Key Points
Question
What is the role of restaging magnetic resonance imaging (MRI) after chemoradiotherapy or radiotherapy, and which specific patients might benefit from a lateral lymph node dissection (LLND)?
Findings
In this multicenter pooled cohort study including 741 patients with low rectal cancer after chemoradiotherapy or radiotherapy, shrinkage of lateral nodes from a short-axis node size of 7 mm or greater on primary MRI to a short-axis node size of 4 mm or less on restaging MRI abolished the risk of lateral local recurrence (LLR). However, in persistently enlarged nodes (greater than 4 mm) in the internal iliac compartment on restaging MRI, the risk of LLR was high, and an LLND lowered this risk significantly.
Meaning
Persistently enlarged nodes in the internal iliac compartment indicate a high risk of LLR, and an LLND should be seriously considered in these patients.
This cohort study investigates the factors on primary and restaging magnetic resonance imaging that are associated with lateral local recurrence in low rectal cancer after chemoradiotherapy or radiotherapy and to formulate specific guidelines on which patients might benefit from a lateral lymph node dissection.
Abstract
Importance
Previously, it was shown in patients with low rectal cancer that a short-axis (SA) lateral node size of 7 mm or greater on primary magnetic resonance imaging (MRI) resulted in a high lateral local recurrence (LLR) rate after chemoradiotherapy or radiotherapy ([C]RT) with total mesorectal excision (TME) and that this risk was lowered by a lateral lymph node dissection (LLND). The role of restaging MRI after (C)RT with regard to LLR risk and which specific patients might benefit from an LLND is not fully understood.
Objective
To determine the factors on primary and restaging MRI that are associated with LLR in low rectal cancer after (C)RT and to formulate specific guidelines on which patients might benefit from an LLND.
Design, Setting, and Participants
In this retrospective, multicenter, pooled cohort study, patients who underwent surgery for cT3 or cT4 low rectal cancer with a curative intent from 12 centers in 7 countries from January 2009 to December 2013 were included. All patients’ MRIs were rereviewed according to a standardized protocol, with specific attention to lateral nodal features. The original cohort included 1216 patients. For this study, patients who underwent (C)RT and had a restaging MRI were selected, leaving 741 for analyses across 10 institutions, including 651 who underwent (C)RT with TME and 90 who underwent (C)RT with TME and LLND.
Main Outcomes and Measures
The main purpose was to identify the factors on primary and restaging MRI associated with LLR after (C)RT with TME. Whether high-risk patients might benefit in terms of LLR reduction from an LLND was also studied.
Results
Of the 741 included patients, 480 (64.8%) were male, and the mean (SD) age was 60.4 (12.0) years. An SA lateral node size of 7 mm or greater on primary MRI resulted in a 5-year LLR rate of 17.9% after (C)RT with TME. At 3 years, there were no LLRs in 28 patients (29.2%) with lateral nodes that were 4 mm or less on restaging MRI. Nodes that were 7 mm or greater on primary MRI and greater than 4 mm on restaging MRI in the internal iliac compartment resulted in a 5-year LLR rate of 52.3%, significantly higher compared with nodes in the obturator compartment of that size (9.5%; hazard ratio, 5.8; 95% CI, 1.6-21.3; P = .003). Compared with (C)RT with TME alone, treatment with (C)RT with TME and LLND in these unresponsive internal nodes resulted in a significantly lower LLR rate of 8.7% (hazard ratio, 6.2; 95% CI, 1.4-28.5; P = .007).
Conclusions and Relevance
Restaging MRI is important in clinical decision making in lateral nodal disease. In patients with shrinkage of lateral nodes from an SA node size of 7 mm or greater on primary MRI to an SA node size of 4 mm or less on restaging MRI, which occurs in about 30% of cases, LLND can be avoided. However, persistently enlarged nodes in the internal iliac compartment indicate an extremely high risk of LLR, and an LLND lowered LLR in these cases.
Introduction
Local recurrence rates in rectal cancer have reduced dramatically since the introduction of the total mesorectal excision (TME) technique.1 These rates have been lowered further with the use of neoadjuvant chemoradiotherapy or radiotherapy ([C]RT) regimens in appropriate cases, decreasing overall rates of 5-year local recurrence to 5% to 10%.2,3,4 Western surgeons have always relied on (C)RT to sterilize the lateral compartment, containing internal iliac and obturator lymph nodes, and to alleviate fears of operative morbidity and nerve function disorders associated with a lateral lymph node dissection (LLND), which is mainly performed in the East.5 Furthermore, most Western clinicians consider lateral nodal disease to represent metastatic disease not amendable to cure.5,6
Single-center studies in 20157 and 20178 have shown that (C)RT with TME is not sufficient to eradicate lateral nodal disease in enlarged nodes, resulting in 30% to 40% 5-year lateral local recurrence (LLR) rates in nodes that are 10 mm or greater, with about half of patients presenting with only localized disease at the time of local recurrence diagnosis. Also, some Japanese centers that combine (C)RT with TME and LLND show excellent disease-free survival rates, suggesting that patients with lateral nodal disease can be cured.9,10
The Lateral Node Study Consortium11 undertook a multicenter study including 12 centers from 7 countries, collecting data over a 5-year period and including all consecutive patients who underwent an operation for cT3 or cT4 low rectal cancer. In all patients, every series of magnetic resonance imaging (MRI) was rereviewed by a standardized protocol, examining lateral pelvic nodes and defining these according to size and the presence of malignant features and relating these to the development of locally recurrent disease. In the first publication of the Lateral Node Study Consortium with a total of 1216 patients,11 it was shown that pretreatment lateral lymph node (LLN) size of 7 mm or greater resulted in an unacceptably high incidence of LLR of 19.5%, despite (C)RT with TME. Within the Lateral Node Study Consortium, several centers performed LLNDs after (C)RT, which resulted in a significantly lower rate of LLR of 5.7% in nodes of 7 mm or greater (P = .04).
In this multicenter study, 75% of the patients who had received (C)RT underwent restaging MRI following treatment. To our knowledge, there is no consensus in the literature on whether the risk of recurrence should be determined by the primary MRI (pre-[C]RT MRI) or the restaging MRI. The goal of the current study is to assess which factors on primary and restaging MRI are associated with lateral nodal recurrence and to formulate specific guidelines on which patients might benefit from an LLND.
Methods
Study Participants and Patient Selection
This study included patients from 12 centers in 7 countries. All participating hospitals were asked to collect the data and to rereview the MRI scans of all consecutive patients who underwent an operation for cT3 or cT4 rectal cancer within 8 cm from the anal verge measured on MRI from January 2009 to December 2013. Exclusion criteria were the absence of (high-quality) MRI scans, the presence of distant metastases, or a noncurative resection (R2 resection status). The treatment regimens and initial results of the 1216 patients regarding primary MRI staging have been published previously.11 As stated previously, each center received institutional review board approval according to local policies. For the current analyses, patients who had not received neoadjuvant (C)RT (248 patients) or had no restaging MRI (227 patients) were excluded, leaving 741 patients (60.9%) for analyses across 10 institutions (eFigure 1 in the Supplement). Informed consent was not obtained, as deidentified data were used.
Reassessment of MRIs
Magnetic resonance imaging reassessment guidelines have been described previously.11 In short, each center used a specific protocol with a color map atlas of the pelvis for reevaluation of pretreatment and posttreatment MRIs by a local expert radiologist (eFigure 2 in the Supplement). In addition to the standard American Joint Committee on Cancer TNM staging, circumferential resection, and tumor height assessment, radiologists were asked to assess LLN status. This was based on the largest LLN identified on pretreatment MRI, of which short-axis (SA) and long-axis node size and location (internal iliac, external iliac, or obturator compartment) were recorded. The stretched benign lymph nodes, located just behind the distal part of the external iliac vein, were specifically not included in the assessment. Furthermore, the presence of malignant features, eg, internal heterogeneity or border irregularity, was also noted.
The assessment was repeated by the same radiologist on restaging MRI, recording the SA and long-axis node sizes and the presence of malignant features on the same lateral nodes after (C)RT. Shrinkage was defined as any reduction in SA node size, with shrinkage size defined as the difference in millimeters and disappearance defined as no visible node left in the compartment after (C)RT. Shrinkage rate was defined as the rate of reduction in SA size of a lymph node on the restaging MRI compared with the SA node size on primary MRI. In patients with local recurrence, imaging was rereviewed, and the recurrent site was categorized into 1 of 5 types: lateral, presacral, anastomotic site, anterior, or perineal, of which the definitions have been described previously.12,13
Statistical Analyses
Statistical analyses were conducted using SPSS Statistics version 23 (IBM) and the survival ROC package of R version 3.4.3 (The R Foundation).14,15 For median values, interquartile ranges (IQRs) were given. Individual variables were compared with Mann-Whitney U tests, t tests, and χ2 tests, as appropriate; a 2-tailed P value of less than .05 was considered significant. Time-dependent receiver operating characteristic (ROC) curves for survival data and area under the ROC curves (AUCs) at 3 and 5 years after surgery were used to evaluate the predictive value of LLN size variables in relation to LLR. Survival curves for LLR, overall local recurrence, distant recurrence rates, and cancer-specific survival were calculated using the Kaplan-Meier method. To determine the risk factors, the effects of covariates were analyzed using a univariate Cox regression model. Subsequently, a multivariate analysis using covariates with a significant effect (P < .10) was performed, in which a P value of less than .05 was considered significant. Regarding response classification, no response was defined as similar outcomes in pathological T staging as in clinical T staging; tumor response was defined as any reduction in pathological T stage compared with primary clinical T stage, as long as there was still tumor left; and complete response was defined as a pathologic complete response with no (viable) tumor tissue identified.
Results
Patients
Of the 741 included patients, 480 (64.8%) were male, and the mean (SD) age was 60.4 (12.0) years. A total 65 patients (8.8%) had a local recurrence, 185 (25.0%) had a distant recurrence, and 107 (14.4%) died of cancer recurrence over a median (IQR) follow-up duration of 52 (37-64) months after surgery. Restaging MRIs were performed a median (IQR) of 35 (29-42) days after the final date of neoadjuvant (C)RT, and resection was performed a median (IQR) of 54 (46-71) days after (C)RT. Baseline characteristics and pathological results are shown in Table 1.
Table 1. Patient Characteristics and Pathological Results.
| Variable | No. (%) |
|---|---|
| Total, No. | 741 |
| Sex | |
| Male | 480 (64.8) |
| Female | 261 (35.2) |
| Age, mean (SD), y | 60.4 (12.0) |
| cT stage | |
| cT3 | 515 (69.5) |
| cT4 | 226 (30.5) |
| cN stage | |
| cN0 | 215 (29.0) |
| cN1 | 289 (39.0) |
| cN2 | 237 (32.0) |
| Location of lateral lymph node | |
| None visible | 256 (34.5) |
| External iliac | 32 (4.3) |
| Obturator | 304 (41.1) |
| Internal iliac | 149 (20.1) |
| Preoperative radiotherapy | |
| Short course | 89 (12.0) |
| Long course | 652 (88.0) |
| Operation | |
| Low anterior resection | 322 (43.4) |
| Hartmann operation | 16 (2.2) |
| Intersphincteric resection | 102 (13.8) |
| (Extended) abdominoperineal resection | 292 (39.4) |
| Pelvic exenteration | 9 (1.2) |
| LLND | |
| No | 651 (87.9) |
| Yes | 90 (12.1) |
| Adjuvant chemotherapy | |
| No | 433 (58.4) |
| Yes | 262 (35.4) |
| Missing | 46 (6.2) |
| ypT stage | |
| ypT0 | 120 (16.2) |
| ypT1 | 45 (6.1) |
| ypT2 | 191 (25.8) |
| ypT3 | 330 (44.5) |
| ypT4 | 55 (7.4) |
| ypN stage | |
| ypN0 | 513 (69.3) |
| ypN1 | 150 (20.2) |
| ypN2 | 78 (10.5) |
| R status | |
| R0 | 697 (94.1) |
| R1 | 44 (5.9) |
Abbreviations: LLND, lateral lymph node dissection; R, residual tumor.
LLN Sizes on Primary and Restaging MRI
At least 1 visible LLN was identified on primary MRI in 485 patients (65.5%). The median (IQR) SA node size of the largest LLN on primary MRI was 5.0 (4.0-7.0) mm. The LLN had disappeared on restaging MRI in 64 patients (13.2%); the chance of disappearance was 15.9% (54 of 340) in patients with pretreatment nodes smaller than 7 mm, while the chance of disappearance was only 6.9% (10 of 145) in patients with pretreatment nodes of 7 mm or greater (risk ratio, 2.3; 95% CI, 1.2-4.4; P = .01). Of 421 patients in whom the LLN remained visible on restaging MRI, the median (IQR) SA node size on restaging MRI was 4.0 (3.0-5.6) mm.
Predictive Performance of SA Node Size Variables on LLR After (C)RT With TME
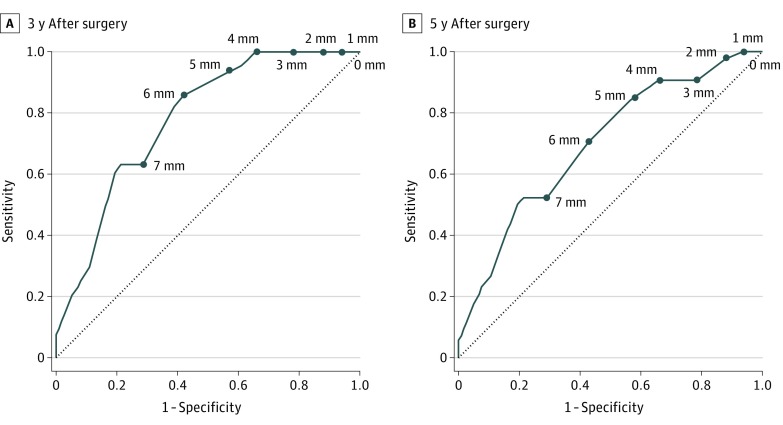
Of 651 patients who underwent (C)RT with TME, 33 (5.1%) developed an LLR (5-year LLR rate, 6.1%); of these, 26 (79%) occurred in the first 3 years after surgery. In 96 patients (14.7%) with an SA node size of 7 mm or greater on primary MRI, the 5-year LLR rate was 17.9%, significantly higher than the 5-year LLR rate in the 555 patients (85.3%) with an SA node size smaller than 7 mm (4.1%; hazard ratio [HR], 4.6; 95% CI, 2.3-9.2; P < .001). At 3 years after surgery, there were no LLRs in the 28 patients (29%) with an SA node size of 4 mm or less on reimaging MRI. eFigure 3 in the Supplement shows the time-dependent ROC curves for SA node size on restaging MRI, shrinkage rate, and shrinkage size at 3 and 5 years after surgery. The AUC value was the highest for SA nodes on restaging MRI; thus, this was chosen as the posttreatment reference. Figure 1 shows the time-dependent ROC curves for SA node size on restaging MRI at 3 and 5 years after surgery; in SA nodes greater than 4 mm on restaging MRI, the AUC value decreased, leaving an uncertainty in assessing risk of LLR.
Figure 1. Time-Dependent Receiver Operating Characteristic Curves for Short-Axis Node Size on Restaging Magnetic Resonance Imaging at 3 and 5 Years After Surgery in 651 Patients Who Underwent Chemoradiotherapy or Radiotherapy With Total Mesorectal Excision.
The dotted line indicates the baseline of receiver operating characteristic curve analysis. The data points indicate the cutoff values of lateral lymph node short-axis sizes. The distance from this line to the data points indicates the capacity of the cutoff value to distinguish positive from negative lateral local recurrence. A, The area under the receiver operating characteristic curve was 0.780. B, The area under the receiver operating characteristic curve was 0.698.
Size vs Malignant Features
In the previous Lateral Node Study Consortium publication,11 it was shown that malignant features on primary MRI were not associated with LLR after multivariate analyses. In this study, malignant features on restaging MRI were present in 68 of 342 patients (19.8%) with visible nodes that underwent (C)RT with TME; they were more common in patients with an SA node size of 7 mm or greater on primary MRI (48 of 91 [53%]) than in the patients with an SA node size less than 7 mm on primary MRI (20 of 251 [8.0%]; risk ratio, 1.9; 95% CI, 1.6-2.4; P < .001). None of the LLNs that had an SA node size of 4 mm or less on restaging MRI had any malignant features, whereas malignant features were present in 48 of 68 patients (71%) with an SA node size greater than 4 mm on restaging MRI. Univariate and multivariate analyses showed that malignant features on restaging MRI increased the risk of LLR further in LLNs with an SA node size greater than 4 mm on restaging MRI in patients that underwent (C)RT with TME (Table 2; eTable in the Supplement).
Table 2. Multivariate Analyses of Risk Factors for Lateral Local Recurrence, Local Recurrence, Distant Recurrence, and Cancer-Specific Survival Among 651 Patients Who Underwent Chemoradiotherapy or Radiotherapy With Total Mesorectal Excisiona.
| Variable | Lateral Local Recurrence | Local Recurrence | Distant Recurrence | Cancer-Specific Survival | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Sex | ||||||||
| Male | NA | NA | 1 [Reference] | .04 | NA | NA | NA | NA |
| Female | NA | 1.7 (1.0-3.0) | NA | NA | ||||
| Age, y | ||||||||
| <62 | NA | NA | NA | NA | 1 [Reference] | .049 | 1 [Reference] | .01 |
| ≥62 | NA | NA | 1.4 (1.4-1.8) | 1.7 (1.1-2.5) | ||||
| cT stage | ||||||||
| cT3 | 1 [Reference] | .64 | 1 [Reference] | .31 | 1 [Reference] | .29 | 1 [Reference] | .19 |
| cT4 | 1.2 (0.6-2.5) | 1.3 (0.8-2.4) | 1.2 (0.8-1.7) | 1.4 (0.9-2.1) | ||||
| cN stage | ||||||||
| cN0 | NA | NA | 1 [Reference] | .08 | 1 [Reference] | .001 | 1 [Reference] | .02 |
| cN1 | NA | 1.1 (0.5-2.3) | 1.5 (1.0-2.3) | 1.1 (0.7-1.9) | ||||
| cN2 | NA | 2.0 (1.0-3.9) | 2.2 (1.4-3.3) | 1.9 (1.1-3.1) | ||||
| Location of lateral lymph node | ||||||||
| None visible | 1 [Reference] | .01 | 1 [Reference] | .08 | 1 [Reference] | .007 | NA | NA |
| External iliac | 1.6 (0.2-14.5) | 2.6 (0.9-7.0) | 2.5 (1.4-4.4) | NA | ||||
| Obturator | 2.4 (0.8-7.6) | 0.9 (0.5-1.8) | 1.0 (0.7-1.4) | NA | ||||
| Internal iliac | 5.9 (1.8-19.4) | 1.7 (0.8-3.8) | 0.8 (0.5-1.4) | NA | ||||
| SA node size and malignant features | ||||||||
| <7 mm on Primary MRI | 1 [Reference] | .01 | 1 [Reference] | .17 | NA | NA | NA | NA |
| ≥7 mm on Primary MRI and ≤4 mm on restaging MRI with no malignant features | 0.6 (0.1-4.9) | 1.0 (0.3-3.3) | NA | NA | ||||
| ≥7 mm on Primary MRI and >4 mm on restaging MRI with no malignant features | 2.8 (0.8-9.9) | 2.1 (0.8-5.4) | NA | NA | ||||
| ≥7 mm on Primary MRI and >4 mm on restaging MRI with malignant features | 4.0 (1.7-9.5) | 2.1 (1.0-4.6) | NA | NA | ||||
| Operation | ||||||||
| Sphincter preserving | NA | NA | NA | NA | 1 [Reference] | .18 | 1 [Reference] | .06 |
| Non–sphincter preserving | NA | NA | 1.2 (0.9-1.7) | 1.5 (1.0-2.3) | ||||
| R status | ||||||||
| R0 | 1 [Reference] | .21 | 1 [Reference] | .003 | 1 [Reference] | .001 | 1 [Reference] | <.001 |
| R1 | 2.0 (0.7-5.9) | 3.1 (1.5-6.6) | 2.4 (1.4-4.0) | 3.9 (2.1-6.6) | ||||
| Response | ||||||||
| Complete response | NA | NA | 1 [Reference] | <.001 | 1 [Reference] | <.001 | 1 [Reference] | <.001 |
| Tumor response | NA | 2.5 (0.8-8.5) | 2.6 (1.4-4.9) | 2.4 (1.0-5.9) | ||||
| No response | NA | 5.7 (1.7-18.7) | 4.9 (2.6-9.2) | 5.1 (2.1-12.1) | ||||
Abbreviations: HR, hazard ratio; MRI, magnetic resonance imaging; NA, not applicable; R, residual tumor; SA, short-axis.
Comparisons that were not significant on univariate analysis did not undergo multivariate analyses.
Location of Enlarged Lateral Nodes
The location of the LLN was a significant risk factor for LLR after (C)RT with TME (Table 2; eTable in the Supplement). Enlarged external iliac nodes did not result in any LLRs (0% LLR rate) but significantly influenced distant recurrence, with a more than 2-fold risk.
Short-axis node size of 7 mm or greater on primary MRI was significantly more common in the internal iliac compartment (32 of 94 [34%]) than in the obturator compartment (56 of 274 [20.4%]; risk ratio, 1.2; 95% CI, 1.0-1.5; P = .008). Table 3 shows LLR rates for different cutoff values in SA node size on restaging MRI for patients with LLNs of 7 mm or greater on primary MRI, separated by obturator vs internal iliac compartment. In obturator nodes, the risk of LLR was 0% in LLNs with an SA node size of 6 mm or less on restaging MRI. However, in internal iliac nodes, the 3-year LLR risk increased rapidly if SA node size was greater than 4 mm on restaging MRI. Short-axis node size of 7 mm or greater on primary MRI and greater than 4 mm on restaging MRI in the internal iliac compartment resulted in a 5-year LLR rate of 52.3%, significantly higher compared with nodes of that size in the obturator compartment (9.5%; HR, 5.8; 95% CI, 1.6-21.3; P = .003).
Table 3. Lateral Local Recurrence (LLR) Rates and Overall Recurrence (OAR) Rates for Different Cutoff Values in Short-Axis (SA) Node Size on Restaging Magnetic Resonance Imaging (MRI) Among Patients With an SA Node Size of 7 mm or Greater on Primary MRI Who Underwent Chemoradiotherapy or Radiotherapy With Total Mesorectal Excision, Separated by Compartment.
| SA Node Size Cutoff on Restaging MRI | Obturator | Internal Iliac | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. (%) | LLR, % | OAR, % | No. (%) | LLR, % | OAR, % | |||||
| 3-y Rate | 5-y Rate | 3-y Rate | 5-y Rate | 3-y Rate | 5-y Rate | 3-y Rate | 5-y Rate | |||
| 0 mm | ||||||||||
| 0 mm | 3 (5) | 0 | 0 | 33.3 | 33.3 | 1 (3) | 0 | 0 | 0 | 0 |
| >0 mm | 53 (95) | 6.1 | 6.1 | 21.2 | 26.9 | 31 (97) | 29.9 | 44.8 | 43.1 | 55.6 |
| 1 mm | ||||||||||
| ≤1 mm | 3 (5) | 0 | 0 | 33.3 | 33.3 | 1 (3) | 0 | 0 | 0 | 0 |
| >1 mm | 53 (95) | 6.1 | 6.1 | 21.2 | 26.9 | 31 (97) | 29.9 | 44.8 | 43.1 | 55.6 |
| 2 mm | ||||||||||
| ≤2 mm | 7 (13) | 0 | 0 | 14.7 | 14.7 | 2 (6) | 0 | 0 | 50.0 | 50.0 |
| >2 mm | 49 (88) | 6.7 | 6.7 | 23.0 | 29.1 | 30 (94) | 31.1 | 46.9 | 44.7 | 47.9 |
| 3 mm | ||||||||||
| ≤3 mm | 11 (20) | 0 | 0 | 9.1 | 9.1 | 6 (19) | 0 | 20.0 | 16.7 | 33.3 |
| >3 mm | 45 (80) | 7.4 | 7.4 | 25.1 | 31.9 | 26 (81) | 37.0 | 52.3 | 52.8 | 64.6 |
| 4 mm | ||||||||||
| ≤4 mm | 20 (36) | 0 | 0 | 10.0 | 15.3 | 7 (22) | 0 | 20.0 | 16.7 | 33.3 |
| >4 mm | 36 (64) | 9.5 | 9.5 | 28.8 | 34.3 | 25 (78) | 37.0 | 52.3 | 52.8 | 64.6 |
| 5 mm | ||||||||||
| ≤5 mm | 26 (46) | 0 | 0 | 11.5 | 15.8 | 9 (28) | 12.5 | 30.0 | 25.0 | 40.0 |
| >5 mm | 30 (54) | 11.7 | 11.7 | 31.4 | 37.2 | 23 (72) | 34.7 | 50.6 | 51.5 | 63.6 |
| 6 mm | ||||||||||
| ≤6 mm | 35 (63) | 0 | 0 | 11.4 | 14.5 | 12 (38) | 20.5 | 45.5 | 28.4 | 50.9 |
| >6 mm | 21 (38) | 17.8 | 17.8 | 40.4 | 47.9 | 20 (63) | 34.5 | 41.8 | 54.6 | 60.1 |
| 7 mm | ||||||||||
| ≤7 mm | 41 (73) | 4.9 | 4.9 | 17.1 | 19.7 | 18 (56) | 21.2 | 40.0 | 38.6 | 53.2 |
| >7 mm | 15 (27) | 11.1 | 11.1 | 36.5 | 47.1 | 14 (44) | 38.9 | 47.6 | 52.4 | 60.3 |
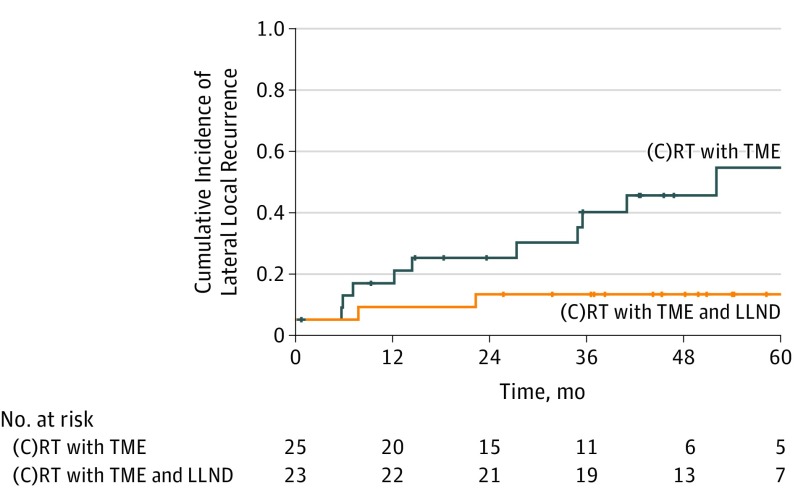
To assess the value of LLND, outcomes of patients with SA node size of 7 mm or greater on primary MRI and greater than 4 mm on restaging MRI in the internal iliac compartment who underwent (C)RT with TME were compared with outcomes of patients with similar SA node sizes treated with (C)RT with TME and LLND. Treatment including LLND resulted in a significantly lower 5-year LLR rate of 8.7% compared with treatment with (C)RT with TME alone (52.3%; HR, 6.2; 95% CI, 1.4-28.5; P = .007) (Figure 2). A total of 17 of 23 patients (74%) who underwent LLND had pathologically positive LLN. This difference was still significant when comparing patients with an SA node size of 7 mm or greater on primary MRI and greater than 4 mm on restaging MRI with malignant features in the internal compartment treated with (C)RT with TME alone with patients with similar LLR rates who underwent (C)RT with TME and LLND (HR, 4.3; 95% CI, 0.9-20.7; P = .048). There was no difference in (C)RT with TME alone vs (C)RT with TME and LLND in those with an SA node size of 7 mm or greater on primary MRI and greater than 4 mm on restaging MRI in the obturator compartment (5-year LLR: 9.5% vs 0%; HR, 31.6; 95% CI, 0-∞; P = .32), although 5 of 11 patients (45%) had positive LLNs, and there was a 0% LLR rate in the LLND group.
Figure 2. Kaplan-Meier Analysis of Lateral Lymph Node Dissection .
Kaplan-Meier plot of patients with a short-axis node size of 7 mm or greater on primary magnetic resonance imaging and greater than 4 mm on restaging magnetic resonance imaging located in the internal iliac compartment among patients who received chemoradiotherapy or radiotherapy ([C]RT) with total mesorectal excision (TME) alone and patients who received (C)RT with TME and lateral lymph node dissection (LLND). Crosses indicate censored events.
Discussion
This study, including 741 patients from 10 centers who underwent operations over a 5-year period for cT3 or cT4 low rectal cancer, demonstrated that both primary and restaging MRI are important in clinical decision making in lateral nodal disease. Shrinkage of nodes from an SA node size of 7 mm or greater on primary MRI to 4 mm or less on restaging MRI abolishes the risk of LLR at 3 years. This occurs in 30% of patients and defines an important group in whom LLND can be avoided, as it probably offers no benefit. However, in persistently enlarged nodes in the internal iliac compartment, the risk of LLR is extremely high (52.3%), and LLND has an important role, as it lowers LLR significantly in these cases.
The first conclusion that can be made is that size matters. It was shown in the first Lateral Node Study Consortium publication11 that relatively small lymph nodes (SA node size of 7 mm or greater) have to raise red flags with clinicians. In this study, it was shown that posttreatment size was a better predictor of LLR than the shrinkage rate or size. Shrinkage to an SA node size of 4 mm or less on restaging MRI is a safe cutoff value that keeps the risk of recurrence at 0% after 3 years. In this study, the focus was mainly on 3-year recurrence rates, as the median follow-up duration was shorter than 5 years and because of the censoring of cases, which resulted in the AUC of the ROC curves becoming smaller and thus making risk prediction less accurate (eFigure 3 in the Supplement). When LLNs with an SA node size of 7 mm or greater on primary MRI were 4 mm or less on restaging MRI after (C)RT treatment, which occurred in 30% of the cases, surgeons probably do not need to consider LLND. However, in 70% of the cases when nodes are still present, clinicians need to start weighing the options.
The second main finding from this study is that besides size of the lateral nodes, the location is a major factor of influence. Although only around 30% of the visible nodes in this study were located in the internal iliac compartment (Table 1), the percentage of LLNs with an SA node size of 7 mm or greater on primary MRI was significantly higher in this compartment. Also, these nodes had a higher chance of being unresponsive (SA node size greater than 4 mm on restaging MRI), and they tended to behave much more aggressively than obturator nodes, with a 5-year LLR rate of 52.3%. To our knowledge, this is a new finding that has not been published previously. It is known from Japanese studies that malignant LLNs were most frequently located in the internal iliac compartment after LLND,16 the rationale behind this being that they are the first basin directly from the lateral ligament.17 However, why similarly large, unresponsive nodes in the obturator compartment result in a much lower 9.5% 5-year LLR rate remains a mystery. One theory to consider is that obturator nodes generally may behave more reactively, while the internal nodes act as sentinel nodes and are more likely to contain viable tumor tissue. Another theory would be that the internal iliac nodes would not have received the full irradiation dose. As described before,11 all centers have stated that in general, both the obturator and the internal iliac compartments were included in the standard irradiated fields for these low cT3 and cT4 tumors. However, in this retrospective study, it was impossible to verify this for each individual patient, but we think this latter theory is less likely, as the internal iliac nodes are closest to the mesorectum. The only way to verify this would be a prospective trial with standardized radiotherapy protocols; the Lateral Nodal Recurrence in Rectal Cancer (LaNoReC) trial is currently being prepared in the Netherlands.
The literature varies on whether persistence (using varying criteria) of lateral nodes on restaging MRI predicts involvement of nodes. These are mainly Eastern studies where LLNDs were performed18,19,20; persistence tends to result in more pathologic metastases in the resected nodes, but it does not tell us whether leaving these nodes behind would actually result in LLR. A study conducted by Kim et al20 including 31 patients showed that even responsive nodes can lead to LLRs after (C)RT with TME, but it does not define in which compartment the nodes were located, and more than 72% of the nodes were responsive, which suggests a selection bias.
In Japan, it is standard to perform an LLND of both the internal iliac and obturator compartment, irrespective of where the involved node is located. This is probably a pragmatic approach, as resecting the internal iliac compartment laparoscopically is more difficult if the obturator compartment is not resected first. Also, in this study, only the largest node was assessed; it might be possible that in some patients, there was a very large node in the obturator compartment and a smaller but more significant node in the internal iliac compartment. The method of using the largest node irrespective of compartment in this study is a representation of clinical decision making as it is currently done in Japan.
Regarding external iliac nodes, as Japanese studies have shown before,16,21 involvement of these nodes is predictive for metastatic but not for local recurrence. These patients do not benefit from an LLND and might need induction chemotherapy to address systemic disease.
Limitations
This study has limitations. As previously stated, this exploratory and hypothesis-generating study is retrospective and multi-institutional in nature, leading to a heterogeneity in patients and treatments, so the results have to be interpreted with caution.11 Additionally, sample size calculation and power analyses were not performed initially, as the study was set up retrospectively, and all centers were asked to analyze all consecutive patients who underwent operations in a 5-year period. Further, to formulate practical clinical guidelines, subgroup analyses had to be performed.
Conclusions
In conclusion, size and location of the lateral pelvic sidewall nodes on primary and restaging MRI are important and may be the key to clinical decision making. These data may provide some insight into the appropriate use of LLND in an effort to abolish preventable local recurrences after rectal cancer surgery.
eTable. Univariate analyses for risk factors for lateral local recurrence, local recurrence, distant recurrence, and cancer-specific survival in 651 patients who underwent (C)RT with TME.
eFigure 1. Flow diagram of study population adhering to the STROBE criteria.
eFigure 2. Color map atlas of the pelvis for rereview of MRIs.
eFigure 3. Time-dependent ROC curves for short-axis size on restaging MRI (post-SA), shrinkage rate, and shrinkage size at 3 and 5 years after surgery in 651 patients who underwent (C)RT with TME.
References
- 1.Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1(8496):-. doi: 10.1016/S0140-6736(86)91510-2 [DOI] [PubMed] [Google Scholar]
- 2.Cedermark B, Dahlberg M, Glimelius B, Påhlman L, Rutqvist LE, Wilking N; Swedish Rectal Cancer Trial . Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997;336(14):980-987. doi: 10.1056/NEJM199704033361402 [DOI] [PubMed] [Google Scholar]
- 3.Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. ; Dutch Colorectal Cancer Group . Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345(9):638-646. doi: 10.1056/NEJMoa010580 [DOI] [PubMed] [Google Scholar]
- 4.Sauer R, Becker H, Hohenberger W, et al. ; German Rectal Cancer Study Group . Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731-1740. doi: 10.1056/NEJMoa040694 [DOI] [PubMed] [Google Scholar]
- 5.Georgiou P, Tan E, Gouvas N, et al. Extended lymphadenectomy versus conventional surgery for rectal cancer: a meta-analysis. Lancet Oncol. 2009;10(11):1053-1062. doi: 10.1016/S1470-2045(09)70224-4 [DOI] [PubMed] [Google Scholar]
- 6.Yano H, Moran BJ. The incidence of lateral pelvic side-wall nodal involvement in low rectal cancer may be similar in Japan and the West. Br J Surg. 2008;95(1):33-49. doi: 10.1002/bjs.6061 [DOI] [PubMed] [Google Scholar]
- 7.Kim MJ, Kim TH, Kim DY, et al. Can chemoradiation allow for omission of lateral pelvic node dissection for locally advanced rectal cancer? J Surg Oncol. 2015;111(4):459-464. doi: 10.1002/jso.23852 [DOI] [PubMed] [Google Scholar]
- 8.Kusters M, Slater A, Muirhead R, et al. What to do with lateral nodal disease in low locally advanced rectal cancer? a call for further reflection and research. Dis Colon Rectum. 2017;60(6):577-585. doi: 10.1097/DCR.0000000000000834 [DOI] [PubMed] [Google Scholar]
- 9.Akiyoshi T, Ueno M, Matsueda K, et al. Selective lateral pelvic lymph node dissection in patients with advanced low rectal cancer treated with preoperative chemoradiotherapy based on pretreatment imaging. Ann Surg Oncol. 2014;21(1):189-196. doi: 10.1245/s10434-013-3216-y [DOI] [PubMed] [Google Scholar]
- 10.Matsuda T, Sumi Y, Yamashita K, et al. Outcomes and prognostic factors of selective lateral pelvic lymph node dissection with preoperative chemoradiotherapy for locally advanced rectal cancer. Int J Colorectal Dis. 2018;33(4):367-374. doi: 10.1007/s00384-018-2974-1 [DOI] [PubMed] [Google Scholar]
- 11.Ogura A, Konishi T, Cunningham C, et al. ; Lateral Node Study Consortium . Neoadjuvant (chemo)radiotherapy with total mesorectal excision only is not sufficient to prevent lateral local recurrence in enlarged nodes: results of the multicenter lateral node study of patients with low cT3/4 rectal cancer. J Clin Oncol. 2019;37(1):33-43. doi: 10.1200/JCO.18.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kusters M, Holman FA, Martijn H, et al. Patterns of local recurrence in locally advanced rectal cancer after intra-operative radiotherapy containing multimodality treatment. Radiother Oncol. 2009;92(2):221-225. doi: 10.1016/j.radonc.2009.03.002 [DOI] [PubMed] [Google Scholar]
- 13.Kusters M, Marijnen CA, van de Velde CJ, et al. Patterns of local recurrence in rectal cancer; a study of the Dutch TME trial. Eur J Surg Oncol. 2010;36(5):470-476. doi: 10.1016/j.ejso.2009.11.011 [DOI] [PubMed] [Google Scholar]
- 14.Heagerty PJ, Zheng Y. Survival model predictive accuracy and ROC curves. Biometrics. 2005;61(1):92-105. doi: 10.1111/j.0006-341X.2005.030814.x [DOI] [PubMed] [Google Scholar]
- 15.Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56(2):337-344. doi: 10.1111/j.0006-341X.2000.00337.x [DOI] [PubMed] [Google Scholar]
- 16.Kanemitsu Y, Komori K, Shida D, et al. Potential impact of lateral lymph node dissection (LLND) for low rectal cancer on prognoses and local control: a comparison of 2 high-volume centers in Japan that employ different policies concerning LLND. Surgery. 2017;162(2):303-314. doi: 10.1016/j.surg.2017.02.005 [DOI] [PubMed] [Google Scholar]
- 17.Takahashi T, Ueno M, Azekura K, Ohta H. Lateral node dissection and total mesorectal excision for rectal cancer. Dis Colon Rectum. 2000;43(10, suppl):S59-S68. doi: 10.1007/BF02237228 [DOI] [PubMed] [Google Scholar]
- 18.Oh HK, Kang SB, Lee SM, et al. Neoadjuvant chemoradiotherapy affects the indications for lateral pelvic node dissection in mid/low rectal cancer with clinically suspected lateral node involvement: a multicenter retrospective cohort study. Ann Surg Oncol. 2014;21(7):2280-2287. doi: 10.1245/s10434-014-3559-z [DOI] [PubMed] [Google Scholar]
- 19.Liang JT. Technical feasibility of laparoscopic lateral pelvic lymph node dissection for patients with low rectal cancer after concurrent chemoradiation therapy. Ann Surg Oncol. 2011;18(1):153-159. doi: 10.1245/s10434-010-1238-2 [DOI] [PubMed] [Google Scholar]
- 20.Kim HJ, Choi GS, Park JS, et al. Optimal treatment strategies for clinically suspicious lateral pelvic lymph node metastasis in rectal cancer. Oncotarget. 2017;8(59):100724-100733. doi: 10.18632/oncotarget.20121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yokoyama S, Takifuji K, Hotta T, et al. Survival benefit of lateral lymph node dissection according to the region of involvement and the number of lateral lymph nodes involved. Surg Today. 2014;44(6):1097-1103. doi: 10.1007/s00595-013-0815-y [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Univariate analyses for risk factors for lateral local recurrence, local recurrence, distant recurrence, and cancer-specific survival in 651 patients who underwent (C)RT with TME.
eFigure 1. Flow diagram of study population adhering to the STROBE criteria.
eFigure 2. Color map atlas of the pelvis for rereview of MRIs.
eFigure 3. Time-dependent ROC curves for short-axis size on restaging MRI (post-SA), shrinkage rate, and shrinkage size at 3 and 5 years after surgery in 651 patients who underwent (C)RT with TME.