Summary
Objective
Firstly, to investigate whether polycystic ovary syndrome (PCOS) shows a continuum of severity with increasing number of phenotypic features comprising the Rotterdam criteria for PCOS and secondly, to explore relationships of these phenotypes to the circadian biomarkers, cortisol and melatonin.
Background
Studies characterizing the spectrum of PCOS subphenotypes give little emphasis to the distinction among women who manifest zero, one or two of the three phenotypic features comprising the Rotterdam criteria. The relationship of circadian biomarkers to PCOS phenotypes is unclear.
Design
Cross‐sectional study of 321 participants from 2011 to 2016 conducted at the National University Hospital (NUH), Singapore.
Participants
Participants included women who attended a health screen for NUH staff, volunteers from the university community, and women referred for possible PCOS from gynaecological clinics at NUH and KK Women's and Children's Hospital (Singapore).
Methods
All participants underwent a physical examination, ovarian ultrasound scan and follicular‐phase blood testing, and completed a health and lifestyle questionnaire.
Results
A significant positive linear trend in all clinical and biochemical characteristics of PCOS with increasing number of phenotypic features comprising the Rotterdam criteria. We observed a similar trend in serum cortisol and melatonin, two biomarkers of the circadian rhythm.
Conclusion
PCOS may not be an “all‐or‐none” condition, but rather a continuous spectrum. The positive relationship between number of PCOS criteria with melatonin and cortisol merits further investigation on the role of circadian biorhythms in the pathogenesis of PCOS.
Keywords: cortisol, melatonin, phenotypic spectrum, polycystic ovary syndrome
1. INTRODUCTION
Studies to elucidate the aetiology of polycystic ovary syndrome (PCOS) are complicated by variations in its definition. The strictest diagnostic criteria, proposed by an expert panel from the National Institute of Child Health and Human Development (NICHD) of the US National Institutes of Health, require the presence of both hyperandrogenism (clinical and/or biochemical) and oligo/anovulation.1 In 2003, a consensus meeting in Rotterdam also included another phenotypic feature: polycystic ovarian morphology, as measured by transvaginal ultrasound. The Rotterdam 2003 criteria for PCOS require the presence of two out of three of the following phenotypic features: (a) oligo/anovulation, (b) hyperandrogenism and (c) polycystic ovarian morphology.2 In 2009, the Androgen Excess and PCOS (AE‐PCOS) Society considered that hyperandrogenism, with its strong associations with both reproductive and metabolic abnormalities, is a key to the pathophysiology of PCOS. Hence, the AE‐PCOS criteria require the presence of hyperandrogenism for the diagnosis of PCOS, in addition to the evidence of ovarian dysfunction in the form of either oligo/anovulation or polycystic ovarian morphology on ultrasonography.3 However, in 2013, a guideline published based on expert consensus recommended the continued use of the Rotterdam criteria, which remain the most widely used.4
The Rotterdam criteria create four PCOS subphenotypes: oligomenorrhoea with hyperandrogenism (OA‐HA), oligomenorrhoea with polycystic ovarian morphology (OA‐PCOM), hyperandrogenism with polycystic ovarian morphology (HA‐PCOM) and all three features (OA‐HA‐PCOM). Hence, the introduction of the Rotterdam criteria for the diagnosis of PCOS in 2003 sparked a flurry of studies characterizing this spectrum of subphenotypes, focused mostly on comparing their risks of cardiometabolic diseases5, 6, 7 and obstetric and neonatal complications.8, 9, 10 Notably, little emphasis has been given to the distinction among women who manifest zero, one or two of the three phenotypic features comprising the Rotterdam criteria (PCOS features). In particular, it is unclear whether women with a single feature are more similar to those with none than to those with two features.
The aetiology of polycystic ovary syndrome (PCOS) has challenged reproductive scientists and clinicians since the syndrome was first described by Stein and Leventhal more than80 years ago.11 Cortisol and melatonin (N‐acetyl‐5‐methoxytryptamine) are two well‐recognized biomarkers of the circadian rhythm which are crucial for regulating sleep‐wake cycles. In humans, these hormones are secreted in a distinct diurnal pattern where cortisol peaks in the early morning12 and melatonin is highest in the early night.13 Few studies have been carried out to explore the potential roles of melatonin and cortisol in the pathogenesis of PCOS, and results of those studies have been inconsistent, with some reports of increased night‐time melatonin levels,14, 15 one study reporting higher mean values over 24 hours16 and another which reported higher morning but not night melatonin levels. One study found increased morning and night cortisol secretion in women with PCOS,17 yet another study reported no significant differences.18
We hypothesized that PCOS would show a continuum of severity according to the number of phenotypic features. Therefore, our primary aim was to evaluate the relationship between the androgenic, ovarian, pituitary and metabolic characteristics typically associated with PCOS with increasing number of PCOS features. Additionally, we were also interested in exploring the associations between circadian biomarkers and the phenotypic spectrum of PCOS.
2. METHODS
2.1. Study design, setting and population
This is a cross‐sectional study carried out at the National University Hospital (NUH), Singapore, from 2011 to 2016. We recruited women 21 to 45 years of age, including NUH staff who underwent an annual corporate health screen, volunteers from the university community, and cases referred from gynaecological clinics at NUH and KK Women's and Children's Hospital (KKH), Singapore, as possible cases of PCOS. Exclusion criteria were pregnancy, breastfeeding, usage of lipid‐lowering or diabetic medications and/or other medications known to affect reproductive function within 60 days of study entry, premature ovarian failure (previously diagnosed or with either of the following: FSH >25.8 IUL, oestrogen <70 pmol/L and/or AMH <0.6 pmol/L), hyperprolactinaemia (previously diagnosed or with prolactin >1000 mIU/L), congenital adrenal hyperplasia, adrenal tumours, androgen‐secreting tumours, thyroid disease, established disease, severe cardiovascular disease, or history of hysterectomy and/or oophorectomy.
A total of 395 women including staff from the health screen or volunteers from the university community (N = 208) and women referred for possible PCOS from NUH (N = 87) or KKH (N = 100) clinics completed a health and lifestyle questionnaire, underwent anthropometric evaluation, transvaginal ultrasonography of the ovaries, and blood sampling for androgenic and circadian biomarkers and other biochemical variables, between menstrual cycle days 2 to 5 at 7:30 to 8:30 am after an overnight fast. Women with eight or fewer menstrual cycles per year were assigned an arbitrary date for examination. After applying the exclusion criteria, the final study population consisted of 321 women, of which 163 women (51%) were recruited from the health screen and the university community and 158 (49%) were referred from the NUH or KKH gynaecology clinics Figure 1.
Figure 1.
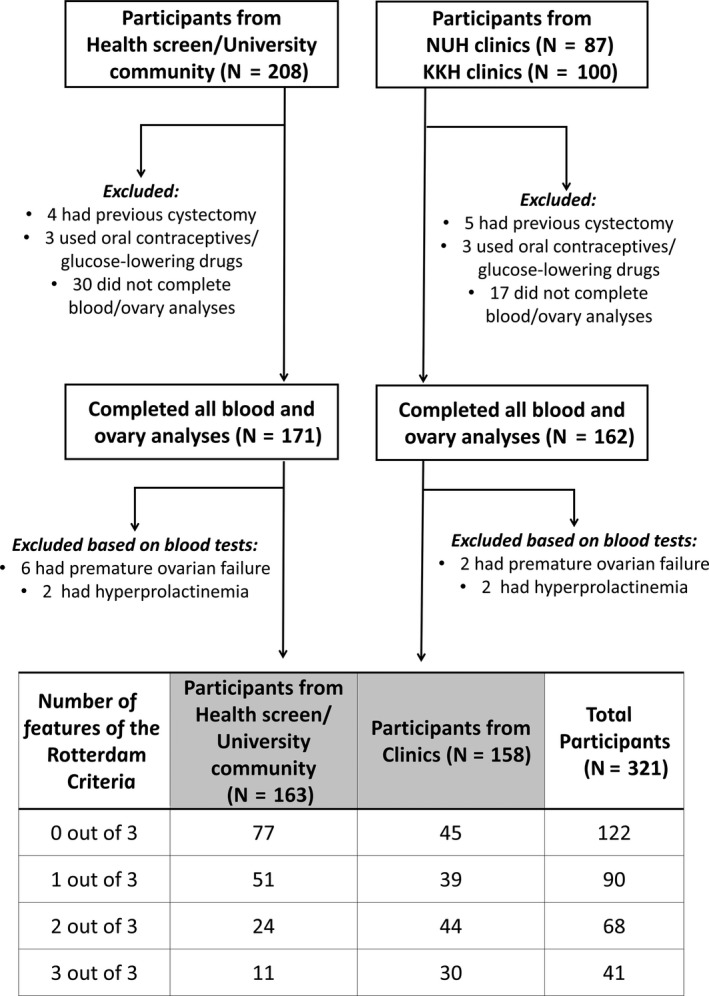
Flow chart of exclusion criteria, derivation of the final study population and classification by number of PCOS criteria
Participants were classified as having either zero, one, two or all three of the following phenotypic features of PCOS: hyperandrogenism, oligo/amenorrhoea or polycystic ovarian morphology. Hyperandrogenism was defined as having either one of the following: modified Ferriman‐Gallwey score (mFG) ≥5,19 androstenedione (ADT) ≥13.7 nmol/L, dehydroepiandrosterone‐sulphate (DHEAS) ≥8.30 mol/L, testosterone ≥1.89 nmol/L. Oligo/amenorrhoea was defined as having an average menstrual cycle length ≥35 days. Polycystic ovarian morphology was defined as having 22 or more follicles in either ovary, and/or increased ovarian volume (≥8.44 mL) in either ovary. Testosterone and ovarian thresholds for PCOS were obtained using sensitivity/specificity analyses and receiver operating characteristic curves that best predicted oligomenorrhoea/anovulation in a healthy non‐PCOS population.20 ADT and DHEAS were NUH laboratory thresholds for women.
2.2. Menstrual cycle length
Menstrual cycle length was classified into three categories for analysis: <25 days (short), 25‐34 days (normal) or ≥35 days (long).21
2.3. Hyperandrogenism
Assessment of hirsutism by modified Ferriman‐Gallwey (mFG) scoring and measurement of circulating testosterone, DHEAS, ADT and sex hormone‐binding globulin (SHBG) were performed as previously described.21, 22
2.4. Ovarian morphology
Morphometric analysis of the ovaries was performed by transvaginal ultrasonography as described previously.21 Women with at least one ovarian cyst or follicle exceeding a diameter of 10 mm, even after repeated evaluation in their following cycle, were excluded to eliminate the possibility of a spuriously large ovarian volume and endocrine effects from a developing dominant follicle. Derivation of antral follicle counts and ovarian volume were as described previously.21, 22
2.5. Anthropometric and metabolic variables
Physical measurements included height, weight, waist circumference and blood pressure. Glucose, insulin and lipids (cholesterol, triglycerides, high‐density lipoprotein [HDL] and low‐density lipoprotein [LDL]) were measured as described previously.21, 22 Body mass index (BMI), waist to hip ratio (WHR), the homoeostasis model assessment‐estimated insulin resistance (HOMA‐IR) and presence of metabolic syndrome were determined from those measurements as described previously.22
2.6. Other biochemical analysis
Prolactin, oestradiol, AMH, LH and FSH were measured using immunoassays, as described previously.21, 22 In addition, we investigated whether the number of PCOS phenotypic features was associated with serum cortisol and melatonin, two well‐recognized biomarkers of the circadian rhythm crucial for regulating sleep‐wake cycles. Serum melatonin and cortisol were measured by ELISA (IBL, Germany), according to the manufacturer's instructions.
Apart from AMH which was assayed weekly, and melatonin and cortisol which were batch‐analysed at the end of the study, all other biochemical analyses were performed within the same day of blood sampling. Biochemical analyses and assay performance characteristics are summarized in Table S1.
2.7. Health and lifestyle questionnaire
Study women were required to report on lifestyle factors that potentially influence circadian rhythms and reproductive health. Details of the questionnaire were as described previously.22 Briefly, lifestyle factors were dichotomized for shift work (yes/no), sleep length (<6 hours/≥6 hours), smoking (yes/no), exercise (regularly vs not), alcohol consumption (never or occasionally/once a week or more) and coffee consumption (<1 cup per day/≥1 cup per day). Stress was recorded on a scale of increasing magnitude ranging from 1 to 10. Additionally, participants were asked to state any previous medical history of depression, anxiety, stress or sleep disorders.
2.8. Statistical analysis
One‐way ANOVA with linear contrast and the Cochran‐Armitage chi‐squared test were used to analyse trends in continuous and categorical variables, respectively, among women with increasing number of PCOS features. Multivariable linear regression was used to adjust for confounding factors that could confound associations between biomarkers and other PCOS phenotypic characteristics and number of PCOS features. They included age, race, education, BMI, medical history of depression, anxiety, stress and sleep disorders, self‐perceived stress levels, and lifestyle factors including shift work, sleep length, exercise frequency, smoking, frequency of alcohol consumption and frequency of coffee consumption. The Cochran‐Armitage chi‐squared test was carried out in R, while all other statistical analyses used SPSS Version 20.
3. RESULTS
As shown in Figure 1, 122 (38%) of the 321 total participants had no phenotypic features of PCOS, 90 (28%) had one of the three features, 68 (21%) had two of the three features and 41 (13%) had all three features. Among women from the health screen and university community, 77 (47%) had no PCOS features, 51 (31%) had one, 24 (15%) had two and 11 (7%) had all three. Among women referred from the clinics, 45 (28%) had no PCOS features, 39 (25%) had one, 44 (28%) had two and 30 (19%) had all three. Of the 90 total participants who had only one of the three PCOS features, 29% had HA only, 23% had OA only and 47% had PCOM only. Of the 68 women who had two out of the three phenotypic features, 9% had HA‐OA, 53% had HA‐PCOM and 38% had OA‐PCOM.
Basic characteristics, lifestyle factors, and relevant medical history of the study women are shown in Table 1. Overall, in the total study sample of 321 women, the majority were Chinese (71%), had at least university education (56%), held executive or professional jobs (55%), did not do shift work (81%), had at least 6 hours of sleep (79%), did not exercise regularly (79%), did not smoke (92%) and consumed neither alcohol (97%) nor (71%) coffee frequently. Less than 10% of the total sample reported a history of depression, anxiety, stress and/or sleep disorders. A highly significant negative association was observed between age and number of PCOS phenotypic features (P < 0.001),women with all three features were 5.6 years younger than those with none (28.2 ± 3.8 vs 33.8 ± 5.1 years, respectively).
Table 1.
Basic characteristics of the study population by number of phenotypic features comprising the Rotterdam criteria for PCOS
| Total (N = 321) | Number of PCOS features | P‐trend | ||||
|---|---|---|---|---|---|---|
| 0 out 3 (N = 122) | 1 out of 3 (N = 90) | 2 out of 3 (N = 68) | 3 out of 3 (N = 41) | |||
| Basic characteristics | ||||||
| Age | 31.6 ± 5.0 | 33.8 ± 5.1 | 31.3 ± 4.7 | 30.0 ± 4.0 | 28.2 ± 3.8 | <0.001 |
| Race | ||||||
| Chinese | 228 (71) | 91 (75) | 65 (72) | 48 (71) | 24 (59) | 0.104 |
| Non‐Chinese | 93 (29) | 31 (25) | 25 (28) | 20 (29) | 17 (42) | |
| Education | ||||||
| Below university | 180 (56) | 60 (49) | 38 (42) | 23 (34) | 20 (49) | 0.354 |
| University | 141 (44) | 62 (51) | 52 (58) | 45 (66) | 21 (51) | |
| Occupation | ||||||
| Unemployed | 26 (8) | 9 (8) | 9 (10) | 2 (3) | 6 (15) | 0.999a |
| Exec/Professional | 176 (55) | 67 (55) | 49 (54) | 39 (57) | 21 (51) | |
| Non‐Exec/Professional | 119 (37) | 46 (38) | 32 (36) | 27 (40) | 14 (34) | |
| Shift work | ||||||
| No | 260 (81) | 102 (84) | 68 (76) | 57 (84) | 33 (81) | 0.832 |
| Yes | 61 (19) | 20 (16) | 22 (24) | 11 (16) | 8 (19) | |
| Sleep length | ||||||
| ≥ 6 h | 253 (79) | 93 (76) | 78 (87) | 53 (78) | 29 (71) | 0.621 |
| <6 h | 68 (21) | 29 (24) | 12 (13) | 15 (22) | 12 (29) | |
| Exercise frequency | ||||||
| Regular | 69 (21) | 22 (18) | 26 (29) | 12 (18) | 9 (22) | 0.811 |
| Non‐regular | 252 (79) | 100 (82) | 64 (71) | 56 (82) | 32 (78) | |
| Smoker | ||||||
| No | 297 (92) | 114 (93) | 84 (93) | 62 (91) | 37 (90) | 0.461 |
| Yes | 24 (8) | 8 (7) | 6 (7) | 6 (9) | 4 (10) | |
| Alcohol frequency | ||||||
| Never/occasionally | 313 (98) | 119 (98) | 88 (98) | 65 (96) | 40 (98) | 0.715 |
| Once a week or more | 9 (3) | 3 (3) | 2 (2) | 3 (4) | 1 (2) | |
| Coffee frequency | ||||||
| <1 cup per day | 228 (71) | 87 (71) | 63 (70) | 51 (75) | 27 (66) | 0.836 |
| ≥ 1 cup per day | 93 (29) | 35 (28) | 27 (30) | 17 (25) | 14 (34) | |
| Self‐perceived stress levels | 5.3 ± 1.9 | 5.2 ± 1.8 | 5.2 ± 1.8 | 5.5 ± 1.9 | 5.2 ± 2.0 | 0.624 |
| Medical history | ||||||
| Depression | ||||||
| No | 313 (98) | 120 (98) | 88 (98) | 65 (96) | 40 (98) | 0.464 |
| Yes | 8 (3) | 2 (2) | 2 (2) | 3 (4) | 1 (2) | |
| Anxiety | ||||||
| No | 312 (98) | 120 (98) | 88 (98) | 66 (97) | 38 (93) | 0.448 |
| Yes | 9 (3) | 2 (2) | 2 (2) | 2 (3) | 3 (7) | |
| Stress | ||||||
| No | 295 (92) | 113 (93) | 82 (91) | 62 (91) | 38 (93) | 0.938 |
| Yes | 26 (8) | 9 (7) | 8 (9) | 6 (9) | 3 (7) | |
| Sleep disorders | ||||||
| No | 308 (96) | 116 (95) | 88 (98) | 66 (97) | 38 (93) | 0.827 |
| Yes | 13 (4) | 6 (5) | 2 (2) | 2 (3) | 3 (7) | |
Data are presented as n (%) or mean ± SD.
Frequencies may not add up due to rounding off.
Average of left and right ovaries.
For analysis of trend occupation is dichotomized to Executive/Professional and Unemployed/Non‐executive/Professional.
Table 2 compares androgenic, ovarian, metabolic and pituitary variables among women having none, one, two or three phenotypic features comprising the Rotterdam criteria. Remarkably, all variables that characterize PCOS, including androgenic hormones (mFG score, testosterone, ADT, DHEAS), ovarian morphometry (follicle counts, ovarian volume) and long menstrual cycles, were positively associated with the number of PCOS features. In contrast, no significant associations were observed between lifestyle factors or medical history variables and number of PCOS features. Other variables associated with PCOS, including AMH, LH, BMI, insulin and HOMA‐IR, also showed a highly significant linear trend with an increasing number of PCOS features.
Table 2.
Clinical and biochemical manifestations of PCOS by number of phenotypic features comprising the Rotterdam criteria
| Total (N = 321) | Number of PCOS features | P‐trend | ||||
|---|---|---|---|---|---|---|
| 0 out 3 (N = 122) | 1 out of 3 (N = 90) | 2 out of 3 (N = 68) | 3 out of 3 (N = 41) | |||
| Androgenic characteristics | ||||||
| Acne score | 1.8 ± 3.7 | 1.5 ± 3.1 | 2.2 ± 5.1 | 1.8 ± 2.6 | 2.0 ± 3.0 | 0.626 |
| mFG score | 1.6 ± 2.4 | 1.0 ± 1.2 | 1.4 ± 2.2 | 2.3 ± 2.8 | 2.7 ± 3.6 | <0.001 |
| Testosterone (nmol/L) | 1.37 ± 0.72 | 1.02 ± 0.38 | 1.19 ± 1.65 | 1.65 ± 0.81 | 2.36 ± 0.57 | <0.001 |
| Androstenedione (nmol/L) | 8.29 ± 6.36 | 6.72 ± 3.04 | 7.50 ± 3.94 | 8.80 ± 3.88 | 13.96 ± 2.18 | <0.001 |
| DHEAS (µmol/L) | 5.42 ± 2.17 | 4.70 ± 1.52 | 5.35 ± 2.18 | 6.07 ± 2.46 | 6.63 ± 2.51 | <0.001 |
| SHBG (nmol/L) | 55.6 ± 31.1 | 62.4 ± 31.9 | 54.7 ± 26.2 | 53.5 ± 35.9 | 40.6 ± 24.5 | <0.001 |
| Free Androgen Index | 3.67 ± 3.88 | 2.20 ± 1.96 | 2.76 ± 2.38 | 2.88 ± 4.67 | 8.45 ± 5.32 | <0.001 |
| Ovarian characteristics | ||||||
| Antral follicle counta | 18.5 ± 12.2 | 10.2 ± 4.9 | 17.6 ± 8.2 | 25.3 ± 10.3 | 34.0 ± 16.0 | <0.001 |
| Ovarian volume (mL)a | 5.76 ± 2.79 | 4.02 ± 1.18 | 5.54 ± 1.84 | 7.14 ± 2.77 | 9.12 ± 3.66 | <0.001 |
| Anti‐Müllerian hormone (pmol/L) | 48.0 ± 36.5 | 22.4 ± 15.6 | 43.4 ± 26.6 | 71.4 ± 33.0 | 94.9 ± 35.6 | <0.001 |
| Menstrual cycle length | ||||||
| <25 days | 15 (5) | 8 (7) | 4 (4) | 3 (4) | 0 (0) | <0.001b |
| 25 to 34 days | 212 (66) | 114 (93) | 65 (72) | 33 (49) | 0 (0) | |
| ≥ 35 days | 94 (29) | 0 (0) | 21 (23) | 32 (47) | 41 (100) | |
| Metabolic characteristics | ||||||
| BMI | 23.4 ± 5.0 | 22.8 ± 3.7 | 23.2 ± 4.5 | 23.3 ± 5.1 | 25.7 ± 7.7 | 0.001 |
| WHR | 0.79 ± 0.06 | 0.79 ± 0.06 | 0.78 ± 0.06 | 0.79 ± 0.07 | 0.79 ± 0.07 | 0.750 |
| Insulin (mu/L) | 8.33 ± 7.93 | 7.62 ± 5.60 | 6.46 ± 3.70 | 8.79 ± 6.75 | 13.9 ± 16.3 | <0.001 |
| Glucose (mmol/L) | 4.77 ± 0.63 | 4.74 ± 0.45 | 4.66 ± 0.43 | 4.92 ± 1.03 | 4.81 ± 0.54 | 0.199 |
| HOMA‐IR | 1.82 ± 1.96 | 1.63 ± 1.34 | 1.36 ± 0.84 | 1.97 ± 1.63 | 3.15 ± 4.15 | <0.001 |
| Systolic BP (mm/Hg) | 100 ± 25 | 101 ± 24 | 102 ± 24 | 94 ± 27 | 101 ± 25 | 0.422 |
| Diastolic BP (mm/Hg) | 60 ± 16 | 61 ± 16 | 62 ± 15 | 58 ± 17 | 59 ± 16 | 0.331 |
| Triglycerides (mmol/L) | 1.00 ± 0.73 | 0.93 ± 0.46 | 1.03 ± 1.09 | 1.02 ± 0.50 | 1.10 ± 0.68 | 0.235 |
| Cholesterol (mmol/L) | 4.83 ± 0.81 | 4.77 ± 0.80 | 4.86 ± 0.84 | 4.82 ± 0.78 | 4.99 ± 0.87 | 0.168 |
| HDL (mmol/L) | 1.48 ± 0.36 | 1.48 ± 0.36 | 1.51 ± 0.37 | 1.45 ± 0.33 | 1.47 ± 0.39 | 0.695 |
| LDL (mmol/L) | 2.91 ± 0.76 | 2.85 ± 0.73 | 2.94 ± 0.85 | 2.91 ± 0.72 | 3.02 ± 0.65 | 0.261 |
| Metabolic syndrome | ||||||
| No | 281 (90) | 112 (92) | 82 (93) | 58 (89) | 29 (76) | 0.198 |
| Yes | 32 (10) | 10 (8) | 6 (7) | 7 (11) | 9 (24) | |
| Pituitary characteristics | ||||||
| LH (IU/L) | 5.75 ± 4.25 | 4.29 ± 2.00 | 4.42 ± 2.33 | 6.27 ± 3.96 | 11.9 ± 6.50 | <0.001 |
| FSH (IU/L) | 7.49 ± 2.34 | 8.21 ± 2.46 | 7.48 ± 2.59 | 6.70 ± 1.62 | 6.69 ± 1.67 | <0.001 |
| Prolactin (mIU/L) | 269 ± 137 | 262 ± 123 | 276 ± 14.7 | 282 ± 151 | 269 ± 137 | 0.487 |
Data are presented as n (%) or mean ±SD.
Frequencies may not add up due to rounding off.
BMI, body mass index; BP, blood pressure; DHEAS, dehydroepiandrosterone‐sulphate; FSH, follicle‐stimulating hormone; mFG, modified Ferriman‐Gallwey; LH, luteinizing hormone; SHBG, sex hormone‐binding globulin; WHR, waist‐hip ratio
Average of left and right ovaries.
For analysis of trend menstrual cycle length dichotomized to <35 days and ≥35 days.
We observed a highly significant positive linear trend in both melatonin (P < 0.001) and cortisol levels (P = 0.003) with an increasing number of PCOS features. Similar results were obtained when we restricted the analysis to women from the health screen and university community (ie, excluding those recruited from the gynaecology clinics), although the differences did not achieve statistical significance, likely owing to the reduced sample size Table 3.
Table 3.
Comparison of melatonin and cortisol levels with increasing number of phenotypic features of the Rotterdam criteria
| Number of PCOS features | N | Mean ±SD | Unadjusted mean difference | 95% CI | Adjusteda mean difference | 95% CI |
|---|---|---|---|---|---|---|
| Melatonin | ||||||
| 0 out of 3 | 122 | 37.5 ± 26.1 | ‐ | ‐ | ‐ | ‐ |
| 1 out of 3 | 90 | 41.7 ± 28.5 | 4.11 | ‐3.59, 11.8 | 2.42 | ‐5.73, 10.6 |
| 2 out of 3 | 68 | 44.4 ± 26.0 | 6.87 | ‐1.52, 15.3 | 3.32 | ‐5.65, 12.3 |
| 3 out of 3 | 41 | 56.0 ± 35.9 | 18.4 | 8.43, 28.4 | 16.0 | 4.85, 27.2 |
| P‐trend: <0.001 | ||||||
| Cortisol | ||||||
| 0 out of 3 | 122 | 103 ± 35 | ‐ | ‐ | ||
| 1 out of 3 | 90 | 107 ± 40 | 3.49 | ‐7.21, 14.2 | 0.784 | ‐10.3, 11.9 |
| 2 out of 3 | 68 | 108 ± 44 | 4.86 | ‐6.79, 16.5 | ‐0.174 | ‐12.4, 12.0 |
| 3 out of 3 | 41 | 125 ± 39 | 21.2 | 7.36, 35.1 | 17.5 | 2.27, 32.6 |
| P‐trend: 0.003 | ||||||
Adjusted for age, BMI, education, medical history of depression, anxiety, stress or sleep disorders, shift work, sleep duration, exercise frequency, smoking, alcohol consumption, coffee consumption and self‐perceived stress levels.
No significant associations between other cardiometabolic outcomes (blood pressure, serum lipid levels or frequency of metabolic syndrome) and the number of PCOS features were observed.
4. DISCUSSION
We observed a highly significant linear trend between the number of phenotypic features comprising the Rotterdam criteria and every clinical and biochemical manifestation of PCOS we examined, including androgenic characteristics (mFG score, testosterone, ADT, DHEAS, free androgen index), ovarian measures (antral follicle count, ovarian volume) and oligomenorrhea. AMH and LH, which are not features included in the Rotterdam criteria but play a role in the pathophysiology of PCOS, also showed a strong positive relationship with the number of PCOS features. Obesity and insulin resistance (as measured by BMI and HOMA‐IR, respectively), which are often observed in women with PCOS, also showed a positive linear trend between the increasing number of PCOS features. Age exhibited the opposite linear trend, consistent with previous reports that the reproductive features of PCOS improve with age.23, 24 Acne score was not associated with number of PCOS features, suggesting that at least in the Asian ethnic context of Singapore, acne may be more reflective of non‐endocrine (perhaps genetic) factors than of hyperandrogenism.
The phenotypic severity of PCOS remains a subject of much debate. PCOS is a reproductive disorder associated with increased metabolic risk, and infertility is therefore a logical candidate for assessing severity. Studies on fertility outcomes across the PCOS phenotypic spectrum are scarce, although a handful of studies have reported on pregnancy and neonatal complications across the four Rotterdam phenotypes.8, 9, 10 Current classification of PCOS severity is more often based on long‐term metabolic risks.25 The strong association we observed between the number of PCOS features and severity of PCOS (as measured by androgenic, ovarian and menstrual characteristics, as well as BMI, insulin and HOMA‐IR) suggests that this continuous measure may better represent the phenotypic spectrum of PCOS than the traditional dichotomous classification.
Additionally, our study is the first to show a strong positive relationship between number of PCOS features and two biomarkers of circadian rhythm, melatonin and cortisol. In particular, the most severe group (manifesting all three PCOS features) had significantly higher mean melatonin and cortisol levels compared with women with none of the features, even after adjustment for potential confounders. In humans, these hormones are secreted in a distinct diurnal pattern: cortisol peaks in the early morning12 and melatonin peaks in the early night.13 Few studies have been carried out to explore the potential roles of melatonin and cortisol in the pathogenesis of PCOS, and results of those studies have been inconsistent.14, 15, 16, 17, 18 Since morning melatonin levels are expected to be low at daylight, our finding of high melatonin levels in the morning suggests a smaller night‐day difference, possibly due to perturbed circadian rhythms in women with PCOS. Unlike melatonin, which is principally regulated by photic stimuli and light‐dark cycles, glucocorticoid secretion is low in the late afternoon and evening but rises markedly when the subject awakens and becomes active. Cortisol secretion rises steadily through the second half of the night, but exhibits a further marked increase during the first hour after waking from sleep.26, 27 This response can be heightened among individuals experiencing job stress, overload, and low self‐esteem.28 In this respect, elevated cortisol in our PCOS subjects may be a sign of stress‐induced hyperactivity of the hypothalamic‐pituitary‐adrenal (HPA) axis, which may accentuate adrenal androgen excess and thereby lead to the more severe PCOS phenotypes we observed with higher cortisol levels. Taken together, the high morning melatonin and cortisol levels we observed in PCOS may be a sign of disrupted circadian rhythms and stress. Further studies involving multiple daily blood sampling would help to confirm the possible circadian rhythm disruption in PCOS women.
An important strength of our study is that all participants underwent standardized tests and were properly assessed for the widely accepted Rotterdam criteria. One limitation of our study, however, was an insufficient sample size to identify which of the PCOS features—HA, OA or PCOM—made a larger contribution to the reproductive features and metabolic outcomes we studied. Larger studies across different societies and cultures, and studies on the extent to which fertility outcomes are associated with the number of PCOS features would shed additional light on the PCOS spectrum.
CONFLICT OF INTEREST
The authors have no conflict of interests to declare.
AUTHOR CONTRIBUTION
E. L. Yong was the principal investigator involved in directing the implementation of the study. All authors contributed intellectually to the conception and design of study, and interpretation of analyses. A. J. R. Lim, M. S. Kramer and E. L. Yong contributed to the drafting and critical revision of this article. Statistical analyses were conducted by A. J. R. Lim. The final version of this article has been approved by all authors.
ETHICS STATEMENT
The study protocol was approved by the National Healthcare Group Domain Specific Review Board, Singapore. Informed written consent was obtained from all participants.
Supporting information
ACKNOWLEDGEMENTS
The authors wish to thank the women who participated in this study, and we would also like to express our gratitude to the following staff members at the National University of Singapore: S.E. Chua, S.K. Eng and P.C. Poon for study coordination; E.M. Tan and the clinicians at NUH and KKH for assistance with data acquisition. This study was supported by the Bedside & Bench Grant from the Singapore National Medical Research Council (NMRC/BnB/0007c/2013).
Lim AJR, Indran IR, Kramer MS, Yong E‐L. Phenotypic spectrum of polycystic ovary syndrome and their relationship to the circadian biomarkers, melatonin and cortisol. Endocrinol Diab Metab. 2019;2:e00047 10.1002/edm2.47
DATA ACCESSIBILITY
The data supporting the findings of this study are available within the article.
REFERENCES
- 1. Zawadski J, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach In: Dunaif A, Givens J, Haseltine F, Merriam G, eds. Polycystic Ovary Syndrome. Boston, MA: Blackwell Scientific Publications; 1992. [Google Scholar]
- 2. Rotterdam EA‐SPCWG . Revised 2003 consensus on diagnostic criteria and long‐term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19‐25. [DOI] [PubMed] [Google Scholar]
- 3. Azziz R, Carmina E, Dewailly D et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91(2):456‐488. [DOI] [PubMed] [Google Scholar]
- 4. Legro RS, Arslanian SA, Ehrmann DA et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(12):4565‐4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Daan NM, Louwers YV, Koster MP et al. Cardiovascular and metabolic profiles amongst different polycystic ovary syndrome phenotypes: who is really at risk? Fertil Steril. 2014;102(5):1444‐1451.e1443. [DOI] [PubMed] [Google Scholar]
- 6. Shroff R, Syrop CH, Davis W, Van Voorhis BJ, Dokras A. Risk of metabolic complications in the new PCOS phenotypes based on the Rotterdam criteria. Fertil Steril. 2007;88(5):1389‐1395. [DOI] [PubMed] [Google Scholar]
- 7. Zhang HY, Zhu FF, Xiong J, Shi XB, Fu SX. Characteristics of different phenotypes of polycystic ovary syndrome based on the Rotterdam criteria in a large‐scale Chinese population. BJOG. 2009;116(12):1633‐1639. [DOI] [PubMed] [Google Scholar]
- 8. de Wilde MA, Lamain‐de Ruiter M, Veltman‐Verhulst SM et al. Increased rates of complications in singleton pregnancies of women previously diagnosed with polycystic ovary syndrome predominantly in the hyperandrogenic phenotype. Fertil Steril. 2017;108(2):333‐340. [DOI] [PubMed] [Google Scholar]
- 9. Naver KV, Grinsted J, Larsen SO et al. Increased risk of preterm delivery and pre‐eclampsia in women with polycystic ovary syndrome and hyperandrogenaemia. BJOG. 2014;121(5):575‐581. [DOI] [PubMed] [Google Scholar]
- 10. Palomba S, Falbo A, Russo T, Tolino A, Orio F, Zullo F. Pregnancy in women with polycystic ovary syndrome: the effect of different phenotypes and features on obstetric and neonatal outcomes. Fertil Steril. 2010;94(5):1805‐1811. [DOI] [PubMed] [Google Scholar]
- 11. Stein I, Leventhal M. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol. 1935;29:181‐191. [Google Scholar]
- 12. Marcheva B, Ramsey KM, Peek CB, Affinati A, Maury E, Bass J. Circadian clocks and metabolism. Handb Exp Pharmacol. 2013;(217):127‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gooley JJ, Chamberlain K, Smith KA et al. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J Clin Endocrinol Metab. 2011;96(3):E463–E472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jain P, Jain M, Haldar C, Singh TB, Jain S. Melatonin and its correlation with testosterone in polycystic ovarian syndrome. J Hum Reprod Sci. 2013;6(4):253‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shreeve N, Cagampang F, Sadek K et al. Poor sleep in PCOS; is melatonin the culprit? Hum Reprod. 2013;28(5):1348‐1353. [DOI] [PubMed] [Google Scholar]
- 16. Luboshitzky R, Qupti G, Ishay A, Shen‐Orr Z, Futerman B, Linn S. Increased 6‐sulfatoxymelatonin excretion in women with polycystic ovary syndrome. Fertil Steril. 2001;76(3):506‐510. [DOI] [PubMed] [Google Scholar]
- 17. Invitti C, De Martin M, Delitala G, Veldhuis JD, Cavagnini F. Altered morning and nighttime pulsatile corticotropin and cortisol release in polycystic ovary syndrome. Metabolism. 1998;47(2):143‐148. [DOI] [PubMed] [Google Scholar]
- 18. Terzieva DD, Orbetzova MM, Mitkov MD, Mateva NG. Serum melatonin in women with polycystic ovary syndrome. Folia Med (Plovdiv). 2013;55(2):10‐15. [DOI] [PubMed] [Google Scholar]
- 19. Zhao X, Ni R, Li L et al. Defining hirsutism in Chinese women: a cross‐sectional study. Fertil Steril. 2011;96(3):792‐796. [DOI] [PubMed] [Google Scholar]
- 20. Indran IR, Huang Z, Khin LW, Chan J, Viardot‐Foucault V, Yong EL. Simplified 4‐item criteria for polycystic ovary syndrome: a bridge too far?. Clin Endocrinol (Oxf) 2018;89(2):202‐211. [DOI] [PubMed] [Google Scholar]
- 21. Zhu R, Lee BH, Huang Z et al. Antimullerian hormone, antral follicle count and ovarian volume predict menstrual cycle length in healthy women. Clin Endocrinol (Oxf). 2016;84(6):870‐877. [DOI] [PubMed] [Google Scholar]
- 22. Lim AJ, Huang Z, Chua SE, Kramer MS, Yong EL. Sleep duration, exercise, shift work and polycystic ovarian syndrome‐related outcomes in a healthy population: a cross‐sectional study. PLoS ONE. 2016;11(11):e0167048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carmina E, Campagna AM, Lobo RA. A 20‐year follow‐up of young women with polycystic ovary syndrome. Obstet Gynecol. 2012;119(2 Pt 1):263‐269. [DOI] [PubMed] [Google Scholar]
- 24. Panidis D, Tziomalos K, Macut D et al. Cross‐sectional analysis of the effects of age on the hormonal, metabolic, and ultrasonographic features and the prevalence of the different phenotypes of polycystic ovary syndrome. Fertil Steril. 2012;97(2):494‐500. [DOI] [PubMed] [Google Scholar]
- 25. Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370(9588):685‐697. [DOI] [PubMed] [Google Scholar]
- 26. Clow A, Thorn L, Evans P, Hucklebridge F. The awakening cortisol response: methodological issues and significance. Stress. 2004;7(1):29‐37. [DOI] [PubMed] [Google Scholar]
- 27. Steptoe A. Cortisol awakening response In: Fink G, ed. Encyclopedia of Stress (2 ed., pp. 649–653). Oxford, UK: Oxford Academic Press; 2000. [Google Scholar]
- 28. Stalder T, Kirschbaum C, Kudielka BM et al. Assessment of the cortisol awakening response: expert consensus guidelines. Psychoneuroendocrinology. 2016;63:414–432. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available within the article.


