Significance
Our understanding of the bacterial transcription machinery is largely based on studies in Escherichia coli. However, the human pathogen Mycobacterium tuberculosis (Mtb) and numerous other bacteria require an additional RNA polymerase binding protein called CarD, which is absent from E. coli, to form stable transcription initiation complexes. On the basis of CarD’s ability to stabilize transcription initiation complexes, it was predicted to function as a transcription activator. Here, we find that CarD serves as a global transcription regulator in Mtb that can either activate or repress gene expression, depending on the intrinsic properties of a given promoter. These studies elucidate paradigms of transcription regulation in the many bacteria phyla that encode homologs of CarD.
Keywords: tuberculosis, transcription, RNA polymerase
Abstract
The ability to regulate gene expression through transcription initiation underlies the adaptability and survival of all bacteria. Recent work has revealed that the transcription machinery in many bacteria diverges from the paradigm that has been established in Escherichia coli. Mycobacterium tuberculosis (Mtb) encodes the RNA polymerase (RNAP)-binding protein CarD, which is absent in E. coli but is required to form stable RNAP-promoter open complexes (RPo) and is essential for viability in Mtb. The stabilization of RPo by CarD has been proposed to result in activation of gene expression; however, CarD has only been examined on limited promoters that do not represent the typical promoter structure in Mtb. In this study, we investigate the outcome of CarD activity on gene expression from Mtb promoters genome-wide by performing RNA sequencing on a panel of mutants that differentially affect CarD’s ability to stabilize RPo. In all CarD mutants, the majority of Mtb protein encoding transcripts were differentially expressed, demonstrating that CarD had a global effect on gene expression. Contrary to the expected role of CarD as a transcriptional activator, mutation of CarD led to both up- and down-regulation of gene expression, suggesting that CarD can also act as a transcriptional repressor. Furthermore, we present evidence that stabilization of RPo by CarD could lead to transcriptional repression by inhibiting promoter escape, and the outcome of CarD activity is dependent on the intrinsic kinetic properties of a given promoter region. Collectively, our data support CarD’s genome-wide role of regulating diverse transcription outcomes.
Bacterial pathogens must coordinate diverse transcriptional responses to survive the multitude of host-derived stresses that they encounter during infection. All bacteria encode a single, multisubunit RNA polymerase (RNAP) core enzyme that consists of the β, β′, α2, and ω subunits. Transcription initiation requires the binding of a dissociable σ factor to form the RNAP holoenzyme, which recognizes and binds promoter sequences upstream of the +1 transcription start site (TSS). Initially, the holoenzyme binds to closed, double-stranded DNA to form the RNAP-promoter closed complex (RPc). The RPc then undergoes a number of reversible conformational changes to form the RNAP-promoter open complex (RPo), in which the promoter DNA is melted into a transcription bubble and the +1 TSS is positioned in the RNAP active site and accessible to the initiating nucleotide. To complete transcription initiation and form a full-length RNA product, the RNAP must break interactions with DNA and escape the promoter. Our understanding of bacterial transcription initiation is largely formed by studies in Escherichia coli, but recent studies of these processes in other bacteria suggest that some paradigms do not hold true in all species. For example, in vitro kinetics analyses show that RNAP from Bacillus subtilis, Thermus, and Mycobacterium species form unstable RPo compared with E. coli RNAP (1–4). Furthermore, mycobacterial genomes possess divergent promoter architecture from E. coli promoters and are not well expressed in E. coli (5, 6). Thus, to obtain a holistic understanding of gene expression in bacteria, transcription initiation events should be studied in their native contexts.
Mycobacterium tuberculosis (Mtb) infection currently results in more deaths each year than any other single infectious agent (7). To develop effective therapeutics for Mtb infection and combat this epidemic, there is a need to better understand how Mtb regulates its gene expression to facilitate its survival in the host. Mycobacteria encode an essential RNAP-associated transcription factor, CarD, that is widely distributed across many eubacteria phyla, but not conserved in E. coli (8, 9). In mycobacteria, carD expression is induced by genotoxic, oxidative, starvation, and antibiotic stresses (8), suggesting that CarD may serve as a stress-responsive transcriptional regulator in addition to its essential function. CarD homologs in other bacteria have also been shown to respond to diverse environmental stimuli (10, 11) and regulate critical processes such as metabolic homeostasis and cell division (12, 13).
Chromatin immunoprecipitation-sequencing experiments in mycobacteria revealed that CarD localizes with RNAP holoenzyme at promoters throughout the mycobacterial genome, but not within gene coding sequences (CDS), indicating that CarD specifically functions during transcription initiation (14, 15). Depletion or mutation of CarD has been shown to lead to changes in ribosomal RNA (rRNA) levels (8, 16, 17), and therefore, initial studies of CarD have focused on rRNA promoters. CarD stabilizes RPo formed by mycobacterial RNAP-σA holoenzyme at the rRNA rrnAP3 promoter (3, 4, 15–17). CarD consists of 2 functional domains that are both required for stabilization of RNAP-σA-rrnAP3 RPo (17). The N terminus of CarD comprises an RNAP-interacting domain (RID) that interacts directly with the β1-lobe of the RNAP-β subunit (8, 16). CarD also possesses a C-terminal DNA-binding domain (DBD) containing a patch of basic residues that is predicted to interact with promoter DNA near the upstream edge of the transcription bubble (15, 17, 18). In addition, a conserved tryptophan (W85) within the C-terminal basic patch is also important for CarD’s effects on RNAP-σA-rrnAP3 RPo and, based on structural studies, has been proposed to wedge into the upstream edge of the transcription bubble to prevent bubble collapse (17, 18). Mtb strains encoding mutations in the CarD RID or DBD are attenuated in a mouse aerosol infection model and exhibit increased sensitivity to killing by oxidative stress and the antibiotics rifampin, streptomycin, and ciprofloxacin (16, 17, 19).
Investigations of CarD’s activity thus far have been limited to a handful of promoters with architectures similar to promoters in E. coli, including rrnAP3. Therefore, the in vivo effect of CarD on mycobacterial gene expression genome-wide remains unknown. To gain a comprehensive view of how CarD regulates transcription globally at all Mtb promoters, we performed RNA-sequencing (RNA-seq) on strains of Mtb expressing mutants of CarD that either disrupt or enhance CarD activity. Our model of CarD activity based on studies of rrnAP3 predicts that CarD activates transcription by stabilizing RPo. If this model held true for all Mtb promoters that CarD regulates, then we would expect that impairing CarD’s activity would lead to global down-regulation of gene expression. On the contrary, we found that CarD mutants show an equal number of down-regulated transcripts and up-regulated transcripts, suggesting that CarD is capable of repressing transcription from certain Mtb promoters, and that its role is more complex than that of a monotonic transcriptional activator. Our comparison of gene expression patterns shows that CarD mutants with similar effects on RPo stability possess similar transcriptional profiles, indicating that the stabilization of RPo by CarD is responsible for gene expression outcomes, which can be diverse and depend on the promoter. Our studies broaden our understanding of how CarD regulates transcription of numerous promoters in vivo to coordinate a gene expression profile that promotes bacterial viability.
Results
Point Mutations in CarD Result in Global Changes in Gene Expression in Mycobacteria.
The effect of CarD activity on global gene expression in Mtb has yet to be investigated, and previous microarray experiments performed in the nonpathogenic model organism Mycobacterium smegmatis during CarD depletion were confounded by cell death (8). Therefore, to measure the effects of CarD activity on expression profiles in Mtb, we performed RNA-seq with RNA collected from Mtb strains that express wild-type (WT) CarD (CarDWT), CarDR47E [a RID mutant with weakened affinity for RNAP (16)], CarDI27F or CarDI27W [RID mutants with increased affinity to RNAP (19)], or CarDK125A [mutation within the DBD predicted to weaken the affinity to DNA (17)] as the only carD allele (Fig. 1A). In vitro, mutations that weaken the CarD-RNAP or CarD-DNA interaction impair the ability of CarD to stabilize RNAP-σA-rrnAP3 RPo (4, 17), whereas mutations that increase the apparent affinity of CarD to RNAP potentiate its RPo-stabilizing activity at lower concentrations of CarD (4, 19). For our RNA-seq analyses, RNA was collected from cultures growing exponentially in unstressed conditions where all of the Mtb strains are viable.
Fig. 1.
Mtb strains encoding point mutants of CarD show genome-wide differences in gene expression. (A) Diagram showing the protein domain structure of CarD, which consists of a N-terminal RID (amino acids 1–66) and a C-terminal DBD (amino acids 67–162). The table lists the 4 CarD point mutations that were used in this study and their effects on CarD function. (B) Pie charts display the proportion of 4,016 Mtb protein encoding genes with nonzero reads that were significantly (Padj < 0.05) up-regulated (fold-change > 0), down-regulated (fold-change < 0), or not significantly differentially expressed (N.S., Padj ≥ 0.05) in each CarD mutant relative to their expression in CarDWT. The bars under FC > 2 show the distribution of up- and down-regulated genes for the subset of Mtb genes that were significantly differentially expressed greater than 2-fold in each mutant genotype. These data are enumerated in the table below. Differential expression testing was performed using RNA-seq data from 3 replicates of each genotype.
In all 4 Mtb strains with mutations in carD, more than half of the genome was significantly (Padj < 0.05) differentially expressed in comparison with the CarDWT strain (Fig. 1B), consistent with CarD’s global presence at mycobacterial promoters (15). Notably, in all 4 CarD mutants, there were a similar number of genes that were up-regulated as there were down-regulated (Fig. 1B). When we focused on the genes that were differentially expressed greater than 2-fold (Dataset S1), we found that in CarDK125A, there were twice as many genes that were up-regulated (n = 106) compared with down-regulated (n = 58), whereas in CarDI27W, the opposite trend was true, where there were twice as many genes down-regulated (n = 79) as there were up-regulated (n = 37). In CarDR47E and CarDI27F, the number of genes up-regulated more than 2-fold was similar to the number of genes down-regulated more than 2-fold. When we categorized differentially expressed genes based on Tuberculist functional annotations (20), they fell into diverse functional categories (Dataset S1), indicating that CarD activity affects multiple cellular processes.
Given that the ability of CarD to interact with the RNAP and DNA is required to stabilize the RNAP-σA-rrnAP3 RPo in vitro (4, 17), one may expect that the RID and DBD work in tandem to stabilize RPo and activate transcription at all promoters that CarD regulates. In this model, the R47E and K125A point mutations would cause a general decrease in transcript production, whereas the I27F and I27W point mutations that enhance the ability of CarD to stabilize RPo may cause a general increase in transcript production. However, this model is not supported by our RNA-seq data, which shows that all 4 mutants exhibit similar numbers of up-regulated and down-regulated transcripts (Fig. 1B). These data could mean that CarD is capable of promoting both activation and repression of gene expression in vivo. However, another possible explanation for the RNA-seq data is that mutation of CarD caused a global up- or down-regulation of transcript production that we were unable to detect because of the submission of equal amounts of RNA from each sample for the RNA-seq reactions. To explore this possibility, we performed spike-in control experiments (21) in which we isolated RNA from log-phase cultures of Mtb expressing CarDR47E, CarDK125A, and CarDWT and added 1 ng MS2 bacteriophage RNA (Roche) per 1 billion cells to the RNA samples to serve as a proxy for cell number. cDNA was generated for each sample, and quantitative reverse transcription PCR was performed to determine the transcript level per cell of 8 Mtb genes that were either up- or down-regulated in the CarDR47E and CarDK125A RNA-seq samples. The quantitative reverse transcription-PCR results largely recapitulated our RNA-seq results (SI Appendix, Fig. S1), supporting that transcripts can be up- or down-regulated in the CarD mutants on a per cell basis. The fold-change differences detected by quantitative reverse transcription PCR had greater variance than those detected by RNA-seq, which may reflect the additional experimental variability introduced when estimating cell number and adding spike-in RNA transcript. The presence of up-regulated genes in the CarD mutant strains provides experimental evidence that CarD may repress gene expression at specific promoters in the Mtb genome.
CarD Delays Promoter Escape and Could Lead to Repression of Gene Expression Depending on the Kinetics of Transcription Initiation.
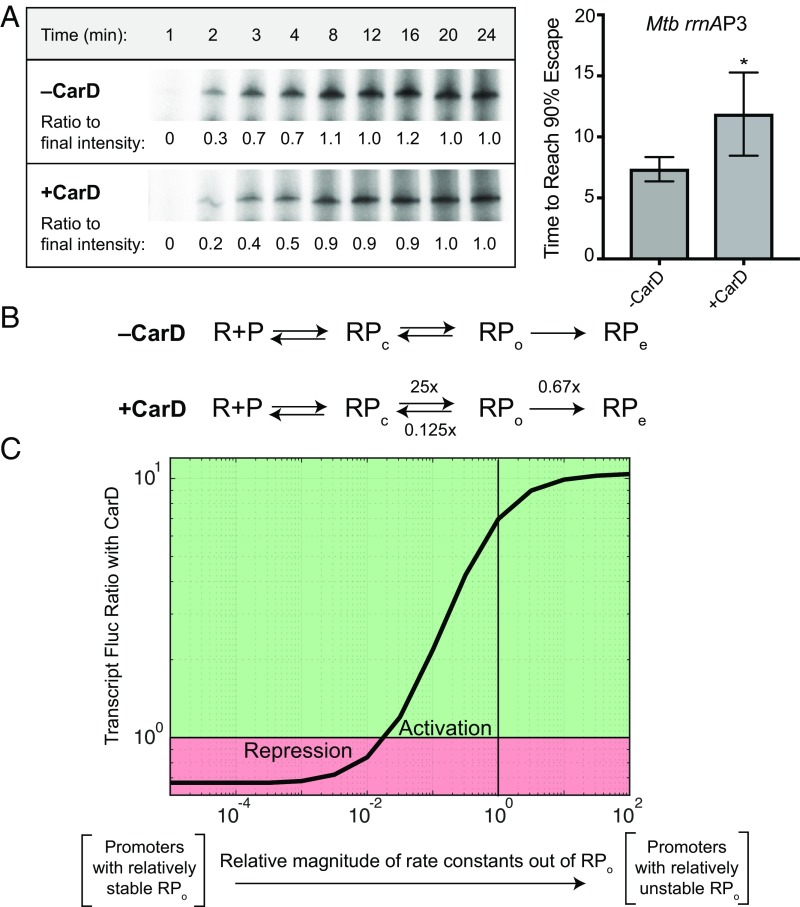
The ability of a single factor that affects RPo stability to lead to both activation and repression of gene expression is not unprecedented. For example, ppGpp and the proteobacterial transcription factor DksA have been shown to activate transcription from amino acid synthesis genes and repress transcription from genes encoding stable RNAs in E. coli by altering the transition rates between RPc and RPo (22–24). Transcript flux modeling suggests that CarD could lead to repression of transcript production via its ability to stabilize RPo if there were a resulting decrease in promoter escape (25), which is predicted to occur, based on the known correlation between RPo stability and promoter escape rate (26). To determine whether CarD can lead to a delay in promoter escape, we monitored the rate of formation of full-length transcript by Mycobacterium bovis RNAP-σA holoenzyme from the Mtb rrnAP3 promoter, using single round in vitro transcription assays. In these assays, we added double-stranded DNA competitor and NTPs at the same time to preformed RNAP-σA-rrnAP3 complexes in the presence or absence of CarD. The NTPs initiate transcription from the RNAP-σA-rrnAP3 complexes, whereas the competitor prevents formation of new complexes, thereby allowing for only one round of transcription to occur. To estimate the rate of promoter escape, the time required for 90% of the final amount of transcript to be formed was calculated (Fig. 2A). In these assays, CarDWT significantly delayed promoter escape from the rrnAP3 promoter from 8.52 to 13.07 min (Fig. 2A), demonstrating that CarD activity leads to both an increase in RPo stability and a decrease in the rate of promoter escape.
Fig. 2.
CarD slows promoter escape from the Mtb rrnAP3 promoter. (A) Single-round in vitro promoter escape assay results with representative gel images showing the time-dependent increase in 32P-labeled RNA transcripts formed by M. bovis RNAP from the Mtb rrnAP3 promoter construct in the presence and absence of CarD. Promoter escape rate is quantified by the time until 90% of the final transcript intensity is reached (t90%). The graph shows the mean t90% ± SEM (−CarD n = 3; +CarD n = 7). Statistical significance was analyzed by Welch’s t test. *P < 0.05. (B) A proposed kinetic model in which CarD accelerates the rate of transcription bubble formation (RPc→RPo) 25-fold, slows the rate of bubble collapse (RPo→RPc) 8-fold, and slows the rate of promoter escape (RPo→RPe) 1.5-fold. Effects of CarD on rates were chosen based on experimentally determined values. (C) Graph showing the mRNA flux ratio from a given Mtb promoter on the addition of CarD. The X-axis represents a titration of the rate constants out of RPo relative to the kinetic model for rrnAP3. Calculations were performed with the kinetic model of CarD activity on a set of hypothetical promoters with a titration of RPo stability, using the web-based tool described in ref. 25. The green region represents promoters that would be activated by CarD; the red region represents promoters that would be repressed.
In the context of this model, whether transcription is activated or repressed by CarD depends on the basal kinetics of transcription initiation from that promoter. Using an expression for the steady-state flux of transcript production (25), the experimentally measured rates of RPo formation (4), and the rate of promoter escape determined earlier, we calculated the ratio of RNA production in the presence and absence of CarD across sets of promoters in which RPo stability varied over 4 orders of magnitude. More specifically, the rate constants leading out of RPo (kclose and kescape) were systematically titrated together such that low rates represent relatively stable RPo and high rates represent relatively unstable RPo. In the presence of CarD, the rate constant of promoter opening (kopen) was increased 25-fold, and the rate of bubble collapse (kclose) was decreased 8-fold, based on our previously published kinetic model (4). Furthermore, the rate of escape (kescape) was decreased 1.5-fold based on the ratio of escape rates determined experimentally from our gel-based assay (Fig. 2 A and B).
The results of our calculations show that the regulatory outcome of CarD does in fact depend on the basal energy landscape (i.e., set of rate constants) of a promoter (Fig. 2C). More specifically, the model predicts that promoters with relatively unstable RPo (i.e., rapid rates of bubble collapse and escape) will be activated by CarD whereas promoters with relatively stable RPo (i.e., slow rates of bubble collapse and escape) will be repressed (Fig. 2C). On the basis of these analyses, we conclude that CarD has the potential to directly activate or repress transcript production, as observed in the RNA-seq data.
Gene Expression Patterns in CarD Mutants Recapitulate Changes in CarD’s RPo Stabilizing Activity.
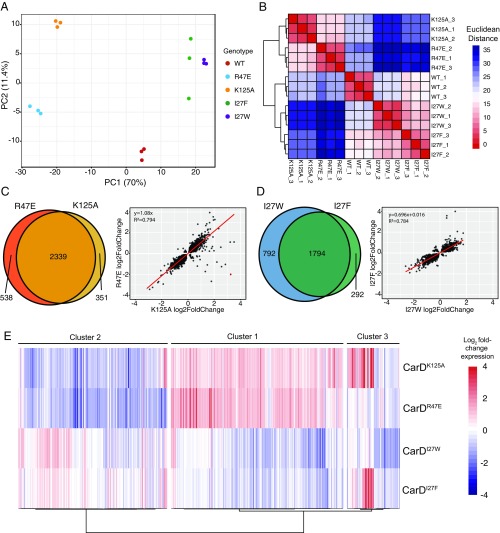
Our flux-based modeling predicts that the transcriptional outcome of a CarD-regulated promoter depends on both the intrinsic RPo stability and the RPo stabilization afforded by CarD. Thus, for a given promoter, any mutation that impairs CarD’s RPo stabilizing activity should yield the same effect on transcript production. To test this prediction, we compared the changes in expression profiles in each of the CarD mutants. Principal component analysis of the Mtb RNA-seq samples was performed and provided a general overview of how gene expression patterns among the CarD mutants clustered in relationship to each other. Sample replicates grouped tightly based on genotype, demonstrating interreplicate consistency in our RNA-seq data (Fig. 3A). To further examine the gene expression relationships between samples, we performed sample-based hierarchical clustering based on Euclidean distance of variance stabilizing transformation transformed counts of all transcripts and found that the samples divided roughly into 3 groups (Fig. 3B). The first group included the CarDWT replicates, the second group included the CarDR47E and CarDK125A replicates, and the third group included the replicates from the CarDI27F and CarDI27W strains.
Fig. 3.
CarD mutants with similar effects on RPo stability show similar gene expression profiles. (A) A principal component analysis of RNA sequencing samples based on read counts generated by mapping reads to a library of protein encoding sequences from the Mtb H37Rv genome. Each point represents one sequencing sample colored by genotype, and the distance between 2 points reflects the variance in gene expression between the 2 samples. The first 2 principle components, PC1 and PC2, define the x- and y-axes, respectively, and account for 70% and 11.4% of the variance, respectively. (B) Hierarchical clustering of RNAseq samples based on read count data in which each column and row represents one sample. The color of each cell represents the Euclidean distance, calculated according to relative expression of all Mtb protein encoding genes, between each sample pair. (C and D) Venn diagrams and scatter plots show the overlap in the lists of Mtb genes that were significantly differentially expressed (Padj < 0.05) between (C) CarDR47E and CarDK125A, and (D) CarDI27F and CarDI27W. Scatter plots compare gene expression changes for each pair of mutants. Each point represents an Mtb gene that was significantly differentially expressed in one or both of the mutant strains being compared. The position of the dot along the axes represents the log2 fold change relative to CarDWT, with one mutant on the x-axis and the other on the y-axis. Red lines represent a linear regression line calculated for each pair. (E) Unsupervised hierarchical clustering (Pearson distance, Ward’s method linkage) of patterns of gene expression changes in a subset of 432 Mtb genes that were significantly differentially expressed greater than 2-fold in at least one CarD mutant Mtb strain relative to CarDWT. Each column represents a different gene in the set, and each row represents a different CarD mutant strain. The coloring of each cell shows the log2 fold change in expression for each gene in a given CarD mutant strain relative to CarDWT.
Mutants from the same expression profile group showed differential expression of a similar subset of genes (hypergeometric test of overenrichment, P < 0.05; Fig. 3 C and D). Furthermore, pairwise linear regression of expression fold-changes show that the direction and magnitude of gene expression changes within these groups showed a strong, positive correlation (P < 0.05; Fig. 3 C and D). These data demonstrate that genes that are up- or down-regulated in CarDR47E are likely to be similarly up- or down-regulated in CarDK125A, and the same is true for CarDI27F and CarDI27W. These similarities in transcriptional profiles are consistent with data showing that CarDR47E is phenotypically similar to CarDK125A (16, 17), and CarDI27F is phenotypically similar to CarDI27W (19). In addition, the magnitudes of expression changes in CarDI27W were larger than those of CarDI27F (Fig. 3D), consistent with previously published data showing that the I27W mutation results in a larger increase in affinity to RNAP-β than the I27F mutation (19). Thus, mutants with the same effect on CarD’s RPo-stabilizing activity group with each other based on gene expression patterns, suggesting that the gene expression changes observed in the mutant bacteria are a consequence of altered RPo stability.
CarD mutants belonging to different expression profile groups also showed a significant overlap in the subsets of genes that were differentially expressed (hypergeometric test of overenrichment, P < 0.05; SI Appendix, Fig. S2), despite not clustering together based on gene expression patterns (Fig. 3B). When we performed pairwise linear regressions, we did not observe a strong correlation, positive nor negative, when comparing the direction of gene expression changes of the genes significantly differentially expressed between the CarDR47E/CarDK125A and the CarDI27F/CarDI27W mutants (SI Appendix, Fig. S2). These data demonstrate that mutations that impair (R47E/K125A) or enhance (I27F/I27W) CarD activity cause differential expression of a similar subset of Mtb genes, but the direction and magnitude of expression changes depend on how the specific mutation affects CarD’s RPo stabilizing activity.
Although pairwise comparisons revealed information about the gene expression profile relationships between the different CarD mutants, we sought to understand the patterns of expression of individual genes across the 4 CarD mutant strains. We chose to focus on genes that were most strongly affected by CarD mutation and examined a subset of 432 Mtb genes that were significantly differentially expressed 2-fold or greater in at least one mutant genotype. We performed gene-based hierarchical clustering of these 432 genes, based on Pearson correlation of their log2 fold-change in expression compared with the CarDWT strain across the 4 CarD mutants, and found that the genes separated into 3 clusters with 2 predominant expression trends (Fig. 3E and Dataset S2). Cluster 1 contained the largest number of genes (199/432), 127 of which were up-regulated in CarDR47E and CarDK125A and down-regulated in CarDI27F and CarDI27W. Cluster 2 was the second largest (172/432), where most genes were down-regulated in CarDR47E and CarDK125A. 65 of the 172 genes in cluster 2 were also up-regulated in CarDI27F and CarDI27W. Almost all (198/199) of the genes in cluster 1 showed higher expression in CarDR47E and CarDK125A compared with CarDI27F and CarDI27W, whereas the opposite trend is true for almost all (171/172) of the genes in cluster 2. Therefore, clusters 1 and 2 reveal a prominent trend in which mutants with impaired CarD activity (CarDR47E and CarDK125A) and mutants with enhanced CarD activity (CarDI27F and CarDI27W) show opposite changes in gene expression. Thus, many of the observed expression changes are likely the outcome of relatively stronger or weaker RPo stabilizing activity by CarD.
The pairwise comparisons and the gene expression patterns in clusters 1 and 2 from the hierarchical clustering analysis demonstrate that the CarDR47E and CarDK125A mutants result in very similar transcriptional profiles. This indicates that despite facilitating distinct macromolecular interactions, CarD’s RID and DBD usually work in concert to potentiate a single transcriptional outcome. Genes in cluster 3, which was the smallest of the 3 clusters (62/432), represent an exception to this relationship. Cluster 3 included a subset of 28 genes that were more than 2-fold up-regulated in CarDK125A but were either unchanged or down-regulated in CarDR47E. This gene expression pattern is unusual, given the positive correlation generally observed between CarDR47E and CarDK125A (Fig. 3C), and suggests that CarD’s RID and DBD may have independent effects on transcription at a smaller subset of promoters. Nonetheless, our data indicate that in the majority of cases, the effect of a CarD mutant on gene expression outcome correlates with the effect of that mutation on CarD-mediated stabilization of RPo, as predicted in the flux-based modeling.
Gene Expression Changes in CarD Mutants Correlate with DNA Sequences in the −10 Element and Discriminator Region of Promoters.
Our flux-based modeling predicts that the outcome of CarD activity on gene expression from different promoters depends on the intrinsic RPo stability of the promoter. Because DNA sequences within a promoter recognition region (PRR) can influence the kinetics of transcription complex formation (27–29), we hypothesized that different sequence elements within the PRR would correlate with the outcome of CarD activity. In E. coli, the −10 and −35 regions, the extended −10 element, UP elements upstream of the −35 region, and the discriminator region between the −10 and TSS can affect RPo stability (2, 30). Mycobacterial promoters contain highly variable sequences in the −35 region (5), and UP elements have not been reported. However, consensus “TANNNT” −10 sequence motifs and extended −10 sequences are prevalent in mycobacterial promoters (31) and have been shown to influence RPo stability (32, 33).
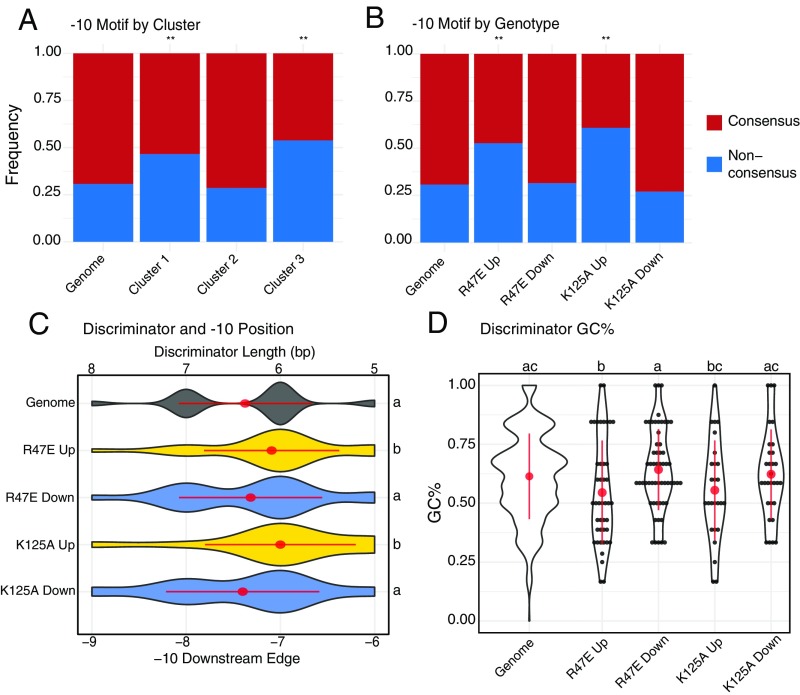
To investigate whether promoter sequences correlated with the outcome of CarD activity, we used a published dataset of Mtb primary TSSs (31) and scanned a 50-bp range upstream of the TSS for −10 element motifs. To avoid counting a promoter multiple times, we only considered genes that are directly downstream of a primary TSS. We assigned the Mtb promoters into 1 of 4 classes: extended consensus −10 “GNTANNNT,” nonextended consensus −10 “TANNNT,” extended nonconsensus −10 “GNVANNNT,” or “No Motif” if none of these motifs were detected within the −10 region (Dataset S3). Across 1,778 primary TSS, 593 (33.3%) contained “GNTANNNT,” 638 (35.9%) contained “TANNNT,” 277 (15.6%) contained “GNVANNNT,” and 270 (15.2%) contained “No Motif” (SI Appendix, Fig. S3 and Table S1), similar to what was previously reported (31). To determine whether there was a relationship between −10 promoter sequence and gene expression profiles in the CarD mutants, we compared the proportions of −10 classes between the differentially expressed gene clusters described in Fig. 3E. We observed that clusters 1 and 3 were significantly enriched (hypergeometric test, P < 0.05) for promoters lacking a consensus −10 motif (“GNVANNT” and “No Motif”; Fig. 4A). Most of the genes in cluster 1 were up-regulated in CarDR47E and CarDK125A, and most of the genes in cluster 3 were up-regulated in CarDK125A (Fig. 3E), suggesting that the direction of gene expression changes in these mutants may also correlate with the −10 motif sequence. We examined the proportions of −10 classes for subsets of genes that were more than 2-fold significantly up- or down-regulated in CarDR47E or CarDK!25A (SI Appendix, Fig. S3B). Our promoter analysis revealed that genes up-regulated in CarDR47E or CarDK125A were significantly enriched for nonconsensus promoters lacking a “TANNNT” hexamer in their −10 region (Fig. 4B; hypergeometric test, P < 0.05). In contrast, the proportion of promoter motifs for genes down-regulated in CarDR47E or CarDK125A were similar to the genome as a whole. When we scanned the promoter regions for −35 elements, we were unable to identify consensus motifs, similar to other studies that have concluded that −35 elements in mycobacteria are highly variable (5, 34).
Fig. 4.
Genes that are up-regulated in RPo-destabilizing CarD mutant Mtb strains are associated with promoters lacking a consensus “TANNNT” −10 element motif and containing shorter and less GC-rich discriminator. Mtb genes directly downstream of a primary TSS identified in Cortes et al. (31) were classified on the basis of the presence of “TANNNT” DNA sequence motif in their promoter −10 element. (A and B) The relative proportions of these promoter classes are shown for the entire Mtb genome (“Genome”), (A) in gene clusters defined in Fig. 3E, and (B) in subsets of genes that were significantly up- or down-regulated in CarDR47E or CarDK125A. Nonconsensus promoters were significantly overrepresented (hypergeometric test; *P < 0.05; **P < 0.005; n.s. = not significant) in genes up-regulated in CarDR47E and CarDK125A. (C) Violin plots showing the distribution of discriminator lengths for Mtb promoter subsets. Promoters without an identifiable −10 element were not included. (D) Violin and dot plots showing distribution of discriminator GC% for Mtb promoter subsets. Red dots and lines represent mean discriminator length or GC% ± SD. Statistically significant differences in discriminator length and GC% were detected using a Kruskal-Wallis rank sum test followed by post hoc pairwise Dunn’s tests in which groups with different letters (a, b, or c) are significantly different from each other (unadjusted P < 0.05). Genome, n = 1,778; R47E Up, n = 55; R47E Down, n = 73; K125A Up, n = 46; K125A Down, n = 37; cluster 1, n = 88; cluster 2, n= 91; cluster 3, n = 26.
The GC content and length of the discriminator sequence between the TSS and the downstream edge of the −10 element has also been shown to influence transcription initiation kinetics (30). We examined the discriminator length and GC content for promoters significantly differentially expressed more than 2-fold in CarDR47E or CarDK125A (SI Appendix, Table S2 and Dataset S3). We omitted promoters lacking an identifiable −10 element (“No Motif”) because the discriminator could not be accurately positioned for these promoters. We found that genes up-regulated in CarDR47E and CarDK125A contained promoters with significantly shorter discriminator regions (Kruskal-Wallis P < 0.05, followed by pairwise rank sum test) than promoters across the genome or promoters down-regulated in those mutants (Fig. 4C). In addition, the promoters of CarDR47E and CarDK125A up-regulated genes had a lower GC% in their discriminator regions (Fig. 4D).
Notably, a separate study by Shell et al. (35) mapped TSS in Mtb and identified numerous unique TSS that were not found by Cortes et al. (31). Although both datasets identified more than 4,000 TSS, only 43.5% (2166/4798) TSS identified by Shell et al. were also mapped by Cortes et al. (SI Appendix, Fig. S4). Therefore, we also performed our sequence analyses using the Shell et al. dataset, and observed similar trends (SI Appendix, Fig. S5 and Table S3). Collectively, our data suggest that genes that are up-regulated when CarD activity is impaired are enriched for promoters that lack a consensus −10 “TANNNT” hexamer and contain shorter, less GC-rich discriminator regions. These data support our model that DNA sequences in the promoter that influence intrinsic RPo stability may also influence the transcriptional outcome of CarD activity.
Discussion
Previous mechanistic studies of CarD’s function on mycobacterial promoters have been mostly limited to the Mtb rrnAP3 promoter. However, the rrnAP3 promoter is similar in architecture to the consensus E. coli promoter and is not representative of most Mtb promoters, which are notably diverse in structure (5). In this study, we generated RNA-seq data using 4 different CarD mutant Mtb strains to investigate CarD’s activity in the context of native Mtb promoter sequences genome-wide. Our results show that when CarD activity is perturbed, approximately two-thirds of the Mtb genome is differentially expressed, highlighting CarD’s broad importance for coordinating Mtb gene expression. The genes affected by mutation of CarD fell into multiple diverse functional categories, indicating that CarD does not regulate a specific regulon of genes similar to a traditional transcription factor, but rather participates in transcription initiation as a basic part of the mycobacterial RNAP machinery. Therefore, CarD’s essentiality in Mtb is likely a result of its broad importance in maintaining global gene expression patterns that support viability.
We also discovered that mutating CarD results in a roughly equal number of up-regulated and down-regulated genes, indicating that CarD is capable of activating and repressing transcription from different Mtb promoters. The ability of CarD to repress gene expression had not been appreciated in previous studies with the rrnAP3 promoter. In principle, this observation could be explained by indirect effects, where CarD enhances the expression of a transcription factor that represses the expression of a set of genes. However, there is also precedent for factors that directly modify RPo stability being able to up-regulate the expression of one gene and down-regulate another, depending on the kinetic properties of the transcription initiation complexes at that promoter (22, 24, 25). Our previous kinetic model of CarD activity only considered CarD’s effect on the rates of RPo formation and RPo collapse (4), and could not adequately explain how CarD’s RPo stabilizing activity could result in differential transcription activation or repression. Here, we show that CarD slows promoter escape from the rrnAP3 promoter, which we hypothesize occurs via the additional RNAP-promoter DNA interactions that result from CarD association. By including CarD’s ability to inhibit promoter escape in a flux-based model of CarD’s effect on transcript production (25), our measurements support that stabilization of RPo by CarD could lead to up- and down-regulation of gene expression, thus explaining the RNA-seq data. Our data comparing mutations that impair CarD’s ability to stabilize RPo (R47E and K125A) with mutations that enhance CarD’s activity (I27F and I27W) (4, 19) supported this model, in which mutations with opposite effects on RPo stability often resulted in opposite changes in gene expression.
Our flux-based model implies that the outcome of CarD’s activity at a promoter depends on intrinsic kinetics of the transcription initiation complexes that form at a particular promoter. In E. coli, RPo stability and promoter escape rates are highly dependent on sequences in the PRR (27, 30, 36). In addition, the small alarmone ppGpp binds to RNAP in E. coli to elicit bidirectional transcriptional responses by altering initiation kinetics (37), and the outcome of ppGpp activity is correlated with certain promoter DNA sequences (24). Most promoters within the GC-rich mycobacterial species do not resemble E. coli promoters (5, 32, 34, 38), and the effect of PRR sequences in Mtb on transcription initiation kinetics has yet to be investigated in detail. We found that genes up-regulated in CarDR47E and CarDK125A were significantly enriched for promoters lacking a consensus “TANNNT” motif in their −10 element and contain shorter, less GC-rich discriminator regions. On the basis of our data, none of these sequence elements are the sole determinant of the outcome of CarD activity because many promoters containing consensus −10 elements and longer GC-rich discriminator regions were also up-regulated, and many nonconsensus promoters were also down-regulated. Therefore, a combination of sequence elements likely affects the outcome of CarD activity, dependent on the overall effect of the PRR sequences on transcription initiation kinetics. A caveat of our analyses based on in vivo expression profiles is that genes that are indirectly differentially expressed as a result of CarD mutation may be masking our ability to discern specific promoter sequences that contribute to the outcome of CarD activity. Future work will focus on performing in vitro assays to isolate only the direct effects of CarD activity on transcription from different promoter sequences.
A previous study suggested that a thymine base at the −12 position (T-12) within the −10 element is important for CarD’s conserved W85 residue to inhibit bubble collapse (18). The T-12 identified in that study likely corresponds to the upstream thymine residue found in consensus “TANNNT” −10 elements. Our promoter analysis suggests that nonconsensus promoters lacking this thymine are enriched in the genes up-regulated in Mtb mutants with impaired CarD activity (Fig. 4 A and B), suggesting that a T-12 is not required for CarD to affect expression. However, given that the Mtb CarDW85A mutant is not viable (17), we were unable to directly interrogate the importance of this interaction between W85 and the upstream thymine in the conserved −10 hexamer.
In addition to cis-acting PRR sequences, transacting factors, such as other transcription factors, could also contribute to the effect of CarD on transcription of a particular gene. Mtb encodes another essential RNAP-interacting protein, RbpA (39, 40), which is also capable of stabilizing RPo formed by mycobacterial RNAP in vitro (41–43). Structural studies have shown that association of CarD and RbpA with the same RNAP holoenzyme is feasible (43, 44), and we have shown that CarD and RbpA can function cooperatively to stabilize RPo (41). In addition to CarD and RbpA, Mtb also encodes numerous 2-component systems and other transcription factors that allow the bacteria to alter its gene expression in response to environmental cues (9). Future studies will focus on elucidating how RbpA and other transcriptional regulators affect the outcome of CarD activity at particular promoters.
Collectively, our data show that CarD coordinates global gene expression patterns in Mtb by activating and repressing transcription via stabilization of RNAP-promoter complexes. A recent study highlighted the potential of prokaryotic transcription factors with single regulatory mechanisms, such as CarD, to potentiate diverse regulatory outcomes in a promoter-specific manner (25). In this article, we demonstrate in vivo that CarD activity leads to diverse gene expression outcomes and does not solely act as a transcriptional activator. Importantly, CarD homologs are conserved in many bacterial phyla (8, 9), and regulate transcription to mediate diverse processes including stress responses, cell division, and metabolic homeostasis (10–13). Therefore, these studies shed light on the maintenance of optimal gene expression patterns broadly across bacteria.
Materials and Methods
Described in detail in the SI Appendix.
Bacterial Strains and Growth Conditions.
All Mtb strains were grown at 37 °C in Sauton’s broth media and derived from the Erdman strain, as described in previous publications (16, 17, 19).
RNA-Seq.
RNA was prepared from log-phase Mtb from CarDWT, CarDR47E, CarDK125A, CarDI27F, and CarDI27W in triplicate, as previously described (8). DNase-treated RNA was submitted to the Washington University Genome Technology Access Center for Illumina sequencing. Single-end RNA-seq reads were pseudoaligned to an index of Mtb H37Rv CDS (GenBank assembly GCA_000195955.2) and counted using kallisto (45). Unnormalized counts were imported using the tximport package, and differential expression analysis was performed using the DESeq2 package (46, 47).
Promoter Escape Assays.
Double-stranded DNA competitor and NTPs were added to preformed RNAP-σA-rrnAP3 complexes in the presence or absence of CarD. Aliquots of the reactions were collected over time after the addition of competitor and NTPs, and the amount of transcript produced at each point was quantified (Image Gauge Program).
Supplementary Material
Acknowledgments
This work was supported by Grant GM107544 from the National Institutes of Health (NIH; to C.L.S. and E.A.G.), NIH National Institute of Allergy and Infectious Diseases Award T32A1007172 (to D.X.Z.), National Institute of General Medical Sciences Cell and Molecular Biology Training Grant GM007067 (to A.L.G.), and the Stephen I. Morse Graduate Fellowship (A.L.G.). C.L.S. is also supported by a Burroughs Wellcome Fund Investigator in the Pathogenesis of Infectious Disease Award. Sequencing was performed at the Genome Technology Access Center in the Department of Genetics at Washington University School of Medicine. The Genome Technology Access Center is partially supported by National Cancer Institute Cancer Center Support Grant P30CA91842 to Siteman Cancer Center and by Institute of Clinical and Translational Sciences/Clinical and Translational Science Award Grant UL1TR000448 from the National Center for Research Resources, a component of the NIH, and NIH Roadmap for Medical Research. This publication is solely the responsibility of the authors and does not necessarily represent the official view of the National Center for Research Resources or NIH.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. E.N. is a guest editor invited by the Editorial Board.
Data deposition: Data have been deposited in Gene Expression Omnibus (accession no. GSE131043).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1900176116/-/DCSupplemental.
References
- 1.Whipple F. W., Sonenshein A. L., Mechanism of initiation of transcription by Bacillus subtilis RNA polymerase at several promoters. J. Mol. Biol. 223, 399–414 (1992). [DOI] [PubMed] [Google Scholar]
- 2.Mekler V., Minakhin L., Kuznedelov K., Mukhamedyarov D., Severinov K., RNA polymerase-promoter interactions determining different stability of the Escherichia coli and Thermus aquaticus transcription initiation complexes. Nucleic Acids Res. 40, 11352–11362 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis E., Chen J., Leon K., Darst S. A., Campbell E. A., Mycobacterial RNA polymerase forms unstable open promoter complexes that are stabilized by CarD. Nucleic Acids Res. 43, 433–445 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rammohan J., Ruiz Manzano A., Garner A. L., Stallings C. L., Galburt E. A., CarD stabilizes mycobacterial open complexes via a 2-tiered kinetic mechanism. Nucleic Acids Res. 43, 3272–3285 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newton-Foot M., Gey van Pittius N. C., The complex architecture of mycobacterial promoters. Tuberculosis (Edinb.) 93, 60–74 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Gomez M., Smith I., “Determinants of mycobacterial gene expression” in Molecular Genetics of Mycobacteria, Hatfull G. F., Jacobs W. R. Jr., Eds. (ASM Press, Washington, DC, 2000), pp. 111–129. [Google Scholar]
- 7.World Health Organization , Global Tuberculosis Report 2017 (World Health Organization, Geneva, 2017). [Google Scholar]
- 8.Stallings C. L., et al. , CarD is an essential regulator of rRNA transcription required for Mycobacterium tuberculosis persistence. Cell 138, 146–159 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flentie K., Garner A. L., Stallings C. L., Mycobacterium tuberculosis transcription machinery: Ready to respond to host attacks. J. Bacteriol. 198, 1360–1373 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan L., et al. , The ferredoxin-like protein FerR regulates PrbP activity in Liberibacter asiaticus. Appl. Environ. Microbiol. 85, e02605–e02618 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen T., et al. , LtpA, a CdnL-type CarD regulator, is important for the enzootic cycle of the Lyme disease pathogen. Emerg. Microbes Infect. 7, 126 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.García-Moreno D., et al. , CdnL, a member of the large CarD-like family of bacterial proteins, is vital for Myxococcus xanthus and differs functionally from the global transcriptional regulator CarD. Nucleic Acids Res. 38, 4586–4598 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woldemeskel S. A., et al. , The conserved transcriptional regulator CdnL is required for metabolic homeostasis and morphogenesis in Caulobacter. bioRxiv:10.1101/557637 (22 February 2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landick R., Krek A., Glickman M. S., Socci N. D., Stallings C. L., Genome-wide mapping of the distribution of CarD, RNAP σA, and RNAP β on the Mycobacterium smegmatis chromosome using chromatin immunoprecipitation sequencing. Genom. Data 2, 110–113 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srivastava D. B., et al. , Structure and function of CarD, an essential mycobacterial transcription factor. Proc. Natl. Acad. Sci. U.S.A. 110, 12619–12624 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiss L. A., et al. , Interaction of CarD with RNA polymerase mediates Mycobacterium tuberculosis viability, rifampin resistance, and pathogenesis. J. Bacteriol. 194, 5621–5631 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garner A. L., Weiss L. A., Manzano A. R., Galburt E. A., Stallings C. L., CarD integrates three functional modules to promote efficient transcription, antibiotic tolerance, and pathogenesis in mycobacteria. Mol. Microbiol. 93, 682–697 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bae B., et al. , CarD uses a minor groove wedge mechanism to stabilize the RNA polymerase open promoter complex. eLife 4, 1–19 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garner A. L., et al. , Effects of increasing the affinity of CarD for RNA polymerase on Mycobacterium tuberculosis growth, rRNA transcription, and virulence. J. Bacteriol. 199, e00698-16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lew J. M., Kapopoulou A., Jones L. M., Cole S. T., TubercuList–10 years after. Tuberculosis (Edinb). 91, 1–7 (2011). [DOI] [PubMed] [Google Scholar]
- 21.Chen K., et al. , The overlooked fact: Fundamental need for spike-in control for virtually all genome-wide analyses. Mol. Cell. Biol. 36, 662–667 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paul B. J., Berkmen M. B., Gourse R. L., DksA potentiates direct activation of amino acid promoters by ppGpp. Proc. Natl. Acad. Sci. U.S.A. 102, 7823–7828 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doniselli N., et al. , New insights into the regulatory mechanisms of ppGpp and DksA on Escherichia coli RNA polymerase-promoter complex. Nucleic Acids Res. 43, 5249–5262 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez-Vazquez P., Dewey C. N., Kitten N., Ross W., Gourse R. L., Genome-wide effects on Escherichia coli transcription from ppGpp binding to its two sites on RNA polymerase. Proc. Natl. Acad. Sci. U.S.A. 116, 8310–8319 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galburt E. A., The calculation of transcript flux ratios reveals single regulatory mechanisms capable of activation and repression. Proc. Natl. Acad. Sci. U.S.A. 115, E11604–E11613 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu L. M., Promoter clearance and escape in prokaryotes. Biochim. Biophys. Acta 1577, 191–207 (2002). [DOI] [PubMed] [Google Scholar]
- 27.Siwo G., et al. , Prediction of fine-tuned promoter activity from DNA sequence. F1000 Res. 5, 158 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ross W., Gourse R. L., Analysis of RNA polymerase-promoter complex formation. Methods 47, 13–24 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Djordjevic M., Bundschuh R., Formation of the open complex by bacterial RNA polymerase–A quantitative model. Biophys. J. 94, 4233–4248 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henderson K. L., et al. , Mechanism of transcription initiation and promoter escape by E. coli RNA polymerase. Proc. Natl. Acad. Sci. U.S.A. 114, E3032–E3040 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cortes T., et al. , Genome-wide mapping of transcriptional start sites defines an extensive leaderless transcriptome in Mycobacterium tuberculosis. Cell Rep. 5, 1121–1131 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bashyam M. D., Tyagi A. K., Identification and analysis of “extended -10” promoters from mycobacteria. J. Bacteriol. 180, 2568–2573 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agarwal N., Tyagi A. K., Role of 5′-TGN-3′ motif in the interaction of mycobacterial RNA polymerase with a promoter of ‘extended -10’ class. FEMS Microbiol. Lett. 225, 75–83 (2003). [DOI] [PubMed] [Google Scholar]
- 34.Agarwal N., Tyagi A. K., Mycobacterial transcriptional signals: Requirements for recognition by RNA polymerase and optimal transcriptional activity. Nucleic Acids Res. 34, 4245–4257 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shell S. S., et al. , Leaderless transcripts and small proteins are common features of the mycobacterial translational landscape. PLoS Genet. 11, e1005641 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heyduk E., Heyduk T., DNA template sequence control of bacterial RNA polymerase escape from the promoter. Nucleic Acids Res. 46, 4469–4486 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gourse R. L., et al. , Transcriptional responses to ppGpp and DksA. Annu. Rev. Microbiol. 72, 163–184 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bashyam M. D., Kaushal D., Dasgupta S. K., Tyagi A. K., A study of mycobacterial transcriptional apparatus: Identification of novel features in promoter elements. J. Bacteriol. 178, 4847–4853 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bortoluzzi A., et al. , Mycobacterium tuberculosis RNA polymerase-binding protein A (RbpA) and its interactions with sigma factors. J. Biol. Chem. 288, 14438–14450 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hubin E. A., et al. , Structural, functional, and genetic analyses of the actinobacterial transcription factor RbpA. Proc. Natl. Acad. Sci. U.S.A. 112, 7171–7176 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rammohan J., et al. , Cooperative stabilization of Mycobacterium tuberculosis rrnAP3 promoter open complexes by RbpA and CarD. Nucleic Acids Res. 44, 7304–7313 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prusa J., et al. , Domains within RbpA serve specific functional roles that regulate the expression of distinct mycobacterial gene subsets. J. Bacteriol. 200, e00690-17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hubin E. A., et al. , Structure and function of the mycobacterial transcription initiation complex with the essential regulator RbpA. eLife 6, 1–40 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boyaci H., Chen J., Jansen R., Darst S. A., Campbell E. A., Structures of an RNA polymerase promoter melting intermediate elucidate DNA unwinding. Nature 565, 382–385 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bray N. L., Pimentel H., Melsted P., Pachter L., Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 34, 525–527 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Love M. I., Huber W., Anders S., Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soneson C., Love M. I., Robinson M. D., Differential analyses for RNA-seq : Transcript-level estimates improve gene-level inferences. F1000 Res. 4, 1521 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.