Summary
Introduction
Hypogonadism is more prevalent in men with type 2 diabetes (T2DM) (25%‐40%) than in men without T2DM. Hypogonadism has been associated with poorer glycaemic outcomes and increased cardiovascular morbidity/mortality. We report a 14‐year follow‐up study to evaluate the influence of baseline testosterone level on T2DM outcomes.
Research design and methods
A total of 550 men with T2DM underwent baseline total testosterone and dihydrotestosterone measurement by tandem mass spectrometry. Mean age of the men was 59.7 ± 12 (mean ± SD) years. Sex hormone‐binding globulin (SHBG) was measured and free testosterone estimated. Patients were followed up between 2002 and 2016. Mean follow‐up period was 12.2 ± 4 years using the Salford (UK) Integrated Health Records system.
Results
Mean baseline total testosterone was 13.7 ± 5.8 nmol/L, and mean free testosterone was 245.7 ± 88.0 pmol/L. Mean for low total testosterone (<10 nmol/L) was 7.6 ± 2.0 nmol/L (n = 154) and 142 men had a free testosterone <190 pmol/L. During the 14‐year duration follow‐up, 22% of men experienced a myocardial infarction, 18% experienced a stroke, 11% developed angina, 14% underwent coronary revascularization. About 38% of the men initially recruited died. A lower total testosterone was associated with a higher body mass index (kg/m2) at follow‐up: regression coefficient −0.30 (95% CI −0.445 to −0.157), P = 0.0001. The mortality rate was higher in patients with lower total testosterone compared to normal baseline total testosterone (5.0% vs 2.8% per year, P < 0.0001). A similar phenomenon was seen for dihydrotestosterone (4.3% vs 2.9% per year, P = 0.002) for normal vs low dihydrotestosterone) and for lower SHBG. Over the whole follow‐up period 36.1% (143/396), men with normal baseline testosterone died vs 55.8% (86/154) of hypogonadal men at baseline. In Cox regression, the age‐adjusted hazard ratio (HR) for higher mortality associated with low total testosterone was 1.54 (95% CI: 1.2‐2.0, P < 0.002), corresponding to a 3.2 year reduced life expectancy for hypogonadal T2DM men.
Conclusion
Low testosterone and dihydrotestosterone levels are associated with higher all‐cause mortality in T2DM men. Hypogonadal men with T2DM should be considered as very high risk for cardiovascular events/death.
Keywords: BMI, mortality, testosterone, type 2 diabetes
Novelty statement.
In this study, 550 men with type 2 diabetes (T2DM) underwent baseline total testosterone and dihydrotestosterone measurement by tandem mass spectrometry. Mean age of the men at baseline was 59.7 ± 12 (mean ± SD) years.
We found that low baseline testosterone and dihydrotestosterone levels were associated with higher all‐cause mortality in T2DM men over a 14‐year follow‐up period.
Hypogonadal men with T2DM should be viewed as very high risk for cardiovascular (CVD) death.
Consideration should be given to include screening for hypogonadism as part of routine T2DM patient reviews in men.
1. INTRODUCTION
Type 2 diabetes (T2DM) is a growing health issue around the world. Several studies have demonstrated a higher prevalence of hypogonadism in T2DM men than those with normal glucose regulation and with type 1 diabetes (T1DM).1, 2, 3 In men with T2DM, low serum levels of testosterone have been associated with a more adverse cardiometabolic risk profile3 and with increased mortality.4
According to the hypogonadal‐obesity cycle, first described by Cohen in 1999,5 a hypogonadal state causes an increase in abdominal adipose tissue deposition, eventually leading to further reduction in total testosterone levels through actions at a hypothalamic level, including increased hypothalamic leptin receptor resistance. Visceral obesity, commonly measured with body mass index (BMI) and waist circumference,1 is one of the main risk factors associated with the development of insulin resistance and consequently T2DM.6 Furthermore, hypogonadism has been associated with higher rates of cardiovascular disease and of cardiovascular death.7
The androgen receptor mediates the peripheral effects of testosterone. The main mechanism of action for the androgen receptor is direct regulation of gene transcription.8 The androgen receptor dimer binds to a specific sequence of DNA, known as a hormone response element, thereby up‐ or down‐regulating specific gene transcription. Testosterone effect via the androgen receptor occurs through its more active metabolite, dihydrotestosterone, to which it is converted by the enzyme 5‐α reductase.9
Exon 1 of the androgen receptor gene contains a polymorphic sequence of CAG repeats, which usually varies in number from 10 to 35.10 Many findings suggest that CAG number is negatively correlated with the transcriptional activity of androgen receptor.11
In this 14‐year follow‐up study, our aims were as follows: To measure baseline testosterone, dihydrotestosterone and sex hormone‐binding globulin (SHBG), and to ascertain the relation between initial androgen status with a (a) change in HbA1C/BMI over time, (b) cardiovascular events and (c) all‐cause mortality.
2. METHODS
The Salford prospective type 2 diabetes cohort provides a unique opportunity to study the relation between baseline androgen profile at recruitment and cardiometabolic outcomes over time. The cohort was established in 2002. We have serum samples and extracted DNA for 550 men collected between 2002 and 2004 with detailed cardiometabolic phenotypic follow‐up data including up to date mortality data until the year 2016. An additional 36 men were lost to follow‐up because they moved out of the Salford area. About 94% of men were of White European origin with the other 6% split between South Asian, Arab, African and Oriental origin.
Furthermore, we have details as to all medication changes including initiation of testosterone replacement with subsequent testosterone levels, where this was done as part of routine care.
Participants donated a blood sample for DNA extraction and circulating hormone/biomarker measurement. Blood samples were collected only once (at baseline)/initial screening and were non‐fasting, being collected before 11.00 in the morning. The project described here is part of a larger long‐term naturalistic T2DM cohort study which aims to provide genetic and epigenetic data to inform our understanding of the outcomes for T2DM patients in a longer‐term follow‐up model. We have explicit consent from the participants to obtain follow‐up health outcome data for them.
We have recently obtained (with full ethics permission) the complete follow‐up cardiometabolic data set for the men from 2002 up to the end of 2016, including mortality data. This dataset includes changes in weight, BMI blood pressure, and cardiometabolic/biochemical profile over time, plus cardiovascular complications/neoplasia from GP and hospital coded diagnoses and death. A full baseline cardiometabolic profile was determined in the blood at recruitment including novel markers of cardiometabolic risk and full insulin‐like growth factor (IGF) system characterization plus insulin C‐peptide.12, 13
We estimated baseline androgen status by measuring serum testosterone and dihydrotestosterone using tandem mass spectrometry at University Hospital of South Manchester and SHBG immunoassay also at the University Hospital of South Manchester.
Testosterone, dihydrotestosterone and SHBG plus IGF‐II were assessed at baseline, that is, at the point of recruitment only. Other metabolic parameters were monitored longitudinally until the end of 2016. The data collection went up to the end of 2016. The dataset includes data collected in all the years from recruitment until 2016.
2.1. Laboratory methods and assays
2.1.1. Hormone assays
Baseline androgen profile was determined by measuring serum testosterone using tandem mass spectrometry at University Hospital of South Manchester14 and SHBG, by immunoassay at the same laboratory.
2.1.2. Principle and method of procedure used for Androgen panel
Samples were prepared for analysis by protein precipitation using zinc sulphate and acetonitrile. Analysis was carried out using on‐line SPE with XBridge C18 cartridges on a Waters OSM system coupled to a Waters Xevo TQS mass spectrometer.
2.1.3. Sex hormone‐binding globulin
The ARCHITECT SHBG assay is a two‐step immunoassay to determine the presence of SHBG in human serum and plasma using CMIA technology with flexible assay protocols, referred to as Chemiflex.
Free testosterone was estimated using the calculator available at http://www.issam.ch/freetesto.htm which is based on the Vermeulen Equation.15
IGF‐II was measured using an enzyme‐linked immunosorbent assay (ELISA) that was developed using antibodies. This assay had an analytical sensitivity of <10 ng/mL, with intra‐assay and inter‐assay, sensitivities were <6% and <10%, respectively.13
2.2. Statistical analysis
The data were analysed using the statistical package stata version 13.1 (Stata Corp, TX, USA). Multiple Linear regression was employed to explore the predictors of BMI and other longitudinal outcome variables. Logistic regression was used to investigate predictors of mortality. Kaplan‐Meier curves were used to summarize survival analysis. Kaplan‐Meier curves compared survival probabilities for men with low total, free testosterone and dihydrotestosterone. Cox regression was applied to estimate adjusted risk of death for low total testosterone.
In order to investigate the association between androgen status and HbA1c, a cross‐sectional multiple linear regression model for HbA1c was fitted. The hormones (testosterone, free testosterone, dihydrotestosterone) plus SHBG were included as either continuous predictors or as dichotomous variables (normal//low).
A longitudinal fixed effect model was applied to the repeated measure data with 16 time‐points and unstructured correlation structure to investigate whether the baseline androgen status predicts future BMI and glycaemic control and lipid profile. A single measure for each androgen was included to determine the best possible prediction of mean BMI, HbA1c and lipid levels over the period 2002‐2016 in a model adjusted for age, factored time interaction and lipid‐lowering therapy. Interactions between the predictors and time were then added to determine whether there is an effect on the rate of change of HbA1c/BMI over time.
A similar modelling procedure was followed to determine the effect of androgen status on mortality and on the risk of cardiovascular events. However, in this case, a survival analysis was used (time to death or time to first cardiovascular event as an outcome).
3. RESULTS
3.1. Descriptive characteristics
Quartiles for follow‐up period and mean follow‐up period were 9.0, 15.0, 15.0 and 12.2 ± 4.03 (mean ± SD) years using the Salford (UK) Integrated Health Records system. Baseline characteristics are given in Table 1. The mean age was 59.7 ± 12.4 years. Mean baseline BMI was 30.0 ± 5.2 kg/m2. Mean baseline HbA1c was 8.2 ± 1.8% (66.1 ± 19.7 mol/mol). Fourteen‐year follow‐up data were available for 348 participants taking into account the mortality rate of the men and the fact that some men had moved out of area. The number of men with follow‐up data does vary slightly between specific analyses.
Table 1.
Baseline Characteristics by Groups & whole Cohort
| Groups | ||||
|---|---|---|---|---|
| Normal TT | Low TT | All cohort | ||
| Mean (SD) | Mean (SD) | Mean (SD) | Range | |
| Age (y) | 58.3(12.6) | 63.3 (11.2) | 59.7 (12.4) | 19‐89 |
| BMI | 29.3 (4.8) | 31.7 (5.6) | 30.0 (5.2) | 18.4‐52.9 |
| HbA1C (%) | 8.3 (1.8) | 8.1 (1.6) | 8.2 (1.8) | Up to 17.8 |
| Cholesterol (mmol/L) | 4.70 (1.0) | 4.70 (1.1) | 4.70 (1.0) | 2.4‐11.7 |
| HDL‐cholesterol (mmol/L) | 1.2 (0.4) | 1.1 (0.3) | 1.2 (0.4) | 0.6‐3.5 |
| LDL‐cholesterol (mmol/L) | 2.6 (0.8) | 2.5 (1.0) | 2.6 (0.8) | 0.8‐5.8 |
| Triglycerides (mmol/L) | 2.0 (1.6) | 2.5 (1.9) | 2.2 (1.7) | 0.3‐13.9 |
| Systolic BP (mm Hg) | 137.9 (16.8) | 137.9 (16.8) | 137.9 (16.8) | 84‐198 |
| Diastolic BP (mm Hg) | 76.2 (10.9) | 76.8 (10.6) | 76.3 (10.8) | 40‐137 |
| Testosterone (nmol/L) | 16.1 (5.0) | 7.6 (2.0) | 13.7 (5.8) | 0.1‐42.1 |
| Free testosterone (pmol/L) (estimated) | 277.2 (77.8) | 164.9 (55.5) | 245.8 (88.1) | 4.1‐701.5 |
| Dihydrotestosterone (DHT) (nmol/L) | 1.4 (0.7) | 0.6 (0.3) | 1.2 (0.7) | 0.1‐4.8 |
| SHBG (nmol/L) | 45.5 (18.2) | 30.8 (20.6) | 41.4 (20.0) | 6.1‐212.3 |
BMI, body mass index; SD, standard deviation; SHBG, sex hormone‐binding globulin.
All measures were taken in the morning before midday but not necessarily fasting.
3.2. Distribution of androgens
Mean total testosterone was 13.7 ± 5.8 nmol/L, and mean free testosterone was 245.7 ± 88.0 pmol/L. About 154 (28.0%) of men had low total testosterone (defined as total testosterone <10 nmol/L; mean for low total testosterone was 7.6 ± 2.0 nmol/L) and 142 (25.8%) men had an estimated free testosterone <190 pmol/L, compatible with hypogonadism (mean for low free testosterone was 146.3 pmol/L). Mean dihydrotestosterone was 1.2 ± 0.7 nmol, while mean SHBG was 41.4 ± 20.0 nmol/L. The distribution of total testosterone is shown in Figure 1.
Figure 1.
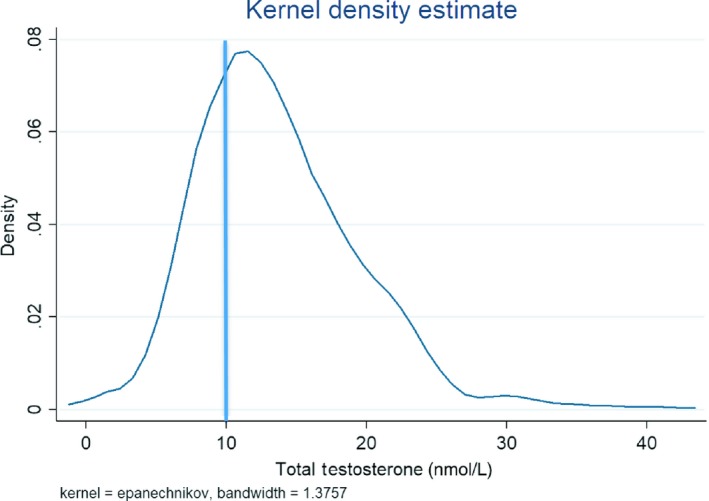
Distribution of total testosterone. 28.0% of men had a total testosterone <10 nmol/L
3.3. Testosterone vs age at baseline
Both total testosterone (regression coefficient −0.12, P < 0.0001) and dihydrotestosterone (regression coefficient −0.006, P = 0.006) were lower in older men with total testosterone being lower by 0.12 nmol/L for every 1 year increase in age at baseline.
3.4. Relation between baseline androgen levels and cardiovascular events/death
During follow‐up, 22% of men (n = 121) experienced a myocardial infarction, 18% experienced a stroke (n = 99), 11% developed angina (n = 61), 15% underwent coronary revascularization (n = 83) and 38% of those recruited to the study died (n = 209).
3.5. Mortality
The all‐cause mortality rate was higher in patients with lower total testosterone compared to normal baseline total testosterone (5.0% vs 2.8% per year, P < 0.0001; Figure 2A) and also for lower free testosterone (Figure 2C). Over the whole follow‐up period, 36.1% (143/396) men with normal baseline testosterone died vs 55.8% (86/154) of hypogonadal men at baseline.
Figure 2.
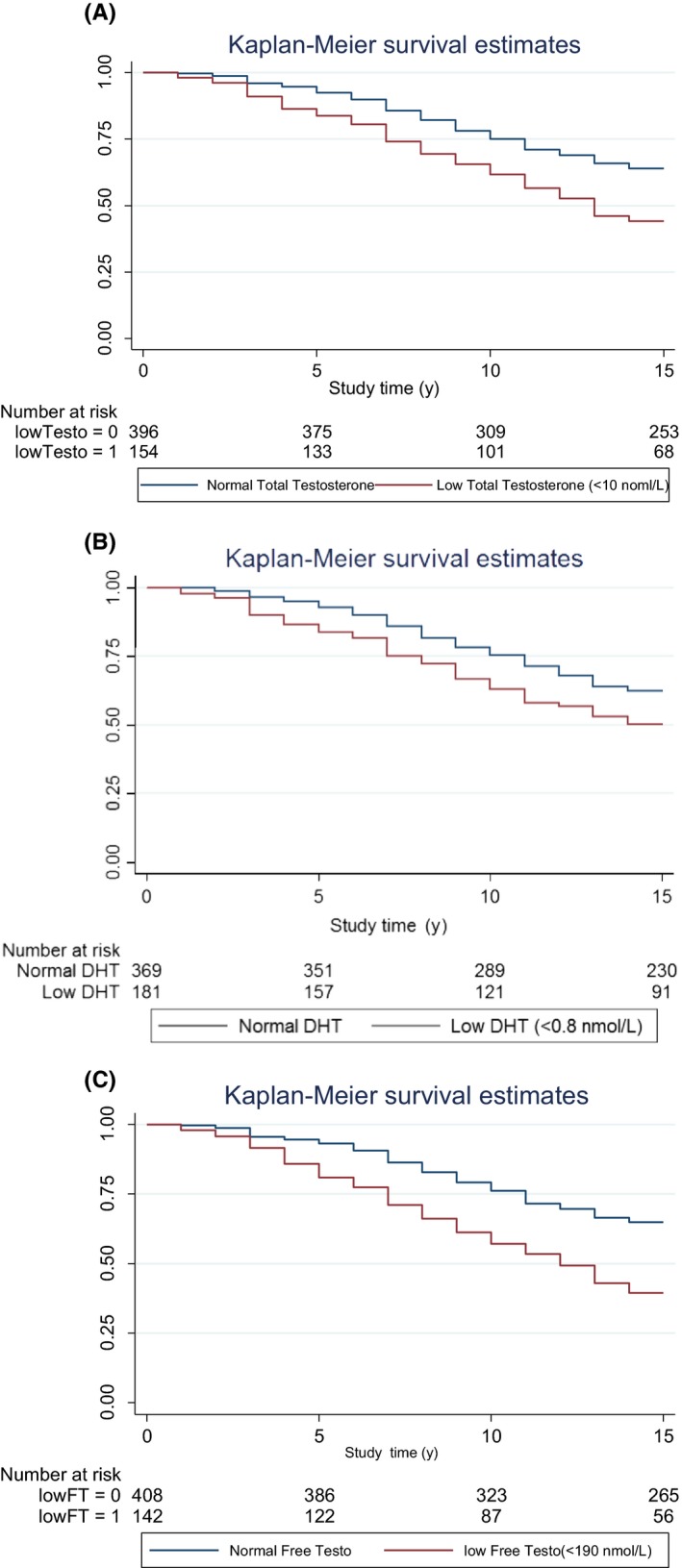
A, All‐cause mortality rate higher in patients with lower baseline total testosterone compared to normal baseline total testosterone. B, All‐cause mortality rate higher in patients with lower baseline dihydrotestosterone compared to normal baseline dihydrotestosterone. C, All‐cause mortality rate higher in patients with lower baseline free testosterone compared to normal baseline free testosterone
A similar phenomenon was seen for lower dihydrotestosterone (4.3% vs 2.9% per year, P < 0.0025; Figure 2B; the 0.8 nmol/L cut point was based on the lowest 15% of DHT readings). In Cox regression, the age‐adjusted hazard ratio (HR) for higher mortality associated with low total testosterone was 1.54 (95% CI: 1.2‐2.0, P < 0.002). This corresponded to a 3.2 year reduced life expectancy (mean age of death in the low total testosterone group [total total testosterone <10 nmol/L was 72.2 [70.5‐73.9] years vs 75.4 [73.3‐77.5]] years for the normal total testosterone group). However, this relation was positively confounded by BMI and SHBG (adjusted HR = 1.24, 95% CI: 0.88‐1.78, P = 0.216).
Logistic regression indicated that there was a 1% excess risk of death by increasing one unit in SHBG (HR = 1.013, 95% CI: 1.003‐1.023, P = 0.010) and 175% excess risk of death for individuals with low testosterone (HR = 2.75, 95% CI: 1.82‐4.18, P < 0.001). However, the age‐adjusted HR did not show any excess risk of death for one unit change in SHBG. There was still a 93% excess risk of death for individuals with low testosterone.
3.6. Coronary artery disease/stroke
Considering IHD as an endpoint, both unadjusted and age and BMI adjusted OR indicated that higher baseline SHBG was a protective factor for cardiovascular events with unadjusted OR = 0.99 (95% CI: 0.97‐1.00, P = 0.033) and adjusted OR = 0.98 (95% CI: 0.97‐1.00, P = 0.041). Age‐adjusted logistic regression indicated that SHBG was protective for angina but there was no relation with coronary revascularization events for SHBG or for testosterone. Unadjusted age‐adjusted logistic regression indicated that both SHBG and low testosterone increased the risk of stroke. However, this risk was not kept in the age‐adjusted model.
In this analysis, both age and BMI were significant predictors of IHD.
3.7. Relation between metabolic/anthropometric variables at baseline and follow‐up
3.7.1. IGF‐II
A low free testosterone was a predictor of low IGF‐II (regression coefficient 0.41 [95%CI 0.076 to 0.738, P = 0.016]). Adjusting for age and BMI attenuated the association slightly, but it remained statistically significant (regression coefficient 0.35 [95% CI 0.012 to 0.698, P = 0.043]).
3.7.2. BMI
A lower baseline total testosterone was strongly associated with a higher BMI at baseline (Figure 3A), with a regression coefficient after adjusting for age of −0.29 (95% CI −0.37 to −0.19, P = 0.001). This negative association of baseline testosterone level persisted with BMI even at follow‐up in 2016 with regression coefficient of −0.30 (95% CI −0.445 to −0.157, P = 0.0001; Figure 3B). However, this association decreased indicating confounding by BMI at baseline (β = −0.10, 95% CI: −0.20 to 0.004, P = 0.062). An even stronger association was seen for dihydrotestosterone with lower baseline levels associated with higher BMI at 2016 follow‐up (age‐adjusted regression coefficient −4.48. (95% CI −5.60 to −3.35, P < 0.0001; Figure 3C).
Figure 3.
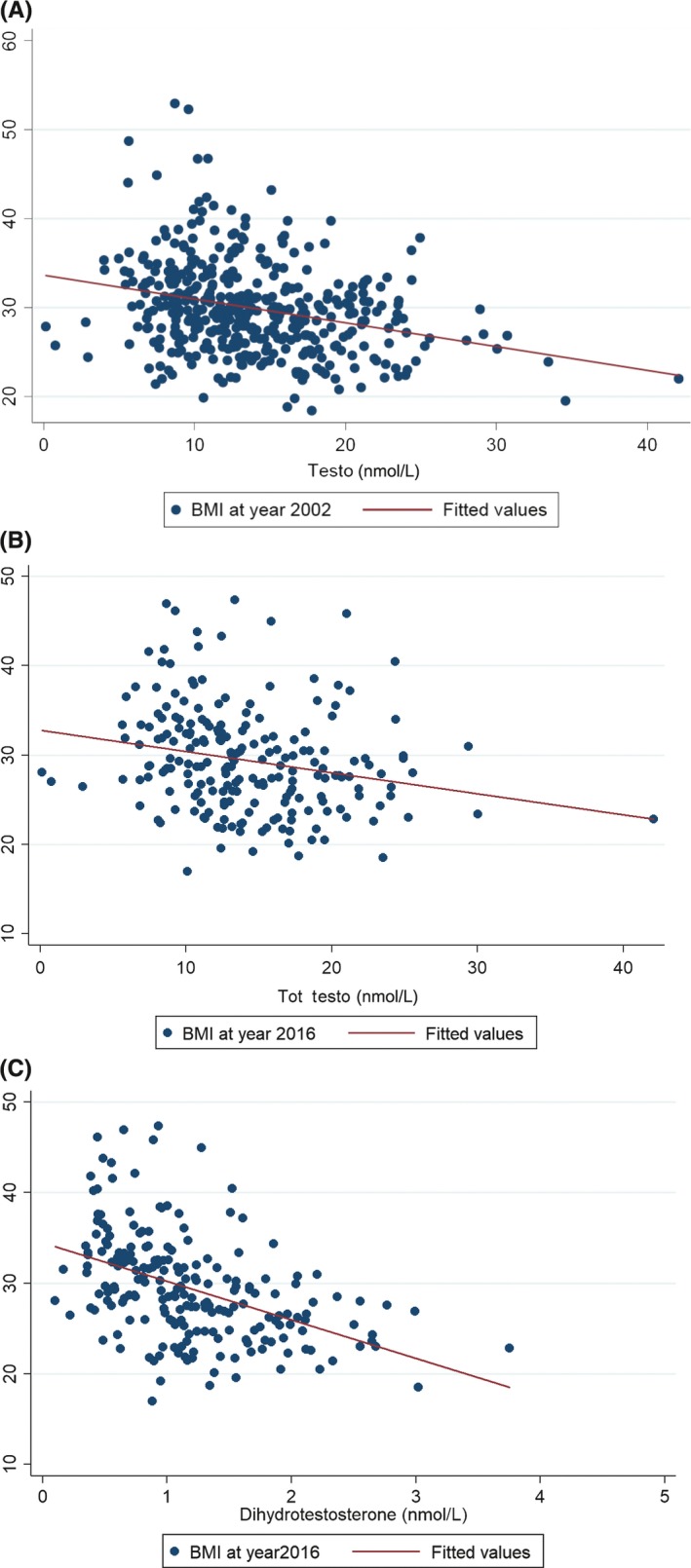
A, Lower baseline total testosterone was associated with lower baseline body mass index (BMI) at recruitment. B, Lower baseline total testosterone related to lower BMI at follow‐up in 2016. C, Lower baseline dihydrotestosterone related to lower BMI at follow‐up in 2016
A lower total testosterone by 0.3 nmol/L and lower dihydrotestosterone by 0.045 nmol/L was associated respectively with a higher BMI by one unit (kg/m2).
3.7.3. Lipids
A higher baseline total testosterone was strongly associated with a higher HDL‐Cholesterol at baseline year and at follow‐up year 2016 after adjusting for age and BMI (regression coefficient 0.014 [95% CI 0.007 to 0.021, P < 0.0001] and 0.016 [95% CI 0.006 to 0.027, P = 0.002], respectively). An even stronger association was seen for dihydrotestosterone with higher HDL levels at baseline and at 2016 follow‐up with HDL‐Cholesterol (regression coefficient 0.17 [95% CI 0.11 to 0.23, P < 0.0001] and 0.15 [95% CI 0.06 to 0.23, P = 0.001]).
We also noticed a strong negative association of both total testosterone and dihydrotestosterone with triglycerides at baseline (regression coefficient −0.078 [95% CI −0.11 to −0.046, P < 0.0001] and −0.81 [95% CI −1.08 to −0.54, P < 0.0001], respectively).
3.8. Trends over time
We found that lower total testosterone concentrations at baseline were predictive of longitudinal increases in BMI over the period 2002‐2016 in a model adjusted for age, diabetes duration, factored time interaction and lipid‐lowering therapy (beta coef = −0.27, CI −0.35 to −0.19, P < 0.0001). The same was true for free testosterone and dihydrotestosterone.
There was a statistically significant positive association with HDL change and negative association with triglyceride change over time after adjustment for age, diabetes duration and lipid‐lowering therapy. Specifically, a higher total testosterone was predictive of lower triglycerides (beta coef = −0.03. 95% CI −0.045 to −0.012, P = 0.001) and higher HDL concentrations over time (beta coef = 0.012; 95% CI 0.006‐0.018, P < 0.0001).
No other significant associations of baseline testosterone level were seen with HbA1c, creatinine, eGFR and/or with the blood pressure measurements over time.
4. DISCUSSION
In this study, we have found that low baseline testosterone and dihydrotestosterone levels were associated with higher all‐cause mortality in T2DM men (overall 55.8% hypogonadal men vs 36.1% eugonadal men), as was lower SHBG. Hypogonadal men with T2DM should be considered as very high risk for cardiovascular (CVD) death and at even higher risk for premature mortality than eugonadal T2DM men. Previously, studies have indicated that lower testosterone both in non‐diabetes populations of older men in the Rancho Bernardo Study16 and in the European Male Aging Study (EMAS),17 and also in men with T2DM5 was associated with a higher all‐cause mortality.
Here, we showed that a lower total testosterone anddihydrotestosterone at baseline were associated with a higher BMI at baseline and at 14‐year follow‐up, with even stronger association between dihydrotestosterone and these measures than for total testosterone. The cross‐sectional relation of low BMI with low testosterone was previously shown by Kapoor et al.1 With regard to the matter of low testosterone and mortality, we are not claiming that the association is independent of BMI, rather that a low testosterone baseline was associated with a higher BMI and that these factors together influenced the higher long‐term mortality rate in hypogonadal men.
We have also shown that there was a statistically significant positive association of total testosterone with HDL‐cholesterol and a negative association with triglycerides and this similar relation is even stronger with dihydrotestosterone. This finding accords with previous studies as reviewed by Shabsigh et al.18 This is the first study to look at the association of dihydrotestosterone with cardiometabolic markers over such a long period of time in men.
A body of evidence exists indicating that hypogonadism is associated with a worse metabolic profile and cardiovascular risk.19 However, other evidence shows that hypogonadism could represent a protective mechanism in unhealthy conditions, such as in people with a history of previous cardiovascular events.20 We have here shown that hypogonadism in T2DM men is associated with an adverse course from a cardiometabolic point of view.
Important functions of testosterone in modulating adiposity, insulin resistance, and T2DM have been postulated.4, 8 It has been known for some time that total testosterone and SHBG levels are lower in men with T2DM compared with healthy controls21 and lower than in men with T1DM.3 The factors that cause low circulating testosterone also cause low SHBG—that is central obesity, insulin resistance and lack of exercise to mention a few. The action of testosterone to improve insulin sensitivity was shown very clearly in the TIMES2 study22 using HOMA modelling and by Dhindsa et al23 in a clamp study.
The association between low circulating free testosterone and low IGF‐II (both associated in this and other studies with a tendency to put on weight) has not been reported before.
With regard to the question around the CAG repeats, a longer androgen receptor CAG repeat was associated with increased body fat and leptin levels in a study of 106 healthy men24 and in a study of 233 men with T2DM.9 The latter study demonstrated that increased central adiposity as measured by waist circumference was associated with a less sensitive androgen receptor. Longer androgen receptor CAG sequences are also associated with higher serum insulin levels in healthy men24 and with obesity and leptin9 although they were not shown to correlate with HbA1c levels in this study. The influence of CAG repeat number (not measured here) must be borne in mind when understanding the biological effects of circulating testosterone at a tissue level in terms of short‐term actions and long‐term consequences.
There is an increasing body of evidence that testosterone replacement may improve survival in hypogonadal men with T2DM4 with increasing benefit with age.7 Furthermore, treatment with phosphodiesterase type‐5 inhibitors may also reduce cardiovascular event rates and mortality, even if only used on an as required basis.25, 26 Finally, we would like to record that the first description of hypogonadotropic hypogonadism in diabetes was by Dhindsa et al27. They also described the inverse relationship of circulating testosterone with BMI27 and concluded that hypogonadotropic hypogonadism occurs commonly in type 2 diabetes.
4.1. Strengths and weaknesses
The major strengths of the study are firstly the extensive characterization of the participants at baseline for conventional and novel biomarkers of cardiovascular risk and secondly the long duration of follow‐up (14 years) with collection of prospective biochemical, anthropometric and health event (including death) data through an integrated digital health record that covered all those years.
A weakness is that we have not been able to go back to the individual participants to obtain follow‐up blood samples or other measures beyond those collected as part of routine care. A further weakness is the non‐fasting measurement of testosterone. This could result in lower testosterone readings.28
5. CONCLUSION
In this study, we have found that low baseline testosterone and dihydrotestosterone levels are associated with higher all‐cause mortality in T2DM men. Hypogonadal men with T2DM should be considered as at very high risk for cardiovascular (CVD) death. Consideration should be given to include screening for hypogonadism as part of routine T2DM patient reviews.
CONFLICT OF INTEREST
Nothing to declare.
AUTHOR CONTRIBUTION
All authors contributed equally to the writing of this paper.
ETHICAL APPROVAL
This study had full ethics approval.
ACKNOWLEDGEMENTS
None.
Malipatil NS, Yadegarfar G, Lunt M, et al. Male hypogonadism: 14‐year prospective outcome in 550 men with type 2 diabetes. Endocrinol Diab Metab. 2019;2:e00064 10.1002/edm2.64
DATA ACCESSIBILITY
The data as utilized for this paper are available on request.
DATA ACCESSIBILITY
The data as utilized for this paper are available on request.
REFERENCES
- 1. Kapoor D, Aldred H, Clark S, Channer KS, Jones TH. Clinical and biochemical assessment of hypogonadism in men with type 2 diabetes: correlations with bioavailable testosterone and visceral adiposity. Diabetes Care. 2007;30(4):911‐917. [DOI] [PubMed] [Google Scholar]
- 2. Heald AH, Patel J, Anderson SG, et al. Migration is associated with lower total, but not free testosterone levels in South Asian men. Clin Endocrinol (Oxf). 2007;67(5):651‐655. [DOI] [PubMed] [Google Scholar]
- 3. Anderson SG, Heald AH, Younger N, et al. Screening for hypogonadism in diabetes 2008/9: results from the cheshire primary care cohort. Primary Care Diabetes. 2012;6(2):143‐148. [DOI] [PubMed] [Google Scholar]
- 4. Muraleedharan V, Marsh H, Kapoor D, Channer KS, Jones TH. Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. Eur J Endocrinol. 2013;169(6):725‐733. [DOI] [PubMed] [Google Scholar]
- 5. Cohen PG. The hypogonadal‐obesity cycle: role of aromatase in modulating the testosterone‐estradiol shunt–a major factor in the genesis of morbid obesity. Med Hypotheses. 1999;52(1):49‐51. [DOI] [PubMed] [Google Scholar]
- 6. Kapoor D, Malkin CJ, Channer KS, Jones TH. Androgens, insulin resistance and vascular disease in men. Clin Endocrinol (Oxf). 2005;63(3):239‐250. [DOI] [PubMed] [Google Scholar]
- 7. Hackett G, Heald AH, Sinclair A, Jones PW, Strange RC, Ramachandran S. Serum testosterone, testosterone replacement therapy and all‐ cause mortality in men with type 2 diabetes: retrospective consideration of the impact of PDE5 inhibitors and statins. Int J Clin Pract. 2016;70(3):244‐253. [DOI] [PubMed] [Google Scholar]
- 8. Ozanne DM, Brady ME, Cook S, Gaughan L, Neal DE, Robson CN. Androgen receptor nuclear translocation is facilitated by the f‐actin cross‐linking protein filamin. Mol Endocrinol. 2000;14(10):1618‐1626. [DOI] [PubMed] [Google Scholar]
- 9. Zitzmann M, Nieschlag M. The CAG repeat polymorphism within the androgen receptor gene and maleness. Int J Androl. 2003;26(2):76‐83. [DOI] [PubMed] [Google Scholar]
- 10. Stanworth RD, Kapoor D, Channer KS, Jones TH. Androgen receptor CAG repeat polymorphism is associated with serum testosterone levels, obesity and serum leptin in men with type 2 diabetes. Eur J Endocrinol. 2008;159(6):739‐746. [DOI] [PubMed] [Google Scholar]
- 11. Stanworth RD, Kapoor D, Channer KS, Jones TH. Dyslipidaemia is associated with testosterone, oestradiol and androgen receptor CAG repeat polymorphism in men with type 2 diabetes. Clin Endocrinol (Oxf). 2011;74(5):624‐630. [DOI] [PubMed] [Google Scholar]
- 12. Stephens RH, McElduff P, Heald AH, et al. Polymorphisms in insulin‐like growth factor‐binding protein 1 (IGFBP1) are associated with impaired renal function in type‐2 diabetes mellitus. Diabetes. 2005;54(12):3547‐3553. [DOI] [PubMed] [Google Scholar]
- 13. Narayanan RP, Fu B, Payton A, et al. IGF2 gene polymorphisms and IGF‐II concentration are determinants of longitudinal weight trends in type 2 diabetes. Exp Clin Endocrinol Diabetes. 2013;121(6):361‐367. [DOI] [PubMed] [Google Scholar]
- 14. Thienpont LM, Van Uytfanghe K, Blincko S, et al. State‐of‐the‐art of serum testosterone measurement by isotope dilution‐liquid chromatography‐tandem mass spectrometry. Clin Chem. 2008;54(8):1290‐1297. [DOI] [PubMed] [Google Scholar]
- 15. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666‐3672. [DOI] [PubMed] [Google Scholar]
- 16. Laughlin GA, Barrett‐Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93(1):68‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pye SR, Huhtaniemi IT, Finn JD, et al. Late‐onset hypogonadism and mortality in aging men. J Clin Endocrinol Metab. 2014;99(4):1357‐1366. [DOI] [PubMed] [Google Scholar]
- 18. Shabsigh R, Katz M, Yan G, Makhsida N. Cardiovascular issues in hypogonadism and testosterone therapy. Am J Cardiol. 2005;26(96):67M‐72M. [DOI] [PubMed] [Google Scholar]
- 19. Jones TH. Testosterone deficiency: a risk factor for cardiovascular disease? Trends Endocrinol Metab. 2010;21(8):496‐503. [DOI] [PubMed] [Google Scholar]
- 20. Corona G, Rastrelli G, Maseroli E, et al. Low testosterone syndrome protects subjects with high cardiovascular risk burden from major adverse cardiovascular events. Andrology. 2014;2(5):741‐747. [DOI] [PubMed] [Google Scholar]
- 21. Andersson B, Marin P, Lissner L, Vermeulen A, Bjorntorp P. Testosterone concentrations in women and men with NIDDM. Diabetes Care. 1994;17(5):405‐411. [DOI] [PubMed] [Google Scholar]
- 22. Jones TH, Arver S, Behre HM, et al. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (the TIMES2 study). Diabetes Care. 2011;34(4):828‐837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dhindsa S, Ghanim H, Batra M, et al. Insulin resistance and inflammation in hypogonadotropic hypogonadism and their reduction after testosterone replacement in men with type 2 diabetes. Diabetes Care. 2016;39(1):82‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zitzmann M, Gromoll J, von Eckardstein A, Nieschlag E. The CAG repeat polymorphism in the androgen receptor gene modulates body fat mass and serum concentrations of leptin and insulin in men. Diabetologia. 2003;46(1):31‐39. [DOI] [PubMed] [Google Scholar]
- 25. Anderson SG, Hutchings DC, Woodward M, et al. Phosphodiesterase type‐5 inhibitor use in type 2 diabetes is associated with a reduction in all‐cause mortality. Heart. 2016;102:1750‐1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Andersson DP, Lagerros YT, Grotta A, Bellocco R, Lehtihet M, Holtzmann MJ. Association between treatment for erectile dysfunction and death or cardiovascular outcomes after myocardial infarction. Heart. 2017;103:1264‐1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dhindsa S, Prabhakar S, Sethi M, Bandyopadhyay A, Chaudhuri A, Dandona P. Frequent occurrence of hypogonadotropic hypogonadism in type 2 diabetes. J Clin Endocrinol Metab. 2004;89:5462‐5468. [DOI] [PubMed] [Google Scholar]
- 28. Bhasin S, Brito JP, Cunningham GR, et al. Testosterone therapy in men with hypogonadism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2018;103:1715‐1744. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data as utilized for this paper are available on request.


