Abstract
Objective
To evaluate the effects of four drug (quadruple) versus three drug (triple) combination antiretroviral therapies in treatment naive people with HIV, and explore the implications of existing trials for clinical practice and research.
Design
Systematic review and meta-analysis of randomised controlled trials.
Data sources
PubMed, EMBASE, CENTRAL, Web of Science, and the Cumulative Index to Nursing and Allied Health Literature from March 2001 to December 2016 (updated search in PubMed and EMBASE up to June 2018); and reference lists of eligible studies and related reviews.
Study selection
Randomised controlled trials comparing quadruple with triple combination antiretroviral therapies in treatment naive people with HIV and evaluating at least one effectiveness or safety outcome.
Review methods
Outcomes of interest included undetectable HIV-1 RNA, CD4 T cell count, virological failure, new AIDS defining events, death, and severe adverse effects. Random effects meta-analyses were conducted.
Results
Twelve trials (including 4251 people with HIV) were eligible. Quadruple and triple combination antiretroviral therapies had similar effects on all relevant effectiveness and safety outcomes, with no point estimates favouring quadruple therapy. With the triple therapy as the reference group, the risk ratio was 0.99 (95% confidence interval 0.93 to 1.05) for undetectable HIV-1 RNA, 1.00 (0.90 to 1.11) for virological failure, 1.17 (0.84 to 1.63) for new AIDS defining events, 1.23 (0.74 to 2.05) for death, and 1.09 (0.89 to 1.33) for severe adverse effects. The mean difference in CD4 T cell count increase between the two groups was −19.55 cells/μL (−43.02 to 3.92). In general, the results were similar, regardless of the specific regimens of combination antiretroviral therapies, and were robust in all subgroup and sensitivity analyses.
Conclusion
In this study, effects of quadruple combination antiretroviral therapy were not better than triple combination antiretroviral therapy in treatment naive people with HIV. This finding lends support to current guidelines recommending the triple regimen as first line treatment. Further trials on this topic should be conducted only when new research is justified by adequate systematic reviews of the existing evidence. However, this study cannot exclude the possibility that quadruple cART would be better than triple cART when new classes of antiretroviral drugs are made available.
Introduction
HIV infection is responsible for a substantial disease burden. With an estimated 36.7 million people living with HIV/AIDS, 1.8 million new cases of HIV infection, and 1.0 million AIDS related deaths in 2016, the disease is one of the five leading causes of total years of life lost globally.1 2 The use of antiretroviral therapy for people with HIV is effective in suppressing viral replication, decreasing viral load, reconstructing immune system, preventing progression to advanced stages and death, prolonging life expectancy, improving quality of life, and reducing risk of transmission to others.3 4 5 6 7 8 9
Antiretroviral drugs have six classes: C-C chemokine receptor type 5 (CCR5) antagonists, nucleoside reverse transcriptase inhibitors (NRTI), non-nucleoside reverse transcriptase inhibitors (NNRTIs), fusion inhibitors, protease inhibitors, and integrase strand transfer inhibitors.10 Theoretically, they can be used as either monotherapy or combination treatments. In 2002, a systematic review by Jordan and colleagues11 showed that triple therapy was more effective than double therapy, and that double therapy was more effective than monotherapy in first line treatment. Possibly as a result, an increased number of drugs was hypothesised to be associated with enhanced effectiveness of antiretroviral therapy. Trials comparing quadruple with triple combination antiretroviral therapies (cART) have been conducted in the past two decades.12 13
However, during the same period, practice guidelines14 15 16 17 have consistently recommended triple cART as first line treatment for most treatment naive people with HIV, consisting of two NRTI drugs as the so-called backbone plus another drug (an NNRTI, integrase strand transfer inhibitor, protease inhibitor, or CCR5 antagonist). These guidelines have rarely mentioned quadruple cART, which has naturally led to the question of whether quadruple cART is more favourable than triple cART. If so, guidelines should be updated to reflect this finding. If not, whether and how further trials on this topic should be conducted would be important to know, so that limited resources can be allocated to areas where genuine uncertainties exist and used in a more efficient way. This question is especially relevant because of the idea of evidence based research, which advocates that no new studies should be conducted without an adequate systematic review of existing evidence justifying new research, and is increasingly accepted by the community of biomedical research.18
However, the above question could not be readily answered by individual trials because their findings were often inconsistent. For example, Gulick and collegaues19 found that people with HIV receiving quadruple therapy seemed more likely to have undetectable HIV-1 RNA (sample size 765; odds ratio 1.38, 95% confidence interval 0.96 to 1.98) but a lower mean increase in CD4 T cell count (mean difference −8.00 cells/μL, −33.62 to 17.62) than those receiving triple cART. However, Orkin and colleagues20 had the opposite results (sample size 53; 0.51, 0.15 to 1.96, for undetectable HIV-1 RNA; 21.00 cells/μL, −52.90 to 94.90, for increase in CD4 T cell count).
With most existing trials small in sample size, it remained uncertain whether the inconsistency between studies was caused by insufficient statistical power, true difference in the effects of different cART, or both. To dissect this issue, a comprehensive analysis of existing evidence was needed. Therefore, we conducted a systematic review and meta-analysis to compare the effects of quadruple with triple cART in treatment naive people with HIV and explore the implications of evidence from existing trials for clinical practice and research.
Methods
This systematic review was conducted in accordance with the recommendations of the Cochrane handbook for systematic reviews of interventions (version 5.1.0) 21 and reported following the PRISMA statement.22
Literature search
We conducted a search in PubMed, EMBASE, Cochrane Central Register of Controlled Trials, Web of Science, and the Cumulative Index to Nursing and Allied Health Literature via EBSCO from March 2001 to December 2016, and updated the search in PubMed and EMBASE up to June 2018. March 2001 was set as the starting point because Jordan and colleagues conducted a comprehensive literature search11 in February 2001 and identified only one study comparing quadruple cART with triple cART,23 which has been included in the present systematic review. Four groups of keywords (together with their synonyms and derivatives) were used in the literature search, including those for HIV/AIDS; randomised controlled trial; treatment and therapy; and abacavir, didanosine, emtricitabine, lamivudine, stavudine, tenofovir, zidovudine, NRTI, triple, and quadruple (supplement 1 shows the PubMed search strategy). We used the names of seven available NRTI drugs as keywords because the NRTI drugs are the so-called backbones in all cART. The reference list of included studies and relevant reviews were manually searched for additional studies.
Eligibility criteria and study selection
Randomised controlled trials that compared quadruple cART with triple cART for the first line treatment of people with HIV and evaluated at least one outcome of effectiveness or safety were considered eligible for the present systematic review. Measures of effectiveness included undetectable HIV-1 RNA (<50 copies/mL), CD4 T cell count, virological failure, new AIDS defining events, and all cause mortality, and the measure of safety was severe adverse effects (≥grade 3) as a composite outcome. We examined the composite outcome instead of specific adverse effects, because the composite was reported consistently by studies and thus combinable.
In determining the number of drugs, boosted protease inhibitor was counted as one drug, because the booster (ritonavir, cobicistat) is used in low doses to inhibit a particular liver enzyme that normally metabolises protease inhibitors and enhances the pharmacokinetic profile of other protease inhibitors rather than for its own antiretroviral activity.24 We excluded studies with a follow-up length shorter than 48 weeks, which was not sufficient for observing clinically significant outcomes for a lifelong intervention. This timeframe had already been adopted by previous systematic reviews on HIV treatment.25 26 27 If multiple publications from the same study were found, only the one with most complete information was included.
All records retrieved by literature search were assessed by two reviewers (QF and AZ) independently for inclusion. The titles and abstracts were first screened to examine their potential eligibility. Full texts of potentially eligible studies were then reviewed to determine their final eligibility. All disagreements were resolved by discussion or referring to a third reviewer (ZY).
Data extraction and quality assessment
A pre-designed form was used to extract study bibliographic information (eg, first author, publication year, study country), treatment information (eg, treatment duration, regimen), patient characteristics (eg, sample size, age, proportion of male patients, baseline count of CD4 T cells, baseline viral load of HIV-1 RNA), main results (eg, risk ratio, mean difference), and information related to quality assessment from eligible studies. Two reviewers (QF and AZ) extracted data and assessed the methodological quality of included studies independently. All disagreements were resolved through discussion.
The Cochrane risk of bias tool was used for quality assessment.28 This tool evaluated biases from seven domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and others. The risk of bias in each domain was judged as low, high, or unclear. The overall risk of bias in a study was classified as low if all domains had low risk, as high if one or more domains had high risk, or as unclear otherwise.28 Because the study outcomes included both subjective (certain severe adverse effects such as pain, fatigue, and psychological symptoms require subjective assessments for grading) and objective (that is, other outcomes confirmed with standardised laboratory techniques or objective records) ones, the risk of bias in data on different types of outcomes was assessed separately according to Cochrane’s guidelines.28
Data analysis
Triple cART was used as the reference group in meta-analysis. For continuous outcomes (that is, CD4 T cell count) in this systematic review, the mean change from baseline and standard deviation were extracted or calculated for each group in each study (supplement 2). We then calculated the difference in mean change between groups, and subsequently pooled them across the studies included in the meta-analysis. A difference in mean change greater than 0 favours quadruple therapy. For the other outcomes, which were binary, a risk ratio with 95% confidence interval was calculated on the basis of the number of people with HIV and number of events in each treatment group within each study, and then combined across studies to obtain an overall estimate. A risk ratio greater than 1 favours triple cART for all binary outcomes except for undetectable HIV-1 RNA.
The outcome data reported at the longest follow-up were used for primary analysis. This practice differed from previous reviews that used a uniform time frame of 48 weeks,25 27 because we wanted to include as many eligible studies in the analysis as possible. The studies reporting data at 48 weeks were also combined in the present systematic review, but as a sensitivity analysis.
For those eligible trials that had more than one triple cART arm or quadruple cART arm within the same study, we first combined the arms with the same number of drugs (eg, combining multiple triple cART arms into one single triple cART arm) using the method recommended in the Cochrane handbook,29 and then compared the merged arm with the comparative arm.
The random effects model was used for all meta-analyses. Statistical heterogeneity among the studies was measured by the Cochrane Q test and I2 statistic. A P value of 0.10 or less or an I2 of 50% or more suggested substantial heterogeneity, in which case we used subgroup analyses and meta-regression, if appropriate, to analyse potential sources of heterogeneity.30 31 The prespecified subgroup factors included CD4 T cell counts at baseline (<200 cells/μL or ≥200 cells/μL) and the class of the fourth drug in the quadruple cART arm. We conducted sensitivity analyses by removing the studies with high risk of bias, using the outcome data reported at 48 weeks only, or replacing the standard deviations of increase in CD4 T cell counts with those estimated by different methods. We planned to assess potential publication bias by funnel plots and Egger’s test but actually did not do so, because the number of studies included in every meta-analysis was fewer than 10, in which case the funnel plots and Egger’s test could yield misleading results and were not recommended.32 All data analyses were performed with the meta package (version 4.9-2) in R software (version 3.4.3).
Patient and public involvement
No people with HIV were involved in the design or implementation of the study, measurement of the outcome, interpretation of results, or writing or editing of the manuscript. There are no plans to disseminate the results of the research to study participants or the relevant patient community.
Results
As shown in the figure 1, 13 710 records were retrieved from initial electronic search and one additional record from a later manual search. After screening titles, abstracts, and full texts, 12 studies including 4251 people with HIV were finally included in this systematic review.12 19 20 33 34 35 36 37 38 39 40 41
Fig 1.
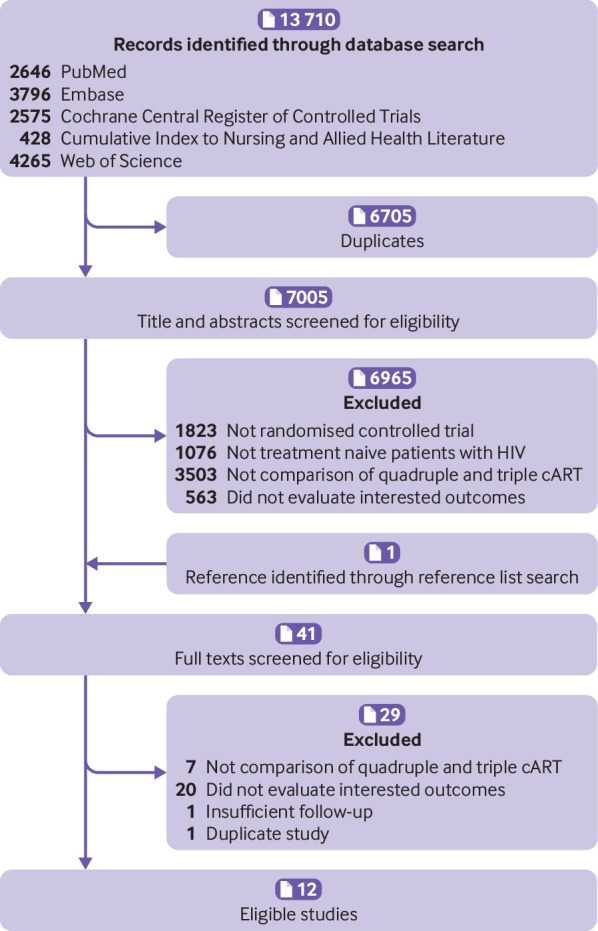
The flowchart of study selection
Study characteristics
Characteristics of 12 eligible studies are shown in table 1. Of 4251 patients who were randomised, 1693 were assigned to receive quadruple cART. One study12 included one triple cART arm and two quadruple cART arms, one study33 included two triple cART arms and one quadruple cART arm, one study40 included three triple cART arms and one quadruple cART arm, while the other nine studies included two arms (that is, one triple cART arm and one quadruple cART arm). In 10 studies, the quadruple cART consisted of three drugs that were used in the triple cART arm plus a fourth drug, which was most frequently an NNRTI, followed by an NRTI, protease inhibitor, CCR5 antagonist, and fusion inhibitor. In another study,35 the triple cART and quadruple cART had only two drugs in common, while the two cART groups in the remaining study41 had no drugs in common. The median sample size was 214 (range 30-1216). The mean age was 37.1 years (range 32.9-43.5) and the median proportion of male individuals was 77.1% (range 58-100%). The follow-up length varied from 48 to 144 weeks (median 48). Four studies19 33 38 41 recruited people with HIV with a mean CD4 T cell count above 200 cells/μL at baseline.
Table 1.
Characteristics of included studies comparing quadruple with triple combination antiretroviral therapy as first line treatment for people with HIV
| Study ID (country) | Sample size (quadruple/triple) | Age | Male patients (%) | Baseline | Follow-up (weeks) | Antiretroviral therapy | Risk of bias | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CD4 count (cells/µL) | HIV viral load (log10/mL) |
Quadruple combination | Triple combination | Objective outcomes | Subjective outcomes | ||||||
| Fischl 200312 (US, Italy) | 517 (349/168) | 38.2 | 81.4 | 161.00 | 5.42 | 108 | 3TC+AZT+IDV+EFV; 3TC+AZT+IDV+NFV |
3TC+AZT+IDV | Low | High | |
| Kirk 200335 (Denmark) | 233 (118/115) | 37 | 75.5 | 137.50 | 5.00 | 48 | 2 NRTIs+NFV+NVP | 2 NRTIs+SQV/r | Low | NA | |
| van Leth 200440 (US, Australia, Europe, South Africa, Thailand) | 1216 (209/1007) | 34.1 | 63.4 | 190.00 | 4.70 | 48 | d4T+3TC+NVP+EFV | d4T+3TC+EFV; d4T+3TC+NVP (once daily); d4T+3TC+NVP (twice daily) | High | High | |
| Orkin 200520 (UK) | 53 (27/26) | NA | NA | 118.50 | 5.50 | 48 | AZT+3TC+EFV+ABC | AZT+3TC+EFV | Unclear | NA | |
| Portilla 200537 (Spain) | 30 (15/15) | 36.6 | 76.7 | 53.65 | 5.39 | 48 | SQV+2 NRTIs+NFV | SQV+2 NRTIs | Unclear | NA | |
| INITIO 200633 (Australia, Brazil, Canada, New Zealand, and 17 European countries) | 764 (250/514) | 38.6 | 79 | 223.33 | 4.93 | 144 | ddl+d4T+EFV+NFV | ddl+d4T+EFV; ddl+d4T+NFV |
Low | Low | |
| Gulick 200619 (US) | 765 (383/382) | 38 | 81 | 215.00 | 4.77 | 144 | AZT+3TC+EFV+ABC | AZT+3TC+EFV | Unclear | NA | |
| Moyle 200636 (UK) | 113 (57/56) | 39.5 | 91.2 | 173.50 | 5.20 | 48 | AZT+3TC+ABC+TDF | AZT+3TC+EFV | Unclear | Unclear | |
| Joly 201334 (France) | 194 (100/94) | 43.5 | 77.8 | 32.00 | 5.40 | 48 | FTC+TDF+LPV/r (or EFV)+ENF | FTC+TDF+LPV/r (or EFV) | Low | Unclear | |
| Puertas 201438 (Spain) | 30 (15/15) | 32.9 | 100 | 424.50 | 4.95 | 48 | RAL+TDF+FTC+MRV | RAL+TDF+FTC | Unclear | NA | |
| Sierra-Madero 201439 (Mexico, South Africa) | 276 (140/136) | 37.1 | 64.5 | 34.00 | 5.35 | 48 | EFV+TDF+FTC+MRV | EFV+TDF+FTC | Low | NA | |
| Mora-Peris 201841 (UK) | 60 (30/30) | 33 | 58 | 441.00 | 4.67 | 48 | ABC+3TC+DRV/r+MRV | TDF+FTC+ATV/r | Unclear | NA | |
3TC=lamivudine; ABC=abacavir; AZT=zidovudine; d4T=stavudine; ddl=didanosine; EFV=efavirenz; ENF=enfuvirtide; FTC=emtricitabine; IDV=indivavir; LPV=lopinavir; MRV=maraviroc; NFV=nelfinavir; NRTI=nucleoside reverse-transcriptase inhibitor; NVP=nevirapine; RAL=raltegravir; SQV=saquinavir; TDF=tenofovir disoproxil fumarate; DRV=darunavir; ATV=atazanavir; /r=boosted with ritonavir; NA=not available or not applicable.
All 12 studies reported on objective outcomes (table 2). Five studies12 33 34 35 39 were at low risk of bias, one study40 that reported data on undetectable HIV-1 RNA and virological failure was at high risk of bias due to incomplete outcome data, and six studies19 20 36 37 38 41 were at unclear risk owing to insufficient reporting of randomisation and allocation concealment. Five studies reported on severe adverse events: one study33 was at low risk of bias, two12 40 were at high risk of bias because of no blinding of outcome assessment and incomplete outcome data, and another two34 36 were at unclear risk of bias because of insufficient reporting of randomisation and blinding of outcome assessment.
Table 2.
Results of risk of bias assessment of included studies comparing quadruple with triple combination antiretroviral therapy as first line treatment for people with HIV
| Study (first author and year) | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias | Overall risk of bias | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| For subjective outcomes | For objective outcomes | For subjective outcomes | For objective outcomes | For subjective outcomes | For objective outcomes | |||||||
| Fischl 200312 | Low | Low | Low | Low | High | Low | Low | Low | Low | High | Low | |
| Kirk 200335 | Low | Unclear | NA | Low | NA | Low | Low | Low | Low | NA | Low | |
| van Leth 200440 | Unclear | Low | Low | Low | High | Low | High | Low | Low | High | High | |
| Orkin 200520 | Unclear | Unclear | NA | Low | NA | Low | Unclear | Low | Low | NA | Unclear | |
| Portilla 200537 | Unclear | Unclear | NA | Low | NA | Low | Unclear | Low | Low | NA | Unclear | |
| INITIO 200633 | Low | Low | Low | Low | Low | Low | Low | Low | Low | Low | Low | |
| Gulick 200619 | Unclear | Unclear | NA | Low | NA | Low | Low | Low | Low | NA | Unclear | |
| Moyle 200636 | Unclear | Low | Low | Low | Unclear | Low | Low | Low | Low | Unclear | Unclear | |
| Joly 201334 | Low | Low | Low | Low | Unclear | Low | Low | Low | Low | Unclear | Low | |
| Puertas 201438 | Unclear | Unclear | NA | Low | NA | Low | Low | Low | Low | NA | Unclear | |
| Sierra-Madero 201439 | Low | Low | NA | Low | NA | Low | Low | Low | Low | NA | Low | |
| Mora-Peris 201841 | Unclear | Unclear | NA | Low | NA | Low | Low | Low | Low | NA | Unclear | |
NA=study did not report subjective outcome (severe adverse effects).
Comparative effectiveness and safety of quadruple versus triple therapy
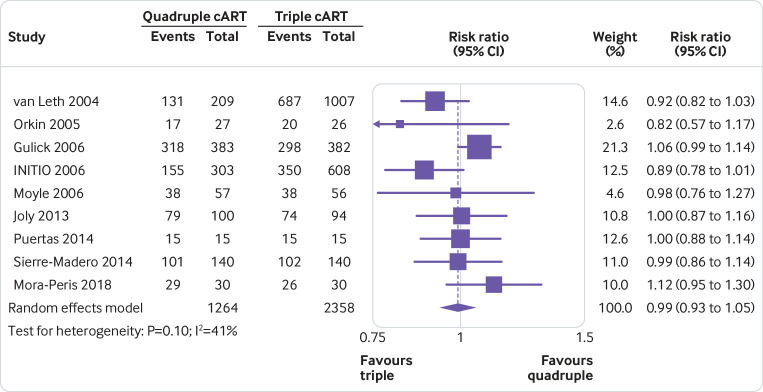
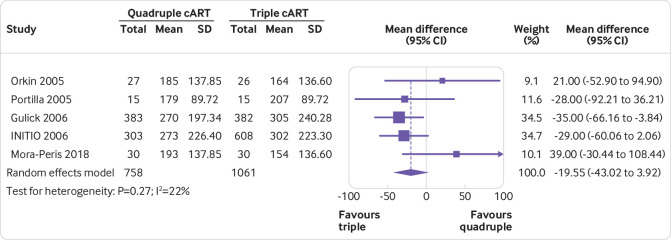
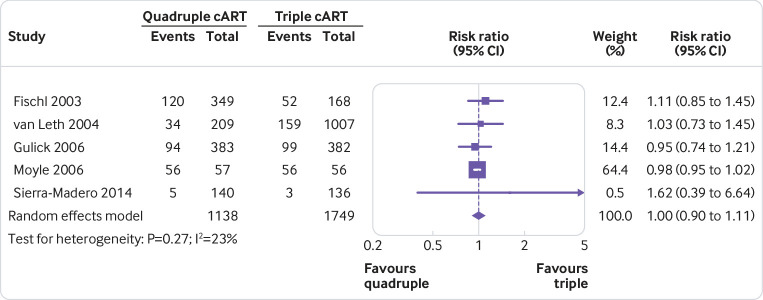
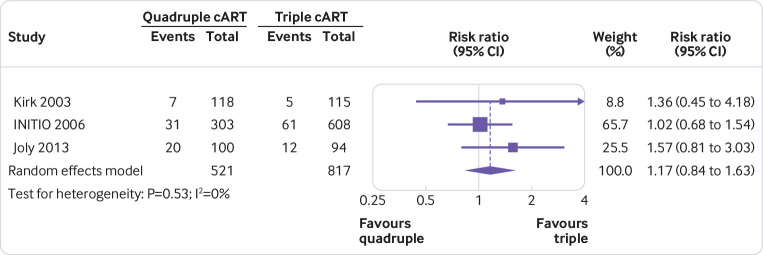
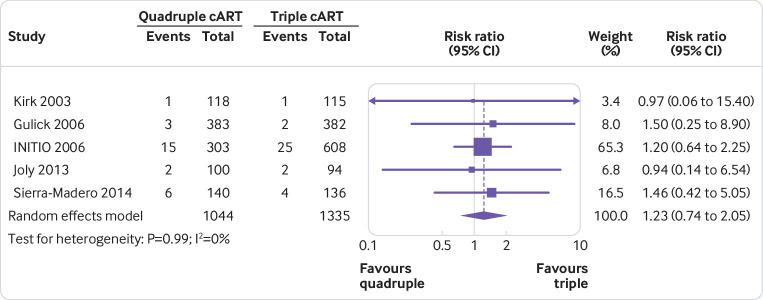
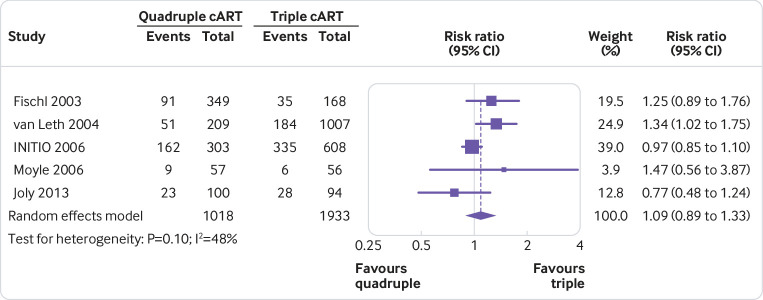
None of the quadruple therapies was significantly better than any of the triple therapies in any trial on any of the outcomes assessed (fig 2, fig 3, fig 4, fig 5, fig 6, fig 7). Meta-analyses showed that quadruple and triple cART had similar effects on all interested outcomes, with none of the point estimates favouring quadruple cART. Specifically, nine studies (n=3622 people with HIV) reported on undetectable HIV-1 RNA, and the overall risk ratio was 0.99 (95% confidence interval 0.93 to 1.05; heterogeneity test I2=41%, P=0.10; fig 2). Five studies (n=1819) reported on change of CD4 T cell count; the overall mean difference was −19.55 cells/μL (−43.02 to 3.92; I2=22%, P=0.27; fig 3). Five studies (n=2887) reported on virological failure; the pooled risk ratio was 1.00 (0.90 to 1.11; I2=23%, P=0.27; fig 4). Three studies (n=1338) reported on new AIDS defining events; the pooled risk ratio was 1.17 (0.84 to 1.63; I2=0%, P=0.53; fig 5). Five studies (n=2379) reported on death; the pooled risk ratio was 1.23 (0.74 to 2.05; I2=0%, P=0.99; fig 6). Five studies (n=2951) reported on severe adverse effects; the pooled risk ratio was 1.09 (0.89 to 1.33; I2=48%, P=0.10; fig 7).
Fig 2.
Meta-analysis of comparative effects between quadruple and triple combination antiretroviral therapies (cART) as first line treatment for people with HIV, on undetectable HIV-1 RNA
Fig 3.
Meta-analysis of comparative effects between quadruple and triple combination antiretroviral therapies (cART) as first line treatment for people with HIV, on increase in CD4 T cell count (cells/μL). SD=standard deviation
Fig 4.
Meta-analysis of comparative effects between quadruple and triple combination antiretroviral therapies (cART) as first line treatment for people with HIV, on virological failure
Fig 5.
Meta-analysis of comparative effects between quadruple and triple combination antiretroviral therapies (cART) as first line treatment for people with HIV, on new AIDS defining events
Fig 6.
Meta-analysis of comparative effects between quadruple and triple combination antiretroviral therapies (cART) as first line treatment for people with HIV, on death
Fig 7.
Meta-analysis of comparative effects between quadruple and triple combination antiretroviral therapies (cART) as first line treatment for people with HIV, on severe adverse effects
Subgroup and sensitivity analyses
The P value for Cochrane’s Q test suggested presence of substantial heterogeneity in figure 2 and figure 7. Prespecified subgroup analyses showed that the results did not vary considerably according to baseline CD4 T cell count and the class of the fourth drug in the quadruple cART arm (supplement 3A-D). Because the number of studies included in the two meta-analyses was small, meta-regression was not performed. Sensitivity analyses that removed studies with potential bias showed consistent results with the primary meta-analyses (risk ratio 1.00 for undetectable HIV-1 RNA, 1.00 for virological failure, 0.98 for severe adverse effects, and 1.02 for AIDS defining events; supplement 3E, 3F, 3H, and 3I, respectively). Such sensitivity analyses were not performed for other outcomes because none of the studies reporting them was at a high risk of bias. Sensitivity analysis that pooled the outcome data reported at 48 weeks, which also showed consistent results, was performed for undetectable HIV-1 RNA and increase in CD4 T cell count only (supplement 3J and 3K) and not for other outcomes owing to lack of relevant data. When the standard deviations for increase in CD4 T cell count were replaced by those estimated by different methods, the results of figure 3 either remained similar (that is, quadruple and triple arms not statistically different) or favoured triple therapies (supplement 2). As explained in the methods section, potential publication bias was not assessed, because the number of studies was small (<10) in all of the above meta-analyses.
Discussion
Principal findings
This systematic review pooled data from 12 studies and showed that the effects of quadruple cART were not better than standard triple cART for first line treatment of people with HIV. Although marginally substantial heterogeneity was observed for two outcomes (undetectable HIV-1 RNA and severe adverse effects), prespecified subgroup and sensitivity analyses suggested that the primary results were robust across various scenarios. The study13 that was excluded from this systematic review owing to insufficient follow-up time showed that the addition of a fourth drug did not lead to a greater increase in the CD4 T cell count at 36 weeks, which was consistent with our findings. The findings were also supported by mechanism studies conducted in animals.42
Strengths, weaknesses, and implications of the study
The systematic review by Jordan and colleagues11 showed that escalating the number of antiretroviral drugs was an effective strategy to improve clinical outcomes. However, it did not evaluate quadruple cART because of the sparse data. The present systematic review is an important addition in this regard. Taking the results from the two systematic reviews together, we found that the effectiveness of cART increases as the number of drugs escalates up to three, but does not continue increasing at four, while the risk of adverse effects seems to keep increasing as the number of drugs increases.
In the included studies, the triple therapies consisted of two NRTI drugs plus one NNRTI, protease inhibitor, or integrase strand transfer inhibitor as recommended, and the added fourth drug varied across studies, involving five classes of antiretroviral drugs. Although different drug combinations could influence the effects of quadruple or triple cART themselves, they would not necessarily lead to a big difference between the two types of regimen,11 43 as shown by the subgroup analyses of this systematic review. In addition, the meta-analyses with no or very low heterogeneity (that is, fig 3, fig 4, fig 5, fig 6) showed that the effects of quadruple cART were not better than those of triple cART. Furthermore, adding a fourth drug to first line treatment not only increases financial and pill burden, which might lead to lower adherence to treatment and consequently drug resistance and treatment failure, but also limits drug options for second line treatments and beyond.44 45 Thus, triple cART regimens could be seen as being superior to quadruple regimens. This finding lends support to current guidelines recommending triple therapy as first line treatment.14 15 16 17
The generalisability of the findings could be a concern, owing to the many potential drug combinations for quadruple and triple cART, with only a few of these combinations evaluated in this systematic review. However, the tested cART regimens have been designed according to good evidence, beliefs, and scientific theories, so they are more likely to be better than other possible combinations. Given this assumption, it is unlikely that other combinations would be better than those tested in the trials. In fact, none of the tested quadruple therapies was statistically significantly better than any of the triple therapies in any trial on any of the outcomes assessed (fig 2, fig 3, fig 4, fig 5, fig 6, fig 7). This finding suggests strongly that these quadruple therapies be highly consistently not better than triple ones.
If the assumption does not hold that the tested cART regimens are better than any untested possible cART regimens, we would need new guiding rules that are better than current ones in designing combination therapies to increase the chance of success and improve cost effectiveness. Without a new guiding rule, there is only a faint possibility that more effective combinations have not been designed and tested so far. In addition, the cost of proving this belief would be huge, because of the large number of possible four drug and three drug combinations; the number of possible comparisons (pairs) between these combinations would be even larger. Given the currently available evidence shown in this study, further testing of new combinations might not be worthwhile.
Even if future studies comparing other drug combinations show a difference, quadruple cART regimens are more likely to be inferior to triple cART regimens, rather than the other way around, because none of the point estimates of effect from our meta-analyses favoured quadruple cART. This argument is especially tenable in view of the additional financial and pill burden and consequent issues associated with quadruple cART. Thus, the cost effectiveness of huge investment in such trials could be a concern. However, this systematic review cannot exclude the possibility that quadruple therapies would be better than triple ones when new classes of antiretroviral drugs are made available.
The above findings have important implications for future research. According to clinical trial registries, large trials to compare quadruple cART with triple cART are still being conducted, with primary interest in surrogate outcomes.46 Some researchers have gone even further and compared quintuple cART regimens with triple cART regimens, which has unsurprisingly shown no difference in effects between the two regimens.47 48 Thus, the idea of evidence based research deserves more emphasis in the proposal of further trials, which are generally expensive and pose potential harms to study participants. Network analysis could also be used to compare regimens across different trials in a principled and cost effective way.
This systematic review had a few limitations. Firstly, it was not pre-registered. However, the review was conducted and reported following the widely accepted guidelines to reduce manipulation and increase transparency. Furthermore, as all major clinical outcomes were studied and reported, the impact of potential incomplete reporting in this review (if any) on the main conclusions was reduced to minimum. Therefore, the validity of this systematic review is unlikely to have been influenced by the lack of pre-registration. Secondly, some of the included studies had potential bias because of no blinding of outcome assessment and incomplete outcome data, which might have undermined the reliability of the results. However, the effect estimates remained unchanged in sensitivity analyses that removed those studies, indicating that the potential bias in studies did not affect the results much. Finally, due to the small number of studies, funnel plots and Egger’s test were not performed. Thus, we cannot rule out the possibility of publication bias.
Conclusion
In our review, the effects of quadruple cART were not better than triple cART in treatment naive people with HIV. This finding lends support to current guidelines recommending triple cART as first line treatment, especially considering the financial and pill burden and consequent issues introduced by a fourth drug. As the chance that quadruple cART turns out to be more favourable than triple cART is low, the idea of evidence based research deserves more emphasis in proposing further trials on this topic. However, this study does not exclude the possibility that quadruple cART would be better than triple ones when new classes of antiretroviral drugs are made available.
What is already known on this topic
Randomised controlled trials comparing quadruple with triple combination antiretroviral therapies for treatment naive people with HIV have continued to be conducted over the past two decades
But during the same period, practice guidelines have also continued to recommend triple combination antiretroviral therapies as standard first line treatment, and have rarely mentioned quadruple therapies
What this study adds
This study clarified that the effects of quadruple therapies were not better than those of standard triple therapies
The finding lends support to current guidelines and suggests no need to update recommendations of triple therapy on first line treatment for people with HIV in general
As the chance that quadruple therapies turn out to be more favourable than triple ones is low, the idea of evidence based research deserves more emphasis in proposing further trials, which are generally expensive and pose potential harms to study participants
Web extra.
Extra material supplied by authors
Web appendix: Supplementary materials
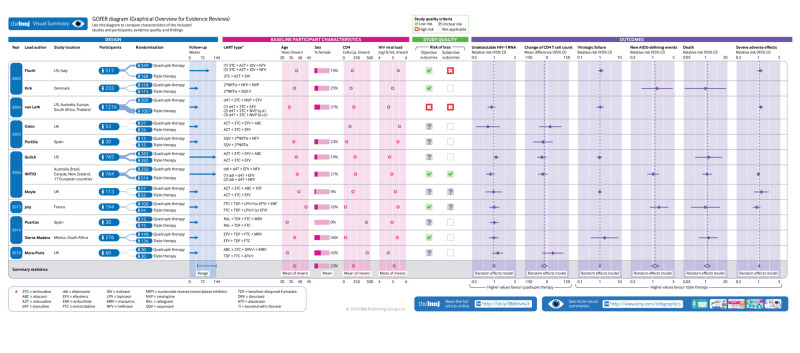
Infographic: Visual summary of trial and participant characteristics
Contributors: ZY and JT conceived the study idea and coordinated the systematic review. QF, AZ and ZY designed the search strategy. QF, AZ, and ZY screened abstracts and full texts, extracted the data, and judged risk of bias in the studies. QF and ZY performed the data analysis. QF wrote the first draft of the manuscript. All authors were involved in interpretation of results and critical revision of manuscript. ZY is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Competing interests: All authors have completed the ICMJE uniform disclosure forms at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; MTM received grants from NIAAA US and NIHR HPRU UK; HZ received grants from the National Science Foundation of China Young Scientist Fund (81703278), National Science and Technology Major Project (China; 2018ZX10721102), Sanming Project of Medicine (Shenzhen; SZSM201811071), and Australian National Health and Medical Research Council Early Career Fellowship (APP1092621); the other authors had no financial relationships with any organisations that might have an interest in the submitted work in the precious three years; no other relationship or activities that could appear to have influenced the submitted work.
Ethical approval: Not required.
Data sharing: All data are freely available on request.
The lead author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
References
- 1. GBD 2016 Causes of Death Collaborators Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1151-210. 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Summary of the global HIV epidemic (2017). https://www.who.int/hiv/data/en/ Accessed 05 Dec 2018.
- 3. Abbas UL, Glaubius R, Mubayi A, Hood G, Mellors JW. Antiretroviral therapy and pre-exposure prophylaxis: combined impact on HIV transmission and drug resistance in South Africa. J Infect Dis 2013;208:224-34. 10.1093/infdis/jit150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Antiretroviral Therapy Cohort Collaboration Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet 2008;372:293-9. 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jin Y, Liu Z, Wang X, et al. A systematic review of cohort studies of the quality of life in HIV/AIDS patients after antiretroviral therapy. Int J STD AIDS 2014;25:771-7. 10.1177/0956462414525769. [DOI] [PubMed] [Google Scholar]
- 6. Montaner JS, Lima VD, Barrios R, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet 2010;376:532-9. 10.1016/S0140-6736(10)60936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Naftalin CM, Wong NS, Chan DP, Wong KH, Reidpath DD, Lee SS. Three different patterns of CD4 recovery in a cohort of Chinese HIV patients following antiretroviral therapy - a five-year observational study. Int J STD AIDS 2015;26:803-9. 10.1177/0956462414553826. [DOI] [PubMed] [Google Scholar]
- 8. Teeraananchai S, Kerr SJ, Amin J, Ruxrungtham K, Law MG. Life expectancy of HIV-positive people after starting combination antiretroviral therapy: a meta-analysis. HIV Med 2017;18:256-66. 10.1111/hiv.12421. [DOI] [PubMed] [Google Scholar]
- 9. Wandeler G, Johnson LF, Egger M. Trends in life expectancy of HIV-positive adults on antiretroviral therapy across the globe: comparisons with general population. Curr Opin HIV AIDS 2016;11:492-500. 10.1097/COH.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pau AK, George JM. Antiretroviral therapy: current drugs. Infect Dis Clin North Am 2014;28:371-402. 10.1016/j.idc.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jordan R, Gold L, Cummins C, Hyde C. Systematic review and meta-analysis of evidence for increasing numbers of drugs in antiretroviral combination therapy. BMJ 2002;324:757. 10.1136/bmj.324.7340.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fischl MA, Ribaudo HJ, Collier AC, et al. Adult AIDS Clinical Trials Group 388 Study Team A randomized trial of 2 different 4-drug antiretroviral regimens versus a 3-drug regimen, in advanced human immunodeficiency virus disease. J Infect Dis 2003;188:625-34. 10.1086/377311. [DOI] [PubMed] [Google Scholar]
- 13. Serrano-Villar S, Sainz T, Ma ZM, et al. Effects of combined CCR5/integrase inhibitors-based regimen on mucosal immunity in HIV-Infected patients naïve to antiretroviral therapy: a pilot randomized trial [correction in: PLoS Pathog 2016;12:e1005540 and 2017;13:e1006368]. PLoS Pathog 2016;12:e1005381. 10.1371/journal.ppat.1005381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. World Health Organization Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2nd ed WHO, 2016. https://www.who.int/hiv/pub/arv/arv-2016/en/ Accessed 05 Dec 2018. [PubMed] [Google Scholar]
- 15. Günthard HF, Aberg JA, Eron JJ, et al. International Antiviral Society-USA Panel Antiretroviral treatment of adult HIV infection: 2014 recommendations of the International Antiviral Society-USA Panel. JAMA 2014;312:410-25. 10.1001/jama.2014.8722. [DOI] [PubMed] [Google Scholar]
- 16. Panel on Antiretroviral Guidelines for Adults and Adolescents Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. Department of Health and Human Services, 2017. https://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf Accessed 05 Dec 2018. [Google Scholar]
- 17.European AIDS Clinical Society. Guidelines. Version 9.0. Oct 2017. www.eacsociety.org/guidelines/eacs-guidelines/eacs-guidelines.html Accessed 05 Dec 2018.
- 18. Lund H, Brunnhuber K, Juhl C, et al. Towards evidence based research. BMJ 2016;355:i5440. 10.1136/bmj.i5440. [DOI] [PubMed] [Google Scholar]
- 19. Gulick RM, Ribaudo HJ, Shikuma CM, et al. AIDS Clinical Trials Group (ACTG) A5095 Study Team Three- vs four-drug antiretroviral regimens for the initial treatment of HIV-1 infection: a randomized controlled trial. JAMA 2006;296:769-81. 10.1001/jama.296.7.769. [DOI] [PubMed] [Google Scholar]
- 20. Orkin C, Stebbing J, Nelson M, et al. A randomized study comparing a three- and four-drug HAART regimen in first-line therapy (QUAD study). J Antimicrob Chemother 2005;55:246-51. 10.1093/jac/dkh515 [DOI] [PubMed] [Google Scholar]
- 21. Higgins J, Green S. Cochrane handbook for systematic review of interventions. [Version 5.1.0] Cochrane Collaboration, 2011. [Google Scholar]
- 22. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1-34. 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 23. Kirk O, Katzenstein TL, Gerstoft J, et al. Combination therapy containing ritonavir plus saquinavir has superior short-term antiretroviral efficacy: a randomized trial. AIDS 1999;13:F9-16. 10.1097/00002030-199901140-00002 [DOI] [PubMed] [Google Scholar]
- 24. Zeldin RK, Petruschke RA. Pharmacological and therapeutic properties of ritonavir-boosted protease inhibitor therapy in HIV-infected patients. J Antimicrob Chemother 2004;53:4-9. 10.1093/jac/dkh029. [DOI] [PubMed] [Google Scholar]
- 25. Ciaranello AL, Chang Y, Margulis AV, et al. Effectiveness of pediatric antiretroviral therapy in resource-limited settings: a systematic review and meta-analysis. Clin Infect Dis 2009;49:1915-27. 10.1086/648079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ewald H, Santini-Oliveira M, Bühler JE, et al. Comparative effectiveness of tenofovir in HIV-infected treatment-experienced patients: systematic review and meta-analysis. HIV Clin Trials 2017;18:17-27. 10.1080/15284336.2016.1261073. [DOI] [PubMed] [Google Scholar]
- 27. Hemkens LG, Ewald H, Santini-Oliveira M, et al. Comparative effectiveness of tenofovir in treatment-naïve HIV-infected patients: systematic review and meta-analysis. HIV Clin Trials 2015;16:178-89. 10.1179/1945577115Y.0000000004. [DOI] [PubMed] [Google Scholar]
- 28. Higgins JP, Altman DG, Gøtzsche PC, et al. Cochrane Bias Methods Group. Cochrane Statistical Methods Group The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Higgins J, Green S. 7.7.3.8 Combining groups. In: Higgins J, Green S, eds. Cochrane handbook for systematic review of interventions. [Version 5.1.0] Cochrane Collabration, 2011. [Google Scholar]
- 30. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Higgins J, Green S. 9.5 heterogeneity. In: Higgins J, Green S, eds. Cochrane handbook for systematic review of interventions. [Version 5.1.0] Cochrane Collabration, 2011. [Google Scholar]
- 32. Higgins J, Green S. 10.4.3.1 Recommendations on testing for funnel plot asymmetry. In: Higgins J, Green S, eds. Cochrane handbook for systematic review of interventions. [Version 5.1.0] Cochrane Collabration, 2011. [Google Scholar]
- 33. Yeni P, Cooper DA, Aboulker JP, et al. INITIO Trial International Co-ordinating Committee Virological and immunological outcomes at 3 years after starting antiretroviral therapy with regimens containing non-nucleoside reverse transcriptase inhibitor, protease inhibitor, or both in INITIO: open-label randomised trial. Lancet 2006;368:287-98. 10.1016/S0140-6736(06)69074-0. [DOI] [PubMed] [Google Scholar]
- 34. Joly V, Fagard C, Grondin C, et al. S 130 Apollo Trial Group Intensification of antiretroviral therapy through addition of enfuvirtide in naive HIV-1-infected patients with severe immunosuppression does not improve immunological response: results of a randomized multicenter trial (ANRS 130 Apollo). Antimicrob Agents Chemother 2013;57:758-65. 10.1128/AAC.01662-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kirk O, Lundgren JD, Pedersen C, et al. A randomized trial comparing initial HAART regimens of nelfinavir/nevirapine and ritonavir/saquinavir in combination with two nucleoside reverse transcriptase inhibitors. Antivir Ther 2003;8:595-602. [PubMed] [Google Scholar]
- 36. Moyle G, Higgs C, Teague A, et al. An open-label, randomized comparative pilot study of a single-class quadruple therapy regimen versus a 2-class triple therapy regimen for individuals initiating antiretroviral therapy. Antivir Ther 2006;11:73-8. [PubMed] [Google Scholar]
- 37. Portilla J, Boix V, Garcia-Henarejos JA, et al. Quadruple-2 protease inhibitors (PI)-therapy does not accelerate viral decay and suppression in PI-naive HIV-1 infected patients with severe immunosuppression and high viral load as compared with standard triple therapy. Int J STD AIDS 2005;16:807-10. 10.1258/095646205774988082. [DOI] [PubMed] [Google Scholar]
- 38. Puertas MC, Massanella M, Llibre JM, et al. MaraviBoost Collaborative Group Intensification of a raltegravir-based regimen with maraviroc in early HIV-1 infection. AIDS 2014;28:325-34. 10.1097/QAD.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 39. Sierra-Madero JG, Ellenberg S, Rassool MS, et al. CADIRIS study team A randomized, double-blind, placebo-controlled clinical trial of a chemokine receptor 5 (CCR5) antagonist to decrease the occurrence of immune reconstitution inflammatory syndrome in HIV-infection: the CADIRIS Study. Lancet HIV 2014;1:e60-7. 10.1016/S2352-3018(14)70027-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van Leth F, Phanuphak P, Ruxrungtham K, et al. 2NN Study team Comparison of first-line antiretroviral therapy with regimens including nevirapine, efavirenz, or both drugs, plus stavudine and lamivudine: a randomised open-label trial, the 2NN Study. Lancet 2004;363:1253-63. 10.1016/S0140-6736(04)15997-7 [DOI] [PubMed] [Google Scholar]
- 41. Mora-Peris B, Bouliotis G, Ranjababu K, et al. Changes in cerebral function parameters with maraviroc-intensified antiretroviral therapy in treatment naive HIV-positive individuals. AIDS 2018;32:1007-15. 10.1097/QAD.0000000000001786. [DOI] [PubMed] [Google Scholar]
- 42. Del Prete GQ, Smedley J, Macallister R, et al. Short communication: comparative evaluation of coformulated injectable combination antiretroviral therapy regimens in simian immunodeficiency virus-infected rhesus macaques. AIDS Res Hum Retroviruses 2016;32:163-8. 10.1089/aid.2015.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kanters S, Vitoria M, Doherty M, et al. Comparative efficacy and safety of first-line antiretroviral therapy for the treatment of HIV infection: a systematic review and network meta-analysis. Lancet HIV 2016;3:e510-20. 10.1016/S2352-3018(16)30091-1. [DOI] [PubMed] [Google Scholar]
- 44. Sethi AK, Celentano DD, Gange SJ, Moore RD, Gallant JE. Association between adherence to antiretroviral therapy and human immunodeficiency virus drug resistance. Clin Infect Dis 2003;37:1112-8. 10.1086/378301. [DOI] [PubMed] [Google Scholar]
- 45. Nachega JB, Marconi VC, van Zyl GU, et al. HIV treatment adherence, drug resistance, virologic failure: evolving concepts. Infect Disord Drug Targets 2011;11:167-74. 10.2174/187152611795589663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.ClinicalTrial.gov. Antiretroviral therapy for acute and chronic HIV infection (NCT00796263) 2018 https://www.clinicaltrials.gov/ct2/history/NCT00796263?V_12=View#StudyPageTop.
- 47. Markowitz M, Evering TH, Garmon D, et al. A randomized open-label study of 3- versus 5-drug combination antiretroviral therapy in newly HIV-1-infected individuals. J Acquir Immune Defic Syndr 2014;66:140-7. 10.1097/QAI.0000000000000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ananworanich J, Chomont N, Fletcher JL, et al. Markers of HIV reservoir size and immune activation after treatment in acute HIV infection with and without raltegravir and maraviroc intensification. J Virus Erad 2015;1:116-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary materials
Infographic: Visual summary of trial and participant characteristics