Key Points
Question
Is maintenance therapy with single-agent panitumumab noninferior to panitumumab plus fluorouracil and leucovorin after a 4-month induction treatment with panitumumab plus FOLFOX-4 in patients with previously untreated RAS wild-type metastatic colorectal cancer?
Findings
In this open-label, phase 2 randomized clinical trial of 229 patients, maintenance therapy with single-agent panitumumab alone was inferior to panitumumab plus fluorouracil-leucovorin in terms of 10-month progression-free survival (49.0% vs 59.9%).
Meaning
The continuation of single-agent anti–epidermal growth factor receptor treatment in the maintenance setting will likely achieve inferior progression-free survival compared with the continuation of chemotherapy plus an anti–epidermal growth factor receptor agent in a phase 3 confirmation trial.
This open-label, phase 2 randomized clinical trial assesses whether maintenance therapy with single-agent panitumumab was noninferior to panitumumab plus combined fluorouracil and leucovorin calcium among patients with RAS wild-type metastatic colorectal cancer.
Abstract
Importance
Few studies are available on the role of maintenance strategies after induction treatment regimens based on anti–epidermal growth factor receptors, and the optimal regimen for an anti–epidermal growth factor receptors–based maintenance treatment in patients with RAS wild-type metastatic colorectal cancer is still to be defined.
Objective
To determine whether maintenance therapy with single-agent panitumumab was noninferior to panitumumab plus fluorouracil and leucovorin after a 4-month induction treatment regimen.
Design, Setting, and Participants
This open-label, randomized phase 2 noninferiority trial was conducted from July 7, 2015, through October 27, 2017, at multiple Italian centers. Patients with RAS wild-type, unresectable metastatic colorectal adenocarcinoma who had not received previous treatment for metastatic disease were eligible. Induction therapy consisted of panitumumab plus FOLFOX-4 (panitumumab, 6 mg/kg, oxaliplatin, 85 mg/m2 at day 1, leucovorin calcium, 200 mg/m2, and fluorouracil, 400-mg/m2 bolus, followed by 600-mg/m2 continuous 24-hour infusion at days 1 and 2, every 2 weeks). Cutoff date for analyses was July 30, 2018.
Interventions
Patients were randomized (1:1) to first-line panitumumab plus FOLFOX-4 for 8 cycles followed by maintenance therapy with panitumumab plus fluorouracil-leucovorin (arm A) or panitumumab (arm B) until progressive disease, unacceptable toxic effects, or consent withdrawal. The minimization method was used to stratify randomization by previous adjuvant treatment and number of metastatic sites.
Main Outcomes and Measures
The prespecified primary end point was 10-month progression-free survival (PFS) analyzed on an intention-to-treat basis with a noninferiority margin of 1.515 for the upper limit of the 1-sided 90% CI of the hazard ratio (HR) of arm B vs A.
Results
Overall, 229 patients (153 male [66.8%]; median age, 64 years [interquartile range (IQR), 56-70 years]) were randomly assigned to arm A (n = 117) or arm B (n = 112). At a median follow-up of 18.0 months (IQR, 13.1-23.3 months]), a total of 169 disease progression or death events occurred. Arm B was inferior (upper limit of 1-sided 90% CI of the HR, 1.857). Ten-month PFS was 59.9% (95% CI, 51.5%-69.8%) in arm A vs 49.0% (95% CI, 40.5%-59.4%) in arm B (HR, 1.51; 95% CI, 1.11-2.07; P = .01). During maintenance, arm A had a higher incidence of grade 3 or greater treatment-related adverse events (36 [42.4%] vs 16 [20.3%]) and panitumumab-related adverse events (27 [31.8%] vs 13 [16.4%]), compared with arm B.
Conclusions and Relevance
In patients with RAS wild-type metastatic colorectal cancer, maintenance therapy with single-agent panitumumab was inferior in terms of PFS compared with panitumumab plus fluorouracil-leucovorin, which slightly increased the treatment toxic effects.
Trial Registration
ClinicalTrials.gov identifier: NCT02476045
Introduction
The treatment landscape of unresectable metastatic colorectal cancer (mCRC) has relevantly changed after the introduction of biological agents and the development of continuum of care strategies. The decision-making process on the optimal intensity and duration of first-line treatment in patients with mCRC is based on literature data, treatment tolerability, patients’ preferences, and costs.1
In pivotal trials of chemotherapy doublets with or without biological agents,2,3,4,5 treatment continuation was scheduled until progressive disease or unacceptable toxic effects. A negative effect on quality of life of this approach is caused by oxaliplatin-related cumulative neurotoxicity.6,7 This evidence paved the way to maintenance or intermittent strategies in patients achieving disease control after combination chemotherapy with or without bevacizumab.6,7,8,9,10,11,12 Available evidence indicates that de-escalating chemotherapy intensity in the maintenance setting significantly reduces the toxicity burden, without impairing survival outcomes.6,7 Indeed, fluorouracil and leucovorin–based maintenance treatment achieves noninferior progression-free survival (PFS) compared with no de-escalation,6 while improving the duration of disease control compared with treatment holidays.8,10,12 According to current guidelines,1 after a 4- to 6-month induction treatment with bevacizumab plus doublet or triplet13 regimens, bevacizumab plus a fluoropyrimidine is regarded as the optimal maintenance regimen.
An anti–epidermal growth factor receptor (EGFR) agent added to doublet chemotherapy is currently recommended as a first-line treatment option for patients with RAS wild-type mCRC.1,14,15,16 However, the optimal duration of anti-EGFR–based upfront treatment is still to be defined because, to date, little evidence-based data are available on the role of maintenance17,18 or intermittent19,20 strategies after anti-EGFR–based induction regimens. We investigated whether, after 4-month induction therapy with panitumumab plus oxaliplatin-based doublet chemotherapy, maintenance treatment with panitumumab was noninferior to panitumumab plus fluorouracil-leucovorin in patients with RAS wild-type mCRC.
Methods
Study Design and Patient Selection
The VALENTINO study was an open-label, randomized, multicenter phase 2 noninferiority trial whose participants were similar to those in trials that established efficacy of first-line doublets plus anti-EGFRs.2,3,4,5 The study protocol is found in Supplement 1. Inclusion criteria consisted of histologically confirmed colorectal adenocarcinoma; being 18 years or older; Eastern Cooperative Oncology Group performance status of 0 to 1; no previous treatment for advanced disease; RAS wild-type status locally assessed at local centers according to European Union–approved standard methods; disease judged unresectable by the local multidisciplinary team; measurable or evaluable lesions according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1; and adequate bone marrow, liver, and renal function. We excluded patients who had received adjuvant oxaliplatin-based chemotherapy and experienced a relapse during treatment or within 12 months from treatment completion (or within 6 months in the case of adjuvant fluoropyrimidine monotherapy). Other exclusion criteria consisted of clinically relevant cardiovascular disease, concurrent active malignant neoplasms (except for those disease free for more than 5 years), or other significant comorbidities that could affect patients’ outcomes; pregnancy, lactation, or child-bearing potential; and not using or not willing to use medically approved contraception. Institutional review board and ethics committee approval was obtained from all participating centers (eMethods in Supplement 2). All the patients provided written informed consent before any study-related procedures. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Treatment Plan and Randomization
Data were collected from July 7, 2015, through October 27, 2017. Patients were randomly assigned (1:1) to panitumumab plus FOLFOX-4 (panitumumab, 6 mg/kg [1-hour infusion for the first administration; 30-minute infusion thereafter], oxaliplatin, 85 mg/m2 at day 1, and leucovorin calcium, 200 mg/m2, and fluorouracil, 400-mg/m2 bolus followed by 600-mg/m2 continuous 24-hour infusion at days 1 and 2, every 2 weeks), for 8 cycles, followed by panitumumab plus fluorouracil-leucovorin (arm A) or panitumumab (arm B). Both maintenance treatments were continued until progressive disease, consent withdrawal, unacceptable toxic effects, or death (eFigure 1 in Supplement 2). Treatment group allocation was performed with a minimization algorithm implementing a random component. Stratification factors were number of metastatic sites (1 vs ≥2) and previous adjuvant therapy (no vs yes).
Study Assessments
Disease evaluation was performed by means of computed tomography of the thorax and abdomen or magnetic resonance imaging scans at screening and every 8 weeks thereafter until progressive disease or death. Tumor response was classified according to RECIST, version 1.1, as complete response, partial response, stable disease, or progressive disease. Complete response and partial response should have been confirmed by 2 assessments not less than 4 weeks apart. Patients who discontinued study treatment without progressive disease had tumor assessments every 8 weeks until progressive disease or study withdrawal. Treatment safety was assessed during the induction and maintenance phases according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03. The protocol recommended preemptive treatment of skin rash with doxycycline at the dose of 100 mg/d for 5 consecutive days, given every other cycle starting at cycle 1, day 1. Quality of life was investigated through the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaires C30 and CR29 and EuroQOL (European quality of life) 5-dimension questionnaires at baseline and every 8 weeks until progressive disease. Quality-of-life data will be analyzed when most patients will conclude treatment and will be presented in a separate publication. BRAF codon 600 mutations were centrally confirmed, as previously described.21
Outcomes
The primary end point was PFS. The aim was to compare 10-month PFS in the 2 treatment arms. Progression-free survival was assessed locally as the interval from randomization to first objective documentation of progressive disease or death due to any cause, whichever occurred first (censoring at last follow-up for patients alive and without progressive disease). Secondary end points were safety, overall survival, overall response rate, disease control rate, duration of response, and quality of life. Overall survival was the interval from randomization to death due to any cause (censoring at last follow-up for patients alive). Overall response rate was defined as the proportion of patients achieving an objective response (complete or partial), and disease control rate was defined as the proportion of patients achieving a complete or a partial response or stable disease. Duration of response was defined as the interval from first documented response to progressive disease or death due to any cause, whichever occurred first (censoring at the date of last follow-up for patients alive and without progressive disease).
Statistical Analysis
Activity and efficacy analyses were performed for the intention-to-treat population (all randomized patients) and for the per protocol population (including patients with complete or partial response or stable disease after induction who received ≥1 maintenance cycle). Safety analyses were performed for patients who received at least 1 induction cycle. Sample size was calculated taking into account the 10.1-month median PFS reported by the PRIME trial (Panitumumab Randomized Trial In Combination With Chemotherapy for Metastatic Colorectal Cancer to Determine Efficacy) in the patient subgroup with RAS wild-type tumors.2 A sample of 224 patients, 112 per arm, recruited during a 2-year period would achieve 90% power to detect 50% PFS in arm A and a maximum difference of 15% less in arm B (1-sided α = 0.1, with a 15% dropout rate taken into account). These assumptions would imply a noninferiority limit equal to 1.515 for the PFS hazard ratio (HR) of arm B vs A, that is, how much A can exceed B, with B still being considered noninferior to A. The null hypothesis for proving noninferiority was HR (B vs A) of at least 1.515 (B is inferior to A); the alternative hypothesis, was HR (B vs A) of less than 1.515 (B is not inferior to A). The null hypothesis would have been rejected in favor of the alternative if the upper limit of the 1-sided 90% CI for HR, estimated in a Cox proportional hazards regression model stratified by prior adjuvant therapy (no or yes) and number of disease sites (1 or ≥2), was lower than 1.515. Overall survival and PFS curves were estimated in the 2 treatment arms by the Kaplan-Meier method and compared using the log-rank test. Binomial 2-sided 95% CIs were calculated for overall response rate and disease control rate. The Mantel-Haenszel method was applied to estimate the pooled odds ratio (OR) of response in arm B vs A across the strata determined according to the stratification factors and tested using the Cochran Mantel-Haenszel test. Duration of response and median duration of response were estimated by the Kaplan-Meier method and compared using the log-rank test. Subgroup analyses were performed by estimating Kaplan-Meier curves according to treatment arm in each subgroup; Cox proportional hazards regression models with interaction treatment × subgroup were fitted to estimate HRs and corresponding 95% CIs. Median follow-up was calculated by the reverse Kaplan-Meier approach. A stratified univariable Cox proportional hazards regression model was fitted to estimate the HR of arm B vs A for overall survival.
All tests were 2 sided at α = .05, with the exception of the 1-sided test of noninferiority for PFS. The analyses were performed using SAS software, version 9.1 (SAS Institute, Inc), and R software, version 3.4.1 (R Foundation for Statistical Computing).
Results
Patient Populations
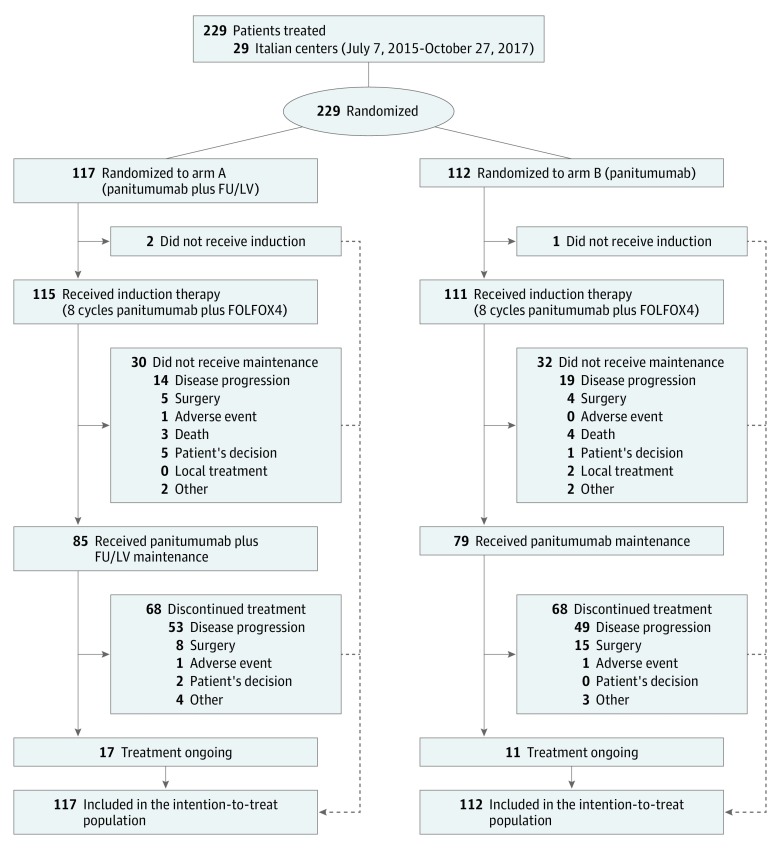
From July 7, 2015, through October 27, 2017, 229 patients (153 male [66.8%] and 76 female [33.2%]; median age, 64 years [interquartile range (IQR), 56-70 years]) were randomized to arm A (n = 117) or arm B (n = 112). Patients’ demographic and disease characteristics were well balanced in both arms (eTable 1 in Supplement 2). Three patients were randomized but did not receive study treatment. The main reasons for treatment discontinuation were progressive disease in 135 (59.7%), resection for metastases in 32 (14.2%), adverse events in 3 (1.3%), deaths in 7 (3.1%), and patient or investigator decision or other reasons in 21 (9.3%) (Figure 1).
Figure 1. Consort Diagram of the Study.
FOLFOX4 indicates panitumumab, 6 mg/kg (1-hour infusion for the first administration, 30-minute infusion thereafter), oxaliplatin, 85 mg/m2 at day 1, leucovorin calcium, 200 mg/m2, and fluorouracil, 400-mg/m2 bolus, followed by 600-mg/m2 continuous 24-hour infusion at days 1 and 2, every 2 weeks. FU indicates fluorouracil; LV, leucovorin.
Efficacy
The cutoff date for analyses was July 30, 2018. The median treatment duration was 7.4 months (IQR, 4.3-12.4 months) for the overall population, 8.4 months (IQR, 4.7-14.0 months) for arm A, and 7.0 months (IQR, 4.0-10.5 months) for arm B. Progression-free survival events occurred in 169 (including 15 deaths in the absence of progression), consisting of 83 in arm A (including 6 deaths) and 86 in arm B (including 9 deaths); median follow-up was 18.0 months (IQR, 13.1-23.3 months).
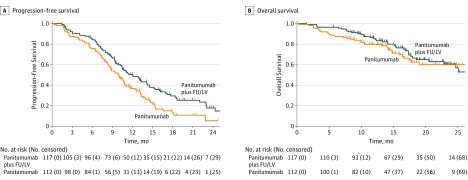
The null hypothesis of inferiority of arm B vs arm A could not be rejected because the upper limit of the 1-sided 90% CI of the HR was 1.857 and exceeded the 1.515 noninferiority boundary. In arm A, 10-month PFS was 59.9% (95% CI, 51.5%-69.8%) and median PFS was 12.0 months (95% CI, 10.4-14.5 months). In comparison, 10-month PFS was 49.0% (95% CI, 40.5%-59.4%) and median PFS was 9.9 months (95% CI, 8.4-11.0 months) in arm B (log-rank test, P = .006) (Figure 2A and eTable 2 in Supplement 2). The HR of arm B vs arm A from the univariable Cox proportional hazards regression model stratified by prior adjuvant therapy and number of disease sites was 1.51 (95% CI, 1.11-2.07; P = .009) (eTable 2 in Supplement 2).
Figure 2. Kaplan-Meier Curves for Progression-Free Survival (PFS) and Overall Survival According to Treatment Arm.
Data are shown in the intention-to-treat population at the date of first data cutoff (July 30, 2018). Ten-month PFS was 59.9% (95% CI, 51.5%-69.8%) vs 49.0% (95% CI, 40.5%-59.4%) in arm A vs arm B. The hazard ratio (HR) of arm B vs arm A was 1.51 (95% CI, 1.11-2.07; P = .009). Overall survival at the median follow-up of 18 months was 66.4% (95% CI, 57.1%-77.2%) vs 62.4% (52.3%-74.4%) in arm A vs arm B (HR, 1.13; 95% CI, 0.71-1.81; P = .60). FU indicates fluorouracil; LV, leucovorin.
The overall number of deaths was 74, including 40 in arm A and 34 in arm B. Overall survival at the median follow-up of 18 months was 66.4% (95% CI, 57.1%-77.2%) in arm A and 62.4% (95% CI, 52.3%-74.4%) in arm B (HR, 1.13; 95% CI, 0.71-1.81; P = .60) (Figure 2B and eTable 2 in Supplement 2).
There was no significant difference between arms in terms of overall response rate and disease control rate (eTable 2 in Supplement 2). Overall response rate was 66.7% (95% CI, 57.4%-75.1%) in arm A and 67.0% (95% CI, 57.4%-75.6%) in arm B; the pooled OR for overall response rate in arm B vs A was 1.07 (95% CI, 0.61-1.86) (P = .82). Disease control rate was 82.9% (95% CI, 74.8%-89.2%) in arm A and 83.9% (95% CI, 75.8%-90.2%) in arm B; the pooled OR for disease control rate in arm B vs A was 1.06 (95% CI, 0.52-2.15; P = .87). In the 153 patients achieving RECIST response, median duration of response was 10.9 months (IQR, 5.8-21.0 months) in arm A and 9.0 months (IQR, 5.9-14.7 months) in arm B (P = .16) (eFigure 2 in Supplement 2).
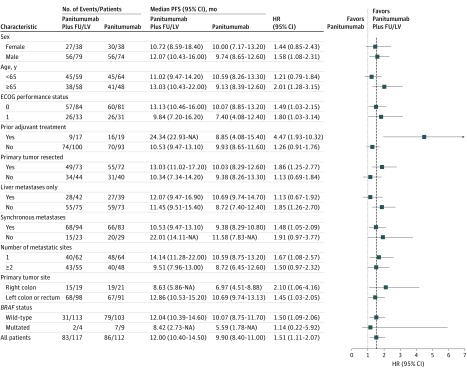
The forest plots showing treatment HR and 95% CI according to stratification factors and other prognostic variables are shown in Figure 3 (for PFS) and eFigure 3 in Supplement 2 (for overall survival). The per protocol population included 164 patients, 85 in arm A and 79 in arm B. Median follow-up was 17.9 months (IQR, 13.1-23.3 months). In arm A, 10-month PFS was 70.3% (95% CI, 61.2%-80.8%) and median PFS was 14.1 months (95% CI, 11.3-17.2 months). In arm B, 10-month PFS was 59.0% (95% CI, 49.0%-71.0%); median PFS was 10.8 months (95% CI, 9.9-13.3 months); and HR was 1.50 (95% CI, 1.03-2.19; P = .04) (eFigure 4A in Supplement 2). For overall survival, HR was 0.95 (95% CI, 0.50-1.82; P = .88) (eFigure 4B in Supplement 2).
Figure 3. Forest Plots of Progression-Free Survival by Patient Subgroups Within the Intention-to-Treat Population.
ECOG indicates Eastern Cooperative Oncology Group; FU, fluorouracil; HR, hazard ratio; LV, leucovorin. Dashed line indicates predefined noninferiority boundary (HR, 1.515).
Safety
Dose delays and reductions are reported in eMethods in Supplement 2. In the safety population of 226 patients, adverse events of at least grade 3 occurred in 52 patients (31.7%), including 36 (42.4%) in arm A and 16 (20.3%) in arm B. In arm A, an increased incidence of diarrhea (any grade, 21 [24.7%] vs 8 [10.1%]; grade ≥3, 4 [4.7%] vs 1 [1.3%]) and stomatitis (any grade, 28 [32.9%] vs 6 [7.6%]; grade ≥3, 6 [7.1%] vs 1 [1.3%]) occurred compared with arm B. Panitumumab-related toxic effects (mainly skin rash, paronychia, hypomagnesemia, and conjunctivitis) were reported in most patients, although the rates were higher in arm A vs B (any grade, 65 [76.5%] vs 33 [41.8%]; grade ≥3, 27 [31.8%] vs 13 [16.5%]). Patients experienced neurotoxic effects of any grade in 84 cases (37.2%) and of grade 3 or higher in 7 (3.1%). Notably, 3 patients died of sepsis during the induction phase (eTable 2 in Supplement 2).
Discussion
The rationale of a maintenance strategy with anti-EGFRs in absence of the fluoropyrimidine backbone is supported by the single-agent efficacy of such drugs in patients with molecularly selected mCRC. The MACRO-2 trial17 suggested the noninferiority in terms of 9-month PFS of maintenance treatment with cetuximab alone compared with continuation of modified FOLFOX-6 plus cetuximab, even though the trial was conducted in patients with KRAS exon 2 wild-type mCRC. Such limitation and the small sample size highlighted the need for larger studies restricted to RAS wild-type mCRC. The VALENTINO phase 2 study prospectively enrolled patients with RAS wild-type mCRC and showed that maintenance therapy with single-agent panitumumab was inferior in terms of PFS compared with panitumumab plus fluorouracil-leucovorin, which slightly increased the treatment toxicity. Even if we adopted a large noninferiority margin as high as 1.515, this reinforces the clear PFS inferiority of single-agent panitumumab as maintenance treatment, so that a subsequent phase 3 trial would have an extremely low probability of meeting a primary end point of noninferiority.
The PFS benefit of adding fluorouracil-leucovorin to panitumumab in the maintenance setting did not differ according to main subgroups, including those with poorer prognosis and primary resistance to anti-EGFR agents, such as right-sided primary tumors or BRAF-mutated ones.16,22 The prevalence of right-sided tumors was only 17%, and that of BRAF-mutated tumors was only 4%, therefore lower than expected in the RAS wild-type population, showing an increased refinement of patients’ selection in the clinical practice. Even if the absence of a panitumumab-free arm did not allow us to perform predictive analyses, PFS in these 2 subgroups was particularly poor in arm B (Figure 2). The clinical importance of further investigating anti-EGFR–based maintenance in these patient subgroups is modest because anti-EGFR agents are not commonly used in the upfront treatment of most patients with BRAF-mutated or right-sided (RAS wild-type) mCRC.1,14
Our results are biologically sound for at least 2 reasons. First, several patients with RAS wild-type mCRC are primarily resistant to EGFR inhibition owing to the presence of EGFR-independent oncogenic drivers other than RAS and BRAF mutations, specific gene expression profiles, and overexpression of specific microRNAs such as microRNA 31-3p.16,22,23,24 Therefore, in this clinically and biologically heterogeneous patient population, single-agent anti-EGFRs may represent a suboptimal maintenance treatment option compared with chemotherapy continuation. Second, in tumors with initial sensitivity to EGFR inhibition, acquired resistance may be delayed by the synergistic effect of the combined chemotherapeutic agent. In our study, the duration of response curves were similar in both arms to 6 months but progressively diverged over time (eFigure 2 in Supplement 2), suggesting the presence of a patient subgroup achieving long-term response with the combination of panitumumab and fluorouracil-leucovorin.
Maintenance treatment with single-agent panitumumab had less toxic effects, although the safety profile was clearly manageable in both treatment arms. Indeed, preemptive therapy for toxic effects on the skin was recommended by our study to decrease the incidence of skin rash of grade 3 or higher according to the literature.24 The incidence of 26.5% (60 patients) in the VALENTINO study favorably compares with 37% reported in the PRIME trial.2 Most importantly, the extremely low incidence of grade 3 neurotoxic effects (3.1%) is in line with other academic trials scheduling early discontinuation of oxaliplatin treatment after a preplanned number of cycles.8,13,19,25
Among postinduction strategies aimed at improving quality of life, intermittent treatments have been investigated by dedicated clinical trials. These strategies may have a remarkable clinical value in patients with low disease burden and in those who achieve the rapid and deep tumor responses typically associated with anti-EGFR–based first-line regimens.26 Among the few available evidences for such strategies in the era of biological agents,19,20 the randomized noncomparative phase 2 COIN-B study19 was conducted in patients with KRAS exon 2 wild-type mCRC and showed that treatment holidays followed by reinduction therapy at progressive disease and maintenance therapy with cetuximab alone may achieve noninferior failure-free survival compared with historical data. However, in the comparative analysis, a treatment break was associated with inferior overall survival and postinduction PFS compared with maintenance therapy with cetuximab.
Limitations
Our study has some limitations. First, the control arm (panitumumab plus fluorouracil-leucovorin) could have been regarded as experimental. Indeed, discontinuing oxaliplatin treatment after a preplanned number of cycles has improved tolerability, with clearly acceptable loss of efficacy.6,7 The available evidence on anti-EGFR–based treatments is limited to the randomized phase 2 SAPPHIRE study,18 showing similar 9-month PFS when de-escalating to panitumumab plus fluorouracil-leucovorin or continuing panitumumab plus modified FOLFOX-6 therapy. Second, the lack of a single-agent fluorouracil-leucovorin arm hampered the chance of assessing the specific effect of continuing panitumumab in the maintenance setting, as investigated by the ongoing randomized phase 2 PanaMa study.27 Third, in our study, randomization was planned before starting induction treatment, and patients who could not proceed to the randomly assigned maintenance strategy were included in the intention-to-treat analysis. However, the results obtained in the per protocol and intention-to-treat populations were highly consistent. Finally, the reintroduction of FOLFOX-4 plus panitumumab after progressive disease during the maintenance phase was not scheduled by our study. Indeed, the negative data on continuation of anti-EGFR–based treatment beyond progression are consistent with the well-described emergence of tumor clones with genomic resistance to EGFR inhibition.28,29
Conclusions
Despite the noninferiority design and the sample size, this study clearly demonstrates the inferiority of maintenance treatment with single-agent panitumumab compared with panitumumab plus fluorouracil-leucovorin. Even if panitumumab plus fluorouracil-leucovorin were to be regarded as the optimal maintenance strategy after an oxaliplatin-based induction treatment, this conclusion cannot be generalized for a combination chemotherapy including irinotecan, because the issue of cumulative toxic effects is less clinically significant. Most important, even if the VALENTINO study planned the discontinuation of oxaliplatin treatment after a fixed 4-month induction, the clinical decision making on treatment de-escalation and its timing should be dynamically managed by physicians by taking into account several factors, such as available evidence, patient preference, baseline patient- and disease-related characteristics, specific drug toxicities, and response to induction treatment.
Trial Protocol
eTable 1. Baseline Characteristics
eTable 2. Efficacy and Activity Measures in Intention-to-Treat Population
eTable 3. Safety Analysis: Adverse Events During Induction and Maintenance and During Maintenance
eFigure 1. Trial Design
eFigure 2. Duration of Response According to Treatment Arm in the Subgroup of Patients With Objective Tumor Response According to RECIST, Version 1.1
eFigure 3. Forest Plots of Overall Survival by Patient Subgroups Within the Intention-to-Treat Population
eFigure 4. Kaplan-Meier Curves for Progression Free-Survival and Overall Survival According to Treatment Arm in the Per Protocol Population
eMethods. Details on Dose Reductions During Study Treatment and Study Centers
Data Sharing Statement
References
- 1.Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386-1422. doi: 10.1093/annonc/mdw235 [DOI] [PubMed] [Google Scholar]
- 2.Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369(11):1023-1034. doi: 10.1056/NEJMoa1305275 [DOI] [PubMed] [Google Scholar]
- 3.Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360(14):1408-1417. doi: 10.1056/NEJMoa0805019 [DOI] [PubMed] [Google Scholar]
- 4.Venook AP, Niedzwiecki D, Lenz HJ, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: a randomized clinical trial. JAMA. 2017;317(23):2392-2401. doi: 10.1001/jama.2017.7105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1065-1075. doi: 10.1016/S1470-2045(14)70330-4 [DOI] [PubMed] [Google Scholar]
- 6.Tournigand C, Cervantes A, Figer A, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-go fashion in advanced colorectal cancer: a GERCOR study. J Clin Oncol. 2006;24(3):394-400. doi: 10.1200/JCO.2005.03.0106 [DOI] [PubMed] [Google Scholar]
- 7.Díaz-Rubio E, Gómez-España A, Massutí B, et al. ; Spanish Cooperative Group for the Treatment of Digestive Tumors . First-line XELOX plus bevacizumab followed by XELOX plus bevacizumab or single-agent bevacizumab as maintenance therapy in patients with metastatic colorectal cancer: the phase III MACRO TTD study. Oncologist. 2012;17(1):15-25. doi: 10.1634/theoncologist.2011-0249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chibaudel B, Maindrault-Goebel F, Lledo G, et al. Can chemotherapy be discontinued in unresectable metastatic colorectal cancer? the GERCOR OPTIMOX2 Study. J Clin Oncol. 2009;27(34):5727-5733. doi: 10.1200/JCO.2009.23.4344 [DOI] [PubMed] [Google Scholar]
- 9.Adams RA, Meade AM, Seymour MT, et al. ; MRC COIN Trial Investigators . Intermittent versus continuous oxaliplatin and fluoropyrimidine combination chemotherapy for first-line treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet Oncol. 2011;12(7):642-653. doi: 10.1016/S1470-2045(11)70102-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hegewisch-Becker S, Graeven U, Lerchenmüller CA, et al. Maintenance strategies after first-line oxaliplatin plus fluoropyrimidine plus bevacizumab for patients with metastatic colorectal cancer (AIO 0207): a randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol. 2015;16(13):1355-1369. doi: 10.1016/S1470-2045(15)00042-X [DOI] [PubMed] [Google Scholar]
- 11.Koeberle D, Betticher DC, von Moos R, et al. Bevacizumab continuation versus no continuation after first-line chemotherapy plus bevacizumab in patients with metastatic colorectal cancer: a randomized phase III non-inferiority trial (SAKK 41/06). Ann Oncol. 2015;26(4):709-714. doi: 10.1093/annonc/mdv011 [DOI] [PubMed] [Google Scholar]
- 12.Simkens LH, van Tinteren H, May A, et al. Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): a phase 3 randomised controlled trial of the Dutch Colorectal Cancer Group. Lancet. 2015;385(9980):1843-1852. doi: 10.1016/S0140-6736(14)62004-3 [DOI] [PubMed] [Google Scholar]
- 13.Loupakis F, Cremolini C, Masi G, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371(17):1609-1618. doi: 10.1056/NEJMoa1403108 [DOI] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network. Colon cancer (version 3). https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Published 2018. Accessed September 4, 2018.
- 15.Pietrantonio F, Cremolini C, Petrelli F, et al. First-line anti-EGFR monoclonal antibodies in panRAS wild-type metastatic colorectal cancer: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2015;96(1):156-166. doi: 10.1016/j.critrevonc.2015.05.016 [DOI] [PubMed] [Google Scholar]
- 16.Arnold D, Lueza B, Douillard JY, et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol. 2017;28(8):1713-1729. doi: 10.1093/annonc/mdx175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aranda E, García-Alfonso P, Benavides M, et al. ; Spanish Cooperative Group for the Treatment of Digestive Tumours (TTD) . First-line mFOLFOX plus cetuximab followed by mFOLFOX plus cetuximab or single-agent cetuximab as maintenance therapy in patients with metastatic colorectal cancer: phase II randomised MACRO2 TTD study. Eur J Cancer. 2018;101:263-272. doi: 10.1016/j.ejca.2018.06.024 [DOI] [PubMed] [Google Scholar]
- 18.Nakamura M, Munemoto Y, Takahashi M, et al. SAPPHIRE: A randomized phase II study of mFOLFOX6 + panitumumab versus 5-FU/LV + panitumumab after 6 cycles of frontline mFOLFOX6 + panitumumab in patients with colorectal cancer. J Clin Oncol. 2018;36(4 suppl):729. doi: 10.1200/JCO.2018.36.4_suppl.729 [DOI] [Google Scholar]
- 19.Wasan H, Meade AM, Adams R, et al. ; COIN-B investigators . Intermittent chemotherapy plus either intermittent or continuous cetuximab for first-line treatment of patients with KRAS wild-type advanced colorectal cancer (COIN-B): a randomised phase 2 trial. Lancet Oncol. 2014;15(6):631-639. doi: 10.1016/S1470-2045(14)70106-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfeiffer P, Sorbye H, Qvortrup C, et al. Maintenance therapy with cetuximab every second week in the first-line treatment of metastatic colorectal cancer: the NORDIC-7.5 study by the Nordic Colorectal Cancer Biomodulation Group. Clin Colorectal Cancer. 2015;14(3):170-176. doi: 10.1016/j.clcc.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 21.Di Bartolomeo M, Pietrantonio F, Perrone F, et al. ; Italian Trials in Medical Oncology Group . Lack of KRAS, NRAS, BRAF and TP53 mutations improves outcome of elderly metastatic colorectal cancer patients treated with cetuximab, oxaliplatin and UFT. Target Oncol. 2014;9(2):155-162. doi: 10.1007/s11523-013-0283-8 [DOI] [PubMed] [Google Scholar]
- 22.Pietrantonio F, Petrelli F, Coinu A, et al. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: a meta-analysis. Eur J Cancer. 2015;51(5):587-594. doi: 10.1016/j.ejca.2015.01.054 [DOI] [PubMed] [Google Scholar]
- 23.Cremolini C, Morano F, Moretto R, et al. Negative hyper-selection of metastatic colorectal cancer patients for anti-EGFR monoclonal antibodies: the PRESSING case-control study. Ann Oncol. 2017;28(12):3009-3014. doi: 10.1093/annonc/mdx546 [DOI] [PubMed] [Google Scholar]
- 24.Laurent-Puig P, Grisoni ML, Heinemann V, et al. Validation of miR-31-3p expression to predict cetuximab efficacy when used as first-line treatment in RAS wild-type metastatic colorectal cancer. Clin Cancer Res. 2019;25(1):134-141. doi: 10.1158/1078-0432.CCR-18-1324 [DOI] [PubMed] [Google Scholar]
- 25.Grothey A, Sobrero AF, Shields AF, et al. Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med. 2018;378(13):1177-1188. doi: 10.1056/NEJMoa1713709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petrelli F, Pietrantonio F, Cremolini C, et al. Early tumour shrinkage as a prognostic factor and surrogate end-point in colorectal cancer: a systematic review and pooled-analysis. Eur J Cancer. 2015;51(7):800-807. doi: 10.1016/j.ejca.2015.02.011 [DOI] [PubMed] [Google Scholar]
- 27.Maintenance therapy with 5-FU/FA plus panitumumab vs. 5-FU/FA alone after prior induction and re-induction after progress for 1st-line treatment of metastatic colorectal cancer (PanaMa). Clinicaltrials.gov identifier: NCT01991873. https://clinicaltrials.gov/ct2/show/NCT01991873. Updated March 6, 2019. Accessed May 31, 2019.
- 28.Pietrantonio F, Vernieri C, Siravegna G, et al. Heterogeneity of acquired resistance to anti-EGFR monoclonal antibodies in patients with metastatic colorectal cancer. Clin Cancer Res. 2017;23(10):2414-2422. doi: 10.1158/1078-0432.CCR-16-1863 [DOI] [PubMed] [Google Scholar]
- 29.Ciardiello F, Normanno N, Martinelli E, et al. ; CAPRI-GOIM Investigators . Cetuximab continuation after first progression in metastatic colorectal cancer (CAPRI-GOIM): a randomized phase II trial of FOLFOX plus cetuximab versus FOLFOX. Ann Oncol. 2016;27(6):1055-1061. doi: 10.1093/annonc/mdw136 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Baseline Characteristics
eTable 2. Efficacy and Activity Measures in Intention-to-Treat Population
eTable 3. Safety Analysis: Adverse Events During Induction and Maintenance and During Maintenance
eFigure 1. Trial Design
eFigure 2. Duration of Response According to Treatment Arm in the Subgroup of Patients With Objective Tumor Response According to RECIST, Version 1.1
eFigure 3. Forest Plots of Overall Survival by Patient Subgroups Within the Intention-to-Treat Population
eFigure 4. Kaplan-Meier Curves for Progression Free-Survival and Overall Survival According to Treatment Arm in the Per Protocol Population
eMethods. Details on Dose Reductions During Study Treatment and Study Centers
Data Sharing Statement