Abstract
STUDY QUESTION
Are melatonin receptors (melatonin receptor 1A (MR1A) and melatonin receptor 1B (MR1B)) expressed in human endometrium and endometriotic tissue, and does melatonin affect endometrial cell proliferation?
SUMMARY ANSWER
Melatonin receptors are expressed in human eutopic endometrium, endometriomas and peritoneal lesions, although to different extents, and melatonin treatment attenuated estradiol-induced endometrial epithelial cell proliferation in culture.
WHAT IS KNOWN ALREADY
Melatonin decreased endometriotic lesion volume in a rat model of endometriosis. Melatonin treatment reduced pain scores in and analgesic use by women with endometriosis.
STUDY DESIGN, SIZE, DURATION
Basic science study using human endometrial tissue and an endometrial epithelial cell line.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Measurement of melatonin receptor expression (mRNA and protein) in women with surgically confirmed endometriosis (endometrioma (n = 20) or peritoneal lesion (n = 11) alone) and women without surgical evidence of endometriosis (control, n = 15). Collection of endometrial and endometriotic tissue samples, gynecologic history and demographic information. Quantification of estradiol (1.0 nM) and melatonin (0.1 nM–1.0 μM) ± estradiol-induced endometrial epithelial cell proliferation in cultures of endometrial epithelial cells (CRL-1671) following 24 and 48 hours of culture.
MAIN RESULTS AND THE ROLE OF CHANCE
MR1A and MR1B were localized by immunohistochemistry in glandular epithelial cells of endometrial biopsies from women with and without endometriosis. Both receptors were expressed in eutopic and ectopic endometrial tissue. mRNA expression of MR1A and MR1B was significantly greater in peritoneal lesions than in either endometriomas or eutopic endometrium. However, protein expression of MR1A was decreased in peritoneal lesions compared to control eutopic endometrium, whereas MR1B expression did not differ between the groups. Melatonin (0.1 nM–1.0 μM) treatment inhibited estradiol (1.0 nM)-induced endometrial epithelial cell proliferation at 48 hours but not 24 hours of culture.
LIMITATIONS, REASONS FOR CAUTION
Beneficial effects of melatonin seen in culture have yet to be comprehensively evaluated in women with endometriosis.
WIDER IMPLICATIONS OF THE FINDINGS
Our data suggest that melatonin may be useful as an adjunct to current endometriosis treatments.
STUDY FUNDING/COMPETING INTEREST(S)
This study was supported by the Canadian Institutes of Health Research (grant MOP142230 to W.G.F.). A.A.M. is supported by a resident research grant through the Physicians Services Incorporated Foundation. The authors have no conflicts of interest.
Keywords: endometriosis, melatonin, melatonin receptors, endometrium, CRL1671 cells, MR1A, MR1B, epithelial
Introduction
Endometriosis is a chronic, estrogen-dependent benign inflammatory disease characterized by the presence of endometrial tissue located outside the uterine cavity. Endometriosis is a prevalent gynecological disease affecting ~10% of women of reproductive age and up to 50% of women with pelvic pain (Giudice and Kao, 2004) that continues to be a major clinical challenge to diagnose and treat. The etiology of endometriosis remains unclear but is believed to involve retrograde menstruation with an abnormal immune response that allows the ectopic endometrial implants to invade into the peritoneum. Two central biochemical disturbances in endometriosis are overproduction of prostaglandins by cyclo-oxygenase 2 and overproduction of estrogen by aromatase (reviewed in (Bulun, 2009)). Thus, the majority of existing therapeutic approaches target inflammation and ovarian suppression. The mainstay of treatment is hormonal suppression of menstruation and ovarian function, the surgical excision of ectopic endometriotic lesions or surgical ablation of more superficial lesions; however, there is a high recurrence rate for both interventions (Guo, 2009). Unfortunately, existing therapeutic interventions remain sub-optimal, leaving women who wish to conceive with the choice between pursuing pregnancy and managing their pelvic pain. Hence, pursuit of novel treatment options for women with endometriosis continues.
Melatonin (N-acetyl-5-methoxytryptamine) is a hormone produced primarily by the pineal gland, and several disparate lines of evidence lead us to postulate a beneficial effect of this hormone for the treatment of endometriosis. Specifically, animal studies have shown that treatment with melatonin decreased the volume and weight of endometriotic lesions (Güney et al., 2008; Yildirim et al., 2010; Kocadal et al., 2013; Cetinkaya et al., 2015; Yilmaz et al., 2015; Yesildaglar et al., 2016). Additionally, melatonin decreased markers of oxidative stress (Güney et al., 2008; Yildirim et al., 2010; Yilmaz et al., 2015), matrix metalloproteinases and markers of angiogenesis (Yilmaz et al., 2015). More severe endometriosis with larger lesion volume and higher histopathological scores was induced by pinealectomy in rats compared to controls, an effect that was reversed by administration of melatonin (Koc et al., 2010). Risk of endometriosis was increased (odds ratio of 1.98 with 95% confidence interval of 1.01–3.85) in women with a history of shift work (Marino et al., 2008) suggesting a potential link between melatonin and endometriosis. Moreover, a randomized, double-blind, placebo-controlled trial with 20 women/arm investigated the effect of melatonin (10 mg/day) in women with endometriosis (Schwertner et al., 2013). Overall pain scores were decreased by 39.8% and analgesic use decreased by 80% after 8 weeks of treatment. The treatment group also had lower levels of brain-derived neurotrophic factor, a clinical biomarker of endometriosis (Giannini et al., 2010; Wessels et al., 2016), compared to the placebo-treated group. Taken together, these data implicate melatonin in the pathophysiology of endometriosis and suggest that it may be an effective and safe therapeutic intervention that addresses their pain while allowing them to attempt to conceive. Consequently, better understanding of melatonin effects in the human endometrium is needed.
Although melatonin treatment decreased endometriotic lesion volume in animal models of endometriosis (Güney et al., 2008; Yildirim et al., 2010; Kocadal et al., 2013; Cetinkaya et al., 2015; Yilmaz et al., 2015) and encouraging results have been demonstrated in a small, randomized, double-blind, placebo-controlled study of women with endometriosis (Schwertner et al., 2013), the underlying mechanism(s) remains unclear. It is known that melatonin is regulated by light–dark cycles and acts through two G protein-coupled receptors, melatonin receptors 1A and 1B (MR1A and MR1B) (Dubocovich and Markowska, 2005); however, expression of these receptors in human endometrium has not be demonstrated previously. Furthermore, the effects of melatonin on endometrial epithelial cell physiology remain to be elucidated. Nocturnal physiological concentrations of melatonin (1.0 nM) attenuated estradiol-induced proliferation of MCF-7 cells, an estrogen-dependent breast cancer cell line (Proietti et al., 2013). Melatonin treatment also dysregulates estrogen receptor type 1 (EsR1) expression in MCF-7 cells (Molis et al., 1994) and inhibited estrogen signaling in breast cancer stem cells (Lopes et al., 2016) further suggesting that melatonin has potentially beneficial effects in estrogen-dependent diseases. Therefore, the objectives of the current study were to investigate melatonin receptor expression in the eutopic endometrium of women (control group) and the eutopic and ectopic endometrium of women with endometriosis (cases) as well as document the effect of melatonin on estrogen-induced endometrial epithelial cell proliferation in vitro.
Materials and Methods
Study participants
Women (n = 46) undergoing a laparoscopic surgery at McMaster University Medical Centre for suspected endometriosis, pelvic pain and fertility care were invited to participate in this study. Exclusion criteria included all women unable to consent, those under the age of 18, currently pregnant, malignancy of any kind, acute inflammatory disease or infection and systemic autoimmune disease. This study was approved by the Hamilton Integrated Research Ethics Board, McMaster University (REB# 12-083-T). All women provided written informed consent and completed a questionnaire assessing demographics, menstrual cycle length, last menstrual period and pelvic pain. During laparoscopic surgery, women were categorized as a case (endometriosis, n = 31) or control (no endometriosis, n = 15) by gynecological surgeons and the stage of endometriosis in the case group was assigned during surgery according to the revised American Society for Reproductive Medicine classification system. In women with endometriosis, the surgeons also noted the location of lesions (endometriomas and peritoneal lesions). Diagnosis was confirmed through review of histological pathology reports. Women with adenomyosis confirmed by pathology were excluded. Study participants were not currently receiving any hormonal therapies and menstrual cycle stage was further confirmed by histopathology.
Sample collection
Eutopic endometrial samples from both controls and cases were collected via pipelle biopsy at the time of surgery prior to the first incision. Ectopic samples were collected upon removal of lesions. Both eutopic and ectopic endometrial samples were placed on ice, transferred to the laboratory and processed for preservation of RNA in RNAlater® (R0901; Sigma-Aldrich, Oakville, Canada) according to the manufacturer’s instructions and subsequently stored at −80°C until required for analysis. A portion of the sample was processed for histology and menstrual cycle staging in the usual fashion (Noyes and Haman, 1953) via 10% (w/v) formalin fixation, processing and embedding in paraffin. Sections (4 μm) were cut on a rotary microtome and stained with hematoxylin and eosin or immunohistochemistry.
Immunohistochemistry
Endometrial sections were de-paraffinized in xylene (3 × 5 minutes), rehydrated through a graded ethanol series (100, 90 and 70% EtOH) followed by PBS and immersed in 1% H2O2 (H325-500; Fisher Chemical, Ottawa, Canada) in PBS to inhibit endogenous peroxidases. Positive staining was assessed using sections of the pancreas (Söderquist et al., 2015). Sections were washed in PBS and incubated in citrate buffer (pH 6.0; C9999; Sigma-Aldrich, Oakville, Canada) at 90°C for 12 minutes followed by 20 minutes at room temperature. Sections were subsequently washed in PBS, blocked for 30 minutes with 5% bovine serum albumin (BSA), fraction V (OmniPur®, LC2960, Calbiochem, San Diego, CA) in PBS, and incubated with the primary antibody (Table I) (anti-MR1A antibody (1:500; abcam ab116337) or anti-MR1B antibody (1:1000; abcam ab128469)) in 1% BSA overnight at 4°C in a humidified chamber. Sections were washed in PBS, incubated with biotinylated secondary antibodies and avidin/horseradish peroxidase complex in a commercially available staining kit (VECTASTAIN® Elite ABC kit, PK-6101; Vector Laboratories, Burlington, Canada) for 1 hour at room temperature in a humidified chamber. Sections were washed and incubated with 1 mg 3,3′-Diaminobenzidine (DAB)/ml, 0.02% v/v H2O2 in water (DAKO® DAB chromogen tablets, Dako, S3000, Santa Clara, CA). The slides were washed, counterstained with Harris modified hematoxylin (HHS32; Sigma-Aldrich, Oakville, Canada) and eosin and cover slipped with permount. For negative controls, the primary antibody was substituted with 1% BSA in PBS for one adjacent tissue section on each slide that underwent all subsequent staining steps. Digital photomicrographs were captured with an Infinity1 camera (Lumenera Corp., Ottawa, Canada) and an Olympus IX81 microscope (Olympus, Richmond Hill, Canada).
Table I.
Antibodies.
| Human protein | Antibody | Protein size (kDa) |
|---|---|---|
| MR1A | Rabbit anti-melatonin receptor 1A antibody (Abcam ab116337) | 39 |
| MR1B | Rabbit anti-melatonin receptor 1B antibody (Abcam ab128469) | 40 |
RNA and protein extraction
Total RNA was extracted from both eutopic and ectopic samples using the RNA/Protein Purification Plus Kit (Norgen Biotek, Thorold, Canada). Briefly, ~10 mg of sample was minced with a scalpel, placed in 500 μl of lysis reagent from the kit and disrupted on ice using a tissue homogenizer (ProScientific, Oxford, CT, USA) for roughly 10 seconds. Samples were centrifuged at 4°C at 14000g for 2 minutes. Genomic DNA was removed using a column separator. RNA quantity and purity were analyzed using a NanoDrop 2000 spectrophotometer using NanoDrop 2000/2000c software (Thermo Scientific, Canada). All RNA samples were stored at −80°C until required. Protein extraction from ~10 mg of sample was performed in 300 μl of RIPA buffer (89900; Thermo Scientific, Canada) supplemented with Halt™ Protease and Phosphatase Inhibitor Cocktail (78440; Thermo Scientific, Canada). The tissue was disrupted on ice using a tissue homogenizer for roughly 10 seconds. Samples were then agitated at 4°C for 2 hours using a rocker followed by centrifugation at 2000g for 20 minutes at 4°C and collection of the supernatant. Total protein content of samples was quantified using a commercially available bicinchoninic acid kit (Pierce™, 23225; Thermo Scientific, Canada). Emission absorbance values were detected at 562 nm on a Synergy™ H4 Hybrid Multi-Detection Microplate Reader using Gen5™ software (Bio-Tek®, Winooski, VT). All protein samples were stored at −20°C until required for analysis.
qPCR
A total of 500 ng of total RNA was reverse transcribed using the iScript™ cDNA Synthesis Kit (1708891; Bio-Rad) according to the manufacturer’s instructions and using a standard thermocycler (Bio-Rad iCycler, Mississauga, Ontario, Canada). cDNA was stored at −20°C until quantitative polymerase chain reaction (qPCR) assays were performed. Quantitative PCR assay was performed using the Roche Lightcycler® 480 System (Roche Diagnostics, Wellington, New Zealand) and LightCycler® 480 SYBR Green I Master (Roche Diagnostics, cat. 04707516001). Primers for all genes were designed using Primer BLAST software available at the NCBI website. For both MR1A and MR1B, the forward primers bind in exon I, whereas the reverse primers bind in exon II over the splice site between exon I and II. Primers (Table II) were obtained from Invitrogen (Life Technologies, Canada). Optimal primer conditions were adjusted to the following: length, 20 bp (range, 17–23 bp); Tm, 60°C (range, 58–62°C); and amplicon length, 50–200 bp. Dissociation analyses were performed to ensure specificity and samples producing a single peak in dissociation curves were used. All qPCR assays were carried out with an initial denaturation at 95°C for 5 minutes, followed by amplification of the gene product through 60 successive cycles of 95°C for 10 seconds, 60°C for 10 seconds and 72°C for 10 seconds. Each sample was run in triplicate. All mRNA data are expressed relative to the reference gene Ywhaz (tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta), whose expression did not differ significantly between groups.
Table II.
Primers.
| Human gene | Forward primer | Reverse primer | Amplicon length (bp) | GenBank accession number |
|---|---|---|---|---|
| Ywhaz | ACTTGACATTGTGGACATCGGA | GTGGGACAGCATGGATGACA | 140 | NM_003406.3 |
| MR1A | CTGGTCATCCTGTCGGTGTA | AGATGTTTCCTGCGTTCCTGAG | 54 | NM_005958.4 |
| MR1B | ACCTCCTGGTGATCCTCTCC | AGAACAAATTACCTGCGTTCCG | 62 | NM_005959.3 |
Western blotting
A total of 15 μg of protein lysate in 1× Laemmli sample buffer (161-0747; Bio-Rad) with β-mercaptoethanol was heated to 95°C for 5 minutes and then resolved on 12% Mini-PROTEAN® TGX Stain-Free™ protein gels (4568046; Bio-Rad) at 50 V for 5 minutes followed by 40 minutes at 200 V and transferred to low-fluorescence polyvinylidene difluoride (LF PVDF) membrane using the Trans-Blot® Turbo™ RTA Midi LF PVDF Transfer Kit (1704275; Bio-Rad) and the Trans-Blot® Turbo™ Transfer System (Bio-Rad) according to the manufacturer’s instructions. Membranes were blocked in 5% non-fat skim milk (NFSM) in phosphate-buffered saline supplemented with 0.05% Tween-20 (PBS-T) for 1 hour at room temperature on a rocker. Membranes were then incubated with anti-MR1A antibody or anti-MR1B antibody (the same antibodies used for immunohistochemistry) in 5% NFSM in PBS-T for 16 hours at 4°C followed by 1 rinse in PBS-T and then 4× 5 minutes PBS-T washes. All membranes were then incubated with anti-rabbit IgG secondary antibody (1:10000, NA934; GE Healthcare, Mississauga, Ontario, Canada) for 1 hour in 5% NFSM/PBS-T and then washed with PBS-T as above. Total protein in each lane on each blot was imaged prior to enhanced chemiluminescence (ECL) detection of bands. Blots were developed using Clarity Max™ Western ECL Substrate-Luminol Solution (1705062; Bio-Rad), images captured using Bio-Rad ChemiDoc MP System and densitometric quantification was carried out using ImageLab Software (Bio-Rad) through normalization to total protein in each lane as per Stain-Free technology normalization protocol. A pooled quality control sample was included on each blot and used to adjust for blot-to-blot variability.
Tissue culture
Endometrial epithelial cells (CRL-1671), an endometrial epithelial adenocarcinoma cell line, were purchased from American Type Culture Collection (ATCC; Manassas, VA) and grown in DMEM/F12 with 10% fetal bovine serum (FBS; Sigma-Aldrich Chemical Co., St. Louis, MO) with 0.1% insulin, transferrin and selenium (ITS; Sigma-Aldrich Chemical Co., St. Louis, MO) and 1.0% penicillin and streptomycin (Sigma-Aldrich Chemical Co., St. Louis, MO). To test the effect of melatonin on cell proliferation, 20 000 endometrial epithelial cells/well were cultured in 96-well plastic culture plates in phenol red-free DMEM/F12 media containing charcoal-stripped 10% FBS and 0.1% ITS. When the cells reached ~70% confluence, the media was replaced with media containing a physiological concentration of estradiol (1.0 nM), melatonin (0.1 nM–1.0 μM) or melatonin (0.1 nM–1.0 μM) + estradiol (1.0 nM). Media was replaced with fresh treatment media after 24 hours of culture. Cell proliferation was evaluated by a commercial MTT tetrazolium cell viability assay (Abcam, Cambridge, UK) at 24 and 48 hours of culture according to the manufacturer’s directions. Briefly, the reduction of the tetrazolium compound MTS to its formazan product was measured by absorbance at 590 nm in a plate reader after 3 hrs of incubation. The quantity of formazan product is proportional to the reductive capacity and metabolic activity of viable cells in culture. While traditional proliferation assays give an absolute measure of proliferation by quantifying cell number, this assay quantifies metabolic activity as a measure of cell viability and activity. Data are expressed as mean absorbance at 590 nm ± standard error of eight (8) replicates/treatment group from 3 different experiments.
Statistical analysis
Multiple logistic regression using SPSS software (Version 24; IBM, Chicago, IL, USA) was employed to assess the relationship between categorical predictor variables (ethnicity, occupational status and smoking status) and a categorical dependent variable (lesion type). Western blot data that were not normally distributed were log transformed in order to achieve normality before using a one-way ANOVA with Tukey’s multiple comparison test. Age, duration of bleeding, age at first menstruation, protein and mRNA levels (with non-detects included) between lesion types were compared using a one-way ANOVA with Tukey’s multiple comparison test when data was normally distributed according to the D’Agostino and Pearson omnibus normality test. When this data was not normally distributed, Kruskal–Wallis one-way ANOVA with Dunn’s multiple comparison test was used. Statistically significant differences between groups were determined by post hoc tests and denoted with different letters above the graphs. Statistical outliers as determined by Grubb’s test (GraphPad QuickCalcs—free online) were not included in analyses. Statistical analyses for one-way ANOVAs were carried out using GraphPad Prism 5.01 (GraphPad Software Inc., La Jolla, CA, USA). Data were checked for outliers (Grubbs test) and failing to detect any, the data are presented as box plots, where the whiskers above and below the box represents 5th and 95th percentiles, and within which is shown the 50th percentile (the median). The whiskers indicate the upper and lower values not classified as statistical outliers by Grubb’s test. Data are presented as the mean ± SEM when normally distributed. Data are reported as medians with 25th and 75th percentiles in results section. Results were considered statistically significant for P ≤ 0.05.
Results
Study participant characteristics
All women included in this study (n = 46) underwent laparoscopic surgery, from which 31 cases of endometriosis and 15 controls were identified. Of the 31 cases of endometriosis, endometriomas alone were present in 20 cases and 11 women had peritoneal lesions only (Table III). Cases with endometriomas consisted of cases with only stage III (n = 2) and stage IV (n = 18) disease, whereas cases with peritoneal lesions consisted of cases with stage I (n = 2), stage II (n = 3), stage III (n = 4) and stage IV (n = 2) disease. All study participants reported regular menstrual cycles and there were no differences in prior use of ovarian suppressing medications between the groups. The average age and median duration of bleeding did not differ statistically between groups. Age of menarche was statistically significant between groups (P = 0.038) according to a Kruskal–Wallis one-way ANOVA; however, Dunn’s Multiple Comparison post hoc test revealed no statistically significant differences between groups (Table III). Multinomial logistic regression analyses revealed no statistically significant relationship between any of the categorical predictor variables—ethnicity, occupational status and smoking status—and the dependent variable—patient grouping based on lesion type.
Table III.
Patient characteristics.
| Characteristic | Control (15) | Cases with endometriomas (20) | Cases with peritoneal lesions (11) | P-value |
|---|---|---|---|---|
| Age (y), mean ± SD | 35.13 ± 8.50 | 33.35 ± 6.73 | 31.30 ± 6.53 | P = 0.444 |
| Stage n (%) | NA | |||
| Minimal 1 | 0 (0) | 0 (0) | 2 (18) | |
| Mild 2 | 0 (0) | 0 (0) | 3 (27) | |
| Moderate 3 | 0 (0) | 2 (10) | 4 (36) | |
| Severe 4 | 0 (0) | 18 (90) | 2 (18) | |
| Duration of bleeding, (d) median (25–75%) | 7 (5.5–7) | 6 (5–6.25) | 6 (5–7.75) | P = 0.505 |
| Age of menarche, (y) median (25–75%) | 12 (11–13) | 13 (12–14) | 13 (12–13.5) | P = 0.038 |
| Ethnicity n (%) | P = 0.084 | |||
| Caucasian | 9 (60) | 8 (40) | 5 (45) | |
| Asian | 1 (7) | 6 (30) | 0 (0) | |
| Black | 1 (7) | 0 (0) | 0 (0) | |
| Aboriginal | 1 (7) | 0 (0) | 0 (0) | |
| Unknown | 3 (20) | 6 (30) | 6 (55) | |
| Occupational status n (%) | P = 0.243 | |||
| Employed | 8 (53) | 12 (60) | 2 (18) | |
| Unemployed | 0 (0) | 1 (5) | 0 (0) | |
| Other | 1 (7) | 1 (5) | 1 (9) | |
| Unknown | 6 (40) | 6 (30) | 8 (73) | |
| Smoking status | P = 0.691 | |||
| Yes | 4 (27) | 3 (15) | 2 (18) | |
| No | 10 (67) | 16 (80) | 7 (64) | |
| Unknown | 1 (7) | 1 (5) | 2 (18) |
Immunohistochemical localization of melatonin receptors
MR1A and MR1B immunostaining were observed in epithelial cells across the menstrual cycle. MR1A immunostaining was predominantly found in collapsed glands in the menstrual phase and the cytoplasm of the glandular epithelial cells in the proliferative and secretory phase (Fig. 1). Similarly, MR1B immunostaining was observed in epithelial cells of glands of menstrual phase and was predominantly localized in epithelial cells of glands in the proliferative and secretory phase (Fig. 2). Specific MR1A and MR1B immunostaining was not observed in stromal cells of any menstrual cycle stage examined.
Figure 1.
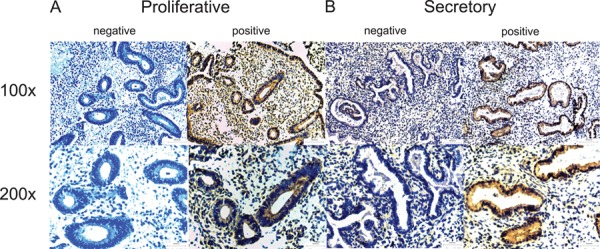
MR1A immunohistochemical localization in endometrium across the menstrual cycle. Representative photomicrographs of MR1A expression at medium (×100) and high (×200) power magnification during the proliferative (A) and secretory (B) phases along with negative (without primary antibody) controls.
Figure 2.
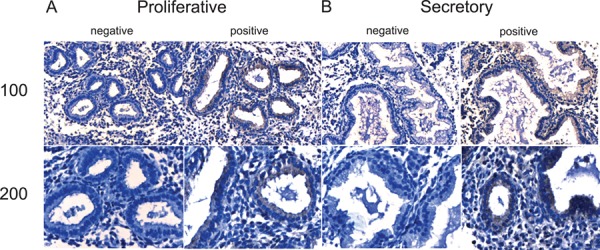
Immunohistochemical localization of MR1B in the endometrium across the menstrual cycle. Representative photomicrographs of MR1B expression at medium (×100) and high (×200) power magnification during the proliferative (A) and secretory (B) phases along with negative (without primary antibody) controls.
Melatonin receptor expression
MR1A mRNA expression was significantly elevated (P < 0.0001) in ectopic peritoneal lesions (4.78 [4.31–6.71, 95% confidence interval]) relative to control endometrium (0.57 [0.08–1.84]), eutopic case endometrium (0.98 [0.17–2.32]) and endometriomas (0.49 [0.12–2.28]; Fig. 3A). Similarly, MR1B mRNA expression was significantly elevated (P < 0.01) in ectopic peritoneal lesions (2.32 [2.06–2.87]) relative to control endometrium (0.82 [0.08–1.82]), eutopic case endometrium (1.13 [0.28–1.72]) and endometriomas (0.830 [0.38–2.12]; Fig. 3B). MR1A protein expression was significantly decreased (P < 0.05) in ectopic peritoneal lesions (0.07 [0.02–0.13]) relative to control endometrium (0.50 [0.36–1.44]); however, ectopic peritoneal lesions did not differ significantly from eutopic case endometrium (0.18 [0.06–2.48]) and endometriomas (0.60 [0.20–2.12]; Fig. 3C and E). MR1B protein expression was not significantly different (P = 0.333) between groups (control endometrium, 0.35 [0.02–2.54]; eutopic case endometrium, 0.218 [0.04–1.64]; endometriomas 0.081 [0.03–0.31]; or ectopic peritoneal lesions 0.18 [0.13–0.85]; Fig. 3D and F).
Figure 3.
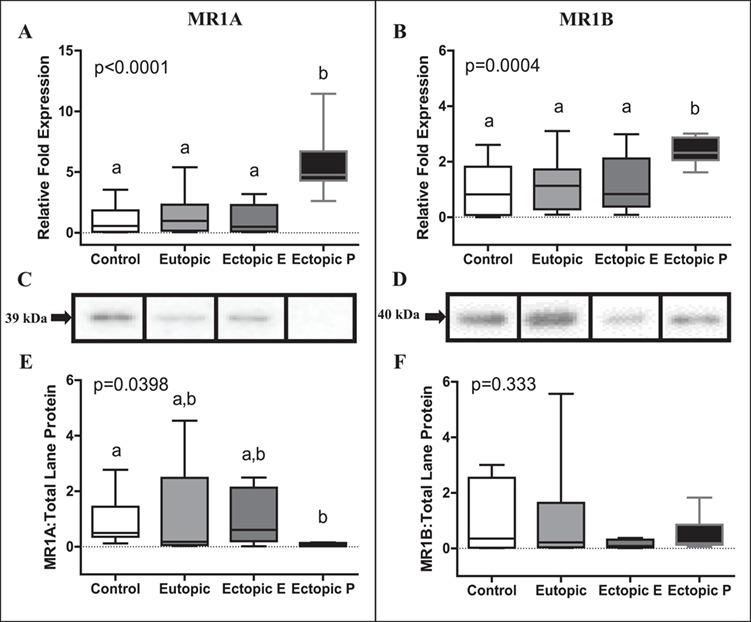
MR1A and MR1B expression in control endometrium, eutopic case endometrium, ectopic endometriomas (E) and ectopic peritoneal (P) lesions. MR1A mRNA expression (A) was significantly elevated in ectopic peritoneal lesions (n = 11) relative to control endometrium (n = 14), eutopic case endometrium (n = 23) and ectopic endometriomas (n = 19). MR1B mRNA expression (B) was also significantly higher in ectopic peritoneal lesions (n = 11) relative to control endometrium (n = 15), eutopic case endometrium (n = 28) and ectopic endometriomas (n = 20). mRNA fold expression was relative to the housekeeping mRNA (YWHaz) used. MR1A protein expression (C, representative bands; E, densitometry) was significantly decreased in ectopic peritoneal lesions (n = 6) relative to control endometrium (n = 7) (eutopic case endometrium, n = 14; ectopic endometriomas, n = 10). MR1B protein expression (D, representative bands; F, densitometry) did not significantly differ between groups (control endometrium, n = 7; eutopic case endometrium, n = 14; ectopic endometriomas, n = 10; ectopic peritoneal lesions, n = 6). Statistically significantly different groups based on post hoc tests are denoted with different letters above a particular group. All data are presented as box plots, where the box represents 25th and 75th percentiles, and within which is shown the 50th percentile (the median). The whiskers represent the 5th and 95th percentiles.
Melatonin-induced attenuation of cell proliferation
After 24 hours of culture neither estradiol nor melatonin had a discernable effect on endometrial epithelial cell viability and proliferation as measured by the MTT assay after (Fig. 4). In contrast, after 48 hours of culture, estradiol alone (used as a positive control) significantly increased cell proliferation (P < 0.01), whereas melatonin alone had no significant effect on either cell proliferation or survival as compared to the vehicle control (EtOH) of culture. However, when melatonin and estradiol were added in combination, all concentrations of melatonin tested (0.1 nM–1.0 μM) at 48 hours of culture significantly (P < 0.01) attenuated estradiol (1.0 nM)-induced endometrial epithelial cell proliferation without affecting cell viability.
Figure 4.
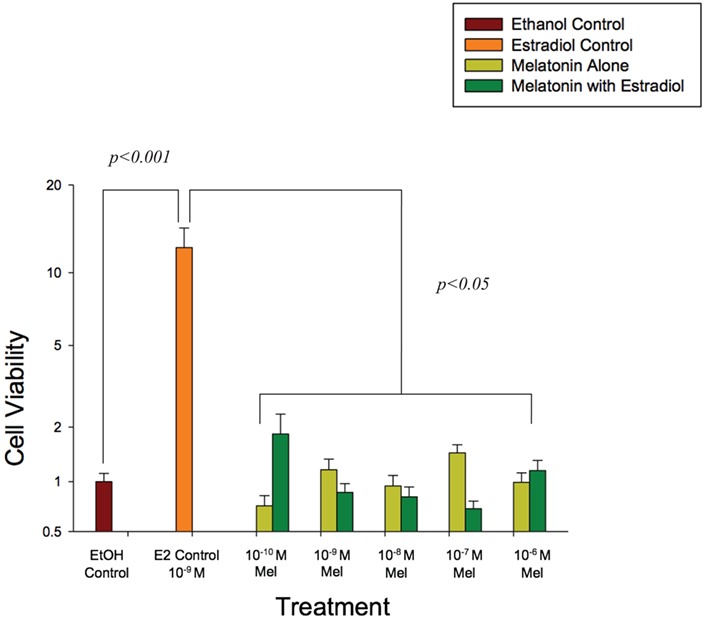
Increasing concentrations of melatonin (0.1 nM–1.0 μM) alone and in the presence of estradiol (1.0 nM) attenuated endometrial epithelial cell proliferation at 48 hours of culture. Cell viability in the control wells was set to 1, and data are expressed on a log scale. Endometrial epithelial cells (CRL1671) were incubated in phenol red-free DMEM/F12 media supplemented with 10% charcoal-stripped FBS and 0.1% ITS. Results are the mean ± SEM of three separate experiments with eight replicates/concentration in each experiment. Data were analyzed by two-way ANOVA and Duncan’s multiple range test. A P < 0.05 was considered significant.
Discussion
Results of the present study reveal that melatonin receptors, MR1A and MR1B are present in epithelial cells of endometrial glands throughout the menstrual cycle. Furthermore, we demonstrated that MR1A and MR1B mRNA and protein are expressed in both eutopic endometrium and ectopic lesions of women with endometriosis. We also examined for differences in melatonin receptor mRNA and protein expression in different lesion types and eutopic endometrium. While mRNA expression for both MR1A and MR1B was increased in peritoneal lesions compared to the eutopic endometrium, protein expression for MR1A was decreased suggesting uncoupling of mRNA and protein expression in these implants. Finally, our results demonstrate that physiologically relevant concentrations of melatonin inhibit estradiol-induced proliferation of endometrial epithelial cells in culture. Taken together, our data demonstrate that the endometrium and endometriotic lesions possess an intact melatonin receptor signaling pathway and that melatonin inhibits estradiol-induced cell proliferation supporting the hypothesis that melatonin modulates endometrial epithelial cell function and thus could have beneficial effects for the management of endometriosis.
In the current study, we used immunohistochemical methods to demonstrate MR1A and MR1B staining in endometrial epithelial cells and endometrial glands. Furthermore, the staining pattern was similar across all stages of the menstrual cycle and thus is not likely to be regulated by gonadal steroids. To our knowledge, melatonin receptor expression in the human endometrium has not been described previously; however, melatonin receptors have previously been detected in ovarian granulosa cells (Niles et al., 1999) and in the pregnant and non-pregnant myometrium (Schlabritz-Loutsevitch et al., 2003), suggesting a potential role of melatonin in reproductive physiology.
The immunohistochemistry results in the current study were corroborated by evidence of MR1A and MR1B mRNA and protein expression in the eutopic endometrium. We also compared mRNA and protein expression in different endometriotic lesions. Our results revealed greater MR1A and MR1B mRNA expression in peritoneal lesions compared to endometriomas and eutopic endometrium. However, we report that differences in mRNA expression were not carried through to protein expression. Specifically, MR1A protein expression was significantly decreased in peritoneal lesions compared to eutopic endometrium from women without endometriosis, whereas no differences in its expression were found between eutopic endometrium from women with endometriosis, endometriomas and peritoneal lesions. No significant difference in MR1B protein expression was found between any of the tissue groups studied. We postulate that although MR1A mRNA is expressed in peritoneal lesions, it is not being translated to protein in peritoneal lesions only. The mechanisms underlying uncoupling of MR1A mRNA and protein expression in the peritoneal lesions is unknown; however, we speculate that tissue specific post-transcriptional regulation of MR1A through dysregulation of non-coding RNAs could explain our observations (Esposito et al., 2011, Zhu et al., 2014). We further postulate that differences in protein expression in peritoneal lesions could reflect differences in the influence of the local tissue milieu in peritoneal lesions compared to other endometriotic lesions and the eutopic endometrium. Regardless, the functional consequences of lower MR1A protein expression are unclear but, if our findings can be reproduced, they could suggest that these lesions are less sensitive to melatonin signaling compared to the eutopic endometrium and endometriomas.
To evaluate the functional significance of melatonin and melatonin receptor expression in the endometrium, we tested the effect of physiologically relevant (0.1 nM) through to pharmacological (1.0 μM) concentrations of melatonin alone and in the presence of a physiological concentration of estradiol (1.0 nM) on endometrial epithelial cell viability and proliferation. At 48 hours of culture, melatonin treatment, at all concentrations tested, inhibited estradiol-induced cell proliferation, whereas melatonin alone had no effect on cell viability or proliferation. Thus, our results are harmonious with a prior study in which melatonin treatment inhibited cell growth in an endometrial cancer cell line (Kanishi et al., 2000) and consistent with results from human umbilical vein endothelial cells (Alvarez-García et al., 2013) and MCF-7 cells, an estrogen-dependent cell line (Proietti et al., 2013). Furthermore, melatonin treatment inhibited estradiol-induced endometriotic epithelial cell migration, invasion and epithelial–mesenchymal transition (EMT) (Qi et al., 2018). Moreover, EMT is thought to be a critical step in the development of endometriosis (Yang and Yang, 2017; Liu et al., 2018). Taken together, our results suggest that melatonin may be beneficial in blocking endometrial EMT. However, the mechanism(s) of melatonin action in the endometrium and endometriotic tissue remains unclear. Melatonin receptors are not known to be expressed in CRL1671 cells; however, preliminary data from our lab (data not shown) revealed that both MR1A and MR1B are expressed in this cell line. Furthermore, while melatonin is not known to bind with estrogen receptors, it has previously been shown to dysregulate estrogen signaling in breast cancer cells through multiple pathways including decreased estrogen synthesis and increased metabolism. Specifically, melatonin inhibits aromatase expression and activity and upregulates 17 β-hydroxysteroid dehydrogenase type I and estrogen sulfatase in breast cancer cells (Gonzalez-Gonzalez et al., 2018). In human umbilical vein endothelial cells, treatment with melatonin inhibited estradiol-induced cell proliferation and decreased aromatase mRNA expression and activity (Alvarez-García et al., 2013). Melatonin also decreased EsR1 expression in MCF-7 cells, an estrogen-dependent breast cancer cell line (Molis et al., 1994), and in breast cancer stem cells (Lopes et al., 2016). Moreover, melatonin destabilizes the calmodulin–estrogen–ER complex inhibiting binding with the estrogen response element in breast cancer cells (Molis et al., 1994; Rato et al., 1999). Consequently, we suggest that melatonin treatment inhibited estradiol-induced cell proliferation in endometrial epithelial cells through either down regulation of estrogen receptor expression or destabilization of the estradiol–ER complex that interferes with binding to the ER response element. Therefore, our results together with data from animal studies (Güney et al., 2008; Yildirim et al., 2010; Kocadal et al., 2013; Cetinkaya et al., 2015; Yilmaz et al., 2015; Yesildaglar et al., 2016) and a single clinical trial in humans (Schwertner et al., 2013) suggest that melatonin may be a useful adjuvant therapy for the management of endometriosis.
Overall, results of the present study together with experimental animal studies and a single clinical trial support the concept that melatonin antagonizes estrogen-driven proliferation of ectopic endometrial tissues. While the findings of the present study lend support to the use of melatonin as an adjuvant therapy in the management of endometriosis, we note that the effect of melatonin may vary depending on endometriotic lesion type. Specifically, lower MR1A protein expression in peritoneal lesions compared to eutopic endometrium and endometriomas suggests that these lesion types may be less sensitive or unresponsive to melatonin treatment. In conclusion, melatonin receptors, MR1A and MR1B, are expressed in human eutopic and ectopic endometrium. Finally, melatonin treatment, at all concentrations tested, inhibited estradiol-induced endometrial epithelial cell proliferation. Taken together, our results add mechanistic insight in support of the use of melatonin as an adjuvant therapy in the management of endometriosis.
Acknowledgements
We thank the study participants and staff of the Endo@Mac program for their assistance and selfless contributions to this project. The authors gratefully acknowledge the assistance of Annette Bullen with study participant recruitment, sample collection and data extraction. Sample collection by the gynecological surgeons Drs. Nicholas Leyland, Sarah Scattalon and Dustin Costescu are gratefully acknowledged.
Authors’ roles
A.A.M., W.G.F. and S.K.A. designed and oversaw all phases of the experiments, data interpretation and co-wrote the final version of the paper. W.G.F. obtained funding, ethics approval for the study, data acquisition and analysis. N.L. was responsible for patient recruitment, sample collection and rAFS staging. M.T. processed clinical samples, performed the immnunohistochemistry, qRT-PCR and western blot experiments and wrote the first draft of the paper. J.L. and C.T. performed the tissue culture experiments described in this report. All authors contributed to and approved the final manuscript for submission.
Funding
Canadian Institutes of Health Research (MOP142230 to W.G.F.); Physicians Services Incorporated Foundation (A.A.M.).
Conflict of interest
None.
REFERENCES
- Alvarez-García V, González A, Martínez-Campa C, Alonso-González C, Cos S. Melatonin modulates aromatase activity and expression in endothelial cells. Oncol Rep 2013;29:2058–2064. [DOI] [PubMed] [Google Scholar]
- Bulun SE. Endometriosis. N Engl J Med 2009;360:268–279. [DOI] [PubMed] [Google Scholar]
- Cetinkaya N, Attar R, Yildirim G, Ficicioglu C, Ozkan F, Yilmaz B, Yesildaglar N. The effects of different doses of melatonin treatment on endometrial implants in an oophorectomized rat endometriosis model. Arch Gynecol Obstet 2015;291:591–598. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML, Markowska M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine 2005;27:101–110. [DOI] [PubMed] [Google Scholar]
- Esposito T, Magliocca S, Formicola D, Gianfrancesco F. piR_015520 belongs to Piwi-associated RNAs regulates expression of the human melatonin receptor 1A gene. PLoS One 2011;6:e22727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannini A, Bucci F, Luisi S, Cela V, Pluchino N, Merlini S, Casarosa E, Russo M, Cubeddu A, Daino D et al. Brain-derived neurotrophic factor in plasma of women with endometriosis. J Endometr Pelvic Pain Disord 2010;2:144–150. [Google Scholar]
- Giudice LC, Kao LC. Endometriosis. Lancet 2004;364:1789–1799. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gonzalez A, Mediavilla MD, Sanchez-Barcelo EJ. Melatonin: a molecule for reducing breast cancer risk. Molecules 2018;23:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo SW. Recurrence of endometriosis and its control. Hum Reprod Update 2009;15:441–461. [DOI] [PubMed] [Google Scholar]
- Güney M, Oral B, Karahan N, Mungan T. Regression of endometrial explants in a rat model of endometriosis treated with melatonin. Fertil Steril 2008;89:934–942. [DOI] [PubMed] [Google Scholar]
- Kanishi Y, Kobayashi Y, Noda S, Ishizuka B, Saito K. Differential growth inhibitory effect of melatonin on two endometrial cancer cell lines. J Pineal Res 2000;28:227–233. [DOI] [PubMed] [Google Scholar]
- Koc O, Gunduz B, Topcuoglu A, Bugdayci G, Yilmaz F, Duran B. Effects of pinealectomy and melatonin supplementation on endometrial explants in a rat model. Eur J Obstet Gynecol Reprod Biol 2010;153:72–76. [DOI] [PubMed] [Google Scholar]
- Kocadal N, Attar R, Yıldırım G, Fıçıcıoğlu C, Ozkan F, Yılmaz B, Yesildaglar N. Melatonin treatment results in regression of endometriotic lesions in an ooferectomized rat endometriosis model. J Turk Ger Gynecol Assoc 2013;14:81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Du Y, Zhang Z, Lv L, Xiong W, Zhang L, Li N, He H, Li Q, Liu Y. Autophagy contributes to the hypoxia-induced epithelial to mesenchymal transition of endometrial epithelial cell in endometriosis. Biol Reprod 2018;99:968–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes J, Arnosti D, Trosko JE, Tai MH, Zuccari D. Melatonin decreases estrogen receptor binding to estrogen response elements sites on the OCT4 gene in human breast cancer stem cells. Genes Cancer 2016;7:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino JL, Holt VL, Chen C, Davis S. Shift work, hCLOCK T3111C polymorphism, and endometriosis risk. Epidemiology 2008;19:477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molis TM, Spriggs LL, Hill SM. Modulation of estrogen receptor mRNA expression by melatonin in MCF-7 human breast cancer cells. Mol Endocrinol 1994;8:1681–1690. [DOI] [PubMed] [Google Scholar]
- Niles LP, Wang J, Shen L, Lobb DK, Younglai EV. Melatonin receptor mRNA expression in human granulosa cells. Mol Cell Endocrinol 1999;156:107–110. [DOI] [PubMed] [Google Scholar]
- Noyes RW, Haman JO. Accuracy of endometrial dating; correlation of endometrial dating with basal body temperature and menses. Fertil Steril 1953;4:504–517. [DOI] [PubMed] [Google Scholar]
- Proietti S, Cucina A, Reiter RJ, Bizzarri M. Molecular mechanisms of melatonin's inhibitory actions on breast cancers. Cell Mol Life Sci 2013;70:2139–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi S, Yan L, Liu Z, Mu YL, Li M, Zhao X, Chen ZJ, Zhang H. Melatonin inhibits 17β-estradiol-induced migration, invasion and epithelial-mesenchymal transition in normal and endometriotic endometrial epithelial cells. Reprod Biol Endocrinol 2018;16:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rato AG, Pedrero JG, Martinez MA, del Rio B, Lazo PS, Ramos S. Melatonin blocks the activation of estrogen receptor for DNA binding. FASEB J 1999;13:857–868. [DOI] [PubMed] [Google Scholar]
- Schlabritz-Loutsevitch N, Hellner N, Middendorf R, Müller D, Olcese J. The human myometrium as a target for melatonin. J Clin Endocrinol Metab 2003;88:908–913. [DOI] [PubMed] [Google Scholar]
- Schwertner A, Conceicao Dos Santos CC, Costa GD, Deitos A, de Souza A, de Souza IC, Torres IL, da Cunha Filho JS, Caumo W. Efficacy of melatonin in the treatment of endometriosis: a phase II, randomized, double-blind, placebo-controlled trial. Pain 2013;154:874–881. [DOI] [PubMed] [Google Scholar]
- Söderquist F, Hellström PM, Cunningham JL. Human gastroenteropancreatic expression of melatonin and its receptors MT1 and MT2. PLoS One 2015;10:e0120195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels JM, Kay VR, Leyland NA, Agarwal SK, Foster WG. Assessing brain-derived neurotrophic factor as a novel clinical marker of endometriosis. Fertil Steril 2016;105:119–128 e115. [DOI] [PubMed] [Google Scholar]
- Yang YM, Yang WX. Epithelial-to-mesenchymal transition in the development of endometriosis. Oncotarget 2017;8:41679–41689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesildaglar N, Yildirim G, Yildirim OK, Attar R, Ozkan F, Akkaya H, Yilmaz B. The effects of melatonin on endometriotic lesions induced by implanting human endometriotic cells in the first SCID-mouse endometriosis-model developed in Turkey. Clin Exp Obstet Gynecol 2016;43:25–30. [PubMed] [Google Scholar]
- Yildirim G, Attar R, Ozkan F, Kumbak B, Ficicioglu C, Yesildaglar N. The effects of letrozole and melatonin on surgically induced endometriosis in a rat model: a preliminary study. Fertil Steril 2010;93:1787–1792. [DOI] [PubMed] [Google Scholar]
- Yilmaz B, Kilic S, Aksakal O, Ertas IE, Tanrisever GG, Aksoy Y, Lortlar N, Kelekci S, Gungor T. Melatonin causes regression of endometriotic implants in rats by modulating angiogenesis, tissue levels of antioxidants and matrix metalloproteinases. Arch Gynecol Obstet 2015;292:209–216. [DOI] [PubMed] [Google Scholar]
- Zhu HQ, Li Q, Dong LY, Zhou Q, Wang H, Wang Y. MicroRNA-29b promotes high-fat diet-stimulated endothelial permeability and apoptosis in apoE knock-out mice by down-regulating MT1 expression. Int J Cardiol 2014;176:764–770. [DOI] [PubMed] [Google Scholar]


