Abstract
Aim:
Analyse the effects of professional flash glucose monitoring system (FreeStyle Libre Pro™) on glycaemic control in insulin-treated type 2 diabetes.
Methods:
Primary (n = 17) and secondary care centres (n = 5) randomised 148 type 2 diabetes patients into three groups: (A) self-monitoring of blood glucose (n = 52), (B) self-monitoring of blood glucose and two Libre Pro sensor wears (n = 46) or (C) self-monitoring of blood glucose and four sensor wears (n = 50). Primary endpoint was time in range (glucose 3.9–10 mmol/L) within group C comparing baseline with days 172–187. Predefined secondary endpoints included HbA1c, hypoglycaemia and quality of life measures analysed within and between groups (clinicaltrials.gov, NCT02434315).
Results:
In group C, time in range in the first 14 days (baseline) and days 172–187 was similar at 15.0 ± 5.0 and 14.1 ± 4.7 h/day (mean ± SD), respectively, (p = 0.1589). In contrast, HbA1c reduced from baseline to study end within group C by 4.9 ± 8.8 mmol/mol (0.44% ± 0.81%; p = 0.0003). HbA1c was also lower in group C compared with A at study end by 5.4 ± 1.79 mmol/mol (0.48% ± 0.16%; p = 0.0041, adjusted mean ± SE), without increased time in hypoglycaemia (p = 0.1795). Treatment satisfaction scores improved in group C compared with A (p = 0.0225) and no device-related serious adverse events were reported.
Conclusions:
Libre Pro can improve HbA1c and treatment satisfaction without increasing hypoglycaemic exposure in insulin-treated type 2 diabetes individuals managed in primary/secondary care centres.
Keywords: Continuous glucose monitoring, flash glucose monitoring, HbA1c, hypoglycaemia, hyperglycaemia, glycaemic variability
Introduction
Improving glycaemic control remains a key target to reduce complications in individuals with diabetes.1–3 However, lowering glucose levels can be challenging, with less than 65% of individuals with type 2 diabetes reaching optimal HbA1c targets,4,5 decreasing further to 24% in those taking insulin.4 An important barrier for lowering glucose levels is the precipitation of hypoglycaemia, a potentially life-threatening complication of insulin therapy.1
Continuous glucose monitoring (CGM) is an established method for improving glucose levels and reducing the risk of hypoglycaemia in type 1 diabetes.6 Although the evidence for the benefits of CGM in type 2 diabetes is generally limited,7–12 current guidance endorses the use of retrospective glucose data and intermittent sensor application in the management of insulin-treated type 2 diabetes13,14 However, the ability of primary care clinicians to embrace complex glucose monitoring strategies has been questioned15,16 despite the majority of clinical management for type 2 diabetes occurring in primary rather than secondary care. For professional CGM technology to be viable in primary care, implementation must be straightforward with glucose data reports that can be rapidly generated and interpreted to aide communication with patients and therapy adjustments. Therefore, the aim of this study was to understand the role of new professional sensor technology and retrospective glucose data in improving glycaemic outcomes using real-life settings with the majority of participants recruited from primary care centres.
Methods
We used a blinded professional sensor-based glucose monitoring system to perform retrospective glucose data analysis (FreeStyle Libre Pro™; Abbott Diabetes Care, Witney, Oxon, UK). The sensor, worn on the upper arm, is factory calibrated and requires no participant or healthcare professional intervention during the 14-day wear. The pro-reader is retained by the healthcare professional and data are not visible to participants or investigators during sensor wear. The sensor automatically captures and stores glucose data every 15 min (96 glucose readings/day). At follow-up visits, scanning the sensor will wirelessly transfer glucose data to the pro-reader. Summary glucose reports (including ambulatory glucose profile) for review and analysis by the healthcare professional are generated using the system software.17
Study design and participants
This pilot, prospective, randomised controlled trial was conducted at 18 general primary healthcare centres and five secondary care diabetes centres in England (total centres n = 23). Eligible participants were ⩾18 years of age with type 2 diabetes treated with insulin therapy for at least 6 months and an HbA1c level between 58 and 108 mmol/mol (7.5%–12.0%). Exclusion criteria included use of animal insulin, total daily dose of insulin (TDD) > 1.75 iu/kg on study entry, insulin pump therapy, CGM use in the last 6 months (including professional CGM), steroid therapy for any condition, pregnant or planning pregnancy, allergy to medical grade adhesives or considered unsuitable to participate by the investigator.
Ethics Committee approval was granted prior to the study and all procedures were in accordance with the Helsinki Declaration of 1964, as revised in 2013. Informed, written consent was obtained from all participants in the study. This trial is registered with ClinicalTrials.gov (NCT02434315).
Randomisation and masking
At each centre, participants were randomly assigned to one of three groups for clinical review with readings from self-monitoring of blood glucose (SMBG) as per standard care (group A, control arm), additional use of two Libre Pro sensor wears (group B) or four Libre Pro sensor wears (group C). Randomisation was by permuted block with varying block sizes in a 1:1:1 ratio using randomly generated opaque, sealed envelopes. Participants and investigators were not masked to group allocation.
Procedures
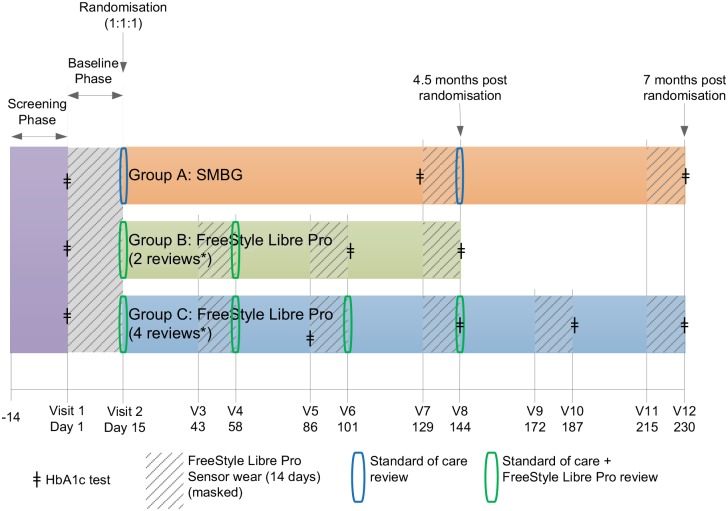
Following consent, screening and enrolment, all participants had a professional flash sensor applied to the upper arm for the 14-day baseline period. Glucose management was supported by their usual regimen of SMBG using their personal device. During each sensor wear, participants were asked to record SMBG results, meals, insulin doses and any physical activity in a diary. Participants with baseline sensor data of ⩾500 sensor glucose readings were randomised to one of the three study groups detailed above (Figure 1). Post randomisation, sensor glucose data were uploaded for intervention participants (group B and group C) and glucose reports (e.g. the ambulatory glucose report) were generated using the system software.17 This information was used by the healthcare professional to support an individualised glucose management plan while participants continued SMBG testing during the treatment phase. Intervention groups B and C had one and three further sensor wear periods, respectively, 1 month apart, followed by review with the healthcare professional (Figure 1).
Figure 1.
Trial design. Following consent, screening and enrolment, all participants had a professional flash sensor applied to the upper arm for the 14-day baseline period. Participants with baseline sensor data of ⩾500 sensor glucose readings were randomised to one of the three study groups as shown.
For group A (control) participants, only SMBG results were used by the healthcare professional to support an individualised glucose management plan, as per standard diabetes care in the United Kingdom. This included continuing to use their personal device for SMBG testing during the treatment phase (Figure 1).
There were no pre-set insulin algorithm or treatment protocols used in the study. HbA1c was measured in all participants using local laboratory testing (Figure 1).
Patient-recorded outcome measures for the Diabetes Treatment Satisfaction Questionnaire (DTSQ)18 were assessed at baseline and at the penultimate visit for each study arm (Figure 1). A user questionnaire (intervention participants) and a healthcare professional user questionnaire (clinicians) were completed when intervention group participants completed their second clinical review for the study (day 58).
Outcomes
The primary outcome was change in sensor derived time in range (glucose 3.9–10.0 mmol/L [70–180 mg/dL]) within intervention group C between the baseline phase and penultimate sensor wear (days 172–187). Pre-specified secondary endpoints included difference in HbA1c and sensor derived glycaemic measurements, TDD, insulin regimen changes, body mass index (BMI)/weight and self-reported SMBG frequency, analysed within and between groups. Glycaemic measurements included number and duration of hypoglycaemic events (<3.9 mmol/L, [70 mg/dL]), number and duration of hyperglycaemic events (>10 mmol/L, [180 mg/dL]; >13.3 mmol/L [240 mg/dL]), mean glucose and glucose variability measurements. An event was defined as at least two consecutive readings at 15-min intervals, outside the predefined sensor glucose range (an event ended with one reading returning within the predefined range).
Pre-specified sub-group analysis of endpoints included the location of diabetes management, type of insulin regimen and glycaemic measures for day- (06.00–23.00) and night-time (23.00–06.00). Safety endpoints incorporated monitoring of all adverse events throughout the study including severe hypoglycaemia19 and sensor insertion or sensor wear–related symptoms. Validated quality of life questionnaires investigated patient satisfaction with the glucose monitoring strategy and health care questionnaire addressed satisfaction of the professional team looking after the patient.
Statistical analysis
Differences within group, including the primary endpoint analysis, were assessed by a paired t-test. Missing values were imputed by last observation carried forward. This included the baseline value if no measurements after baseline were available. Differences between groups were considered by analysis of covariance on the baseline values with site as a covariate. Confidence intervals were calculated for the group least squares mean of each measure and the difference between group least squares means. To detect a change of 1.5 h/day in time in range (TIR; 3.9–10.0 mmol/L) within group C with a power of 80% (at p < 0.05), a total of 40 individuals are needed, based on SD of TIR of 3.5 h/day.20 Assuming a drop out of 20%, we aimed to recruit 48 participants into each study group.
Data analysis was performed by qualified statisticians at Abbott Diabetes Care. Version 9·2 of SAS (or higher) was used for all analyses.
Results
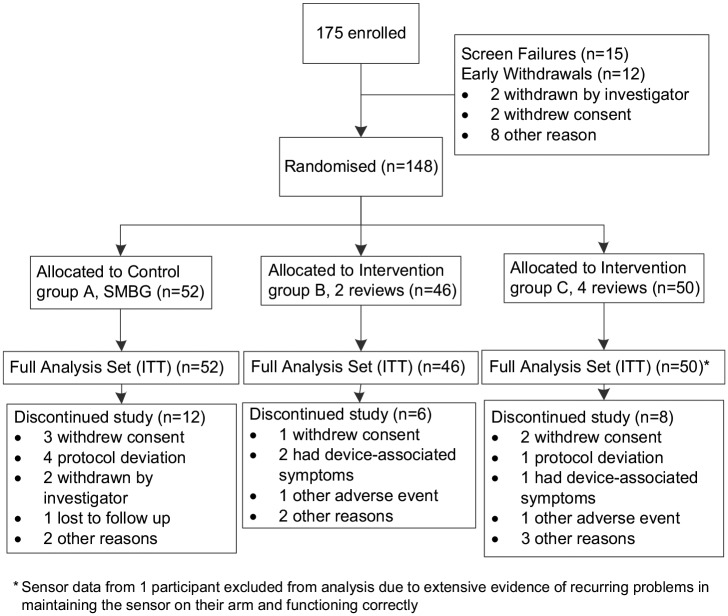
We enrolled 175 participants between 27 April 2015 and 13 October 2015; 148 participants were eligible for randomisation following the baseline phase and were assigned to control group A (n = 52), intervention group B (n = 46) or intervention group C (n = 50); 70.9% (n = 105) of participants were from primary care. Baseline characteristics are shown in Table 1.
Table 1.
Baseline characteristics of participants.
| Control group A (n = 52) |
Intervention group B (n = 46) |
Intervention group C (n = 50) |
All (N = 148) |
|
|---|---|---|---|---|
| Age (years) | 65.0 ± 11.2 | 63.9 ± 10.7 | 61.7 ± 11.1 | 63.6 ± 11.0 |
| Weight (kg) | 92.1 ± 16.7 | 94.5 ± 19.6 (n = 44) |
95.5 ± 18.6 | 94.0 ± 18.2 (n = 146) |
| BMI (kg/m2) | 32.1 ± 5.5 | 32.3 ± 5.8 (n = 44) |
33.2 ± 6.5 | 32.6 ± 5.9 (n = 146) |
| Insulin therapy | ||||
| Basal only | 21 (40.4%) | 24 (52.2%) | 21 (42.0%) | 66 (44.6%) |
| Basal/bolus | 26 (50.0%) | 19 (41.3%) | 25 (50.0%) | 70 (47.3%) |
| Biphasic | 5 (9.6%) | 3 (6.5%) | 4 (8.0%) | 12 (8.1%) |
| Duration of insulin use (years) | 9.5 ± 6.5 | 7.3 ± 4.8 | 8.2 ± 6.3 | 8.4 ± 6.0 |
| Duration of current insulin regimen (years) | 8.0 ± 5.7 | 5.8 ± 4.1 | 6.5 ± 5.0 | 6.8 ± 5.0 |
| Self-reported SMBG frequency per day | 2.3 ± 1.3 | 2.2 ± 1.6 | 2.2 ± 1.5 | 2.3 ± 1.5 |
| Screening HbA1c (%) | 8.7 ± 1.0 | 8.7 ± 1.1 | 8.7 ± 0.8 (n = 49) |
8.7 ± 1.0 (n = 147) |
| Screening HbA1c (mmol/mol) | 71.4 ± 11.3 | 71.5 ± 12.2 | 71.3 ± 9.3 (n = 49) |
71.4 ± 10.9 (n = 147) |
| Male | 28 (53.8%) | 28 (60.9%) | 28 (56.0%) | 84 (56.8%) |
| Female | 24 (46.2%) | 18 (39.1%) | 22 (44.0%) | 64 (43.2%) |
| Employment status | ||||
| Full-time | 16 (30.8%) | 9 (19.6%) | 12 (24.0%) | 37 (25.0%) |
| Part-time | 4 (7.7%) | 3 (6.5%) | 4 (8.0%) | 11 (7.4%) |
| Full-time student | 0 | 0 | 2 (4.0%) | 2 (1.4%) |
| Not employed/retired | 32 (61.5%) | 32 (69.6%) | 32 (64.0%) | 96 (64.9%) |
| Other | 0 | 2 (4.3%) | 0 | 2 (1.4%) |
| Race | ||||
| White | 50 (96.2%) | 44 (95.7%) | 44 (88.0%) | 138 (93.2%) |
| Asian | 1 (1.9%) | 2 (4.3%) | 4 (8.0%) | 7 (4.7%) |
| Mixed | 1 (1.9%) | 0 | 1 (2.0%) | 2 (1.4%) |
| Black | 0 | 0 | 1 (2.0%) | 1 (0.7%) |
Data are n (%) or mean ± SD. There were no statistically significant differences between groups. BMI: body mass index; SMBG: self-monitoring of blood glucose; SD: standard deviation.
The full analysis set included all 148 randomised participants. There were 27 withdrawals before randomisation and 26 participants discontinued the study after randomisation (Figure 2).
Figure 2.
Trial profile demonstrating withdrawal rate.
Time in range
There was no change in TIR (sensor glucose 3.9–10.0 mmol/L [70–180 mg/dL]) within group C comparing baseline (15.0 ± 5.0 h/day [mean ± SD]) with days 172–187 (14.1 ± 4.7, p = 0.1589). No difference was found compared with the control group at study end (days 215–230; Table 2, Figure 3).
Table 2.
Glycaemic and glucose variability measures.
| Control group A (n = 52) Intervention group C (n = 49) Glycaemic measure |
Baseline mean (SD) |
Day 215–230 mean (SD) |
Difference in adjusted means vs control (SE) | p vs control | |||
|---|---|---|---|---|---|---|---|
| Intervention group C | Control group | Intervention group C | Control group | ||||
| Glucose 3·9–10.0 mmol/L (70–180 mg/dL) |
Time, hours | 15.0 (5.0) | 14.5 (4.8) | 13.7 (4.5) | 12.4 (4.8) | 0.96 (0.77) | 0.2193 |
| HbA1c (mmol/mol) | 70.7 (9.8) | 70.8 (11.6) | 65.8 (9.8) | 71.8 (12.8) | −5.4 (1.79) | 0.0041 | |
| HbA1c (%) | 8.6 (0.9) | 8.6 (1.1) | 8.2 (0.9) | 8.7 (1.2) | −0.48 (0.16) | 0.0041 | |
| Glucose <3·9 mmol/L (70 mg/dL) |
Time, hours | 1.66 (1.64) | 1.77 (2.08) | 1.76 (2.35) | 1.33 (1.51) | 0.51 (0.38) | 0.1795 |
| Events | 0.74 (0.61) | 0.75 (0.70) | 0.70 (0.77) | 0.61(0.61) | 0.09 (0.13) | 0.4660 | |
| Glucose >13·3 mmol/L (240 mg/dL) |
Time, hours | 2.74 (3.64) | 2.71 (3.35) | 3.39 (3.69) | 4.60 (4.85) | −1.03 (0.70) | 0.1458 |
| Events | 0.87 (0.77) | 0.92 (0.81) | 1.12 (0.79) | 1.25 (0.75) | −0.12 (0.13) | 0.3640 | |
| Glucose > 10.0 mmol/L (180 mg/dL) |
Time, hours | 7.35 (5.42) | 7.70 (5.18) | 8.55 (5.32) | 10.29 (5.41) | −1.50 (0.89) | 0.0970 |
| Events | 1.89 (0.75) | 1.82 (0.71) | 2.07 (0.82) | 2.11 (0.70) | −0.10 (0.14) | 0.4891 | |
| Mean glucose mmol/L | 8.7 (2.1) | 8.7 (1.9) | 9.1 (2.2) | 9.9 (2.6) | −0.71 (0.41) | 0.0866 | |
| SD glucose mmol/L | 3.0 (0.81) | 3.1 (0.91) | 3.1 (0.87) | 3.4 (0.84) | −0.13 (0.12) | 0.2510 | |
| CV glucose (%) | 34.4 (7.7) | 35.3 (7.7) | 34.8 (6.2) | 35.0 (8.0) | 0.29 (0.92) | 0.7547 | |
| LGI | 1.45 (1.25) | 1.53 (1.57) | 1.39 (1.56) | 1.15 (1.22) | 0.29 (0.27) | 0.2851 | |
| HGI | 7.20 (6.04) | 7.41 (5.52) | 8.42 (6.16) | 10.78 (8.36) | −1.98 (1.25) | 0.1180 | |
| SD of glucose rate of change (mg/dL/min) | 0.73 (0.14) | 0.72 (0.14) | 0.75 (0.15) | 0.78 (0.14) | −0.04 (0.02) | 0.0545 | |
| CONGA | 1 h (mmol/L) | 1.84 (0.40) | 1.80 (0.37) | 1.92 (0.44) | 1.99 (0.38) | −0.10 (0.06) | 0.1076 |
| 2 h (mmol/L) | 2.80 (0.64) | 2.77 (0.57) | 2.95 (0.72) | 3.06 (0.62) | −0.12 (0.10) | 0.2300 | |
| 4 h (mmol/L) | 3.72 (0.95) | 3.73 (0.86) | 3.94 (1.07) | 4.13 (0.90) | −0.16 (0.14) | 0.2754 | |
SD: standard deviation, CV: coefficient of variation, LGI: low glucose index, HGI: high glucose index, CONGA: continuous overall net glycaemic action. Time and events are per day.
Figure 3.
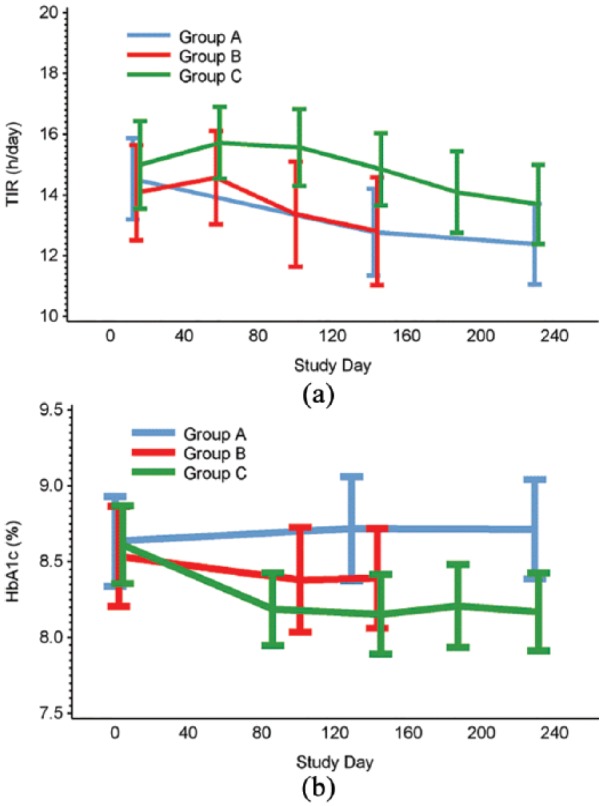
Time in (a) glucose range 3.9–10 mmol/L and (b) HbA1c, during the baseline and treatment phases.
There was no change in TIR in group B compared with the control group at days 129–144 (0.3 ± 0.86 h/day [adjusted mean ± standard error (SE)], p = 0.6891).
HbA1c measurements
HbA1c level reduced in group C (from 70.7 ± 9.8 mmol/L mean ± SD [8.6% ± 0.9%] at baseline to 65.8 ± 9.8 mmol/L [8.2% ± 0.9%] at day 230), while this did not change in the control group (70.8 ± 11.6 mmol/mol [8.6% ± 1.1%] at baseline to 71.8 ± 12.8 [8.7% ± 1.2%]), Table 2, Figure 3). A significant difference was also detected comparing HbA1c in group C with A at study end (adjusted mean ± SE: –5.4 ± 1.8 [–0.48% ± 0.16%], p = 0.0041).
There was no difference in HbA1c in group B compared with the control group (–2.9 ± 1.9 mmol/mol [–0.26% ± 0.17%], p = 0.1331, day 144).
Other glycaemic parameters
At study end (day 215–230), time in hyperglycaemia >180 mg/dL (> 10.0 mmol/mol) was 8.6 ± 5.3 h/day (mean ± SD) in group C and 10.3 ± 5.4 h/day in group A (control group) but this difference of –1.50 ± 0.89 h/day (adjusted mean ± SE) failed to reach statistical significance (p = 0.097, Table 2, Figure 4).
Figure 4.
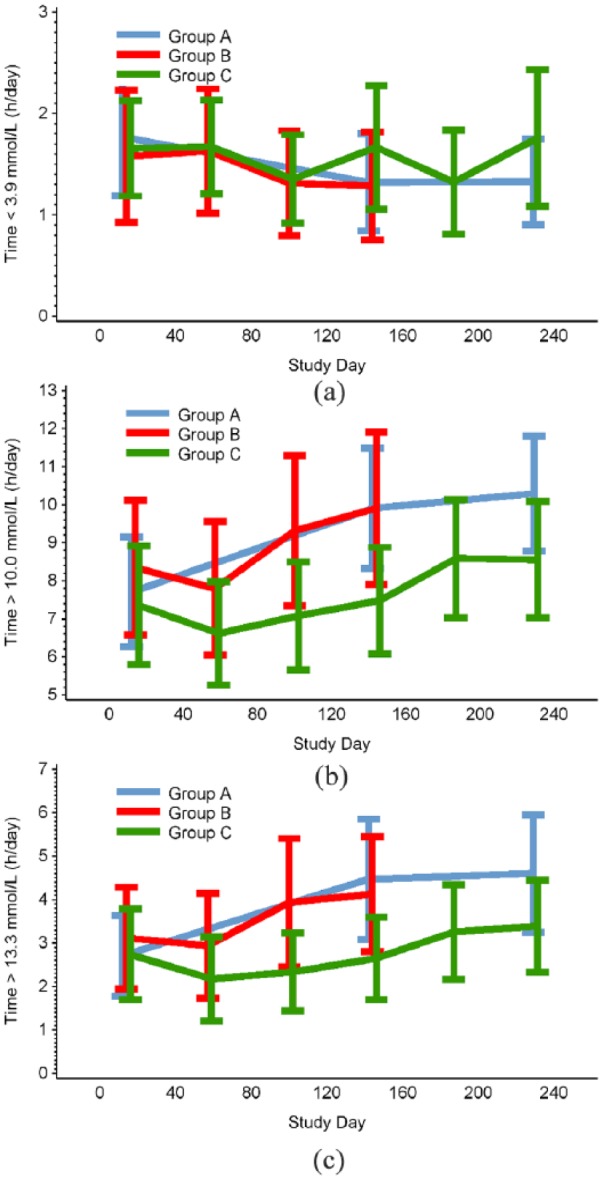
Time in (a) hypoglycaemia <3.9 mmol/L, (b) hyperglycaemia >10 mmol/L and (c) hyperglycemia >13.3 mmol/L, during the baseline and treatment phases.
Hypoglycaemic measures (time, number of episodes and AUC) <3.9 mmol/L (70 mg/dL) were unaffected in group C compared with group A (p = 0.1795, p = 0.4660 and p = 0.1098, respectively; Table 2, Figure 4).
A trend towards lower mean glucose in group C compared with group A was shown (p = 0.0866). The difference in SD of glucose rate of change for group C compared to group A was nearing significance (p = 0.0545), whereas other measures of glycaemic variability were unchanged (Table 2).
There was no observed change in time in hyperglycaemia (>180 mg/dL [>10.0 mmol/mol], >13.3 mmol/L [240 mg/dL]; p = 0.6404, p = 0.3038, respectively), hypoglycaemia (<3.9 mmol/L [70 mg/dL], p = 0.8530) and mean glucose (p = 0.3350) for group B compared with the control group at days 129–144.
In group C, 48% of participants who improved their time in hypoglycaemia by ⩾30% also improved their HbA1c by ⩾0.5%, compared with 17% in the control group (p = 0.0327).
Pre-specified sub-group analysis showed no difference in TIR within group C or when compared to control participants (group A), for participants managed within primary care (105/148) or in secondary care (43/148).
Role of insulin regimen
Pre-specified sub-group analysis of HbA1c level by insulin regimen demonstrated significant improvement at day 230 for group C participants using multiple daily injection (MDI) or biphasic insulin therapy (n = 29) of –0.67% ± 0.19% (adjusted mean ± SE, p = 0.0010) compared with group A (n = 31). For participants using basal insulin only, change in HbA1c (0.41% ± 0.39%) was not significant (p = 0.3011).
There was no change in the TDD for intervention participants compared with the control group. Five participants changed from a basal only insulin regimen to include bolus or biphasic insulin, two participants from the control group, two from intervention group B and one from intervention group C.
Group C participants showed no difference in change in weight at day 230 compared with controls (0.22 ± 0.95 kg [adjusted mean ± SE]; p = 0.8215).
Treatment satisfaction
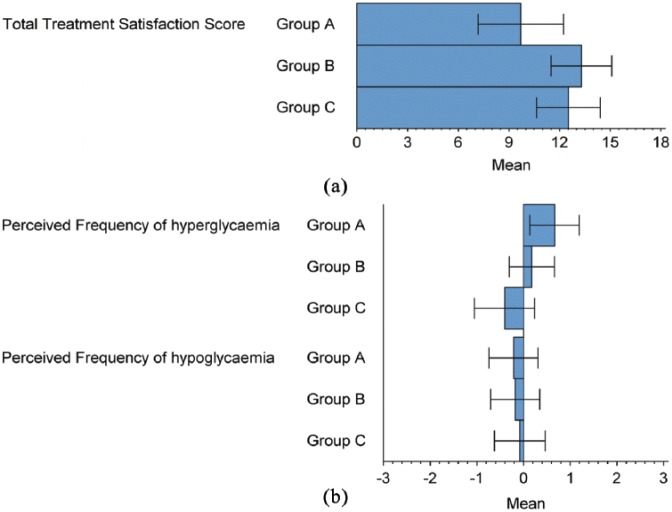
DTSQ scores showed increased overall treatment satisfaction for intervention group B and C participants compared to control (3.45 ± 1.54 [adjusted mean ± SE], p = 0.0277 and 3.54 ± 1.52, p = 0.0225, respectively, Figure 5).
Figure 5.
Scores from the DTSQ Questionnaire for (a) total treatment satisfaction score and (b) perceived frequency of hyperglycaemia and hypoglycaemia.
User (Table 3) and healthcare professional questionnaire (Supplemental Tables S1–S3) responses to using the device and the glucose reports were equally positive. Intervention participants (n = 85) reviewing glucose reports with their healthcare professional agreed the reports were easy to understand (94%) and by using them the healthcare professional was able to explain how current therapies worked (99%), identify abnormalities in glucose levels (96%) and the rationale for treatment changes (98%). As a consequence, understanding of self-management needs improved in all participants (100%), thereby aiding learning about glucose control (95%) and supporting self-management (93%). Both primary and secondary care healthcare professionals (n = 47) agreed the visual nature of the glucose reports supported effective communication with the patient (96%); were easy to read (91%) and understand (85%) to quickly determine abnormal glucose levels (>80%) and what therapy changes were needed (>77%).
Table 3.
User questionnaire responses.
| Indicate your level of agreement with the following statements: | Strongly agree | Agree | Neither agree or disagree | Disagree | Strongly disagree | No response provided |
|---|---|---|---|---|---|---|
| The sensor was comfortable to wear | 60 (70.6%) | 21 (24.7%) | 1 (1.2%) | 1 (1.2%) | 2 (2.4%) | |
| The sensor was easy to wear due to its small size | 58 (68.2%) | 20 (23.5%) | 5 (5.9%) | 1 (1.2%) | 1 (1.2%) | |
| I believe that other people did not notice that I was wearing the sensor | 48 (56.5%) | 18 (21.2%) | 13 (15.3%) | 4 (4.7%) | 2 (2.4%) | |
| While wearing the sensor, I did not feel any discomfort under my skin | 61 (71.8%) | 17 (20.0%) | 4 (4.7%) | 2 (2.4%) | 1 (1.2%) | |
| The sensor did not get in the way of my daily activities | 59 (69.4%) | 20 (23.5%) | 3 (3.5%) | 2 (2.4%) | 1 (1.2%) | |
| The reports were easy to understand | 42 (49.4%) | 38 (44.7%) | 3 (3.5%) | 1 (1.2%) | 1 (1.2%) | |
| My doctor/nurse was able to use the reports effectively to explain how my current diabetes treatment plan is working | 63 (74.1%) | 21 (24.7%) | 1 (1.2%) | |||
| My doctor/nurse was able to use the reports effectively to indicate areas where my glucose management could be improved | 59 (69.4%) | 23 (27.1%) | 2 (2.4%) | 1 (1.2%) | ||
| My doctor/nurse was able to use the reports effectively to explain why he or she suggested changes to my glucose management | 59 (69.4%) | 24 (28.2%) | 2 (2.4%) | |||
| My doctor/nurse used the reports to personalise my diabetes treatment plan for me | 55 (64.7%) | 24 (28.2%) | 5 (5.9%) | 1 (1.2%) | ||
| I think that it would be useful to repeat sensor wear at intervals to review my progress in managing my diabetes | 66 (77.6%) | 19 (22.4%) | ||||
| Seeing my complete glucose profile helps me to understand my glucose levels | 66 (77.6%) | 19 (22.4%) | ||||
| Seeing my complete glucose profile helps me to manage my diabetes better | 59 (69.4%) | 20 (23.5%) | 6 (7.1%) | |||
| I learned something about my diabetes by reviewing the reports with my doctor/nurse | 60 (70.6%) | 21 (24.7%) | 3 (3.5%) | 1 (1.2%) | ||
| I would recommend this system to others | 67 (78.8%) | 18 (21.2%) |
Adverse events
A total of 248 adverse events were experienced by 102 participants (Table 4). This included 26 serious adverse events for 22 participants; none of which were device-related.
Table 4.
Adverse events.
| Control group A (n = 52) |
Intervention group B (n = 46) |
Intervention group C (n = 50) |
Not randomised (n = 27) | Total (N = 175) | |
|---|---|---|---|---|---|
| Participants with adverse or serious adverse events | 29 (55.8%) | 30 (65.2%) | 41 (82.0%) | 2 (7.4%) | 102 (58.3%) |
| Number of adverse or serious adverse events | 61 | 64 | 121 | 2 | 248 |
| Participants with serious adverse events | 8 (15.4%) | 7 (15.2%) | 7 (14.0%) | 0 | 22 (12.6%) |
| Number of serious adverse events | 9 | 9 | 8 | 0 | 26 |
| Participants with hypoglycaemia serious adverse eventsa | 1 (1.9%) | 0 | 0 | 0 | 1 (0.6%) |
| Number of hypoglycaemia serious adverse events | 1 | 0 | 0 | 0 | 1 |
| Participants with hypoglycaemia adverse eventsa (excluding serious) | 0 | 1 (2.2%) | 3 (6.0%) | 0 | 4 (2.3%) |
| Number of hypoglycaemia adverse events (excluding serious) | 0 | 1 | 3 | 0 | 4 |
| Participants with device-related adverse eventsb | 0 | 0 | 2 (4.0%) | 0 | 2 (1.1%) |
| Number of device-related adverse eventsb | 0 | 0 | 2 | 0 | 2 |
| Participants with study procedure-related adverse eventsb | 0 | 0 | 1 (2.0%) | 0 | 1 (0.6%) |
| Number of study procedure-related adverse eventsb | 0 | 0 | 1 | 0 | 1 |
Table includes the full analysis set. No serious adverse events were related to the study device or procedure.
No hypoglycaemic events were related to the device.
Device and/or study procedure adverse events were reported as ‘possibly related’ to sensor wear: one patient with superficial thrombophlebitis (mild) and one with redness (mild).
One control group participant experienced one severe hypoglycaemic event.18 In addition, four intervention participants reported four adverse events related to symptomatic hypoglycaemia. No mild or moderate hypoglycaemic events were related to the study device or procedure.
Two mild adverse events from two intervention participants were possibly related to wearing the sensor (superficial thrombophlebitis, resolved without treatment; redness, partially resolved with non-prescription creams). These all resolved.
There were no diabetic ketoacidosis or hyperosmolar hyperglycaemic state events reported during the study.
Sensor insertion/wear symptoms
Sensor insertion-site signs and symptoms (n = 42) experienced by 29 participants (36 mild and 6 moderate) were primarily associated with sensor wear; erythema (16), itching (10) and rash (4). Those related to sensor insertion were bleeding (5), bruising (4), pain (2), oedema (1) and induration (0). All resolved quickly, primarily without topical treatment (Supplemental Table S4).
Three intervention participants withdrew due to repeated mild and moderate sensor wear–related symptoms of rash (n = 1), itching (n = 1) and erythema (n = 1)
Discussion
To our knowledge, this is the first randomised controlled trial investigating the role of Libre Pro in modulating glycaemia in insulin-treated type 2 diabetes managed in both primary and secondary care settings.11,21–23
Although the primary endpoint of change in TIR was not achieved, the study showed a significant reduction in HbA1c in the intervention arm associated with improved quality of life measures. The reduction in HbA1c was evident within and between group analyses, suggesting this is not a spurious finding. As sensor glycaemic readings, patterns and trends could not be seen by any participant in the present study and given they were aware their glucose data were monitored, the likely explanation for the discrepancy between TIR and HbA1c is behaviour modification of study participants while wearing the sensor during the baseline phase. Thus, the decreasing trend in TIR over the study duration for participants randomised to the control arm was likely due to these participants returning to their usual lifestyle/behaviours after the baseline phase was completed and not having a sensor glucose data review included in subsequent clinic visits. The extent of the fall in TIR (i.e. worsening glycaemic control) for the control group was unexpected and more than previously observed in a similar population further supporting the notion of a ‘study effect’.9
The blinded nature of the study, possibly giving participants less incentive to continue, may have been a factor in the participant drop-out rate of 18%. Although this was slightly higher than observed in studies using unblinded sensors in type 2 diabetes,9 it was accounted for in our power calculations (which were based on 20% drop-out rate).
It is documented that lifestyle modification can markedly influence TIR regardless of monitoring technology.10 As an isolated clinical marker, TIR has the advantage of reflecting intra and inter-day glycaemic changes24 but may be misleading when individuals modify their lifestyle over a short period of time.25 Therefore, having HbA1c as an additional glycaemic marker gives invaluable data as to whether failing to improve TIR is indicative of a lack of effect for the intervention.
Type 2 diabetes disease progression is marked by escalating therapies and deteriorating glycaemic control26 which may not respond to intensification of insulin regimens.15 Studies using professional continuous monitoring in insulin-treated type 2 diabetes have generally failed to report improvement in HbA1c.7,27,28 The clinically significant reduction in HbA1c documented in this study, without increasing hypoglycaemic exposure, is a key finding. Moreover, this was achieved in patients treated in both secondary and primary care settings, supporting generalizability of the results. In particular, the large proportion of participants from primary care sites, who had improvement in HbA1c, supports the use of modern glucose monitoring strategies outside secondary care settings.
There was no change in TDD, however, smaller insulin dose changes may have occurred without any noticeable overall change in total insulin.9 As sensor glucose data were not visible in real-time, the fall in time in hyperglycaemia is likely a direct result of reviewing and discussing retrospective sensor data with the healthcare professional.8 The intrinsic value and advantage of clinical dialogue between patient and clinician supported by ‘pictorial’ examples of glycaemic issues and improvements shown in the glucose reports is the least measurable factor. The benefit of this was evident by improved treatment satisfaction supporting the intermittent use of real-time CGM. In contrast, Beck et al.10 found no improvement in treatment satisfaction in a similar population using real-time-CGM over 6 months. The difference between the two studies may be related to easier application of flash technology compared with CGM or may simply be due to differences in study design and population investigated.
To date, the number of days of professional or intermittent glucose sensor wear for studies in insulin-treated type 2 has been varied and is usually less than seven days.7,11,27 Our findings add to the body of evidence for CGM use in type 2 diabetes and recommendations that 14 days of CGM data is effective at providing enough glucose data to enable review of glucose control to target lowering of HbA1c.29–31
With regard to safety, the few events relating to sensor insertion or wear were typical of medical adhesive use and similar to those observed in other cohorts.10,32
This was a pilot study and it has a number of strengths that should be highlighted. First, the choice of study population adds to the limited published evidence on the use of CGM in type 2 diabetes. Second, this is the first piece of work to include patients from primary and secondary care settings, therefore, study findings are not limited to a particular group of health care professionals. Third, ‘real-world’ glucose management was used regardless of location of care and there was no mandated treatment protocol. This lack of more directed glucose management may have also been a limitation as glycaemic benefits might have been observed earlier and with fewer sensor wears had an insulin titration algorithm been in place. It is possible that the observed changes in HbA1c for group C may have been due to additional clinical contact and dialogue alone and the changes observed were not related to Libre Pro use. However, this is unlikely to be the case given that these patients were recruited because of poor glycaemic control with previous clinical intervention failing to improve glucose levels.
Other limitations include the apparent strong study effect observed in the control group which is a concern, although it is unclear how best to tackle this in future professional CGM studies. Moreover, non-blinding of either participants or clinicians can be regarded as a drawback and is a recognised issue with device studies. Finally, the choice of TIR as the primary endpoint, may not have been ideal given the study effect and HbA1c is possibly a better overall glycaemic marker as it is less affected by short-term behavioural modifications.
Conclusion
To summarise, use of professional flash technology in insulin-treated type 2 diabetes managed within primary and secondary care sites was associated with a significant reduction in HbA1c level and improved satisfaction with treatment. TIR and time in hypoglycaemia were unaffected. These data suggest that this novel professional flash sensor-based glucose monitoring technology has the ability to introduce clinically meaningful changes in HbA1c with a favourable safety profile when used in primary and secondary care settings.
Supplemental Material
Supplemental material, Ajjan_et_al_supp_appendix_5_July_2018 for Reduction in HbA1c using professional flash glucose monitoring in insulin-treated type 2 diabetes patients managed in primary and secondary care settings: A pilot, multicentre, randomised controlled trial by Ramzi A Ajjan, Neil Jackson and Scott A Thomson in Diabetes & Vascular Disease Research
Acknowledgments
The Authors thank all the participants in this study and the many individuals involved in the collection of data at the study sites (Supplementary material p. 7). The Authors thank Katie Cranfield and Zoe Welsh (Abbott Diabetes Care) for statistical support and Amanda Cartmale (Abbott Diabetes Care) for assistance with manuscript preparation. Abbott Diabetes Care designed the study protocol in collaboration with the principal investigator and provided all study materials. Abbott Diabetes Care was involved in data collection, analysis and reporting but was not involved in the Authors’ interpretation of the results. All Authors were investigators for the study and collected data. The Corresponding Author had full access to all the study data and, together with the other Authors, had final responsibility for the decision to submit for publication.
Footnotes
Data availability: The datasets during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: RA Ajjan has received honoraria and research support from Abbott Diabetes Care. N Jackson reports grants from Abbott Diabetes Care during the conduct of the study. SA Thomson reports personal fees from Abbott Diabetes Care during the conduct of the study, personal fees from Sanofi and personal fees from the Clinical Research Practice Datalink (CPRD) outside the submitted work.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Supplemental material: Supplemental material for this article is available online.
References
- 1. American Diabetes Association. Standards of medical care in diabetes – 2017. Diabetes Care 2017; 40: S1–S135.27979885 [Google Scholar]
- 2. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015; 38: 140–149. [DOI] [PubMed] [Google Scholar]
- 3. National Institute for Health and Care Excellence (NICE). Type 2 diabetes in adults: management NICE guideline [NG28], https://www.nice.org.uk/guidance/ng28 (accessed July 2017).
- 4. Casagrande SS, Fradkin JE, Saydah SH, et al. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010. Diabetes Care 2013; 36: 2271–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. National Diabetes Audit, 2015–2016: report 1: care processes and treatment targets, http://www.content.digital.nhs.uk/catalogue/PUB23241/nati-diab-rep1-audi-2015-16.pdf (accessed 26 July 2017).
- 6. Petrie JR, Peters AL, Bergenstal RM, et al. Improving the clinical value and utility of CGM systems: issues and recommendations. A joint statement of the European Association for the Study of Diabetes and the American Diabetes Association Diabetes Technology Working Group. Diabetes Care 2017; 40: 1614–1621. [DOI] [PubMed] [Google Scholar]
- 7. Cosson E, Hamo-Tchatchouang E, Dufaitre-Patouraux L, et al. Multicentre randomised, controlled study of the impact of continuous sub-cutaneous glucose monitoring (Glucoday) on glycaemic control in type 1 and type 2 diabetes patients. Diabetes Metab 2009; 35: 312–318. [DOI] [PubMed] [Google Scholar]
- 8. Vigersky RA, Fonda SJ, Chellappa M, et al. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care 2012; 35: 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haak T, Hanaire H, Ajjan RA, et al. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther 2017; 8: 55–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beck RW, Riddlesworth TD, Ruedy K, et al. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections. A randomized trial. Ann Intern Med 2017; 167: 365–374. [DOI] [PubMed] [Google Scholar]
- 11. Leinung M, Nardacci E, Patel N, et al. Benefits of short-term professional continuous glucose monitoring in clinical practice. Diabetes Technol Ther 2013; 15: 744–747. [DOI] [PubMed] [Google Scholar]
- 12. Sato J, Kanazawa A, Ikeda F, et al. Effect of treatment guidance using retrospective continuous glucose monitoring system on glycaemic control in outpatients with type 2 diabetes mellitus: a randomized controlled trial. J Int Med Res 2016; 44: 109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fonseca VA, Grunberger G, Anhalt H, et al. Continuous glucose monitoring: a consensus conference of the American Association of Clinical Endocrinologists and American College of Endocrinology. Endocr Pract 2016; 22: 1008–1021. [DOI] [PubMed] [Google Scholar]
- 14. Peters AL, Ahmann AJ, Battelino T, et al. Diabetes technology – continuous subcutaneous insulin infusion therapy and continuous glucose monitoring in adults: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2016; 101: 3922–3937. [DOI] [PubMed] [Google Scholar]
- 15. Bhide M, Grey JMS, Moser EG, et al. A primary care perspective on use of continuous glucose monitoring in clinical practice. Diabetes Technol Ther 2013; 15: 533–537. [DOI] [PubMed] [Google Scholar]
- 16. Dale J, Martin S, Gadsby R. Insulin initiation in primary care for patients with type 2 diabetes: 3-year follow-up study. Prim Care Diabetes 2010; 4: 85–89. [DOI] [PubMed] [Google Scholar]
- 17. FreeStyle Libre Professional software, https://www.myfreestyle.com/provider/freestyle-libre-pro-resources (accessed 17 January 2018).
- 18. Bradley C. Diabetes treatment satisfaction questionnaire. In: Bradley C. (ed.) Handbook of psychology and diabetes. Chur: Harwood Academic Publishers, 1994, pp. 111–132. [Google Scholar]
- 19. Workgroup on Hypoglycemia and American Diabetes Association. Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycaemia. Diabetes Care 2005; 28: 1245–1249. [DOI] [PubMed] [Google Scholar]
- 20. Ajjan RA, Abougila K, Bellary S, et al. Sensor and software use for the glycaemic management of insulin-treated type 1 and type 2 diabetes patients. Diab Vasc Dis Res 2016; 13: 211–219. [DOI] [PubMed] [Google Scholar]
- 21. Mohan V, Jain S, Kesavadev J, et al. Use of retrospective continuous glucose monitoring for optimizing management of type 2 diabetes in India. J Assoc Physicians India 2016; 64: 16–21. [PubMed] [Google Scholar]
- 22. Distiller LA, Cranston I, Mazze R. First clinical experience with retrospective flash glucose monitoring (FGM) analysis in South Africa: characterizing glycemic control with ambulatory glucose profile. J Diabetes Sci Technol 2016; 10: 1294–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hulse A, Rai S, Prasanna Kumar KM. Evaluation of accuracy of ambulatory glucose profile in an outpatient setting in children with type 1 diabetes. Indian J Endocrinol Metab 2016; 20: 643–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Danne T, Nimri R, Battelino T, et al. International consensus on the use of continuous glucose monitoring. Diabetes Care 2017; 40: 1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ahn D, Pettus J, Edelman S. Unblinded CGM should replace blinded CGM in the clinical management of diabetes. J Diabetes Sci Technol 2016; 10: 793–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care 2009; 32: S151–S156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Murphy HR, Rayman G, Lewis K, et al. Effectiveness of continuous glucose monitoring in pregnant women. BMJ 2008; 337: a1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Furler J, O’Neal D, Speight J, et al. Supporting insulin initiation in type 2 diabetes in primary care: results of the Stepping Up pragmatic cluster randomised controlled clinical trial. BMJ 2017; 356: j783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bergenstal RM, Danne T, Heller SR, et al. Reaching an international consensus on standardizing continuous glucose monitoring (CGM) outcomes – aligning clinicians, researchers, patients and regulators. In: Presented at the American Diabetes Association 77th scientific sessions, San Diego, CA, 9–18 June 2017. [Google Scholar]
- 30. Evans M, Cranston I, Bailey CJ. Ambulatory glucose profile (AGP): utility in UK clinical practice. Br J Diabetes 2017; 17: 26–33. [Google Scholar]
- 31. Rodbard D. Continuous glucose monitoring: a review of recent studies demonstrating improved glycemic outcomes. Diabetes Technol Ther 2017; 19: S25–S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bolinder J, Antuna R, Geelhoed P, et al. Using novel flash glucose-sensing technology reduces hypoglycemia in individuals with type 1 diabetes. Lancet 2016; 388: 2254–2263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Ajjan_et_al_supp_appendix_5_July_2018 for Reduction in HbA1c using professional flash glucose monitoring in insulin-treated type 2 diabetes patients managed in primary and secondary care settings: A pilot, multicentre, randomised controlled trial by Ramzi A Ajjan, Neil Jackson and Scott A Thomson in Diabetes & Vascular Disease Research