Abstract
The stress-induced activation of the hypothalamic-pituitary-adrenal (HPA) axis is normally suppressed during pregnancy. Dysregulation of the HPA axis has been proposed to play a role in postpartum depression. However, direct investigation into the relationship between the HPA axis and postpartum depression has been hindered by the lack of useful animal models. Building on our discovery of a role for the K+/Cl− co-transporter, KCC2, in the GABAergic regulation of CRH neurons in the paraventricular nucleus of the hypothalamus (PVN), critical for mounting the body’s physiological response to stress, we assessed the role of KCC2 in the regulation of the HPA axis during pregnancy and the postpartum period. Here we demonstrate that the normal suppression of the stress-induced activation of the HPA axis during the peripartum period involves maintenance of KCC2 in the PVN. Mice lacking KCC2 specifically in corticosterone-releasing hormone (CRH) neurons, which govern the activity of the HPA axis (KCC2/Crh mice), exhibit dysregulation of the HPA axis and abnormal postpartum behaviors. Loss of KCC2 specifically in CRH neurons in the PVN is sufficient to reproduce the depression-like phenotype and deficits in maternal behaviors during the postpartum period. Similarly, chemogenetic activation of CRH neurons in the PVN is sufficient to induce abnormal postpartum behaviors and chemogenetic silencing of CRH neurons in the PVN can ameliorate abnormal postpartum behaviors observed in KCC2/Crh mice. This study demonstrates that dysregulation of the HPA axis is sufficient to induce abnormal postpartum behaviors and deficits in maternal behaviors in mice, providing empirical support for a role of HPA axis dysfunction in the pathophysiology of postpartum depression
Keywords: postpartum depression, stress, HPA axis, corticosterone, KCC2, GABA
1. Introduction
The risk of depression in women becomes significantly increased during the postpartum period, with nearly 20% of mothers suffering from postpartum depression (Gavin et al. 2005;Steiner 1998) and a much larger percentage (up to 75%) suffering from postpartum blues (Robertson 2008). Though antenatal depression is a strong predictor of postpartum depression (Milgrom et al. 2008), antenatal anxiety is also associated with an increased risk of postpartum depression (Heron et al. 2004). Indeed, over two-thirds of women with postpartum depression experience anxiety-related symptoms (Wisner et al. 2013) and antenatal anxiety symptoms may even be a stronger predictor of PPD than antenatal depressive symptoms (Matthey et al. 2003).
Stress has been identified as a significant risk factor for postpartum depression (O’hara and Swain 1996;Pfost et al. 1990;Rich-Edwards et al. 2006); for review see (Stowe and Nemeroff 1995). These clinical observations led to the hypothesis that abnormalities in stress reactivity, mediated by the HPA axis, may contribute to postpartum depression. Experimentally, chronic stress during pregnancy (Maguire and Mody 2016) or during lactation (Carini et al. 2013) induces abnormal maternal behaviors and depression-like behaviors in postpartum rodents. The effects of stress on abnormal postpartum behaviors are thought to be mediated by corticosterone since these behaviors can be mimicked by administration of exogenous corticosterone during pregnancy and/or the postpartum period (Brummelte and Galea 2010;Brummelte et al. 2006;Maguire and Mody 2016), suggesting a role for the HPA axis in the pathophysiology of postpartum depression. Interestingly, a recent study demonstrated that early life stress can also negatively impact maternal behaviors, which is associated with HPA axis dysfunction (Morrison et al. 2017).
The stress-induced activation of the HPA axis is normally blunted during pregnancy (Brunton et al. 2008;Brunton and Russell 2008;Brunton and Russell 2011;de and Buitelaar 2005;Kammerer et al. 2002;Schulte et al. 1990). Expression of CRH mRNA in the PVN (Johnstone et al. 2000) and CRH peptide in the median eminence (Ma et al. 2005) are both reduced during late pregnancy and decreased CRF signaling is associated with appropriate maternal care (Klampfl et al. 2013;Klampfl et al. 2014;Klampfl et al. 2016). Adrenocorticotropic hormone (ACTH) and corticosterone secretion are decreased in non-human animal models following stress (Brunton and Russell 2008). In women, exogenous CRH does not elicit an increase in ACTH or cortisol (Schulte et al. 1990) and stress-induced cortisol levels are reduced (Altemus et al. 1995;Heinrichs et al. 2001;Kammerer et al. 2002) during late pregnancy, consistent with HPA axis hypofunction. Inability to suppress the stress-induced activation of the HPA axis during pregnancy and the postpartum period has been proposed to play a role in postpartum depression (for review see (Bloch et al. 2003;Chrousos et al. 1998). Evidence of altered levels of cortisol, ACTH, and CRH in patients suffering from postpartum depression support the proposed role for HPA axis dysfunction in contributing to postpartum depression (for review see (Bloch et al. 2003;Chrousos et al. 1998). In fact, elevated levels of CRH have been suggested to be a diagnostic criteria for postpartum depression (Yim et al. 2009). However, the involvement of the HPA axis in postpartum depression remains controversial (for review see (Meltzer-Brody 2011). One of the major limitations in determining the relationship between the HPA axis and postpartum depression has been the reliance on correlational studies in humans given the relative lack of animal models of this complex disorder.
Our research program previously characterized a mouse model lacking neurosteroid-sensitive GABAA receptors (GABAARs) which exhibit depression-like behaviors restricted to the postpartum period and deficits in maternal behaviors (Maguire and Mody 2008). Through a series of hypothesis-driven studies, we discovered that the abnormal postpartum behaviors in Gabrd−/− mice involve hyperexcitability of the HPA axis during the postpartum period (Maguire and Mody 2016), leading us to investigate the mechanisms of HPA axis regulation during pregnancy and the postpartum period.
Previous studies from our laboratory and others demonstrated a critical role for the K+/Cl− co-transporter, KCC2, on CRH neurons in the PVN in the regulation of the stress-induced activation of the HPA axis (Hewitt et al. 2009;Sarkar et al. 2011). These studies demonstrated a role for chloride homeostasis in regulating the physiological response to stress (Gunn et al. 2013;Hewitt et al. 2009;Sarkar et al. 2011;Ye et al. 2012). Specifically, our laboratory demonstrated that dephosphorylation of KCC2 at residue Ser940 and downregulation of KCC2 plays a role in the activation of the HPA axis (Sarkar et al. 2011). Here we investigate whether KCC2 plays a role in the regulation of the HPA axis during pregnancy and the postpartum period. Our data demonstrate that the suppression of the HPA axis during pregnancy and the postpartum period involves maintenance of KCC2 expression in the PVN. Selective loss of KCC2 in CRH neurons (KCC2/Crh mice; lenti-CRH-Cre mice) results in the inability to suppress the HPA axis during pregnancy and the postpartum period, which is associated with increased anxiety- and depression-like behaviors during the postpartum period and deficits in maternal behaviors. Consistent with a role for hypothalamic CRH activity as a contributing factor in abnormal peripartum stress reactivity and pathological postpartum behaviors, chemogenetic activation of CRH neurons in the PVN induced an abnormal postpartum phenotype in wildtype (CRH-Cre) dams. Further, inhibition of CRH neurons in the PVN reversed the abnormal postpartum phenotype observed in KCC2/Crh dams. These findings support a role for KCC2 in peripartum stress hyporeactivity necessary for adaptive maternal behaviors.
2. Methods
2.1. Animals
Adult (>P60) female mice were bred and housed at Tufts University School of Medicine’s Division of Laboratory Animal Medicine facility under a 14/10 light schedule (lights on at 7:00am). Experiments were performed in virgin, pregnant (day 18), and postpartum (48–72 hours) females. Females were housed in groups of 2–4 and were mated with a receptive male three days after soiled-male bedding was introduced to the cage. The presence of a vaginal plug was used to time pregnancy (Positive plug=day 0) and at approximately day 14 of pregnancy, dams were individually housed to enable quantification of pup survival. The estrous cycle was not monitored in the experimental virgin animals used in this study. However, under these housing conditions, which include ventilated racks and gender isolation, we have determined that the female mice are acyclic. Animals had ad libitum access to food and water. All procedures were approved by the Tufts University Institutional Animal Care and Use Committee and adhered to the ethical guidelines presented in the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Research Council 2011).
We generated mice lacking functional KCC2 specifically in CRH neurons by crossing a floxed KCC2 (KCC2f/f) mouse line (a generous gift from Dr. Stephen J. Moss) with CRH-Cre mice originally obtained from the Mutant Mouse Regional Research Center (Stock # 030850-UCD) and characterized in a previous manuscript from our laboratory (Sarkar et al. 2011).
The floxed KCC2 mice, KCC2/Crh mice, and Cre−/− littermates are maintained on a 129/Sv background. The CRH-Cre mice used for the DREADD experiments have been backcrossed onto a C57Bl6/J background for over 12 generations. Thus, these experiments will assess the role that dysregulation of the HPA axis plays in abnormal postpartum behavior in two different strains of mice. Genotyping of the KCC2/Crh mice was carried out in-house using the following primers:
| KCC2 | 5’: ATGAGTAGCAGATCCCATAGGCGAACC |
| 3’: CTGCCAAGAGCCATTACTACAGTGGATG | |
| CRH-Cre | 5’: CTGTCTTGTCTGTGGGTGTCCGAT |
| 3’: CGGCAAACGGACAGAAGCATT |
The expected PCR product size is 426 bp for wild type and 543 bp for floxed KCC2 mice, which is sufficient to discriminate between wild type mice and floxed KCC2 mice. The expected PCR product size is 310 bp for CRH-Cre.
2.2. Acute Restraint Stress
To demonstrate postpartum stress hyporeactivity in mice, restraint stress was performed by placing each mouse into a 50mL falcon tube modified with breathing holes. Following this 30 minute restraint stress, mice were anesthetized with isoflurane and rapidly decapitated. Trunk blood was collected for corticosterone measurements, and the PVN was rapidly microdissected for Western blot analysis.
2.3. DREADD and LV-pCRF3.0-Cre experiments
Designer Receptors Exclusively Activated by Designer Drugs (DREADD) were employed to selectively activate (Gq DREADD) or inhibit (Gi DREADD) CRH neurons in the PVN (Alexander et al, 2009; Krashes et al, 2011). An LV-pCRF3.0-Cre virus (provided by Dr. Kerry Ressler and the Emory University Viral Vector Core Facility) was employed in floxed KCC2 mice to specifically eliminate KCC2 in CRH neurons confined to the PVN. CRH-Cre mice were stereotaxically injected bilaterally with 250nl of AAV-hSyn-DIO-hM3D(Gq)-mCherry (Gq DREADD) into the PVN using the following stereotaxic coordinates (0.45mm posterior, ±0.05mm lateral, and 5.0mm depth). KCC2/Crh mice were stereotaxically injected bilaterally into the PVN with 250nl of AAV-hSyn-DIO-hM4D(Gi)-mCherry (Gi DREADD) to suppress the HPA axis hyperexcitability in these mice during the postpartum period. Both CRH-Cre and KCC2/Crh mice were bilaterally injected with 250nl of AAV-GFP (Vector Biolabs, Serotype 2) into the PVN to serve as corresponding controls and also treated with clozapine-N-oxide (CNO; Sigma-Aldrich) to control for any off-target effects (Gomez et al. 2017). LV-pCRF3.0-Cre was bilaterally injected into the PVN of floxed KCC2 mice using these same coordinates. All DREADD, LV-pCRF3.0-Cre, and control injections were done 5 days prior to breeding, leaving approximately 28 days for optimal expression prior to experimentation. Accurate targeting and specific expression of the DREADD viruses and LV-pCRF3.0-Cre virus in CRH neurons was confirmed by post-hoc analysis using immunohistochemistry to confirm colocalization with the endogenous CRH peptide. At 48–72 hours postpartum, dam behaviors were assessed using the forced swim test and maternal approach test. For the DREADD experiments, DREADD and GFP controls were injected with 3 mg/kg CNO 30 minutes prior to assessing postpartum behaviors. CNO was prepared by dissolving 3 mg CNO in 50 μL of DMSO and suspending this in 1 mL of injection saline. Mice were injected with a volume of 100 μL per 10 g of body weight.
2.4. Behavioral Tests
Behavioral tests were conducted on virgin and postpartum (48–72 hrs) KCC2/Crh mice and Cre−/− littermates in at least three separate cohorts. All behavioral tests occurred between 9am and 4pm, following at least 30 minutes of habituation in the behavioral testing room. Postpartum mice remained with their litters between testing. Pregnant mice were excluded from behavioral testing to minimize distress to the dams. Lactation can also influence behavior and maternal separation can cause stress to the dams and pups, which the authors considered when designing the experiments and interpreting the data focusing on comparisons between postpartum mice of each experimental group. Littermates (KCC2/Crh and Cre−/− controls) were used for all behavioral experiments. For the FST and Maternal Approach tests, the behavioral tests were videotaped and scored by two different investigators, one blinded to the experimental condition, to confirm an unbiased analysis and reproducibility of the results. The independent analysis by different investigators obtained similar results between experimental groups.
Elevated Plus Maze
The elevated plus maze is composed of two opposing 38cm × 6.5cm wide arms, standing 75cm from the ground. The closed arms included 10cm high walls allowing an average Δlux of 250lx between the open and closed arms. All four arms comprised 48 equally spaced photocells. To initiate the test, the mice were placed individually in the central 5cm × 5cm square, facing the open arms. The entries, distance traveled, and total time spent in the open arm were measured during the 10 minute test using the automated MotorMonitor software (Hamilton-Kinder). The ratio of both time spent in open arms and open arm entries (reported as percentage) were used as indices of anxiety-like behavior.
Light/Dark Box
Mice were individually placed in the dark side of a 2 compartment light/dark box with equally sized compartments and an average Δlux of 300lx. The box was contained in a 22cm × 43cm photobeam frame (4×6 equally spaced photo emitters and detectors). These frames interfaced with the MotorMonitor software (Hamilton-Kinder) which coded the distance traveled in the light and dark sides of the box. The percent of time spent in the light side of the box was measured during the 10 minute test as a measure of anxiety-like behavior.
Forced Swim Test
Mice were placed into a plastic beaker (21cm diameter) containing 15cm of water (23– 25°C) for 6 minutes. A video camera was used to record each session. Two different investigators scored the videos to measure the latency to the first bout of immobility and the total time spent immobile during the 6 minute test.
Maternal Approach Test
To investigate the impact of stress during the postpartum period on maternal behavior, immediately following the forced swim stress, the mice were tested in the maternal approach test. The dams were returned to their homecage, to the far corner opposite their nest and litter, and the dam’s behavior in the homecage was videorecorded for 30 minutes. The latency to approach the pups and the total time spent in contact with pups were measured as indices of maternal behavior following a stressful event.
2.5. Corticosterone Measurements
Trunk blood was collected in CAPIJECT® (T-MG) tubes at baseline, at the end of a 30-minute restraint stress or 30 minutes following the 6-minute forced swim test. All samples were collected between 10am and 12pm. The blood was spun down and serum collected and stored at −20°C prior to subjection to the ELISA analysis. Corticosterone was measured using a commercially available enzyme immunoassay kit, according to the manufacturer’s instructions (Enzo Pharmaceuticals, New Jersey). Briefly, samples were run in duplicate and compared to a standard curve of known corticosterone concentrations. In our hands, the corticosterone assay has an inter-assay variability of 6.3 ng/ml. Different experimental groups were run in parallel in 4 separate cohorts.
2.6. Immunohistochemistry
Mice were anesthetized with isoflurane, sacrificed by guillotine-assisted decapitation, and the brain was rapidly harvested and placed into ice-cold 4% paraformaldehyde. Brains were cryoprotected using successive overnight incubations in 10% and 30% sucrose, frozen in dry-ice chilled isopentane and sectioned at 40μm. Free-floating sections containing the hypothalamus were blocked for 1h with 10% NGS (in 0.3% TritonX100) and incubated with either a rabbit anti-KCC2 antibody (Millipore, 1:1,000) or a rabbit anti-CRH antibody (kindly provided by Dr. Paul Sawchenko on behalf of Dr. Wylie Vale, Salk Institute for Biological Studies, 1:10,000) for 4 days at 4°C. Sections were then washed in PBS and incubated with a biotinylated anti-rabbit secondary antibody (VectaStain Elite ABC Kit, Vector Labs, 1:200) for 2 hours at room temperature (RT). Sections were washed in PBS and incubated with streptavidin conjugated AlexaFluor 488 or AlexaFluor 568 (Molecular probes, 1:1,000) for 2 hours at RT. Following a final series of PBS washes, sections were quickly rinsed with ddH2O, mounted (Vectashield HardSet mounting medium with DAPI, Vector Labs), and imaged with a Nikon A1R confocal. For the CRH immunohistochemistry, colchicine (50μg in a 2μl volume) was injected unilaterally into the lateral ventricle using the following stereotaxic coordinates (0.5mm posterior, 1mm lateral, 2mm depth) 24 hours prior to tissue processing as previously described by our laboratory (Hooper and Maguire 2016).
2.7. Western blot
Western blots were carried out as previously described (MacKenzie and Maguire 2015;O’Toole et al. 2013;Sarkar et al. 2011). Briefly, mice were anesthetized between 10am and 12pm with isoflurane, sacrificed by guillotine-assisted decapitation, the brain was rapidly removed, and the PVN was grossly microdissected and placed in ice-cold homogenization buffer (10mM NaPO4, 100mM NaCl, 10mM Na pyrophosphate, 25 mM NaF, 5 mM EDTA, 5 mM EGTA, 2% Triton X-100, 0.5% Deoxycholate, 1 mM Na vanadate, pH 7.4), in the presence of protease inhibitors (complete mini, Roche, in fresh 100mM PMSF dissolved in ethanol). The tissue was briefly sonicated and the lysate was incubated on ice for 30 minutes. The supernatant was collected following centrifugation at 14,000 rpm for 5min at 4°C. Protein concentrations were determined using DC Protein Assay (Bio-Rad, Hercules, CA). Total protein (25 μg) was loaded onto a 12% SDS-polyacrylamide gel, subjected to electrophoresis and transferred to a PDVF membrane (Immobilon P, Millipore, Temecula, CA), blocked in 10% nonfat milk, and probed with a polyclonal antibody specific for KCC2 (1:1,000, Millipore, Temecula, CA), a polyclonal antibody specific for the phosphorylated Ser940 residue on KCC2 (1:1,000, a generous gift from Dr. Stephen J. Moss), or a monoclonal β-tubulin antibody (1:10,000, Sigma Aldrich, St. Louis, MO). The blots were then incubated with either peroxidase labeled anti-rabbit IgG (1:2,500, GE Healthcare) or peroxidase labeled anti-mouse IgG (1:2,500, GE Healthcare) and immunoreactive proteins were visualized using enhanced chemiluminescence (Amersham/GE Healthcare). All experimental groups were run in parallel. Optical density measurements were performed using NIH ImageJ software and normalized to total protein levels (25μg total protein) rather than a housekeeping protein, which have shown variability in expression levels (Li and Shen 2013).
2.8. Electrophysiological recordings
Mice were anesthetized with isoflurane, sacrificed by guillotine-assisted decapitation, and the brain was rapidly removed and immersed in ice cold artificial cerebrospinal fluid (ACSF) containing (in mM) 126 NaCl, 26 NaHCO3, 1.5 NaH2PO4, 5 KCl, 2 CaCl2, 10 dextrose (300–310 mOsm) and 3 mM kynurenic acid. Coronal sections (350 μm) were cut using a Leica vibratome and incubated at 33°C in normal ACSF for at least one hour prior to transferring to the recording chamber. The recording chamber was maintained at 33°C (in line heater, Warner Instruments) and continuously perfused at a rate of ≥ 4 ml/minute with ACSF throughout the experiment. Solutions were continuously bubbled with 95% O2 and 5% CO2. For all electrophysiological recordings, series resistance and whole-cell capacitance were continually monitored and compensated throughout the course of the experiment.
EGABA measurements
Kynurenic acid (3 mM) was added to the extracellular solution to isolate GABAergic currents. Perforated patch clamp recordings were performed on CRH neurons in the PVN to measure the equilibrium potential for evoked GABAergic currents (EGABA). An internal solution containing (in mM): 140 KCl, 4 NaCl, 10 HEPES, 0.1 EGTA, 2 Mg-ATP, 0.3 Na-GTP (pH = 7.25, 280–290 mOsm) with 50 μg/ml gramicidin (ABCD, Sigma) was used to easily determine if the perforated patch was ruptured. A > 1GΩ seal was achieved and perforation was continuously monitored by the decrease in input resistance, the gradual increase in the capacitive transient following a −5mV voltage step, and Vpipette reaching the resting membrane potential (RMP). Recordings were immediately rejected if the membrane potential and/or input resistance suddenly changed or if the perforated patch ruptured, evident by a sudden switch to inwardly directed IPSCs. At the end of each experiment, the perforated patch was ruptured, and the average whole-cell EGABA was measured to be −12.7 ± 9.3 mV.
Inhibitory postsynaptic currents were evoked by stimulating the peri-PVN region known to be enriched in GABAergic neurons and were recorded in CRH neurons in the PVN. The evoked IPSCs were recorded at holding voltages from −100mV to 0mV in 10mV steps and the currents generated at each holding voltage were used to plot the current-voltage (I-V) relationship. Data were excluded if the GABAa mediated I-V relationship failed to cross the x-axis between −90 and −30 mV. The I-V relationship was fit with a linear regression and the x-axis intercept was calculated as the EGABA value for each cell.
Chemogenetic modulation of CRH neurons
Whole-cell patch clamp recordings were performed on CRH neurons from Gq DREADD and Gi DREADD mice to confirm chemogenetic modulation of CRH neurons. An internal solution containing (in mM): 130 K-gluconate, 10 KCl, 4 NaCl, 10 HEPES, 0.1 EGTA, 2 Mg-ATP, 0.3 Na-GTP (pH = 7.25, 280 – 290 mosm) and electrodes with DC resistance of 5–8MΩ were used to record changes in the firing rate and resting membrane potential (RMP) following bath application of CNO (500nM). The average firing rate was measured over a 60 s segment before and a 60 s segment after the application of CNO. The RMP was measured over a 100 ms, action potential-free period immediately before application of CNO and compared to the RMP after stabilization following CNO administration.
2.9. Statistical Analyses
All data were analyzed using GraphPad Prism 6 or Excel. Comparison between KCC2/Crh vs. Cre−/− littermates, DREADD vs. GFP, and lenti-CRH-Cre vs. GFP was carried out using a Student’s t-test. Comparison between reproductive status (virgin, pregnant, and postpartum) was conducted using a one-way ANOVA with a Bonferroni postdoc test for multiple comparisons. Comparison between reproductive status (virgin, pregnant, and postpartum) and genotype (Cre−/− and KCC2/Crh) or state (control vs. stress) was conducted using a two-way ANOVA with a Sidak posthoc test for multiple comparisons. A Kruskal-Wallis test was also performed to verify statistical significance in cases where heterogeneity of variance was violated. All data are represented as the average ± SEM. Statistical significance was defined as p <0.05. A timeline of the experimental approach is provided (Figure 1).
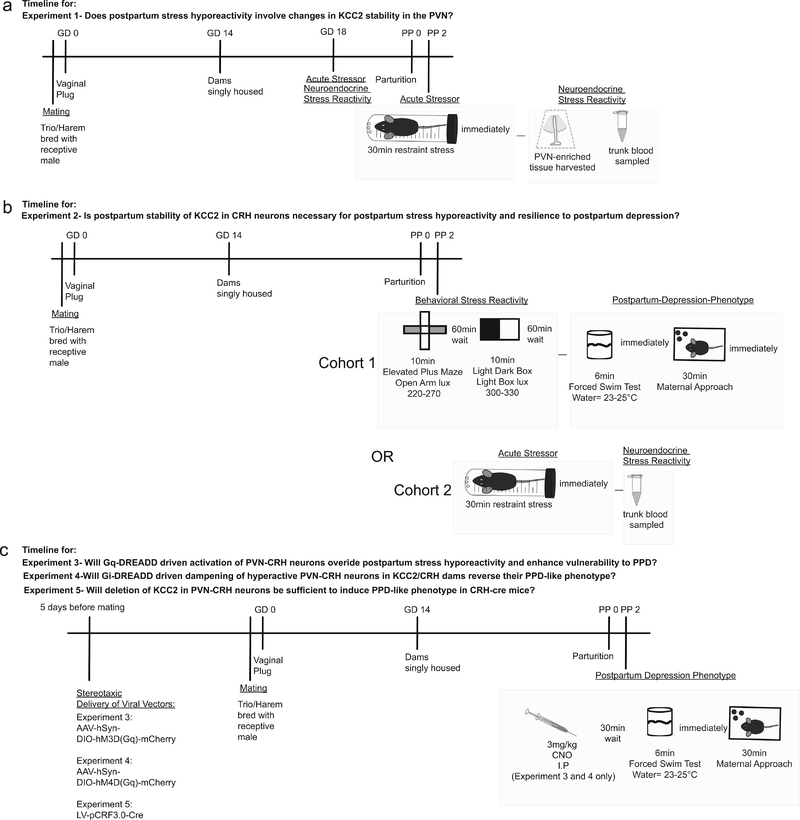
Figure 1: Timeline of the experimental procedures.
a, Trunk blood was collected and the PVN was microdissected from virgin, pregnant (day 18), and postpartum (48 hrs postpartum) mice following a 30 min restraint stress or in unstressed control mice to measure circulating corticosterone levels and KCC2 expression in the PVN, respectively. b, Anxiety-like, depression-like, and stress-induced changes in maternal behaviors were assessed in KCC2/Crh mice and Cre−/− littermates at 48–72 hrs postpartum. In parallel, trunk blood was collected from virgin, pregnant, and postpartum KCC2/Crh and Cre−/− littermates following a single, 30 min restraint stress or in unstressed controls. c, AAV-hSyn-DIO-hM3D(Gq)-mCherry, AAV-hSyn-DIO-hM4D(Gq)-mCherry, LV-pCRF3.0-Cre, or AAV-GFP was stereotaxically injected into the PVN of KCC2/Crh mice or Cre−/− mice 5 days prior to mating and depression-like behaviors and stress-induced changes in maternal care were assessed at 48–72 hrs postpartum.
3. Results
3.1. The stress-induced dephosphorylation and downregulation of KCC2 in the PVN is prevented during pregnancy and the postpartum period
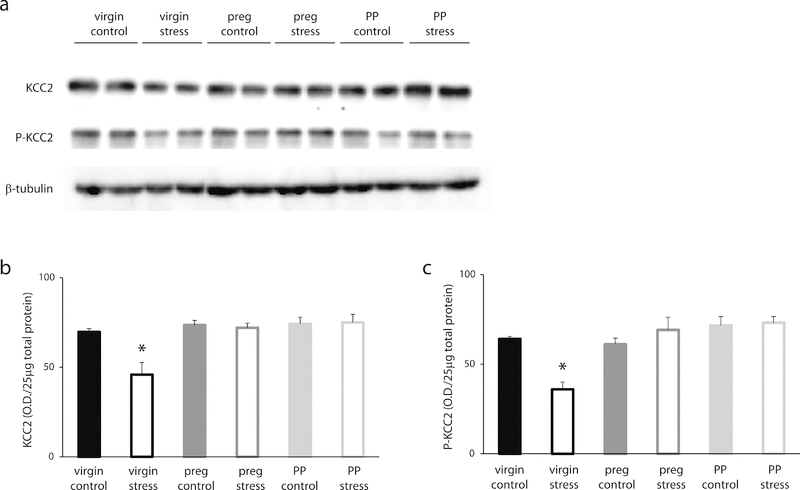
To investigate the role of KCC2 in HPA axis regulation during the peripartum period, we examined the phosphorylation state of KCC2 at residue Ser940, which has been shown to regulate the surface expression and function of the transporter (Lee et al. 2007), and total KCC2 expression in the PVN in virgin, pregnant, and postpartum mice with or without stress exposure. Here, we demonstrate interactions between the phosphorylation state of KCC2 at residue Ser940 and reproductive status [F (2, 48) = 9.543, p<0.05], and between total KCC2 expression and reproductive status [F (2, 55) = 6.475, p<0.05]. Total KCC2 expression and the phosphorylation of KCC2 at residue Ser940 are decreased in virgin females following a single, 30 min restraint stress compared to non-stressed controls (Figure 2a–c; p values <0.05). This stress-induced dephosphorylation and downregulation of KCC2 is not observed in pregnant or postpartum mice. There is no difference in total KCC2 expression in pregnant or postpartum mice subjected to acute restraint stress compared to non-stressed controls (Figure 2a,b). Similarly, there is no difference in the phosphorylation at KCC2 residue Ser940 in pregnant or postpartum mice subjected to acute restraint stress compared to non-stressed controls (Figure 2a,c). These data demonstrate that there is a maintenance of the phosphorylation of KCC2 at residue Ser940 and total KCC2 expression in the PVN following stress during the peripartum period.
Figure 2: Suppression of the stress-induced activation of the HPA axis involves maintenance of KCC2 in the PVN.
a) Representative Western Blots of total KCC2 expression, the phosphorylation of KCC2 at residue Ser940, and β-tubulin in total protein isolated from the PVN of control and stressed virgin, pregnant, and postpartum wild type mice. The average optical density of total KCC2 expression (b) and the phosphorylation at KCC2 residue Ser940 (c) is decreased in virgin mice following acute restraint stress (n = 10–14 mice per experimental group; * denotes p values < 0.05 using a two-way ANOVA). The stress-induced dephosphorylation of KCC2 at residue Ser940 and downregulation of total KCC2 expression was not observed in pregnant or postpartum mice.
3.2. Selective deletion of KCC2 from CRH neurons increases CRH activity via depolarizing EGABA
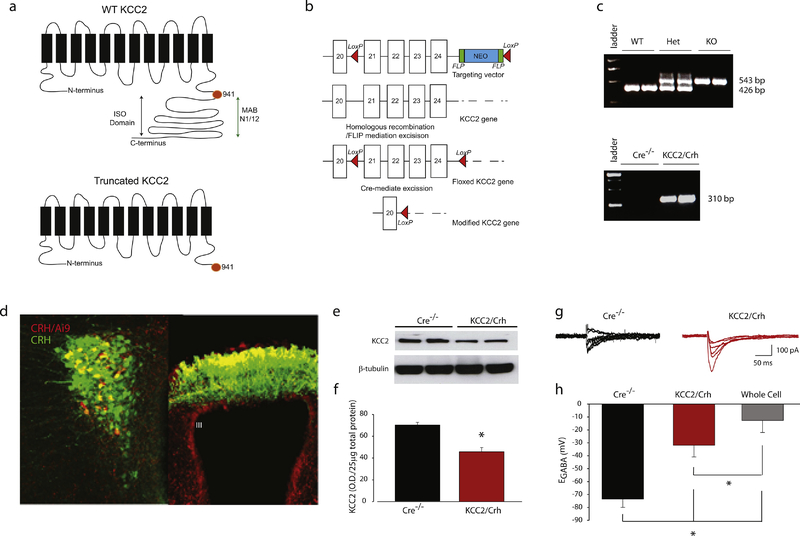
To confirm a role of KCC2 in the PVN in peripartum stress reactivity, we generated mice which lack KCC2 specifically in CRH neurons by crossing a floxed KCC2 (KCC2fl/fl) mouse line with a CRH-Cre line. The floxed KCC2 mice were generated by GenOway (GenOway, France) and provided by Dr. Steve Moss (Tufts University School of Medicine). A schematic representation of the targeting strategy is depicted in Figure 3a,b. A 772-nt region encompassing exons 21 through 24 are flanked by LoxP sites (Figure 3a,b), encoding for 186 amino acids in the intracellular, C-terminal domain of the protein, a region with numerous sites for posttranslational modifications (for review see (Kahle et al. 2013) and the ISO domain which is critical for isotonic transport function (Acton et al. 2012). The CRH-Cre line used in the current study was previously characterized by our laboratory (Sarkar et al. 2011). Further confirmation of the fidelity of the expression of Cre recombinase with the endogenous CRH peptide was accomplished by crossing the CRH-Cre mice with floxed Ai9 reporter mice (Jax Stock #007909). The tdTomato reporter expression (red) colocalized with the endogenous CRH peptide (green) in the PVN (Figure 3d, left panel) and in the median eminence (Figure 3d, right panel). In fact, although the reporter is not expressed in all neurons expressing the endogenous CRH peptide (57.6 ± 8.3 %), the endogenous CRH peptide is expressed in 87.7 ± 3.1% of neurons expressing Cre recombinase (n = 36 sections, 6 mice), highlighting the fidelity of Cre recombinase expression in PVN CRH neurons in this line.
Figure 3: Characterization of floxed KCC2 and KCC2/Crh mice.
a, A diagram of the predicted structure of wild type KCC2 (top panel), with a predicted topology of 12 membrane spanning segments, and the truncated KCC2 protein (bottom panel), which has a truncated C-terminal domains and lacks numerous sites for posttranslational modifications as well as the ISO domain which is critical for transport function. b, The targeting strategy for homologous recombination to generate the floxed KCC2 mouse strain. Line diagrams representing the targeting construct with the Neo cassettes with the red arrowheads indicating the locations of the LoxP sites, the targeted allele following homologous recombination and FLIP mediated excision of the Neo cassette. c, PCR products demonstrating the ability to discriminate between mice which are wild type, heterozygous, and homozygous for the floxed KCC2 gene (top panel) and those expressing CRH-Cre (bottom panel). d, Ai9 reporter expression in CRH/Ai9 mice (red) colocalizes with the endogenous CRH peptide in the PVN (left panel) and median eminence (right panel), confirming the fidelity of Cre recombinase expression in CRH neurons. e, Representative Western blot of KCC2 expression in two independent KCC2/Crh mice and Cre−/− littermates. f, The average optical density of KCC2 expression in the PVN is decreased in KCC2/Crh mice compared to Cre−/− littermates (n = 5 mice per group; * denotes p < 0.05 using a Student’s t-test). g, Representative evoked GABAergic currents in CRH neurons in the PVN of KCC2/Crh mice and Cre−/− littermates recorded from −100 - −50 mV in 10mV steps. h, The average EGABA value is depolarized in KCC2/Crh mice compared to Cre−/− littermates, consistent with the lack of KCC2 function. The EGABA value recorded in the whole-cell configuration is provided for comparison (n = 11–12 cells, 3 mice per experimental group; * denotes p values < 0.05 using a one-way ANOVA).
A reduction of KCC2 in CRH neurons was confirmed by Western blot analysis of KCC2 in the total protein isolated from the PVN. KCC2 expression is significantly decreased in the PVN from KCC2/Crh mice compared to Cre−/− littermates (Figure 3e–f), although not completely eliminated since the PVN is a heterogeneous nucleus. The reduction in KCC2 was sufficient to shift the equilibrium potential for GABAergic currents (EGABA) in KCC2/Crh mice compared to Cre−/− littermates (Figure 3g,h; t(21) = −3.82; p<0.05), consistent with a decrease of KCC2 function in CRH neurons. We calculated that this dramatic shift in EGABA in KCC2/Crh mice equates to a rise in intracellular chloride levels from approximately 13.7 ± 8.9 mM (Glykys et al. 2014) to 28 mM. The shift in EGABA in KCC2/Crh mice was significantly different from the EGABA values obtained in the whole-cell patch clamp configuration (Figure 3h; t(21) = −1.48; p<0.05), demonstrating the integrity of the perforated patch clamp recordings. These data demonstrate that KCC2/Crh mice are a useful model for studying the role of KCC2 in CRH neurons in mediating the neuroendocrine and behavioral responses to stress during the peripartum period.
3.3. KCC2/Crh mice have the inability to suppress the stress-induced activation of the HPA axis during pregnancy and the postpartum period
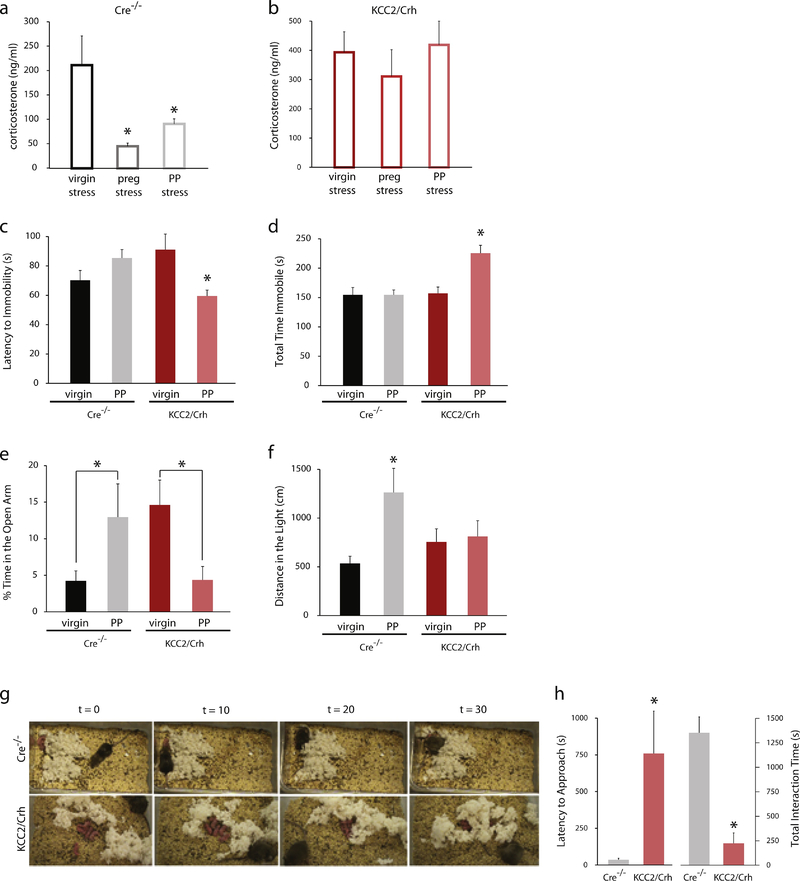
The stress-induced activation of the HPA axis is suppressed during pregnancy and the postpartum period. Corticosterone levels following a 30 min restraint stress are significantly increased in virgin females (211.8 ± 60.0 ng/ml) compared to non-stressed controls (31.5 ± 6.0 ng/ml; n= 12 mice per experimental group; t(22) = −2.99; p<0.05). The stress-induced increase in circulating corticosterone is suppressed in pregnant and postpartum dams compared to virgin (Figure 4a; [F(2, 57)=4.16, p<0.05). These data demonstrate that the stress-induced activation of the HPA axis is suppressed during pregnancy and the postpartum period in mice. To assess peripartum stress reactivity in KCC2/Crh dams, stress-induced elevations in corticosterone levels were measured in virgin, pregnant, and postpartum KCC2/Crh mice following a single, 30 min restraint stress. Unexpectedly, there is no difference in baseline corticosterone levels between unstressed, virgin KCC2/Crh mice (31.1 ± 8.5 ng/ml) and Cre−/− littermates (31.5 ± 6.0 ng/ml; data not shown; n = 8 – 12 mice per experimental group; ns determined using a Student’s t-test; t(18) = 0.04; p<0.05)). Whereas, the stress-induced corticosterone levels are decreased in pregnant and postpartum Cre−/− littermates compared to virgin (Figure 4a), there is no difference in stress-induced elevations in corticosterone following acute restraint stress in virgin, pregnant, or postpartum KCC2/Crh mice, demonstrating their inability to suppress the stress-induced activation of the HPA axis (Figure 4b; ns determined using a one-way ANOVA; [F(2, 17)=0.48). However, there is a significant difference between stress-induced corticosterone levels in pregnant and postpartum KCC2/Crh mice compared to pregnant and postpartum Cre−/− littermates (p values<0.05 using a two-way ANOVA with Sidak’s multiple comparison test; [F (1, 45) = 27.43].
Figure 4: KCC2/Crh mice exhibit abnormal postpartum behaviors.
a) The average serum corticosterone levels in Cre−/− controls following subjection to a 30 minute restraint stress in virgin, pregnant, and postpartum mice (n = 8–12 mice per experimental group; * denotes significance using a one-way ANOVA). b, Following a single, 30 min restraint stress, the average serum corticosterone levels are elevated in pregnant and postpartum KCC2/Crh mice pregnant and postpartum Cre−/− littermates (n = 6–8 mice per experimental group; * denotes p values < 0.05 using a one-way ANOVA). Postpartum KCC2/Crh mice exhibit a decreased latency to the first bout of immobility (c) and an increased total time spent immobile (d) in the forced swim test, suggesting increased depression-like behaviors compared to virgin mice and postpartum Cre−/− littermates. Postpartum wild type mice exhibit anxiolysis during the postpartum period, evident by the increased percent time spent in the open arm of the elevated plus maze (e) and the increased distance traveled in the light compartment of the light/dark box (f). KCC2/Crh mice exhibit deficits in maternal behaviors in the maternal approach test. g, Representative time-lapse images of Cre−/− and KCC2/Crh dams in the maternal approach test demonstrate interactions of the Cre−/− dams with their pups while the KCC2/Crh dams remain distant from their litter. h, KCC2/Crh mice exhibit an increased latency to approach their pups and spending less time interacting with their pups in the maternal approach test (for all experiments, n = 6–15 mice per experimental group; * denotes p values < 0.05 using a two-way ANOVA).
3.4. KCC2/Crh mice exhibit depression-like behaviors during the postpartum period
Inability to suppress the HPA axis during pregnancy and the postpartum period has been implicated in postpartum depression (for review see (Bloch et al. 2003;Chrousos et al. 1998). Consistent with a role of HPA axis dysfunction in postpartum depression, the inability to suppress the stress-induced activation of the HPA axis in KCC2/Crh mice (Figure 4b) is associated with depression-like behaviors during the postpartum period. The data support a significant interaction between reproductive status and genotype on both the latency to the first bout of immobility: [F (1, 37) = 11.06, p <0.05] and total time spent immobile [F (1, 37) = 8.729, p<0.05] in the forced swim task. Posthoc analysis confirms that the latency to the first bout of immobility is decreased and the total time spent immobile in the forced swim test is increased in postpartum KCC2/Crh mice compared to postpartum Cre−/− littermates, virgin Cre−/− mice, and virgin KCC2/Crh mice (Figure 4c,d; p values<0.05).
3.5. KCC2/Crh mice do not exhibit the anxiolytic effect of the postpartum period
The attenuated neuroendocrine response to stress in wild type mice during the postpartum period is associated with anxiolysis assessed using the elevated plus maze and light-dark box. Data support an interaction of reproductive status and genotype [F (1, 25) = 10.13), p<0.05]). Posthoc analysis confirm that postpartum wild type dams spend a greater percent of time in the open arms of the elevated plus maze compared to wild type virgins (Figure 4e, p<0.05). This postpartum anxiolysis was not observed in postpartum KCC2/Crh dams when compared to their virgin counterparts (Figure 4e).
Similarly, postpartum wild type (Cre−/−) dams exhibit a decrease in anxiety-like behaviors in the light/dark box. Data support an interaction of reproductive status and genotype [F (1, 25) = 4.917, p<0.05]). Posthoc analysis confirm that postpartum wild type dams exhibit an increase in distance traveled in the light compartment of the light/dark box compared to wild type virgin mice (Figure 4f, p<0.05). In contrast, there was no difference in the distance traveled in the light compartment of the light/dark box between virgin and postpartum KCC2/Crh mice (Figure 4f). However, there was no difference in the total distance traveled in the dark compartment across groups (virgin Cre−/−: 2489 ± 255.1 cm; virgin KCC2/Crh 2471± 360.8 cm; postpartum Cre−/− dams: 3071± 329.3 cm; postpartum KCC2/Crh dams: 2264 ± 268.9 cm; data not shown; ns determined using a two-way ANOVA).
These data demonstrate that KCC2/Crh mice do not exhibit the anxiolytic effects of the postpartum period, an effect that is observed in wild type (Cre−/− mice; Figure 4e,f).
3.6. KCC2/Crh mice exhibit deficits in maternal behaviors
The impact of HPA axis dysregulation on maternal behaviors was assessed using the maternal approach test. Representative time lapse images are shown in Figure 4g. KCC2/Crh mice exhibit deficits in maternal behaviors, evident from a dramatic increase in the latency to approach their pups and a decrease in the amount of time spent interacting with their pups during the 30 min maternal approach test compared to Cre−/− littermates (Figure 4g,h; latency t(17) = −2.95, total time t(17) = 6.03; p values<0.05). Consistent with deficits in maternal behaviors, there is an increased mortality rate in pups born to KCC2/Crh dams (24.8 ± 8.4 %) compared to Cre−/− littermates (5.6 ± 4.6 %; data not shown; n = 13 – 14 dams, 97 – 104 pups; p<0.05 using a Students t-test; t(25) = −1.96; p<0.05). These data suggest that the inability to suppress the stress-induced activation of the HPA axis during the peripartum period is associated with deficits in maternal behaviors.
3.7. Activation of CRH neurons in the PVN induces abnormal postpartum behaviors
Activation of CRH neurons in Gq DREADD mice was confirmed using whole-cell electrophysiological recordings. CNO application causes a depolarization in the RMP and an increase in the firing rate compared to the RMP recorded in the same cells in nACSF (Figure 5a–d; RMP t(8) = −4.95, firing rate t(7) = −3.88; p values<0.05).
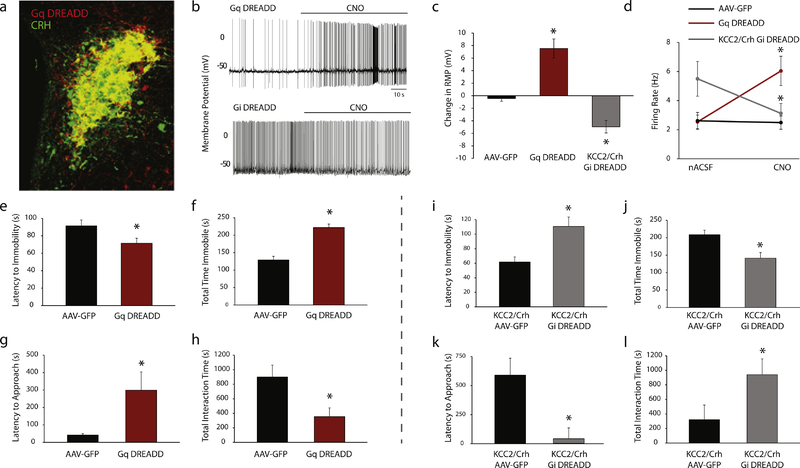
Figure 5: Chemogenetic modulation of CRH neurons in the PVN alters postpartum behaviors.
a, A representative image demonstrating colocalization of DREADD expression (mCherry, red) with the endogenous CRH peptide (AlexaFluor-488, green). b, Representative electrophysiological recordings demonstrating the effect of CNO administration on the firing rate and RMP of CRH neurons from Gq DREADD and Gi DREADD mice. c, The average change in the RMP after CNO application in mice expressing either AAV-GFP, Gq DREADD, or Gi DREADD compared to the RMP recorded in the presence of nACSF (n = 7 – 10 cells, 2 mice per experimental group; * denotes p < 0.05 compared to AAV-GFP using a paired Student’s t-test). d, The average firing rate is increased upon bath application of CNO in CRH neurons expressing Gq DREADD and decreased in CRH neurons expressing Gi DREADD. CNO had no effect in CRH neurons expressing AAV-GFP Postpartum wild type mice (Cre−/−) expressing Gq DREADDs in CRH neurons in the PVN exhibit a decreased latency to the first bout of immobility (e) and spend an increased total time immobile (f) in the forced swim test (n = 11–14 mice per experimental group; * denotes p<0.05 using a Student’s t-test). Wild type dams expressing Gq DREADDs exhibit an increased latency to approach their pups (g) and spend less time interacting with their pups (h) in the maternal approach test (n = 11–14 mice per experimental group; * denotes p < 0.05 using a Student’s t-test). Silencing of CRH neurons in the PVN of KCC2/Crh mice increases the latency to the first bout of immobility (i) and decreases the total time spent immobile in the forced swim test (j) compared to KCC2/Crh AAV-GFP controls (n = 8–14 mice per experimental group; * denotes p < 0.05 using a Student’s t-test). KCC2/Crh dams expressing Gi DREADDs exhibit a decreased latency to approach their pups (k) and spend more time interacting with their pups (l) in the maternal approach test (n = 8–18 mice per experimental group; * denotes p<0.05 using a Student’s t-test).
Activation of CRH neurons in postpartum Gq DREADD dams increases depression-like behaviors. Gq DREADD mice exhibit a decrease in the latency to the first bout of immobility and an increase in the total time spent immobile in the forced swim test compared to AAV-GFP controls (Figure 5e,f; latency t(23) = −2.19, total time t(23) = −6.24; p values<0.05).
Activation of CRH neurons in postpartum Gq DREADD dams also induces deficits in maternal behaviors. Gq DREADD mice exhibit an increase in the latency to approach and spend less time interacting with their pups in the maternal approach test compared to AAV-GFP controls (Figure 5g,h; latency t(22) = −2.66, total time t(22) = 2.62; p values<0.05). The differences in postpartum behaviors cannot be attributed to changes in locomotor activity since Gq DREADD mice exhibit a similar distance traveled in the open field (2243.8 ± 319.3 cm) compared to AAV-GFP control (2667.3 ± 218.6 cm; data not shown, ns determined using a Student’s t-test; t(15) = 1.12).
These data demonstrate that inappropriate activation of CRH neurons in the PVN during the postpartum period is sufficient to induce depression-like behaviors and deficits in maternal behaviors.
3.8. Silencing CRH neurons ameliorates abnormal postpartum behaviors in KCC2/Crh mice
Inhibition of CRH neurons in KCC2/Crh mice expressing Gi DREADD was evident from the hyperpolarization in the RMP of CRH neurons in the PVN following CNO administration and a decrease in the firing rate compared to nACSF (Figure 5a–d; RMP t(9) = 5.00, firing rate t(9) = 3.38; p values<0.05). In contrast, CNO has no effect on the RMP in mice expressing AAV-GFP and does not cause a change in the firing rate (Figure 5a–d; ns determined using a paired Student’s t-test; RMP t(6) = 1.07, firing rate t(6) = 0.49).
Silencing CRH neurons in postpartum KCC2/Crh dams increases the latency to the first bout of immobility and decreases the total time spent immobile in the forced swim test compared to KCC2/Crh controls (Figure 5i,j; latency t(20) = −3.66, total time t(20) = 3.17; p values<0.05).
Silencing CRH neurons in postpartum KCC2/Crh dams is also sufficient to improve the observed deficits in maternal behaviors. Gi DREADD mice exhibit an decrease in the latency to approach and spend more time interacting with their pups in the maternal approach test compared to AAV-GFP controls (Figure 5k,l; latency t(23) = 2.30, total time t(23) = −3.09; p values<0.05).
These data demonstrate that suppressing CRH neuron activation in the PVN is sufficient to reduce depression-like behaviors and deficits in maternal behaviors in postpartum KCC2/Crh mice.
3.9. Selective deletion of KCC2 from CRH neurons in the PVN induces abnormal postpartum behaviors
Reduction of KCC2 specifically in CRH neurons in the PVN of mice by stereotaxic injection of the LV-pCRF3.0-Cre virus was confirmed using immunohistochemistry and Western blot analysis. The intensity of KCC2 immunoreactivity in CRH neurons in the PVN expressing the LV-pCRF3.0-Cre virus is decreased compared to GFP controls (Figure 6a,b; t(7) = 3.86; p<0.05). Similarly, the expression of KCC2 assessed using Western blot analysis in total protein isolated from the PVN of mice bilaterally injected with the LV-pCRF3.0-Cre virus is decreased compared to GFP controls (Figure 6c,d; t(15) = 2.42; p<0.05). Similar to KCC2/Crh mice, there is not a complete loss of KCC2 within the PVN since this is a heterogeneous nucleus.
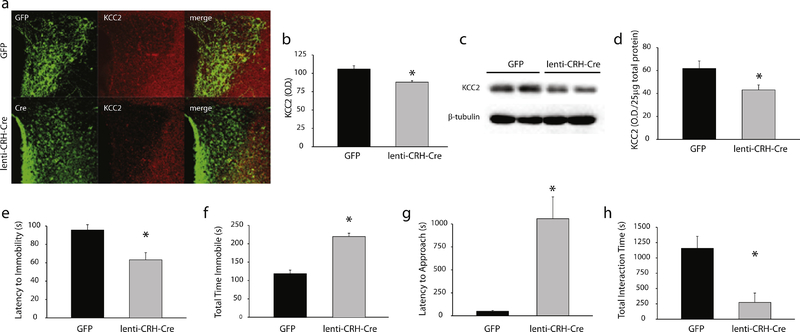
Figure 6: Elimination of KCC2 from CRH neurons exclusively in the PVN is sufficient to induce abnormal postpartum behaviors.
a, A representative image of GFP or lenti-CRH-Cre expression in the PVN (green) and KCC2 expression (red). b, The average KCC2 optical density in the PVN of mice stereotaxically injected with either GFP or lenti-CRH-Cre (n = 4–5 mice per experimental group; * denotes p < 0.05 using a Student’s t-test). c, Representative Western blots of total protein isolated from the PVN and probed for KCC2 or β-tubulin. d, The average KCC2 optical density determined using Western blot analysis in mice stereotaxically injected with either GFP or lenti-CRH-Cre (n = 8–9 mice per experimental group; * denotes p < 0.05 using a Student’s t-test). There is an increase in depression-like behaviors in postpartum mice (48–72 hrs) with a loss of KCC2 expression specifically in CRH neurons in the PVN (lenti-CRH-Cre), evident from a decrease in the latency to immobility (e) and an increase in the total time spent immobile in the forced swim test (f), compared to GFP postpartum controls. Deficits in maternal behaviors, such as an increase in the latency to approach their pups (g) and a decrease in the total interaction time (h) in the maternal approach test, are also observed in mice stereotaxically injected with lenti-CRH-Cre into the PVN compared to GFP controls. (n = 9–12 mice per experimental group for all behavioral experiments; * denotes p < 0.05 using a Student’s t-test).
Alterations in stress reactivity during the postpartum period resulting from the loss of KCC2 expression in CRH neurons in the PVN of mice injected with the LV-pCRF3.0-Cre virus was assessed by measuring corticosterone levels following a single 30 min restraint stress in mice at 48–72 hours postpartum. The stress-induced elevation in corticosterone is increased in postpartum mice injected with the LV-pCRF3.0-Cre virus (193.0 ± 13.9 ng/ml) compared to GFP controls (86.8 ± 19.1 ng/ml; n = 6–9 mice per experimental group; data not shown; p < 0.05 using a Student’s t-test; t(13) = −4.07; p<0.05). These data demonstrate that elimination of KCC2 in CRH neurons in the PVN prevents the suppression of the stress-induced activation of the HPA axis during the postpartum period.
The loss of KCC2 in CRH neurons specifically in the PVN (LV-pCRF3.0-Cre mice) is sufficient to induce abnormal postpartum behaviors. LV-pCRF3.0-Cre dams exhibit depression-like behaviors at 48–72 hours postpartum, evident by a decrease in the latency to the first bout of immobility and an increase in the total time spent immobile in the forced swim test compared to GFP controls (Figure 6e,f; latency t(19) = 3.44, total time t(19) = −7.29; p values<0.05).
The loss of KCC2 in CRH neurons in the PVN is also sufficient to induce deficits in maternal behaviors. LV-pCRF3.0-Cre dams exhibit an increase in the latency to approach their pups and spend less time interacting with their pups in the maternal approach test compared to GFP controls (Figure 6g,h; latency t(18) = −5.00, total time t(18) = 3.50; p values<0.05).
These data demonstrate a specific role for KCC2 in CRH neurons in the PVN in regulating HPA axis function during the postpartum period and influencing depression-like and maternal behaviors.
4. Discussion
Here we demonstrate that dysregulation of the HPA axis during the postpartum period, either in KCC2/Crh mice, Gq DREADD mice, or lenti-CRH-Cre mice, is sufficient to induce depression-like behaviors during the postpartum period and deficits in maternal behaviors. These findings are consistent with clinical findings suggesting that HPA axis dysfunction plays a role in postpartum depression (see (Bloch et al. 2003;Chrousos et al. 1998) and previous experimental evidence in rodents demonstrating that chronic stress is sufficient to induce abnormal maternal behaviors (Carini et al. 2013;Maguire and Mody 2016). Further, our ability to reverse this dysfunction by using Gi DREADDs to dampen CRH activity in the PVN specifically implicate this neuronal population in mediating these abnormal behaviors during the postpartum period.
The data presented herein also demonstrates a role for KCC2 regulation of CRH neurons in maintaining peripartum stress hyporeactivity. Further, we demonstrate that hyporeactivity of CRH neurons in the PVN is essential for adaptive behaviors and appropriate maternal behaviors during the postpartum period. These data support stress hyporeactivity as an important neuroendocrine adaptation that reduces the vulnerability to display maladaptive postpartum behaviors and supports regulation of KCC2 activity as a potential novel target for the treatment of postpartum depression.
Our findings demonstrate a specific role for CRH neurons in the PVN in mediating abnormal postpartum behaviors. HPA axis hyperexcitability and excessive glucocorticoid production during the peripartum period have been suggested to contribute to abnormal postpartum behaviors, due to the evidence that administration of exogenous glucocorticoids induces abnormal postpartum behaviors in rodents (Brummelte and Galea 2010;Maguire and Mody 2016). However, recently, a novel neural circuit involving CRH neurons projecting from the PVN to the lateral hypothalamus has been demonstrated to play a role in the behavioral manifestations of stress (Fuzesi et al. 2016). Thus, it is possible that activation of this neural circuit contributes to depression-like behaviors during the postpartum period and deficits in maternal behaviors. Future studies are required to dissociate the role of glucocorticoids versus CRH-specific neural circuits in mediating abnormal postpartum behaviors.
The data generated in the current study relied on the use of a CRH-Cre line whose fidelity was recently questioned. Therefore, we found it necessary to validate this tool prior to use. To this end, we crossed our CRH-Cre line with an Ai9 reporter mouse and quantified the degree of colocalization with the endogenous CRH peptide in the PVN. Our findings contradict previous reports questioning the specificity of Cre recombinase expression in the PVN of these CRH-Cre mice (Chen et al. 2015). Instead, we demonstrate a high degree of colocalization of the reporter with endogenous CRH immunoreactivity in both the PVN and the median eminence in Figure 3d.
In this study, we also discovered a novel role for KCC2-mediated regulation of CRH neurons in the PVN in the suppression of the stress-induced activation of the HPA axis during the peripartum period. Previous studies convincingly demonstrate a role for neurosteroids in the suppression of the HPA axis during the postpartum period (Brunton et al. 2009;Brunton and Russell 2008;Brunton and Russell 2011). However, the mechanisms whereby neurosteroids mediate suppression of the HPA axis during the peripartum period are unknown, but are thought to involve a GABAergic mechanism (Brunton and Russell 2008;Schiller et al. 2014). Here we demonstrate that the phosphorylation of KCC2 at residue Ser940 and total KCC2 expression is maintained in the PVN following subjection to an acute stressor in pregnant and postpartum mice, in contrast to virgin animals where the transporter is rapidly dephosphorylated and downregulated. However, the mechanisms maintaining KCC2 expression in the PVN are unknown, but hormonal changes during the peripartum period are obvious potential candidates. Future studies are required to investigate the mechanisms regulating KCC2 expression in the PVN during pregnancy and the postpartum period as well as the potential relationship with neurosteroid-mediated regulation of the HPA axis during the postpartum period.
Interestingly, changes in ovarian hormones and neurosteroids have also been implicated in the pathophysiology of peripartum depression (Schiller et al. 2014). For example, a negative correlation was found between serum allopregnanolone concentrations and depression in late pregnancy (Hellgren et al. 2014) and the postpartum period (Nappi et al. 2001). However, deficits in absolute levels of progesterone and/or allopregnanolone in the pathophysiology of postpartum depression remain controversial, which may be in part due to the likely diversity in underlying etiologies, which confound correlational studies in humans. However, changes in the levels of ovarian hormones and ovarian hormone-derived neurosteroids may contribute to vulnerability to postpartum mood disorders in susceptible women (for review see (Schiller et al. 2014). In fact, a recent open-label study using a positive allosteric modulator (PAM) targeting GABAA receptors has shown promise in patients with severe postpartum depression (Kanes et al. 2017).
It is interesting to note that the loss of KCC2 in CRH neurons does not alter baseline corticosterone levels, which is somewhat surprising given that CRH neurons in the PVN are tightly controlled by GABAergic inhibition (for review see (Levy and Tasker 2012) and KCC2 is critical for the inhibitory effects of GABA (for review see (Ben-Ari 2002). Our studies demonstrate that the loss of KCC2 in CRH neurons is not sufficient to disrupt the activity of the HPA axis under basal conditions, but rather the phenotype of these animals is only evident during the postpartum period or following stress exposure. A recent study suggested that impermeant anions play a role in establishing the chloride gradient in neurons (Glykys et al. 2014). Perhaps under baseline conditions, the chloride gradient is effectively maintained by impermeant anions, but when the system is challenged, such as following stress, KCC2 is required to maintain chloride homeostasis. Another potential explanation is that KCC2 functions at equilibrium near the normal resting membrane potential (Kaila et al. 2014) and disruptions which push the system away from equilibrium are required to appreciate deficits in KCC2 function. Regardless, the unique phenotype of the KCC2/Crh mice, including changes in anxiety- and depression-like behaviors restricted to the postpartum period, makes these animals useful for investigating the pathophysiological mechanisms contributing to postpartum depression.
5. Conclusions
Our data support stress hyporeactivity as an important neuroendocrine adaptation that reduces the vulnerability to maladaptive postpartum behaviors. Further, these data suggest that KCC2 plays a role in the normal suppression of the HPA axis during the peripartum period and may be a useful target for the treatment of postpartum depression. However, there remains much we need to learn about how KCC2 is regulated during the peripartum period and how dysregulation of the HPA axis mediates the development of mood disorders and deficits in maternal behaviors.
Highlights.
Suppression of the stress-induced activation of the HPA axis during the peripartum period involves maintenance of KCC2 expression in the PVN.
The inability to suppress the HPA axis during the peripartum period induces abnormal postpartum behaviors.
Inappropriate engagement of CRH neurons is sufficient to induce abnormal postpartum behaviors.
A reduction in KCC2 specifically in CRH neurons in the PVN induces HPA axis dysfunction and abnormal postpartum behaviors.
Acknowledgments
J.M. is supported by NIH-NINDS grant R01 NS073574 (J.M.) and NS102937 (J.M.). S.M. is supported by NS087662 and MH106954. L.C.M is supported by NIH-NIGMS grant K12GM074869; an IRACDA postdoctoral training grant to Tufts University, Training in Education and Critical Research Skills (TEACRS). Dr. Stephen J. Moss generated and supplied the floxed KCC2 mice essential for the current study for which we are extremely grateful. The behavioral and imaging studies were conducted in the Tufts Center for Neuroscience Research, P30 NS047243. The authors would also like to thank Dr. Kerry Ressler and the Emory University Viral Vector Core for providing the LV-pCRF3.0-Cre virus.
Abbreviations
- HPA
hypothalamic-pituitary-adrenal
- KCC2
K+/Cl− co-transporter 2
- CRH
corticotropin releasing hormone
- PVN
paraventricular nucleus of the hypothalamus
- DREADD
Designer Receptors Exclusively Activated by Designer Drugs
- CORT
corticosterone
- ACTH
adrenocorticotropic hormone
- GABAARs
GABAA receptors
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Acton BA, Mahadevan V, Mercado A, Uvarov P, Ding Y, Pressey J, Airaksinen MS, Mount DB, Woodin MA (2012) Hyperpolarizing GABAergic Transmission Requires the KCC2 C-terminal ISO Domain. J Neurosci 32:8746–8751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altemus M, Deuster PA, Galliven E, Carter CS, Gold PW (1995) Suppression of hypothalmic-pituitary-adrenal axis responses to stress in lactating women. The Journal of Clinical Endocrinology & Metabolism 80:2954–2959 [DOI] [PubMed] [Google Scholar]
- 3.Ben-Ari Y (2002) Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci 3:728–739 [DOI] [PubMed] [Google Scholar]
- 4.Bloch M, Daly RC, Rubinow DR (2003) Endocrine factors in the etiology of postpartum depression. Compr Psychiatry 44:234–246 [DOI] [PubMed] [Google Scholar]
- 5.Brummelte S, Galea LA (2010) Chronic corticosterone during pregnancy and postpartum affects maternal care, cell proliferation and depressive-like behavior in the dam. Horm Behav 58:769–779 [DOI] [PubMed] [Google Scholar]
- 6.Brummelte S, Pawluski JL, Galea LAM (2006) High post-partum levels of corticosterone given to dams influence postnatal hippocampal cell proliferation and behavior of offspring: A model of post-partum stress and possible depression. Hormones and Behavior 50:370–382 [DOI] [PubMed] [Google Scholar]
- 7.Brunton PJ, McKay AJ, Ochedalski T, Piastowska A, Rebas E, Lachowicz A, Russell JA (2009) Central opioid inhibition of neuroendocrine stress responses in pregnancy in the rat is induced by the neurosteroid allopregnanolone. J Neurosci 29:6449–6460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunton PJ, Russell JA (2008) Attenuated hypothalamo-pituitary-adrenal axis responses to immune challenge during pregnancy: the neurosteroid opioid connection. J Physiol 586:369–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunton PJ, Russell JA (2011) Allopregnanolone and suppressed hypothalamo-pituitary-adrenal axis stress responses in late pregnancy in the rat. Stress 14:6–12 [DOI] [PubMed] [Google Scholar]
- 10.Brunton PJ, Russell JA, Douglas AJ (2008) Adaptive responses of the maternal hypothalamic-pituitary-adrenal axis during pregnancy and lactation. J Neuroendocrinol 20:764–776 [DOI] [PubMed] [Google Scholar]
- 11.Carini LM, Murgatroyd CA, Nephew BC (2013) Using Chronic Social Stress to Model Postpartum Depression in Lactating Rodents. Journal of Visualized Experiments : JoVE50324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Molet J, Gunn BG, Ressler K, Baram TZ (2015) Diversity of Reporter Expression Patterns in Transgenic Mouse Lines Targeting Corticotropin-Releasing Hormone-Expressing Neurons. Endocrinology 156:4769–4780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chrousos GP, Torpy DJ, Gold PW (1998) Interactions between the hypothalamic-pituitary-adrenal axis and the female reproductive system: clinical implications. Ann Intern Med 129:229–240 [DOI] [PubMed] [Google Scholar]
- 14.de WC, Buitelaar JK (2005) Cortisol awakening response in pregnant women. Psychoneuroendocrinology 30:902–907 [DOI] [PubMed] [Google Scholar]
- 15.Fuzesi T, Daviu N, Wamsteeker Cusulin JI, Bonin RP, Bains JS (2016) Hypothalamic CRH neurons orchestrate complex behaviours after stress. Nature Communications 7:11937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T (2005) Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol 106:1071–1083 [DOI] [PubMed] [Google Scholar]
- 17.Glykys J, Dzhala V, Egawa K, Balena T, Saponjian Y, Kuchibhotla KV, Bacskai BJ, Kahle KT, Zeuthen T, Staley KJ (2014) Local Impermeant Anions Establish the Neuronal Chloride Concentration. Science 343:670–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, Ellis RJ, Richie CT, Harvey BK, Dannals RF, Pomper MG, Bonci A, Michaelides M (2017) Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 357:503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gunn BG, Cunningham L, Cooper MA, Corteen NL, Seifi M, Swinny JD, Lambert JJ, Belelli D (2013) Dysfunctional astrocytic and synaptic regulation of hypothalamic glutamatergic transmission in a mouse model of early-life adversity: relevance to neurosteroids and programming of the stress response. J Neurosci 33:19534–19554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heinrichs M, Meinlschmidt G, Neumann I, Wagner S, Kirschbaum C, Ehlert U, Hellhammer DH (2001) Effects of Suckling on Hypothalamic-Pituitary-Adrenal Axis Responses to Psychosocial Stress in Postpartum Lactating Women. The Journal of Clinical Endocrinology & Metabolism 86:4798–4804 [DOI] [PubMed] [Google Scholar]
- 21.Hellgren C, Akerud H, Skalkidou A, Backstrom T, Sundstrom-Poromaa I (2014) Low serum allopregnanolone is associated with symptoms of depression in late pregnancy. Neuropsychobiology 69:147–153 [DOI] [PubMed] [Google Scholar]
- 22.Heron J, O’Connor TG, Evans J, Golding J, Glover V (2004) The course of anxiety and depression through pregnancy and the postpartum in a community sample. Journal of Affective Disorders 80:65–73 [DOI] [PubMed] [Google Scholar]
- 23.Hewitt SA, Wamsteeker JI, Kurz EU, Bains JS (2009) Altered chloride homeostasis removes synaptic inhibitory constraint of the stress axis. Nat Neurosci 12:438–443 [DOI] [PubMed] [Google Scholar]
- 24.Hooper A, Maguire J (2016) Characterization of a novel subtype of hippocampal interneurons that express corticotropin-releasing hormone. Hippocampus 26:41–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnstone HA, Wigger A, Douglas AJ, Neumann ID, Landgraf R, Seckl JR, Russell JA (2000) Attenuation of hypothalamic-pituitary-adrenal axis stress responses in late pregnancy: changes in feedforward and feedback mechanisms. J Neuroendocrinol 12:811–822 [DOI] [PubMed] [Google Scholar]
- 26.Kahle KT, Deeb TZ, Puskarjov M, Silayeva L, Liang B, Kaila K, Moss SJ (2013) Modulation of neuronal activity by phosphorylation of the KΓÇôCl cotransporter KCC2. Trends Neurosci 36:726–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaila K, Price TJ, Payne JA, Puskarjov M, Voipio J (2014) Cation-chloride cotransporters in neuronal development, plasticity and disease. Nat Rev Neurosci 15:637–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kammerer M, Adams D, Castelberg Bv BV, Glover V (2002) Pregnant women become insensitive to cold stress. BMC Pregnancy Childbirth 2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanes SJ, Colquhoun H, Doherty J, Raines S, Hoffmann E, Rubinow DR, MeltzerΓÇÉBrody S (2017) OpenΓÇÉlabel, proofΓÇÉofΓÇÉconcept study of brexanolone in the treatment of severe postpartum depression. Human Psychopharmacology 32:e2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klampfl SM, Brunton PJ, Bayerl DS, Bosch OJ (2014) Hypoactivation of CRF Receptors, Predominantly Type 2, in the Medial-Posterior BNST Is Vital for Adequate Maternal Behavior in Lactating Rats. The Journal of Neuroscience 34:9665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klampfl SM, Neumann ID, Bosch OJ (2013) Reduced brain corticotropin-releasing factor receptor activation is required for adequate maternal care and maternal aggression in lactating rats. Eur J Neurosci 38:2742–2750 [DOI] [PubMed] [Google Scholar]
- 32.Klampfl SM, Schramm MM, Stinnett GS, Bayerl DS, Seasholtz AF, Bosch OJ (2016) Brain CRF-binding protein modulates aspects of maternal behavior under stressful conditions and supports a hypo-anxious state in lactating rats. Hormones and Behavior 84:136–144 [DOI] [PubMed] [Google Scholar]
- 33.Lee HH, Walker JA, Williams JR, Goodier RJ, Payne JA, Moss SJ (2007) Direct protein kinase C-dependent phosphorylation regulates the cell surface stability and activity of the potassium chloride cotransporter KCC2. J Biol Chem 282:29777–29784 [DOI] [PubMed] [Google Scholar]
- 34.Levy BH, Tasker JG (2012) Synaptic regulation of the hypothalamic-pituitary-adrenal axis and its modulation by glucocorticoids and stress. Front Cell Neurosci 6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li R, Shen Y (2013) An old method facing a new challenge: re-visiting housekeeping proteins as internal reference control for neuroscience research. Life Sciences 92:747–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma S, Shipston MJ, Morilak D, Russell JA (2005) Reduced hypothalamic vasopressin secretion underlies attenuated adrenocorticotropin stress responses in pregnant rats. Endocrinology 146:1626–1637 [DOI] [PubMed] [Google Scholar]
- 37.MacKenzie G, Maguire J (2015) Chronic stress shifts the GABA reversal potential in the hippocampus and increases seizure susceptibility. Epilepsy Research 109:13–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maguire J, Mody I (2008) GABA(A)R plasticity during pregnancy: relevance to postpartum depression. Neuron 59:207–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maguire J, Mody I (2016) Behavioral Deficits in Juveniles Mediated by Maternal Stress Hormones in Mice. Neural Plasticity 2016:2762518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matthey S, Barnett B, Howie P, Kavanagh DJ (2003) Diagnosing postpartum depression in mothers and fathers: whatever happened to anxiety? Journal of Affective Disorders 74:139–147 [DOI] [PubMed] [Google Scholar]
- 41.Meltzer-Brody S (2011) New insights into perinatal depression: pathogenesis and treatment during pregnancy and postpartum. Dialogues in Clinical Neuroscience 13:89–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Milgrom J, Gemmill AW, Bilszta JL, Hayes B, Barnett B, Brooks J, Ericksen J, Ellwood D, Buist A (2008) Antenatal risk factors for postnatal depression: A large prospective study. Journal of Affective Disorders 108:147–157 [DOI] [PubMed] [Google Scholar]
- 43.Morrison KE, Epperson CN, Sammel MD, Ewing G, Podcasy JS, Hantsoo L, Kim DR, Bale TL (2017) Preadolescent Adversity Programs a Disrupted Maternal Stress Reactivity-ain Humans and Mice. Biological Psychiatry 81:693–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nappi RE, Petraglia F, Luisi S, Polatti F, Farina C, Genazzani AR (2001) Serum allopregnanolone in women with postpartum “blues”. Obstet Gynecol 97:77–80 [DOI] [PubMed] [Google Scholar]
- 45.National Research Council (2011) Guide for the care and use of laboratory animals. National Academies Press; [PubMed] [Google Scholar]
- 46.O’hara MW, Swain AM (1996) Rates and risk of postpartum depressionΓÇöa meta-analysis. International Review of Psychiatry 8:37–54 [Google Scholar]
- 47.O’Toole KK, Hooper A, Wakefield S, Maguire J (2013) Seizure-induced disinhibition of the HPA axis increases seizure susceptibility. Epilepsy Research 108(1):29–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pfost KS, Stevens MJ, Lum CU (1990) The relationship of demographic variables, antepartum depression, and stress to postpartum depression. J Clin Psychol 46:588–592 [DOI] [PubMed] [Google Scholar]
- 49.Rich-Edwards JW, Kleinman K, Abrams A, Harlow BL, McLaughlin TJ, Joffe H, Gillman MW (2006) Sociodemographic predictors of antenatal and postpartum depressive symptoms among women in a medical group practice. J Epidemiol Community Health 60:221–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robertson ECNaSDE (2008) Risk factors for postpartum depression. World Health Organization: Literature review of risk factors and interventions on postpartum depressio [Google Scholar]
- 51.Sarkar J, Wakefield S, Mackenzie G, Moss SJ, Maguire J (2011) Neurosteroidogenesis Is Required for the Physiological Response to Stress: Role of Neurosteroid-Sensitive GABAA Receptors. J Neurosci 31:18198–18210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schiller CE, Schmidt PJ, Rubinow DR (2014) Allopregnanolone as a Mediator of Affective Switching in Reproductive Mood Disorders. Psychopharmacology (Berl) 231:3557–3567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schulte HM, Weisner D, Allolio B (1990) The corticotrophin releasing hormone test in late pregnancy: lack of adrenocorticotrophin and cortisol response. Clin Endocrinol (Oxf) 33:99–106 [DOI] [PubMed] [Google Scholar]
- 54.Steiner M (1998) Perinatal mood disorders: position paper. Psychopharmacol Bull 34:301–306 [PubMed] [Google Scholar]
- 55.Stowe ZN, Nemeroff CB (1995) Women at risk for postpartum-onset major depression. Am J Obstet Gynecol 173:639–645 [DOI] [PubMed] [Google Scholar]
- 56.Wisner KL, Sit DKY, McShea MC, Rizzo DM, Zoretich RA, Hughes CL, Eng HF, Luther JF, Wisniewski SR, Costantino ML, Confer AL, Moses-Kolko EL, Famy CS, Hanusa BH (2013) Onset Timing, Thoughts of Self-harm, and Diagnoses in Postpartum Women With Screen-Positive Depression Findings. JAMA Psychiatry 70:490–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ye ZY, Li DP, Byun HS, Li L, Pan HL (2012) NKCC1 upregulation disrupts chloride homeostasis in the hypothalamus and increases neuronal activityΓÇôsympathetic drive in hypertension. J Neurosci 32:8560–8568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yim IS, Glynn LM, Dunkel-Schetter C, Hobel CJ, Chicz-DeMet A, Sandman CA (2009) Risk of postpartum depressive symptoms with elevated corticotropinreleasing hormone in human pregnancy. Arch Gen Psychiatry 66:162–169 [DOI] [PMC free article] [PubMed] [Google Scholar]