This secondary analysis of a randomized clinical trial assesses whether a 12-week endurance or resistance training intervention can regulate epicardial and pericardial adipose tissue mass.
Key Points
Question
Are epicardial and pericardial adipose tissues regulated by both endurance and resistance training?
Findings
In this secondary analysis of a randomized clinical trial including 50 individuals with abdominal obesity, endurance and resistance training significantly reduced epicardial adipose tissue mass by 32% and 24%, respectively. While resistance training reduced pericardial adipose tissue mass by 32%, there was no effect of endurance training on pericardial adipose tissue.
Meaning
Endurance and resistance training have the potential to reduce cardiac adipose tissue mass and may have clinical potential given that excessive cardiac adipose tissue is associated with an increased incidence of cardiovascular disease.
Abstract
Importance
Epicardial and pericardial adipose tissues are emerging as important risk factors for cardiovascular disease, and there is a growing interest in discovering strategies to reduce the accumulation of fat in these depots.
Objective
To investigate whether a 12-week endurance or resistance training intervention regulates epicardial and pericardial adipose tissue mass.
Design, Setting, and Participants
Secondary analysis of a randomized, assessor-blinded clinical trial initiated on August 2016 and completed April 2018. This single-center, community-based study included 50 physically inactive participants with abdominal obesity.
Interventions
Participants were randomized to a supervised high-intensity interval endurance training (3 times a week for 45 minutes), resistance training (3 times a week for 45 minutes), or no exercise (control group).
Main Outcomes and Measures
Change in epicardial and pericardial adipose tissue mass assessed by magnetic resonance imaging, based on a prespecified secondary analysis plan including 3 of 5 parallel groups.
Results
Of 50 participants (mean [SD] age, 41 [14] years, 10 men [26%]; mean [SD] body mass index [calculated as weight in kilograms divided by height in meters squared], 32 [5]), 39 [78%] completed the study. Endurance training and resistance training reduced epicardial adipose tissue mass by 32% (95% CI, 10%-53%) and 24% (95% CI, 1%-46%), respectively, compared with the no exercise control group (56% [95% CI, 24%-88%]; P = .001 and 48% [95% CI, 15%-81%]; P < .001, respectively). While there was a nonsignificant reduction in pericardial adipose tissue mass after endurance training (11% [95% CI, −5% to 27%]; P = .17), resistance training significantly reduced pericardial adipose tissue mass by 31% (95% CI, 16%-47%; P < .001) when compared with the no exercise control group. Compared with the no exercise control group, there was an increase in left ventricular mass by endurance (20 g [95% CI, 11%-30%]; P < .001) and resistance training (18 g [95% CI, 8%-28%]; P < .001). Other cardiometabolic outcomes remained unchanged after the 12-week trial period.
Conclusions and Relevance
In individuals with abdominal obesity, both endurance and resistance training reduced epicardial adipose tissue mass, while only resistance training reduced pericardial adipose tissue mass. These data highlight the potential preventive importance of different exercise modalities as means to reduce cardiac fat in individuals with abdominal obesity.
Trial Registration
ClinicalTrials.gov identifier: NCT02901496
Introduction
Cardiovascular disease is the leading cause of death globally.1 Among the risk factors for developing cardiovascular disease is obesity,2,3 in particular obesity associated with visceral adipose tissue accumulation.4 As follows, excessive amounts of cardiac adipose tissue is reported to be associated with cardiovascular disease.5,6,7,8,9
Cardiac adipose tissue consists of 2 distinct depots, the epicardial depot located immediately next to the myocardium and the pericardial and outermost depot located within and on the pericardium.4 Epicardial adipose tissue is proposed to play a cardioprotective role owing to its ability to take up and metabolize large amounts of fatty acids and thereby preventing the formation of atherosclerotic plaques.10,11 However, increased release of inflammatory adipokines accompanying a pathophysiological expansion of epicardial adipose tissue is proposed to provoke atherosclerosis.5 The pericardial adipose depot is less well studied and although its effects on myocardial function are not fully clarified,12 pericardial adipose tissue is exclusively associated with cardiovascular risk factors, coronary calcification, and incident of coronary heart disease.7,12,13
Pharmaceutical drugs, such as the glucagon-like peptide 1 analogs,14 sodium-glucose transporter 2 inhibitors,15,16 and lipid-lowering drugs,17 successfully reduce epicardial adipose tissue mass and the risk of developing cardiovascular disease.10 Also, bariatric surgery promotes loss of epicardial adipose tissue.18,19 In contrast to these invasive approaches, exercise training may serve as a noninvasive strategy to reduce excessive cardiac adipose tissue. Until now, few studies have investigated the effect of exercise on epicardial and pericardial adipose tissue mass and the existing literature is inconsistent, to our knowledge.20,21,22,23,24 For instance, it is unclear whether exercise training, both endurance and resistance training, facilitates changes in both epicardial and pericardial adipose tissue.
Based on the evidence that exercise targets visceral adipose tissue and lowers cardiovascular risk,3,25,26,27 we set out to investigate the effects of a 12-week endurance exercise and resistance exercise intervention on epicardial and pericardial adipose tissue mass in physically inactive individuals with abdominal obesity. We hypothesized that both endurance and resistance training would reduce epicardial and pericardial fat compared with an inactive control group. This study is explorative as the analysis is based on data from a larger study exploring a different objective.26,28
Methods
This study was part of a larger randomized exercise intervention clinical trial26,28 performed between August 2016 to April 2018 at the Centre for Physical Activity Research in Copenhagen, Denmark. The study was approved by the ethical committee of the Capital Region of Denmark (H-16018062) and reported to the Danish Data Protection Agency (2012-58-0004). The study was conducted in accordance with the Declaration of Helsinki.29 All participants provided written informed consent.
Participants, Randomization, and Blinding
The complete study protocol has been previously published.28 Briefly, participants were eligible if they were inactive and had abdominal obesity (waist to height ratio was ≥0.5 and/or waist circumference was ≥88 cm for women and ≥102 cm for men) and older than 18 years. Exclusion criteria were ischemic heart disease, diabetes, atrial fibrillation, pregnancy, treatment with immunotherapy or other biological rheumatic drugs, and health conditions that prevented magnetic resonance imaging (MRI) scans and exercise to be performed. Eligible participants were block randomized (1:1:1:1:1) into 5 groups. The randomized sequence was generated by 1 of us (K.K.) using computer-generated block randomization and concealed from all other researchers. To maintain blinding, the randomized sequence number was delivered concealed to a training instructor. The training instructor informed the participant and if assigned to the training intervention, the instructor organized the subsequent training sessions with the participant. The principal investigators thus remained blinded to the training modality. The 5 randomized groups were (1) no exercise plus placebo, (2) no exercise plus tocilizumab, (3) exercise plus placebo, (4) exercise plus tocilizumab, or (5) resistance exercise plus placebo. As described in the protocol,28 participants allocated to no exercise plus placebo, exercise plus placebo, and resistance training plus placebo groups were included in this secondary analysis of this particular study. The no exercise plus placebo and exercise plus placebo groups were also included in the primary study.26
Baseline and Postintervention Measurements
Cardiorespiratory Measurements
To determine the maximal oxygen consumption rate (Vo2 max), a graded exercise test on a bicycle ergometer was performed at baseline and after the 12-week intervention as previously described.28 At baseline, 2 Vo2 max tests were performed to allow familiarization. A 1–repetition maximum (RM) test was used to assess muscle strength during knee extensions (right and left leg separately) and chest press.
Anthropometric Measurements and Blood Samples
Body weight, height, and body mass index (calculated as weight in kilograms divided by height in meters squared) were determined as previously described.26 Dual-energy x-ray absorptiometry (version 8.8; Lunar Prodigy, GE Medical Systems) was performed to evaluate changes in body composition, including total fat mass, android and gynoid fat mass, and lean body mass. Fasting blood samples were analyzed for cardiometabolic and inflammatory markers as previously described.26
Cardiac Adipose Tissue Mass and Function by MRI
The MRI scan was performed on a 1.5-T whole-body MRI scanner (Ingenia; Philips). The cardiac MRI was supervised by a senior cardiologist (J.B.R.).28 Steady-state free precession cine images were obtained during repeated breath holds in 2 long axes and in a stack of short axes covering the left ventricle (LV) to rule out wall motion abnormalities and allow for cardiac chamber and mass quantification. Sagittal, axial, and horizontal planes were obtained from 5 retrospective cardiac cycles with a slice thickness of 8 mm (interslice gap 2 mm for the short axis stack), with temporal resolution 30 to 50 milliseconds depending on heart rate (25 acquired phases). The number of short-axis cines acquired was participant specific, depending on ventricular size using retrospective gating in all participants when possible.
We also performed short-TI Inversion Recovery sequences, which are used to null the signal from fat to distinguish water (pericardial effusion) from cardiac fat. These images were compared with the cine images to rule out water. Semiautomatic calculations using cardiovascular imaging software cvi42, version 5.2, were applied for postprocessing using endocardial and epicardial contours to calculate LV ejection fraction, stroke volume, end-diastolic volume, end-systolic volume, and myocardial mass. Epicardial and pericardial adipose tissue volumes were quantified from cine images in the end-diastolic short axis by drawing contours around the epicardial and pericardial fat layers of the entire right ventricle and LV on each slice. Fat areas were generated automatically by the cvi42 software. Each slice area was multiplied by the slice thickness to yield a volume. The total mass of epicardial and pericardial adipose tissue was obtained by summation of all slice volumes and conversion to mass by the specific weight of fat (0.92 g/cm3).30
The MRI scans were analyzed by the same investigator in a blinded manner. A cardiologist (J.B.R.) reanalyzed a subset of the cardiac MRI slices (n = 10) to assess accuracy and interreader variability. The degree of agreement between the 2 assessors (intraclass correlation) was found to be 0.87 (95% CI, 0.30-0.97) for epicardial adipose tissue and 0.98 (95% CI, 0.86-0.99) for pericardial adipose tissue.31
Exercise Training
Educated personnel supervised all exercise sessions. Participants performed 3 weekly training sessions of 45 minutes during a 12-week period.28 The endurance exercise was high-intensive interval exercise performed on an ergometer bicycle. The resistance exercise was designed as a 45-minute interval-type, medium-load, high-repetition, time-based training. Participants performed 3 to 5 sets of 10 exercises. The resistance exercise load was 60% of 1 RM and increased throughout the 12 weeks to 80%.28
Lifestyle Monitoring
Participants were instructed to maintain their habitual lifestyle during the study. On a monthly basis, self-reported 3-day dietary intake was recorded and during a 4-day period free-living physical activity levels were monitored using accelerometry (AX3; sampling frequency 100 Hz). Postprocessing was conducted as described previously.26,32
Analysis Population
The study was designed to focus on the per-protocol population, where participants were included in the analysis if they completed the trial with a satisfactory training adherence. Completing a minimum of 29 of 36 training sessions (80%) was satisfactory.
Statistical Analyses
A prespecified sample size of 14 individuals in each group was obtained from a power calculation based on the primary study outcome.26,28 Because the training effects on cardiac adipose tissue were exploratory, no power calculation was performed related to this outcome.
Statistical analyses were performed using SAS software, version 9.3 (SAS Institute) and GraphPad Prism, version 7.02 (GraphPad Software). A 2-sided P < .05 was considered statistically significant and reported with 2-sided 95% CIs, with no default adjustment for multiplicity. Baseline characteristics with normal or nonnormal distribution were reported as mean and SD or median and interquartile range. Postintervention levels of continuous variables adjusted for baseline levels were compared using analysis of covariance performed with postmeasure or absolute or relative change from baseline measure as the dependent outcome, with a fixed factor for group (3 levels) and using the baseline value as a covariate; for each continuous outcome group, means were adjusted for baseline (least squares means). Independent of the result of the overall analysis of covariance model, contrasts between groups were performed. Intervention-induced changes in epicardial and pericardial adipose within groups was evaluated by Wilcoxon matched pairs signed-rank test.
Results
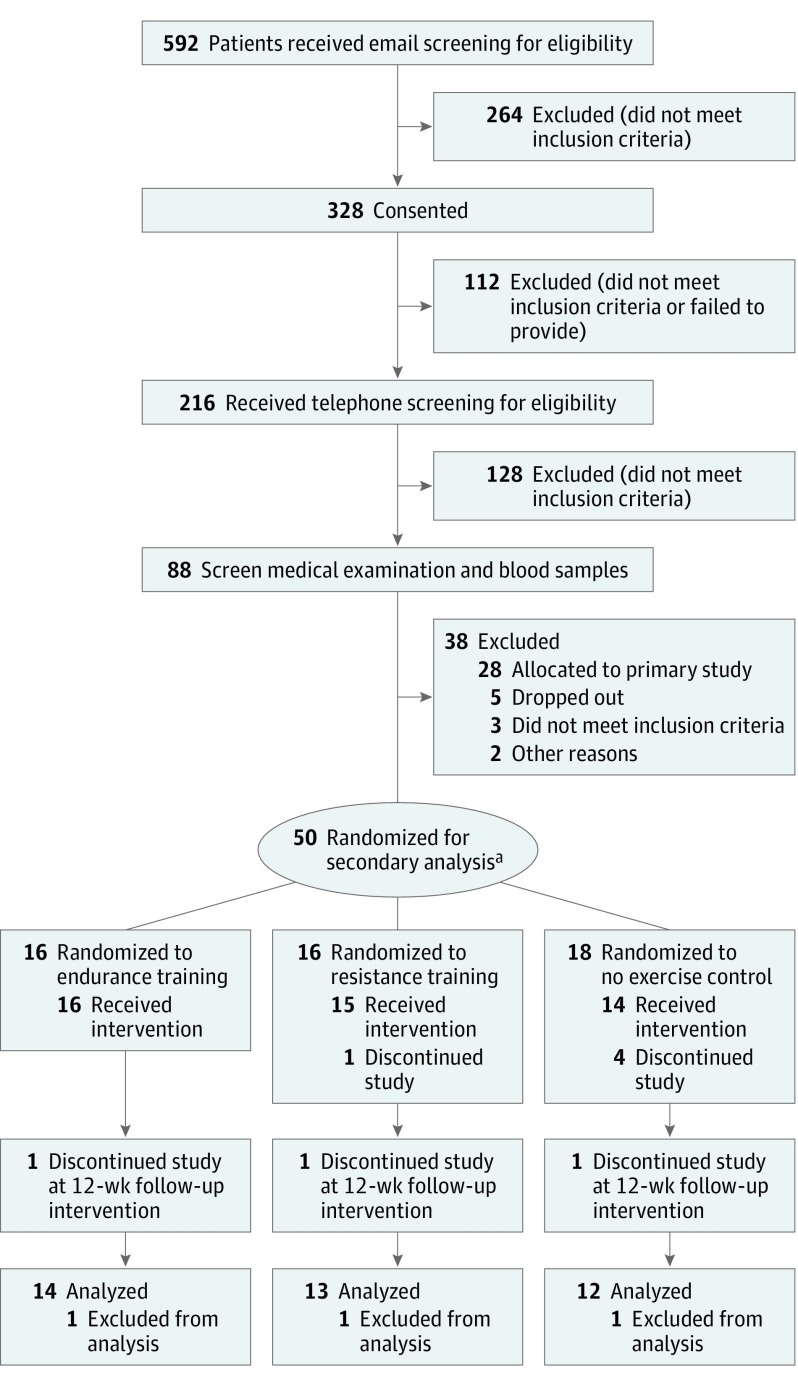
Of the 50 randomized participants, 11 participants discontinued or were excluded from the study. Eight of them discontinued without providing a reason (n = 6) or owing to lack of time (n = 2). Three participants were excluded from the analysis. The reasons for exclusion were initiation of medical anticontraceptive/anti-inflammatory treatment during the study (n = 2) and the lack of cardiac MRI follow-up scan (n = 1) (Figure 1). The final study population comprised 39 participants distributed across 3 groups: no exercise control (n = 12), endurance training (n = 14), and resistance training (n = 13) (Figure 1). No individuals were lost to follow-up. Baseline characteristics appeared to be similar between groups (Table 1).
Figure 1. CONSORT Flow Diagram.
aEighty-three participants were randomized in the primary analysis.
Table 1. Baseline Characteristics of Study Population.
| Characteristic | No. (%) | |||
|---|---|---|---|---|
| Total (N = 39) | Endurance (n = 14) | Resistance (n = 13) | Control (n = 12) | |
| Male | 10 (26) | 3 (21) | 5 (38) | 2 (17) |
| Age, y | 41 (14) | 39 (14) | 38 (14) | 47 (12) |
| Current and previous smokers | 16 (41) | 6 (43) | 6 (46) | 4 (33) |
| Fitness | ||||
| Vo2 max, mL/min | 2655 (641) | 2550 (566) | 2871 (791) | 2543 (524) |
| 1-RM chest press, kg | 38 (15) | 38 (15) | 40 (20) | 35 (10) |
| 1-RM knee extension right, kg | 32 (11) | 31 (10) | 33 (13) | 31 (10) |
| Body composition | ||||
| Waist circumference, cm | 104 (12) | 104 (14) | 102 (9) | 108 (12) |
| Hip circumference, cm | 116 (11) | 115 (11) | 114 (8) | 119 (13) |
| Waist:height ratio | 0.6 (0.07) | 0.6 (0.08) | 0.6 (0.04) | 0.6 (0.08) |
| Body weight, kg | 95 (16) | 92 (14) | 94 (17) | 97 (18) |
| BMI | 32 (5) | 33 (5) | 30 (4) | 34 (6) |
| Whole body fat, % | 45.8 (7.4) | 48.0 (6.6) | 42.6 (6.7) | 46.6 (8.2) |
| Android, kg | 4.2 (1.3) | 4.2 (1.3) | 3.8 (1.0) | 4.6 (1.5) |
| Gynoid, kg | 7.3 (1.9) | 7.5 (1.7) | 6.8 (1.7) | 7.4 (2.4) |
| Whole body lean mass, kg | 48.7 (9.8) | 45.4 (7.4) | 51.8 (12.4) | 49.0 (8.6) |
| Cardiac adipose tissue, median (IQR), g | 166 (125-228) | 153 (137-174) | 164 (125-228) | 193 (122-253) |
| Epicardial adipose tissue, median (IQR), g | 23 (13-29) | 23 (13-26) | 22 (15-29) | 26 (16-34) |
| Pericardial adipose tissue, median (IQR), g | 148 (101-184) | 140 (113-160) | 154 (101-195) | 167 (91-227) |
| Blood pressure | ||||
| Systolic, mm Hg | 124 (26) | 128 (21) | 127 (16) | 124 (20) |
| Diastolic, mm Hg | 84 (9) | 87 (10) | 81 (9) | 85 (9) |
| Resting heart rate, bpm | 69 (11) | 68 (7) | 68 (12) | 71 (13) |
| Cardiometabolic profile | ||||
| Total cholesterol, mg/dL | 189.5 (30.9) | 185.6 (34.8) | 185.6 (30.9) | 197.2 (30.9) |
| LDL, mg/dL | 123.7 (27.1) | 119.9 (23.2) | 119.9 (27.1) | 127.6 (30.9) |
| HDL, mg/dL | 50.3 (11.6) | 54.1 (11.6) | 50.3 (7.7) | 46.4 (11.6) |
| Atherogenic indexa | 3.9 (1.0) | 3.6 (0.9) | 3.9 (0.9) | 4.4 (1.1) |
| HbA1c, % of total hemoglobin | 5.9 (0.5) | 5.8 (0.4) | 5.7 (0.5) | 6.2 (0.6) |
| HbA1c, mmol/mol | 34.5 (3.6) | 33.6 (3.0) | 33.5 (3.6) | 36.6 (3.7) |
| Fasting glucose, mg/dL | 88.2 (9.0) | 91.8 (9.0) | 84.6 (7.2) | 91.8 (9.0) |
| Pro-BNP, median (IQR), pg/mL | 50 (50-60) | 50 (50-81) | 50 (50-50) | 50 (50-50) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); bpm, beats per minute; IQR, interquartile range; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein; pro-BNP, pro-B-type natriuretic peptide; RM, repetition maximum; Vo2 max, maximal oxygen consumption.
SI conversion factors: To convert cholesterol to millimoles per liter, multiply values by 0.0259; glucose to millimoles per liter, multiply by 0.0555; pro-BNP to nanograms per liter, multiply by 1.
Total cholesterol divided by HDL.
Epicardial and Pericardial Adipose Tissue and Exercise Training
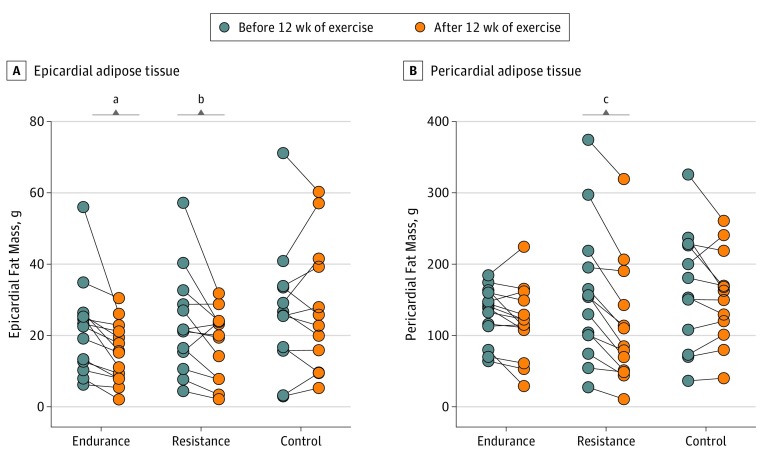
Changes in epicardial adipose tissue mass differed across the groups after the training intervention (P = .005) (Table 2 and eFigure in the Supplement). Whereas epicardial adipose tissue mass remained unchanged in the no exercise control group (2 g [95% CI, −2 to 6]), endurance training reduced epicardial adipose tissue mass by 8 g (95% CI, 4-11) (Figure 2A). Comparing the changes between groups showed that endurance exercise reduced epicardial adipose tissue more than the no exercise control group (9 g [95% CI, 4-15], P = .002) (Table 2). The resistance training group also reduced epicardial adipose tissue mass more than the control group (8 g [95% CI, 2-13], P = .009) (Table 2). The effect of resistance vs endurance training on epicardial adipose tissue mass were not different (1 g [95% CI, −4 to 7], P = .58).
Table 2. Summary of Anthropometric and Fitness Outcome Measures.
| Characteristic | Least Square Means (95% CI)a | ||||
|---|---|---|---|---|---|
| Endurance | Resistance | Control | Difference Control vs Endurance | Difference Control vs Resistance | |
| Epicardial Fat Mass, g | |||||
| Total No. | 14 | 13 | 12 | NA | NA |
| Postintervention | 17 (13 to 30)b | 18 (14 to 22)b | 26 (22 to 30) | −9 (−15 to −4) | −8 (−13 to −2) |
| Change | −8 (−11 to −4)b | −6 (−10 to −2)b | 2 (−2 to 6) | −9 (−15 to −4) | −8 (−13 to −2) |
| Relative change, % | −32 (−53 to −10)b | −24 (−46 to −1)b | 24 (1 to 24) | −56 (−88 to −24) | −48 (−81 to −15) |
| Pericardial Fat Mass, g | |||||
| 12 wk | 135 (121 to 150)c | 107 (92 to 122)b,d | 141 (126 to 156) | −6 (−27 to 15) | −34 (−55 to −12) |
| Change | −15 (−30 to −1)c | −43 (−58 to −29)b,d | −10 (−25 to 6) | −6 (−27 to 15) | −34 (−55 to −12) |
| Relative change, % | −12 (−22 to −1)c | −32 (−43 to −21)b,d | −1 (−12 to 11) | −11 (−27 to 5) | −31 (−47 to −16) |
| Fat Mass, g | |||||
| 12 wk | 40 719 (39 917 to 41 520) | 40 575 (39 728 to 41 422) | 41 666 (40 749 to 42 583) | −948 (−2162 to 267) | −1091 (−2359 to 177) |
| Change | −1053 (−1854 to −251) | −1196 (−2043 to −350) | −105 (−1022 to 812) | −948 (−2162 to 267) | −1091 (−2359 to 177) |
| Relative change, % | −3 (−5 to 0) | −4 (−6 to −1) | 0 (−3 to 2) | −2 (−6 to 1) | −3 (−7 to 0) |
| Android Fat Mass, g | |||||
| 12 wk | 4108 (3979 to 4237) | 4049 (3913 to 4186) | 4210 (4061 to 4360) | −103 (−300 to 95) | −161 (−367 to 45) |
| Change | −115 (−244 to 14) | −174 (−310 to −37) | −12 (−161 to 137) | −103 (−300 to 95) | −161 (−367 to 45) |
| Relative change, % | −3 (−7 to 1) | −5 (−9 to −1) | 0 (−4 to 4) | −3 (−9 to 3) | −5 (−11 to 1) |
| Gynoid Fat Mass, g | |||||
| 12 wk | 7257 (7066 to 7448) | 7200 (7000 to 7401) | 7251 (7034 to 7467) | 6 (−282 to 291) | −50 (−347 to 247) |
| Change | −76 (−268 to 115) | −133 (−333 to 68) | −83 (−299 to 134) | 6 (−282 to 291) | −50 (−347 to 247) |
| Relative change, % | −1 (−5 to 3) | −2 (−5 to 1) | −1 (−4 to 2) | 0 (−4 to 4) | −1 (−5 to 3) |
| Lean Body Mass, g | |||||
| 12 wk | 49 532 (486 08 to 50 456) | 49 989 (49 028 to 50 950) | 49 132 (48 109 to 50 155) | 400 (−978 to 1778) | 857 (−546 to 2261) |
| Change | 1070 (145 to 1994) | 1527 (566 to 2488) | 669 (−353 to 1692) | 400 (−978 to 1778) | 857 (−546 to 2261) |
| Relative change, % | 2 (0 to 4) | 4 (2 to 6) | 1 (−1 to 3) | −1 (3 to 1) | 0 (−2 to 2) |
| Body Weight, kg | |||||
| 12 wk | 94.1 (92.9 to 95.2) | 95.0 (93.8 to 96.2) | 95.0 (93.8 to 96.2) | −1 (−3 to 1) | 0 (−2 to 2) |
| Change | −0.6 (−1.7 to 0.6) | 0.4 (−0.8 to 1.5) | 0.4 (−0.9 to 1.6) | −1 (−3 to 1) | 0 (−2 to 2) |
| Relative change, % | −0.6 (−1.9 to 0.7) | 0.3 (−1.1 to 1.6) | 0.4 (−1.1 to 1.8) | −1 (−3 to 1) | 0 (−2 to 2) |
| BMI | |||||
| 12 wk | 32.2 (31.8 to 32.6) | 32.6 (32.1 to 33.0) | 32.5 (32.0 to 33.0) | −0.3 (−0.9 to 0.3) | 0.1 (−0.6 to 0.7) |
| Change | −0.2 (−0.6 to 0.2) | 0.2 (−0.3 to 0.6) | 0.1 (−0.4 to 0.6) | −0.3 (−0.9 to 0.3) | 0.1 (−0.6 to 0.7) |
| Relative change, % | −0.7 (−2.0 to 0.6) | 0.4 (−1.0 to 1.8) | 0.3 (−1.2 to 1.8) | −0.9 (−2.9 to 1.0) | 0.1 (−2.0 to 2.2) |
| Waist | |||||
| 12 wk, cm | 102 (100 to 105) | 100 (97 to 103)b | 105 (102 to 108) | −2 (−6 to 2) | −5 (−9 to −1) |
| Change, kg/m2 | −1 (−4 to 1) | −4 (−7 to −1)b | 1 (−2 to 4) | −2 (−6 to 2) | −5 (−9 to −1) |
| Relative change, % | −1 (−4 to 1) | −4 (−7 to −1)b | 1 (−2 to 4) | −2 (−6 to 2) | −5 (−9 to −1) |
| Vo2 max, mL/min | |||||
| 12 wk | 2859 (2744 to 2974)b | 2819 (2693 to 2945)b | 2547 (2411 to 2683) | 312 (134 to 489) | 271 (84 to 460) |
| Change | 219 (104 to 334)b | 179 (53 to 305)b | −92 (−229 to 44) | 312 (134 to 489) | 271 (84 to 460) |
| Relative change, % | 9 (4 to 13) | 8 (3 to 12) | −4 (−9 to 2) | 12 (6 to 19) | 11 (4 to 18) |
| RM Chest, kg | |||||
| 12 wk | 37 (33 to 40) | 49 (46 to 53)b,d | 38 (34 to 42) | −1 (−7 to 4) | 11 (6 to 17) |
| Change | −1 (−4 to 3) | 12 (8 to 16)b,d | 1 (−4 to 5) | −1 (−7 to 4) | 11 (6 to 17) |
| Relative change, % | 1 (−12 to 14) | 42 (28 to 55)b,d | −4 (−19 to 11) | 5 (−15 to 25) | 46 (25 to 66) |
| RM Left Knee Extension, kg | |||||
| 12 wk | 32 (29 to 36) | 42 (37 to 46)b,d | 29 (25 to 33) | 3 (−2 to 9) | 12 (6 to 18) |
| Change | 1 (−3 to 4) | 10 (6 to 14)b,d | −3 (−7 to 2) | 3 (−2 to 9) | 12 (6 to 18) |
| Relative change, % | 2 (−9 to 13) | 36 (24 to 49)b,d | −9 (−23 to 4) | 11 (−6 to 29) | 45 (27 to 64) |
| RM Right Knee Extension, kg | |||||
| 12 wk | 32 (28 to 37) | 41 (37 to 46)b,d | 29 (24 to 33) | 4 (−2 to 10) | 13 (6 to 19) |
| Change | 1 (−3 to 5) | 10 (6 to 14)b,d | −3 (−7 to 2) | 4 (−2 to 10) | 13 (6 to 19) |
| Relative change, % | 7 (−7 to 20) | 33 (18 to 48)b,d | −10 (−26 to 5) | 17 (−4 to 38) | 43 (22 to 65) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); NA, not applicable; RM, repetition maximum; Vo2 max, maximal oxygen consumption.
Values are presented as least square means (means adjusted for baseline) with 95% CI by treatment group.
P < .05 compared with control group.
P < .05 compared with resistance training.
P < .05 compared with endurance training.
Figure 2. Epicardial and Pericardial Adipose Tissue Before and After 12 Weeks of Exercise.
Epicardial (A) and pericardial (B) adipose tissue mass assessed by magnetic resonance imaging before and after 12 weeks of exercise. Within-group assessments by Wilcoxon matched pairs signed-rank test.
aP = .005.
bP = .04.
cP < .001.
Changes in the pericardial adipose tissue mass differed between groups (P = .005) (Table 2). Endurance training reduced pericardial adipose tissue by 15 g (95% CI, 1-30) (Figure 2B), but the reduction did not differ significantly from the no exercise control group (6 g [95% CI, −15 to 27], P = .58) (Table 2). In contrast and compared with the no exercise control group, resistance training reduced pericardial adipose tissue mass by 34 g (95% CI, 12-55; P = .003) (Table 2). Moreover, comparing the changes in pericardial adipose tissue induced by endurance vs resistance training revealed a more pronounced effect of resistance training (28 g [95% CI, 7-49], P = .01). Other anthropometric measures changed nonsignificantly in response to training (Table 2). An intention-to-treat analysis using baseline carried forward imputation showed that the per-protocol findings were robust (eTable 1 in the Supplement).
Cardiorespiratory Fitness and Muscle Strength
Changes in Vo2 max differed between groups (P = .003) (Table 2); Vo2 max increased 219 mL/min (95% CI, 104-334) in the endurance training group and remained unchanged in the no exercise control group (−92 mL/min [95% CI, −229 to 44]). Comparing the changes between these 2 groups showed a significant improvement in the Vo2 max after endurance training vs no exercise (312 mL/min [95% CI, 134-489], P = .001). Vo2 max also increased in response to resistance training compared with the no exercise control group (271 mL/min [95% CI, 84-460], P = .006). Improvements in Vo2 max did not differ between the endurance and resistance training groups (40 mL/min [95% CI, −132 to 212], P = .64).
Training modality affected muscle strength (Table 2). There was no change in the 1-RM chest press in the no training control group (1 kg [95% CI, −4 to 3]); in contrast, resistance training increased 1-RM chest press by 12 kg (95% CI, 8-16), which was significantly different from the no training control group (11 kg [95% CI, 6-17], P < .001) (Table 2). One-RM chest press was unaffected by endurance training (−1 kg [95% CI, −4 to 3]) and not different from the no training control group (−1 kg [95% CI, −7 to 4], P = .64). Changes in left and right quadriceps strength (knee extension) in the 2 training groups were comparable with those observed during chest press (Table 2).
Cardiac Function and Cardiometabolic Parameters
All participants had normal cardiac function at baseline with a mean (SD) LV ejection fraction of 69% (7.2%). While measures of systolic function and LV volumes did not change between the 3 groups after the intervention, LV mass changed across groups (P < .001) (Table 3). Compared with the no training control, there was an increase in LV mass by both endurance (20 g [95% CI, 11-30], P < .001) and resistance training (18 g [95% CI, 8-28], P < .001). There was no difference in the effect of resistance vs endurance training on LV mass increase (2 g [95% CI, −7 to 12], P = .63).
Table 3. Summary of Cardiac Measures.
| Characteristic | Least Square Means (95% CI)a | ||||
|---|---|---|---|---|---|
| Endurance | Resistance | Control | Difference Control vs Endurance | Difference Control vs Resistance | |
| LVEF | |||||
| Total No. | 14 | 13 | 12 | NA | NA |
| Postintervention | 69 (66 to 72) | 70 (67 to 73) | 71 (68 to 75) | −2 (−7 to 2) | −1 (−7 to 4) |
| Change | 1 (−2 to 4) | 2 (−1 to 5) | 3 (−0.2 to 7) | −2 (−7 to 2) | −1 (−7 to 4) |
| Relative change, % | 2 (−3 to 7) | 4 (−1 to 9) | 6 (0 to 12) | −4 (−11 to 4) | −2 (−11 to 6) |
| LVEDM, g | |||||
| 12 wk | 133 (127 to 140) | 131 (124 to 138) | 113 (106 to 120) | 20 (11 to 30) | 18 (8 to 28) |
| Change | 12 (5 to 18) | 9 (2 to 16) | −9 (−15 to −2) | 20 (11 to 30) | 18 (8 to 28) |
| Relative change, % | 10 (5 to 15) | 8 (3 to 13) | −7 (−12 to −1) | 16 (9 to 24) | 15 (7 to 22) |
| LVESV, mL | |||||
| 12 wk | 44 (35 to 54) | 45 (34 to 56) | 38 (27 to 49) | 6 (−9 to 21) | 7 (−9 to 23) |
| Change | 1 (−9 to 11) | 2 (−9 to 13) | −5 (−16 to 6) | 6 (−9 to 21) | 7 (−9 to 23) |
| Relative change, % | 4 (−20 to 27) | 7 (−19 to 33) | −4 (−31 to 33) | 8 (−27 to 43) | 11 (−28 to 50) |
| LVEDV, mL | |||||
| 12 wk | 141 (133 to 150) | 135 (126 to 144) | 133 (124 to 142) | 14 (0 to 28) | 3 (−12 to 18) |
| Change | 8 (−2 to 18) | −3 (−13 to 8) | −6 (−16 to 4) | 14 (0 to 28) | 3 (−12 to 18) |
| Relative change, % | 7 (−2 to 15) | 5 (−0.2 to −9) | −4 (−13 to 5) | 11 (−1 to 22) | 4 (−9 to 16) |
| LV Stroke Volume, mL | |||||
| 12 wk | 97 (89 to 106) | 94 (86 to 103) | 90 (80 to 99) | 7 (−5 to 20) | 5 (−8 to 17) |
| Change | 6 (−2 to 14) | 3 (−5 to 12) | −1 (−10 to 8) | 7 (−5 to 20) | 5 (−8 to 17) |
| Relative change, % | 9 (−1 to 18) | 6 (−4 to 15) | 1 (−9 to 11) | 8 (−6 to 21) | 5 (−9 to 19) |
| Cardiac Index, L/min/m2 | |||||
| 12 wk | 3.0 (2.7 to 3.4) | 2.8 (2.4 to 3.2) | 2.7 (2.3 to 3.1) | 0.3 (−0.3 to 0.8) | 0.1 (−0.5 to 0.6) |
| Change | 0.1 (−0.2 to 0.4) | −0.1 (−0.5 to 0.3) | −0.2 (−0.6 to 0.2) | 0.3 (−0.3 to 0.8) | 0.1 (−0.5 to 0.6) |
| Relative change, % | 8 (−6 to 24) | −1 (−17 to 15) | −2 (−18 to 15) | ||
| SBP, mm HG | |||||
| 12 wk | 127 (120 to 134) | 124 (116 to 131) | 125 (117 to 133) | −2 (−12 to 9) | −1 (−12 to 10) |
| Change | 0 (−7 to 7) | −3 (−10 to 4) | −2 (−10 to 6) | 2 (−9 to 12) | −1 (−12 to 10) |
| Relative change, % | 2 (−4 to 8) | −3 (−9 to 3) | 0 (−6 to 7) | 2 (−7 to 11) | −3 (−12 to 6) |
| Heart Rate, bpm | |||||
| 12 wk | 67 (62 to 71) | 70 (65 to 75) | 66 (61 to 71) | 1 (−6 to 7) | 4 (−3 to 11) |
| Change | −2 (−7 to 2) | 1 (−3 to 6) | −3 (−8 to 2) | 1 (−6 to 7) | 4 (−3 to 11) |
| Relative change, % | −3 (−10 to 4) | 3 (−4 to 10) | −3 (−10 to 4) | 0 (−10 to 10) | 6 (−4 to 16) |
Abbreviations: bpm, beats per minute; EF, ejection fraction; EDM, end-diastolic mass; EDV, end-diastolic volume; ESV, end-systolic volume; HR, heart rate; LV, Left ventricular; NA, not applicable; SBP, systolic blood pressure.
Values are presented as least square means (means adjusted for baseline) with 95% CI by treatment group.
There were no changes between the groups in systolic blood pressure, heart rate (Table 3), or plasma levels of total cholesterol, low-density lipoprotein and high-density lipoprotein cholesterol, triglyceride level, glucose, hemoglobin A1c, C-reactive protein, tumor necrosis factor α, interleukins 6, 1β, 8, and 10, and adiponectin after the intervention (eTable 2 in the Supplement).
Compliance, Training Intensity, and Energy Expenditure
The compliance to training was comparable between the 2 groups: 89% vs 93% in the endurance and resistance training groups, respectively (P = .07) (eTable 3 in the Supplement). Average energy intake or free-living physical activity, assessed monthly, did not differ between groups nor did it change during the intervention (eTable 4 in the Supplement).
Discussion
This study showed that endurance and resistance training reduced epicardial adipose tissue mass in physically inactive people with abdominal obesity and moreover that pericardial adipose tissue mass was only reduced by resistance training. Because epicardial and pericardial adipose tissues are emerging as risk factors for cardiovascular disease, there is a growing interest in preventive strategies to reduce fat accumulation in these depots.10 Here we demonstrate that 2 training modalities are capable of reducing cardiac adipose tissue mass. Most studies20,21 but not all33 report reduced epicardial adipose tissue after endurance training. Our study contributes by demonstrating that not only endurance, but also resistance training, can reduce epicardial adipose tissue.
Three months of resistance and endurance training were associated with reduced epicardial adipose tissue by 24% and 32%, respectively. These effects represent the isolated effect of training because food intake remained unchanged. Dietary restrictions are reported to reduce epicardial adipose tissue by 32%,19 so it is possible that combining training and dietary restriction may have a greater effect on epicardial adipose tissue mass than exercise alone. Furthermore, in comparison, treatment with sodium-glucose transporter 2 inhibitors for 6 months16 and treatment with glucagon-like peptide 1 analogs for 3 months14 resulted in 14% and 29% reductions in epicardial adipose tissue, respectively. Considering that pharmacologic treatment can be associated with adverse effects and is associated with increased economic costs compared with training-based treatment strategies, our study highlights the importance of exercise training as a means to reduce cardiac adipose tissues.
Although pericardial adipose tissue has been suggested to be a stronger cardiovascular risk marker than epicardial adipose tissue,12 the effect of exercise training on pericardial adipose tissue has largely been neglected. Our study showed that pericardial adipose tissue mass was reduced by resistance training but not endurance training. Overall, our results suggest that resistance training may be superior to endurance training as resistance training reduced pericardial adipose tissue and improved fitness and strength, while endurance training only improved fitness. Nevertheless, both exercise modalities were associated with reduced epicardial adipose tissue, suggesting that people with specific training preferences or requirements can benefit from both training modalities.
Both training modalities increased LV mass. Exercise is known to stimulate cardiac remodeling including mild cardiac hypertrophy; hence, these results were expected.34,35 Given the healthy study population without heart disease and the relative short training duration (12 weeks), we did not expect to find alterations in systolic cardiac function. Previous studies primarily report increased diastolic function or a small increase in stroke volume after 6 months of exercise training.36,37 Moreover, previous studies have shown that antidiabetic drugs, such as glucagon-like peptide 1 analogs14 and sodium-glucose transporter 2 inhibitors,16 reduced epicardial adipose tissue volume and nonfatal and fatal cardiac events.38 Whether the reduction in epicardial adipose tissue directly improves cardiac function and protects against cardiovascular disease, or is merely a biomarker, remains unclear. Further studies are needed to clarify whether training interventions can improve cardiac function via a reduction in epicardial and pericardial adipose tissue mass in a cardiovascular disease population.
Given that excessive epicardial and pericardial adipose tissue are risk factors of cardiovascular disease,6,7 our results may have clinical potential as a means to reduce cardiac adipose tissue mass. The beneficial cardiovascular-lowering risk of current antidiabetic therapeutics has been hypothesized to be in part driven by a reduction in epicardial and pericardial volume.14,16,39,40 Hence, this study shows that both endurance and resistance training may have the potential as preventive strategies.
Strengths and Limitations
Strengths of this study include the randomized, placebo-controlled clinical study design, the fact that every exercise session was supervised, and that MRI was used to determine epicardial and pericardial adipose tissue mass. Epicardial and pericardial adipose tissues covering the ventricles and not the entire heart were measured, which may underestimate the true amount of these fat depots. The study followed a per-protocol design and replaced dropouts, and this may introduce a bias (attrition bias) and limit the conclusion to only apply to people who performed exactly the prescribed amount of exercise. The small group sizes, which fell below the numbers prespecified by the power calculation, limit the interpretations of the findings and introduce a risk of type 2 errors. However, an intention-to-treat analysis using baseline carried forward imputation showed that the per-protocol findings were robust (eTable 1 in the Supplement). Importantly, the training effect on epicardial and pericardial adipose tissue was not the primary objective of the main study26,28 rendering this analysis exploratory, and thus the findings in this study need replication. We did not combine resistance and endurance training, which would have been interesting to reveal their potential additive effects.
Conclusions
In this secondary analysis of a 12-week randomized clinical trial, including physically inactive people with abdominal obesity, supervised high-intensity interval endurance training and resistance training reduced epicardial adipose tissue, while only resistance training reduced pericardial adipose tissue mass.
eFigure. Relative changes in cardiac fat mass
eTable 1. Intention to treat analysis
eTable 2. Measures of cardiometabolic and inflammatory profile
eTable 3. Training compliance and intensity
eTable 4. Free-living physical activity and energy intake
References
- 1.Pagidipati NJ, Gaziano TA. Estimating deaths from cardiovascular disease: a review of global methodologies of mortality measurement. Circulation. 2013;127(6):749-756. doi: 10.1161/CIRCULATIONAHA.112.128413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67(5):968-977. doi: 10.1161/01.CIR.67.5.968 [DOI] [PubMed] [Google Scholar]
- 3.Lavie CJ, Laddu D, Arena R, Ortega FB, Alpert MA, Kushner RF. Healthy weight and obesity prevention: JACC health promotion series. J Am Coll Cardiol. 2018;72(13):1506-1531. doi: 10.1016/j.jacc.2018.08.1037 [DOI] [PubMed] [Google Scholar]
- 4.Iacobellis G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat Rev Endocrinol. 2015;11(6):363-371. doi: 10.1038/nrendo.2015.58 [DOI] [PubMed] [Google Scholar]
- 5.Mazurek T, Zhang L, Zalewski A, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108(20):2460-2466. doi: 10.1161/01.CIR.0000099542.57313.C5 [DOI] [PubMed] [Google Scholar]
- 6.Christensen RH, von Scholten BJ, Hansen CS, et al. Epicardial, pericardial and total cardiac fat and cardiovascular disease in type 2 diabetic patients with elevated urinary albumin excretion rate. Eur J Prev Cardiol. 2017;24(14):1517-1524. doi: 10.1177/2047487317717820 [DOI] [PubMed] [Google Scholar]
- 7.Ding J, Hsu F-C, Harris TB, et al. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr. 2009;90(3):499-504. doi: 10.3945/ajcn.2008.27358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah RV, Anderson A, Ding J, et al. Pericardial, but not hepatic, fat by CT Is associated with CV outcomes and structure: the Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc Imaging. 2017;10(9):1016-102. doi: 10.1016/j.jcmg.2016.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lavie CJ, Oktay AA, Pandey A. Pericardial fat and CVD: is all fat created equally? JACC Cardiovasc Imaging. 2017;10(9):1028-1030. doi: 10.1016/j.jcmg.2016.11.018 [DOI] [PubMed] [Google Scholar]
- 10.Iacobellis G. Epicardial fat: a new cardiovascular therapeutic target. Curr Opin Pharmacol. 2016;27:13-18. doi: 10.1016/j.coph.2016.01.004 [DOI] [PubMed] [Google Scholar]
- 11.Marchington JM, Mattacks CAPC, Pond CM. Adipose tissue in the mammalian heart and pericardium: structure, foetal development and biochemical properties. Comp Biochem Physiol B. 1989;94(2):225-232. doi: 10.1016/0305-0491(89)90337-4 [DOI] [PubMed] [Google Scholar]
- 12.Sicari R, Sironi AM, Petz R, et al. Pericardial rather than epicardial fat is a cardiometabolic risk marker: an MRI vs echo study. J Am Soc Echocardiogr. 2011;24(10):1156-1162. doi: 10.1016/j.echo.2011.06.013 [DOI] [PubMed] [Google Scholar]
- 13.Ding J, Kritchevsky SB, Harris TB, et al. ; Multi-Ethnic Study of Atherosclerosis . The association of pericardial fat with calcified coronary plaque. Obesity (Silver Spring). 2008;16(8):1914-1919. doi: 10.1038/oby.2008.278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iacobellis G, Mohseni M, Bianco SD, Banga PK. Liraglutide causes large and rapid epicardial fat reduction. Obesity (Silver Spring). 2017;25(2):311-316. doi: 10.1002/oby.21718 [DOI] [PubMed] [Google Scholar]
- 15.Díaz-Rodríguez E, Agra RM, Fernández ÁL, et al. Effects of dapagliflozin on human epicardial adipose tissue: modulation of insulin resistance, inflammatory chemokine production, and differentiation ability. Cardiovasc Res. 2018;114(2):336-346. doi: 10.1093/cvr/cvx186 [DOI] [PubMed] [Google Scholar]
- 16.Sato T, Aizawa Y, Yuasa S, et al. The effect of dapagliflozin treatment on epicardial adipose tissue volume. Cardiovasc Diabetol. 2018;17(1):6. doi: 10.1186/s12933-017-0658-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexopoulos N, Melek BH, Arepalli CD, et al. Effect of intensive versus moderate lipid-lowering therapy on epicardial adipose tissue in hyperlipidemic post-menopausal women: a substudy of the BELLES trial (Beyond Endorsed Lipid Lowering with EBT Scanning). J Am Coll Cardiol. 2013;61(19):1956-1961. doi: 10.1016/j.jacc.2012.12.051 [DOI] [PubMed] [Google Scholar]
- 18.Willens HJ, Byers P, Chirinos JA, Labrador E, Hare JM de ME, de Marchena E. Effects of weight loss after bariatric surgery on epicardial fat measured using echocardiography. Am J Cardiol. 2007;99(9):1242-1245. doi: 10.1016/j.amjcard.2006.12.042 [DOI] [PubMed] [Google Scholar]
- 19.Iacobellis G, Singh N, Wharton S, Sharma AM. Substantial changes in epicardial fat thickness after weight loss in severely obese subjects. Obesity (Silver Spring). 2008;16(7):1693-1697. doi: 10.1038/oby.2008.251 [DOI] [PubMed] [Google Scholar]
- 20.Kim M-K, Tomita T, Kim M-J, Sasai H, Maeda S, Tanaka K. Aerobic exercise training reduces epicardial fat in obese men. J Appl Physiol (1985). 2009;106(1):5-11. doi: 10.1152/japplphysiol.90756.2008 [DOI] [PubMed] [Google Scholar]
- 21.Wilund KR, Tomayko EJ, Wu PT, et al. Intradialytic exercise training reduces oxidative stress and epicardial fat: a pilot study. Nephrol Dial Transplant. 2010;25(8):2695-2701. doi: 10.1093/ndt/gfq106 [DOI] [PubMed] [Google Scholar]
- 22.Rosety MA, Pery MT, Rodriguez-Pareja MA, et al. A short-term circuit resistance programme reduced epicardial fat in obese aged women. Nutr Hosp. 2015;32(5):2193-2197. [DOI] [PubMed] [Google Scholar]
- 23.Fornieles González G, Rosety Rodríguez MA, Rodríguez Pareja MA, et al. A home-based treadmill training reduced epicardial and abdominal fat in postmenopausal women with metabolic syndrome. Nutr Hosp. 2014;30(3):609-613. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez-del-Valle M, Gonzales JU, Kloiber S, Mitra S, Klingensmith J, Larumbe-Zabala E. Effects of resistance training on MRI-derived epicardial fat volume and arterial stiffness in women with obesity: a randomized pilot study. Eur J Appl Physiol. 2018;118(6):1231-1240. doi: 10.1007/s00421-018-3852-9 [DOI] [PubMed] [Google Scholar]
- 25.Lavie CJ, Ozemek C, Carbone S, Katzmarzyk PT, Blair SN. Sedentary behavior, exercise, and cardiovascular health. Circ Res. 2019;124(5):799-815. doi: 10.1161/CIRCRESAHA.118.312669 [DOI] [PubMed] [Google Scholar]
- 26.Wedell-Neergaard AS, Lang Lehrskov L, Christensen RH, et al. Exercise-induced changes in visceral adipose tissue mass are regulated by IL-6 signaling: a randomized controlled trial. Cell Metab. 2019;29(4):844-855.e3. doi: 10.1016/j.cmet.2018.12.007 [DOI] [PubMed] [Google Scholar]
- 27.Fletcher GF, Landolfo C, Niebauer J, Ozemek C, Arena R, Lavie CJ. Reprint of: promoting physical activity and exercise: JACC health promotion series. J Am Coll Cardiol. 2018;72(23 pt B):3053-3070. doi: 10.1016/j.jacc.2018.10.025 [DOI] [PubMed] [Google Scholar]
- 28.Christensen RH, Wedell-Neergaard AS, Lehrskov LL, et al. The role of exercise combined with tocilizumab in visceral and epicardial adipose tissue and gastric emptying rate in abdominally obese participants: protocol for a randomised controlled trial. Trials. 2018;19(1):266. doi: 10.1186/s13063-018-2637-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 30.Doesch C, Streitner F, Bellm S, et al. Epicardial adipose tissue assessed by cardiac magnetic resonance imaging in patients with heart failure due to dilated cardiomyopathy. Obesity (Silver Spring). 2013;21(3):E253-E261. doi: 10.1002/oby.20149 [DOI] [PubMed] [Google Scholar]
- 31.Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155-163. doi: 10.1016/j.jcm.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Treuth MS, Schmitz K, Catellier DJ, et al. Defining accelerometer thresholds for activity intensities in adolescent girls. Med Sci Sports Exerc. 2004;36(7):1259-1266. [PMC free article] [PubMed] [Google Scholar]
- 33.Jonker JT, de Mol P, de Vries ST, et al. Exercise and type 2 diabetes mellitus: changes in tissue-specific fat distribution and cardiac function. Radiology. 2013;269(2):434-442. doi: 10.1148/radiol.13121631 [DOI] [PubMed] [Google Scholar]
- 34.Fulghum K, Hill BG. Metabolic mechanisms of exercise-induced cardiac remodeling. Front Cardiovasc Med. 2018;5(September):127. doi: 10.3389/fcvm.2018.00127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeMaria AN, Neumann A, Lee G, Fowler W, Mason DT. Alterations in ventricular mass and performance induced by exercise training in man evaluated by echocardiography. Circulation. 1978;57(2):237-244. doi: 10.1161/01.CIR.57.2.237 [DOI] [PubMed] [Google Scholar]
- 36.Verboven M, Van Ryckeghem L, Belkhouribchia J, et al. Effect of exercise intervention on cardiac function in type 2 diabetes mellitus: a systematic review. Sports Med. 2019;49(2):255-268. doi: 10.1007/s40279-018-1003-4 [DOI] [PubMed] [Google Scholar]
- 37.Hambrecht R, Gielen S, Linke A, et al. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure: A randomized trial. JAMA. 2000;283(23):3095-3101. doi: 10.1001/jama.283.23.3095 [DOI] [PubMed] [Google Scholar]
- 38.Zinman B, Wanner C, Lachin JM, et al. ; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi: 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 39.Dutour A, Abdesselam I, Ancel P, et al. Exenatide decreases liver fat content and epicardial adipose tissue in patients with obesity and type 2 diabetes: a prospective randomized clinical trial using magnetic resonance imaging and spectroscopy. Diabetes Obes Metab. 2016;18(9):882-891. doi: 10.1111/dom.12680 [DOI] [PubMed] [Google Scholar]
- 40.Yagi S, Hirata Y, Ise T, et al. Canagliflozin reduces epicardial fat in patients with type 2 diabetes mellitus. Diabetol Metab Syndr. 2017;9(1):78. doi: 10.1186/s13098-017-0275-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Relative changes in cardiac fat mass
eTable 1. Intention to treat analysis
eTable 2. Measures of cardiometabolic and inflammatory profile
eTable 3. Training compliance and intensity
eTable 4. Free-living physical activity and energy intake