Abstract
The rapid rise in the prevalence of type 2 diabetes mellitus (T2DM) poses a huge healthcare burden across the world. Although there are several antihyperglycaemic agents (AHAs) available including addition of new drug classes to the treatment algorithm, more than 50% of patients with T2DM do not achieve glycaemic targets, suggesting an urgent need for treatment strategies focusing on prevention and progression of T2DM and its long‐term complications. Lifestyle changes including implementation of healthy diet and physical activity are cornerstones for the management of T2DM. The positive effects of diet and exercise on incretin hormones such as glucagon‐like peptide‐1 (GLP‐1) have been reported. We hypothesize an IDEP concept (Interaction between Diet/Exercise and Pharmacotherapy) aimed at modifying the diet and lifestyle, along with pharmacotherapy to enhance the GLP‐1 levels, would result in good glycaemic control in patients with T2DM. Consuming protein‐rich food, avoiding saturated fatty acids and making small changes in eating habits such as eating slowly with longer mastication time can have a positive impact on the GLP‐1 secretion and insulin levels. Further the type of physical activity (aerobic/resistance training), intensity of exercise, duration, time and frequency of exercise have shown to improve GLP‐1 levels. Apart from AHAs, a few antihypertensive drugs and lipid‐lowering drugs have also shown to increase endogenous GLP‐1 levels, however, due to quick degradation of GLP‐1 by dipeptidyl peptidase‐4 (DPP‐4) enzyme, treatment with DPP‐4 inhibitors would protect GLP‐1 from degradation and prolong its activity. Thus, IDEP concept can be a promising treatment strategy, which positively influences the GLP‐1 levels and provide additive benefits in terms of improving metabolic parameters in patients with T2DM and slowing the progression of T2DM and its associated complications.
Keywords: diet, exercise, glucagon‐like peptide‐1, pharmacotherapy
1. INTRODUCTION
Over the past three decades, the world has witnessed a rapid rise in the prevalence of type 2 diabetes mellitus (T2DM) and expected to have 629 million of diabetes patients with 20‐79 years by 2045. This pandemic of T2DM leads not only to health problem such as diabetic‐related complications but also to economic burden which is estimated to be USD 776 billion by 2045 across the globe.1 An ageing population, changes in lifestyle, dietary patterns, physical inactivity, obesity and stress are the major contributors for the rise in T2DM. Due to the chronic and progressive nature of the disease, undiagnosed or poorly managed T2DM can lead to increased morbidity and mortality.
Despite the availability of several AHAs and addition of new drug classes to the treatment algorithm, more than 50% of patients with T2DM do not achieve glycaemic targets, suggesting an urgent need for treatment strategies focusing on prevention and progression of T2DM and its long‐term complications.2, 3 The updated treatment guidelines (2018) recommend making lifestyle changes in diet and physical activity as part of T2DM management. Nutrition therapy with individualized meal plan including energy balance, eating patterns and macronutrient distribution and each nutrition is indicated based on patient's age, body weight, physical activity, baseline HbA1c levels and diabetic‐related complications.4 For exercise therapy, patients with T2DM are advised to do 150 min/wk of moderate‐to‐vigorous‐intensity physical activity or 75 min/wk of vigorous‐intensity exercise or interval training for a minimum of 3 d/wk to reduce the risk of T2DM‐related complications.4
Landmark studies, which are bases of the guideline, revealed the importance of lifestyle modification including diet and exercise for glycaemic control and prevention of diabetes‐related complications. As intervention for diet, the DIRECT study indicated that Mediterranean and low‐carbohydrate diets may be as effective as low‐fat diets for glycaemic control.5 There are several intervention studies focusing on exercise. The IDES study demonstrated that promoting physical activity with aerobic and resistance training improved HbA1c compared to exercise counselling alone.6 In addition, the Diabetes Prevention Programme study has shown benefits of lifestyle intervention, at least 150 minutes of physical activity per week to achieve a 7% weight loss, in reducing the incidence of T2DM in subjects at risk of diabetes.7 The Look AHEAD study demonstrated that intensive lifestyle intervention through both decreased caloric intake and increased physical activity focusing on weight loss did not reduce the rate of cardiovascular events in overweight or obese adults T2DM patients while achieved better glycaemic control compared to control group.8 In the randomized Steno‐2 study of 21 years follow‐up, multifactorial intervention including lifestyle changes (diet, exercise and weight loss) and pharmacotherapy (control of blood glucose, blood pressure and lipid profile) has been shown to reduce the risk of not only microvascular but also macrovascular events in T2DM.9 The benefits of an intensive multifactorial treatment approach on the risk of cerebrovascular events and macrovascular complications were demonstrated in Japanese patients in the J‐DOIT3 trial.10
Among the available oral AHAs, use of incretin‐based therapies such as DPP‐4 inhibitors has increased recently because of the good glycaemic control with a low risk of hypoglycaemia and weight neutrality associated with these drugs. In Japan, more than 70% of patients with T2DM are being treated with DPP‐4 inhibitors and ~60% of drug‐naïve patients receive DPP‐4 inhibitors as the first‐line treatment.11 In view of recent reports demonstrating positive effects of nutrition and diet on incretin hormones such as GLP‐1,12 we hypothesize an IDEP (Interaction between Diet/Exercise and Pharmacotherapy) concept by comprehensive literature review. The IDEP concept is the active incretin hormone levels, especially GLP‐1, can be enriched through modifications in the diet, physical exercise and pharmacotherapy, which is further protected from degradation by use of DPP‐4 inhibitors, thereby offers good glycaemic control in patients with T2DM. In this review, we discuss the IDEP concept, focusing on whether diet, exercise and pharmacotherapy can affect GLP‐1 secretion.
2. INCRETIN HORMONES
GLP‐1 and glucose‐dependent insulinotropic polypeptide (GIP) are two physiologically important incretin hormones. While GLP‐1 is released within minutes from enteroendocrine L cells by nutritional, hormonal, pharmacological and neural signals, GIP is produced in the duodenal K cells in the proximal small intestine.13 GIP and GLP‐1 stimulate pancreatic beta cells to induce insulin secretion. In detail, GIP and GLP‐1 exert their effects by binding to their specific receptors, the GIP receptor and the GLP‐1 receptor. Binding to each receptor activates and increases the level of intracellular cyclic adenosine monophosphate thereby activating protein kinase A (PKA) and exchange protein activated by cAMP2 (EPAC2). PKA and EPAC2 are involved in a wide variety of intracellular events including altered ion channel activity, elevated cytosolic calcium levels and enhanced exocytosis of insulin‐containing granules, all of which contribute to stimulation of insulin section in a glucose‐dependent manner.14 Incretin effect accounts for approximately 50% to 70% of total insulin secretion in subjects with normal glucose tolerance; however, in patients with T2DM, the incretin effect is diminished and accounts only for 10% to 40% of insulin secretion. Whether or not the decreased incretin effect is due to T2DM itself, or due to decreased β cell function, remains unclear.15 Of the two incretins, we focused on GLP‐1 because it potentiates insulin secretion under hyperglycaemic conditions and reduces blood glucose levels in patients with T2DM along with inhibition of gastric emptying, food intake and glucagon secretion.16 In addition to improved glycaemic control and preserving islet β cell mass, GLP‐1 has demonstrated beneficial effects on such as cardiac function and atherosclerotic plaque.17
Further, the effect of incretins is dependent on the serum level of the soluble DPP‐4 enzyme. In obese patients with T2DM, the plasma DPP‐4 activity is increased resulting in reduced incretin effect and thereby decreasing the efficacy of DPP‐4 inhibitor therapy.18, 19 In addition, bariatric surgery aimed at weight loss in individuals with T2DM shown to alter GLP‐1 dynamics resulting in improved secretory response to nutrient intake. This improved nutrient sensing could be due to the increased nutrient load into the jejunum, bypassing duodenum, because of the direct connection to the stomach.20 The maximum utilization of GLP‐1 could be achieved by the IDEP concept.
3. METHOD TO RETRIEVE ARTICLES
We performed a narrative review as we had three topics integrated in one concept. We searched appropriate articles with the following terms: (Diet OR Food) AND type 2 diabetes AND (DPP‐4 inhibitors AND [GLP‐1 or incretins] or metformin); GLP‐1 AND Food/Diet AND DPP‐4 inhibitors; GLP‐1 AND food/Diet AND metformin; GLP‐1 AND exercise AND metformin; GLP‐1 AND exercise AND DPP‐4 inhibitors and got references both from PubMed and OVID, but as we started working on the draft for individual sections, we have pooled in all the studies that we might have retrieved by hand search as well. And we have also deleted some studies while making the draft short to meet the requirement of the journal.
4. DIET THERAPY
Diet therapy is important not only as a fundamental treatment for diabetes but as a method to induce GLP‐1 which collaborates with pharmacotherapy for better glycaemic control. In the following section, we summarize nutrition, food, dietary patterns and a couple of ways to induce GLP‐1 secretion.
4.1. Nutrition that induces GLP‐1 secretion
4.1.1. Carbohydrates
Glucose is a potent stimulus for the secretion of incretin hormones. Intestinal glucose sensing is mainly facilitated by the sodium‐dependent glucose transporter (SGLT1), which is present abundantly in the absorptive enterocytes and the apical membranes of L and K cells.21 Apart from SGLT1, GLUT2 is essential for glucose sensing and thus plays a role in systemic glucose control.22 In addition to SGLT1 and GLUT2, secretion of GLP‐1 in response to glucose may be mediated, at least in part, by the sweet taste receptors (T1R2 + T1R3)23 even though, unlike glucose, artificial sweeteners do not trigger insulin, GLP‐1 or GIP release in human.24
Sugars including galactose, maltose, sucrose, and 3‐O‐methyl‐D‐glucose (3OMG) and maltitol stimulate GLP‐1 release, whereas fructose, fucose, mannose, xylose and lactose do not stimulate GLP‐1 secretion. A little difference in the molecular structures of sugars could affect stimulation of GLP‐1, however, the mechanism of action is unclear.25 Further a non‐caloric sweetener and rare sugar, D‐allulose (D‐psicose), induced GLP‐1 release in animal models.26 Short‐chain fatty acids (SCFAs) resulting from fermentation of resistant starch, which include acetate, butyrate and propionate, enhance GLP‐1 levels.27, 28
4.1.2. Protein and amino acids
Peptide transporter‐1 (PEPT1), calcium‐sensing receptor (CaSR) and GPR‐C6A, which are all highly expressed in L cells, are involved in GLP‐1 release.29 L‐arginine, which acts as an insulin secretagogue, increases GLP‐1 levels and improve glucose clearance as shown in a mouse study.30 Similarly, glutamine stimulates GLP‐1 secretion in murine GLUTag cells, and Na+‐coupled amino acid transporters are thought to play an important role in GLP‐1 secretion.31 Amino acids such as glutamine, phenylalanine, tryptophan, asparagine, arginine and the di‐peptide glycine‐sarcosine trigger GLP‐1 secretion through CaSR and PEPT1 receptors.32, 33
4.1.3. Lipids
Lipids including monounsaturated fatty acids, α‐linolenic acid and docosahexaenoic acid (DHA) act as potent stimulators for GLP‐1 release from L cells.34, 35 Several G protein‐coupled receptors (GPRs) including GPR40, GPR43, GPR119, GPR120 act as prime sensors for fats, and free fatty acid receptors (FFAR 1/3) play an important role in inducing GLP‐1 release. GPR40 and GPR119 act synergistically and mediate the triglyceride (TG)‐induced secretion of incretins, whereas GPR120 plays a minor role in the regulation of incretin secretion.36, 37
We summarized the effects of nutrition including carbohydrates, amino acids and lipids and the receptors involved in GLP‐1 levels in vitro and in vivo in Table 1. These experimental evidence indicate that GLP‐1 is secreted in response to multiple factors including a variety of nutrients. However, it is not clear what type of nutrition stimulates GLP‐1 secretion effectively.
Table 1.
Effect of nutrients on GLP‐1 secretion in vivo and in vitro
| Study (Cell lines/animal model) | Stimulus | Concentration and duration | Receptors/transporters | Clinical outcomes (GLP‐1 levels) |
|---|---|---|---|---|
| In vitro | ||||
| Gribble et al 2003109 (GLUTag cells) | Glucose and methyl‐α‐glucopyranoside | glucose (0.5 mmol/L) and methyl‐α‐glucopyranoside (100 mmol/L) for 2 h | SGLT1 |
|
| Jang et al 2007110 (Human enteroendocrine NCI‐H716 cells) | Glucose | Indicated concentrations for 10 min | Taste receptors |
|
| Mace et al 201233 (Male Sprague‐Dawley rats, Perfused small intestine) | Gln, Phe, Trp, Asn, Arg | 10 mmol/L for 30 min | CaSR |
|
| Diakogiannaki et al 201332 (Primary intestinal epithelial cell) | Gly‐Sar (Glycine‐Sarcosine) | 10 mmol/L for 2 h | PEPT1 |
|
| Tolhurst et al 2012111 (Primary intestinal epithelial cell) | SCFA | 140 mmol/L for 2 h | GPR43 |
|
| In vivo | ||||
| Gorboulev et al 201221 (wild type (C57BL)/Sglt1−/− mice) | d‐glucose | 5 min after gavage with D‐glucose bolus (6 mg/g body wt) | SGLT1 |
|
| Roder et al 201422 (wild type (C57BL)/glut2−/− mice) | Glucose | 15 min after oral glucose gavage (4 g/kg) | GLUT2 |
|
| Clemmensen et al 201330 (Diet‐induced obese (DIO) mice, Male C57BL/6 mice) | L‐arginine | 15 min after oral gavage of L‐arginine (1 g/kg) | — |
|
| Edfalk et al 200836 (Gpr40 +/+/Gpr40 LacZ/LacZ mice) | Fat | 60 min after oral high‐fat diet | GPR40 |
|
| Tanaka et al 200834 (Rat GPR120) | α‐linolenic acid | Oral α‐linolenic acid 3 μmol/100 μL for 4 weeks | GPR120 |
|
| Shida et al 201335 (diabetic KK‐A(y) mice) | Docosahexaenoic acid (DHA) | Oral DHA (100 nmol/200 μL/40 g body weight) for 4 wk | — |
|
4.2. Foods that affect GLP‐1 secretion
A variety of foods can increase GLP‐1 secretion,38 including tortillas,39 GFO (glutamine, fibre and oligosaccharide),40 probiotics such as Lactobacillus reuteri, 41 flaked sorghum biscuits,42 vinegar,43 extra virgin olive oil,44 capsaicin,45 low glycaemic index beverages,46 soya protein and polydextrose,47 fish protein hydrolysate (Micromesistius poutassou)48 and whey protein49 (Table 2). It was reported that saturation of fatty acids and length of fatty acids can have an effect on GLP‐1 release. Olive oil induces GLP‐1 response better than butter in both healthy individuals and in patients with T2DM, suggesting an inverse correlation between GLP‐1 release and saturation of fatty acids.50 In addition, fatty acid chains with ≥12 carbons increase GLP‐1 levels more than those with <12 carbons.51
Table 2.
Effect of food on GLP‐1 secretion in humans
| Study (population) | Type of nutrient | Duration | GLP‐1 levels | Clinical outcomes (Glucose levels) |
|---|---|---|---|---|
| Almario et al 2017112 (Patients with T2DM) | Whey protein | acute/2 wk | ↑ | ↓ |
| Nobile et al 201648 (Slightly overweight men and women) | Fish protein hydrolysate | 90 d | ↑ | — |
| Kang et al 201645 (Healthy subjects) | Capsaicin | 6 wk | ↑ | — |
| Stefoska‐Needham et al 201642 (Healthy subjects) | Sorghum biscuits | — | ↑ | → |
| Keller et al 201646 (Healthy young men) | Low glycaemic index beverage | 1 wk | ↑ | — |
| Soong et al 201647 (Lean subjects) | Soya protein and polydextrose | — | ↑ | ↓ |
| Ames et al 201539 (Healthy subjects) | Tortillas with high insoluble fibre | 1 wk | ↑ | → |
| Joo et al 201540 (Healthy subjects) | GFO (glutamine, fibre, and oligosaccharide) | — | ↑ | — |
| Violi et al 201544 (Healthy subjects) | Extra virgin olive oil | 1 mo | ↑ | ↓ |
| Simon et al 201541 (Glucose‐tolerant humans) | Lactobacillus reuteri | 4 wk |
Lean: ↑ Obese: → |
— |
| Dalgaard et al 200452 (Patients with T2DM) | A fat‐rich mixed meal with alcohol | — | ↓ | → |
| Mofidi et al 201254 (Overweight or obese males) | Bread made from sourdough | — | ↓ | → |
On the other hand, certain foods can have a negative effect on stimulation of GLP‐1 secretion. For example, consumption of a fat‐rich meal with alcohol in subjects with T2DM results in suppression of GLP‐1 early in the postprandial phase.52 Further, reductions in the amount of sucrose in the meal were demonstrated to markedly decrease GLP‐1 secretion and were not restored by addition of sweeteners to compensate for the sweet taste in healthy individuals.53 Similarly, bread made from sourdough lowered GLP‐1 response when compared with multigrain or sprouted grain bread in subjects at risk for glucose intolerance54 (Table 2).
4.3. Dietary patterns that induce GLP‐1 secretion
4.3.1. Effect of a sequence of a dietary regimen intake on incretin secretion
The type of meal, sequence of meal intake and adjustment in calorie consumption can all play a role in maintaining postprandial glucose homoeostasis. It has been reported that consuming fish/meat before rice enhances GLP‐1 secretion compared with consuming rice before fish/meat in both healthy subjects and in patients with T2DM which leads to amelioration of postprandial glucose excursion.55 Similarly, a whey preload or protein drink before a meal resulted in increased insulin and GLP‐1 secretion, and decreased gastric emptying, in patients with T2DM.56, 57 These results suggest that eating carbohydrates later in a meal after protein might be a good strategy to enhance secretion of GLP‐1 and in regulating postprandial glucose.
In patients with T2DM, adjusting the calorie balance in each meal with a high‐energy breakfast and low‐energy dinner has been shown to increase GLP‐1 levels, and decrease prandial hyperglycaemia compared to a high‐energy dinner and lower‐energy breakfast.58 Therefore, a change in meal timing with regard to calorie consumption could beneficially modulate GLP‐1 secretion.
4.3.2. Effect of mastication frequency per mouthful and eating speed on incretin secretion
It has been reported that mastication frequency, eating speed and eating duration have an impact on incretin secretion. In healthy individuals, chewing 30‐times per bite increased the endogenous GLP‐1 compared with usual eating, without affecting the concentrations of blood glucose or serum insulin.59 Findings from the Watari Study in Japan showed that eating quickly was significantly associated with metabolic syndrome compared with slow eating.60 Further, eating the same meal slowly for 30 minutes instead of 5 minutes resulted in higher concentrations of GLP‐1 and peptide YY (PYY) levels in healthy subjects.61 Therefore, eating slowly and chewing more could contribute to induce GLP‐1 secretion.
4.3.3. Effect of intermittent fasting vs continuous energy restriction on glycaemic control and incretin secretion
A recent report revealed that intermittent energy restriction is comparable for the reduction of HbA1c with continuous energy restriction in patients with type 2 diabetes.62 While a report showed that continuous energy restriction reduced active baseline GLP‐1 from baseline,63 there is few report to reveal the effect of energy restriction on GLP‐1 secretion which means further studies are warranted to elucidate effect on GLP‐1 secretion in these important dietary interventions.
5. EXERCISE THERAPY
5.1. Current situation of exercise therapy for T2DM
Lifestyle modifications, including change in dietary habits and increase in physical activity, are cornerstones for the treatment of T2DM. The effectiveness of exercise, including stretching, aerobic and resistance exercise, depends on the patient's condition such as age, cognitive function and physical activity levels. There is limited evidence on the concrete methods of exercise for optimal glycaemic control and health outcomes, even though adults with T2DM are advised to do these combination exercises. There are some evidence that have shown positive effects of the exercises with several duration time, intensity and methods on glycaemic control. Apart from that, non‐exercise activity thermogenesis (NEAT) can be considered as one of the important energy expenditure activities to get a well control of blood glucose.64 Studies have shown that interrupting prolonged sitting with brief 5 minutes bouts of standing or light‐intensity walking can attenuate postprandial increase in glucose levels, insulin, C‐peptide and TG responses in patients with T2DM and improve prandial glucose and insulin responses in patients at high risk for T2DM.65, 66 Furthermore, in patients with T2DM, interval‐walking training helped maintain insulin secretion and improve insulin sensitivity and disposition index, in contrast to energy expenditure‐matched continuous walking training.67 The timing of exercise, for example, after a meal was shown to be effective in reducing glucose and TG levels in obese patients with T2DM who were on standard treatment. The results showed that while resistance exercise before dinner improved postprandial glycaemic control, resistance exercise after dinner improved both postprandial glucose and TG elevation.68 In addition, a recent report demonstrated that afternoon exercise is more efficacious than morning exercise at improving blood glucose in T2DM individuals.69 Further, calorie restriction and exercise resulted in weight loss, and improved glucose regulation as well as incretin secretion in obese patients with T2DM, suggesting the beneficial effects of combining diet and exercise therapy.70
5.2. Muscle structure in T2DM
Better understanding of skeletal muscles in terms of type of muscle fibres, size, fibre colour, fatigue resistance, metabolism and insulin sensitivity helps the management of obesity and chronic diseases such as T2DM. For example, slow‐twitch type I fibres with high amount of mitochondria have greater insulin sensitivity compared with the fast‐twitch type II fibres. Skeletal muscles exhibit hypertrophy or atrophy depending on the types of fibres under various conditions, such as ageing and T2DM. Type II fibres have higher susceptibility to atrophy with ageing.71 Therefore, muscle fibre twitch transformation could lead to inadequate glycaemic control in elderly patients with T2DM.72 On the other hand, if the induction of slow type I skeletal muscle phenotype is possible, it can be a potential treatment for obesity and T2DM.73 For example, running exercise inhibit diabetes‐associated type shifting of fibres, a higher percentage of type IIB fibres and a lower percentage of type I and type IIA fibres through an OLTEF rat experiment.72
5.3. Myokine, muscle‐derived interleukin‐6 (IL‐6) induces GLP‐1 secretion
Skeletal muscle constitutes 30%−40% of body weight and glucose uptake by the skeletal muscles increases by up to 50‐fold during exercise. As exercise‐induced glucose uptake involves a complex molecular signalling that is different from insulin, the glucose uptake is preserved even in insulin‐resistant muscle emphasizing the therapeutic potential of exercise in the management of chronic metabolic disease.74 GLUT4 translocation during muscle contraction to sarcolemma and t‐tubules is essential for exercise‐induced glucose uptake. Exercise stimulates glucose uptake through enhancing insulin sensitivity and responsiveness. Muscle contraction, muscle remodelling or exercise training produce several secretory factors such as proteins, growth factors and cytokines (also referred to as myokines) that exert beneficial effects of exercise.75 Although the discovery and validation of myokines are underway, a well‐known myokine, IL‐6 plays an important role in accelerating glucose uptake independent of insulin and communicating with central and peripheral organs.76 Both type I and type II fibres express the muscle‐derived IL‐6 during exercise, which enhances glucose uptake and fat oxidation through activation of AMP‐kinase and/or phosphatidylinositol 3‐kinase.77
Findings from several studies reported the benefits of exercise on GLP‐1 release (Table 3). Both in vitro and in vivo analysis showed that IL‐6 or elevated IL‐6 levels during exercise stimulate GLP‐1 secretion from intestinal L cells and α cells via increased proglucagon and prohormone convertase 1/3 expression.78 There are two latest clinical studies demonstrating the role of exercise in GLP‐1 secretion. Firstly, Islam et al,79 demonstrated that acute exercise bout in healthy volunteers regulate the appetite via lactate and IL‐6 which leads to GLP‐1 secretion in the aspect of energy homoeostasis. Acute intensive exercise leads to a transient reduction in appetite, suppressing ghrelin which is an appetite‐stimulating hormone and increasing GLP‐1 concentration. Secondly, Eshghi et al80 demonstrated the effect of long‐duration (90 minutes), moderate‐intensity exercise bouts in patients with T2DM on IL‐6 and GLP‐1 concentration. The results demonstrated that, IL‐6 levels increased after exercise and GLP‐1 and GIP levels remain increased even after 24 hours of exercise, whereas there was no change in prandial insulin or glucagon levels with no effect on prandial glucose levels. Although few studies have examined the association between exercise and GLP‐1 secretion, more than 30 minutes exercise with high intensity (more than 70% of ventilator threshold) could be required to increase GLP‐1 concentration. However, more studies are required to elucidate the optimal exercise for T2DM.
Table 3.
Effect of exercise on GLP‐1 secretion
| Study (subjects) | Exercise and duration | Myokines involved | GLP‐1 levels | Clinical outcomes |
|---|---|---|---|---|
| Ellingsgaard et al 201178 (Mice) |
|
IL‐6 ↑ | ↑ | Glucose ↓ |
| Islam et al 201779 (Healthy subjects) |
|
IL‐6 ↑ | ↑ | Appetite ↓ |
| Eshghi et al 201780 (Patients with T2DM) |
|
IL‐6 ↑ | — | Glucose ↓ |
6. PHARMACOTHERAPY
On top of diet and exercise, pharmacotherapy itself is critical to achieve good glycaemic control. In the meantime, there are several medications which induce GLP‐1 not only lowering blood glucose level. In the following section, we summarize antihyperglycaemic agents, antidyslipidaemia agents and antihypertensive agents as GLP‐1 inducers.
6.1. Antihyperglycaemic agents
6.1.1. Biguanides
Biguanides are oral medications that reduce plasma glucose via multiple mechanisms.81 Advantages of metformin include its high efficacy, low cost, minimal hypoglycaemia risk. Gastrointestinal symptom and lactic acidosis are adverse events to be considered.
Several studies demonstrated the involvement of metformin either directly or indirectly in GLP‐1 secretion (Table 4). It has been hypothesized that metformin can act directly on GLP‐1 biosynthesis by increasing proglucagon expression and processing. Metformin also increases the L cell sensitivity to GLP‐1 secretagogues. Other potential mechanisms by which metformin plays a role in GLP‐1 biosynthesis include as follows.82
modulating bile acids (BAs) reabsorption there by stimulating GLP‐1 secretion
modulating gut microbiota, resulting in increased production of either SCFAs or BAs which in turn increases GLP‐1 secretion
reducing lipotoxicity
activating enteric neuropeptide (gastrin‐releasing peptide)
stimulating the parasympathetic nervous system
Table 4.
Effect of antihyperglycaemic drugs on GLP‐1
| Study (population) | Duration | GLP‐1 levels | Mechanism |
|---|---|---|---|
| Metformin | |||
| Kappe et al 2013113 (murine GLUTag cell line) |
Acute: 0.5 mmol/L for 30 min Long‐term: 0.5 mmol/L for 48 h |
Acute: GLP‐1→ Long‐term: GLP‐1↑ Preproglucagon ↓ |
Metformin direct effect on GLP‐1 secretion |
| Kim et al 201483 (hyperglycaemic db/db mice) | 8 wk |
GLP‐1↑ in serum GLP‐1 receptor expression in islets ↑ |
Metformin direct effect on GLP‐1 secretion |
| Pyra et al 2012103 (human enteroendocrine cells NCI‐H716) | Cells were incubated for 2 h at 37°C | GLP‐1↑ | Metformin direct effect on GLP‐1 secretion |
| SGLT1/2 inhibitors | |||
| Powell et al 201386 (high‐fat diet mice) | Single dose | Total and active GLP‐1↑ | SGLT1 inhibition |
| Zambrowicz et al 2013114 (Healthy subjects) | 12 d | Total and active GLP‐1↑ | SGLT1 inhibition |
| Zambrowicz et al 2013115 (Patients with T2DM) | Single dose | Total and active GLP‐1↑ | SGLT1 inhibition |
| Oguma et al 201587 (Zucker diabetic fatty rats) | Single dose | Active GLP‐1↑ | SGLT1 inhibition |
| Ferrannini et al 201488 (Patients with T2DM) | 4 weeks | GLP‐1↑ | Unknown |
| α‐GIs | |||
| Hamada et al 2013116 (Male Nagoya‐Shibata‐Yasuda (NSY) mice) | 4 or 12 wk | Active GLP‐1↑ | α‐GI enhancement of bile acid levels |
| Lee et al 201591 (Kir6.2 knockout mice) |
Early phase: 5 min Late phase: 10 and 30 min |
Active GLP‐1↑ | α‐GIs delay carbohydrate absorption and potentiate GLP1 secretion. Miglitol activates duodenal EC cells, possibly via SGLT3, & potentiates GLP1 secretion via parasympathetic nervous system |
| Zheng et al 2013117 (Patients with T2DM) | 24 wk | GLP‐1↑ | Large amounts of undigested carbohydrates reach the lower portion of the small intestine which is rich in L cells |
| Amagai et al 2017118 (Suspected late dumping syndrome patients) | Single dose |
Total GIP↓ Active GLP‐1↓ in early phase |
Inhibiting absorption of carbohydrates in the upper gastrointestinal tract |
| Pioglitazone | |||
| Zheng et al 201792 (Sprague Dawley rats) | 14 wk | Active GLP‐1↑ | Improving insulin resistance |
In the NCI‐H716 cell line and in mice, metformin has been shown to activate GLP‐1 secretion, probably by altering calcium mobilization and the insulin‐signalling pathway upstream from the canonical Wnt‐signalling. Further, 48‐hour incubation of GLUTag cells with metformin increased GLP‐1 secretion, in addition, the expression of proglucagon was decreased.83
The gut microbiota is closely associated with energy metabolism and homoeostasis. Recent report demonstrated that metformin affects gut microbiota in diabetic treatment‐naive T2DM patients which contributes to metformin's antidiabetic effect.84 Alteration in microbiota was demonstrated in T2DM patients treated with metformin. The improvement in glucose tolerance was indirectly demonstrated in germ‐free mice transferred human faecal samples obtained from subjects treated with metformin. Metformin increased abundance of Akkermansia muciniphila, which could improve glucose homoeostasis, metabolic features. Metformin improved glucose sensing in upper small intestine via changing microbiota and upregulation of SGLT1, which in turn is associated with glucose‐induced GLP‐1 secretion.85 Sodium‐glucose cotransporter1/2 inhibitors (SGLT1/SGLT2 inhibitors).
SGLT2 inhibitors reduce plasma glucose by enhancing urinary excretion of glucose then the glucose‐lowering efficacy of these medications is dependent on renal function.81 Mycotic genital infections and ketoacidosis are adverse effects to be considered.
LX4211, a dual SGLT1 and SGLT2 inhibitor treatment improved glycaemic control by reducing intestinal glucose absorption, while increasing circulating levels of GLP‐1 and PYY when tested in Sglt1 −/− mice and Sglt2 −/− mice.86 Intestinal SGLT1 inhibition enhances GLP‐1 secretion in normal and diabetic rats. The combined treatment of canagliflozin and teneligliptin increased plasma GLP‐1 levels and improved glucose tolerance compared with either of the monotherapies in Zucker diabetic fatty rats.87 Treatment with empagliflozin, a highly potent and selective SGLT2 inhibitor, improved β cell function and insulin sensitivity thereby reducing prandial glycaemia in patients with T2DM in addition to enhanced GLP‐1 response although not significant88 (Table 4).
6.1.2. α‐glucosidase inhibitors
α‐glucosidase inhibitors are not listed in the latest ADA/EASD guideline,81 while they are drugs that delay the breakdown of carbohydrates in the gut and consequently slow down the absorption of sugars. Gastrointestinal adverse effects are common among α‐glucosidase inhibitors.89
In patients with T2DM treated with miglitol or voglibose for 12‐weeks, GLP‐1 responses increased. The probable mechanism underlying increased GLP‐1 levels could be either an increase in GLP‐1 secretion or inhibition of the DPP‐4 enzyme.90 Miglitol has been shown to activate duodenal enterochromaffin cells possibly via SGLT3, and potentiates GLP‐1 secretion through the parasympathetic nervous system91 (Table 4).
6.1.3. Thiazolidinediones
Thiazolidinediones (TZDs) are oral medications that increase insulin sensitivity and are of high glucose‐lowering efficacy. TZDs are associated with the best evidence among glucose‐lowering medications for glycaemic durability.81 Potential adverse effect of TZDs is regarding fluid retention and congestive heart failure, weight gain and bone fracture.
Insulin resistance is associated with impaired GLP‐1 secretion. Use of pioglitazone improved the insulin resistance developed in rats fed with a high‐fat diet as well as increased GLP‐1 secretion92 (Table 4). Pioglitazone acts as a potent insulin sensitizer via the nuclear receptor peroxisome proliferator‐activated receptor‐γ (PPARγ). The PPARs are superfamily of ligand‐inducible transcription factors and control the gene expression involved in adipogenesis, lipid metabolism and inflammation. Thiazolidinediones could increase GLP‐1 secretion via PPARγ signalling.92
6.1.4. Sulphonylureas
Sulphonylureas lower glucose by stimulating insulin secretion from pancreatic beta cells.81 They are inexpensive, widely available and have high glucose‐lowering efficacy. As adverse effects, sulphonylureas are associated with weight gain and risk for hypoglycaemia.
Sulphonylurea seems not affect the secretion of incretin.93 Sulphonylurea could uncouple the glucose dependence of the insulinotropic effect of GLP‐1.94
6.2. Antidyslipidaemia agents
There is evidence that the hypolipidaemic agent, atorvastatin, competitively inhibits the DPP‐4 enzyme which otherwise inactivates the incretin hormones GLP‐1 and GIP. However, this action on DPP‐4 inhibition is not class specific, but is structure specific.95 Another antihypercholesterolaemia agent, ezetimibe, stimulated GLP‐1 secretion in high‐fat fed mice not by inhibiting the DPP‐4 enzyme but by the activation of the mitogen‐activated protein (MEK)/extracellular signal‐regulated kinase (ERK) pathway.96
6.3. Antihypertensive agents
The loop diuretic, bumetanide impaired GLP‐1 secretory response by glycine without affecting glucose‐triggered secretion suggesting that the bumetanide‐sensitive Na‐K‐Cl cotransporter maintains high intracellular Cl−concentrations in the GLUTag cells.97 The angiotensin receptor blocker (ARB), olmesartan improved glucose intolerance independent of improvements in muscle and/or adipose insulin signalling, which could be attributed to increasing GLP‐1 concentration and pancreatic GLP‐1 receptor expression thereby improving glucose‐dependent insulin secretion.98
7. IDEP
After reviewing diet, exercise and pharmacotherapy, respectively, we move on to interaction among them. Diet and pharmacotherapy interaction is presented in Table 5. Nutrient preloads including whey protein before a meal, decreased postprandial glycaemic excursions partly due to the slowing of gastric emptying and the stimulating secretion of the incretins GLP‐1 and GIP. In a study with the DPP‐4 inhibitor, vildagliptin, whey preload in metformin‐treated patients enhanced the efficacy of vildagliptin by slowing gastric emptying and reducing postprandial glycaemia, in addition to increase in plasma intact GLP‐1 and GIP.99 Similarly, in T2DM patients who consumed a preload drink containing 50 g D‐xylose, level of prandial glycaemia was reduced and the effect of a DPP‐4 inhibitor, sitagliptin, was enhanced.100 Further, glycaemic control improved in patients with T2DM taking AHAs and eating glutinous brown rice (GBR) for 8 weeks. Moreover, in patients on DPP‐4 inhibitor therapy, a significant decrease in glycated haemoglobin (HbA1c) levels and glycoalbumin, in addition to increased active levels of the gut hormones GLP‐1 and PYY, were reported, demonstrating additive benefits of GBR.101 Other than DPP‐4 inhibitor therapy, metformin has shown positive effects on GLP‐1 levels. It was reported that, mice fed with a HF diet for 12 weeks developed insulin resistance along with increased blood glucose and HbA1c levels, and fasting plasma insulin in conjunction with reduced oral glucose tolerance. However, these effects were reversed with metformin due to improved GLP‐1 responses along with reducing prandial plasma free fatty acids.102 The combination of metformin and a prebiotic oligofructose (OFS) improved fat mass, hepatic TG, and decreased plasma DPP‐4 activity and GIP levels in obese rats, thereby improving metabolic outcomes in obesity; however, there was no interactive effect of combining OFS with metformin on GLP‐1 secretion.103 Furthermore, recent study showed that a high‐fibre diet improved HbA1c levels as well as increasing GLP‐1 AUC in T2DM patients with acarbose and α‐glucosidase inhibitors. This could be due to acarbose which transforms some of the starch present in the diet into a fibre thereby reducing its digestion and increasing fermentable carbohydrate in the colon.104
Table 5.
Diet‐pharmacotherapy interaction
| Study (population) | Type of nutrient | Treatment | Clinical outcomes |
|---|---|---|---|
| Wu et al 201699 (Patients with T2DM) | Whey protein | Metformin + vildagliptin |
↓ Glucose excursions ↓ Plasma glucagon ↓ Gastric emptying ↑ Plasma insulin ↑ Plasma GLP‐1 |
| Wu et al 2013100 (Patients with T2DM) | D‐xylose preload | Sitagliptin |
↓ Glucose excursions ↓ Plasma glucose levels ↓ Insulin‐to‐glucose ratio ↓ Gastric emptying ↑ Plasma GLP‐1 |
| Nakayama et al 2017101 (Patients with T2DM) | Glutinous brown rice | Insulin injections with or without oral AHAs |
↓ HbA1c levels ↓ Glycoablumin ↓ Postprandial glucose levels ↑ GLP‐1 and PYY |
| Zhao et al 2018104 (Patients with T2DM) | High‐fibre diet (SCFA producers) | Acarbose |
↓ HbA1c levels ↑ Plasma GLP‐1 |
| Kappe et al 2014102 (Mice study) | High‐fat diet | Metformin |
↓ Plasma glucose levels ↓ HbA1c levels ↓ Plasma free fatty acids ↑ Plasma GLP‐1 |
| Pyra et al 2012103 (Rat study) | Oligofructose | Metformin |
↓ Plasma DPP‐4 activity → GLP‐1 levels ↓ GIP levels ↓ Fat mass |
In Japanese patients with T2DM treated with a DPP‐4 inhibitor as monotherapy for 1 year, fat intake, especially saturated fat was shown to be significantly associated with deterioration of HbA1c levels (≥0.4%).105
There is little evidence regarding exercise and pharmacotherapy interaction. In contrast to the findings demonstrating exercise improves glycaemic and metabolic parameters in patients with T2DM, one study demonstrated that aerobic exercise did not acutely increase total GLP‐1 and GIP levels in patients with T2DM, however, metformin treatment, independent of exercise, significantly increased total plasma GLP‐1 and GIP concentrations.106 On the other hand, a 12‐week aerobic exercise training programme in obese patients with metabolic syndrome reduced plasma DPP‐4 levels, which in turn improved insulin sensitivity and fat oxidation.107 Taken together, aerobic exercise could have an effect on reduction of plasma DPP‐4 levels but not GLP‐1 secretion. Resistance exercise which showed increase GLP‐1 secretion through IL‐6 could have an effect on reduction of appetite. Therefore, different types of exercises shown to have different effects on GLP‐1 utility for patients with T2DM. Therefore, the combination of exercise and DPP‐4 inhibitors are compatible for T2DM treatment.
With respect to pharmacotherapy interactions, AHAs such as metformin, SGLT1/2 inhibitors, α‐glucosidase inhibitors and pioglitazone as well as certain antihypertensive agents and lipid‐lowering drugs have a direct or indirect role in increasing GLP‐1 levels. Therefore, the combination of DPP‐4 inhibitors and other AHAs are effective choice for T2DM treatment.
8. DISCUSSION/CONCLUSIONS
Diet, exercise and diabetes education alongside pharmacotherapy are crucial for the optimal management of T2DM. Changing the diet regimen and including exercise intervention as part of T2DM management can improve glycaemic control and reduce treatment escalation of AHAs, which otherwise can lead to risk of developing potential adverse effects and puts excessive costs on health care.108 The interaction between diet/exercise and pharmacotherapy is limited, and hence we attempted to summarize such studies in the present review.
T2DM is a multifactorial disease affecting multiple pathophysiological pathways, and thus a holistic management of the disease, considering combination of diet and exercise together with pharmacotherapy, can result in better treatment outcomes and improve overall quality of life in patients with T2DM. Despite most antihyperglycaemic drugs enhance GLP‐1 levels, the DPP‐4 enzyme soon degrades the active GLP‐1. However, in the case of treatment with DPP‐4 inhibitors, the effect of enhanced endogenous GLP‐1 levels due to diet or exercise are protected, resulting in prolonged active GLP‐1 levels and good glycaemic control without the need for treatment escalation. Although, there is a lot of evidence regarding effect of diet/exercise and pharmacotherapy on GLP‐1 concentration is available, no direct comparison between studies could be made because of different methodologies used in measuring plasma GLP‐1 levels.
To conclude, the IDEP concept can be a promising treatment strategy by positively influencing GLP‐1 levels and providing additive benefits in terms of improving metabolic parameters in patients with T2DM and slowing the progression of T2DM and its associated complications (Figure 1). The IDEP concept further supports and emphasizes the importance of mandating diet therapy and exercise as part of the prescription in order to reduce the disease burden and improve the quality of life in patients with T2DM.
Figure 1.
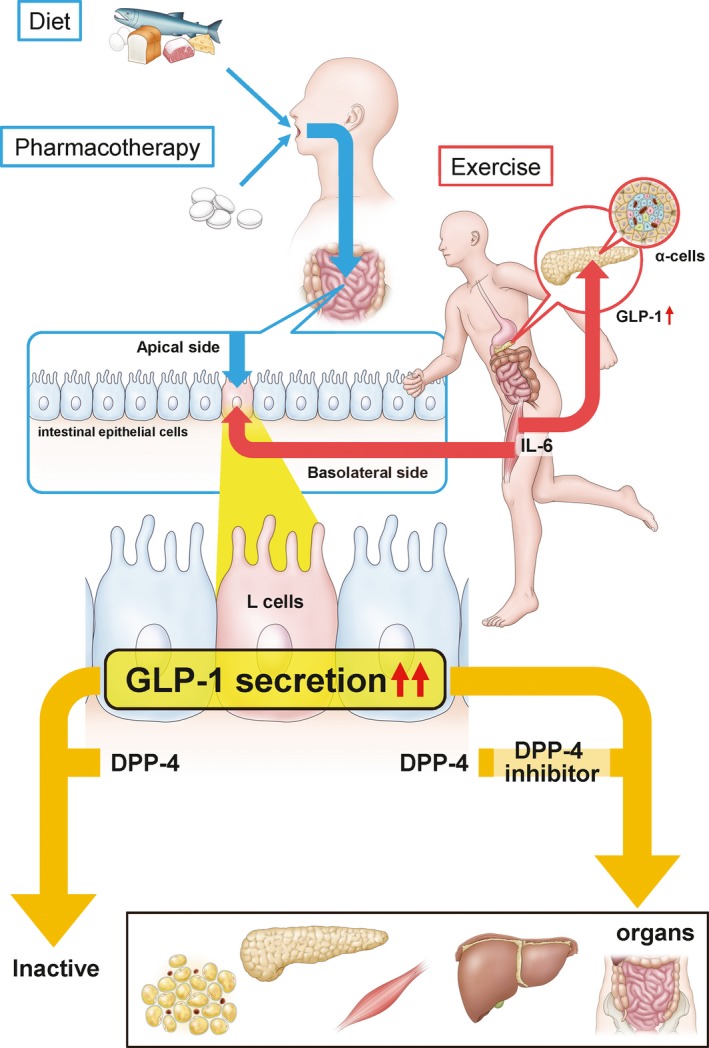
The incretin hormone, glucagon‐like peptide‐1 (GLP‐1) is secreted from intestinal L cells by not only nutrient‐stimulus containing carbohydrate, protein and lipids but also exercise in addition to pharmacotherapy in T2DM patients. The IDEP concept is focused on enhancement of GLP‐1 through changes in the diet, physical exercise and pharmacotherapy. Although GLP‐1 is deactivated by dipeptidyl peptidase‐4 (DPP‐4) except for the neural pathway for the actions of GLP‐1, DPP‐4 inhibitors protect GLP‐1 from degradation. Active GLP‐1 involves positive effects via several mechanisms in pancreas, liver, adipose tissues, muscle and intestines.16 Exercise‐induced interleukin‐6 (IL‐6) concentrations stimulates GLP‐1 secretion from intestinal L cells and pancreatic α cells. Patients with T2DM usually present with comorbidities such as dyslipidemia and hypertension. Therefore, apart from anti‐hyperglycemic drugs, pharmacotherapies with anti‐hyperlipidemia drugs, anti‐hypertensive drugs those have an effect on GLP‐1 release, must be considered for better management of T2DM
CONFLICT OF INTEREST
Yuki Fujiwara, Shunsuke Eguchi, Hiroki Murayama, Yuri Takahashi, Mitsutoshi Toda and Kota Imai are employees of Novartis Pharma. Kinsuke Tsuda has served as an advisory board member for Novartis and has received lecture fees from Novartis.
AUTHOR CONTRIBUTIONS
All the authors researched and analysed the data and were involved in drafting the outline, reviewed all the drafts and approved the final draft of the manuscript.
ETHICAL APPROVAL
Not applicable.
ACKNOWLEDGEMENTS
Sponsorship and article processing charges for this study were funded by Novartis. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole and have given final approval to the version to be published. The authors thank Lakshmi Deepa Gurajala, PhD, of Novartis Healthcare Pvt. Ltd, Hyderabad, India for medical writing support, funded by Novartis Pharma AG, Basel, Switzerland, in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Fujiwara Y, Eguchi S, Murayama H, et al. Relationship between diet/exercise and pharmacotherapy to enhance the GLP‐1 levels in type 2 diabetes. Endocrinol Diab Metab. 2019;2:e00068 10.1002/edm2.68
Yuki Fujiwara and Shunsuke Eguchi contributed equally to this work.
Data Availability Statement: All data are included within the manuscript.
DATA AVAILABILITY
All data are included within the manuscript.
REFERENCES
- 1. Cho NH, Shaw JE, Karuranga S, et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271‐281. [DOI] [PubMed] [Google Scholar]
- 2. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient‐centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140‐149. [DOI] [PubMed] [Google Scholar]
- 3. Ross SA. Breaking down patient and physician barriers to optimize glycemic control in type 2 diabetes. Am J Med. 2013;126:S38‐S48. [DOI] [PubMed] [Google Scholar]
- 4. American Diabetes Association . 4. Lifestyle management: standards of medical care in diabetes‐2018. Diabetes Care. 2018;41:S38‐S50. [DOI] [PubMed] [Google Scholar]
- 5. Shai I, Schwarzfuchs D, Henkin Y, et al. Weight loss with a low‐carbohydrate, Mediterranean, or low‐fat diet. N Engl J Med. 2008;359:229‐241. [DOI] [PubMed] [Google Scholar]
- 6. Balducci S, Zanuso S, Nicolucci A, et al. Effect of an intensive exercise intervention strategy on modifiable cardiovascular risk factors in subjects with type 2 diabetes mellitus: a randomized controlled trial: the Italian Diabetes and Exercise Study (IDES). Arch Intern Med. 2010;170:1794‐1803. [DOI] [PubMed] [Google Scholar]
- 7. Knowler WC, Barrett‐Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wing RR, Bolin P, Brancati FL, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gaede P, Oellgaard J, Carstensen B, et al. Years of life gained by multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: 21 years follow‐up on the Steno‐2 randomised trial. Diabetologia. 2016;59:2298‐2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ueki K, Sasako T, Okazaki Y, et al. Effect of an intensified multifactorial intervention on cardiovascular outcomes and mortality in type 2 diabetes (J‐DOIT3): an open‐label, randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5:951‐964. [DOI] [PubMed] [Google Scholar]
- 11. Seino Y, Kuwata H, Yabe D. Incretin‐based drugs for type 2 diabetes: Focus on East Asian perspectives. J Diabetes Investig. 2016;7(Suppl 1):102‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang X, Liu H, Chen J, Li Y, Qu S. Multiple factors related to the secretion of glucagon‐like peptide‐1. Int J Endocrinol. 2015;2015:651757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153‐165. [DOI] [PubMed] [Google Scholar]
- 14. Seino Y, Fukushima M, Yabe D. GIP and GLP‐1, the two incretin hormones: similarities and differences. J Diabetes Investig. 2010;1:8‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cho YM, Fujita Y, Kieffer TJ. Glucagon‐like peptide‐1: glucose homeostasis and beyond. Annu Rev Physiol. 2014;76:535‐559. [DOI] [PubMed] [Google Scholar]
- 16. Ahren B, Foley JE. Improved glucose regulation in type 2 diabetic patients with DPP‐4 inhibitors: focus on alpha and beta cell function and lipid metabolism. Diabetologia. 2016;59:907‐917. [DOI] [PubMed] [Google Scholar]
- 17. Ussher JR, Drucker DJ. Cardiovascular biology of the incretin system. Endocr Rev. 2012;33:187‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aso Y, Ozeki N, Terasawa T, et al. Serum level of soluble CD26/dipeptidyl peptidase‐4 (DPP‐4) predicts the response to sitagliptin, a DPP‐4 inhibitor, in patients with type 2 diabetes controlled inadequately by metformin and/or sulfonylurea. Transl Res. 2012;159:25‐31. [DOI] [PubMed] [Google Scholar]
- 19. Sell H, Bluher M, Kloting N, et al. Adipose dipeptidyl peptidase‐4 and obesity: correlation with insulin resistance and depot‐specific release from adipose tissue in vivo and in vitro. Diabetes Care. 2013;36:4083‐4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Neff KJ, O'Shea D, le Roux CW. Glucagon like peptide‐1 (GLP‐1) dynamics following bariatric surgery: a Signpost to a new frontier. Curr Diabetes Rev. 2013;9:93‐101. [DOI] [PubMed] [Google Scholar]
- 21. Gorboulev V, Schurmann A, Vallon V, et al. Na(+)‐D‐glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose‐dependent incretin secretion. Diabetes. 2012;61:187‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roder PV, Geillinger KE, Zietek TS, Thorens B, Koepsell H, Daniel H. The role of SGLT1 and GLUT2 in intestinal glucose transport and sensing. PLoS One. 2014;9:e89977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zietek T, Daniel H. Intestinal nutrient sensing and blood glucose control. Curr Opin Clin Nutr Metab Care. 2015;18:381‐388. [DOI] [PubMed] [Google Scholar]
- 24. Zukerman S, Glendinning JI, Margolskee RF, Sclafani A. T1R3 taste receptor is critical for sucrose but not Polycose taste. Am J Physiol Regul Integr Comp Physiol. 2009;296:R866‐R876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shima K, Suda T, Nishimoto K, Yoshimoto S. Relationship between molecular structures of sugars and their ability to stimulate the release of glucagon‐like peptide‐1 from canine ileal loops. Acta Endocrinol (Copenh). 1990;123:464‐470. [DOI] [PubMed] [Google Scholar]
- 26. Iwasaki Y, Sendo M, Dezaki K, et al. GLP‐1 release and vagal afferent activation mediate the beneficial metabolic and chronotherapeutic effects of D‐allulose. Nat Commun. 2018;9:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Raigond P, Ezekiel R, Raigond B. Resistant starch in food: a review. J Sci Food Agric. 2015;95:1968‐1978. [DOI] [PubMed] [Google Scholar]
- 28. Keenan MJ, Zhou J, Hegsted M, et al. Role of resistant starch in improving gut health, adiposity, and insulin resistance. Adv Nutr. 2015;6:198‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gribble FM. The gut endocrine system as a coordinator of postprandial nutrient homoeostasis. Proc Nutr Soc. 2012;71:456‐462. [DOI] [PubMed] [Google Scholar]
- 30. Clemmensen C, Smajilovic S, Smith EP, et al. Oral L‐arginine stimulates GLP‐1 secretion to improve glucose tolerance in male mice. Endocrinology. 2013;154:3978‐3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reimann F, Williams L, da Silva XG, Rutter GA, Gribble FM. Glutamine potently stimulates glucagon‐like peptide‐1 secretion from GLUTag cells. Diabetologia. 2004;47:1592‐1601. [DOI] [PubMed] [Google Scholar]
- 32. Diakogiannaki E, Pais R, Tolhurst G, et al. Oligopeptides stimulate glucagon‐like peptide‐1 secretion in mice through proton‐coupled uptake and the calcium‐sensing receptor. Diabetologia. 2013;56:2688‐2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mace OJ, Schindler M, Patel S. The regulation of K‐ and L‐cell activity by GLUT2 and the calcium‐sensing receptor CasR in rat small intestine. J Physiol. 2012;590:2917‐2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tanaka T, Yano T, Adachi T, Koshimizu TA, Hirasawa A, Tsujimoto G. Cloning and characterization of the rat free fatty acid receptor GPR120: in vivo effect of the natural ligand on GLP‐1 secretion and proliferation of pancreatic beta cells. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:515‐522. [DOI] [PubMed] [Google Scholar]
- 35. Shida T, Kamei N, Takeda‐Morishita M, Isowa K, Takayama K. Colonic delivery of docosahexaenoic acid improves impaired glucose tolerance via GLP‐1 secretion and suppresses pancreatic islet hyperplasia in diabetic KK‐A(y) mice. Int J Pharm. 2013;450:63‐69. [DOI] [PubMed] [Google Scholar]
- 36. Edfalk S, Steneberg P, Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes. 2008;57:2280‐2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ekberg JH, Hauge M, Kristensen LV, et al. GPR119, a major enteroendocrine sensor of dietary triglyceride metabolites coacting in synergy with FFA1 (GPR40). Endocrinology. 2016;157:4561‐4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bodnaruc AM, Prud'homme D, Blanchet R, Giroux I. Nutritional modulation of endogenous glucagon‐like peptide‐1 secretion: a review. Nutr Metab. 2016;13:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ames N, Blewett H, Storsley J, Thandapilly SJ, Zahradka P, Taylor C. A double‐blind randomised controlled trial testing the effect of a barley product containing varying amounts and types of fibre on the postprandial glucose response of healthy volunteers. Br J Nutr. 2015;113:1373‐1383. [DOI] [PubMed] [Google Scholar]
- 40. Joo E, Muraoka A, Hamasaki A, et al. Enteral supplementation with glutamine, fiber, and oligosaccharide modulates incretin and glucagon‐like peptide‐2 secretion. J Diabetes Investig. 2015;6:302‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Simon MC, Strassburger K, Nowotny B, et al. Intake of Lactobacillus reuteri improves incretin and insulin secretion in glucose‐tolerant humans: a proof of concept. Diabetes Care. 2015;38:1827‐1834. [DOI] [PubMed] [Google Scholar]
- 42. Stefoska‐Needham A, Beck EJ, Johnson SK, Chu J, Tapsell LC. Flaked sorghum biscuits increase postprandial GLP‐1 and GIP levels and extend subjective satiety in healthy subjects. Mol Nutr Food Res. 2016;60:1118‐1128. [DOI] [PubMed] [Google Scholar]
- 43. Lim J, Henry CJ, Haldar S. Vinegar as a functional ingredient to improve postprandial glycemic control‐human intervention findings and molecular mechanisms. Mol Nutr Food Res. 2016;60:1837‐1849. [DOI] [PubMed] [Google Scholar]
- 44. Violi F, Loffredo L, Pignatelli P, et al. Extra virgin olive oil use is associated with improved post‐prandial blood glucose and LDL cholesterol in healthy subjects. Nutr Diabetes. 2015;5:e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kang C, Zhang Y, Zhu X, et al. Healthy subjects differentially respond to dietary capsaicin correlating with specific gut enterotypes. J Clin Endocrinol Metab. 2016;101:4681‐4689. [DOI] [PubMed] [Google Scholar]
- 46. Keller J, Kahlhofer J, Peter A, Bosy‐Westphal A. Effects of low versus high glycemic index sugar‐sweetened beverages on postprandial vasodilatation and inactivity‐induced impairment of glucose metabolism in healthy men. Nutrients. 2016;8:E802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Soong YY, Lim WX, Leow MK, Siow PC, Teh AL, Henry CJ. Combination of soya protein and polydextrose reduces energy intake and glycaemic response via modulation of gastric emptying rate, ghrelin and glucagon‐like peptide‐1 in Chinese. Br J Nutr. 2016;115:2130‐2137. [DOI] [PubMed] [Google Scholar]
- 48. Nobile V, Duclos E, Michelotti A, Bizzaro G, Negro M, Soisson F. Supplementation with a fish protein hydrolysate (Micromesistius poutassou): effects on body weight, body composition, and CCK/GLP‐1 secretion. Food Nutr Res. 2016;60:29857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hutchison AT, Piscitelli D, Horowitz M, et al. Acute load‐dependent effects of oral whey protein on gastric emptying, gut hormone release, glycemia, appetite, and energy intake in healthy men. Am J Clin Nutr. 2015;102:1574‐1584. [DOI] [PubMed] [Google Scholar]
- 50. Wu T, Rayner CK, Jones K, Horowitz M. Dietary effects on incretin hormone secretion. Vitam Horm. 2010;84:81‐110. [DOI] [PubMed] [Google Scholar]
- 51. Feltrin KL, Little TJ, Meyer JH, et al. Effects of intraduodenal fatty acids on appetite, antropyloroduodenal motility, and plasma CCK and GLP‐1 in humans vary with their chain length. Am J Physiol Regul Integr Comp Physiol. 2004;287:R524‐R533. [DOI] [PubMed] [Google Scholar]
- 52. Dalgaard M, Thomsen C, Rasmussen BM, Holst JJ, Hermansen K. Ethanol with a mixed meal decreases the incretin levels early postprandially and increases postprandial lipemia in type 2 diabetic patients. Metabolism. 2004;53:77‐83. [DOI] [PubMed] [Google Scholar]
- 53. Sakurai K, Lee EY, Morita A, et al. Glucagon‐like peptide‐1 secretion by direct stimulation of L cells with luminal sugar vs non‐nutritive sweetener. J Diabetes Investig. 2012;3:156‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mofidi A, Ferraro ZM, Stewart KA, et al. The acute impact of ingestion of sourdough and whole‐grain breads on blood glucose, insulin, and incretins in overweight and obese men. J Nutr Metab. 2012;2012:184710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kuwata H, Iwasaki M, Shimizu S, et al. Meal sequence and glucose excursion, gastric emptying and incretin secretion in type 2 diabetes: a randomised, controlled crossover, exploratory trial. Diabetologia. 2016;59:453‐461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gunnerud UJ, Heinzle C, Holst JJ, Ostman EM, Bjorck IM. Effects of pre‐meal drinks with protein and amino acids on glycemic and metabolic responses at a subsequent composite meal. PLoS One. 2012;7:e44731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ma J, Stevens JE, Cukier K, et al. Effects of a protein preload on gastric emptying, glycemia, and gut hormones after a carbohydrate meal in diet‐controlled type 2 diabetes. Diabetes Care. 2009;32:1600‐1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jakubowicz D, Wainstein J, Ahrén BO, et al. High‐energy breakfast with low‐energy dinner decreases overall daily hyperglycaemia in type 2 diabetic patients: a randomised clinical trial. Diabetologia. 2015;58:912‐919. [DOI] [PubMed] [Google Scholar]
- 59. Sonoki K, Iwase M, Takata Y, et al. Effects of thirty‐times chewing per bite on secretion of glucagon‐like peptide‐1 in healthy volunteers and type 2 diabetic patients. Endocr J. 2013;60:311‐319. [DOI] [PubMed] [Google Scholar]
- 60. Hattori T, Konno S, Munakata M. Gender differences in lifestyle factors associated with metabolic syndrome and preliminary metabolic syndrome in the general population: the Watari Study. Intern Med. 2017;56:2253‐2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kokkinos A, le Roux CW, Alexiadou K, et al. Eating slowly increases the postprandial response of the anorexigenic gut hormones, peptide YY and glucagon‐like peptide‐1. J Clin Endocrinol Metab. 2010;95:333‐337. [DOI] [PubMed] [Google Scholar]
- 62. Carter S, Clifton PM, Keogh JB. Effect of intermittent compared with continuous energy restricted diet on glycemic control in patients with type 2 diabetes: a randomized noninferiority trial. JAMA Network Open. 2018;1:e180756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Coutinho SR, Halset EH, Gåsbakk S, et al. Compensatory mechanisms activated with intermittent energy restriction: a randomized control trial. Clin Nutr. 2018;37:815‐823. [DOI] [PubMed] [Google Scholar]
- 64. Ravussin E. Physiology. A NEAT way to control weight? Science. 2005;307:530‐531. [DOI] [PubMed] [Google Scholar]
- 65. Henson J, Davies MJ, Bodicoat DH, et al. Breaking up prolonged sitting with standing or walking attenuates the postprandial metabolic response in postmenopausal women: a randomized acute study. Diabetes Care. 2016;39:130‐138. [DOI] [PubMed] [Google Scholar]
- 66. Dempsey PC, Larsen RN, Sethi P, et al. Benefits for type 2 diabetes of interrupting prolonged sitting with brief bouts of light walking or simple resistance activities. Diabetes Care. 2016;39:964‐972. [DOI] [PubMed] [Google Scholar]
- 67. Karstoft K, Clark MA, Jakobsen I, et al. The effects of 2 weeks of interval vs continuous walking training on glycaemic control and whole‐body oxidative stress in individuals with type 2 diabetes: a controlled, randomised, crossover trial. Diabetologia. 2017;60:508‐517. [DOI] [PubMed] [Google Scholar]
- 68. Heden TD, Winn NC, Mari A, et al. Postdinner resistance exercise improves postprandial risk factors more effectively than predinner resistance exercise in patients with type 2 diabetes. J Appl Physiol. 1985;2015(118):624‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Savikj M, Gabriel BM, Alm PS, et al. Afternoon exercise is more efficacious than morning exercise at improving blood glucose levels in individuals with type 2 diabetes: a randomised crossover trial. Diabetologia. 2019;62:233‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Weiss EP, Albert SG, Reeds DN, et al. Calorie restriction and matched weight loss from exercise: independent and additive effects on glucoregulation and the incretin system in overweight women and men. Diabetes Care. 2015;38:1253‐1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ishihara A, Nagatomo F, Fujino H, Kondo H, Tsuda K. Lifestyle‐related disease and skeletal muscle: a review. J Phys Fit Sports Med. 2012;1:12‐27. [Google Scholar]
- 72. von Haehling S, Ebner N, Dos Santos MR, Springer J, Anker SD. Muscle wasting and cachexia in heart failure: mechanisms and therapies. Nat Rev Cardiol. 2017;14:323‐341. [DOI] [PubMed] [Google Scholar]
- 73. Duan Y, Li F, Tan B, Yao K, Yin Y. Metabolic control of myofibers: promising therapeutic target for obesity and type 2 diabetes. Obes Rev. 2017;18:647‐659. [DOI] [PubMed] [Google Scholar]
- 74. Sylow L, Kleinert M, Richter EA, Jensen TE. Exercise‐stimulated glucose uptake ‐ regulation and implications for glycaemic control. Nat Rev Endocrinol. 2017;13:133‐148. [DOI] [PubMed] [Google Scholar]
- 75. Fiuza‐Luces C, Garatachea N, Berger NA, Lucia A. Exercise is the real polypill. Physiology (Bethesda). 2013;28:330‐358. [DOI] [PubMed] [Google Scholar]
- 76. Whitham M, Febbraio MA. The ever‐expanding myokinome: discovery challenges and therapeutic implications. Nat Rev Drug Discov. 2016;15:719‐729. [DOI] [PubMed] [Google Scholar]
- 77. Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle‐derived interleukin‐6. Physiol Rev. 2008;88:1379‐1406. [DOI] [PubMed] [Google Scholar]
- 78. Ellingsgaard H, Hauselmann I, Schuler B, et al. Interleukin‐6 enhances insulin secretion by increasing glucagon‐like peptide‐1 secretion from L cells and alpha cells. Nat Med. 2011;17:1481‐1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Islam H, Townsend LK, McKie GL, Medeiros PJ, Gurd BJ, Hazell TJ. Potential involvement of lactate and interleukin‐6 in the appetite‐regulatory hormonal response to an acute exercise bout. J Appl Physiol. 1985;2017(123):614‐623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Eshghi SR, Fletcher K, Myette‐Côté É, et al. Glycemic and metabolic effects of two long bouts of moderate‐intensity exercise in men with normal glucose tolerance or type 2 diabetes. Front Endocrinol (Lausanne). 2017;8:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Davies MJ, D'Alessio DA, Fradkin J, et al. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;2018(41):2669‐2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bahne E, Hansen M, Bronden A, Sonne DP, Vilsboll T, Knop FK. Involvement of glucagon‐like peptide‐1 in the glucose‐lowering effect of metformin. Diabetes Obes Metab. 2016;18:955‐961. [DOI] [PubMed] [Google Scholar]
- 83. Kim MH, Jee JH, Park S, Lee MS, Kim KW, Lee MK. Metformin enhances glucagon‐like peptide 1 via cooperation between insulin and Wnt signaling. J Endocrinol. 2014;220:117‐128. [DOI] [PubMed] [Google Scholar]
- 84. Wu H, Esteve E, Tremaroli V, et al. Metformin alters the gut microbiome of individuals with treatment‐naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017;23:850‐858. [DOI] [PubMed] [Google Scholar]
- 85. Bauer PV, Duca FA, Waise T, et al. Metformin alters upper small intestinal microbiota that impact a glucose‐SGLT1‐sensing glucoregulatory pathway. Cell Metab. 2018;27:101‐117 e105. [DOI] [PubMed] [Google Scholar]
- 86. Powell DR, Smith M, Greer J, et al. LX4211 increases serum glucagon‐like peptide 1 and peptide YY levels by reducing sodium/glucose cotransporter 1 (SGLT1)‐mediated absorption of intestinal glucose. J Pharmacol Exp Ther. 2013;345:250‐259. [DOI] [PubMed] [Google Scholar]
- 87. Oguma T, Kuriyama C, Nakayama K, et al. The effect of combined treatment with canagliflozin and teneligliptin on glucose intolerance in Zucker diabetic fatty rats. J Pharmacol Sci. 2015;127:456‐461. [DOI] [PubMed] [Google Scholar]
- 88. Ferrannini E, Muscelli E, Frascerra S, et al. Metabolic response to sodium‐glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest. 2014;124:499‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Aoki K, Muraoka T, Ito Y, Togashi Y, Terauchi Y. Comparison of adverse gastrointestinal effects of acarbose and miglitol in healthy men: a crossover study. Intern Med. 2010;49:1085‐1087. [DOI] [PubMed] [Google Scholar]
- 90. Narita T, Yokoyama H, Yamashita R, et al. Comparisons of the effects of 12‐week administration of miglitol and voglibose on the responses of plasma incretins after a mixed meal in Japanese type 2 diabetic patients. Diabetes Obes Metab. 2012;14:283‐287. [DOI] [PubMed] [Google Scholar]
- 91. Lee EY, Kaneko S, Jutabha P, et al. Distinct action of the alpha‐glucosidase inhibitor miglitol on SGLT3, enteroendocrine cells, and GLP1 secretion. J Endocrinol. 2015;224:205‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zheng J, Xiao KL, Chen L, et al. Insulin sensitizers improve the GLP‐1 secretion and the amount of intestinal L cells on high‐fat‐diet‐induced catch‐up growth. Nutrition. 2017;39‐40:82‐91. [DOI] [PubMed] [Google Scholar]
- 93. Fukase N, Manaka H, Sugiyama K, et al. Response of truncated glucagon‐like peptide‐1 and gastric inhibitory polypeptide to glucose ingestion in non‐insulin dependent diabetes mellitus. Effect of sulfonylurea therapy. Acta Diabetol. 1995;32:165‐169. [DOI] [PubMed] [Google Scholar]
- 94. de Heer J, Holst JJ. Sulfonylurea compounds uncouple the glucose dependence of the insulinotropic effect of glucagon‐like peptide 1. Diabetes. 2007;56:438‐443. [DOI] [PubMed] [Google Scholar]
- 95. Taldone T, Zito SW, Talele TT. Inhibition of dipeptidyl peptidase‐IV (DPP‐IV) by atorvastatin. Bioorg Med Chem Lett. 2008;18:479‐484. [DOI] [PubMed] [Google Scholar]
- 96. Chang E, Kim L, Choi JM, et al. Ezetimibe stimulates intestinal glucagon‐like peptide 1 secretion via the MEK/ERK pathway rather than dipeptidyl peptidase 4 inhibition. Metabolism. 2015;64:633‐641. [DOI] [PubMed] [Google Scholar]
- 97. Gameiro A, Reimann F, Habib AM, et al. The neurotransmitters glycine and GABA stimulate glucagon‐like peptide‐1 release from the GLUTag cell line. J Physiol. 2005;569:761‐772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Rodriguez R, Viscarra JA, Minas JN, Nakano D, Nishiyama A, Ortiz RM. Angiotensin receptor blockade increases pancreatic insulin secretion and decreases glucose intolerance during glucose supplementation in a model of metabolic syndrome. Endocrinology. 2012;153:1684‐1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wu T, Little TJ, Bound MJ, et al. A protein preload enhances the glucose‐lowering efficacy of vildagliptin in type 2 diabetes. Diabetes Care. 2016;39:511‐517. [DOI] [PubMed] [Google Scholar]
- 100. Wu T, Bound MJ, Zhao BR, et al. Effects of a D‐xylose preload with or without sitagliptin on gastric emptying, glucagon‐like peptide‐1, and postprandial glycemia in type 2 diabetes. Diabetes Care. 2013;36:1913‐1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Nakayama T, Nagai Y, Uehara Y, et al. Eating glutinous brown rice twice a day for 8 weeks improves glycemic control in Japanese patients with diabetes mellitus. Nutr Diabetes. 2017;7:e273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kappe C, Zhang Q, Nystrom T, Sjoholm A. Effects of high‐fat diet and the anti‐diabetic drug metformin on circulating GLP‐1 and the relative number of intestinal L‐cells. Diabetol Metab Syndr. 2014;6:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Pyra KA, Saha DC, Reimer RA. Prebiotic fiber increases hepatic acetyl CoA carboxylase phosphorylation and suppresses glucose‐dependent insulinotropic polypeptide secretion more effectively when used with metformin in obese rats. J Nutr. 2012;142:213‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Zhao L, Zhang F, Ding X, et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018;359:1151‐1156. [DOI] [PubMed] [Google Scholar]
- 105. Kuwata H, Okamoto S, Seino Y, et al. Relationship between deterioration of glycated hemoglobin‐lowering effects in dipeptidyl peptidase‐4 inhibitor monotherapy and dietary habits: retrospective analysis of Japanese individuals with type 2 diabetes. J Diabetes Investig. 2017;9(5):1153‐1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Eshghi SR, Bell GJ, Boule NG. Effects of aerobic exercise with or without metformin on plasma incretins in type 2 diabetes. Can J Diabetes. 2013;37:375‐380. [DOI] [PubMed] [Google Scholar]
- 107. Malin SK, Huang H, Mulya A, Kashyap SR, Kirwan JP. Lower dipeptidyl peptidase‐4 following exercise training plus weight loss is related to increased insulin sensitivity in adults with metabolic syndrome. Peptides. 2013;47:142‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Makam AN, Nguyen OK. An evidence‐based medicine approach to antihyperglycemic therapy in diabetes mellitus to overcome overtreatment. Circulation. 2017;135:180‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Gribble FM, Williams L, Simpson AK, Reimann F. A novel glucose‐sensing mechanism contributing to glucagon‐like peptide‐1 secretion from the GLUTag cell line. Diabetes. 2003;52:1147‐1154. [DOI] [PubMed] [Google Scholar]
- 110. Jang H‐J, Kokrashvili Z, Theodorakis MJ, et al. Gut‐expressed gustducin and taste receptors regulate secretion of glucagon‐like peptide‐1. Proc Natl Acad Sci USA. 2007;104:15069‐15074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Tolhurst G, Heffron H, Lam YS, et al. Short‐chain fatty acids stimulate glucagon‐like peptide‐1 secretion via the G‐protein‐coupled receptor FFAR2. Diabetes. 2012;61:364‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Almario RU, Buchan WM, Rocke DM, Karakas SE. Glucose‐lowering effect of whey protein depends upon clinical characteristics of patients with type 2 diabetes. BMJ Open Diabetes Res Care. 2017;5:e000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kappe C, Patrone C, Holst JJ, Zhang Q, Sjoholm A. Metformin protects against lipoapoptosis and enhances GLP‐1 secretion from GLP‐1‐producing cells. J Gastroenterol. 2013;48:322‐332. [DOI] [PubMed] [Google Scholar]
- 114. Zambrowicz B, Ogbaa I, Frazier K, et al. Effects of LX4211, a dual sodium‐dependent glucose cotransporters 1 and 2 inhibitor, on postprandial glucose, insulin, glucagon‐like peptide 1, and peptide tyrosine tyrosine in a dose‐timing study in healthy subjects. Clin Ther. 2013;35:1162‐1173 e1168. [DOI] [PubMed] [Google Scholar]
- 115. Zambrowicz B, Ding ZM, Ogbaa I, et al. Effects of LX4211, a dual SGLT1/SGLT2 inhibitor, plus sitagliptin on postprandial active GLP‐1 and glycemic control in type 2 diabetes. Clin Ther. 2013;35:273‐285 e277. [DOI] [PubMed] [Google Scholar]
- 116. Hamada Y, Nagasaki H, Fuchigami M, et al. The alpha‐glucosidase inhibitor miglitol affects bile acid metabolism and ameliorates obesity and insulin resistance in diabetic mice. Metabolism. 2013;62:734‐742. [DOI] [PubMed] [Google Scholar]
- 117. Zheng M‐Y, Yang J‐H, Shan C‐Y, et al. Effects of 24‐week treatment with acarbose on glucagon‐like peptide 1 in newly diagnosed type 2 diabetic patients: a preliminary report. Cardiovasc Diabetol. 2013;12:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Amagai M, Tsuchiya H, Chiba Y, et al. Incretin kinetics before and after miglitol in Japanese patients with late dumping syndrome. J Clin Med Res. 2017;9:879‐885. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are included within the manuscript.


