Abstract
This review is based on the presentations and deliberations at the 7th John Goldman Chronic Myeloid Leukemia (CML) and Myeloproliferative Neoplasms (MPN) Colloquium which took place in Estoril, Portugal on the 15th October 2017, and the 11th post-ASH International Workshop on CML and MPN which took place on the 6th-7th December 2016, immediately after the 58th American Society of Hematology Annual Meeting. Rather than present a resume of the proceedings, we have elected to address some of the topical translational research and clinically relevant topics in greater detail. We address recent updates in the genetics and epigenetics of MPN, the mechanisms of transformation by mutant calreticulin, advances in the biology and therapy of systemic mastocytosis, clinical updates on JAK2 inhibitors and other therapeutic approaches for patients with MPNs, cardiovascular toxicity related to tyrosine kinase inhibitors and the concept of treatment-free remission for patients with CML.
Keywords: CML, MPN, TFR, Mastocytosis, CALR, Cardiotoxicity, JAK2 inhibitors
1. Introduction
Persons with myeloproliferative neoplasms (MPNs) have been well-served by translational research over the past two decades, and in the instance of chronic myeloid leukemia (CML), for the past half-century. The recent publication of the long-term results of imatinib therapy with a median follow-up of 11 years, confirms the safety and efficacy of this first-generation tyrosine kinase-inhibitor (TKI) [1,2]. Clinical progress for other subtypes of MPNs has been more limited but there have been therapy advances for certain other MPNs [3]. For example, there are encouraging therapies for patients with systematic mastocytosis (SM) and myeloid/lymphoid neoplasms with eosinophilia that are characterized by rearrangements of PDGFRA, PDGFRB, FGFR1 or PCM-JAK2 [4,5]. In contrast, in persons with BCR/ABL1 -negative MPNs, ruxolitinib, a JAK1 and JAK2-inhibitor, is the only newly-approved drug for persons with MPN-associated myelofibrosis (MF) and with polycythemia vera (PV). Most, if not all, other JAK2-inhibitors have been discontinued, principally due to unacceptable toxicity. Here, we address these and other important developments in CML and MPN biology and therapy discussed at the 7th John Goldman CML and MPN Colloquium which took place in Estoril, Portugal on the 15th October 2017, and the 11th Post-ASH CML and MPN workshop, which immediately followed the 58th American Society of Hematology meeting in San Diego, California.
2. What are the current genomic findings which inform the diagnosis, classification, prognosis and therapy-decisions in MPNs?
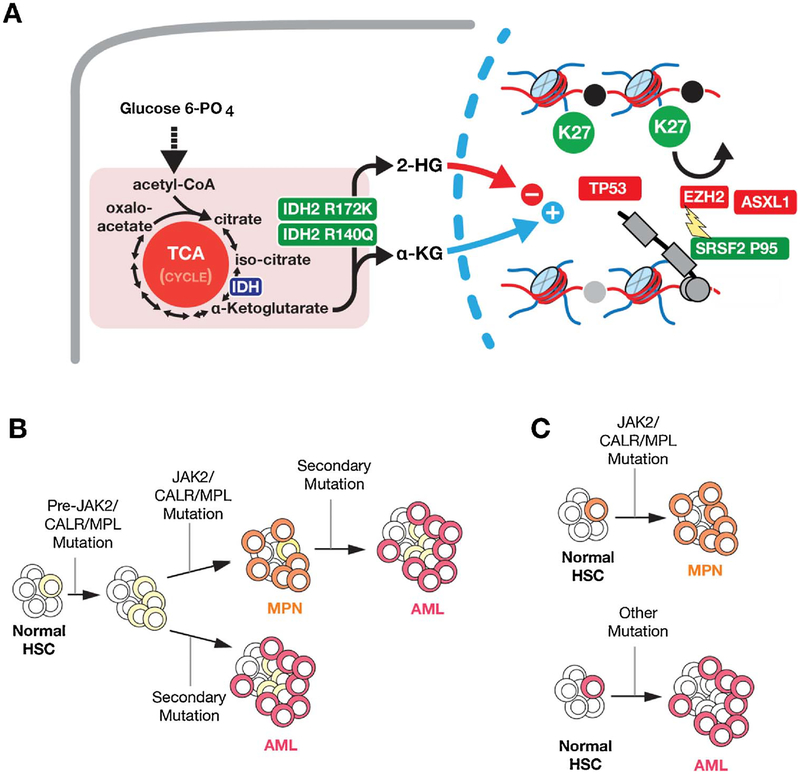
Genomic information has played a major role in the discovery of principal pathogenetic driver mutations in several subtypes of MPNs, beginning with the discovery of the BCR-ABL1 fusion gene in persons with CML [2]. In contrast, the BCR-ABL1 negative MPNs have multiple somatic mutations impacting disease biology and clinical outcome [6–12], typically in JAK2, CALR or MPL. These mutually exclusive alterations inform the WHO 2016 classification [13]. JAK2V617F mutations are present in almost everyone with polycythemia vera (PV) and 50–60% of persons with essential thrombocytosis (ET) and primary myelofibrosis (PMF). Persons with PV but without JAK2V617F often have mutations in JAK2 exon 12 [14]. CALR mutations in are typically absent in PV but present in 20–25% of persons with ET and PMF. Mutations in MPL are also rare in PV but present in about 10 percent, or less, of persons with ET or PMF. At present about 15 percent of persons with ET and PMF lack detectable mutations in JAK2, MPL and CALR mutations and termed ‘triple negative’. It is, however, likely that with the use of next-generation sequencing (NGS), additional mutations will be revealed [15–18]. Several other somatic mutations have been reported in persons with JAK2, MPL or CALR mutations including epigenetic modifiers (ASXL1, TET2, EZH2, IDH1, IDH2, DNMT3A), RNA splicing factors (SRSF2, U2AF1, SF3B1) and transcriptional regulators (TP53, IKZF1, NF-E2, CUX1) [19]. These mutations do not cause myelo-proliferation but influence the phenotype and prognosis. For example, mutations in IDH1, IDH2, EZH2, ASXL1, and SRSF2 have all been shown to predict risk for leukemic transformation of patients with myelofibrosis. Moreover, mutations and deletions in TP53 have been shown to predict leukemic transformation of all classic BCR-ABL1 negative MPNs and promote transformation to acute myeloid leukemia in an experimental model with JAK2V617F expression. Interestingly, mutations in ASXL1, EZH2, and SRSF2 all appear to impinge on methylation of his-tone H3 lysine 27 methylation [20]. Fig. 1 depicts the principal genetic abnormalities associated with evolution of the BCR-ABL1 negative MPNs.
Fig. 1. Genetic alterations and routes to leukemic transformation in BCR-ABL1 negative classic myeloproliferative neoplasms (MPNs).
Mutationsin IDH1, IDH2, EZH2, ASXL1, and SRSF2 have all been shown to predict risk for leukemic transformation of patients with myelofibrosis. Moreover, mutations and deletions in TP53 have been shown to predict leukemic transformation of all classic BCR- ABL1 negative MPNs and promote transformation to acute myeloid leukemia in an experimental model with JAK2V617F expression. Interestingly, mutations in ASXL1, EZH2, and SRSF2 all appear to impinge on methylation of histone H3 lysine 27 methylation. (B) The genetic alterations shown in (A) may occur in a cell with a pre-existing JAK2/CALR/MPL mutation to result in AML or could occur in a cell prior to acquisition of the JAK2/CALR/MPL mutation (in which case the patient may have both CALR/JAK2/MPL mutant MPN and CALR/JAK2/MPL wildtype AML). (C) As a final possibility, occasionally AML may be generated from a hematopoietic stem cell (HSC) unrelated to the HSC which gave rise to MPN.
Unique genetic hallmarks of other specific sub-types of MPNs are also described. For example, activating mutations in CSF3R occur in almost everyone with chronic neutrophilic leukemia (CNL) [21]. Mutations in PDGFRA, PDGFRB and FGFR1, and fusions, such as PCM1- JAK2, are associated in several myeloid and lymphoid neoplasms with eosinophilia [5,22,23]. In addition, the sequences of mutation acquisition may influence biology and clinical features of MPNs [24]. For example, if JAK2V617F occurs before mutations in TET2 or DNMT3A or acquired uniparental disomy (aUPD) of chromosome 14q, a person is more likely to develop PV than ET compared with the contrary [25,26]. Furthermore, an antecedent TET2 mutation seems to prevent JAK2V617F from up-regulating proliferation associated genes and may be associated with an increased risk of transformation to AML. Another alteration which can alter MPN phenotype are mutations in EZH2 [16]. In contrast to JAK2V617F mutations which may occur early or late in the development of MPNs, CALR mutations are thought to always be the initiating mutation in MPNs. There may also be differences in pathological signaling in the JAK-STAT pathway caused by different driver mutations. However, the precise contributions of driver and associated mutations linked to the phenotype of MPNs remains an enigma. Parenthetically, JAK2 or CALR mutations and BCR-ABL1 are reported in very rare cases of atypical myeloid neoplasms with a hybrid CML/MPN phenotype [27]. Familial MPNs are also rare and can be associated with a single-nucleotide germline variant in TERT added to germline mutations in the JAK2 JH1 and JH2 domains and to the MPL transmembrane domain [16,28,29].
3. How do CALR mutant proteins promote transformation?
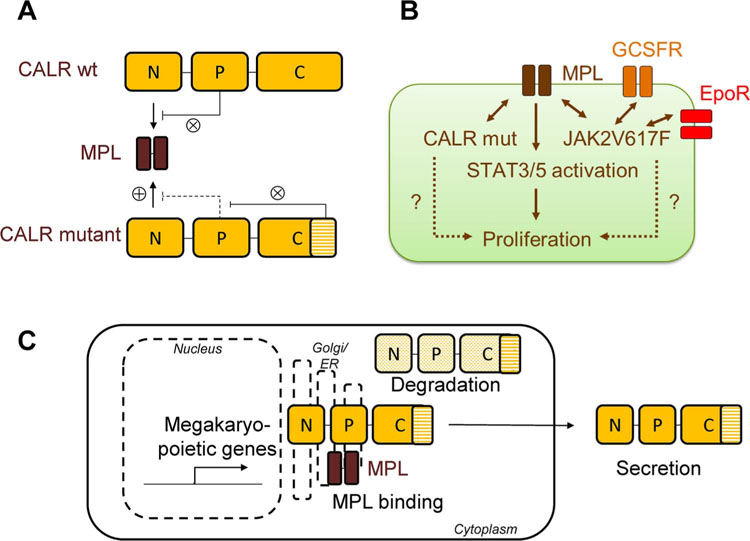
Following the seminal description of CALR mutations in MPNs in 2013, there has been a concerted effort to understand the molecular mechanisms by which mutant CALR proteins promote MPN development [11,12,30]. The fact that JAK2V617F, MPL, and CALR mutations are mutually exclusive in MPNs suggested that transformation occurred through the same pathway. The JAK-STAT pathway had already been shown to mediate the transforming potential of JAK2V617F mutations, where full cellular transformation required the presence of either MPL, EPO, or CSF3 (G-CSF) receptors [31]. Notably, work carried out by Constantinescu and colleagues revealed that the presence of the thrombopoietin receptor encoded by MPL was essential for CALR-mediated transformation [30]. This has now been confirmed by several investigators [32–34]. Transformation of megakaryocytic cells by CALR mutants required the presence of JAK2 protein as well as the interaction of CALR with MPL, occurring between the glycan binding site of MPL and the novel C-terminus of CALR, a domain generated by the MPN-associated mutations in CALR [30]. Further research showed that the charge and sequence of the novel C-terminal portion of CALR were essential for interaction with MPL and for transformation, as was the mutant CALR-mediated autoinhibition of the CALR P domain, which physiologically prevents binding of the N-terminal domain and the MPL receptor [32,33]. CALR mutant proteins by themselves induce low-level expression of megakaryocytic genes, potentially sensitizing 32D cells to CALR-mediated transformation, a finding also confirmed in Ba/F3 cells and human CD34+ cells [34–36]. Moreover, with the KDEL ER retention signal missing in CALR mutant proteins, CALR mutants showed abnormal subcellular localization [11,30,34]. Interestingly, mutations in CALR result in reductions in intracellular levels of CALR mutant protein and increased secretion of CALR [34,35]. While paracrine effects of secreted CALR mutant proteins were not uniformly observed, Dao and colleagues observed that secreted CALR mutant protein may indeed exert activity, enhancing inflammatory cytokine production in normal [37,38]. Further work is needed to elucidate the full spectrum of aberrant characteristics of CALR mutant proteins as well as the question of how these specific characteristics may be exploited therapeutically (Fig. 2).
Fig. 2. Mechanisms of transformation by mutant calreticulin (CALR).
(A) Binding of wildtype (wt) CALR protein to the thrombopoietin receptor (MPL) is inhibited by the P-domain of CALR (⊗). The novel C-terminus of mutant CALR protein antagonizes this inhibition (⊗), and mutant CALR protein can now bind to MPL via its N-terminal domain (⊗). [This figure was modified from Araki et al. Blood [33]. (B) Mutant CALR protein binds almost exclusively to MPL, thus inducing a megakaryocytic MPN pheno-type such as essential thrombocythemia (ET) or primary myelofibrosis (PMF). Conversely, mutant JAK2V617F protein may bind to either MPL or the erythropoietin receptor (EPOR) or the granulocyte-colony stimulating factor receptor (GCSFR) and thus can mediate phenotypes ranging from ET and PMF to polycythemia vera (PV). Both CALR and JAK2V617F mutant proteins lead to activation of the JAK-STAT pathway and ultimately stimulate the proliferation of hematopoietic stem and progenitor cells. (C) Intracellularly, CALR mutants are subject to enhanced protein degradation. MPL binding occurs as early in the Golgi apparatus and endoplasmic reticulum (ER), and this stabilizes mutant CALR protein and leads to JAK-STAT activation. In addition, mutant CALR protein is secreted into the extracellular space where it may activate monocytes and induce inflammation.
4. Does midostaurin treatment accord molecular and clinical responses in patients with systemic mastocytosis?
Systemic mastocytosis (SM) is due to the pathologic accumulation of neoplastic mast cells in one or more extracutaneous organ(s) such as the bone marrow, spleen, liver, lymph nodes, or gastrointestinal tract [13,39]. The canonical KITD816V mutation is found in ~90% of SM patients and is the primary pathogenetic driver of the disease [40]. Advanced forms of SM include aggressive systemic mastocytosis (ASM), SM with an associated hematologic neoplasm (SM-AHN), and mast cell leukemia (MCL). ASM, SM-AHN, and MCL are associated with poor-prognosis [median overall survival (OS) of 3.5 years, 2 years, and ≤ 6 months, respectively], wherein morbidity and mortality often relate to progressive organ damage (so-called “C-findings”) by neoplastic mast cells [41,42]. In contrast to indolent SM, where treatment is geared to control of mast cell activation symptoms, such as flushing and diarrhea, cytoreduction of mast cells is required to reverse organ damage in advanced disease [43]. Off-label therapies including pegylated interferon-alfa and cladribine, but not imatinib, have demonstrated reversion of organopathy in approximately 30–50% of patients, but data for their efficacy are primarily derived from small case series with limited follow-up and a heterogeneous mixture of indolent and advanced patients [44,45]. Midostaurin (N-benzoylstaurosporine; PKC412) is a multikinase inhibitor that demonstrates activity against both wild type and D816V-mutated KIT, as well as FLT3, PDGFR-α/β, PKC, and VEGFR2 [46,47]. In a small study of 26 patients with advanced SM, > 50% improvement or normalization of SM-related organ damage was observed in 69% patients treated with midostaurin [48]. These results provided the impetus for a global, open-label, single-arm study of midostaurin in patients with ASM, MCL, and SM-AHN [49]. Among 89 evaluable patients, the overall response rate was 60%, of which 75% were major responses (MRs; defined by complete reversion of organ damage) [40]. Responses were observed regardless of KITD816V status, prior therapy, or the presence of an AHN. After a median follow-up of 26 months, the median duration of response and median OS were 24.1 and 28.7 months, respectively. Median OS in responders was 44.4 months vs. 15.4 months in non-responders (hazard ratio for death, 0.42; p = 0.005). Of the 16 patients with MCL, 8 responded, including 7 molecular remissions. The median OS was 9.4 months among all patients with MCL, and was not reached among responding MCL patients. Symptoms and quality of life were also significantly improved with midostaurin. The drug’s ability to improve organ damage and symptoms may relate to its blockade of both mast cell proliferation and IgE-dependent mediator release [50]. Midostaurin was generally well tolerated with a manageable toxicity profile consisting mostly of gastrointestinal side effects and myelosuppression. Patients who experienced a response on midostaurin treatment in accordance with the modified Valent criteria or a > 50% reduction in bone marrow mast cell burden, and/or a reduction in the KITD816V allele burden, had an improved survival [50]. A recent study has also defined molecular correlates of response and progression on midostaurin. Patients with SM-AHN, who harbored SRSF2, ASXL1, or RUNX1 mutations, known to adversely impact survival, had inferior response rates, median duration of midostaurin treatment, and OS, compared with those who tested negative [51]. Midostaurin was licensed in April 2017, by the FDA, for patients with ASM, SM-AHN, and MCL [4].
5. How long to continue treatment in patients with CML who have had molecularly undetectable disease for at least 2 years?
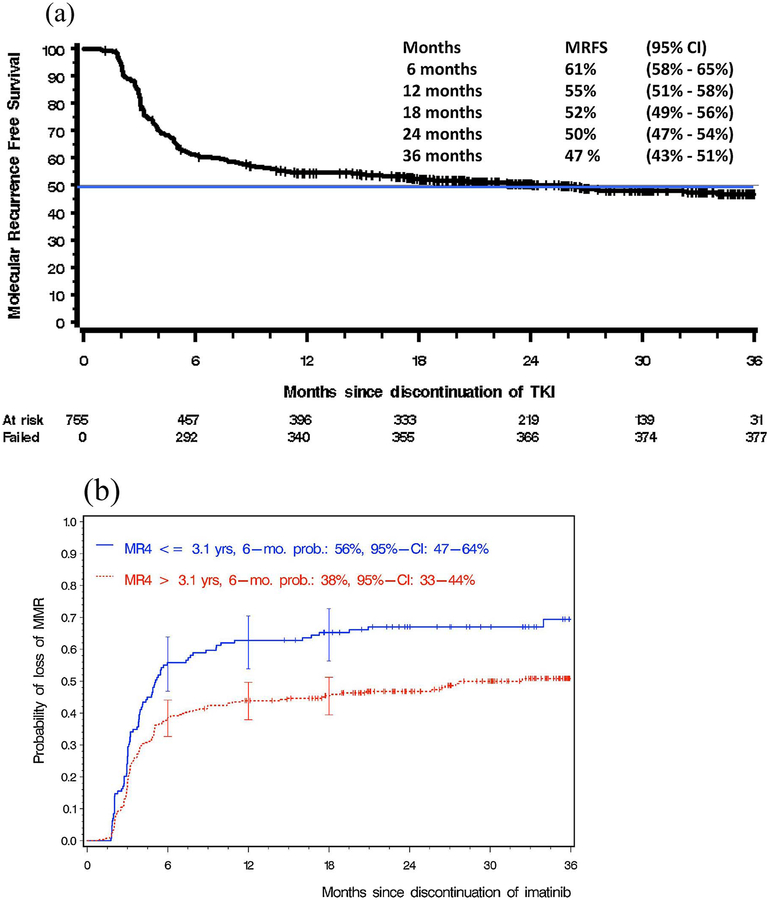
A principal goal of CML therapy with TKIs is the achievement of a stable molecular remission which accords successful discontinuation of therapy, a concept now known as ‘treatment-free remission (TFR). Since second- and third-generation TKIs exhibit greater potency over imatinib, resulting in deeper molecular responses, they are associated with a greater proportion of patients achieving TFR, even though there is no OS superiority [52,53]. An unresolved challenge has been to determine how long to continue treatment in patients who achieve molecularly undetectable disease for at least 2 years [54]. Clearly this is a prudent issue with significant impact not only to the quality-of-life and side effects experienced by patients, but also the financial burdens that affect both patients and society. The concept of TFR has now been addressed in several studies [55–59]. The STIM1 study showed that, following a median follow-up of > 6 years, 38% of the patients who discontinued therapy have not had a molecular recurrence to date, with the rest having a relapse [55]. The Euro-SKI study enrolled 772 persons who were considered eligible to stop imatinib, dasatinib or nilotinib after a median of 91 months and a sustained MR4 or greater for at least 1 year. The molecular relapse-free survival at 12 months was 56%, with patients who had a MR4 of > 3.1 years duration, and no significant safety concerned were noted [Fig. 3(a)(b)] [60]. In patients who relapse, most, if not all, achieve molecular remissions on the re-institution of the previously discontinued TKI. Similar findings have been observed in other dasatinib- and nilotinib-treated persons in clinical trials. As illustration, the ENESTfreedom was a single arm phase 2 study which assessed the potential for TFR following first-line treatment with nilotinib, administered for 2 years followed by a 1-year consolidation [61]. The study cohort comprised of 215 patients, of which 190 could demonstrate sustained deep molecular response and enter the TFR phase of the study. 51.6% of these persons remained in molecular remission, and the median duration of nilotinib therapy in this study was relatively short at 3.6 years. At present, although nilotinib was granted a European regulatory approval in June 2017, for the inclusion of TFR, many specialists believe that, at least for the moment, it is probably best to discontinue the TKI therapy only within the framework of a clinical trial [62]. Most agree that discontinuation is feasible if close clinical and molecular monitoring is readily available [63,64]. Other factors which can be useful as positive predictive factors for TFR include a higher NK-cell count at the time of TKI discontinuation and a lower expression of the T-cell inhibitory receptor-ligand CD86 on plasmacytoid dendritic cells, deep molecular response and duration of TKI treatment [65,66]. Recent findings suggest that the age and gender of the person, as well as intrinsic psychological and cognitive variables, might also be important [67,68]. An alternative strategy for TFR involves de-escalation of the TKI doses for patients who have durable major molecular response or a deep molecular response, such as that demonstrated in the DESTINY study [69]. The observation that lower TKI doses could maintain responses in a significant proportion of the study cohort is attractive and merits further investigation. So far, however, no reliable biomarkers are available to identify patients that will not lose MMR following TKI discontinuation. The notion of achieving TFR successfully is clearly is a significant milestone for the optimal management of persons with CML, most of whom have a life expectancy equivalent to that of the general population, following appropriate treatment with TKIs [70].
Fig. 3.
Results from the Euro-SKI study (ASH 2016) (a) Molecular recurrence-free survival (n = 755 of 772 persons enrolled); Euro-SKI study (ASH 2016) (b) Prognostic modeling (n = 405, imatinib);Euro-SKI study (ASH 2016).
6. What can patients with MPNs expect from JAK2 inhibitors, and what comes next?
In contrast to most myeloid malignancies, MPNs appear to have a remarkably long natural history with many patients with ET and PV having a survival which is like that of the general population [21]. It is therefore important to avoid unnecessary treatment and treatment-related side effects, and all MPNs patients need to be assessed carefully. Ruxolitinib, a type 1 inhibitor of JAK1 and JAK2, was approved, in 2011, for the treatment of patients with intermediate- and high-risk MF, and, in 2016, for persons with PV refractory or intolerant to hydro-xyurea [71–74]. These indications followed the results from randomized studies, which demonstrated the drug’s ability to substantially alleviate symptoms, reduce splenomegaly, and improve blood counts in some patients, but without altering molecular response or reducing risk of transformation to AML. Longer-term data confirms a survival benefit from ruxolitinib treatment in MF, particularly for those patients in which there is histologic improvement in the bone marrow, though neither of the two randomized trails (COMFORT-1 and −2) were powered to measure differences in survival [3,75,76]. Also, ruxolitinib appears to exert no appreciable effect on the malignant clone and the discontinuation rate of the drug appears high. The drug’s major effect appears to be suppression of inflammatory cytokine production, a major feature mediator of MPN-related symptoms, lending some support to the speculation that some of the survival benefit might stem from the anti-inflammatory properties which result in improving symptoms and/or splenomegaly [77]. It is of interest that the survival benefit was observed in both studies despite complete crossover and raises the possibility that the drug might indeed modify the natural history in some cases.
However, clearly not all persons with MF respond to ruxolitinib, responses are not enjoyed indefinitely, and challenges with cytopenias remain [3]. Consequently, there has been considerable interest in investigating the role of selective, potentially non-myelosuppressive type 1 JAK2 inhibitors, such as pacratinib and momelotinib [78,79]. Several other JAK2 inhibitors, such as fedratinib, have also been tested in a randomized trial in persons with MF (JAKARTA), and discontinued due to significant toxicity, including possible Wernicke’s encephalopathy [80]. Pacritinib, a JAK2 and FLT3 inhibitor, has also been studied in randomized phase 3 studies (PERSIST-1 and −2), but accrual of PERSIST-2 was impeded by a FDA suspension because of concerns of safety with hemorrhage in both studies, which has now been lifted [78,81]. PERSIST-2 study includes persons with MF with a platelet count of < 100,000/dl, a cohort with an unmet clinical need. The study evaluated two different doses of pacritinib compared with best alternative and identified that pacritinib 200 mg twice a day was superior to either pacritinib 400 mg a day or best alternative therapy, including ruxolitinib, for endpoints of improving symptom burden and spleen response. Additionally, pacritinib resulted in improvements in red cell transfusions and tolerability, even in patients with significant thrombocytopenia. Further studies with pacritinib are planned to adequately identify the minimally effective dose of this agent for patients with MF.
Long term data from momelotinib, a JAK1 and JAK2 inhibitor, in MF suggest durable responses for cytopenias, as well as splenomegaly and symptoms, but neuropathy was not infrequently observed [79]. Momelotinib is thought to potentially improve anemia through its inhibition of activin A receptor type I and reduction of hepcidin production. The drug has now been tested in two phase 3 studies (SIMPLFY-1 and −2), and though both studies confirmed the drug’s credentials with respect to improving anemia, and SIMPLIFY-2 symptomatic improvement, the studies were negative and further development of the drug for MF discontinued [82]. Other JAK inhibitors in development for MF include the JAK2 inhibitor NS018 (NS Pharma, Japan), which has demonstrated modest activity and safety in phase 2 setting [83]. Type 2 JAK2 inhibitors, such as CHZ868, have also been tested in murine models, but currently clinical studies are not planned [84]. A JAK1-selective inhibitor, INCB039110, a potent inhibitor of several keynote inflammatory cytokines, has also been studied in persons with MF and demonstrates modest activity with minimal myelo-suppression [85].
Rational ruxolitinib-based combinations, such as with pomalidomide, azacytidine, and decitabine, have now been reported [86–88]. In general, though well tolerated, they appear to add little to ruxolitinib’s activity as monotherapy. Preliminary data from a dose seeking study of the PI3Kδ Inhibitor TGR-1202 in combination with ruxolitinib was favorable [89]. Finally, new agents were reported in development for myelofibrosis, including PRM-151, a recombinant human pentraxin-2 that inhibits fibrocyte differentiation, and sotatercept, a novel soluble receptor fusion protein that relieves blockade of terminal erythropoiesis caused by TGF beta and has efficacy in MF-anemia [90,91]. Results from randomized studies of long acting pegylated alpha interferons (2a and 2b) in high-risk PV and ET have confirmed their efficacy and safety, and non-inferior (but not superior) to hydroxyurea regarding the study endpoints of blood count control, prevention of thrombotic complications, and bone marrow response criteria; some ropeginterferon (2b)-treated patients also demonstrated molecular responses following one year of treatment [92–94].
7. Vascular and cardiac toxicity of ABLl-tyrosine kinase inhibitors
Following longer term follow-up, the clinical risk of ABL1-TKI related vascular and cardiac safety is increasingly recognized and has raised concerns, when TFR might not be feasible and long-term therapy is indicated [95]. The risks are greater with the second- and third-generation TKIs, and in the presence of recognized comorbidities. In general, the risks are low, but it is likely that these risks have been underestimated [96]. Several systemic reviews and meta-analysis reveal dasatinib, nilotinib, and ponatinib, but not bosutinib, to increase vascular occlusive events, compared with imatinib [97–99]. In view of these considerations, expert panels, such as the European LeukemiaNet (ELN), have convened to offer recommendations for the management and avoidance of vascular and cardiac adverse events [100]. Since the precise underlying mechanism for these effects remains an enigma, probably related to inhibition of ligand-related signaling pathways and direct effects on vascular and perivascular cells, rationale approaches to minimize the long-term sequelae from such critical adverse events have been proposed. The notion of considering the effect of aspirin and anticoagulation on TKIs induced thrombotic events in CML has been tested and found to decrease the thrombotic events excluding myocardial infarction [101]. Clearly such approaches need further study and validation, as they are crucial for the optimal management of patients with CML.
8. Conclusions
Ongoing studies in MPNs have underlined the remarkable long-term success of TKIs in the therapy of CML. This contrasts with other sub-types of MPNs where, so far, only a qualified success has been noted. With this success of TKIs in CML, the importance of TFR has been recognized as an important future milestone. This has now been achieved with the recent regulatory inclusion of nilotinib for TFR and marks the CML community’s efforts to discontinue TKIs effectively, to reduce the significant therapy-related impact on the quality-of-life, other side-effects, and financial burdens that affect both patients and society, in a responsible manner. And we need rational approaches to reduce the risk of vascular and cardiac toxicities. We have also learned much from the longer-term studies of ruxolitinib’s efficacy and safety and that of emerging JAK2 and other therapies for MPNs. Ruxolitinib affords substantial clinical benefits, such as symptomatic relief, reduction of splenomegaly, and a modest survival benefit. Importantly there is some uncertainty as to precise disease-initiating events in MF and efforts based on targeting multiple molecular pathways, in addition to JAKSTAT, are now assessing the role of combining ruxolitinib with other drugs, including IFN-α. Furthermore, clinical trials assessing the efficacy of the type II JAK inhibitors are now in progress. A principal challenge is to establish the prognostic and predictive impact of the numerous mutations found in BCR-ABL1 negative classic MPN in efforts to better identify drug targets and understand the impact of the microenvironment and inflammation in the pathogenesis of these diseases.
Acknowledgments
The authors and the faculty members at both live events wish to thank Dr. Alpa Parmar for her organization skills, and Incyte Corporation, Novartis Oncology Global and Alpine Oncology Foundation for their support.
Footnotes
Conflict of interest disclosures
O Abdel-Wahab: No relevant disclosures.
T Barbui: No relevant disclosures.
J Cortes: Consultancy: Novartis, Bristol-Myers Squibb, Pfizer. Research
funding: Novartis, Bristol-Myers Squibb, Pfizer.
R Gale: Part-time employee Celgene Corp.
J Gotlib: No relevant disclosures.
R Hehlmann: Research funding: Bristol-Myers Squibb, Novartis.
HJ Khoury: No relevant disclosures.
S Koschmieder: Consultancy: Novartis, Bristol-Myers Squibb, Pfizer, Baxalta/CTI, AOP, Sanofi. Research funding: Novartis, Bristol-Myers Squibb, Novartis Foundation.
R Mesa: Research funding: Incyte, Lilly, NS Pharma, Sanofi, Gilead, CTI, Genentech.
TI Mughal: No relevant disclosures.
G Saglio: Consultancy: Bristol-Myers Squibb; Research funding:
Novartis.
S Saussele: No relevant disclosures.
RA Van Etten: Scientific Advisory Boards: Bristol Myers-Squibb, Pfizer, TEVA, Sunesis, Karyopharm; Research funding: TEVA, Verastem.
S Verstovsek: Research funding: Incyte Corporation, Roche, Astrazeneca, Lilly Oncology, NS Pharma, Bristol Myers Squibb, Celgene, Gilead, Seattle Genetics, Promedior, CTI BioPharma Corp., Galena BioPharma, Pfizer, Genentech, Blueprint Medicines Corp.
References
- [1].Hochhaus A, Larson RA, Guilhot F, et al. , Long-term outcomes of imatinib treatment for chronic myeloid leukemia, N. Engl. J. Med 376 (2017) 917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mughal TI, Radich JP, Deininger MW, et al. , Chronic myeloid leukemia: reminiscences and dreams, Haematologica 101 (2016) 541–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bose P, Verstovsek S, JAK2 inhibitors for myeloproliferative neoplasms: what is next, Blood 130 (2017) 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gotlib J, A molecular roadmap for midostaurin in mastocytosis, Blood 130 (2017) 98–100. [DOI] [PubMed] [Google Scholar]
- [5].Reiter A, Gotlib J, Myeloid neoplasms with eosinophilia, Blood 129 (2017) 704–714. [DOI] [PubMed] [Google Scholar]
- [6].Baxter EJ, Scott LM, Campbell PJ, et al. , Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders, Lancet 365 (2005) 1054–1061. [DOI] [PubMed] [Google Scholar]
- [7].James C, Ugo V, Le Couedic JP, et al. , A unique clonal JAK2 mutation leading to constitutive signaling causes polycythemia vera, Nature 434 (2005) 1144–1148. [DOI] [PubMed] [Google Scholar]
- [8].Kralovics R, Passamonti F, Buser AS, et al. , A gain-of-function mutation of JAK2 in myeloproliferative disorders, N. Engl. J. Med 352 (2005) 1779–1790. [DOI] [PubMed] [Google Scholar]
- [9].Levine RL, Wadleigh M, Cools J, et al. , Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid meta-plasia with myelofibrosis, Cancer Cell 7 (2005) 387–397. [DOI] [PubMed] [Google Scholar]
- [10].Pikman Y, Lee BH, Mercher T, et al. , MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia, PLoS Med. 2 (2006) e270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Klampf T, Gisslinger H, Harutyunyan AS, et al. , Somatic mutations of calreti- culin in myeloproliferative neoplasms, N. Engl. J. Med 369 (2013) 2379–2390. [DOI] [PubMed] [Google Scholar]
- [12].Nangalia J, Massie CE, Baxter EJ, et al. , Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2, N. Engl. Med 369 (2013) 2391–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Arber DA, Orazi A, Hasserjian R, et al. , The 2016 revision to the WHO classification in myeloid neoplasms and acute leukemias, Blood 127 (2016) 2391–2405. [DOI] [PubMed] [Google Scholar]
- [14].Scott LM, Tong W, Levine RL, et al. , JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis, N. Engl. J. Med 356 (2007) 459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Milosevic Feenstra JD, Nivarthi H, Gisslinger H, et al. , Whole-exome sequencing identifies novel MPL and JAK2 mutations in triple negative myeloproliferative neoplasms, Blood 127 (2016) 325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Langabeer SE, Chasing down the triple-negative myeloproliferative neoplasms: implications for molecular diagnostics, JAK-STAT 5 (2–4) (2016) e1248011, 10.1080/21623996.2016.1248011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chang YC, Lin HC, Chiang YH, et al. , Targeted next-generation sequencing identified novel mutations in triple-negative myeloproliferative neoplasms, Med. Oncol 34 (2017) 83. [DOI] [PubMed] [Google Scholar]
- [18].Frawley T, O’Brien CP, Conneally E, et al. , Genetic Testing and Molecular Biomarkers, (2018), 10.1089/gtmb.2017.0203 (pre-pub). [DOI] [PubMed]
- [19].Vainchenker W, Kralovics R, Genetic basis and molecular pathophysiology of classical myeloproliferative neoplasms, Blood 66 (2017) 7–679. [DOI] [PubMed] [Google Scholar]
- [20].Vannucchi AM, Lasho TL, Guglielmelli P, et al. , Mutations and prognosis in primary myelofibrosis, Leukemia 27 (2013) 1861–1869. [DOI] [PubMed] [Google Scholar]
- [21].Maxson JE, Gotlib J, Pollyea DA, et al. , Oncogenic CSF3R mutations in chronic neutrophilic leukemia and atypical CML, N. Engl. J. Med 368 (2013) 1781–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Heilmann AM, Schrock AB, He J, et al. , Novel PDGFRB fusions in childhood B and T acute lymphoblastic leukemia, Leukemia 31 (2017) 1989–1992. [DOI] [PubMed] [Google Scholar]
- [23].Baer CR, Muehlbacher V, Kern W, Haferlach C, Haferlach T, Comprehensive genetic characterization of 63 myeloid/lymphoid neoplasms associated with eo- sinophilia and rearrangement of PDGFR-α, PDGFR-β, FGFR1 or PCM1-JAK2, Blood 130 (2017) 4219. [Google Scholar]
- [24].Nangalia J, Nice FL, Wedge DC, et al. , DNMT3A mutations occur early or late in patients with myeloproliferative neoplasms and mutation order influences phenotype, Haematologica 100 (2015) e438–e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ortmann C, Kent DG, Nangalia J, et al. , Effect of mutation order on myeloproliferative neoplasms, N. Engl. J. Med 372 (2015) 601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chase A, Leung W, Tapper W, et al. , Profound parental bias associated with chromosome 14 acquired uniparental disomy indicates targeting of an imprinted locus, Leukemia 29 (2015) 2069–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sircilla M, Nader K, Ferber A, A case report of chronic myelogenous leukemia with JAK2- and BCR-ABL-positive mutation, AJHO (2017) (in press). [Google Scholar]
- [28].Spivak JL, Myeloproliferative neoplasms N. Engl. J. Med 376 (2017) 2168–2181. [DOI] [PubMed] [Google Scholar]
- [29].Leroy E, Constantinescu SN, Rethinking JAK2 inhibition: towards novel strategies of more specific and versatile janus kinase inhibition, Leukemia 31 (2017) 1023–1038. [DOI] [PubMed] [Google Scholar]
- [30].Chachoua I, Pecquet C, El-Khoury M, et al. , Thrombopoietin receptor activation by myeloproliferative neoplasm associated calreticulin mutants, Blood 127 (2016) 1325–1335. [DOI] [PubMed] [Google Scholar]
- [31].Sangkhae V, Etheridge SL, Kaushansky K, Hitchcock IS, The thrombopoietin receptor, MPL, is critical for development of a JAK2V617F-induced myeloproliferative neoplasm, Blood 124 (2014) 3956–3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lu X, Levine R, Tong W, et al. , Expression of a homodimeric type I cytokine receptor is required for JAK2V617F-mediated transformation, Proc. Natl. Acad. Sci. U. S. A 102 (2005) 18962–18967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Araki M, Yang Y, Masubuchi N, et al. , Activation of the thrombopoietin receptor by mutant calreticulin in CALR-mutant myeloproliferative neoplasms, Blood 127 (2016) 1307–1316. [DOI] [PubMed] [Google Scholar]
- [34].Elf S, Abdelfattah NS, Chen E, et al. , Mutant Calreticulin requires both its mutant C-terminus and the thrombopoietin receptor for oncogenic transformation, Cancer Discov. 6 (2016) 368–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Han L, Schubert C, Köhler J, et al. , Calreticulin-mutant proteins induce mega- karyocytic signaling to transform hematopoietic cells and undergo accelerated degradation and Golgi-mediated secretion, J. Hematol. Oncol 13 (9) (2016) 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Nivarthi H, Chen D, Cleary C, et al. , Thrombopoietin receptor is required for the oncogenic function of CALR mutants, Leukemia 30 (2016) 1759–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kollmann K, Warsch W, Gonzalez-Arias C, et al. , A novel signaling screen demonstrates that CALR mutations activate essential MAPK signalling and facilitate megakaryocyte differentiation, Leukemia 31 (2017) 934–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Garbati MR, Welgan CA, Landefeld SH, et al. , Mutant calreticulin-expressing cells induce monocyte hyperreactivity through a paracrine mechanism, Am. J. Hematol 91 (2016) 211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Horny HP, Metcalfe DD, Bennet JM, et al. , Mastocytosis, in: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H (Eds.), WHO Classification of Tumors of Hematopoietic and Lymphoid Tissues, 4th ed, IARC, Lyon, 2008, pp. 54–63. [Google Scholar]
- [40].Arock M, Sotlar K, Broesby-Olsen S, et al. , KIT mutation analysis in mast cell neoplasms: recommendations of the European Competence Network on Mastocytosis, Leukemia 29 (6) (2015) 1223–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lim KH, Tefferi A, Lasho TL, et al. , Systemic mastocytosis in 342 consecutive adults: survival studies and prognostic factors, Blood 113 (23) (2009) 5727–5736. [DOI] [PubMed] [Google Scholar]
- [42].Valent P, Akin C, Hartmann K, et al. , Advances in the classification and treatment of mastocytosis: current status and outlook toward the future, Cancer Res. 77(6) (2017) 1261–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Pardanani A, Systemic mastocytosis in adults: 2017 update on diagnosis, risk stratification, and management, Am. J. Hematol 91 (11) (2016) 1146–1159. [DOI] [PubMed] [Google Scholar]
- [44].Lim KH, Pardanani A, Butterfield JH, Li CY, Tefferi A, Cytoreductive therapy in 108 adults with systemic mastocytosis: outcome analysis and response prediction during treatment with interferon-alpha, hydroxyurea, imatinib mesylate or 2- chlorodeoxyadenosine, Am. J. Hematol 84 (12) (2009) 790–794. [DOI] [PubMed] [Google Scholar]
- [45].Cools J, DeAngelo DJ, Gotlib J, et al. , A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hy- pereosinophilic syndrome, N. Engl. J. Med 348 (13) (2003) 1201–1214. [DOI] [PubMed] [Google Scholar]
- [46].Growney JD, Cark JJ, Adelsperger J, et al. , Activation mutations of human c- KIT resistant to imatinib mesylate are sensitive to the tyrosine kinase inhibitor PKC412, Blood 106 (2) (2005) 721–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Fabbro D, Ruetz S, Bodis S, et al. , PKC412 — a protein kinase inhibitor with a broad therapeutic potential, Anticancer Drug Des. 15 (1) (2000) 17–28. [PubMed] [Google Scholar]
- [48].DeAngelo DJ, George TI, Linder A, et al. , Efficacy and safety of midostaurin in patients with advanced systemic mastocytosis: 10-year median follow-up of a phase II trial, Leukemia 32 (July) (2018) 470–478, 10.1038/leu.2017.234 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- [49].Gotlib J, Kluin-Nelemans HC, George TI, et al. , Efficacy and safety of mid- ostaurin in advanced systemic mastocytosis, N. Engl. J. Med 374 (26) (2016) 2530–2541. [DOI] [PubMed] [Google Scholar]
- [50].Krauth MT, Mirkina I, Herrmann H, et al. , Midostaurin (PKC412) inhibits immunoglobulin E-dependent activation and mediator release in human blood basophils and mast cells, Clin. Exp. Allergy 39 (11) (2009) 1711–1720. [DOI] [PubMed] [Google Scholar]
- [51].Jawhar M, Schwaab J, Naumann N, et al. , Response and progression on mid- ostaurin in advanced systemic mastocytosis: KIT D816V and other molecular markers, Blood 130 (2017) 137–145. [DOI] [PubMed] [Google Scholar]
- [52].Longo D, Imatinib changed everything, N. Engl. J. Med 376 (2017) 982–983. [DOI] [PubMed] [Google Scholar]
- [53].Dulucq S, Mahon FX, Deep molecular responses for treatment-free remission in chronic myeloid leukemia, Cancer Med. 5 (9) (2016) 2398–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Baccarani M, Deininger MW, Rosti G, et al. , European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013, Blood 122 (2013) 872–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Mahon FX, Rea D, Guilhot J, et al. , Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicenter Stop Imatinib (STIM) trial, Lancet Oncol. 11 (2010) 1029–1035. [DOI] [PubMed] [Google Scholar]
- [56].Etienne G, Guilhot J, Read D, et al. , Long term follow-up of the French Stop Imatinib (STIM1) study in patients with chronic myeloid leukemia, J. Clin. Oncol 35 (2017) 298–305. [DOI] [PubMed] [Google Scholar]
- [57].Ross DM, Branford S, Seymour JF, et al. , Safety and efficacy of cessation for chronic myeloid leukemia patients with stable undetected minimal residual disease -results from TWISTER study, Blood 122 (2013) 515–522. [DOI] [PubMed] [Google Scholar]
- [58].Rousselot P, Charbonnier A, Cony-Makhoul P, et al. , Loss of major molecular response as a trigger for restarting tyrosine kinase inhibitor therapy in patients with chronic-phase chronic myelogenous leukemia who have stopped imatinib after durable undetectable disease, J. Clin. Oncol 32 (5) (2014) 424–430. [DOI] [PubMed] [Google Scholar]
- [59].Mahon FX, Richter J, Guilhot J, et al. , Cessation of tyrosine kinase inhibitors treatment in chronic myeloid leukemia patients with deep molecular response: results of the Euro-Ski trial, Blood 128 (22) (2016) 787. [Google Scholar]
- [60].Hochhaus A, Masszi T, Giles F, et al. , Treatment-free remission following frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: results from the ENESTfreedom study, Leukemia 31 (July (7)) (2017) 1525–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Saussele S, Richter J, Hochhaus A, Mahon FX, The concept of treatment-free remission in chronic myeloid leukemia, Leukemia 30 (2016) 1638–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Cross NCP, White HE, Colomer D, et al. , Laboratory recommendations for scoring deep molecular response following treatment for chronic myeloid leukemia, Leukemia 29 (2015) 999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].National Comprehensive Cancer Network, NCCN Clinical Practice Guidelines in Oncology: Chronic Myeloid Leukemia, v1.2018-July 26, (2017) Available at: https://www.nccn.org/professionals/physician_gls/pdf/cml.pdf (last Accessed 14 September 2017).
- [64].Rea D, Dulphy N, Henry G, et al. , Low natural killer (NK) cell counts and functionality are associated with molecular relapse after imatinib discontinuation in patients (pts) with chronic phase (CP)-chronic myeloid leukemia (CML) with undetectable BCR-ABL transcripts for at least 2 years: preliminary results from immunostim, On Behalf Of STIM Investigators, Blood 122 (21) (2013) 856.23929834 [Google Scholar]
- [65].Hughes TP, Ross DM, Moving treatment-free remission into mainstream clinical practice in CML, Blood 128 (2016) 17–23. [DOI] [PubMed] [Google Scholar]
- [66].Ross D, Villemagne-Sanchez L, Hall K, et al. , Factors that influence patient willingness to attempt treatment-free remission in chronic myeloid leukemia, Haematologica 101 (2016) 238. [Google Scholar]
- [67].Castagnetti F, Gugliotta G, Baccarani M, et al. , Differences among young adults, adults and elderly chronic myeloid leukemia patients, Ann. Oncol 26 (1) (2015) 185–192. [DOI] [PubMed] [Google Scholar]
- [68].Breccia M, Efficace F, Are chronic myeloid leukemia patients ready to stop long-term treatment? Leuk. Lymphoma 58 (2017) 2976–2978. [DOI] [PubMed] [Google Scholar]
- [69].Clark RE, Polydoros F, Apperley J, et al. , De-escalation of tyrosine kinase inhibitor dose in patients with chronic myeloid leukaemia with stable major molecular response (DESTINY); an interim analysis of a non-randomised, phase 2 trial, Lancet (May) (2017), 10.1016/S2352-3026(17)30066-2 (prepub). [DOI] [PubMed] [Google Scholar]
- [70].Bower H, Björkholm M, Dickman PW, Höglund M, Lambert PC,Andersson TM, Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population, J. Clin. Oncol 34 (2016) 2851–2857. [DOI] [PubMed] [Google Scholar]
- [71].Verstovek S, Mesa RA, Gotlib J, et al. , A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis, N. Engl. J. Med 366 (4) (2012) 787–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Harrison C, Kiladjian JJ, Al-Ali HK, et al. , JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis, N. Engl. J. Med 366 (9) (2012) 787–798. [DOI] [PubMed] [Google Scholar]
- [73].Gupta V, Verstovsek S, Paquette R, et al. , Clinical outcomes with ruxolitinib (RUX) in patients with myelofibrosis (MF) stratified by transfusion status: a pooled analysis of the COMFORT-I and -II trials, Blood 128 (22) (2016) 3118–3118. [Google Scholar]
- [74].Vannucchi AM, Kiladjian JJ, Grieshammer M, et al. , Ruxolitinib versus standard therapy for the treatment of polycythemia vera, N. Engl. J. Med 372 (5) (2015) 426–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Verstovsek S, Gupta V, Gotlib JR, et al. , A pooled overall survival (OS) analysis of 5-year data from the COMFORT-I and COMFORT-II trials of ruxolitinib for the treatment of myelofibrosis (MF), Blood 128 (22) (2016) 3110–3110. [Google Scholar]
- [76].Kvasnicka HM, Thiele J, Bueso-Ramos CE, et al. , Effects of long-term ruxolitinib (RUX) on bone marrow (BM) morphology in patients with myelofibrosis (MF) enrolled in the COMFORT-I study, Blood 128 (22) (2016) 1949–1949. [Google Scholar]
- [77].Koschmeider S, Mughal TI, Hasselbalch H, et al. , Myeloproliferayive neoplasms and inflammation: whether to target the malignant clone or the inflammatory process or both, Leukemia 1 (2016) 1–741. [DOI] [PubMed] [Google Scholar]
- [78].Mesa RA, Vannucchi AM, Mead A, et al. , Pacritinib versus best available therapy for the treatment of myeofibrosis irrespective of baseline cytopenias (PERSIST-1): an international randomised, phase 3 trial, Lancet Haematol. 4 (5) (2017) e255–e236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Tefferi A, Barraco D, Lasho TL, et al. , Momelotinib therapy in myelofibrosis: 6- years follow-up data on safety, efficacy and the impact of mutations on overall and relapse-free survival, Blood 128 (22) (2016) 1123–1123. [Google Scholar]
- [80].Pardanani A, Harrison C, Cortes JE, et al. , Safety and efficacy of fedratinib in patients with primary or secondary myelofibrosis: a randomized clinical trail, JAMAOncol. 1 (5) (2015) 643–651 [DOI] [PubMed] [Google Scholar]
- [81].Mascarenhas J, Hoffman R, Talpaz M, et al. , Results of the persist-2 phase 3 study of pacritinib (PAC) versus best available therapy (BAT), including rux- olitinib (RUX), in patients (pts) with myelofibrosis (MF) and platelet counts & lt;100,000/μl, Blood 128 (22) (2016) LBA–5. [Google Scholar]
- [82].Mesa RA, Kiladjian JJ, Catalano JV, et al. , Phase 3 trail of momelotinib (MMB) vs ruxoltinib (RUX) in JAK inhibitor (JAKi) na#xp#ve patients with myelofibrosis,J. Clin. Oncol 35 (2017) (abstract 7000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Verstovsek S, Talpaz M, Ritchie EK, et al. , Phase 1/2 study of NS-018, an oral JAK2 inhibitor, in patients with primary myelofibrosis (PMF), post-polycythemia vera myelofibrosis (postPV MF), or post-essential thrombocythemia myelofibrosis (postET MF), Blood 128 (22) (2016) 1936–1936. [Google Scholar]
- [84].Meyer SC, Keller MD, Chiu S, et al. , CHZ868, a type II JAK2 inhibitor, reverses type JAK inhibitor persistence and demonstrates efficacy in myeloproliferative neoplasms, Cancer Cell 100 (8) (2015) 1058–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Mascarenhas JO, Talpaz M, Guota V, et al. , Primary analysis of a phase II open-label trial of INCB039110, a selective JAK1 inhibitor, in patients with myelofi brosis, Haematologica 102 (2) (2017) 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Tefferi A, Al-Ali HK, Barosi G, et al. , A randomized study of pomalidomide vs placebo in persons with myeloproliferative neoplasm-associated myelofibrosis and RBC-transfusion dependence, Leukemia 31 (4) (2017) 896–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Daver N, Cortes JE, Pemmaraju N, et al. , Ruxolitinib (RUX) in combination with 5-azacytidine (AZA) as therapy for patients (pts) with myelofibrosis (MF), Blood 128 (22) (2016) 1127–1127. [Google Scholar]
- [88].Rampal RK, Mascarenhas JO, Kosiorek HE, et al. , Safety and efficacy of combined ruxolitinib and decitabine in patients with blast-phase MPN and post MPN AML: results of a phase I study (myeloproliferative disorders research consortium 109 trial), Blood 128 (22) (2016) 1124–1124. [Google Scholar]
- [89].Moyo TK, Sochacki A, Ayers GD, et al. , Preliminary results from a phase I dose escalation trial of ruxolitinib and the PI3Kδ inhibitor TGR-1202 in myelofibrosis, Blood 128 (22) (2016) 1125–1125. [Google Scholar]
- [90].Verstovek S, Mesa RA, Foltz LM, et al. , PRM-151 in myelofibrosis: durable efficacy and safety at 72 weeks, Blood 126 (23) (2015) (abstract 56). [Google Scholar]
- [91].Bose P, Daver N, Jabbour EJ, et al. , Phase-2 study of sotatercept (ACE-011) in myeloproliferative neoplasm-associated myelofibrosis and anemia, Blood 128 (22) (2016) 478–478. [Google Scholar]
- [92].Mascarenhas JO, Prchal JT, Rambaldi A, et al. , Interim analysis of the myeloproliferative disorders research consortium (MPD-RC) 112 global phase III trial of front line pegylated interferon alpha-2a vs. hydroxyurea in high risk polycythemia vera and essential thrombocythemia, Blood 128 (22) (2016) 479–479.27207789 [Google Scholar]
- [93].Mesa RA, Hoffman R, Kosiorek HE, et al. , Impact on MPN symptoms and quality of life of front line pegylated interferon alpha-2a vs: hydroxyurea in high risk polycythemia vera and essential thrombocythemia: interim analysis results of myeloproliferative disorders research consortium (MPD-RC) 112 global phase III trial, Blood 128 (22) (2016) 4271–4271. [Google Scholar]
- [94].Gisslinger H, Klade C, Georgiev P, et al. , Final results from PROUD-PV a randomized controlled phase 3 trial comparing ropeginterferon alfa-2b to hydro-xyurea in polycythemia vera patients, Blood 128 (22) (2016) 475–475. [Google Scholar]
- [95].Moslehi JJ, Deininger M, Tyrosine kinase inhibitor-assocaited cardiovascular toxicity in chronic myeloid leukemia, J. Clin. Oncol 33 (2015) 4210–4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Valent P, Hadzijusufovic E, Schernthaner G-H, et al. , Vascular safety issues in CML patients treated with BCR/ABL1 kinase inhibitors, Blood 125 (6) (2015) 901–906. [DOI] [PubMed] [Google Scholar]
- [97].Douxfils J, Haguet H, Mullier F, et al. , Association between BCR-ABL tyrsoine kinase inhibitors for chronic myeloid leukemia and cardiovascular events, major molecular response, and overall survival: a systemic review and meta-analysis, JAMA Oncol. 2 (5) (2017) 625–632. [DOI] [PubMed] [Google Scholar]
- [98].Saglio G, le Coutre P, Cortes J, et al. , Evaluation of cardiovascular ishaemic evnts rates in dasatinib-treted patients using standarize incidence ratios, Ann. Hematol 96 (2017) 1303–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Cortes J, Mauro M, Steegmann JL, et al. , Cardiovascular and pulmonary adverse events in patients treated with BCR-ABL inhibitors: data from the FDA Adverse Event Reporting System, Am. J. Hematol 90 (2015) E66–E72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Steegmann JL, Baccaranni M, Breccia M, et al. , European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeoid leukemia, Leukemia 30 (2016) 1648–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Numan Y, Jamil M, Cortes JE, The effect of aspirin and anticoagulation on tyrosine kinase inhibitors induceed thrombotic events in chronic myeloid leukemia: a retrospective cohort, J. Clin. Oncol 35 (15) (2017) e18550. [Google Scholar]