Key Points
Question
What is the recent national trend in treating fulminant Clostridium difficile colitis with loop ileostomy vs total abdominal colectomy, and do they provide comparable short-term outcomes?
Findings
In this cohort study examining a national administrative database spanning 2011 to 2015, a gradual increase in the frequency of diverting loop ileostomy was observed for treating Clostridium difficile colitis. In-hospital mortality was not associated with the choice of procedure.
Meaning
Loop ileostomy, being a less invasive and organ-preserving procedure, is a viable surgical option for treating fulminant Clostridium difficile colitis and has been used increasingly frequently on a national level; however, the grounds for selection of surgical treatment need clarification.
Abstract
Importance
Diverting loop ileostomy and colonic lavage has generated much interest since it was first reported as a potential alternative to total abdominal colectomy for treating Clostridium difficile colitis in 2011. To our knowledge, few studies have validated the benefit reported in the initial description, and the association of this new approach with practice patterns has not been described.
Objective
To examine the national adoption pattern and outcomes of diverting loop ileostomy vs total abdominal colectomy as treatment for fulminant C difficile colitis.
Design, Setting, and Participants
This retrospective cohort study used data from hospitals participating in the National Inpatient Sample database across the United States from January 2011 to September 2015 and included 3021 adult patients who underwent surgery for C difficile colitis during the study period, comprising 2408 subtotal colectomies and 613 loop ileostomies. The data were analyzed between November 2018 and April 2019.
Exposures
Loop ileostomy as surgery of choice.
Main Outcomes and Measures
In-hospital mortality.
Results
Of 2408 participants, 1416 (58.8%) were women, 1781 (78.4%) were white, and 627 (21.6%) were individuals of color and the mean (SD) age was 68.2 (14.8) years. During the overall study period, 613 patients (20.28%) underwent diverting loop ileostomy without total abdominal colectomy. The annual proportion of patients undergoing only diversion increased from 11.16% in 2011 to 25.30% in 2015. Significantly more loop ileostomies were performed within the first day of hospitalization, in contrast to subtotal colectomies (23.31% vs 12.21%; P < .01). There was no significant difference in in-hospital mortality rates between the 2 groups (25.98% vs 31.18%; P = .28).
Conclusions and Relevance
This study demonstrates the adoption of diverting loop ileostomy to treat C difficile colitis across the United States. While fulminant C difficile colitis remains a condition with high mortality rates, no significant difference in this outcome was observed between loop ileostomy and total abdominal colectomy. Loop ileostomy may represent a viable surgical alternative to total abdominal colectomy, although the grounds for selection of treatment need to be clarified.
This cohort study describes US trends in adopting loop ileostomy for treating fulminant C difficile colitis.
Introduction
Fulminant Clostridium difficile colitis (FCDC), defined as C difficile colitis that is refractory to nonoperative interventions, including antibiotics and rectal lavage, has traditionally been treated with laparotomy, total abdominal colectomy (TAC), and endileostomy. Although colonic lavage to reduce the intraluminal burden of toxins has been under investigation with bench research for at least 3 decades,1,2 diverting loop ileostomy (LI) and intraoperative colonic lavage as a clinically relevant surgical option for FCDC was first reported by Neal et al3 in 2011. They reported a lower mortality than historical controls who have undergone TAC. Other groups4,5 have since used this therapy in patients with FCDC and found varying degrees of success. Interestingly, the relapse of C difficile infection has been described on reversal of LI for FCDC in isolated cases.6 A recent database study of nearly 457 patients with FCDC found no mortality differences between TAC and LI.7
Despite whether there is demonstrable improvement in mortality, LI received a high level of interest in the acute care surgery community, likely in part because of its less invasive and organ-preserving nature compared with TAC. These perceived advantages may have lowered the threshold for surgical intervention for treating FCDC. In fact, some have hypothesized that the survival benefit observed in patients receiving LI may arise from the earlier timing of surgical intervention rather than the intrinsic differences attributable to the procedure used.8
Because of the low incidence of FCDC and novel nature of LI for this indication, currently available evidence for using this therapy is primarily derived from studies with small sample sizes. Similarly, to our knowledge, the adoption rate of LI, its generalized outcomes, and justification for its use in various settings remain uncharacterized. This study examined the national trends in the use of LI for treating FCDC across the United States and compared its outcomes with patients receiving TAC.
Methods
We performed a retrospective cohort study of patients with FCDC undergoing either TAC or LI in the United States from January 2011 to September 2015. Because of the deidentified nature of the data, this study was deemed exempt by the institutional review board of the University of California, Los Angeles. Informed consent was waived because the study used secondary data that had been deidentified.
Nationwide Inpatient Sample Database
The Healthcare Cost and Utilization Project Nationwide Inpatient Sample (NIS) is the largest all-payer health care administrative database in the United States, with a weighted sampling of approximately 20% of hospitalizations from nonfederal acute care hospitals in the sampled states. The sampling algorithm is designed to allow an estimation of nationally representative statistics that pertains to inpatient outcomes. It includes patient-level and hospital-level variables from each hospital discharge, including demographics, up to 15 primary and secondary diagnoses, procedures performed, payment source, length of stay, hospital characteristics, in-hospital mortality, and complications.
Case Identification
While no consensus definition for FCDC exists, the term is commonly used to designate C difficile colitis that is at the most complicated and severe end along its disease severity spectrum and is refractory to medical management.8 In this study, we defined fulminant disease as C difficile colitis that required surgical intervention with either LI or TAC.
We identified adult patients (age >18 years) with a discharge diagnosis of C difficile colitis, who received an International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code of 008.45, and had undergone TAC (ICD-9 procedure code 45.8x) or LI (ICD-9 procedure code 46.01). Instances in which TAC and LI were performed concomitantly were included in the TAC group. To avoid confounding the analyses with surgeries performed for indications other than FCDC, we excluded any patient with concomitant diagnoses of ulcerative colitis, Crohn disease, ischemic colitis, cancer, and lower gastrointestinal hemorrhage. These patient selection criteria were adopted based on previously published work using the NIS.9
Statistical Analysis
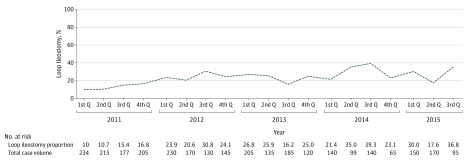
Data were stored and analyzed using Stata, version 13.0 (StataCorp). We summarized and compared the baseline patient and hospital characteristics for patients who underwent TAC or LI for FCDC using NIS definitions.10 Patient-level variables of interest included age, sex, race, payer (Medicare, Medicaid, private insurance including a health maintenance organization, and other), Elixhauser comorbidity index, and median household income. Hospital-level variables included hospital bed size (small, medium, and large), location/teaching status (rural, urban/nonteaching, and urban/teaching), and geographical region (northeast, Midwest, south, and west). The baseline burden of comorbidities was quantified using the Elixhauser index, a previously validated composite marker of chronic illness, as described elsewhere.11 Furthermore, we described the temporal change in the use of LI to treat FCDC since 2011 when the study by Neal et al3 was published. Quarterly proportions of surgeries in which LI was elected were calculated and depicted in Figure 1. A modified Wilcoxon rank sum test for trends across ordered groups was used to assess the significance of temporal trends.
Figure 1. National Trend in Operative Case Volume and Operation of Choice for Fulminant Clostridium difficile Colitis.
We compared the in-hospital outcomes between TAC and LI. The primary outcome of interest was in-hospital mortality. Secondary outcomes included length of stay and surgical complications, such as hemorrhage (ICD-9: 998.1x, 578.9), infection (ICD-9: 998.51, 998.59), and wound disruption (ICD-9: 998.30-32, 998.83). Furthermore, multivariate logistic regression analyses were performed to adjust for potential confounders that arose from baseline differences between patients receiving TAC and LI.
Continuous variables were reported as mean (SD) when the underlying distribution followed a normal distribution and as median (interquartile range) when skewed. The Wilcoxon Mann-Whitney U test was used for continuous variables, whereas the Fisher exact test and Pearson χ2 were used for categorical variables. All tests were unpaired with significance level defined as a 2-tailed α of <.05.
A power analysis was performed to determine the minimal sample size needed to detect meaningful differences in outcomes between patients undergoing TAC and LI. Three levels of absolute risk reduction (15%, 20%, and 25%) were used for patients undergoing LI, using mortality and complication rates with patients undergoing TAC used as the reference. All power analyses were performed with P level of .05 and power of 0.20 using a 2-sided z test with continuity correction and unpooled variance.
Results
Study Cohort
A total of 3021 patients underwent surgery for FCDC during the study period; of these, 2408 (79.7%) received TAC and 613 (20.3%) underwent LI. The mean (SD) age of the overall sample was 66.5 (15.6) years, with 1727 (57.3%) being women and 2231 (73.8%) being white. More than two-thirds (69.9%) of these patients were treated in urban teaching hospitals. Notably, only 15 patients (0.6%) who eventually received TAC also received LI during the same hospitalization, likely representing treatment failure with LI.
Trends in national case volumes and fractions treated with LI are shown in Figure 1. While the total annual operations performed for FCDC declined from 831 in 2011 to 445 cases in 2014, the proportion treated with LI more than doubled (12.9% to 26.5%) during the study period.
Patient and Hospital Characteristics
Patients undergoing LI were younger on average (age, 60.4 [16.6] years vs 68.2 [14.8] years; P < .01) and had fewer comorbidities (Elixhauser index, >5; 9.6% vs 13.3%; P = .03). In addition, LI appeared to occur more often in hospitals with a large bed size (76.3% vs 65.4%; P = .05) and urban teaching hospitals (84.0% vs 66.3%; P < .01) compared with TAC. However, the 2 groups were similar in the distribution of sex, race, median household income, or hospital geographical region. The results of the bivariate analysis are displayed in Table 1, where comparisons were made between patients undergoing LI and TAC.
Table 1. Summary of Patient-Level and Hospital-Level Characteristics of Patients Receiving Surgery for Fulminant Clostridium difficile Colitis.
| Characteristic | No. (%) | P Value | |
|---|---|---|---|
| Total Abdominal Colectomy (n = 2408 [79.7%]) | Loop Ileostomy (n = 613 [20.3%]) | ||
| Patient-Level Variables | |||
| Age, mean (SD), y | 68.2 (14.8) | 60.4 (16.6) | <.01 |
| Sex | |||
| Men | 992 (41.2) | 301 (48.7) | .07 |
| Women | 1416 (58.8) | 312 (51.3) | |
| Race/ethnicity | |||
| White | 1781 (78.4) | 450 (78.3) | .96 |
| Individuals of color | 627 (21.6) | 163 (21.7) | |
| Payer | |||
| Medicare | 1686 (70.3) | 320 (52.3) | <.01 |
| Medicaid | 245 (10.2) | 105 (17.1) | |
| Private insurance | 369 (15.4) | 168 (27.4) | |
| Self-pay | 45 (1.9) | <10 | |
| Others | 54 (2.3) | 15 (2.4) | |
| Elixhauser index | |||
| 0 ~ 2 | 494 (20.5) | 202 (32.9) | .03 |
| 3-5 | 1593 (66.2) | 352 (57.5) | |
| >5 | 321 (13.3) | 59 (9.6) | |
| Median household income | |||
| First quartile (lowest) | 631 (26.5) | 163 (27.3) | .39 |
| Second quartile | 637 (26.8) | 213 (35.7) | |
| Third quartile | 667 (28.0) | 124 (20.7) | |
| Fourth quartile (highest) | 445 (18.7) | 98 (16.4) | |
| Hospital-Level Variables | |||
| Bed size | |||
| Small | 205 (8.5) | 60 (9.8) | .05 |
| Medium | 629 (26.1) | 85 (13.9) | |
| Large | 1574 (65.4) | 467 (76.3) | |
| Location/teaching status | |||
| Rural | 121 (5.0) | 15 (2.4) | <.01 |
| Urban nonteaching | 691 (28.7) | 83 (13.5) | |
| Urban teaching | 1596 (66.3) | 515 (84.0) | |
| Region | |||
| Northeast | 525 (21.8) | 192 (31.4) | .14 |
| Midwest | 567 (23.5) | 158 (25.8) | |
| South | 939 (39.0) | 193 (31.6) | |
| West | 377 (15.7) | 69 (11.3) | |
| Outcome Variables | |||
| Mortality | 749 (31.1) | 159 (26.0) | .28 |
| Length of stay, median (IQR), d | 16 (10-24) | 18 (11-32.5) | NA |
| Postoperative hemorrhage | 39 (1.6) | 10 (1.6) | .99 |
| Wound disruption | 55 (2.3) | 34 (5.6) | .04 |
| Infections complications | 87 (3.6) | 55 (8.9) | .01 |
Abbreviations: IQR, interquartile range; NA, not applicable.
Outcome Comparison Between LI and TAC
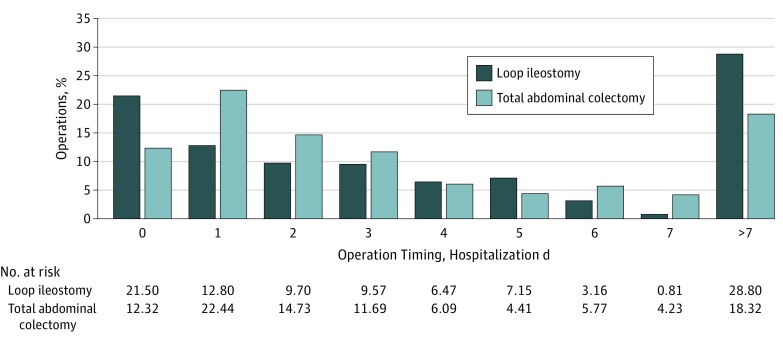
Among those receiving surgery for FCDC, the operations most commonly occurred on hospital day 0 or 1. The differences in operation timing between LI and TAC groups are shown in Figure 2. The median hospital day at which operation occurred was similar (P = .49) for IL (median day 3; 25th and 75th quartiles were days 1 and 8) and TAC (median day 3; 25th and 75th quartiles were days 1 and 7). However, a significantly higher proportion of patients undergoing LI received surgery on hospital day 0 than patients undergoing TAC (21.2% vs 11.4%; P < .01). In addition, we noted that a higher proportion of LI cases also occurred after hospital day 7 than TAC (28.8% vs 13.3%; P < .01).
Figure 2. Operation Timing for Subtotal Colitis and Loop Ileostomy for Fulminant Clostridium difficile Colitis.
The overall in-hospital mortality rate for patients undergoing surgery for FCDC was 30.1%, without significance difference between LI and TAC (26.0% vs 31.1%; P = .28). In addition, no obvious trend in mortality was apparent for surgeries performed on different hospital days (Table 2). There was also no difference observed in the length of stay following LI and TAC. The LI and TAC groups had a similar incidence of postoperative hemorrhage (1.6% vs 1.6%; P = .99). Surprisingly, we observed higher rates of operative wound disruption (5.6% vs 2.3%; P = .04) and surgical site infection (8.9 vs 3.6%, P = .01) among patients receiving LI compared with TAC.
Table 2. Mortality Rate Following Operations Performed on Different Hospital Days.
| Mortality Rate (%) | Time to Surgery (Hospital Day No.) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | >7 | |
| Loop ileostomy | 11.27 | 18.94 | 33.33 | 33.02 | 12.5 | 22.61 | 51.23 | 0 | 39.79 |
| Total abdominal colectomy | 38.04 | 33.8 | 27.2 | 23 | 40 | 29.85 | 35.46 | 16.03 | 30.41 |
| Whole cohort | 29.27 | 31.76 | 28.13 | 24.83 | 33.68 | 27.59 | 37.55 | 15.22 | 32.71 |
In view of the baseline differences observed in age, comorbidities, and hospital teaching status between patients undergoing TAC and LI, we performed several multivariate logistic regressions to further evaluate the association between surgical approach and in-hospital mortality (Table 3). We found no association between the choice of operation and postoperative mortality despite adjusting for the previously described preoperative and postoperative variables. Higher age (adjusted odds ratio [aOR], 1.03; 95% CI, 1.02-1.04; P < .001), Medicaid coverage (aOR, 1.55; 95% CI, 1.01-2.36; P = .04), and a higher Elixhauser comorbidity index score (aOR, 1.13; 95% CI, 1.05-1.21; P = .02) were associated with higher in-hospital mortality. On the other hand, having received surgery at a hospital in the western United States (aOR, 0.58; 95% CI, 0.39-0.85; P = .01) and experiencing wound disruption (aOR, 0.36; 95% CI, 0.17-0.76; P = .01) or infectious complications (aOR, 0.54; 95% CI, 0.31-0.95; P = .03) were associated with lower postoperative mortality. The paradoxical association between complications and lower mortality was likely attributable to the longer time at risk for patients that survived the operation, as patients who died during hospitalization appeared to die early following surgery and thus had a shorter time at risk for experiencing operations.
Table 3. Logistic Regression Models Evaluating for Factors Associated With Mortality.
| Characteristic | Model 1: Univariate Analysis | Model 2: Multivariate Analysis With Preoperative Variables | Model 3: Multivariate Analysis With Preoperative and Postoperative Variables | |||
|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | P Value | Adjusted Odds Ratio (95% CI) | P Value | Adjusted Odds Ratio (95% CI) | P Value | |
| Surgery performed | ||||||
| Loop ileostomy | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| Total abdominal colectomy | 1.32 (0.99-1.75) | .06 | 1.22 (0.90-1.64) | .20 | 1.19 (0.88-1.61) | .25 |
| Age, y | 1.03 (1.02-1.04) | <.001 | 1.03 (1.02-1.04) | <.001 | ||
| Sex | ||||||
| Men | NA | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| Women | 0.84 (0.67-1.05) | .14 | 0.84 (0.67-1.05) | .12 | ||
| Race/ethnicity | ||||||
| White | NA | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| Individuals of color | 1.04 (0.80-1.33) | .79 | 1.03 (0.80-1.33) | .82 | ||
| Payer | ||||||
| Medicare | NA | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| Medicaid | 1.55 (1.01-2.37) | .046 | 1.55 (1.01-2.36) | .04 | ||
| Private insurance | 0.78 (0.55-1.11) | .17 | 0.79 (0.55-1.12) | .19 | ||
| Self-pay | 0.55 (0.16-1.92) | .35 | 0.55 (0.16-1.88) | .34 | ||
| Others | 2.37 (1.06-1.23) | .02 | 2.31 (1.12-4.80) | .02 | ||
| Elixhauser index | ||||||
| 0 ~ 2 | NA | NA | 1.14 (1.07-1.23) | <.001 | 1.13 (1.05-1.21) | .02 |
| Median household income | ||||||
| First quartile (lowest) | NA | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| Second quartile | 1.23 (0.92-1.65) | .17 | 1.26 (0.94-1.69) | .13 | ||
| Third quartile | 1.10 (0.80-1.52) | .56 | 1.09 (0.79-1.52) | .59 | ||
| Fourth quartile (highest) | 0.86 (0.62-1.20) | .39 | 0.86 (0.62-1.21) | .39 | ||
| Hospital-level variables | ||||||
| Bed size | ||||||
| Small | NA | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| Medium | 1.11 (0.73-1.70) | .61 | 1.10 (0.72-1.67) | .67 | ||
| Large | 1.19 (0.82-1.74) | .36 | 1.18 (0.80-1.72) | .40 | ||
| Location/teaching status | ||||||
| Rural | NA | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| Urban nonteaching | 1.14 (0.57-2.26) | .71 | 1.15 (0.57-2.31) | .69 | ||
| Urban teaching | 1.30 (0.67-2.54) | .44 | 1.30 (0.66-2.56) | .45 | ||
| Region | ||||||
| Northeast | NA | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| Midwest | 0.76 (0.55-1.04) | .09 | 0.76 (0.55-1.05) | .10 | ||
| South | 0.91 (0.67-1.24) | .55 | 0.90 (0.66-1.23) | .51 | ||
| West | 0.57 (0.39-0.84) | .004 | 0.58 (0.39-0.85) | .01 | ||
| Outcome variables | ||||||
| Postoperative hemorrhage | NA | NA | NA | NA | 1.39 (0.84-2.30) | .20 |
| Wound disruption | 0.36 (0.17-0.76) | .01 | ||||
| Infectious complication | 0.54 (0.31-0.95) | .03 | ||||
Abbreviation: NA, not applicable.
Power Analysis
Power analyses were calculated for 3 levels of absolute risk reduction (15%, 20%, and 25%) for patients undergoing LI using a fixed mortality rate of 30% for patients undergoing TAC. If the in-hospital mortality rates for patients undergoing LI were assumed to be 25.5% (15% relative risk reduction), 24% (20% relative risk reduction), or 22.5% (relative risk reduction, 25%), and assuming a standard deviation of 10%, then a total of 156, 88, or 56 patients, respectively, would be needed in the comparison groups to detect the desired mortality difference with 80% power. Given the availability of 2408 patients undergoing TAC and 613 patients undergoing LI, this study would have been adequately powered to detect mortality differences as small as a 15% risk reduction.
Discussion
To our knowledge, this is the first description of the national trend in adopting LI for treating FCDC. During the study period, FCDC remained a severe condition, with an in-hospital mortality of more than 30%. We found that since the description of LI and colonic lavage as a viable surgical option for FCDC in 2011, a clear change in procedure choice for FCDC has taken place throughout the United States (Figure 1). Age, burden of comorbidity, and the teaching status of hospitals were all associated with using LI rather than TAC. Furthermore, no obvious difference in in-hospital mortality was observed between TAC and LI. This observation was further validated by a multivariate logistic regression analysis.
The prevalence of in-hospital C difficile infection has been rising throughout the United States,12 with virulent strains like BI/NAP1/027 becoming more widespread.13,14 Among these, only about 1 in 100 patients develop fulminant symptoms that require surgical intervention.12,15 We found that the increasing adoption of LI occurred more rapidly in larger, urban teaching hospitals than smaller, rural, nonteaching hospitals. In addition, LI tends to be used for younger patients with fewer comorbidities. This latter observation is noteworthy in that LI is an organ-preserving operation that may ultimately lead to colon salvage. Prior studies have indicated that many patients undergoing LI for FCDC ultimately keep their colon.5 Future research should investigate the association of these approaches with functional and quality-of-life outcomes, as gastrointestinal function is markedly different between patients with an intact colon vs an ileorectal anastomosis.16
The initial study by Neal et al3 cited a major survival benefit associated with LI (a mortality rate of 19% vs 50%). However, to our knowledge, this survival advantage has never been replicated to a similar extent. A prior American College of Surgeons–National Surgical Quality Improvement Program study by Hall et al7 found no difference in mortality between TAC and LI in a random national sample of 457 patients. Similarly, an institutional case series from Fashandi et al4 (sample size of 23 patients) failed to demonstrate significant mortality differences between LI and TAC. However, the multi-institutional study by Ferrada et al5 (sample size of 98 patients) observed a lower risk-adjusted mortality for LI than TAC (17.2% vs 39.7%; P = .002). In our nationwide study sample, we also found no significant difference in in-hospital mortality between TAC and LI. Two challenges in the surgical management are the lack of clearly defined guidelines regarding the surgery timing17,18,19,20,21,22 and patient selection criteria.8 Some have attributed the previously observed survival advantages associated with LI to earlier surgical intervention because of the surgeon’s readiness to operate, citing a lower threshold for surgical intervention because LI was perceived as being less invasive, less morbid, and potentially reversible compared with TAC.8 In this study, we found that a significantly higher proportion of patients undergoing LI received surgery on hospital day 0 than patients undergoing TAC, although mortality was similar for both procedures regardless of the timing of surgery. It is also possible that surgeons have used LI for less severe cases FCDC instead of continued medical management, thereby biasing the mortality outcomes in LI’s favor. Notably, this study observed a slightly higher wound and infectious complication rate that was associated with LI. This was unexpected, as LI was frequently performed laparoscopically and usually does not require any mobilization. This finding also conflicted with prior studies5,7 in which lower perioperative complication rates for LI were observed. The reason for this discordance is likely because of the inconsistent definition of complications in different databases and may also reflect the inability to capture postdischarge complications.
Limitations
We recognize several limitations to this study. As with all prior publications comparing LI and TAC, this study is retrospective in design, and therefore selection bias limits the generalizability of the results. The existing literature has identified only compromised colonic viability as a contraindication to LI.3,4 However, each surgeon’s choice of procedure was likely influenced by personal experience and comfort level with the procedure, as well as various clinical variables not captured by the database. Most management guidelines recommend early surgical consultation without offering advice on the choice of operation.23 In this study, we do not have information regarding the presence of pneumoperitoneum, use of vasopressors, extent of end organ dysfunction, and other known risk factors for mortality21,24 and can therefore not comprehensively adjust for the severity of colitis at the time of surgery.
This study is also limited by the administrative nature of the database used. As a nationwide sample of 20% of all inpatient admissions, the NIS is ideal for studying the overall population-based adoption of novel surgical interventions, such as LI for FCDC. This is the consideration that motivated its use for this study. Nonetheless, the NIS lacks the coding data to determine whether intraoperative colonic lavage was performed according to the protocol initially proposed by Neal et al.3 The experience from our institution is that this can be an often omitted step, especially with care teams not accustomed to this infrequently applied protocol. The use of postoperative antegrade vancomycin lavage was also not assessable through the NIS. Furthermore, the NIS lacks several important clinical variables, such as the use of vasopressors and other physiologic data, necessary to stratify the severity of FCDC. Finally, the sampled cases in this study do not represent the entire spectrum of FCDC, as we have excluded patients with concurrent inflammatory bowel disease, ischemic colitis, colon cancer, and lower gastrointestinal hemorrhage. To select only cases in which FCDC could be unambiguously attributable for the surgical intervention, we included only patients without other common colectomy and ileostomy indications, as the NIS does not provide information regarding which diagnosis acted as the clinical indication for the surgery. Currently, a randomized clinical trial (NCT02347280) is actively recruiting patient enrollment for evaluating mortality following TAC and LI, and we hope that studying this disease prospectively will help identify clinical predictors that can be used to guide the most appropriate operation for each individual patient.
Conclusions
Fulminant C difficile colitis remains associated with exceptionally high mortality following surgical intervention. The adoption of LI as a valid alternative to conventional surgical interventions, such as TAC, has more than doubled over the past few years. The data in this study corroborated prior findings regarding equivalent outcomes between LI and TAC. While results from randomized clinical trials and a better understanding of functional outcomes are both needed, it appears that LI is viable alternative for acute care surgeons during management of FCDC.
References
- 1.Wellmann W, Fink PC, Benner F, Schmidt FW. Endotoxaemia in active Crohn’s disease: treatment with whole gut irrigation and 5-aminosalicylic acid. Gut. 1986;27(7):814-820. doi: 10.1136/gut.27.7.814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alverdy J, Piano G. Whole gut washout for severe sepsis: review of technique and preliminary results. Surgery. 1997;121(1):89-94. doi: 10.1016/S0039-6060(97)90187-2 [DOI] [PubMed] [Google Scholar]
- 3.Neal MD, Alverdy JC, Hall DE, Simmons RL, Zuckerbraun BS. Diverting loop ileostomy and colonic lavage: an alternative to total abdominal colectomy for the treatment of severe, complicated Clostridium difficile associated disease. Ann Surg. 2011;254(3):423-427. doi: 10.1097/SLA.0b013e31822ade48 [DOI] [PubMed] [Google Scholar]
- 4.Fashandi AZ, Martin AN, Wang PT, et al. An institutional comparison of total abdominal colectomy and diverting loop ileostomy and colonic lavage in the treatment of severe, complicated Clostridium difficile infections. Am J Surg. 2017;213(3):507-511. doi: 10.1016/j.amjsurg.2016.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrada P, Callcut R, Zielinski MD, et al. ; EAST Multi-Institutional Trials Committee . Loop ileostomy versus total colectomy as surgical treatment for Clostridium difficile-associated disease: an Eastern Association for the Surgery of Trauma multicenter trial. J Trauma Acute Care Surg. 2017;83(1):36-40. doi: 10.1097/TA.0000000000001498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fashandi AZ, Ellis SR, Smith PW, Hallowell PT. Overwhelming recurrent Clostridium difficile infection after reversal of diverting loop ileostomy created for prior fulminant C. difficile colitis. Am Surg. 2016;82(8):e194-e195. http://www.ncbi.nlm.nih.gov/pubmed/27657564. [PMC free article] [PubMed] [Google Scholar]
- 7.Hall BR, Leinicke JA, Armijo PR, Smith LM, Langenfeld SJ, Oleynikov D. No survival advantage exists for patients undergoing loop ileostomy for clostridium difficile colitis. Am J Surg. 2019;217(1):34-39. doi: 10.1016/j.amjsurg.2018.09.023 [DOI] [PubMed] [Google Scholar]
- 8.Olivas AD, Umanskiy K, Zuckerbraun B, Alverdy JC. Avoiding colectomy during surgical management of fulminant Clostridium difficile colitis. Surg Infect (Larchmt). 2010;11(3):299-305. doi: 10.1089/sur.2010.026 [DOI] [PubMed] [Google Scholar]
- 9.Halabi WJ, Nguyen VQ, Carmichael JC, Pigazzi A, Stamos MJ, Mills S. Clostridium difficile colitis in the United States: a decade of trends, outcomes, risk factors for colectomy, and mortality after colectomy. J Am Coll Surg. 2013;217(5):802-812. doi: 10.1016/j.jamcollsurg.2013.05.028 [DOI] [PubMed] [Google Scholar]
- 10.Healthcare Cost and Utilization Project . NIS description of data elements. https://www.hcup-us.ahrq.gov/db/nation/nis/nisdde.jsp. Accessed December 1, 2019.
- 11.Austin SR, Wong Y-N, Uzzo RG, Beck JR, Egleston BL. Why summary comorbidity measures such as the Charlson comorbidity index and Elixhauser score work. Med Care. 2015;53(9):e65-e72. doi: 10.1097/MLR.0b013e318297429c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ricciardi R, Rothenberger DA, Madoff RD, Baxter NN. Increasing prevalence and severity of Clostridium difficile colitis in hospitalized patients in the United States. Arch Surg. 2007;142(7):624-631. doi: 10.1001/archsurg.142.7.624 [DOI] [PubMed] [Google Scholar]
- 13.McDonald LC, Killgore GE, Thompson A, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353(23):2433-2441. doi: 10.1056/NEJMoa051590 [DOI] [PubMed] [Google Scholar]
- 14.Loo VG, Poirier L, Miller MA, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353(23):2442-2449. doi: 10.1056/NEJMoa051639 [DOI] [PubMed] [Google Scholar]
- 15.Seder CW, Villalba MR Jr, Robbins J, et al. Early colectomy may be associated with improved survival in fulminant Clostridium difficile colitis: an 8-year experience. Am J Surg. 2009;197(3):302-307. doi: 10.1016/j.amjsurg.2008.11.001 [DOI] [PubMed] [Google Scholar]
- 16.Van Duijvendijk P, Slors JF, Taat CW, et al. Quality of life after total colectomy with ileorectal anastomosis or proctocolectomy and ileal pouch-anal anastomosis for familial adenomatous polyposis. Br J Surg. 2000;87(5):590-596. doi: 10.1046/j.1365-2168.2000.01442.x [DOI] [PubMed] [Google Scholar]
- 17.Synnott K, Mealy K, Merry C, Kyne L, Keane C, Quill R. Timing of surgery for fulminating pseudomembranous colitis. Br J Surg. 1998;85(2):229-231. doi: 10.1046/j.1365-2168.1998.00519.x [DOI] [PubMed] [Google Scholar]
- 18.Ali SO, Welch JP, Dring RJ. Early surgical intervention for fulminant pseudomembranous colitis. Am Surg. 2008;74(1):20-26. [DOI] [PubMed] [Google Scholar]
- 19.Lamontagne F, Labbé AC, Haeck O, et al. Impact of emergency colectomy on survival of patients with fulminant Clostridium difficile colitis during an epidemic caused by a hypervirulent strain. Ann Surg. 2007;245(2):267-272. doi: 10.1097/01.sla.0000236628.79550.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall JF, Berger D. Outcome of colectomy for Clostridium difficile colitis: a plea for early surgical management. Am J Surg. 2008;196(3):384-388. doi: 10.1016/j.amjsurg.2007.11.017 [DOI] [PubMed] [Google Scholar]
- 21.Dudukgian H, Sie E, Gonzalez-Ruiz C, Etzioni DA, Kaiser AM. C. difficile colitis—predictors of fatal outcome. J Gastrointest Surg. 2010;14(2):315-322. doi: 10.1007/s11605-009-1093-2 [DOI] [PubMed] [Google Scholar]
- 22.Byrn JC, Maun DC, Gingold DS, Baril DT, Ozao JJ, Divino CM. Predictors of mortality after colectomy for fulminant Clostridium difficile colitis. Arch Surg. 2008;143(2):150-154. doi: 10.1001/archsurg.2007.46 [DOI] [PubMed] [Google Scholar]
- 23.Fehér C, Mensa J. A comparison of current guidelines of five international societies on Clostridium difficile infection management. Infect Dis Ther. 2016;5(3):207-230. doi: 10.1007/s40121-016-0122-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dallal RM, Harbrecht BG, Boujoukas AJ, et al. Fulminant Clostridium difficile: an underappreciated and increasing cause of death and complications. Ann Surg. 2002;235(3):363-372. doi: 10.1097/00000658-200203000-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]