Abstract
Background and Aims
Determining seed longevity by identifying chemical changes that precede, and may be linked to, seed mortality, is an important but difficult task. The standard assessment, germination proportion, reveals seed longevity by showing that germination proportion declines, but cannot be used to predict when germination will be significantly compromised. Assessment of molecular integrity, such as RNA integrity, may be more informative about changes in seed health that precede viability loss, and has been shown to be useful in soybean.
Methods
A collection of seeds stored at 5 °C and 35–50 % relative humidity for 1–30 years was used to test how germination proportion and RNA integrity are affected by storage time. Similarly, a collection of seeds stored at temperatures from −12 to +32 °C for 59 years was used to manipulate ageing rate. RNA integrity was calculated using total RNA extracted from one to five seeds per sample, analysed on an Agilent Bioanalyzer.
Results
Decreased RNA integrity was usually observed before viability loss. Correlation of RNA integrity with storage time or storage temperature was negative and significant for most species tested. Exceptions were watermelon, for which germination proportion and storage time were poorly correlated, and tomato, which showed electropherogram anomalies that affected RNA integrity number calculation. Temperature dependencies of ageing reactions were not significantly different across species or mode of detection. The overall correlation between germination proportion and RNA integrity, across all experiments, was positive and significant.
Conclusions
Changes in RNA integrity when ageing is asymptomatic can be used to predict onset of viability decline. RNA integrity appears to be a metric of seed ageing that is broadly applicable across species. Time and molecular mobility of the substrate affect both the progress of seed ageing and loss of RNA integrity.
Keywords: Ageing, Arrhenius, collections, degradation, germination, kinetics, longevity, RNA integrity, safflower, seed, viability
INTRODUCTION
Seeds eventually lose the ability to complete germination. The duration that seeds remain alive (i.e. germinable) depends on storage temperature and moisture, as well as circumstances during development and maturation that are not well understood. Before succumbing, germinating seeds occasionally show evidence of age-related damage in the form of delayed germination, abnormalities or stunted growth (Abdul-Baki and Anderson, 1972). However, the transition between being alive and not alive usually occurs discreetly in a dry seed because typical signs of life are only apparent when water, and other germination requirements, are provided. Therefore, reliably predicting the onset of lost viability is difficult because biological factors contributing to survival (or death) are not yet known, and the moment when germination potential is lost cannot be observed. This lack of predictive power costs the seed industry millions of dollars per year in unscheduled losses of quality (McDonald, 1998) and contributes to the high cost of seed bank management (FAO, 2014).
Ageing damage to cellular machinery is likely caused by oxidative reactions, which can break molecules apart or cause molecules to cross-link (Job et al., 2005; Halliwell and Gutteridge, 2015; Waterworth et al., 2015; Green and Speller, 2017). Numerous studies implicate changes in proteins, lipids or nucleic acids as correlates, even causes, of death (Sattler et al., 2004; França et al., 2007; Terskikh et al., 2008; Kranner et al., 2011), or protection from change as a reason for extended survival (Hoekstra et al., 2001; Buitink and Leprince, 2004; Ogé et al., 2008; Charron and Quatrano, 2009; Chatelain et al., 2012; Petla et al., 2016). RNAs are an interesting ageing substrate, as they are essential for seed germination but are nevertheless fragile molecules, easily damaged by mechanisms ranging from fission to base substitutions (Wurtmann and Wolin, 2010; Rajjou et al., 2012; Bai et al., 2017). Recently, we demonstrated that RNA fragmentation correlates with lower viability in ageing soybean seeds; that damage appeared to occur randomly throughout the seed transcriptome (Fleming et al., 2017, 2018). Molecular damage is frequently compared to lost germination potential because experimental design or sample availability dictates this approach. In other words, biochemical or physical differences are sought to flag the culmination of ageing reactions (i.e. death) (Kranner et al., 2010b and references therein). Moreover, a common goal is to link mortality with specific, correlating changes. Since there is a plethora of substrates that may participate in ageing reactions, it is not surprising that we find weak or inconsistent correlations between viability loss and potential ageing substrates in studies that use seeds having broad genetic backgrounds or diverse provenances (Walters et al., 2005).
To account for the apparent stochasticity of molecular flags of ageing, our working model of seed ageing is that it is a process in which all molecules in cells are susceptible to damage and damage accumulates with time. We hypothesize that seeds eventually reach a tipping point at which a minor, stochastic event has a major effect (i.e. the seed loses capacity to germinate), analogous to the ‘straw that broke the camel’s back’. In this perspective, there may be hundreds of specific circumstances leading towards death, and the approach to mortality can be accelerated if essential components are completely degraded or if toxic by-products are generated. In any scenario, the switch from germinable to not germinable occurs after a threshold is crossed. Determining exactly what constitutes the threshold, especially considering that it may depend on the seed’s unique experience of ageing damage, may be futile (Fleming et al., 2018). However, seeking the speed of approach to the transition between germinable and not germinable may be experimentally tractable.
Measuring ageing rate, i.e. how quickly damage accumulates – or how fast the biological clock ticks – requires a quantitative assay of chemical or physical change. In actively metabolizing organisms, both telomere length and abundance of DNA methylation tags reflect the biological clock (Monaghan and Haussmann, 2006; Johnson et al., 2012; Michalak et al., 2015; Ogneva et al., 2016). Changes are more subtle and harder to measure in dry biological systems. A first attempt correlated rates of ethanol and methanol formation, through molecular fissures of diverse molecules, with rates of viability loss (Mira et al., 2010). Our recent studies of the integrity of an entire class of molecules, namely RNA, opened the possibility of developing another molecular clock. Other promising assays include the use of infrared thermography, DNA laddering or protein carbonylation (Kranner et al., 2010a, 2011; Cabiscol et al., 2014).
Deteriorative reactions are slower in dry seeds because they are material solids (Walters et al., 2010), not fluids as in actively metabolizing organisms. Reaction kinetics in material solids depend on structural factors affecting molecular mobility and proximity, as well as the reaction mechanism, e.g. whether intra- or inter-molecular interactions are involved. Because temperature regulates molecular mobility, its effects are key to understanding reaction kinetics in solid-state systems (Fundo et al., 2015; Ballesteros et al., 2017). Moisture plasticizes solids, making it another key parameter regulating molecular mobility and reaction kinetics. In the case of seeds, moisture loosens solidified cytoplasm, which decreases structure and increases fluidity, changing how fast the biological clock ticks.
The primary purpose of this study is to find molecular markers that consistently flag the progress of ageing in dry biological systems, such as seeds. Here, ageing responses are evaluated as loss of viability or decrease in RNA quality, and we ask whether observations made in soybean seed (Fleming et al., 2017, 2018) are consistent in seeds of species exhibiting a range of longevity characteristics. To do this, we make use of unique seed collections that have been kept dry for decades and are now showing evidence of lost viability. One collection, begun in 1987, consists of different cohorts of commercially available seeds purchased in different years and stored together. Another collection, begun in 1959, consists of seeds that were stored at a range of temperatures. We ask whether (1) loss of RNA integrity is observed in stored seeds from diverse species, (2) decreased RNA integrity relates to lost germination potential, storage time and storage temperature, and (3) temperature dependencies of losses in viability or RNA integrity are similar.
MATERIALS AND METHODS
Plant material
Two seed collections were used in these experiments, one begun by Walters in 1987 and the other by Bass in 1959 (Bass et al., 1962, 1963a, b). Walters’ ‘refrigerator’ collection consists of seeds from >40 species that have been stored at 3–5 °C and 35–50 % relative humidity (RH). This collection includes seeds of soybean (Fleming et al., 2017, 2018, not described in this study), lettuce (Lactuca sativa ‘Black Seeded Simpson’), carrot (Daucus carota ‘Danvers Half Long’), onion (Allium cepa ‘Sweet Spanish’), pea (Pisum sativum ‘Early Alaska’), tomato (Solanum lycopersicum ‘Roma’) and watermelon (Citrullus lanatus ‘Sugar Baby’). These seeds were purchased every 2–4 years from commercial seed growers between 1987 and 2017. The Bass ‘sealed can’ experiment consists of single lots of seeds from five species [crimson clover (Trifolium incarnatum ‘Dixie’), lettuce (‘Great Lakes’), sorghum (Sorghum bicolor ‘RS 610’), safflower (Carthamus tinctorius ‘Pacific No. 1’) and sesame (Sesamum indicum ‘Margo’)]. Pre-weighed samples ranging from 3 (lettuce) to 30 g (safflower) per can were adjusted to 4, 7 and 10 % water content (fresh weight basis) and hermetically sealed in 84-mL aluminium cans that were purged, then filled with different gases and then placed at −15 to −12, −5 to −1, 8 to 11, 20 to 22 and 32 to 35 °C (Bass et al., 1962, 1963a, b). Results for seeds adjusted to 4 % and stored in air are presented in this paper. In the refrigerator collection, most accessions of tomato had seeds with trichomes; however, three accessions (harvested in 1987, 1992 and 1999) included trichomeless seeds, indicating a different processing procedure (Raval et al., 2016).
Germination assays and deterioration time courses
Seeds from the refrigerator collection were periodically tested, most recently in July 2017. Samples of 20–100 seeds were rolled in moist germination paper (Anchor, St Paul, MN, USA) and placed in a germinator (Percival Scientific, Perry, IA, USA) at 25 °C with 16 h light/8 h dark. Radicle emergence was assessed after 7 d. Radicle length was also measured when <35 seeds were sown. Germination of seeds in Bass’s sealed can experiment (Bass et al., 1962, 1963a, b) was periodically assayed, most recently in 2015–2017, using at least 100 seeds and AOSA testing rules (AOSA, 2017).
The effect of storage time on germination potential was expressed using deterioration time courses. For the refrigerator collection, germination data from 2017 were used and storage time was calculated as the difference between the germination test date and the year that each cohort was purchased. Germination assays were repeated in 2018 for tomato and watermelon to confirm the poor relationship between harvest year and germination percentage. For Bass’s sealed can experiment, germination percentage was assayed almost yearly by Bass from 1959 to 1983 and again in 2003, 2008–2009 and 2015–2017. Storage time was calculated as the difference between the date when seeds were sown for the germination assay and the start of the experiment. Time-course data were fitted to the Avrami equation to generate a longevity curve, according to methods described previously (Mira et al., 2016), and the calculated time to reach 50 % of initial germination (P50) was used to quantify longevity. We used the reciprocal of P50 (P50−1) to quantify ageing rate. Time course data were also fitted to a logistic function using dose.p in R (Crawley, 2007) to calculate P50 and the standard error around P50.
RNA extraction and characterization
RNA was extracted from 5–30 mg of seed tissues, which required pools of five seeds (lettuce, carrot, tomato), three seeds (crimson clover, onion) or two seeds (sesame), or single seeds of watermelon (seed coat removed) and safflower, or a seed fraction (sorghum, pea). For pea, the embryonic axis, seed coat and ~10 mg of endosperm tissue were assayed separately for each seed. For sorghum, the embryonic axis including adjacent scutellum tissue was used. Seed tissues were pulverized with a hammer and transferred to a 2-mL microcentrifuge tube containing a steel shot #40 BB and 1–2 mg of polyvinylpyrrolidone-40 (Fisher Scientific, Fairlawn, NJ, USA). Tissue samples were ground under liquid nitrogen in a Retsch (Haan, Germany) Bead Mill for 2 min at 30 oscillations s−1. After grinding, the Qiagen (Hilden, Germany) Plant RNeasy kit was used for RNA extraction, following the manufacturer’s protocol with the modification that the column was washed three times with 500 µL of buffer RPE to minimize guanidine hydrochloride contamination.
RNA concentration and purity were assessed using a Nanodrop (Thermo Fisher, Wilmington, DE, USA) 1000 spectrophotometer. RNA samples were diluted to 2 ng µL−1 in nuclease-free water. Integrity of diluted RNA was quantified on an Agilent (Waldbronn, Germany) Bioanalyzer, using Agilent RNA 6000 Pico chips and the Plant RNA Pico assay (Agilent 2100 Expert software version B.02.08.SI648 R3), following the manufacturer’s protocols.
Temperature dependency of seed ageing and RNA degradation
Arrhenius plots were used to quantify the temperature dependency of degradation kinetics for seeds from the Bass sealed can experiment (Walters et al., 2004). Slopes of relationships between ln(rate) and 1/T (where T is temperature in kelvin) were calculated and compared using Excel and R regression modules. The rate that seeds died was expressed as P50−1. The initial RNA integrity number (RIN) was equated to the average of the five highest RIN values across all temperatures. The rate of RNA degradation was calculated from the difference in initial RIN and RIN measured for the storage temperature, divided by a storage time of 59 years.
Statistical analyses
Descriptive statistics were calculated using Excel or R functions. Linear regressions used Excel functions. The effects of germination and species on RIN were modelled in JMP 12 (SAS Institute, Cary, NC, USA) using a two-way mixed-model ANOVA, with germination as a fixed effect and species as a random effect. As no significant correlation between germination and RIN existed for watermelon, these data were excluded from the model.
RESULTS
Crop seeds stored at 5 °C (refrigerator) for different durations
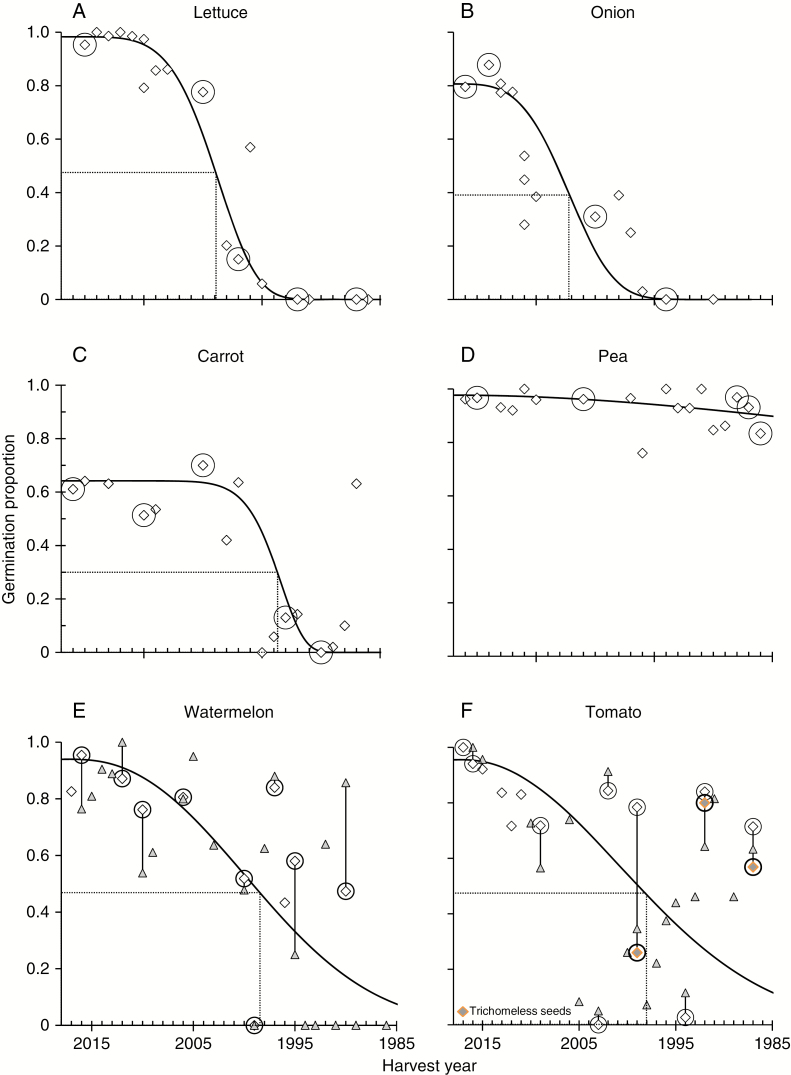
Seeds in Walters’ refrigerator collection, reflecting different cohorts of the same cultivar (lettuce, onion, carrot, pea, tomato, watermelon), had similar appearance and mass, with no visual clues of ageing in the oldest samples (i.e. those purchased in the early 1990s). Germination was tested within 6 months of harvest and all seed lots were initially of high quality, similar to results reported here for the most recently purchased accessions [harvested in 2016 (2016H) or 2017 (2017H, and similarly for all other harvest years) in Fig. 1; initial germination data for other harvest years are not shown].
Fig. 1.
Proportion of seeds germinating in seed lots harvested in the indicated years and stored at 5 °C and 3–50 % RH. Germination was measured in 2018 (diamonds), and samples that were assessed for RNA integrity are indicated by circled points. Data for 2017 germination assessments of watermelon (E) and tomato (F) are also provided (triangles) and these points are connected to 2018 assessments by a vertical line. Some tomato seed lots had seeds lacking normal thin trichomes on seed coats; these are indicated by coloured points. Data for each crop were fitted to the Avrami model (solid curve), which was used to calculate longevity parameters (P50, dashed lines).
Germination proportion of samples recently retested (in 2017 for all species and in 2018 for tomato and watermelon) ranged from 0 to at least 0.80, depending on species and harvest year. Greater deterioration did not always correspond with earlier harvest years, as older seeds sometimes retained high germination while more recently harvested seeds had low germination. For example, germination proportion of the oldest carrot seeds (1992H) was 0.62, compared with 0.61 for 2016H and 0.51 for 2010H. Pea seeds showed only minor evidence of deterioration: germination proportion was high (0.83) in the oldest seeds (1991H), while the lowest germination proportion, for 2001H seeds, was still high at 0.76. Tomato seeds with trichomes had higher germination proportion than their trichomeless counterparts, with the greatest difference observed for 1999H (0.78 versus 0.26), followed by 1987H (0.71 versus 0.57), then 1992H (0.84 versus 0.80). Germination proportion of watermelon and tomato seeds from different cohorts was particularly inconsistent with age, clearly indicated by proportions ranging from 0.1 to 0.91 in tomato seeds harvested before 2005 and from 0 to 0.86 in watermelon seed harvested before 2001. This inconsistency can be partially attributed to experimental error of germination assessments, evident in higher measured germination in 2018 compared with 2017 assays for some cohorts (1995H, 2000H, 2006H, 2010H and 2016H for watermelon and 2009H and 1992H for tomato) (Fig. 1E, F). We purposely included tomato and watermelon seeds in analyses of RNA integrity because harvest year (i.e. storage duration) did not appear to be a strong factor explaining germination proportion; these seeds therefore provided the opportunity to disassociate viability and storage time from RIN decline.
Storage time corresponding to lost viability (estimated as P50) was calculated for each species. Using the dose.p function in R, P50 ranged from 9.9 ± 0.3 years (onion) to 51.9 ± 12.4 years (pea), with most species exhibiting negligible change in germination potential for about 10 years (lettuce, tomato, watermelon, carrot). The P50 values calculated with the Avrami equation were similar, ranging from 9.7 years (onion) to 100.5 years (pea), and highly correlated with dose.p results (R2 = 0.88, P < 0.004). The value of P50 for pea seeds was estimated at about 52 and 101 years from Avrami and logistic functions, respectively; the difference of 50 years between estimates reflects the uncertainty of predicting longevity in pea, where degradation was only slightly apparent in 2018.
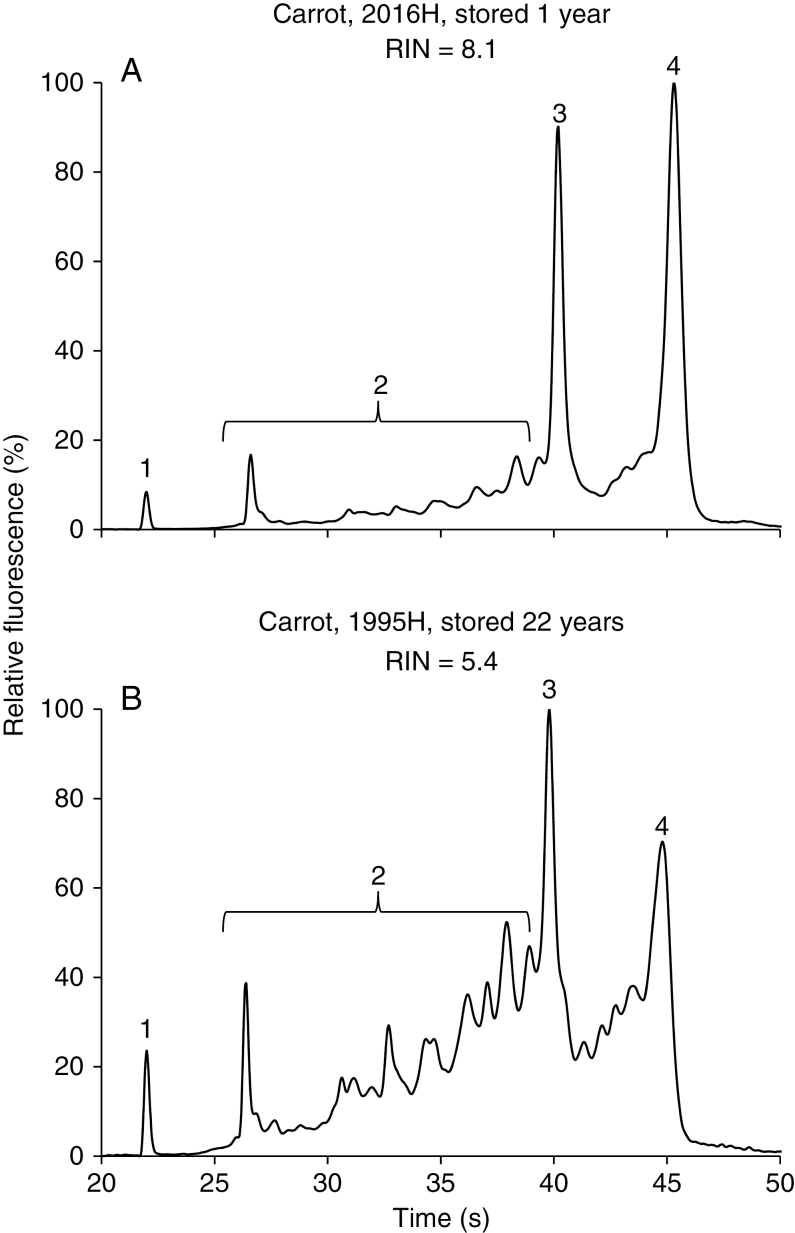
Quality of total RNA extracted from crop seeds varied, usually depending on cohort age or measured germination proportion (representative electropherograms from carrot are shown in Fig. 2). High-quality RNA produced similar-looking electropherograms among the diverse species (Fig. 2A) that featured prominent peaks (relative fluorescence approaching 100 %) for rRNAs [18S (region 3, ~40 s) and 25S (region 4, ~45 s); height of the 25S peak greater than that of the 18S peak] and minimal signal (relative fluorescence <20 %) for peaks in the fast region (region 2, 25–39 s). Calculation of the RIN considers fluorescence intensity and peak area for each of these regions, which produces RIN values >7 for seeds with high-quality RNA (Schroeder et al., 2006). In contrast, low-quality RNA has increased signal in the fast region (region 2, 40–50 % relative fluorescence) relative to the rRNA regions (regions 3 and 4) and reduced signal of the 25S relative to the 18S peak, resulting in RIN values much lower than 7. Fluorescence patterns in the fast region (region 2) varied between species.
Fig. 2.
Electropherograms of RNA extracted from carrot seeds that were harvested in 2016 (A) and 1995 (B) and stored at 5 °C until used. The top panel shows data from a pool of five co-extracted fresh seeds and is an example of high-quality RNA, while the bottom panel of five seeds from a 22-year-old sample presents age-related damage. The electropherograms are divided into four regions: (1) a 25-nt DNA fragment used as the lowest molecular weight marker; (2) a ‘fast’ region of small, early-eluting molecules, between 27 and 39 s; (3) 18S rRNA at about 40 s; and (4) 25S rRNA at about 45 s. Ageing is indicated by a changed ratio between the 18S and 25S rRNA peaks (regions 3 and 4) and increased area occupied in the fast small molecular weight region (region 2). RIN is 8.1 and 5.4 for the high- and low-quality RNA, respectively.
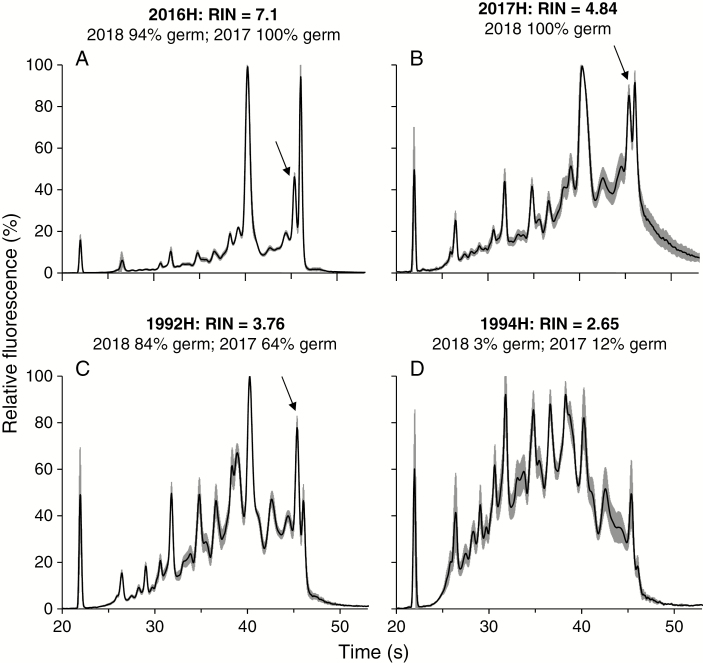
Electropherograms of high-quality total RNA from fresh tomato seeds had dual peaks in the 25S (45–46 s) and 18S (39–40 s) rRNA regions (Fig. 3A). RIN calculation using the manufacturer’s software assumes only one peak in each rRNA region (i.e. regions 3 and 4; Fig. 2) in intact RNA; hence assignment of RIN in tomato appeared unreliable to the extent that samples with relatively intact RNA, as visually assessed on the electropherograms, were nevertheless assigned low RIN values. RNA quality was lower in trichomeless tomato seeds compared with seeds with trichomes from 1999 (RINtrichomeless = 3.06 ± 0.39; RINtrichomes = 3.98 ± 0.61; P = 0.02) and there was no difference between the two seed forms in the 1987 (RINtrichomeless = 3.92 ± 0.64; RINtrichomes = 3.48 ± 0.24; P = 0.19) and 1992 harvests (RINtrichomeless = 3.54 ± 0.76; RINtrichomes = 3.76 ± 1.06; P = 0.72, Student’s t-test).
Fig. 3.
Electropherograms of tomato seeds harvested in the indicated years. Data for average fluorescence (black) and standard deviation (grey) are given for five replicates of five seeds each. Electropherograms are ordered from highest (A) to lowest (D) RIN value and show the general trend of decreasing RNA quality with lower germination, though there is considerable variation in calculated RIN and in germination response to storage time. There are two peaks in the region of 25S rRNA (near 45 s) (arrow), which confounded RIN calculation, resulting in low RIN values despite highly viable material [e.g. 2017H seeds (B)].
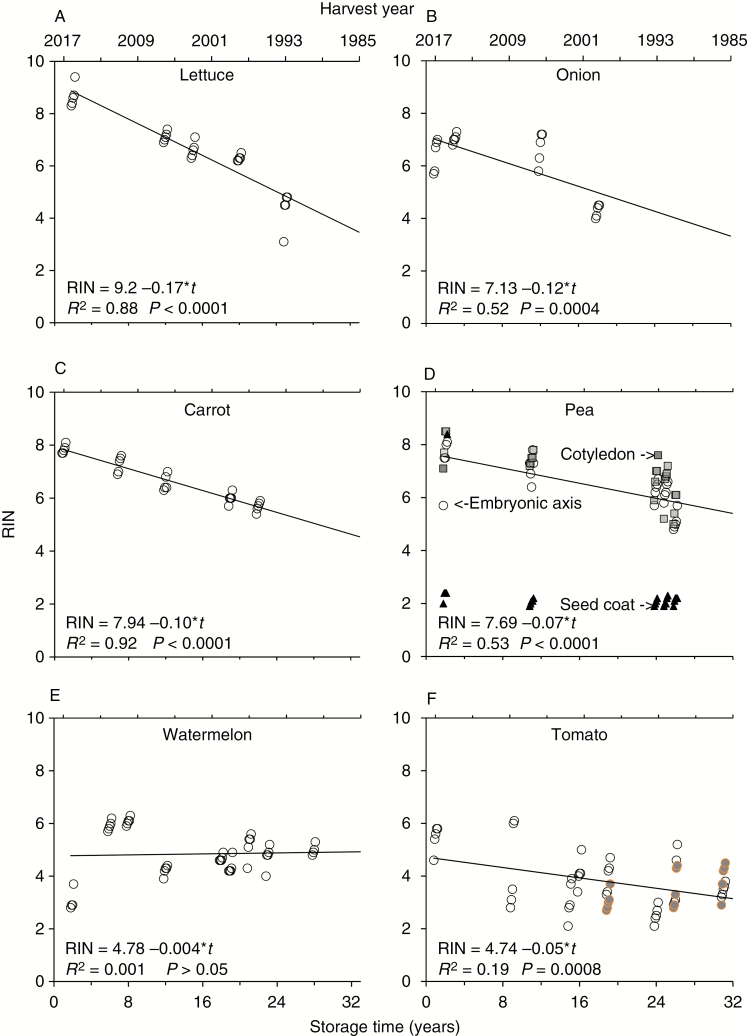
Seeds stored for longer times tended to produce lower RIN values calculated from electropherograms of extracted RNA [Fig. 4; linear correlations were significant (P ≤ 0.0008) in all species except watermelon]. The effect of storage time on RIN was most pronounced for lettuce seeds, which were assigned an average RIN of 8.68 ± 0.43 for seeds stored for 2 years (2015H) and 4.34 ± 0.71 for seeds stored for 25 years (1992H), a 50 % decrease, giving a steep slope of 0.17 RIN units year−1 (Fig. 4A, Table 1). RNA from tomato seeds, which was difficult to assess using RIN software, showed a much smaller change with time and a slope of 0.05 RIN units year−1. The y-intercept [storage time (t) = 0, harvest year = 2017] of the linear regression of RIN versus storage time was >7 for lettuce, onion, carrot and pea, indicating that the quality of RNA extracted from fresh seeds is usually high. Previously measured RIN values for fresh soybean seeds were also >7 (Fleming et al., 2017). On the other hand, initial mean RIN values for RNA extracted from watermelon and tomato were 4.76 and 4.72, respectively, reflecting poor quality of extracted RNA or problems in RIN calculations that confound assessments of change in RNA with time. For watermelon, the regression between RIN and storage time was not significant; its slope was a tenth of observations made for tomato, at 0.004 RIN units year−1.
Fig. 4.
RNA integrity of stored seeds as a function of storage time. Specific cohorts from a larger collection (Fig. 1, circled points) were selected for RIN analysis to provide a range of germination proportions and storage times. RIN values are provided for each of five replicates and data points are jittered as an aid to the eye. The drawn lines represent a linear model of the correlation between time and RIN, and the results of the regression are provided for each species. Sample size for each regression (N) is the number of time points × 5. Each pea seed was separated into embryonic axis (circles), cotyledon (squares) and seed coat (triangles) before RIN assessment (D); regression results are for embryonic axes. Data from the three trichomeless tomato seed cohorts (coloured points) were included in the regression (F). There is a significant negative linear correlation between RIN and storage time (t) for all species except watermelon (E).
Table 1.
Summary of RNA integrity results for seeds from each species and harvest year used in the refrigerator experiment, in which seeds were stored at 3–5 °C and 35–50 % RH. Average RIN values ± s.d. from five independent samples are reported from assays performed in 2017. Each sample included one to five seeds, or a seed fraction, depending on species
| 1987 | 1990 | 1991 | 1992 | 1993 | 1994 | 1995 | 1997 | 1998 | 1999 | 2000 | 2002 | 2003 | 2005 | 2006 | 2009 | 2010 | 2012 | 2014 | 2015 | 2016 | 2017 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lettuce, 5 seeds |
4.34 ± 0.71 | 6.30 ± 0.12 | 6.62 ± 0.31 | 7.12 ± 0.19 | 8.68 ± 0.43 | |||||||||||||||||
| Onion, 3 seeds |
4.30 ± 0.23 | 6.68 ± 0.61 | 7.04 ± 0.18 | 6.42 ± 0.62 | ||||||||||||||||||
| Carrot, 5 seeds |
5.68 ± 0.19 | 6.00 ± 0.21 | 6.58 ± 0.30 | 7.28 ± 0.31 | 7.84 ± 0.17 | |||||||||||||||||
| Pea, embryonic axis | 5.10 ± 0.35 | 6.24 ± 0.32 | 6.30 ± 0.38 | 7.14 ± 0.52 | 7.36 ± 0.97 | |||||||||||||||||
| Pea, cotyledon | 5.65 ± 0.54 | 6.56 ± 0.78 | 6.82 ± 0.63 | 7.46 ± 0.23 | 7.95 ± 0.68 | |||||||||||||||||
| Pea, seed coat |
2.12 ± 0.13 | 2.08 ± 0.16 | 2.05 ± 0.13 | 2.06 ± 0.11 | 3.52 ± 2.73 | |||||||||||||||||
| Tomato, 5 seeds with trichomes |
3.48 ± 0.24 | 3.76 ± 1.06 | 2.54 ± 0.34 | 3.98 ± 0.61 | 4.12 ± 0.57 | 3.08 ± 0.73 | 4.30 ± 1.62 | 5.44 ± 0.50 | ||||||||||||||
| Tomato, 5 seeds without trichomes |
3.92 ± 0.64 | 3.54 ± 0.76 | 3.06 ± 0.39 | |||||||||||||||||||
| Watermelon, 1 seed No seed coat |
5.00 ± 0.22 | 4.74 ± 0.44 | 5.16 ± 0.51 | 4.36 ± 0.30 | 4.68 ± 0.13 | 4.22 ± 0.19 | 6.08 ± 0.15 | 5.92 ± 0.19 | 3.07 ± 0.42 |
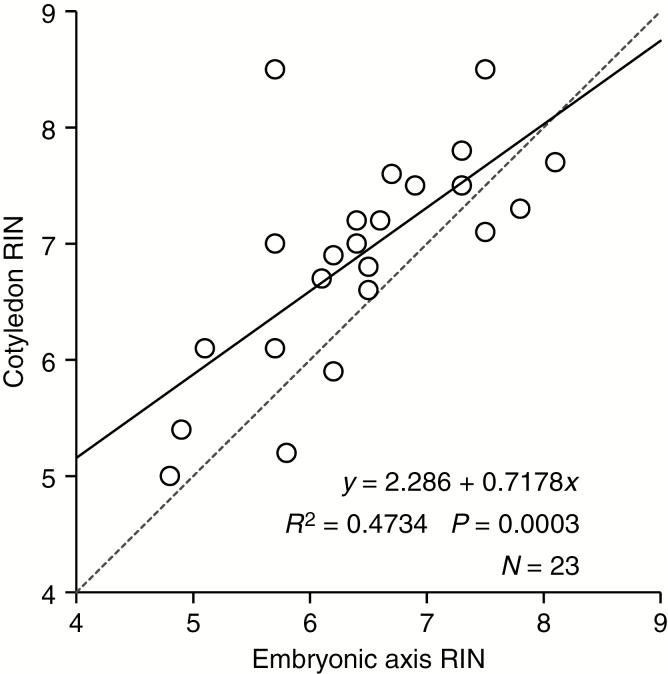
The RIN values calculated from electropherograms of pea embryonic axes or cotyledon sections appeared superimposed; they decreased at the same rate with increasing seed storage time (RINaxis = 7.69 – 0.07 × t, R2 = 0.53, P < 0.0001; RINcotyledon = 8.19 – 0.07 × t, R2 = 0.53, P < 0.0001; Fig. 4D). RIN values of embryonic axes and cotyledon sections from the same seed were significantly correlated (R2 = 0.47, N = 23, P = 0.0003, RINcotyledon = 2.29 + 0.72 × RINaxis; Fig. 5). Although correlated, the values were not identical: the non-zero intercept and fractional slope mean that RIN values from cotyledon tissue tended to be greater than RIN values obtained from axes. This difference appeared greater when the RIN value from an axis was <6.5 (P < 0.05 for intercept ≠ 0; P < 0.001 for slope ≠ 1). In contrast to relatively high RNA quality from embryonic axis and cotyledon tissues, RNA extracted from pea seed coats was usually profoundly degraded, with RIN values ≤2.4 for most samples. There was no significant change in RIN values of pea seed coats with storage time (RINseed coat = 3.23 – 0.05 × t, R2 = 0.14, P = 0.07; Fig. 4D, Table 1).
Fig. 5.
RNA integrity of embryonic axis and cotyledon tissue from the same pea seed. RNA integrity is significantly positively correlated between the two tissues, with higher RIN values measured in cotyledon than axis tissue.
Crop seeds stored in sealed cans at different temperatures for 59 years
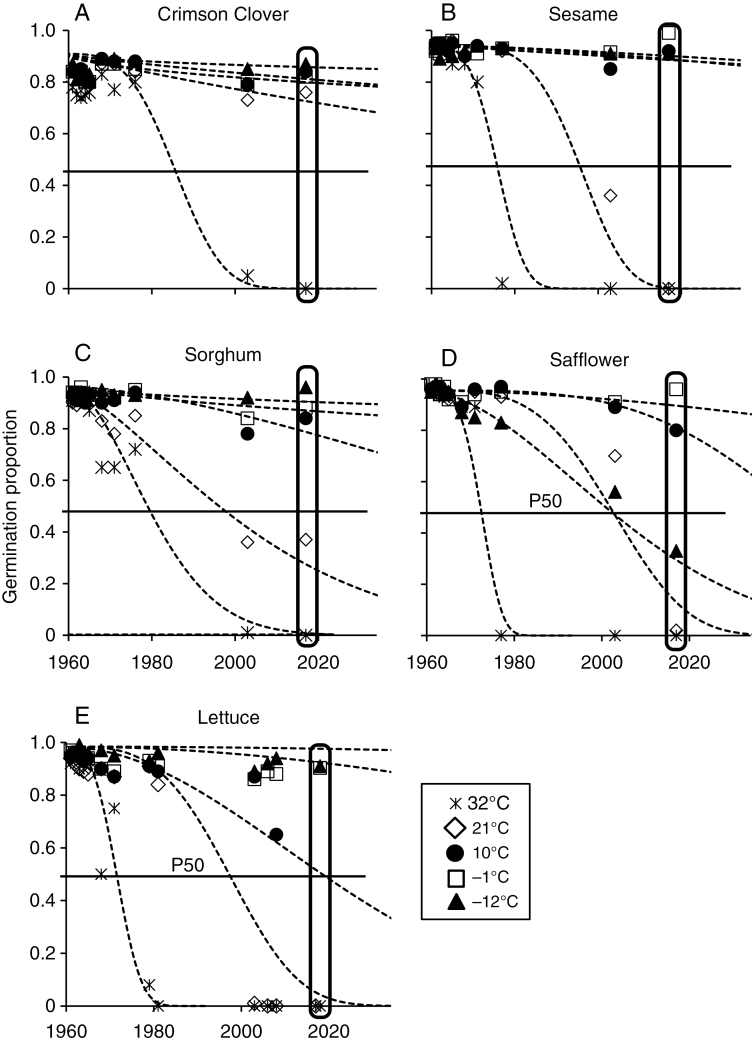
In a different experimental design that varied storage temperature rather than storage time, we compared RNA quality with ageing progress within the same seed lot. Germination of seeds declined over the 59 years of the experiment; viability measured in 2017 ranged from 0 (all seeds stored at 32 °C) to at least 0.8 (seeds stored at −12 or −1 °C) (Fig. 6). Germination time courses, which compiled all germination data collected for this experiment through the years, followed the familiar pattern of a prolonged period of no detectable change followed by a period of rapid mortality. Viability decline was modelled using the Avrami equation and the dose.p function for each species at each storage temperature to calculate P50. Values of P50 varied with temperature and species, ranging from 12 years (lettuce) to 28 years (crimson clover) for seeds stored at 32 °C and 4 % water, and from 45 years (safflower) to >5000 years (crimson clover) for seeds stored at −12 °C. Safflower seeds were exceptional for their 45-year longevity at −12 °C; P50 of >500 years was estimated for seeds of this species stored at −1 °C.
Fig. 6.
Germination data from Bass’s sealed can experiment, initiated in 1959. Seeds of five species were dried to 4 % moisture, sealed in air and placed at temperatures of approximately −12 (closed triangles), −1 (open squares), 10 (closed circles), 21 (open diamonds) and 32 (asterisks) °C, and assayed for viability at the indicated years. Data for each crop and storage temperature were fitted to the Avrami model (curves), which was used to calculate longevity parameters (P50, solid horizontal line). Points enclosed in a capsule for the 2017 germination assay also represent materials used for RIN analyses. Note that, unlike other species, safflower seeds stored at −12 °C had lower germination levels than those stored at −1 or +10 °C. Samples for lettuce at −12 and +10 °C were depleted.
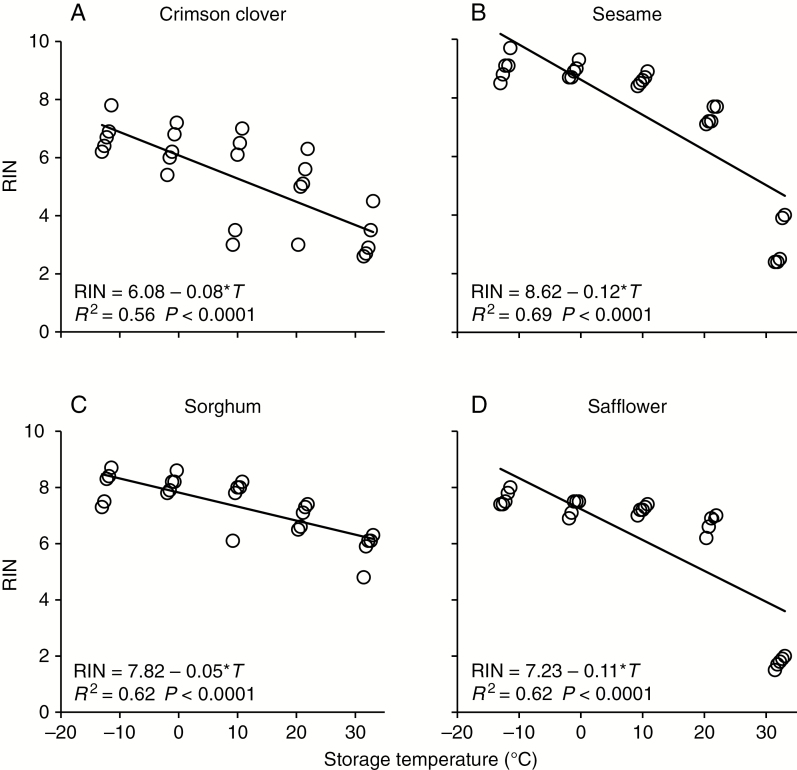
In 2017, RNA integrity of dry seeds was quantified by measuring RIN values (points in a capsule in Fig. 6). Samples of lettuce seed were depleted in 2008 and not available for RIN assessment. RIN values for seeds stored for 59 years at about −12 °C [from 6.80 ± 0.62 (crimson clover) to 9.04 ± 0.44 (sesame); Fig. 7] were comparable to values measured for fresh seeds from the refrigerator collection [from 6.42 ± 0.62 (onion) to 8.68 ± 0.43 (lettuce); Fig. 4A–D]. In contrast, RIN values for seeds stored at about 32 °C had decreased, and ranged from 1.78 ± 0.19 (safflower) to 5.84 ± 0.60 (sorghum) (Fig. 7, Table 2). RNA integrity decreased significantly with increased storage temperature (P < 0.0001).
Fig. 7.
RIN values for seeds in the Bass’s sealed can experiment that were stored for 59 years at 4 % moisture and the indicated temperatures before RNA was extracted and analysed for quality. RIN values are provided for each of five replicates and data points are jittered as an aid to the eye. The drawn lines represent a linear model of the correlation between temperature (T) and RIN value that was significant for each species; results of the regression are provided for each species. Sample size for each regression (N) is 25.
Table 2.
Summary of RNA integrity results in 2017 for seeds from each species and approximate storage temperature used in the sealed can experiment, in which seeds were adjusted to 4 % water content and sealed in cans filled with air, then stored for 59 years. Average RIN values ± s.d. from five independent samples are reported. Each sample included one to three seeds, or a seed fraction, depending on species
| −12 °C | −1 °C | 10 °C | 21 °C | 32 °C | |
|---|---|---|---|---|---|
| Crimson clover, 3 seeds |
6.80 ± 0.62 | 6.32 ± 0.70 | 5.22 ± 1.83 | 5.00 ± 1.23 | 3.24 ± 0.79 |
| Sesame, 2 seeds |
9.04 ± 0.44 | 8.92 ± 0.25 | 8.62 ± 0.19 | 7.38 ± 0.29 | 3.04 ± 0.83 |
| Sorghum, embryonic axis |
8.04 ± 0.61 | 8.14 ± 0.31 | 7.62 ± 0.86 | 6.98 ± 0.41 | 5.84 ± 0.60 |
| Safflower, 1 seed |
7.62 ± 0.27 | 7.30 ± 0.28 | 7.22 ± 0.15 | 6.72 ± 0.33 | 1.78 ± 0.19 |
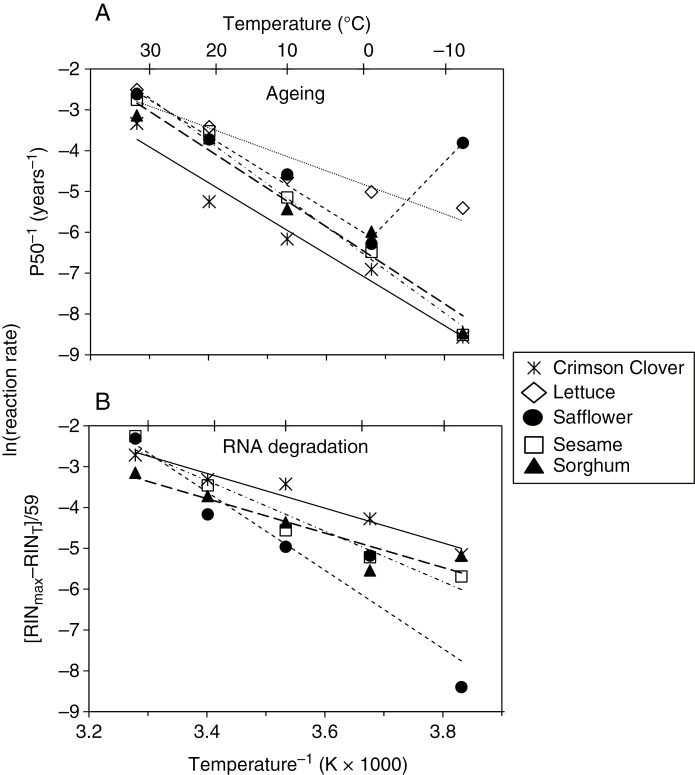
Temperature effects on reaction kinetics in dry systems are typically modelled by the Arrhenius equation, which plots ln(rate) versus T−1 (in kelvin) (Walters et al., 2004; Ballesteros and Walters, 2011) (Fig. 8). We used P50−1 to quantify the rate at which viability was lost in preserved seeds. The anomalous point at −12 °C for safflower germination was excluded from consideration of Arrhenius behaviour. Arrhenius behaviour of germination decline is indicated by the linear relationship between ln(P50−1) and 1/T for all five species (R2 > 0.92; P < 0.01). Arrhenius plots of P50−1 for species appeared nearly parallel, with an average slope of −8588 ± 1970 and ranging from −5284 (lettuce) to −10 523 (sesame); slopes were not significantly different (P > 0.05, ANCOVA) (Fig. 8A). Temperature dependency of RIN decline mirrored that of germination decline, with near-parallel linear relationships and slopes ranging from −4217 (sorghum) to −9590 (safflower). These slopes also were not significantly different (P > 0.05, ANCOVA), with an average of −6062 ± 2523 (Fig. 8B). Furthermore, the calculated slopes for germination and RNA integrity were not significantly different from each other (P > 0.05, ANCOVA). These Arrhenius slopes correspond to an apparent activation energy (Ea) for ageing reactions of about 55 KJ mol−1 (germination) or 57 KJ mol−1 (RIN) (Ea = Arrhenius slope × R, R = 8.314 J mol−1).
Fig. 8.
Arrhenius plots showing the temperature dependency of rates of ageing (P50−1) (A) and RNA degradation (B) of seeds in Bass’s sealed can experiment that were stored for 59 years at 4 % moisture and different temperatures. Ageing rate is the reciprocal of longevity and was calculated by the Avrami model in Fig. 6 for each species and temperature combination. RNA degradation rates were calculated from 2017 measurements of RIN, assuming that there was a linear relationship between RIN and time (Fig. 3) and that the maximum RIN (RINmax, average of the five highest RIN values) would be comparable to the RIN value from fresh seeds, had the technology been available in 1959. The R2 value for all lines is >0.90. Slopes range from −4200 to −10 500 and are not significantly different among species or among ageing and RIN measurements (P > 0.05). In (A), the point for safflower at −12 °C was not included in the linear regression.
Correlation of RNA integrity with germination proportion across species
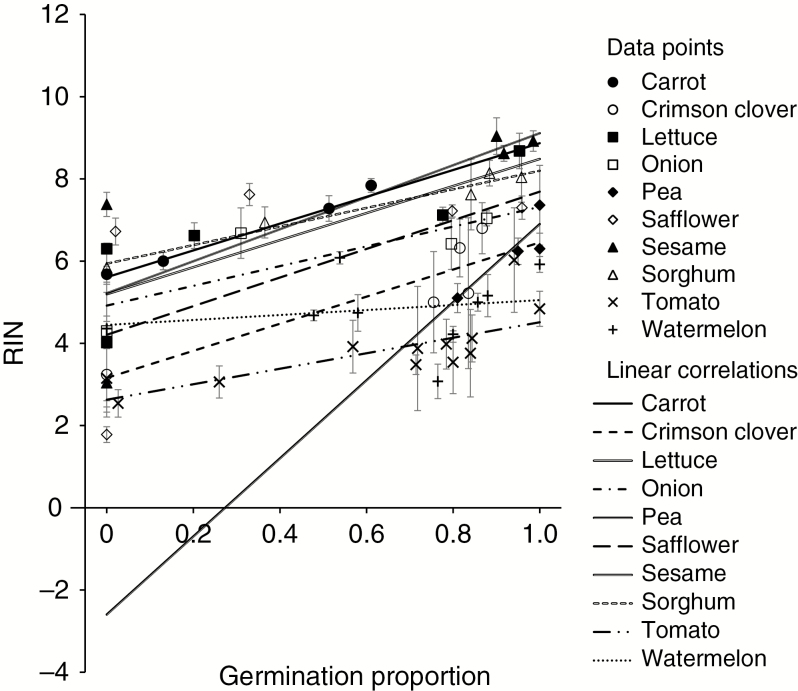
We modelled the effects of germination proportion and species on RIN values obtained from all experiments using a two-way mixed-model ANOVA (Fig. 9). Anomalous data for safflower (at −12 °C) and watermelon were excluded from the model. Overall, RIN values decreased with decreasing germination proportion (R2 = 0.70, P < 0.0001, RMSE = 1.10, N = 257), with a significant interaction between germination proportion (a fixed effect) and species (a random effect) (P = 0.029). The R2 values for linear regressions for each species varied, from 0.23 (pea) to >0.70 (sorghum, carrot) (Table 3). Germination proportion and RIN were poorly correlated for watermelon seeds (RIN = 4.45 + 0. 61 × germination proportion, R2 = 0.04, P = 0.20, d.f. = 42).
Fig. 9.
Linear correlations between germination proportion and RNA integrity for all experiments described in this paper. Significant positive linear correlations hold for all species except watermelon (for details see Table 3). Each point represents the average of five independent measurements; error bars show standard deviations. Linear correlations are based on all measurements, with two exceptions. For pea, only data for cotyledons were used, and for safflower data from −12 °C were excluded from the correlation.
Table 3.
Details of the linear correlations shown in Fig. 9, between each germination proportion and all associated RIN values, for species from the refrigerator and sealed can experiments. The correlation for pea used data from cotyledon tissue only, and the correlation for safflower excluded data from −12 °C
| Experiment | R 2 | P | Slope | Intercept | |
|---|---|---|---|---|---|
| Carrot | Refrigerator | 0.82 | < 0.0001 | 3.259 | 5.609 |
| Crimson clover | Sealed can | 0.46 | 0.0002 | 3.295 | 3.159 |
| Lettuce | Both | 0.58 | < 0.0001 | 3.292 | 5.193 |
| Onion | Refrigerator | 0.57 | 0.0001 | 2.401 | 4.919 |
| Pea (cotyledon) | Refrigerator | 0.23 | 0.0319 | 9.496 | -2.596 |
| Safflower | Sealed can | 0.43 | 0.0016 | 3.481 | 4.208 |
| Sesame | Sealed can | 0.60 | < 0.0001 | 3.893 | 5.218 |
| Sorghum | Sealed can | 0.70 | < 0.0001 | 2.260 | 5.938 |
| Tomato | Refrigerator | 0.32 | < 0.0001 | 1.884 | 2.628 |
| Watermelon | Refrigerator | 0.04 | 0.2022 | 0.606 | 4.447 |
DISCUSSION
We are exploring two ageing reactions in dry-stored seeds, namely RNA degradation and viability loss. Measurement of dry systems is an essential component of the work, which requires experiments lasting decades (Walters et al., 2010; Fleming et al., 2017, 2018). Previously, we demonstrated that RNA integrity in soybean seed declined over 27 years of dry storage, and quantification using RIN assays (Schroeder et al., 2006) strongly and positively correlated with germination potential (Fleming et al., 2017, 2018). Our purpose here is to expand these findings to other seed species and to compare the kinetics of degradation in the two ageing reactions. To meet the requirements of the long experimental time frame, we made use of two legacy seed collections begun in 1987 (refrigerator collection) and 1959 (Bass sealed can experiment). Each used an exemplar cultivar to represent a crop species; ten species were explored. We demonstrate here that (1) RNA integrity degraded with increased time or temperature in nine of ten species (Figs 4 and 7); (2) changes in RNA occurred contemporaneously with declining germination potential, and the effects of temperature on the kinetics of viability and RIN decline were similar (Fig. 8); and (3) RIN positively correlated with germination proportion for all species tested except watermelon (P < 0.05; Fig. 9, Table 3).
The ultimate goal of the work is to develop reliable predictors of seed longevity, i.e. the amount of time that an individual seed retains germination potential or that a seed lot maintains overall high viability (i.e. a high proportion of seeds that complete germination). It is well known that seed longevity varies with storage environment (temperature and moisture) and biological traits expressed at species and seed-lot levels (e.g. Walters et al., 2005; Nguyen et al., 2012; Nagel et al., 2015). Seed longevity, quantified in this paper as P50, is consistent with general, species-level trends (Walters et al., 2005). For example, lettuce and onion seeds are considered short-lived and did, indeed, age faster than pea and sorghum, which are considered long-lived (Walters et al., 2005) (Figs 1 and 6). However, tomato and watermelon seeds were thought to be long-lived, but deteriorated relatively rapidly in our experiment. Moreover, there was considerable unexplained variation in longevity for tomato or watermelon cohorts, which is apparent from the deviation of individual accessions from the Avrami model (Fig. 1) and underscores the need for predictive tools that account for separate ageing trajectories arising from maternal effects and processing factors. Germination was measured differently for the refrigerator and sealed can experiments to maintain continuity with earlier assays, and so P50 estimations from each experiment may be slightly different. We suspect they would be strongly correlated, but are not aware of published studies that directly compare germination method and P50.
In lieu of predicting seed longevity, deterioration is usually monitored through frequent germination tests. Unfortunately, germination tests can only detect ageing after the fact, when mortality is evident. As a snapshot in time, a germination test showing high viability does not indicate when a seed lot will begin to die, nor does a test showing low viability illuminate when a seed lot became ungerminable. For example, it appears that pea seeds harvested in 1991 (Fig. 1D) and sesame seeds stored at 10 °C since 1959 (Fig. 6B) are at the threshold of rapid viability loss, but that prediction can only be tested in years to come. Moreover, none of the seeds stored at about 32 °C for nearly 60 years germinated in 2017; rapid mortality actually began 35–45 years ago (Fig. 6) and recent germination assays provided little new information. Accurate measurement of germination during the period of rapid mortality is difficult. Retests may increase the accuracy of the measurement for a particular storage time (e.g. watermelon and tomato in Fig. 1E, F) or improve resolution of the period between P80 and P20 (times to reach 0.8 and 0.2 of initial germination proportion, respectively), which indicates when the seed lot changes from highly viable to mostly inviable (Figs 1 and 6).
The transition from alive to dead is a large and discrete change for an individual seed. The proportion function for a seed lot may appear continuous with time because it averages the ageing experience of individual seeds. In contrast, RNA integrity is a quantitative feature and it declines continuously with time. Because the ageing kinetics for the two reactions have different shapes (sigmoidal/linear), we can expect germination proportion and RNA quality to be correlated (Fig. 9), but not to track perfectly. The inherent disconnect is further illustrated by low and consistent variation of RIN values among replicates throughout the time series (Fig. 4). If RIN and viability tracked perfectly, we would expect higher variation of RIN in cohorts examined between P80 and P20 (Fig. 1), when there is also higher variation of germination. The observed low variation of RIN values in this time period means that RIN assays cannot detect individual seeds that do and do not have the capacity to germinate (Fleming et al., 2017), and underscores the complexity of events contributing to this physiological transition. Because RNA integrity and germination proportion exhibit profoundly different functions, the correlation between RIN values and germination necessarily depends on sampling within the deterioration time course; saturated sampling before P80 or after P20 is reached will give lower R2 values.
The imperfect correlation between RIN and mortality during ageing is actually advantageous to our search for early markers of ageing progress. Ageing is ‘asymptomatic’ before P80 is reached, meaning that changes in germination do not occur or are difficult to reliably detect. However, changes in RIN values were detectable within this time frame: RIN values were significantly different for cohorts that were either freshly harvested or near P80 (P < 0.01 for species with cohorts near P80, i.e. lettuce, pea, watermelon, tomato; Student’s t-test).
Regression coefficients for the relationship between RIN values and germination proportion differed by species (Fig. 9, Table 3). Differences in slopes arise from several factors, including bias from disproportionate sampling before P80 or after P20 is reached (described above), as well as potential differences in the mechanisms of seed deterioration among species. The different slopes can lead to different interpretations about interacting ageing reactions. For example, in pea the large slope between germination proportion and RIN gives the impression that a lot of damage to RNA occurs before loss in viability (Fig. 9), yet the rate of RIN decline was only slightly slower in pea compared with other species (Fig. 3). In contrast, regression slopes for germination versus RIN are smaller in tomato and watermelon compared with other species (Fig. 9), giving the impression that these seeds succumb with no or minor changes in RNA quality. However, the low initial RIN value (y-intercept of 4.7 in Fig. 4E, F) for these crops suggests low starting quality of RNA, perhaps indicative of considerable RNA damage during processing which may be undetected by us. Collectively, these results imply that RNA integrity declines both before germination is lost and while seeds die, but there is probably not a single threshold value for all species for either high viability or death. Moreover, chemical change, indicated by the different slopes for decline in RIN values with time (Fig. 4) and with germination (Fig. 9), points to both avoidance (damage prevention) and tolerance (damage survival) mechanisms underlying the longevity phenotype. Testing these predictions more robustly will require analysis of fresh data sets.
The single-time-point sampling of RIN values in the sealed can experiment, which varied temperature, provides confirmatory evidence that RIN decrease corresponds to expected damage to seeds during ageing, even when there is no observable difference in viability. For example, all sesame seeds stored at 21 and 32 °C appeared dead (germination proportion = 0) in 2017, but RIN values for RNA extracted from seeds were 7.38 ± 0.29 (21 °C treatment, which was dead near 2010; Fig. 6B) and 3.04 ± 0.83 (32 °C treatment, which was dead near 1985) (Fig. 7B). At the other extreme of seed health, crimson clover seeds stored at −12, −1 and 10 °C all had germination proportions >0.8 in 2017 (Fig. 6A), but RIN values were lower with increasing storage temperature from 6.8 ± 0.6 (−12 °C) to 5.2 ± 1.8 (10 °C) (Fig. 7A).
Temperature dependency of a reaction, quantified by Arrhenius kinetics, indicates how molecular mobility regulates reaction rates (Walters, 2005). Similar Arrhenius slopes among species and between viability loss and RIN decline (Fig. 8) indicate that molecular mobility has a common and predictable negative effect on degradation during dry storage. Differences in the Arrhenius pre-exponential coefficient among germplasm with different longevities provides clear evidence that intrinsic biological factors are critical to longevity. Similarities between Arrhenius temperature coefficients measured for viability loss and RNA degradation point to the possibility of predicting ageing during low-temperature storage from studies that combine RIN assessments and higher temperatures.
A temperature anomaly is indicated by faster ageing of safflower seeds stored at −12 °C (germination proportion = 0.3) compared with counterparts stored at −1 °C (germination proportion = 0.7) (Fig. 6D). Safflower seeds are considered to be orthodox (Royal Botanic Gardens, Kew, 2018), yet they show the classic symptoms of so-called intermediate storage physiology (Walters, 2015), which became evident after about 15 years of storage and were quite apparent after 60 years. We have linked this temperature anomaly to lipid crystallization (Crane et al., 2003, 2006; Hamilton et al., 2009). The phase behaviour of triacylglycerols within safflower is fairly complex, exhibiting considerable recrystallization in the 0 to −20 °C range after nuclei form (differential scanning calorimetry data not shown). The temperature anomaly is not apparent for RNA degradation, indicated by similar RIN values for seeds stored at −12 °C (7.6 ± 0.3) and −1 °C (7.3 ± 0.3). We interpret the continuous effect of temperature on RIN and discontinuous effect on viability as an indication of greater complexity than previously recognized in the regulation of ageing reactions in cytoplasm with both aqueous and lipid domains. These seed germination data present the daunting possibility of widespread occurrence of intermediate-type seeds and difficulty detecting this physiology with short-term exposure tests (Crane et al., 2006).
The RIN values for pea embryonic and cotyledon tissues correlate strongly (P = 0.0003), but are not identical. Embryonic axes have a slightly higher initial RIN than cotyledons and RIN then decays faster, accounting for a fractional regression slope and positive intercept >0 (Fig. 5). These results are similar to those previously reported for soybean seeds using ten cohorts, where RINcotyledon = 2.80 + 0.59 × RINaxis (Fleming et al., 2017). The low R2 between RIN values of embryonic axis and cotyledon tissues (0.47 for pea, 0.28 for soybean) suggests that subsampling tissues will not reliably predict germination. However, pea seeds did not express the effects of ageing through viability loss (Fig. 1D). In other words, while RNA integrity of pea seeds decreases with time, it is premature to correlate RNA integrity in either the embryonic axis or cotyledon tissue with viability. In contrast to RNA extracted from embryonic axis or cotyledon tissues, RNA extracted from pea seed coats was degraded in freshly harvested seeds and did not change appreciably with storage time.
Seeds of watermelon and tomato had the most variable response to storage time among the 40 species in the refrigerator collection (Fig. 1E, F); hence they were selected for RIN assessment with the idea that they represented the worst possible scenarios. Consistently, we found weak or no correlation of RIN values with storage time in these species (Fig. 4E, F). However, RIN values and germination were correlated for tomato, although with relatively low R2 (0.32; Table 3). RNA integrity was hard to characterize in this species (Fig. 3), contributing to some error, but more error comes from the high variability of longevity in tomato seeds within a sample and among harvest years. It is not surprising that RIN values and germination potential did not correlate well in watermelon seeds; both of these ageing reactions were weakly associated with time (Figs 1E and 4E), suggesting that low quality of watermelon seeds may not be attributable to ageing per se. We find it interesting that these two exceptional species both produce fleshy fruits; they may be subject to different water relations that cue expression of longevity genes (Chatelain et al., 2012; Verdier et al., 2013; Pereira Lima et al., 2017).
Conclusions
These experiments demonstrate that RNA integrity declines with storage time in dry seeds in nine of ten species studied. Here, RNA integrity was quantified by an RIN calculation that is based predominantly on comparisons of 18S and 25S rRNA. RIN values decline linearly with time and so complement information from germination assays, especially at storage times before viability loss is detectable as well as times well after all seeds have died. The kinetics of RIN decline and viability loss are profoundly and similarly affected by storage temperature among species, indicating that molecular mobility within the material solid of a dry seed is a general and major factor regulating ageing reactions. Accurately assessing ageing kinetics in dry systems required extremely long storage times (on the order of decades) and so the methods and materials used in this study provide a unique perspective on mechanisms that contribute to changing seed quality under dry storage conditions.
ACKNOWLEDGEMENTS
M.B.F. gratefully acknowledges the support of a USDA-ARS postdoctoral research fellowship. The authors acknowledge L. Bass and L. Wheeler for their foresight and dedication in initiating and continuing the sealed can experiments, and E. Dorr for assistance with germination assays.
LITERATURE CITED
- Abdul-Baki AA, Anderson JD. 1972. Physiological and biochemical deterioration of seeds. In: Kozlowski T, ed. Seed biology. Vol.II New York: Academic Press, 203–215. [Google Scholar]
- AOSA. 2017. Rules for testing seeds. Washington, DC: Association of Official Seed Analysts. [Google Scholar]
- Bai B, Peviani A, van der Horst S, et al. 2017. Extensive translational regulation during seed germination revealed by polysomal profiling. New Phytologist 214: 233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros D, Walters C. 2011. Detailed characterization of mechanical properties and molecular mobility within dry seed glasses: relevance to the physiology of dry biological systems. Plant Journal 68: 607–619. [DOI] [PubMed] [Google Scholar]
- Ballesteros D, Hill LM, Walters C. 2017. Variation of desiccation tolerance and longevity in fern spores. Journal of Plant Physiology 211: 53–62. [DOI] [PubMed] [Google Scholar]
- Bass LN, Clark DC, James E. 1962. Vacuum and inert-gas storage of lettuce seed. Proceedings of the Association of Official Seed Analysts 52: 116–122. [Google Scholar]
- Bass LN, Clark DC, James E. 1963a Vacuum and inert-gas storage of safflower and sesame seeds. Crop Science 3: 237–240. [Google Scholar]
- Bass LN, Clark DC, James E. 1963b Vacuum and inert-gas storage of crimson clover and sorghum seeds. Crop Science 3: 425–428. [Google Scholar]
- Buitink J, Leprince O. 2004. Glass formation in plant anhydrobiotes: survival in the dry state. Cryobiology 48: 215–228. [DOI] [PubMed] [Google Scholar]
- Cabiscol E, Tamarit J, Ros J. 2014. Protein carbonylation: proteomics, specificity and relevance to aging. Mass Spectrometry Reviews 33: 21–48. [DOI] [PubMed] [Google Scholar]
- Charron AJ, Quatrano RS. 2009. Between a rock and a dry place: the water-stressed moss. Molecular Plant 2: 478–486. [DOI] [PubMed] [Google Scholar]
- Chatelain E, Hundertmark M, Leprince O, et al. 2012. Temporal profiling of the heat-stable proteome during late maturation of Medicago truncatula seeds identifies a restricted subset of late embryogenesis abundant proteins associated with longevity. Plant, Cell and Environment 35: 1440–1455. [DOI] [PubMed] [Google Scholar]
- Crane J, Miller AL, Van Roekel JW, Walters C. 2003. Triacylglycerols determine the unusual storage physiology of Cuphea seed. Planta 217: 699–708. [DOI] [PubMed] [Google Scholar]
- Crane J, Kovach D, Gardner C, Walters C. 2006. Triacylglycerol phase and “intermediate” seed storage physiology: a study of Cuphea carthagenensis. Planta 223: 1081–1089. [DOI] [PubMed] [Google Scholar]
- Crawley MJ. 2007. The R book. Chichester: Wiley. [Google Scholar]
- FAO. 2014. Genebank standards for plant genetic resources for food and agriculture. Rome: FAO. [Google Scholar]
- Fleming MB, Richards CM, Walters C. 2017. Decline in RNA integrity of dry-stored soybean seeds correlates with loss of germination potential. Journal of Experimental Botany 44: 34–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming MB, Patterson EL, Reeves PA, Richards CM, Gaines TA, Walters C. 2018. Exploring the fate of mRNA in aging seeds: protection, destruction, or slow decay? Journal of Experimental Botany 69: 4309–4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- França MB, Panek AD, Eleutherio ECA. 2007. Oxidative stress and its effects during dehydration. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 146: 621–631. [DOI] [PubMed] [Google Scholar]
- Fundo JF, Quintas MAC, Silva CLM. 2015. Molecular dynamics and structure in physical properties and stability of food systems. Food Engineering Reviews 7: 384–392. [Google Scholar]
- Green EJ, Speller CF. 2017. Novel substrates as sources of ancient DNA: prospects and hurdles. Genes 8: 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. 2015. Free radicals in biology and medicine. Oxford: Oxford University Press. [Google Scholar]
- Hamilton KN, Ashmore SE, Pritchard HW. 2009. Thermal analysis and cryopreservation of seeds of Australian wild Citrus species (Rutaceae): Citrus australasica, C. inodora and C. garrawayi. CryoLetters 30: 268–279. [PubMed] [Google Scholar]
- Hoekstra FA, Golovina EA, Buitink J. 2001. Mechanisms of plant desiccation tolerance. Trends in Plant Science 6: 431–438. [DOI] [PubMed] [Google Scholar]
- Job C, Rajjou L, Lovigny Y, Belghazi M, Job D. 2005. Patterns of protein oxidation in Arabidopsis seeds and during germination. Plant Physiology 138: 790–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AA, Akman K, Calimport SRG, Wuttke D, Stolzing A, de Magalhães JP. 2012. The role of DNA methylation in aging, rejuvenation, and age-related disease. Rejuvenation Research 15: 483–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranner I, Kastberger G, Hartbauer M, Pritchard HW. 2010a Noninvasive diagnosis of seed viability using infrared thermography. Proceedings of the National Academy of Sciences of the USA 107: 3912–3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranner I, Minibayeva FV, Beckett RP, Seal CE. 2010b What is stress? Concepts, definitions and applications in seed science. New Phytologist 188: 655–673. [DOI] [PubMed] [Google Scholar]
- Kranner I, Chen H, Pritchard HW, Pearce SR, Birtić S. 2011. Inter-nucleosomal DNA fragmentation and loss of RNA integrity during seed ageing. Plant Growth Regulation 63: 63–72. [Google Scholar]
- McDonald MB. 1998. Seed quality assessment. Seed Science Research 8: 265–275. [Google Scholar]
- Michalak M, Plitta-Michalak BP, Naskręt-Barciszewska M, Barciszewski J, Bujarska-Borkowska B, Chmielarz P. 2015. Global 5-methylcytosine alterations in DNA during ageing of Quercus robur seeds. Annals of Botany 116: 369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mira S, González-Benito ME, Hill LM, Walters C. 2010. Characterization of volatile production during storage of lettuce (Lactuca sativa) seed. Journal of Experimental Botany 61: 3915–24. [DOI] [PubMed] [Google Scholar]
- Mira S, Hill LM, González-Benito ME, Ibáñez MA, Walters C. 2016. Volatile emission in dry seeds as a way to probe chemical reactions during initial asymptomatic deterioration. Journal of Experimental Botany 67: 1783–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan P, Haussmann MF. 2006. Do telomere dynamics link lifestyle and lifespan? Trends in Ecology and Evolution 21: 47–53. [DOI] [PubMed] [Google Scholar]
- Nagel M, Kranner I, Neumann K, et al. 2015. Genome-wide association mapping and biochemical markers reveal that seed ageing and longevity are intricately affected by genetic background and developmental and environmental conditions in barley. Plant, Cell and Environment 38: 1011–1022. [DOI] [PubMed] [Google Scholar]
- Nguyen T-P, Keizer P, van Eeuwijk F, Smeekens S, Bentsink L. 2012. Natural variation for seed longevity and seed dormancy are negatively correlated in Arabidopsis. Plant Physiology 160: 2083–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogé L, Bourdais G, Bove J, et al. 2008. Protein repair L-isoaspartyl methyltransferase1 is involved in both seed longevity and germination vigor in Arabidopsis. Plant Cell 20: 3022–3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogneva ZV, Dubrovina AS, Kiselev KV. 2016. Age-associated alterations in DNA methylation and expression of methyltransferase and demethylase genes in Arabidopsis thaliana. Biologia Plantarum 60: 628–634. [Google Scholar]
- Pereira Lima JJ, Buitink J, Lalanne D, et al. 2017. Molecular characterization of the acquisition of longevity during seed maturation in soybean. PLoS ONE 12: e0180282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petla BP, Kamble NU, Kumar M, et al. 2016. Rice PROTEIN L-ISOASPARTYL METHYLTRANSFERASE isoforms differentially accumulate during seed maturation to restrict deleterious isoAsp and reactive oxygen species accumulation and are implicated in seed vigor and longevity. New Phytologist 211: 627–645. [DOI] [PubMed] [Google Scholar]
- Rajjou L, Duval M, Gallardo K, et al. 2012. Seed germination and vigor. Annual Review of Plant Biology 63: 507–533. [DOI] [PubMed] [Google Scholar]
- Raval A, Sasidharan N, Rao K. 2016. Effect of seed extraction procedures on seed quality parameters in tomato. Advances in Life Sciences 5: 9020–9024. [Google Scholar]
- Royal Botanic Gardens, Kew. 2018. Seed Information Database (SID). Version 7.1 http://data.kew.org/sid/ (October 2018).
- Sattler SE, Gilliland LU, Magallanes-Lundback M, Pollard M, DellaPenna D. 2004. Vitamin E is essential for seed longevity and for preventing lipid peroxidation during germination. Plant Cell 16: 1419–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder A, Mueller O, Stocker S, et al. 2006. The RIN: an RNA integrity number for assigning integrity values to RNA measurements. BMC Molecular Biology 7: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terskikh VV, Zeng Y, Feurtado JA, Giblin M, Abrams SR, Kermode AR. 2008. Deterioration of western redcedar (Thuja plicata Donn ex D. Don) seeds: protein oxidation and in vivo NMR monitoring of storage oils. Journal of Experimental Botany 59: 765–777. [DOI] [PubMed] [Google Scholar]
- Verdier J, Lalanne D, Pelletier S, et al. 2013. A regulatory network-based approach dissects late maturation processes related to the acquisition of desiccation tolerance and longevity of Medicago truncatula seeds. Plant Physiology 163: 757–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters C. 2005. Dying while dry: kinetics and mechanisms of deterioration in desiccated organisms. Integrative and Comparative Biology 45: 751–758. [DOI] [PubMed] [Google Scholar]
- Walters C. 2015. Orthodoxy, recalcitrance and in-between: describing variation in seed storage characteristics using threshold responses to water loss. Planta 242: 397–406. [DOI] [PubMed] [Google Scholar]
- Walters C, Wheeler L, Stanwood PC. 2004. Longevity of cryogenically stored seeds. Cryobiology 48: 229–244. [DOI] [PubMed] [Google Scholar]
- Walters C, Wheeler LM, Grotenhuis JM. 2005. Longevity of seeds stored in a genebank: species characteristics. Seed Science Research 15: 1–20. [Google Scholar]
- Walters C, Ballesteros D, Vertucci VA. 2010. Structural mechanics of seed deterioration: standing the test of time. Plant Science 179: 565–573. [Google Scholar]
- Waterworth WM, Bray CM, West CE. 2015. The importance of safeguarding genome integrity in germination and seed longevity. Journal of Experimental Botany 66: 3549–3558. [DOI] [PubMed] [Google Scholar]
- Wurtmann EJ, Wolin SL. 2010. RNA under attack: cellular handling of RNA damage. Critical Reviews in Biochemistry and Molecular Biology 44: 34–49. [DOI] [PMC free article] [PubMed] [Google Scholar]