Abstract
Background
Development of oligonucleotide probes facilitates chromosome identification via fluorescence in situ hybridization (FISH) in many organisms.
Results
We report a high throughput and economical method of chromosome identification based on the development of a dye solution containing 2 × saline-sodium citrate (SSC) and oligonucleotide probes. Based on the concentration, staining time, and sequence effects of oligonucleotides, an efficient probe dye of peanut was developed for chromosome identification. To validate the effects of this solution, 200 slides derived from 21 accessions of the cultivated peanut and 30 wild Arachis species were painted to identify Arachis genomes and establish karyotypes. The results showed that one jar of dye could be used to paint 10 chromosome preparations and recycled at least 10 times to efficiently dye more than 100 slides. The A, B, K, F, E, and H genomes showed unique staining karyotype patterns and signal colors.
Conclusions
Based on the karyotype patterns of Arachis genomes, we revealed the relationships among the A, B, K, F, E, and H genomes in genus Arachis, and demonstrated the potential for adoption of this oligonucleotide dye solution in practice.
Electronic supplementary material
The online version of this article (10.1186/s13007-019-0451-7) contains supplementary material, which is available to authorized users.
Keywords: Arachis species, Oligonucleotide dye solution, FISH, Karyotype
Background
The chromosome is a carrier of genetic materials, and plays important roles in genetic transmission, recombination, and modification. Thus, the development of chromosome identification technology is important for a better understanding of chromosome structure and function. Previously, both chromosome banding and fluorescence in situ hybridization (FISH) have considerably facilitated chromosome identification in many organisms [1, 2]. However, both have disadvantages, as they are time-consuming and exhibit low efficiency. The recent development of single-strand oligonucleotide FISH (SSON FISH) has demonstrated its efficiency as a powerful new tool [3–5] that has been successfully used in humans [3], Drosophila [6], cucumber [7], wheat [5, 8, 9], peanut [10], rye [11, 12], and maize [13].
In comparison with conventional FISH, SSON FISH has not only produced signals in denaturing chromosomes but also in non-denaturing chromosomes [14]. To date, several SSONs have been developed for non-denaturing FISH (ND-FISH) in wheat, rye, barley, and maize [4, 5, 13, 15]. This means that many SSONs possibly have the ability to invade double stranded DNA in chromosomes [4]. Interestingly, Du et al. [5] found that some SSONs even at very low concentrations, as low as 0.01 ng/µL of (GAA)10 for example, could still produce clear signals. This is indicative of the efficient length and concentration of some SSONs, which also makes it possible to develop a type of SSON dye solution with which chromosomes can be painted.
In this study, based on concentration, staining time, and sequence analyses, efficient SSONs were identified and used to develop a SSON dye solution. An efficient method of chromosome identification was thus developed and validated by analyzing Arachis accessions. As a result, all genomes were clearly distinguished, and their composition and relationships were revealed, indicating the potential of this method for chromosome identification in peanut and other species.
Materials and methods
Plant materials
Twenty-one accessions of cultivated peanut, 30 wild species, and one unknown wild species were used in this study. All cultivated peanut accessions and five wild species (Zw64, Zw65, Zw68, Zw70, and Zw74) were obtained from the Industrial Crops Research Institute, Henan Academy of Agricultural Sciences. The other wild species were kindly provided by the Plant Genetic Resources Conservation Unit, Griffin, GA, USA. The accession numbers, provenance, section, and genome of each plant material are listed in Table 1.
Table 1.
Arachis accessions used in this study
| Accession no. | Plant introduction (PI) no. | Species/variety name | Provenance | Section/type | Genome | References |
|---|---|---|---|---|---|---|
| SLH | Silihong | Jilin, China | var. fastigiata | AhyAhyBhyBhy | ||
| N477 | PI240560 | ND | South Africa | var. hypogae | AhyAhyBhyBhy | |
| N602 | PI471954 | ND | Zimbabwe | var. vulgaris | AhyAhyBhyBhy | |
| N606 | Jiyou4hao | Hebei, China | var. hypogaea | AhyAhyBhyBhy | ||
| N610 | Guihuahong95 | Guangxi, China | var. fastigiata | AhyAhyBhyBhy | ||
| N613 | PI482189 | ND | Zimbabwe | var. vulgaris | AhyAhyBhyBhy | |
| N614 | Nanyanghuasheng | Henan, China | var. hirsuta | AhyAhyBhyBhy | ||
| N618 | PI468250 | ND | ND | var. fastigiata | AhyAhyBhyBhy | |
| N622 | PI429420 | ND | Zimbabwe | var. fastigiata | AhyAhyBhyBhy | |
| N640 | Lougezhuangbanman | Shandong, China | var. hypogaea | AhyAhyBhyBhy | ||
| N642 | Xingshanzihuasheng | Hubei, China | var. hypogaea | AhyAhyBhyBhy | ||
| N644 | PI290536 | ND | India | var. hypogaea | AhyAhyBhyBhy | |
| N646 | PI295250 | ND | Israel | var. hypogaea | AhyAhyBhyBhy | |
| N651 | Fushandalidun | Shandong, China | var. hypogaea | AhyAhyBhyBhy | ||
| N654 | PI461434 | ND | China | var. vulgaris | AhyAhyBhyBhy | |
| N656 | Beijingdalidun | Beijing, China | var. hypogaea | AhyAhyBhyBhy | ||
| N658 | Dongmingbenhuasheng | Henan, China | var. hypogaea | AhyAhyBhyBhy | ||
| N659 | PI290566 | ND | India | var. hypogaea | AhyAhyBhyBhy | |
| N666 | PI355271 | ND | Mexico | var. hypogaea | AhyAhyBhyBhy | |
| N667 | PI259851 | ND | Malawi | var. hypogaea | AhyAhyBhyBhy | |
| N689 | Yuhua9327 | Henan, China | var. hypogaea | AhyAhyBhyBhy | ||
| Zw02 | PI 666101 | Arachis trinitensis | Trinidad, Bolivia | Arachis | FtrFtr | Robledo and Seijo [17] |
| Zw03 | Grif 14534 | A. simpsonii | Mato Grosso, Brazil | Arachis | AsiAsi | Robledo et al. [16] |
| Zw04 | PI 468155 | A. paraguariensis | Aquidauana, Brazil | Erectoides | EpaEpa | Valls and Simpson [22] |
| Zw07 | PI 263393 | A. monticola | Sao Paulo, Brazil | Arachis | AmoAmoBmoBmo | Robledo et al. [16] |
| Zw12 | PI 666103 | A. valida | Brazil | Arachis | BvaBva | Robledo and Seijo [17] |
| Zw13 | PI 476003 | A. cruziana | Santa Cruz, Bolivia | Arachis | KcrKcr | Robledo and Seijo [17] |
| Zw14 | PI 604844 | A. archeri | Brazil | Erectoides | EarEar | Krapovickas and Gregory [21] |
| Zw15 | PI 674407 | A. microsperma | Concepcion, Paraguay | Arachis | AmiAmi | Krapovickas and Gregory [21] |
| Zw16 | PI 468337 | A. magna | ND | Arachis | BmaBma | Robledo and Seijo [17] |
| Zw17 | PI 666092 | A. lutescens | Mato Grosso, Brazil | Exteanervosae | ExluExlu | Krapovickas and Gregory [21] |
| Zw18 | Grif 7571 | A. kuhlmannii | Brazil | Arachis | AkuAku | Robledo et al. [16] |
| Zw19 | PI 468330 | A. kempff-mercadoi | Portachuelo, Bolivia | Arachis | AkeAke | Robledo et al. [16] |
| Zw20 | PI 475882 | A. duranensis | El Tunal, Argentina | Arachis | AduAdu | Robledo et al. [16] |
| Zw21 | Grif 7682 | A. hoehnei | Brazil | Arachis | BhoBho | Krapovickas and Gregory [21] |
| Zw22 | PI 475877 | A. benensis | Trinidad, Bolivia | Arachis | FbeFbe | Robledo and Seijo [17] |
| Zw24 | PI 497262 | A. duranensis | Salta, Argentina | Arachis | AduAdu | Robledo et al. [16] |
| Zw39 | PI 475844 | A. duranensis | Yacuiba, Bolivia | Arachis | AduAdu | Robledo et al. [16] |
| Zw48 | PI 468200 | A. duranensis | Rio Perico, Argentina | Arachis | AduAdu | Robledo et al. [16] |
| Zw53 | PI 468322 | A. ipaensis | Santa Cruz, Bolivia | Arachis | BipBip | Robledo and Seijo [17] |
| Zw54 | PI 468150 | A. hoehnei | BaiaVermelho, Brazil | Arachis | BhoBho | Krapovickas and Gregory [21] |
| Zw55 | PI 219823 | A. duranensis | Argentina | Arachis | AduAdu | Robledo et al. [16] |
| Zw56 | PI 298636 | A. villosa | Argentina | Arachis | AviAvi | Robledo et al. [16] |
| Zw57 | PI 276235 | A. diogoi | Paraguay | Arachis | AdiAdi | Robledo et al. [16] |
| Zw61 | PI 468178 | A. stenophylla | Porto Murtinho, Brazil | Erectoides | EstEst | Krapovickas and Gregory [21] |
| Zw62 | PI 468162 | A. benthamii | Rio Verde, Brazil | Erectoides | EbeEbe | Krapovickas and Gregory [21] |
| Zw64 | A. batizocoi | Bolivia | Arachis | KbaKba | Robledo and Seijo [17] | |
| Zw65 | A. pusilla | Brazil | Heteranthae | HpuHpu | Krapovickas and Gregory [21] | |
| Zw68 | A. dardani | Brazil | Heteranthae | HdaHda | Krapovickas and Gregory [21] | |
| Zw70 | ND | Argentina | ND | ND | ||
| Zw74 | A. monticola | United States | Arachis | AmoAmoBmoBmo | Robledo et al. [16] |
ND represents species or provenance of an unknown name
Chromosome preparation
All plant materials were cultured in culture medium at 25 °C. Healthy lateral root tips were excised and pretreated with 2 mmol/L 8-hydroxyquinoline for 3–4 h at 25 °C, and then fixed in absolute ethanol (3 parts) : glacial acetic acid (1 part) for 12 h at 4 °C to kill cells. The meristem of the root tips (0.3–0.5 mm in length) was excised and squashed in 45% glacial acetic acid, and frozen at − 80 °C for 12 h. The spread chromosomes were then dehydrated in 100% ethanol and air-dried after the cover slips were removed.
Probes and FISH
Six oligonucleotides of peanut (DP-1, DP-2, DP-4, DP-5, DP-7, and DP-8), as reported by Du et al. [10], were used to optimize the probe dye. All oligonucleotides were modified at the 5′-ends with tetramethylrhodamine (TAMRA) or 6-carboxyfluorescein (FAM) by the General Biosystems Company (Anhui, China).
The oligonucleotide staining procedure comprised three steps. First, the preparation of the formulated probe dye containing 40 mL 2 × saline-sodium citrate (SSC) and X µL of each probe (1 µg/µL). Second, chromosome slides were stained in the probe dye under dim light conditions. Third, slides were washed 8–10 times with 2 × SSC at 20–25 °C, and mounted with Vectashield Mounting Medium, after which they were stained with 4’, 6-diamidino-2-phenylindole (DAPI).
In order to determine the optimum conditions for staining, four concentrations of the dye (2.5 × 10−3 ng/µL; 2.5 × 10−4 ng/µL; 2.5 × 10−5 ng/µL; and 2.5 × 10−6 ng/µL) were used to stain chromosome slides for 6 h at 37 °C. Four durations (15 min, 0.5 h, 1.5 h, and 3 h) were set to determine the optimum time. Furthermore, FISH and ND-FISH were performed, to compare the differences between them and the oligonucleotide dye, according to methods of Zhu et al. [13].
To establish a control karyotype of peanut, the genome and rDNA FISH were performed after chromosome staining. Genomic DNA probes were labeled using total genomic DNA of Arachis duranensis and Arachis ipaensis. In addition, rDNA probes were labeled using 5S and 45S rDNA of wheat (Triticum aestivum L.), which were provided by Dr. Bikram S. Gill, Kansas State University, USA. The 5S rDNA clone and total genomic DNA of A. duranensis were labeled with biotin-16-dUTP (Roche, Germany) by nick translation and detected with fluorescein anti-biotin (Roche); whereas 45S rDNA and total genomic DNA of A. ipaensis were labeled with digoxigenin-11-dUTP (Roche) and detected with anti-digoxigenin-rhodamine (Roche). The sequential FISH was performed according to the methods of Du et al. [10]. Briefly, slides were denatured in 70% formamide at 75 °C, after which they were submerged in the oligonucleotide dye, and then washed in 2 × SSC to remove all signals. Genomic in situ hybridization (GISH) was then performed using total genomic DNA of A. duranensis and A. ipaensis to identify chromosomes of the A and B genomes, respectively. A second FISH using 45S and 5S rDNA probes was conducted on the same slides to determine specific karyotypes.
To validate the efficiency of the oligonucleotide dye, two jars of the dye solution were used as two repetitions. Each jar accommodated 10 slides each time, and 10 batches were stained in each jar. In total, 200 slides of 21 accessions of cultivated peanut and 30 wild species (at least three slides of each accession) were dyed using the two jars of recycled dye solution.
Image acquisition and analysis
Slides were examined using a Leica DM6000 fluorescence microscope (Leica). Separate images from each filter set were captured using a cooled charge-coupled device (CCD) camera (Leica). Images were optimized for contrast and brightness using Adobe Photoshop. For karyotyping and chromosome diversity analysis, 3–5 cells of each accession were observed. Most karyotypes were developed from a single cell, unless overlapping chromosomes were involved. Chromosomes of each species were primarily ordered based on morphology, size, and unique patterns, as reported by Du et al. [10].
To reveal and compare the genomic relationships of Arachis, genomes of 21 peanut cultivars and two tetraploid wild species (Zw07 and Zw74) were divided into independent genomes A and B. These genomes together with genomes of other 28 diploid species were clustered for a total of 74 genomes. Specifically, the long arm and short arm of each chromosome were divided equally into six blocks. Chromosome polymorphic information was then read based on whether the unique signal pattern of the recycled oligonucleotide dye was present or not in various blocks. Block types were scored as present (1) or absent (0) for each of the 74 genomes. The NTsys 2.10e software was then used to calculate genetic distance and construct a neighbor-joining tree.
Results
Identification of SSONs for development of the dye solution
Four concentrations (2.5 × 10−3 ng/µL; 2.5 × 10−4 ng/µL; 2.5 × 10−5 ng/µL; and 2.5 × 10−6 ng/µL) of oligonucleotide DP-8 were used to compare signals produced on chromosomes in the dead cell of cultivar Silihong (SLH) with the oligonucleotide dye. The results showed there were no detectable signals at a concentration of 2.5 × 10−6 ng/µL (Fig. 1). However, when the concentration was increased to 2.5 × 10−4 ng/µL and 2.5 × 10−5 ng/µL, weak signals could be detected. When the concentration rose to 2.5 × 10−3 ng/µL, clear signals were observed (Fig. 1).
Fig. 1.
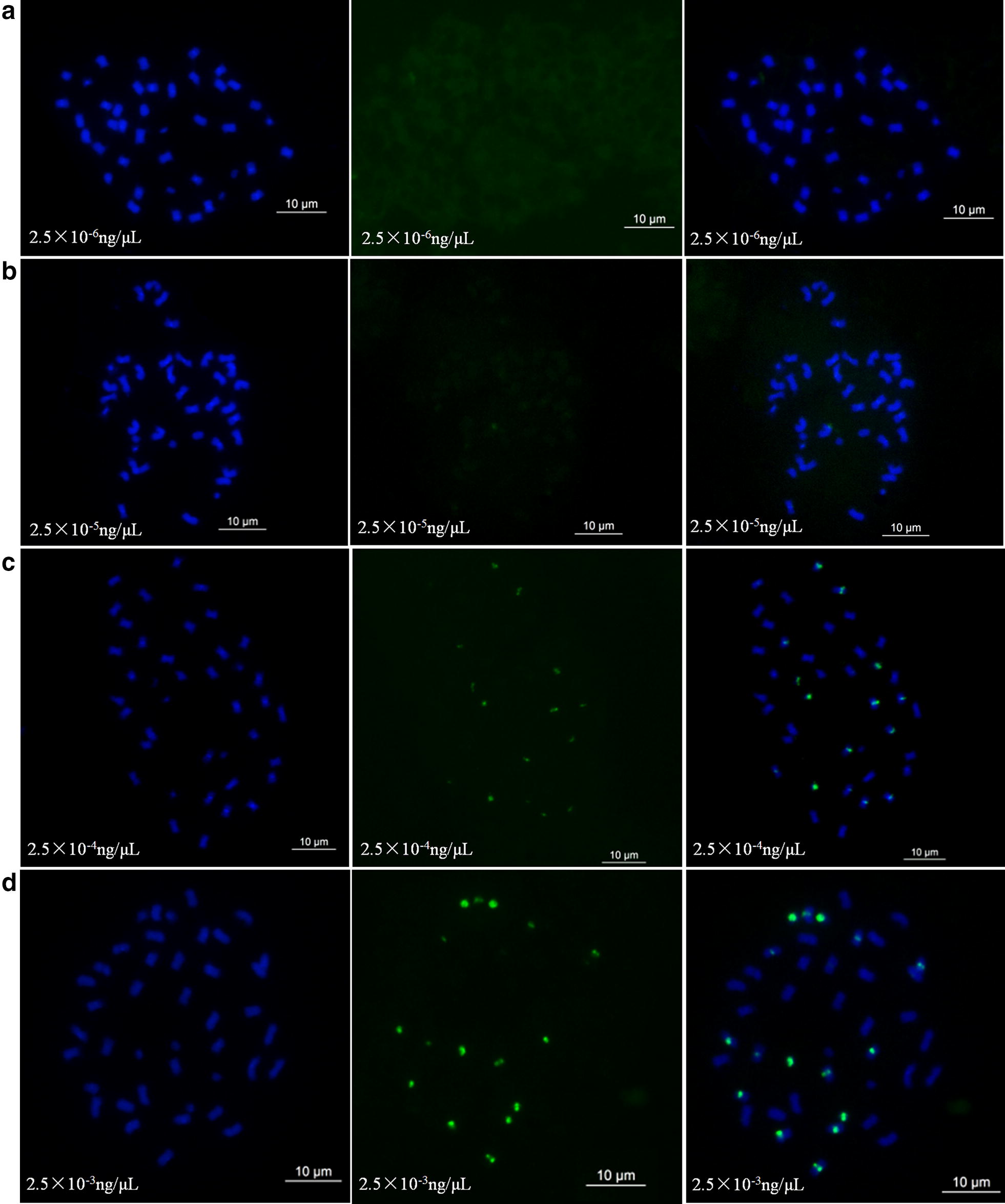
Concentration comparison analysis of oligonucleotide dye using DP-8 as a probe in Arachis hypogea cv. Silihong (SLH). Green signals show oligonucleotides that are DP-8 modified with FAM. a–d Show the results using oligonucleotide dye concentrations of 2.5 × 10−3 ng/µL, 2.5 × 10−4 ng/µL, 2.5 × 10−5 ng/µL, and 2.5 × 10−6 ng/µL, respectively
To determine the optimum duration of staining, chromosome slides of SLH were treated in the dye at 2.5 × 10−3 ng/µL for different durations at 37 °C in an incubator. We detected no signals at 15 min, but detectable signals were produced when the time was increased to 0.5 h. As the staining time increased, the signals became increasingly brighter. Furthermore, the signals in SLH were almost stable after 3 h (Additional file 1: Fig. S1 and Additional file 2: Fig. S2).
Based on the aforementioned results, considering weak signals probably occurred in different accessions, we thus used 0.075 ng/µL and 6 h as the minimal requirements to identify candidate probes to produce oligonucleotide dyes. Five different oligonucleotides, DP-1, DP-2, DP-4, DP-5, and DP-7 were used to detect the effects of various sequences on the oligonucleotide dye. Generally, the oligonucleotides were able to produce stable signals, with the exception of DP-4 and DP-2 (Additional file 3: Fig. S3 and Additional file 4: Fig. S4). We further performed FISH and ND-FISH using DP-2 at a concentration of 200 ng/µL as the probe. The results showed whether FISH or ND-FISH were able to produce fourteen strong or weak signals on chromosomes (Additional file 4: Fig. S4). We then used the DNAMAN software to analyze the oligonucleotides. We found that the max complementarities of DP-2 and DP-4 were both 66.7%. However, the highest complementarities of other oligonucleotides, such as DP-8, were 51.6%, which was significantly lower than those of DP-2 and DP-4 (Additional file 5: Fig. S5). These results indicate that oligonucleotides with higher complementarities or formed polymers required higher concentrations to invade dsDNA, which were difficult to achieve with the oligonucleotide dye.
Validation of the dye solution
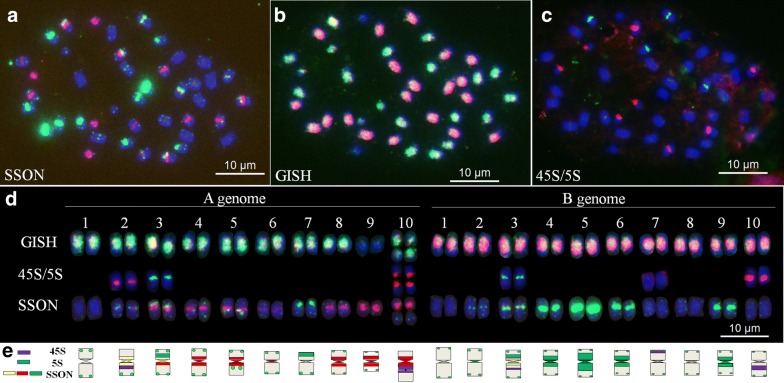
Based on the foregoing results, we developed a probe dye of peanut that comprised 2 × SSC, TAMRA-modified oligonucleotides DP-1 and DP-8, and FAM-modified oligonucleotides DP-5 and DP-7 (Table 2). The procedure first entailed preparation of the dye solution and chromosomes, followed by staining in the solution, washing with 2 × SSC, and then staining with DAPI (Additional file 6: Fig. S6). The oligonucleotide staining procedure was combined with sequential FISH using 45S rDNA, 5S rDNA, and A. duranensis and A. ipaensis DNA as probes (Fig. 2a–c). Thus, we established a reference karyotype of the cultivar SLH (Fig. 2d, e). In this karyotype, the A and B genomes of peanut could be easily identified. For example, the A genome is dominated by red signals, and the B genome is dominated by green signals (Fig. 2).
Table 2.
Cost of each staining jar probe dye in this study
| Probe dye | Modification | Volume (µL) | Concentration (ng/µL) | Price of each staining jar ($)a |
|---|---|---|---|---|
| 2 × SSC | 40000 | 0.1 | ||
| Peanut | TAMRA- DP-1 | 3 | 0.075 | 1.2 |
| TAMRA- DP-8 | 3 | 0.075 | 1.2 | |
| FAM-DP-5 | 1 | 0.025 | 0.2 | |
| FAM-DP-7 | 1 | 0.025 | 0.2 |
aCost is based on present market price in China
Fig. 2.
Sequential FISH and ideogram of karyotypes of Arachis hypogea cv. Silihong (SLH) using 45S rDNA (red), 5S rDNA (green), and A. duranensis (green) and A. ipaensis (red) total genomic DNA as probes, after using the oligonucleotide probe dye of peanut. a–c Sequential FISH of SLH; d FISH karyotypes of SLH; e Ideogram of SLH. Blue color represents chromosomes counterstained with 4′, 6-diamidino-2-phenylindole (DAPI); SSON: oligonucleotide probe dye signals; 45S/5S: 45S rDNA and 5S rDNA signals; GISH: A. duranensis and A. ipaensis total genomic DNA signals
To determine the stability of the probe dye solution, ten slides at a time were dyed within the same jar of dye solution every 3 days, and a total of 10 batches were carried out. The results showed that the 10th batch stained on the 30th day produced stable and clear signals, which were similar to those produced at the first staining (Additional file 7: Fig. S7). The cost of the probe dye was very economical. Each staining jar of the probe dye contained a volume of 40 mL, costs less than $3, and could be recycled to dye hundreds of slides (Table 2).
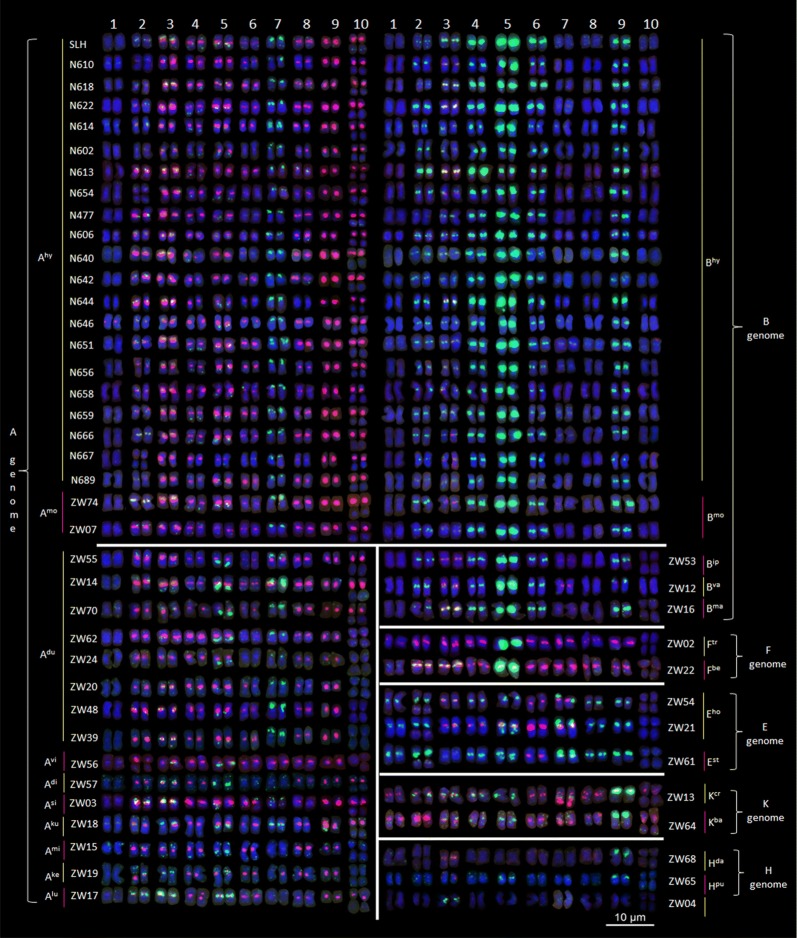
High throughput chromosome identification using the oligonucleotide dye solution
Based on the reference karyotype of SLH, we recycled each jar of the dye solution 10 times to paint 100 chromosome slides, and two jars of the dye solution were used to dye 200 slides of 21 accessions of cultivated peanut and 30 wild species. As a result, karyotypes of these species were established (Fig. 3). From those karyotypes, we determined that the genomes of these species had bands that differed significantly and were easy to identify. Based on these features and previous genome classifications [16, 17], the various species were identified and divided into six genomes (A, B, F, E, K, and H). The A genome was dominated by red signals and contained a small chromosome A9. The B genome was dominated by green signals and contained chromosome B5 with significantly strong green signals in the centromeric region. Chromosome F5 in the F genome was also observed to have similar signals to chromosome B5. However, in contrast to the B genome, the other chromosomes in the F genome were dominated by red signals. Both E and K genomes had a submetacentric chromosome 9, but the E genomes had a characteristic chromosome 7 with large green signals in the telomeric region on the short arm that the K genome did not. The remaining chromosomes in genomes E and K were dominated by green signals and red signals respectively. In the H genome, only a few chromosomes had signals (Fig. 3).
Fig. 3.
Oligonucleotide dye karyotypes and genome classification of 21 accessions of cultivated peanut and 30 wild species using the oligonucleotide probe dye of peanut
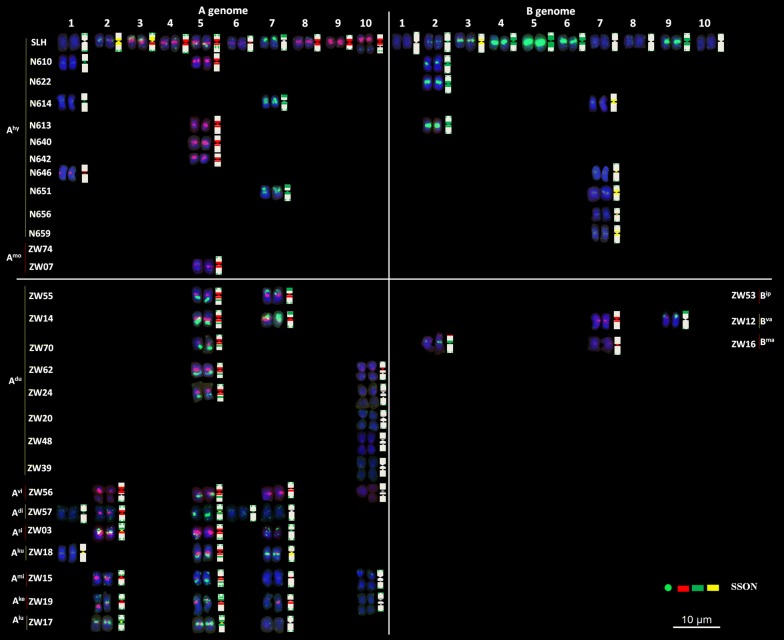
The results of cultivar staining showed that except for the detection of additional signals in: the centromeric region of chromosome A1 in N614 and N646; the centromeric region of chromosome A7 in N614 and N651; the centromeric region of chromosome B7 in N614, N651, N656, and N659; and the lack of signals in the long arm of chromosome A5 in N610, N613, N640, and N642, only a few other chromosomes with differences in signal intensity, showed similar karyotypes. The two tetraploid wild species were almost identical to the cultivar. Moreover, among all species in the A genome, A. duranensis had the highest similarity to the karyotypes of the A genome in the cultivars; however, no accession was identical in karyotypes to those of the cultivars. The genomic karyotype of A. ipaensis was almost identical to those of other species in the B genome (Fig. 4).
Fig. 4.
Chromosome structural rearrangements detected in cultivated peanuts, A. monticola, and diploid wild species with A and B genomes using the oligonucleotide probe dye of peanut
The consistent genome karyotypes were constructed, based on the genomic features of wild species (Additional file 8: Fig. S8). We found that the classifications of most genomes were consistent with the findings of previous studies. However, six accessions (ZW 04, ZW 14, ZW 17, ZW 21, ZW 54, and ZW 62) may have been incorrectly designated. For example, the species accession ZW 04 with a genome previously labeled as E was probably a member belonging to the H genome (or a new genome), because only a few of its chromosomes showed signals. The other E genome species accession ZW 62 should be in the A genome owing to the small chromosome A9. Similarly, the previous B genome species ZW 21 and ZW 54, which contained the characteristic chromosome 7 with large green signals in the telomeric region, should instead be classified in the E genome. In addition, Zw17 in the Ex genome should be correctly labeled as part of the A genome, because of the presence of the small chromosome A9, which is present in all A genome species. One previously unknown wild species, ZW70, should also be included in the A genome, based on its unique patterns (Fig. 3).
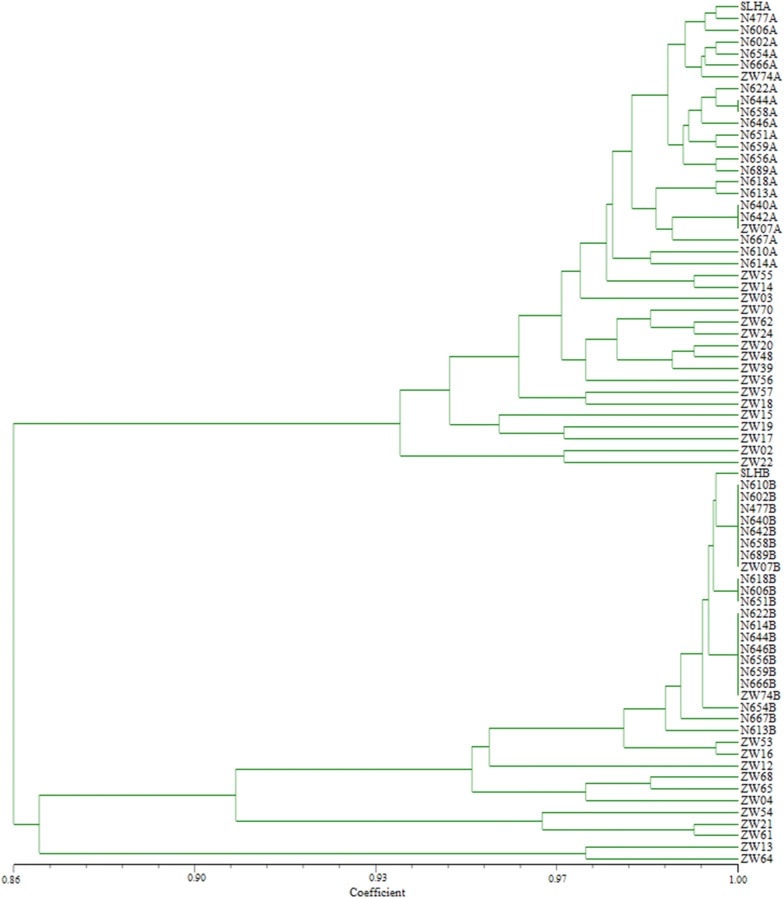
The chromosome bands of the various species facilitated the clustering of genomes into two major groups and four subgroups, according to chromosome blocks (Fig. 5). The first subgroup contained species in the A and F genomes, the second subgroup contained species in the K genome, the third subgroup consisted of species in the B and H genomes, and the fourth subgroup included species in the E genome.
Fig. 5.
Cluster analysis of 74 genomes of Arachis species based on structural rearrangements and polymorphic blocks
Discussion
Chromosome invasion by oligonucleotides
Cuadrado and Jouve [4] found that simple sequence repeat (SSR) oligonucleotides could invade chromosomes through triplex DNA or some other unknown way in non-denaturing chromosomes. In addition, non-SSR oligonucleotide probes could be used for ND-FISH assays of plant chromosomes [9, 11, 13, 18]. Furthermore, Du et al. [5] found that oligonucleotide probes of a suitable length had a stronger ability to invade chromosomes. In the present study, we found that chromosomes could produce signals in concentrations of 2.5 × 10−5 ng/µL, using a 31-bp oligonucleotide probe dye, and probes could invade chromosomes within 30 min. These properties of oligonucleotides permitted the development of an efficient technique for genome evolution and chromosome identification. Nevertheless, not all oligonucleotides were able to invade chromosomes. Some were affected by their sequence structures and the duration for invasion, among other factors. However, what kind of oligonucleotides is easier to stain, even what the dyeing mechanism is, and more research is needed.
SSON dye solution provides a new technique for automatic FISH and chromosome sorting
The chromosome banding technique and FISH have played important roles in developing distant hybrids over the past few decades [1, 2]. However, chromosome banding is susceptible to experimental conditions, and FISH is both expensive and cumbersome, which limits large-scale application in the cytologically distinguishing identification of genetic materials. In this study, we demonstrated that oligonucleotides with lower levels of complementarities and without polymerization could invade dsDNA in dead cells at a very low concentration. This facilitated the development of a new, low-cost, simple oligonucleotide dye technique. Based on these characteristics, we developed the probe dye technique for peanut, which could effectively identify various genomes, similar to previous studies. Our findings have considerably simplified the procedures of conventional FISH using plasmid clones and oligonucleotide FISH [10, 19].
In addition, if used to dye 10 slides at each trial, the solution could be recycled to dye hundreds of slides. Staining 10 slides at a time is only an example used in the present study. We could have dyed more slides at any given time. The more batches that can be dyed, the greater the likelihood of further development of commercial kits and possibilities for the automation of FISH and chromosome analysis. More importantly, this method has been extended to wheat, rye, and maize by our team, and not only provides powerful tools for chromosome engineering and evolutionary analysis, but also benefits chromosome sorting by flow cytometry based on different fluorescence signal intensities [20]. However, some of the SSON sequences that easily form polymers cannot be used as a probe dye. Therefore, it was necessary to develop effective probes to ensure a highly efficient SSON dye.
Genome evolution in Arachis species
Based on oligonucleotide dye technology, we developed a consistent genome karyotype of Arachis species, which provides a reference for the rapid identification of its genome. With these karyotypes, this study identified some misclassified species and provided cytological evidence.
The genus Arachis contains approximately 80 species. Based on morphology, geographic distribution, cytogenetics, and cross compatibilities, it has been divided into nine sections: Arachis (A, B, D, K, F, and G genomes); Caulorrhizae (C genome); Erectoides (E genome); Extranervosae (Ex genome); Heteranthae (H genome); Procumbentes (Pr genome); Rhizomatosae (R1, R2 genome); Trierectoides (Te genome); and Triseminatae (T genome) [16, 17, 21–23]. The present study involved four sections of eight genomic types: AB, A, B, K, F, E, Ex, and H.
The results showed that very few signals were produced in the H genome using repeat sequence oligonucleotide probes, and those karyotypes were very different from those of other genomes. Previously, extensive hybridizations have shown that species in the H genome could only be crossed successfully with species in the Ex genome and were incompatible when crossed with species in the section Arachis and other sections [24, 25]. Moreover, internal transcribed spacer variation analysis showed large genetic differences between the H and A, B, K, F, and E genomes [26]. The aforementioned observations indicate that the H genome might have diverged from the other genomes at an early stage of the evolution of the genus Arachis.
Only one species in the Ex genome, Zw17, was included in this study. However, its karyotype showed great similarity to the A genome, as evidenced by the presence of the small chromosome A9. Thus, it is hard to determine if the species is a member of the A genome owing to the lack of a sufficient number of species in the Ex genome.
For genomes of the section Arachis, the signal color of the two genomes A and B were completely different. This showed that repeated sequences in the two genomes were also considerably different, and further indicated that the two genomes diverged at a relatively earlier stage in the evolution of Arachis genomes. Furthermore, these findings also suggest the existence of fewer chromosome exchanges during the evolution process.
Most chromosomes of Ftr and Fbe had red signals in the centromeric regions. However, chromosome F5 had a large number of amplifications in the centromeric region, which is a characteristic feature of chromosome B5 that is believed to exist only in the B genome. The geographic distribution of A. trinitensis and A. benensis has shown their similarity to the B genome species of A. williamsii, and affinity for B genome species [17, 24, 27]. Therefore, we speculated that the B and F genomes might be derived from the same genome after genome divergence of the genus Arachis.
Previous studies have suggested that E genome species could hybridize with most species of the section Arachis [25]. Cytological evidence shows that A. batizocoi and A. stenophylla contain similar DAPI bands and the inversion chromosome A10 [28]. In the present study, all the E genome and K genome species shared similar chromosomes 9 with large arm ratios, indicating the E genome might be closer to the K genome than other genomes in the genus Arachis.
A. duranensis and A. ipaensis have been considered the donors of cultivated peanut [16, 17, 19, 29–31]. Moreover, A. monticola is considered a distinct species from A. hypogaea, based mainly on sequencing, cytogenetic analysis, and fruit structure [32–35]. Based on the dyed karyotypes in the present study, we found that chromosome variation was minimal among cultivars. In addition, the karyotypes of A. monticola were also almost identical to those of the cultivar, indicating that cultivated peanut might have domesticated from A. monticola. Moreover, the karyotypes of A. duranensis and A. ipaensis showed the most similarity to those of the A and B genomes of A. monticola and cultivars among all wild species under investigation. The karyotype of A. ipaensis was identical to those of the B genome of the cultivars, proving it is the donor of the B genome, as previously described [16, 17, 19, 29–31, 36]. However, none of the A. duranensis accessions used in the present study shared identical karyotypes with the A genome of the cultivars, which is consistent with those of previous studies [19, 29–31, 36]. Based on those results, it is quite obvious that variations at both chromosome and molecular level have taken place among different accessions of A. duranensis. Therefore, more evidence is required to confirm which accession of A. duranensis or even which species is the direct donor of the A genome of the cultivars. Further identification of more accessions of A. duranensis and more species of Arachis will provide more indirect information. However, distant hybridization between A. ipaensis and candidate accessions of A. duranensis, or other species of Arachis, and investigation of the fertility of hybrid F1 will provide direct evidence on the origin of the A genome of cultivars.
Conclusion
Lower levels of complementarities without the polymerization of repeated sequence oligonucleotides facilitates their invasion of the chromosomes of dead cells through triplex DNA or some other unknown process in non-denaturing chromosomes. This facilitated the development of a new, low-cost, simple oligonucleotide dye technique. Based on the identification of karyotype patterns of Arachis genomes by the oligonucleotide dye technique, we revealed the genome relationships of Arachis. These findings demonstrate the potential for the adoption of this oligonucleotide dye solution in practice. Furthermore, the oligonucleotide dye technique may provide a powerful tool in the future that could help to accelerate chromosome sorting by flow cytometry based on different fluorescence signal intensities.
Additional files
Additional file 1: Fig. S1. Effects of staining time of oligonucleotide dye. Green signals show DP-8 oligonucleotides modified with FAM. a–d show the oligonucleotide dye results at 2.5 × 10−3 ng/µL for 15 min, 0.5 h, 1.5 h, and 3.0 h, respectively
Additional file 2: Fig. S2. Signal intensity variation over time in oligonucleotide dye using DP-8
Additional file 3: Fig. S3. Effects of oligonucleotide sequences on oligonucleotide dye. a–d: Green signals show oligonucleotides DP-1, DP-7, DP-5, and DP-4 modified with FAM, respectively
Additional file 4: Fig. S4. Comparison of analyses among FISH (a), ND-FISH (b), and oligonucleotide dye (c) using TAMRA-DP-2 as the probe
Additional file 5: Fig. S5. Oligo structure and similarity of oligonucleotides DP-2 and DP-8
Additional file 6: Fig. S6. Staining procedure using oligonucleotide probe dye
Additional file 7: Fig. S7. Staining results of 10 batches of the peanut cultivar, Silihong (SLH), using the same jar of probe dye solution of peanut
Additional file 8: Fig. S8. Consistent ideogram karyotypes of Arachis genomes using modified probe dyes of peanut
Acknowledgements
We thank Ms. Kassandra Semrau (MYcroarray, Ann Arbor, Michigan, USA) for designing the chromosome-specific oligonucleotide library and genomic repeat sequence-based oligonucleotide probes for this study. We thank Petal Smart, DVM (Hons) (https://peerwith.expert/drpetalsmart) for editing the English text of a draft of this manuscript.
Abbreviations
- FISH
fluorescence in situ hybridization
- SSC
saline-sodium citrate
- SSON FISH
single-strand oligonucleotides FISH
- ND-FISH
non-denaturing FISH
- FAM
6-carboxyfluorescein
- TAMRA
6-carboxytetramethylrhodamine; 4′, 6-diamidino-2-phenylindole (DAPI)
- GISH
genomic in situ hybridization
- SLH
silihong
- SSR
simple sequence repeat
Authors’ contributions
XZ, ZQ, WD, BH, and FT designed the experiments; PD, CC, HL, FL, LL, XD, LQ, SW, SH, and JX performed the probe design and FISH, and analyzed the data; PD, XZ, ZQ, and WD wrote the manuscript. All authors read and approved the manuscript.
Funding
This research was supported by Major R&D and Promotion Projects in Henan, China (0071); National Natural Science Foundation of China (31801397); China Agriculture Research System (CARS-13); and the Henan Provincial Agriculture Research System, China (S2012-05).
Availability of data and materials
All the data pertaining to the present study have been included in table and/or figure form in the manuscript and authors are pleased to share analyzed/raw data and plant materials upon reasonable request. Other datasets supporting the conclusions of this article are included within the article and its additional files.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Pei Du, Caihong Cui and Hua Liu contributed equally to this study
Contributor Information
Wenzhao Dong, Phone: +86-371-65739143, Email: dongwzh@126.com.
Zengjun Qi, Phone: +86-25-84399029, Email: zjqi@njau.edu.cn.
Xinyou Zhang, Phone: +86-371-65729560, Email: haasz@126.com.
References
- 1.Gill BS, Friebe B, Endo TR. Standard karyotype and nomenclature system for description of chromosome bands and structural aberration in wheat (Triticum aestivum) Genome. 1991;34:830–839. doi: 10.1139/g91-128. [DOI] [Google Scholar]
- 2.Jiang JM, Gill BS. Current status and the future of fluorescence in situ hybridization (FISH) in plant genome research. Genome. 2006;49:1057–1068. doi: 10.1139/g06-076. [DOI] [PubMed] [Google Scholar]
- 3.Beliveau BJ, Joyce EF, Apostolopoulos N, Yilmaz F, Fonseka CY, McCole RB, et al. Versatile design and synthesis platform for visualizing genomes with oligopaint FISH probes. Proc Natl Acad Sci USA. 2012;109:21301–21306. doi: 10.1073/pnas.1213818110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuadrado A, Jouve N. Chromosomal detection of simple sequence repeats (SSRs) using nondenaturing FISH (ND-FISH) Chromosoma. 2010;119:495–503. doi: 10.1007/s00412-010-0273-x. [DOI] [PubMed] [Google Scholar]
- 5.Du P, Zhuang LF, Wang YZ, Yuan L, Wang Q, Wang DR, et al. Development of oligonucleotides and multiplex probes for quick and accurate identification of wheat and Thinopyrum bessarabicum chromosomes. Genome. 2017;60:93–103. doi: 10.1139/gen-2016-0095. [DOI] [PubMed] [Google Scholar]
- 6.Beliveau BJ, Boettiger AN, Avendaño MS, Jungmann R, McCole RB, Joyce EF, et al. Single-molecule super-resolution imaging of chromosomes and in situ haplotype visualization using Oligopaint FISH probes. Nat Commun. 2015;6:7147. doi: 10.1038/ncomms8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han YH, Zhang T, Thammapichai P, Weng YQ, Jiang JM. Chromosome-specific painting in Cucumis species using bulked oligonucleotides. Genetics. 2015;200:771–779. doi: 10.1534/genetics.115.177642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang XY, Zhu MQ, Zhuang LF, Zhang SY, Wang JJ, Chen XJ, et al. Structural chromosome rearrangements and polymorphisms identified in Chinese wheat cultivars by high-resolution multiplex oligonucleotide FISH. Theor Appl Genet. 2018;131:1967–1986. doi: 10.1007/s00122-018-3126-2. [DOI] [PubMed] [Google Scholar]
- 9.Tang SY, Tang ZX, Qiu L, Yang ZJ, Li GR, Lang T, et al. Developing new oligo probes to distinguish specific chromosomal segments and the A, B, D genomes of wheat (Triticum aestivum L.) using ND-FISH. Front Plant Sci. 2018;9:1104. [DOI] [PMC free article] [PubMed]
- 10.Du P, Li LN, Liu H, Fu LY, Sun ZQ, Qin L, et al. High-resolution painting using multiplex and chromosome specific-oligonucleotides identifies chromosome rearrangements and genome differentiation in peanut. BMC Plant Biol. 2018;18:240–253. doi: 10.1186/s12870-018-1468-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu SL, Chen L, Wang YY, Li M, Yang Z. Oligonucleotide probes for ND-FISH analysis to identify rye and wheat chromosomes. Sci Rep. 2015;5:10552. doi: 10.1038/srep10552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang SY, Qiu L, Xiao ZQ, Fu SL, Tang ZX. New oligonucleotide probes for ND-FISH analysis to identify barley chromosomes and to investigate polymorphisms of wheat chromosomes. Genes. 2016;7:118. doi: 10.3390/genes7120118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu MQ, Du P, Zhuang LF, Chu CG, Zhao H, Qi ZJ. A simple and efficient non-denaturing FISH method for maize chromosome differentiation using single-strand oligonucleotide probes. Genome. 2017;60:657–664. doi: 10.1139/gen-2016-0167. [DOI] [PubMed] [Google Scholar]
- 14.Cuadrado A, Golczyk H, Jouve N. A novel, simple and rapid nondenaturing FISH (ND-FISH) technique for the detection of plant telomeres. Potential use and possible target structures detected. Chromosome Res. 2009;17:755–762. doi: 10.1007/s10577-009-9060-z. [DOI] [PubMed] [Google Scholar]
- 15.Tang ZX, Yang ZJ, Fu SL. Oligonucleotides replacing the roles of repetitive sequences pAs1, pSc119.2, pTa-535, pTa71, CCS1, and pAWRC.1 for FISH analysis. J Appl Genet. 2014;55:313–318. doi: 10.1007/s13353-014-0215-z. [DOI] [PubMed] [Google Scholar]
- 16.Robledo G, Lavia GI, Seijo JG. Species relations among wild Arachis species with the A genome as revealed by FISH mapping of rDNA loci and heterochromatin detection. Theor Appl Genet. 2009;118:1295–1307. doi: 10.1007/s00122-009-0981-x. [DOI] [PubMed] [Google Scholar]
- 17.Robledo G, Seijo G. Species relationships among the wild B genome of Arachis species (section Arachis) based on FISH mapping of rDNA loci and heterochromatin detection, a new proposal for genome arrangement. Theor Appl Genet. 2010;121:1033–1046. doi: 10.1007/s00122-010-1369-7. [DOI] [PubMed] [Google Scholar]
- 18.Wang YZ. Development and characterization of small segment translocations of Thinopyrum bessarabicum and cytological mapping of interest genes. Master’s thesis, Nanjing Agricultural University, Nanjing, China. 2013. [In Chinese with English abstract].
- 19.Zhang LN, Yang XY, Tian L, Chen L, Yu WC. Identification of peanut (Arachis hypogaea) chromosomes using a fluorescence in situ hybridization system reveals multiple hybridization events during tetraploid peanut formation. New Phytol. 2016;211:1424–1439. doi: 10.1111/nph.13999. [DOI] [PubMed] [Google Scholar]
- 20.Kubaláková M, Kovářová P, Suchánková P, Číhalíková J, Bartoš J, Lucretti S, et al. Chromosome sorting in tetraploid wheat and its potential for genome analysis. Genetics. 2005;170:823–829. doi: 10.1534/genetics.104.039180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krapovickas A, Gregory WC. Taxonomía del género Arachis (Leguminosae) Bonplandia. 1994;8:1–4. [Google Scholar]
- 22.Valls JFM, Simpson CE. New species of Arachis from Brazil. Paraguay and Bolivia. Bonplandia. 2005;14:35–64. [Google Scholar]
- 23.Silvestri MC, Ortiz AM, Lavia GI. rDNA loci and heterochromatin positions support a distinct genome type for ‘x = 9 species’ of section Arachis (Arachis, Leguminosae) Plant Syst Evol. 2015;301:555–562. doi: 10.1007/s00606-014-1092-y. [DOI] [Google Scholar]
- 24.Gregory MP, Gregory WC. Exotic germoplasm of Arachis L. interspecific hybrids. J Hered. 1979;70:185–193. doi: 10.1093/oxfordjournals.jhered.a109231. [DOI] [Google Scholar]
- 25.Stalker HT. Utilizing wild species for peanut improvement. Crop Sci. 2017;57:1102–1120. doi: 10.2135/cropsci2016.09.0824. [DOI] [Google Scholar]
- 26.Bechara Marcelo D, Moretzsohn Márcio C, Palmieri Darío A, Monteiro Jomar P, Bacci Maurício, Martins Joaquim, Valls José FM, Lopes Catalina R, Gimenes Marcos A. Phylogenetic relationships in genus Arachis based on ITS and 5.8S rDNA sequences. BMC Plant Biology. 2010;10(1):255. doi: 10.1186/1471-2229-10-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tallury SP, Hilu KW, Milla SR, Friend SA, Alsaghir M, Stalker HT, et al. Genomic affinities in Arachis section Arachis (Fabaceae): molecular and cytogenetic evidence. Theor Appl Genet. 2005;111:1229–1237. doi: 10.1007/s00122-005-0017-0. [DOI] [PubMed] [Google Scholar]
- 28.Du P, Li LN, Zhang ZX, Liu H, Qin L, Huang BY, et al. Chromosome painting of telomeric repeats reveals new evidence for genome evolution in peanut. J Integr Agr. 2016;15:2488–2496. doi: 10.1016/S2095-3119(16)61423-5. [DOI] [Google Scholar]
- 29.Bertioli DJ, Cannon SB, Froenicke L, Huang GD, Farmer AD, Cannon EK, et al. The genome sequences of Arachis duranensis and Arachis ipaensis, the diploid ancestors of cultivated peanut. Nat Genet. 2016;48:438–446. doi: 10.1038/ng.3517. [DOI] [PubMed] [Google Scholar]
- 30.Chen X, Lu Q, Liu H, Zhang J, Hong Y, Lan H, et al. Sequencing of cultivated peanut, arachis hypogaea, yields insights into genome evolution and oil improvement. Mol Plant. 2019;1–15. [DOI] [PubMed]
- 31.Bertioli DJ, Jenkins J, Clevenger J, Dudchenko O, Gao DY, Seijo G, et al. The genome sequence of segmental allotetraploid peanut Arachis hypogaea. Nat Genet. 2019;51:877–884. doi: 10.1038/s41588-019-0405-z. [DOI] [PubMed] [Google Scholar]
- 32.Koppolu R, Upadhyaya HD, Dwivedi SL, Hoisington DA, Varshney RK. Genetic relationships among seven sections of genus Arachis studied by using SSR markers. BMC Plant Biol. 2010;10:15. doi: 10.1186/1471-2229-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moretzsohn MC, Gouvea EG, Inglis PW, Leal-Bertioli SCM, Valls JFM, Bertioli DJ. A study of the relationships of cultivated peanut (Arachis hypogaea) and its most closely related wild species using intron sequences and microsatellite markers. Ann Bot (Lond). 2013;111:113–126. doi: 10.1093/aob/mcs237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seijo G, Lavia GI, Fernández A, Krapovickas A, Ducasse DA, Bertioli DJ, et al. Genomic relationships between the cultivated peanut (Arachis hypogaea, Leguminosae) and its close relatives revealed by double GISH. Am J Bot. 2007;94:1963–1971. doi: 10.3732/ajb.94.12.1963. [DOI] [PubMed] [Google Scholar]
- 35.Yin D, Ji CM, Ma XL, Li H, Zhang WK, Li S, et al. Genome of an allotetraploid wild peanut Arachis monticola: a de novo assembly. GigaScience. 2018;7:1–9. doi: 10.1093/gigascience/giy066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhuang W, Chen H, Yang M, Wang J, Pandey MK, Zhang C, et al. The genome of cultivated peanut provides insight into legume karyotypes, polyploid evolution and crop domestication. Nat Genet. 2019;51:865–876. doi: 10.1038/s41588-019-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1. Effects of staining time of oligonucleotide dye. Green signals show DP-8 oligonucleotides modified with FAM. a–d show the oligonucleotide dye results at 2.5 × 10−3 ng/µL for 15 min, 0.5 h, 1.5 h, and 3.0 h, respectively
Additional file 2: Fig. S2. Signal intensity variation over time in oligonucleotide dye using DP-8
Additional file 3: Fig. S3. Effects of oligonucleotide sequences on oligonucleotide dye. a–d: Green signals show oligonucleotides DP-1, DP-7, DP-5, and DP-4 modified with FAM, respectively
Additional file 4: Fig. S4. Comparison of analyses among FISH (a), ND-FISH (b), and oligonucleotide dye (c) using TAMRA-DP-2 as the probe
Additional file 5: Fig. S5. Oligo structure and similarity of oligonucleotides DP-2 and DP-8
Additional file 6: Fig. S6. Staining procedure using oligonucleotide probe dye
Additional file 7: Fig. S7. Staining results of 10 batches of the peanut cultivar, Silihong (SLH), using the same jar of probe dye solution of peanut
Additional file 8: Fig. S8. Consistent ideogram karyotypes of Arachis genomes using modified probe dyes of peanut
Data Availability Statement
All the data pertaining to the present study have been included in table and/or figure form in the manuscript and authors are pleased to share analyzed/raw data and plant materials upon reasonable request. Other datasets supporting the conclusions of this article are included within the article and its additional files.