This cohort study assesses changes in nonhyperemic and hyperemic hemodynamic stenosis and resistance indices in nonculprit vessels in patients with ST-segment elevation myocardial infarction (STEMI).
Key Points
Question
Are nonculprit physiology indices to assess stenosis severity associated with altered intracoronary hemodynamics in the acute setting of ST-segment elevation myocardial infarction (STEMI)?
Findings
Among 73 patients with STEMI with multivessel disease, nonculprit fractional flow reserve was increased and coronary flow reserve was decreased in the acute setting. Instantaneous wave-free ratio numerically increased but remained stable from the acute moment of presentation to 1-month follow-up; these changes were accompanied by an increased hyperemic and decreased baseline microcirculatory resistance in the acute setting of STEMI.
Meaning
The findings suggest that intracoronary hemodynamics among patients with STEMI are altered in the acute setting and are associated with the value of nonculprit intracoronary physiologic indices necessary to guide revascularization of intermediate stenoses.
Abstract
Importance
Percutaneous coronary intervention (PCI) of nonculprit vessels among patients with ST-segment elevation myocardial infarction (STEMI) is associated with improved clinical outcome compared with culprit vessel–only PCI. Fractional flow reserve (FFR) and coronary flow reserve are hyperemic indices used to guide revascularization. Recently, instantaneous wave-free ratio was introduced as a nonhyperemic alternative to FFR. Whether these indices can be used in the acute setting of STEMI continues to be investigated.
Objective
To assess the value of hemodynamic indices in nonculprit vessels of patients with STEMI from the index event to 1-month follow-up.
Design, Setting, and Participants
This substudy of the Reducing Micro Vascular Dysfunction in Revascularized STEMI Patients by Off-target Properties of Ticagrelor (REDUCE-MVI) randomized clinical trial enrolled 98 patients with STEMI who had an angiographic intermediate stenosis in at least 1 nonculprit vessel. Patient enrollment was between May 1, 2015, and September 19, 2017. After successful primary PCI, nonculprit intracoronary hemodynamic measurements were performed and repeated at 1-month follow-up. Cardiac magnetic resonance imaging was performed from 2 to 7 days and 1 month after primary PCI.
Main Outcomes and Measures
The value of nonculprit instantaneous wave-free ratio, FFR, coronary flow reserve, hyperemic index of microcirculatory resistance, and resting microcirculatory resistance from the index event to 1-month follow-up.
Results
Of 73 patients with STEMI included in the final analysis, 59 (80.8%) were male, with a mean (SD) age of 60.8 (9.9) years. Instantaneous wave-free ratio (SD) did not change significantly (0.93 [0.07] vs 0.94 [0.06]; P = .12) and there was no change in resting distal pressure/aortic pressure (mean [SD], 0.94 [0.06] vs 0.95 [0.06]; P = .25) from the acute moment to 1-month follow-up. The FFR decreased (mean [SD], 0.88 [0.07] vs 0.86 [0.09]; P = .001) whereas coronary flow reserve increased (mean [SD], 2.9 [1.4] vs 4.1 [2.2]; P < .001). Hyperemic index of microcirculatory resistance decreased and resting microcirculatory resistance increased from the acute moment to follow-up. The decrease in distal pressure from rest to hyperemia was smaller at the acute moment vs follow-up (mean [SD], 10.6 [11.2] mm Hg vs 14.1 [14.2] mm Hg; P = .05). This blunted acute hyperemic response correlated with final infarct size (ρ, –0.29; P = .02). The resistive reserve ratio was lower at the acute moment vs follow-up (mean [SD], 3.4 [1.7] vs 5.0 [2.7]; P < .001).
Conclusions and Relevance
In the acute setting of STEMI, nonculprit coronary flow reserve was reduced and FFR was augmented, whereas instantaneous wave-free ratio was not altered. These results may be explained by an increased hyperemic microvascular resistance and a blunted adenosine responsiveness at the acute moment that was associated with infarct size.
Introduction
Of patients with ST-segment elevation myocardial infarction (STEMI), 52.8% have multivessel disease (at least 1 obstructive nonculprit lesion).1 Patients with STEMI and multivessel disease without cardiogenic shock have a worse short-term prognosis compared with patients with STEMI who have 1-vessel disease.2 Revascularization of nonculprit lesions is associated with an improved clinical outcome.3,4,5,6 Subsequently, multivessel primary percutaneous coronary intervention (pPCI) either at the time of pPCI or in a staged procedure has been assigned a class IIa recommendation.7
Fractional flow reserve (FFR) is an invasive hyperemic hemodynamic physiology index frequently used to guide revascularization of intermediate stenoses.8 Coronary flow reserve (CFR), which also requires the use of adenosine, is mostly used for the noninvasive assessment of suspected or known ischemic heart disease by positron emission tomography,9 but can also be calculated invasively.10 More recently, instantaneous wave-free ratio (iFR) was introduced as an adenosine-free index of stenosis severity.11 Two large clinical trials12,13 showed a similar occurrence of major adverse events in patients with stable coronary artery disease or non–STEMI revascularized based on iFR as compared with FFR. Changes in baseline and hyperemic coronary flow, potentially depending on infarct size, might affect both FFR and iFR.14,15,16,17,18,19 Whether FFR and iFR can also be used interchangeably in the acute setting of STEMI is unknown. To our knowledge, there are no studies available to date that investigate the value of both indices in nonculprit vessels in the acute setting of STEMI compared with that in the late, more stable phase.
The aim of our study was to assess changes in nonhyperemic and hyperemic hemodynamic stenosis and resistance indices in nonculprit vessels of patients with STEMI from the index event to 1-month follow-up. We assessed whether these indices were associated with infarct characteristics as assessed with cardiac magnetic resonance because we hypothesized that hemodynamic acute changes are more pronounced in large infarcts.
Methods
Overview of Study Procedures
This study was a predefined substudy of the Reducing Micro Vascular Dysfunction in Revascularized STEMI Patients by Off-target Properties of Ticagrelor (REDUCE-MVI) trial.20 The primary end point of the REDUCE-MVI study was microvascular resistance at 30 days in patients with ticagrelor vs prasugrel, measured by the index of microcirculatory resistance (IMR), which was similar in both groups. Patients with a first STEMI and 1 or more intermediate lesions (50%-90% diameter stenosis) in a nonculprit vessel were enrolled after successful pPCI of the culprit vessel. For clinical purposes, patients underwent follow-up invasive coronary angiography 1 month after the index event. The cardiac magnetic resonance imaging was performed from 2 to 7 days after pPCI and within 5 days of 1-month follow-up to assess infarct size, left ventricle ejection fraction, and microvascular injury. For a complete description of the cardiac magnetic resonance acquisition and analysis, see eAppendix 1 in the Supplement. An overview of the screened patients during the inclusion period with accompanying inclusion and exclusion reasons was published previously.20 This study was approved by the medical ethics committee at Amsterdam University Medical Center, Vrije Universiteit, Amsterdam, the Netherlands, and all participants gave written informed consent.
Patient Population
Paired hemodynamic data were assessed in patients enrolled between May 1, 2015, and September 19, 2017, at the Amsterdam University Medical Center, Vrije Universiteit, Amsterdam, the Netherlands; Medisch Spectrum Twente, Enschede, the Netherlands; Hospital Clínico San Carlos, Madrid, Spain; and Radboud University Medical Centre, Nijmegen, the Netherlands. All patients were admitted with an acute STEMI, presented fewer than 12 hours after onset of symptoms, and had an intermediate (50%-90%) lesion in a nonculprit vessel. For a complete overview of the inclusion and exclusion criteria, see eAppendix 2 in the Supplement.
Calculation of Physiological Indices
The coronary catheterization procedure and measurement of hemodynamic indices have been published previously.20 All measurements were directly extracted and analyzed offline by a blinded analyst using RadiView Software (Abbott). The FFR was calculated by the ratio of distal coronary pressure (Pd) to aortic pressure (Pa) during a period of stable maximal hyperemia, and the lowest stable FFR value was used. When drift exceeded 0.02, we corrected for the pressure drift.21 The iFR is defined as resting Pd/Pa within the mid- to end-diastolic part (wave-free period) of the cardiac cycle. The iFR was calculated offline by using a dedicated MATLAB script (The MathWorks Inc),22 and CFR is defined as the ratio between resting and hyperemic mean transit time. The product of distal pressure and mean hyperemic transit time was used to calculate IMR, and the product of resting mean transit time and resting distal pressure was used to calculate baseline microvascular resistance (BMR). The resistive reserve ratio was used to determine the vasodilatory microcirculatory response (ratio between BMR and IMR).23 Quantitative coronary angiography was performed to assess diameter stenosis (CAAS II; Pie Medical BV). We measured pressures to calculate FFR and iFR using the RadiAnalyzer Xpress, QUANTIEN console (Abbott), which does not have simultaneous electrocardiography (ECG) recordings.
Statistical Analysis
Means were compared between subgroups using the independent samples t test. Classification agreement (CCA) and the proportion of abnormal values at the acute moment and follow-up were compared using the McNemar test. To assess the difference between parameters at the acute moment and follow-up, a 2-sided, paired t test was used. P < .05 was considered to be statistically significant. For a complete overview of the statistical analyses, see eAppendix 3 in the Supplement.
Results
Patient and Infarct Characteristics
Of the 110 patients included in REDUCE-MVI, a total of 98 patients were enrolled in the current study, with 25 patients excluded for various reasons (eTable 1 in the Supplement). A total of 73 patients remained for final analysis; 59 (80.8%) were male, and the mean (SD) age was 60.8 (9.9) years. Patient, vessel, and infarct characteristics are specified in the Table. One-month follow-up was performed at a mean (SD) of 31.0 (5.5) days after pPCI, and 24 patients (32.9%) presented with an anterior wall STEMI. The distribution of nonculprit vessels was the left anterior descending artery (n = 29; 39.7%), circumflex artery (n = 26; 35.6%), and right coronary artery (n = 18; 24.7%). Baseline characteristics and comparisons of the excluded and included patients are summarized in eTable 2 in the Supplement. Infarct characteristics assessed by cardiac magnetic resonance are presented in eTable 3 in the Supplement.
Table. Patient and Infarct Characteristics.
| Characteristic | Patients (n = 73) |
|---|---|
| Age, mean (SD), y | 60.8 (9.9) |
| Male, No. (%) | 59 (80.8) |
| Body mass index, mean (SD)a | 27.3 (3.7) |
| Medical history, No. (%) | |
| Hypertension | 19 (26.0) |
| Type 2 diabetes | 5 (6.8) |
| Current smoker | 29 (39.7) |
| Family history of CAD | 27 (37.0) |
| Hypercholesterolemia | 11 (15.1) |
| Previous percutaneous intervention | 2 (2.7) |
| History of pulmonary disease | 5 (6.8) |
| Medication before pPCI, No. (%) | |
| Aspirin | 4 (5.5) |
| Lipid-lowering medication | 10 (13.7) |
| Angiotensin-converting enzyme inhibitor | 4 (5.5) |
| Angiotensin receptor blocker | 7 (9.6) |
| β-Blocker | 6 (8.2) |
| Calcium channel blocker | 6 (8.2) |
| Peak laboratory values, median (IQR) | |
| Creatine kinase level, U/L | 1086.0 (467.5-2240.5) |
| CK-MB level, μg/L | 99.2 (43.3-224.0) |
| Troponin level, μg/L | 2.01 (0.53-4.52) |
| Culprit arteries, No. (%) | |
| Left anterior descending | 24 (32.9) |
| Circumflex | 19 (26.0) |
| Right coronary | 30 (41.1) |
| Angiographic and stent characteristics, No. (%) | |
| TIMI flow grade before pPCI 0 | 44 (60.3) |
| 1 | 7 (9.6) |
| 2 | 15 (20.5) |
| 3 | 7 (9.6) |
| TIMI flow grade after pPCI 0 | 0 |
| 1 | 4 (5.5) |
| 2 | 11 (15.1) |
| 3 | 58 (79.5) |
| cTFC | 38 (23-59) |
| Nonculprit arteries, No. (%) | |
| Left anterior descending | 29 (39.7)b |
| Circumflex | 26 (35.6)c |
| Right coronary | 18 (24.7) |
| QCA diameter stenosis, No. (%) | 55.1 (13.3) |
Abbreviations: CAD, coronary artery disease; IQR, interquartile range; cTFC, corrected TIMI frame count; pPCI, primary percutaneous coronary intervention; QCA, quantitative coronary angiography; TIMI, thrombolysis in myocardial infarction.
SI conversion factors: To convert creatine kinase to microkatals per liter, multiply by 0.0167; to convert CK-MB and troponin to micrograms per liter, multiply by 1.0.
Calculated as weight in kilograms divided by height in meters squared.
One patient included with the left anterior descending artery as the culprit vessel and the first diagonal branch as the nonculprit vessel. The culprit location was in the midleft anterior descending artery distal to the first diagonal branch (marked as left anterior descending in the Table).
One patient included with the circumflex artery as the culprit vessel and the intermediate branch (marked as circumflex in the Table) as the nonculprit vessel. The culprit location was midcircumflex.
iFR and FFR Values at the Index Procedure and Follow-up
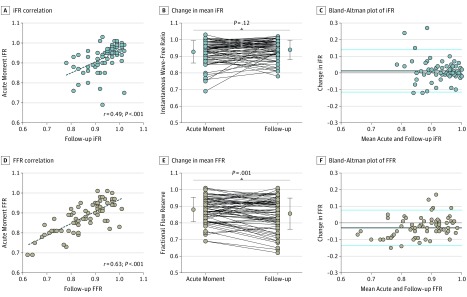
Mean (SD) iFR at the index procedure was 0.93 (0.07) and 0.94 (0.06) at 1-month follow-up (P = .12); Bland-Altman analysis showed a mean (SD) difference of 0.012 (0.064) (Figure 1). The CCA between iFR at the acute moment and follow-up was 82.2% (Figure 2A). The total number of hemodynamically significant iFR (defined as ≤0.89) values was 17 (23.3%) at the acute moment vs 14 (19.2%) at 1-month follow-up (P = .58). Mean (SD) resting Pd/Pa was also not significantly different between the acute moment and follow-up (0.94 [0.06] vs 0.95 [0.06]; P = .25).
Figure 1. Instantaneous Wave-Free Ratio (iFR) and Fractional Flow Reserve (FFR) During the Acute Moment and at 1-Month Follow-up.
A and D, Dashed lines indicate the regession line of acute and follow-up iFR (A) and FFR (D). B and E, Dots and whiskers on both sides indicate means and SDs, respectively. C and F, The differences given were calculated as follow-up values minus acute values. Blue lines indicate within 2 SDs, and black lines indicate bias.
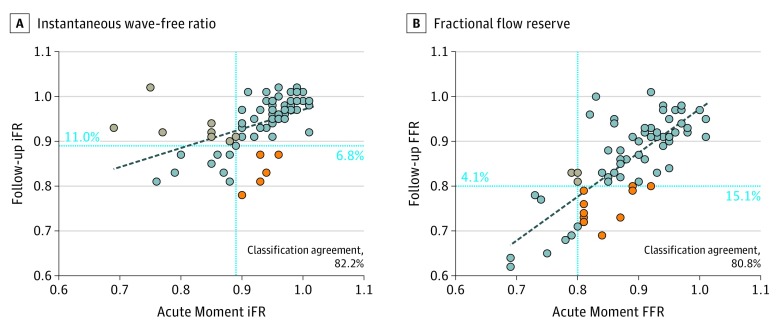
Figure 2. Classification Agreement of Instantaneous Wave-Free Ratio and Fractional Flow Reserve Values Between the Acute Moment and 1-Month Follow-up.
Classification agreement is noted for 73 vessels in both plots. Blue lines represent the established cutoff values of 0.89 (A) and 0.80 (B). Dashed lined represent regression lines between acute and follow-up. Orange dots indicate decrease and gray dots indicate increase between the acute moment and follow-up values.
Mean (SD) FFR at the index procedure was 0.88 (0.07) and at 1-month follow-up was 0.86 (0.09) (P = .001); Bland-Altman analysis showed a mean difference of –0.030 (0.054) (Figure 1). The CCA between FFR at the acute moment and 1-month follow-up was 80.8% (59 of 73 patients) (Figure 2B), which was similar to the CCA of iFR (82.2%) (P > .99). The total number of hemodynamically significant FFR (defined as ≤0.80) values was 11 (15.1%) at the acute moment vs 19 (26.0%) at 1-month follow-up (P = .06).
CFR Values at the Index Procedure and Follow-up
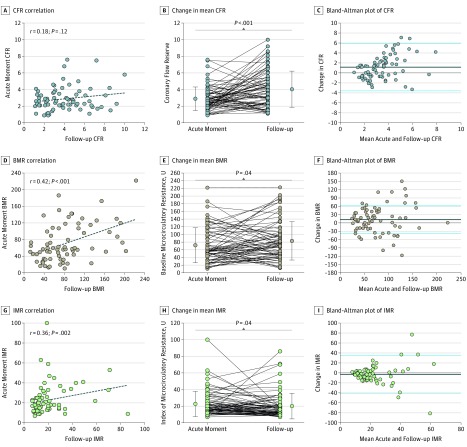
The mean (SD) CFR significantly increased from the index procedure (2.9 [1.4]) to 1-month follow-up (4.1 [2.2]) (P < .001); Bland-Altman analysis showed a mean (SD) difference of 1.16 (2.37) (Figure 3). The CCA between CFR at the acute moment and 1-month follow-up was 63.9% (46 of 72 patients; 1 with missing data). The total number of hemodynamically significant CFR values (defined as <2.0) was 16 (21.9%) at the acute moment vs 12 (16.7%) at follow-up (P = .56).
Figure 3. Coronary Flow Reserve (CFR), Baseline Microcirculatory Resistance (BMR), and the Index of Microcirculatory Resistance (IMR) During the Acute Moment and 1-Month Follow-up.
A, D, and G, Dashed lines indicate the regression line between acute and follow-up values. B, E, and H, Dots and whiskers on both sides indicate means and SDs, respectively. C, F, and I, The differences given were calculated as follow-up values minus acute values. Blue lines indicate within 2 SDs, and black lines indicate bias.
iFR and FFR Values
There was a correlation between FFR and iFR both at the acute moment (ρ, 0.52; P < .001) and at 1-month follow-up (ρ, 0.77; P < .001). The CCA between FFR and iFR at the acute moment was 80.8% (59 of 73 patients) and at 1-month follow-up was 87.7% (64 of 73 patients) (P = .18). At the acute moment, there were 4 abnormal FFR values with normal iFR values (5.5%) and 10 abnormal iFR values with normal FFR values (13.7%) (P = .18). At follow-up, there were 7 abnormal FFR values with normal iFR values (9.6%) and 2 abnormal iFR values with normal FFR values (2.7%) (P = .18).
Resistance Values at the Index Procedure and Follow-up
Median IMR was 18.0 U (interquartile range, 13.5-27.0 U) at the acute moment and decreased to 14.5 U (interquartile range, 11.0-21.0 U) at follow-up (P = .06); Bland-Altman analysis showed a mean (SD) difference of –2.81 (18.98) (Figure 3). Median BMR was 57.3 U (interquartile range, 37.3-107.1 U) at the acute moment and increased to 72.4 U (interquartile range, 46.1-104.9 U) at follow-up (P = .05); Bland-Altman analysis showed a mean (SD) difference of 11.94 (50.06) U (Figure 3).
Distal Pressure and Adenosine Administration at the Acute Moment
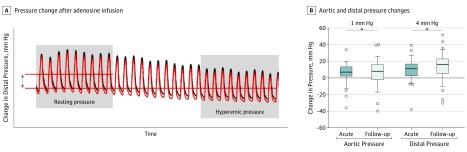
eTable 4 in the Supplement gives an overview of the correlations between heart rate or blood pressure in the acute setting and physiology indices. We did not observe a significant difference in hyperemic response of the aortic pressure between the acute moment and follow-up (eTable 5 in the Supplement). A decreased hyperemic response of distal pressure was found at the acute moment vs follow-up (mean [SD], 10.6 [11.2] mm Hg vs 14.1 [14.2] mm Hg; P = .049) (Figure 4). Patients with low FFR values at follow-up (cutoff value used was the median: 0.87) showed a larger hyperemic response of Pd (mean [SD], 17.9 [9.4] vs 10.2 [17.1]; P = .02). The hyperemic response of Pd at the acute moment or at follow-up did not correlate with percentage of diameter of stenosis (ρ, 0.07; P = .63 and ρ, –0.01; P = .98).
Figure 4. Association Between Adenosine-Induced Hyperemia and Aortic and Distal Pressures at the Acute Moment vs 1-Month Follow-up.
A, Aortic pressure (black line) and distal pressure (red line) results after adenosine infusion. B, A nonsignificant increase in hyperemic response of aortic pressure (resting minus hyperemic aortic pressure) was associated with an absolute difference of 1 mm Hg, and a significant increase in hyperemic response of distal pressure (resting minus hyperemic distal pressure) from the acute moment of presentation to follow-up was associated with an absolute difference of 4 mm Hg. Boxplots represent means, and whiskers represent SDs.
Vasodilatory reserve as measured by resistive reserve ratio was significantly lower at the acute moment vs follow-up (mean [SD], 3.4 [1.7] vs 5.0 [2.7]; P < .001). The difference between acute and follow-up resistive reserve ratio correlated with the difference in hyperemic response of Pd between the acute moment and follow-up (ρ, 0.38; P = .001).
Cardiac Magnetic Resonance Imaging, Infarct Characteristics, and Hemodynamic Measurements
There was no significant association between change in iFR (β, 0.14; P = .25), change in Pd/Pa (β, 0.08; P = .51), or change in CFR (β, –0.21; P = .10) and infarct size reported in grams. There was a significant association between change in FFR and infarct size as reported in grams (β, 0.26; P = .03). Both IMR in the acute setting (ρ, –0.43; P = .001) and change in IMR (ρ, 0.52; P < .001) correlated significantly with myocardial salvage index. There were no significant clinical or hemodynamic predictors of changes in FFR, iFR, or Pd/Pa (eTable 6 in the Supplement). A smaller hyperemic distal pressure decrease at the acute moment, shown by a large difference in Pd between acute and follow-up, correlated with left ventricular ejection fraction (ρ, 0.39; P = .001) and infarct size (reported as left ventricular percentage) (ρ, –0.29; P = .02). For patients with microvascular injury, the difference between acute and follow-up Pd was higher (mean [SD], 10.1 [16.4] mm Hg vs 1.7 [14.1] mm Hg; P = .04) and there was a larger change in IMR compared with patients without microvascular injury (mean [SD], 7.4 [21.4] U vs –1.3 [17.7] U; P = .09).
Discussion
The aim of this study was to assess changes in nonculprit hemodynamic indices in patients with STEMI at the index procedure compared with 1-month follow-up. For the first time, to our knowledge, we performed a paired analysis of both nonculprit FFR and iFR. The hyperemic indices FFR and CFR showed significant changes between the index event and 1-month follow-up, and the nonhyperemic indices Pd/Pa and iFR numerically increased but did not significantly change between the index event and 1-month follow-up. The CCA between the acute moment and follow-up was similar for FFR and iFR. The IMR was increased at the index event compared with follow-up, whereas nonculprit BMR was decreased. The hyperemic vasodilatory response of the microcirculation was lower at the acute moment compared with follow-up. This blunted hyperemic response was associated with elevated FFR values and was most pronounced in patients with a large infarct size, low left ventricular ejection fraction, and microvascular injury.
Previous studies have investigated the temporal development of nonculprit coronary blood flow after STEMI.14,15,16,24 We studied the extent to which physiologic indices are associated with altered intracoronary nonculprit flow in the setting of acute STEMI.
We showed a significant decrease in FFR accompanied by a large increase in CFR from the acute moment to 1-month follow-up. This finding may have been associated with changes in intracoronary hyperemic flow at the acute moment of STEMI. Consistent with our findings, Uren et al24 reported that among patients with STEMI treated with thrombolysis, CFR assessed by [15O]H2O positron emission tomography was decreased 1 week after acute myocardial infarction compared with healthy control patients in both the culprit and remote territory. The attenuated CFR was associated with a slight increase in baseline flow in combination with a substantially decreased hyperemic flow. This finding was confirmed for patients with STEMI treated with pPCI.15,16 Thus, the decrease in CFR at the acute moment in nonculprit vessels was mainly associated with a decrease in hyperemic flow. Under the assumption that stenosis geometry does not change within a month, these hemodynamic changes may explain our findings—an increased FFR and decreased CFR in nonculprit vessels in the acute setting of STEMI.
The changes in epicardial hemodynamic parameters were associated with changes in microvascular resistance. In our study, we showed an increased acute hyperemic microcirculatory resistance in nonculprit vessels. This finding is similar to that of a previous study.16
Nonhyperemic iFR in the Acute Setting of STEMI
In contrast, iFR and resting Pd/Pa were numerically lower at the acute moment but did not significantly change from the acute moment to follow-up. This can be explained by a modest change in resting flow in nonculprit vessels at the acute moment of STEMI. In pigs, coronary nonculprit resting flow assessed by radionuclide-labeled microspheres was increased during the initial 14 days after STEMI.25 In patients with STEMI, a 12% increase in nonculprit resting flow was reported at the acute moment.14 In 73 patients with anterior myocardial infarction, there was a small increase in resting flow assessed by Doppler flow velocity compared with 6-month follow-up.16 The minor increase in acute nonculprit resting flow gradually normalized and was similar at 1 week compared with 3-month follow-up.15 We showed a decreased BMR, which was not significantly associated with a change in acute iFR compared with follow-up. This might have occurred because of the specific diastolic nature of this index.
Hyperemic Flow in Nonculprit Vessels
In culprit vessels, the decreased hyperemic coronary flow was secondary to microvascular injury, extravascular compression due to edema, intramyocardial hemorrhage, increased left ventricular end-diastolic pressure, microvascular plugging, and an increased neurohumoral state.26,27 In culprit vessels, the presence of microvascular injury correlates with elevated FFR values, lower CFR values, and increased IMR values.28 In nonculprit vessels, the causes of altered coronary flow and flow responses are less well described. An important finding in our study was the acute blunted hyperemic microcirculatory response to adenosine, which was similar to results of a previous study in patients with STEMI.23 Under normal circumstances, adenosine leads to a reduction in distal coronary pressure because of a decrease in microvascular resistance caused by arteriolar vasodilation.29 However in the acute setting of STEMI, the sensitivity of the purinergic adenosine receptors was shown to be diminished even in the remote myocardium,30 causing a blunted adenosine response. In future studies, it would be interesting to compare different hyperemic agents to explore the potential advantage of other vasodilators (eg, nitrovasodilators) compared with adenosine in the acute setting. Furthermore, increased neurohumoral activation and elevated levels of endothelin-1,31 elevated left ventricular end-diastolic pressure,32 and myocardial edema33 potentially contribute to a decrease in hyperemic nonculprit flow. Consequently, the changes in flow following myocardial infarction are directly associated with infarct size and the presence of microvascular injury as assessed by cardiac magnetic resonance imaging in patients with STEMI as well as in a porcine model of acute myocardial infarction.14
Resting Flow in Nonculprit Vessels
In culprit vessels, the increased culprit resting flow is attributable to post–ischemic reactive hyperemia. Similarly in nonculprit vessels, a primary increase in myocardial baseline flow has been found directly after pPCI that gradually decreases.17 This is probably attributable to hyperkinesia of noninfarcted myocardium to compensate for the loss of contractile function of the infarcted or stunned myocardium in the culprit territory.34 Also, because of activation of the sympathetic nervous system at the acute moment of STEMI, heart rate is higher, causing increased baseline coronary blood flow.35 We showed a decreased nonculprit BMR at the acute moment compared with 1-month follow-up. In the chronic phase after STEMI, the above-mentioned acute changes somewhat normalize.
Pressure-Based Assessment of Epicardial Stenosis Severity in Nonculprit Vessels
Ntalianis et al19 examined the reliability of solely FFR in patients with myocardial infarction and found no significant difference in FFR from the acute moment to follow-up (mean [SD] FFR was 0.77 [0.13] at both the acute moment and follow-up). However, the exact time of follow-up varied between 4 and 128 days, which was an important confounder in that study. Also, infarct size was small and patients with both STEMI and non-STEMI were included. Of note, when only assessing patients with STEMI, they also observed an absolute 0.02 decrease in FFR. The Nonculprit Stenosis Evaluation Using iFR in Patients With STEMI (iSTEMI) study18 investigated the reliability of iFR in 120 patients with STEMI and 157 with corresponding nonculprit stenoses. The investigators found a significant increase in iFR from the acute event to follow-up (0.89 vs 0.91; P < .001), with a median follow-up of 16 days (range, 5-32 days). We showed a similar absolute increase in iFR of 0.01, and we found a comparable CCA in iFR from the acute event to follow-up (82% in our study vs 78% in the iSTEMI study18). In our study, the difference in mean iFR from the acute moment to follow-up did not significantly change; the correlation between the acute moment and follow-up iFR seemed to be inferior compared with the correlation between the acute moment and follow-up FFR. In the iSTEMI study,18 FFR was not measured at the acute moment, and CFR, IMR, and BMR were not measured. The iFR and FFR for the assessment of nonculprit lesions during the index procedure in patients with ST-segment elevation myocardial infarction (the WAVE study),36 a single center observational registry, is the only study to date to perform both FFR and iFR measurements at the acute moment compared with the subacute phase. The investigators showed that iFR and FFR values were stable between the acute and the subacute moments (FFR: 0.82 vs 0.82; P = .62) and (iFR: 0.90 vs 0.89; P = .64). A major limitation of that study was that follow-up was performed close to the index event (5-8 days after the index procedure). At that moment, hemodynamic changes initiated at the acute moment of STEMI might still exist.
Clinical Relevance
In the present study, we showed for the first time to our knowledge that iFR performed equally to FFR in terms of CCA between the acute moment and late (1-month) follow-up measurements when interrogating the nonculprit vessels in patients with STEMI. In addition, both iFR and FFR were associated with altered coronary hemodynamics in the acute vs late phase. This finding should be kept in mind, especially in the case of large infarcts and values around the cutoff. For these patients, acute intracoronary measurements could be insufficient, and a delayed physiology-guided revascularization of nonculprit vessels may be beneficial. An alternative could be a hybrid approach using both FFR and iFR in the acute setting. As FFR decreases, FFR could guide revascularization in vessels with acute FFR of 0.80 or less, but in patients with FFR greater than 0.80, iFR should guide revascularization of nonculprit vessels in the acute setting of STEMI. The ongoing iFR Guided Multi-vessel Revascularization During Percutaneous Coronary Intervention for Acute Myocardial Infarction (the iMODERN trial),37 a prospective multicenter trial, may shed light on the clinical use of iFR in nonculprit vessels at the acute moment of STEMI.
Limitations
Our study comprised a relatively small number of patients. However, it is the only study to our knowledge that assessed both FFR and iFR during the acute moment and at late (1-month) follow-up. We collected thermodilution-based CFR, IMR, and BMR measurements that were in line with the findings for the pressure-based measurements and complied with the theoretical hemodynamic framework. In line with other contemporary STEMI studies,38 infarct size in our study was relatively preserved. In patients with more myocardial damage, our results could potentially have been more pronounced. We did not measure or correct for collateral supply, and iFR was calculated using the ECG-independent algorithm, which closely correlates with the frequently used ECG-dependent iFR (r = 0.9997).22
Conclusions
In patients with STEMI and with multivessel disease, nonculprit coronary hemodynamics were altered such that FFR was slightly overestimated, CFR was significantly underestimated, and iFR and Pd/Pa were slightly underestimated compared with values remeasured in the stable setting 1 month later. These findings should be taken into account when performing functional assessment of a diseased nonculprit vessel in the acute setting of STEMI.
eAppendix 1. Cardiac Magnetic Resonance Imaging Methods
eAppendix 2. List of Inclusion and Exclusion Criteria
eAppendix 3. Statistical Analysis
eTable 1. Reasons for Exclusion of Patients
eTable 2. Baseline Characteristics of the Excluded Patients
eTable 3. CMR-derived Infarct Characteristics
eTable 4. Influence of Heart Rate and Blood Pressure in the Acute Setting on FFR, iFR and PdPa Values
eTable 5. Effect of Administration of Adenosine on Aortic and Distal Pressure
eTable 6. Clinical and Hemodynamic Predictors of Changes in Hemodynamic Indices
References
- 1.Park DW, Clare RM, Schulte PJ, et al. . Extent, location, and clinical significance of non–infarct-related coronary artery disease among patients with ST-elevation myocardial infarction. JAMA. 2014;312(19):2019-2027. doi: 10.1001/jama.2014.15095 [DOI] [PubMed] [Google Scholar]
- 2.Sorajja P, Gersh BJ, Cox DA, et al. . Impact of multivessel disease on reperfusion success and clinical outcomes in patients undergoing primary percutaneous coronary intervention for acute myocardial infarction. Eur Heart J. 2007;28(14):1709-1716. doi: 10.1093/eurheartj/ehm184 [DOI] [PubMed] [Google Scholar]
- 3.Smits PC, Abdel-Wahab M, Neumann FJ, et al. ; Compare-Acute Investigators . Fractional flow reserve-guided multivessel angioplasty in myocardial infarction. N Engl J Med. 2017;376(13):1234-1244. doi: 10.1056/NEJMoa1701067 [DOI] [PubMed] [Google Scholar]
- 4.Wald DS, Morris JK, Wald NJ, et al. ; PRAMI Investigators . Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013;369(12):1115-1123. doi: 10.1056/NEJMoa1305520 [DOI] [PubMed] [Google Scholar]
- 5.Gershlick AH, Khan JN, Kelly DJ, et al. . Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015;65(10):963-972. doi: 10.1016/j.jacc.2014.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engstrøm T, Kelbæk H, Helqvist S, et al. ; DANAMI-3–PRIMULTI Investigators . Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3–PRIMULTI): an open-label, randomised controlled trial. Lancet. 2015;386(9994):665-671. doi: 10.1016/S0140-6736(15)60648-1 [DOI] [PubMed] [Google Scholar]
- 7.Neumann FJ, Sousa-Uva M. ‘Ten Commandments’ for the 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2018;39(42):3759. doi: 10.1093/eurheartj/ehy658 [DOI] [PubMed] [Google Scholar]
- 8.Pijls NH, van Son JA, Kirkeeide RL, De Bruyne B, Gould KL. Experimental basis of determining maximum coronary, myocardial, and collateral blood flow by pressure measurements for assessing functional stenosis severity before and after percutaneous transluminal coronary angioplasty. Circulation. 1993;87(4):1354-1367. doi: 10.1161/01.CIR.87.4.1354 [DOI] [PubMed] [Google Scholar]
- 9.Driessen RS, Raijmakers PG, Stuijfzand WJ, Knaapen P. Myocardial perfusion imaging with PET. Int J Cardiovasc Imaging. 2017;33(7):1021-1031. doi: 10.1007/s10554-017-1084-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JM, Jung JH, Hwang D, et al. . Coronary flow reserve and microcirculatory resistance in patients with intermediate coronary stenosis. J Am Coll Cardiol. 2016;67(10):1158-1169. doi: 10.1016/j.jacc.2015.12.053 [DOI] [PubMed] [Google Scholar]
- 11.Sen S, Escaned J, Malik IS, et al. . Development and validation of a new adenosine-independent index of stenosis severity from coronary wave-intensity analysis: results of the ADVISE (ADenosine Vasodilator Independent Stenosis Evaluation) study. J Am Coll Cardiol. 2012;59(15):1392-1402. doi: 10.1016/j.jacc.2011.11.003 [DOI] [PubMed] [Google Scholar]
- 12.Davies JE, Sen S, Dehbi HM, et al. . Use of the instantaneous wave-free ratio or fractional flow reserve in PCI. N Engl J Med. 2017;376(19):1824-1834. doi: 10.1056/NEJMoa1700445 [DOI] [PubMed] [Google Scholar]
- 13.Götberg M, Christiansen EH, Gudmundsdottir IJ, et al. ; iFR-SWEDEHEART Investigators . Instantaneous wave-free ratio versus fractional flow reserve to guide PCI. N Engl J Med. 2017;376(19):1813-1823. doi: 10.1056/NEJMoa1616540 [DOI] [PubMed] [Google Scholar]
- 14.de Waard GA, Hollander MR, Teunissen PF, et al. . Changes in coronary blood flow after acute myocardial infarction: insights from a patient study and an experimental porcine model. JACC Cardiovasc Interv. 2016;9(6):602-613. doi: 10.1016/j.jcin.2016.01.001 [DOI] [PubMed] [Google Scholar]
- 15.Teunissen PF, Timmer SA, Danad I, et al. . Coronary vasomotor function in infarcted and remote myocardium after primary percutaneous coronary intervention. Heart. 2015;101(19):1577-1583. doi: 10.1136/heartjnl-2015-307825 [DOI] [PubMed] [Google Scholar]
- 16.Bax M, de Winter RJ, Koch KT, Schotborgh CE, Tijssen JG, Piek JJ. Time course of microvascular resistance of the infarct and noninfarct coronary artery following an anterior wall acute myocardial infarction. Am J Cardiol. 2006;97(8):1131-1136. doi: 10.1016/j.amjcard.2005.11.026 [DOI] [PubMed] [Google Scholar]
- 17.Ambrosio G, Weisman HF, Mannisi JA, Becker LC. Progressive impairment of regional myocardial perfusion after initial restoration of postischemic blood flow. Circulation. 1989;80(6):1846-1861. doi: 10.1161/01.CIR.80.6.1846 [DOI] [PubMed] [Google Scholar]
- 18.Thim T, Götberg M, Fröbert O, et al. . Nonculprit stenosis evaluation using instantaneous wave-free ratio in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2017;10(24):2528-2535. doi: 10.1016/j.jcin.2017.07.021 [DOI] [PubMed] [Google Scholar]
- 19.Ntalianis A, Sels JW, Davidavicius G, et al. . Fractional flow reserve for the assessment of nonculprit coronary artery stenoses in patients with acute myocardial infarction. JACC Cardiovasc Interv. 2010;3(12):1274-1281. doi: 10.1016/j.jcin.2010.08.025 [DOI] [PubMed] [Google Scholar]
- 20.Janssens GN, van Leeuwen MA, van der Hoeven NW, et al. . Reducing microvascular dysfunction in revascularized patients with ST-elevation myocardial infarction by off-target properties of ticagrelor versus prasugrel. Rationale and design of the REDUCE-MVI study. J Cardiovasc Transl Res. 2016;9(3):249-256. doi: 10.1007/s12265-016-9691-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pijls NH, Bruyne BD. Fractional flow reserve, coronary pressure wires, and drift. Circ J. 2016;80(8):1704-1706. doi: 10.1253/circj.CJ-16-0623 [DOI] [PubMed] [Google Scholar]
- 22.Petraco R, Sen S, Nijjer S, et al. . ECG-independent calculation of instantaneous wave-free ratio. JACC Cardiovasc Interv. 2015;8(15):2043-2046. doi: 10.1016/j.jcin.2015.10.005 [DOI] [PubMed] [Google Scholar]
- 23.Layland J, Carrick D, McEntegart M, et al. . Vasodilatory capacity of the coronary microcirculation is preserved in selected patients with non–ST-segment-elevation myocardial infarction. Circ Cardiovasc Interv. 2013;6(3):231-236. doi: 10.1161/CIRCINTERVENTIONS.112.000180 [DOI] [PubMed] [Google Scholar]
- 24.Uren NG, Crake T, Lefroy DC, de Silva R, Davies GJ, Maseri A. Reduced coronary vasodilator function in infarcted and normal myocardium after myocardial infarction. N Engl J Med. 1994;331(4):222-227. doi: 10.1056/NEJM199407283310402 [DOI] [PubMed] [Google Scholar]
- 25.MacLean MJ, Biro GP. Time course of myocardial bloodflow changes during healing of myocardial infarct in pigs. Can J Cardiol. 1992;8(7):749-755. [PubMed] [Google Scholar]
- 26.Betgem RP, de Waard GA, Nijveldt R, Beek AM, Escaned J, van Royen N. Intramyocardial haemorrhage after acute myocardial infarction. Nat Rev Cardiol. 2015;12(3):156-167. doi: 10.1038/nrcardio.2014.188 [DOI] [PubMed] [Google Scholar]
- 27.Robbers LF, Eerenberg ES, Teunissen PF, et al. . Magnetic resonance imaging–defined areas of microvascular obstruction after acute myocardial infarction represent microvascular destruction and haemorrhage. Eur Heart J. 2013;34(30):2346-2353. doi: 10.1093/eurheartj/eht100 [DOI] [PubMed] [Google Scholar]
- 28.Cuculi F, De Maria GL, Meier P, et al. . Impact of microvascular obstruction on the assessment of coronary flow reserve, index of microcirculatory resistance, and fractional flow reserve after ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2014;64(18):1894-1904. doi: 10.1016/j.jacc.2014.07.987 [DOI] [PubMed] [Google Scholar]
- 29.Tarkin JM, Nijjer S, Sen S, et al. . Hemodynamic response to intravenous adenosine and its effect on fractional flow reserve assessment: results of the Adenosine for the Functional Evaluation of Coronary Stenosis Severity (AFFECTS) study. Circ Cardiovasc Interv. 2013;6(6):654-661. doi: 10.1161/CIRCINTERVENTIONS.113.000591 [DOI] [PubMed] [Google Scholar]
- 30.Zhou Z, de Wijs-Meijler D, Lankhuizen I, et al. . Blunted coronary vasodilator response to uridine adenosine tetraphosphate in post-infarct remodeled myocardium is due to reduced P1 receptor activation. Pharmacol Res. 2013;77:22-29. doi: 10.1016/j.phrs.2013.08.007 [DOI] [PubMed] [Google Scholar]
- 31.Kelly RF, Hursey TL, Schaer GL, et al. . Cardiac endothelin release and infarct size, myocardial blood flow, and ventricular function in canine infarction and reperfusion. J Investig Med. 1996;44(9):575-582. [PubMed] [Google Scholar]
- 32.Van Herck PL, Carlier SG, Claeys MJ, et al. . Coronary microvascular dysfunction after myocardial infarction: increased coronary zero flow pressure both in the infarcted and in the remote myocardium is mainly related to left ventricular filling pressure. Heart. 2007;93(10):1231-1237. doi: 10.1136/hrt.2006.100818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biesbroek PS, Amier RP, Teunissen PFA, et al. . Changes in remote myocardial tissue after acute myocardial infarction and its relation to cardiac remodeling: a CMR T1 mapping study. PLoS One. 2017;12(6):e0180115. doi: 10.1371/journal.pone.0180115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daher E, Dione DP, Heller EN, et al. . Acute ischemic dysfunction alters coronary flow reserve in remote nonischemic regions: potential mechanical etiology identified in an acute canine model. J Nucl Cardiol. 2000;7(2):112-122. doi: 10.1016/S1071-3581(00)90031-X [DOI] [PubMed] [Google Scholar]
- 35.Graham LN, Smith PA, Huggett RJ, Stoker JB, Mackintosh AF, Mary DA. Sympathetic drive in anterior and inferior uncomplicated acute myocardial infarction. Circulation. 2004;109(19):2285-2289. doi: 10.1161/01.CIR.0000129252.96341.8B [DOI] [PubMed] [Google Scholar]
- 36.Clinicaltrials.gov Instantaneous wave-free ratio and fractional flow reserve for the assessment of non culprit lesions in patients with ST-segment elevation myocardial infarction (WAVE). NCT02869906 https://clinicaltrials.gov/ct2/show/NCT2869906 Accessed August 17, 2016.
- 37.Clinicaltrials.gov iFR guided multi-vessel revascularization during percutaneous coronary intervention for acute myocardial infarction (iMODERN). NCT03298659. https://clinicaltrials.gov/ct2/show/NCT03298659 Accessed October 2, 2017.
- 38.Roolvink V, Ibáñez B, Ottervanger JP, et al. ; EARLY-BAMI Investigators . Early intravenous beta-blockers in patients with ST-segment elevation myocardial infarction before primary percutaneous coronary intervention. J Am Coll Cardiol. 2016;67(23):2705-2715. doi: 10.1016/j.jacc.2016.03.522 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Cardiac Magnetic Resonance Imaging Methods
eAppendix 2. List of Inclusion and Exclusion Criteria
eAppendix 3. Statistical Analysis
eTable 1. Reasons for Exclusion of Patients
eTable 2. Baseline Characteristics of the Excluded Patients
eTable 3. CMR-derived Infarct Characteristics
eTable 4. Influence of Heart Rate and Blood Pressure in the Acute Setting on FFR, iFR and PdPa Values
eTable 5. Effect of Administration of Adenosine on Aortic and Distal Pressure
eTable 6. Clinical and Hemodynamic Predictors of Changes in Hemodynamic Indices