Abstract
This review of novel oncology drugs approved by the US Food and Drug Administration examines the use of surrogate end points for overall survival, evaluates the use of patient-reported outcomes in trials supporting approvals, and seeks to determine whether drugs initially approved without evidence of overall survival or patient-reported outcome benefits demonstrate improvements in either measure postapproval.
The US Food and Drug Administration (FDA) may approve drugs based on surrogate end points that reasonably predict that a drug provides clinical benefit.1,2 If approved via the accelerated approval pathway, the FDA may require postmarketing studies to confirm the perceived clinical benefit. Assessment of patient-reported outcomes (PROs), defined as any report on a patient’s health that comes directly from the patient, can also play a key role in understanding benefits and tolerability of oncology drugs.3 We sought to examine the use of surrogate end points for overall survival (OS) in new oncology drug approvals, to evaluate the use of PROs in trials supporting approvals, and to determine whether oncology drugs initially approved without evidence of OS or PRO benefits demonstrated improvements in either measure postapproval.
Methods
We reviewed the FDA’s yearly Novel Drug Summary for drugs approved for oncology indications in 2011 through 2017. We accessed the Drugs@FDA database and analyzed pivotal trial data supporting approval, approved indications, approval type, and PROs. For pivotal trials and postapproval studies, clinical trial designs and study results were identified through ClinicalTrials.gov, PubMed, and abstracts of major oncology conferences. This study was exempt from institutional review board approval owing to its exclusive use of publicly available data.
Results
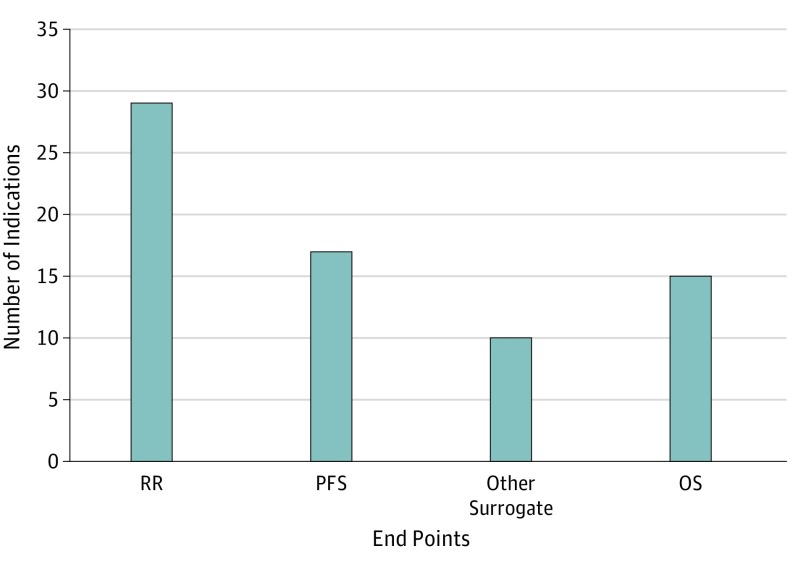
Between 2011 and 2017, 65 drugs were approved for 71 oncology indications. For 15 of the 71 (21%) initial indications, the approval was supported by OS data (median OS gain, 1.7 months [range, 1.4-11.8 months]). For 54 of the 71 (76%) initial indications, the approval was supported by a surrogate end point (Figure 1). For indications approved based on OS data, 14 of the 15 (93%) indications were granted via traditional regulatory pathways vs 23 of the 54 (43%) indications based on surrogate end points.
Figure 1. Overall Survival and Surrogate End Points Used as the Basis of Approval for 69 Initial Indications of 63 Novel Oncology Drugs Approved by the FDA Between 2011 and 2017a,b,c.
FDA indicates the US Food and Drug Administration; OS, overall survival; PFS, progression-free survival; RR, response rate. Other surrogates include event-free survival, invasive disease-free survival, major cytogenetic response, major hematologic response, complete hematologic response, overall hematologic response, complete remission/complete remission with partial hematological recovery, complete response/complete response with partial hematologic recovery, and complete remission/incomplete remission.
aInstead of approval based on OS or a surrogate, ruxolitinib and asparaginase Erwinia chrysanthemi were approved on the basis of spleen volume reduction and asparaginase activity level, respectively.
bResponse rate and PFS were coprimary end points of the pivotal trial for elotuzumab; both end points were reached.
cResponse rate was the primary end point of the abemaciclib monotherapy trial while PFS was the primary end point of the abemaciclib-fulvestrant combination trial.
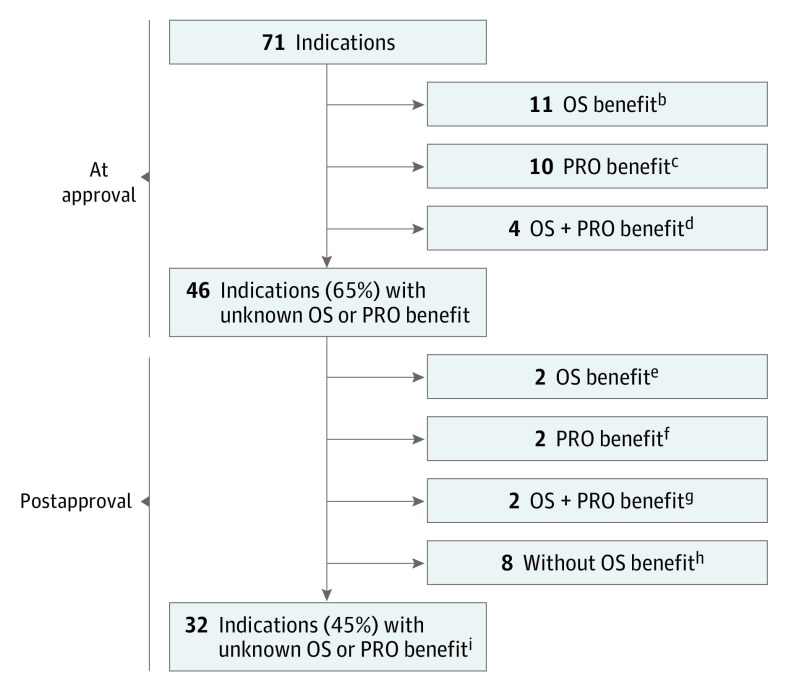
With a median follow-up of 4.1 years postapproval, 8 additional drugs showed improvement in OS for the initial indication. In total, an improvement in OS was shown for 23 of the 71 (32%) initial indications; no improvement was shown for 13 of the 71 (18%) initial indications; and for 35 of the 71 (49%) initial indications, the effect of the drug on OS remains unknown (Figure 2).
Figure 2. OS and PRO Benefits for 71 Initial Indications of 65 Novel Oncology Drugs Approved by the FDA Between 2011 and 2017a.
FDA indicates the US Food and Drug Administration; OS, overall survival; PRO, patient-reported outcome.
aA drug was considered to have demonstrated an OS benefit if OS was a prespecified primary or secondary end point of a pivotal trial (or postapproval study) and a statistically significant difference was observed between the drug and comparator arms. A drug was considered to have demonstrated an improvement in a PRO if a statistically significant difference was reported between the drug and comparator arms for a global score, a subscale, or a specific item from a validated PRO instrument evaluated in the trial(s).
bMidostaurin (indication: acute myeloid leukemia), olaratumab, necitumumab, cobimetinib, trifluridine/tipiracil, ramucirumab, regorafenib, ziv-aflibercept, pertuzumab, vemurafenib, and ipilimumab.
cInotuzumab ozogamicin, pembrolizumab, ceritinib, pomalidomide, dabrafenib, trametinib, afatinib, crizotinib, ruxolitinib, and vandetanib.
dAdo-trastuzumab emtansine, radium 223, enzalutamide, and abiraterone acetate.
eIxazomib and obinutuzumab (OS benefit was demonstrated for a total of 6 indications postapproval; 4 [pembrolizumab, pomalidomide, trametinib, and ruxolitinib] had previously demonstrated a PRO benefit).
fOsimertinib and ibrutinib.
gBlinatumomab and idelalisib (indication: chronic lymphocytic leukemia).
hPanobinostat, trabectedin, lenvatinib, nivolumab, olaparib, cabozantinib, atezolizumab, and axitinib (no OS benefit was shown for a total of 13 indications; 5 [afatinib, crizotinib, vandetanib, ibrutinib, and inotuzumab ozogamicin] had previously demonstrated a PRO benefit). For 5 indications (afatinib, axitinib, inotuzumab ozogamicin, lenvatinib, and trabectedin), the lack of OS benefit was known at the time of initial approval.
iDurvalumab, ribociclib, brigatinib, midostaurin (indication: aggressive systemic mastocytosis), enasidenib, acalabrutinib, copanlisib, avelumab (2 indications), neratinib, abemaciclib, niraparib, rucaparib, venetoclax, daratumumab, alectinib, sonidegib, dinutuximab, belinostat, bosutinib, vismodegib, palbociclib, elotuzumab, ponatinib (2 indications), carfilzomib, omacetaxine, asparaginase Erwinia chrysanthemi, brentuximab (2 indications), and idelalisib (indications: follicular B-cell non–Hodgkin lymphoma and small lymphocytic lymphoma).
We found that drugs for 50 of the 71 (70%) indications evaluated PROs during pivotal trials supporting initial approval. Fourteen drugs demonstrated a statistically significant improvement in at least one PRO; only 1 of the 14, ruxolitinib, was granted a PRO labeling claim at the time of approval. Postapproval, an additional 4 drugs demonstrated a PRO benefit. In total, a statistically significant improvement in PROs was shown for 18 of the 71 (25%) initial indications.
Discussion
We found that the use of surrogate end points has increased in recent years (76% vs 67% during 2008-2012).4 For new oncology drug indications based on OS, OS gains were marginal. In the absence of OS benefit, an argument can be made that novel oncology drugs might provide patients with better quality of life. However, only a quarter of the indications showed a statistically significant improvement in PROs.
More than half of the indications for oncology drugs we evaluated have not demonstrated an OS benefit nor a PRO improvement. A recent review of oncology drugs approved by the European Medicines Agency reported a similar finding.5 While drug costs were not evaluated in our analysis, one study found that costs were comparable among oncology drugs regardless of whether OS or PRO benefits had been demonstrated.6
It is recognized that evaluation of OS can be challenging or unfeasible in some instances and is complicated by factors such as use of crossover trial design. Where possible, the requirement for postmarketing studies confirming an OS benefit, enforcement of timely completion of the studies, and action should those studies fail to show a benefit represents one avenue by which the FDA could improve the number of oncology drugs that provide true meaningful benefits to patients.
References
- 1.US Food and Drug Administration Code of Federal Regulations Title 21. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=314.510. Updated April 1, 2018. Accessed May 30, 2019.
- 2.US Food and Drug Administration Table of surrogate endpoints that were the basis of drug approval or licensure. https://www.fda.gov/drugs/development-resources/table-surrogate-endpoints-were-basis-drug-approval-or-licensure. Accessed May 30, 2019.
- 3.Basch E. Beyond the FDA PRO guidance: steps toward integrating meaningful patient-reported outcomes into regulatory trials and US drug labels. Value Health. 2012;15(3):401-403. doi: 10.1016/j.jval.2012.03.1385 [DOI] [PubMed] [Google Scholar]
- 4.Kim C, Prasad V. Cancer drugs approved on the basis of a surrogate end point and subsequent overall survival: an analysis of 5 years of US Food and Drug Administration approvals. JAMA Intern Med. 2015;175(12):1992-1994. doi: 10.1001/jamainternmed.2015.5868 [DOI] [PubMed] [Google Scholar]
- 5.Davis C, Naci H, Gurpinar E, Poplavska E, Pinto A, Aggarwal A. Availability of evidence of benefits on overall survival and quality of life of cancer drugs approved by European Medicines Agency: retrospective cohort study of drug approvals 2009-13. BMJ. 2017;359:j4530. doi: 10.1136/bmj.j4530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rupp T, Zuckerman D. Quality of life, overall survival, and costs of cancer drugs approved based on surrogate endpoints. JAMA Intern Med. 2017;177(2):276-277. doi: 10.1001/jamainternmed.2016.7761 [DOI] [PubMed] [Google Scholar]