. doi: 10.1162/jocn.1996.8.6.527 [DOI] [PubMed] [Google Scholar]
- Schröger E., Giard M. H., Wolff C. (2000). Auditory distraction: Event-related potential and behavioral indices. Clinical Neurophysiology, 111, 1450–1460. doi: 10.1016/S1388-2457(00)00337-0 [DOI] [PubMed] [Google Scholar]
- Schröger E., Wolff C. (1998. a). Attentional orienting and reorienting is indicated by human event- related brain potentials. NeuroReport, 9, 3355–3358. doi: 10.1097/00001756-199810260-00003 [DOI] [PubMed] [Google Scholar]
- Schröger E., Wolff C. (1998. b). Behavioral and electrophysiological effects of task-irrelevant sound change: A new distraction paradigm. Cognitive Brain Research, 7, 71–87. doi: 10.1016/S0926-6410(98)00013-5 [DOI] [PubMed] [Google Scholar]
- Shtyrov Y., Hauk O., Pulvermuller F. (2004). Distributed neuronal networks for encoding category-specific semantic information: The mismatch negativity to action words. European Journal of Neuroscience, 19, 1083–1092. doi: 10.1111/j.1460-9568.2004.03126.x [DOI] [PubMed] [Google Scholar]
- Shtyrov Y., Pulvermuller F. (2003). Distributed neuronal networks for semantic information: Mismatch negativity to action words. Psychophysiology, 19, 1083–1092. doi: 10.1111/j.1460-9568.2004.03126.x [DOI] [PubMed] [Google Scholar]
- Sokolov E. N. (1963). Higher nervous functions: The orienting reflex. Annual Review of Physiology, 25, 545–580. doi: 10.1146/annurev.ph.25.030163.002553 [DOI] [PubMed] [Google Scholar]
- Van Heuven W. J. B., Mandera P., Keuleers E., Brysbaert M. (2014). SUBTLEX-UK: A new and improved word frequency database for British English. The Quarterly Journal of Experimental Psychology, 67, 1176–1190. doi: 10.1080/17470218.2013.850521 [DOI] [PubMed] [Google Scholar]
- Vasilev M. R., Kirkby J. A., Angele B. (2018). Auditory distraction during reading: A Bayesian meta-analysis of a continuing controversy. Perspectives on Psychological Science, 15, 567–597. doi: 10.1177/1745691617747398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel J. R. (2017). Perceptual surprise aides inhibitory motor control. Journal of Experimental Psychology: Human Perception and Performance, 43, 1585–1593. doi: 10.1037/xhp0000452 [DOI] [PubMed] [Google Scholar]
- Wessel J. R., Aron A. R. (2013). Unexpected events induce motor slowing via a brain mechanism for action-stopping with global suppressive effects. Journal of Neuroscience, 33, 18481–18491. doi: 10.1523/JNEUROSCI.3456-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel J. R., Aron A. R. (2017). On the globality of motor suppression: Unexpected events and their influence on behavior and cognition. Neuron, 93, 259–280. doi: 10.1016/j.neuron.2016.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel N., Scharf F., Widmann A. (2018). Can’t ignore—Distraction by task-irrelevant sounds in early and middle childhood. Child Development. Advance online publication. doi: 10.1111/cdev.13109 [DOI] [PubMed] [Google Scholar]
- Wetzel N., Schröger E., Widmann A. (2013). The dissociation between the P3a event-related potential and behavioral distraction. Psychophysiology, 50, 920–930. doi: 10.1111/psyp.12072 [DOI] [PubMed] [Google Scholar]
- Wetzel N., Schröger E., Widmann A. (2016). Distraction by novel and pitch-deviant sounds in children. Frontiers in Psychology, 7, 1949. doi: 10.3389/fpsyg.2016.01949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan G., Meng Z., Liu N., He L., Paterson K. B. (2018). Effects of irrelevant background speech on eye movements during reading. The Quarterly Journal of Experimental Psychology, 71, 1270–1275. doi: 10.1080/17470218.2017.1339718 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Materials_rev for Distraction by deviant sounds during reading: An eye-movement study by Martin R Vasilev, Fabrice BR Parmentier, Bernhard Angele and Julie A Kirkby in Quarterly Journal of Experimental Psychology