Abstract
STUDY QUESTION
Can of Clinical Genetics, Maastricht University Medical Centre, Maastricht kisspeptin and its analogues regulate the motility of human decidual stromal cells and what intracellular signaling pathways are involved?
SUMMARY ANSWER
Kisspeptin analogue–mediated cell motility in human decidual stromal cells via the focal adhesion kinase (FAK)–steroid receptor coactivator (Src) pathway suggesting that kisspeptin may modulate embryo implantation and decidual programming in human pregnancy.
WHAT IS KNOWN ALREADY
The extravillous trophoblast invades the maternal decidua during embryo implantation and placentation. The motile behavior and invasive potential of decidual stromal cells regulate embryo implantation and programming of human pregnancy.
STUDY DESIGN, SIZE, DURATION
Human decidual stromal cells were isolated from healthy women undergoing elective termination of a normal pregnancy at 6- to 12-week gestation, after informed consent.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Kisspeptin analogues were synthetic peptides. Cell motility was estimated by an invasion and migration assay. Immunoblot analysis was performed to investigate the expression of kisspeptin receptor and the effects of kisspeptin analogues on the phosphorylation of FAK and Src. Small interfering RNAs (siRNAs) were used to knock down the expression of kisspeptin receptor, FAK, Src, matrix metallo-proteinases (MMPs) 2 and 9, and extracellular signal-regulated protein kinase (ERK) 1/2.
MAIN RESULTS AND THE ROLE OF CHANCE
The kisspeptin receptor was expressed in human decidual stromal cells. Kisspeptin agonist decreased, but antagonist increased, cell motility. Kisspeptin agonist decreased the phosphorylation of FAK and Src tyrosine kinases, whereas antagonist increased it. These effects on phosphorylation were abolished by kisspeptin receptor siRNA. The activation of cell motility by kisspeptin analogues was suppressed by siRNA knockdown of endogenous FAK (decreased 66%), Src (decreased 60%), kisspeptin receptor (decreased 26%), MMP-2 (decreased 36%), MMP-9 (decreased 23%), and ERK 1/2 inhibitor (decreased 27%).
LIMITATIONS, REASONS FOR CAUTION
Human decidual stromal cells were obtained from women having terminations after 6–12 weeks of pregnancy and differences in timing could affect their properties.
WIDER IMPLICATIONS OF THE FINDINGS
Kisspeptin acting within the endometrium has a potential modulatory role on embryo implantation and decidual programming of human pregnancy.
STUDY FUNDING/COMPETING INTEREST(S)
This work was supported by grant NSC-104-2314-B-182A-146-MY2 (to H.-M.W.) from the Ministry of Science and Technology, Taiwan, and grants CMRPG3E0401 and CMRPG3E0402 (to H.-M.W.). This work was also supported by grants from the Canadian Institutes of Health Research to P.C.K.L. P.C.K.L. is the recipient of a Child & Family Research Institute Distinguished Investigator Award. The authors have no conflicts of interest to disclose.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: kisspeptin , decidua , motility , endometrium , FAK , Src
Introduction
Migratory and invasive actions of decidual stromal cells play an important role in the encroachment of the maternal decidua by extravillous trophoblast during embryo implantation and programming of human pregnancy (Gellersen et al., 2010; Teklenburg et al., 2010; Wu et al., 2015). Decidual stromal cells of women with recurrent miscarriage demonstrate abnormal decidualization in vitro (Salker et al., 2010; Teklenburg et al., 2010).
The first reported role for kisspeptin was to regulate GnRH secretion by the hypothalamus. The pulsatile secretion of GnRH regulates secretion of gonadotrophins by the pituitary and is critical in maintenance of reproductive function including sex hormone secretion (de Roux et al., 2003). More recently, Baba et al. (2015) demonstrated kisspeptin expression in endometrial cancer stromal cells and its ability to suppress endometrial cancer stromal cell motility. They also demonstrated induction of kisspeptin secretion in stromal cells through decidualization in pregnancy (Baba et al., 2015), suggesting that it might have local physiological effects in the endometrium. Little is known about how kisspeptin controls the motility of decidual stromal cell and what impact it could have on the endometrium and human reproduction.
Focal adhesion kinase (FAK) and steroid receptor coactivator (Src) are intracellular tyrosine kinases that promote cell motility (Guo and Giancotti, 2004; Mitra and Schlaepfer, 2006), although the mechanisms for this are only partially understood. extracellular signal-regulated protein kinase (ERK) 1/2 are critical factors in hormone-mediated signaling pathways in various cell types (Davidson et al., 2006; Kraus et al., 2001; Wu et al., 2009). We have previously demonstrated that the effect of peptide hormones in uterine tissues is regulated by mitogen-activated protein kinases (MAPKs) signaling (Kim et al., 2005; Wu et al., 2009). According to our previous studies, MAPKs and matrix metallo-proteinases (MMPs) signaling have been demonstrated to regulate peptide hormone-regulated cell motility (Wu et al. 2015; Wu et al., 2013). In the present study, we examined the effect of kisspeptin analogues on the motility of decidual stromal cells, and identified the cell signaling mechanisms involved.
Materials and Methods
This study was approved by the Institutional Review Board of Chang Gung Memorial Hospital (CGMH-IRB numbers 103-5320A3 and 104-4726B).
Cell culture
Human decidual tissue was obtained, after informed consent, from women (n = 25) aged 25–38 years undergoing elective surgical termination of a normal pregnancy after 6–8 weeks of gestation.
Enzymatic digestion and mechanical dissociation were performed to isolate decidual stromal cells from human decidual tissue (Chou et al., 2003). Briefly, human decidual tissue was minced and treated with type IV collagenase (Sigma-Aldrich, St. Louis, MO) and DNase type I in a shaking water bath at 37°C for 90 min. The cell digest was then passed through a Falcon Cell Strainer (Falcon, New York, USA). The decidual epithelial and stromal cells that passed through the filter were collected. Next, epithelial cells were separated from stromal cells with a 45-μm filter. The stromal cells in the filtrate were subsequently pelleted by centrifugation at 1000 × g for 5 min at room temperature. The cell pellet was washed once in Dulbecco's Modified Eagle Medium (DMEM), resuspended and plated in DMEM containing 25-mM glucose, 200-mM L-glutamine, and antibiotics (100-U/ml penicillin and 100-μg/ml streptomycin), and supplemented with 10% (v/v) fetal bovine serum (FBS).
Reagents
The kisspeptin analogues, the synthetic multipeptide kisspeptin agonist (KP10) and antagonist (KP234), were purchased from Bachem (San Carlos, CA). MAPK/ERK kinase inhibitor U0126 was purchased from Calbiochem (San Diego, CA).
Immunoblot analysis
Human decidual stromal cells cultured to 70% confluence in 10-cm dishes were treated with kisspeptin agonist (KP10) (500 nM) or kisspeptin antagonist (KP234) (500 nM) or control for h. The cells were lysed in buffer containing 20-mM Tris, pH 7.4, 2-mM EGTA, 2-mM Na2VO3, 2-mM Na4P2O7, 2% (w/v) Triton X-100, 2% (w/v) sodium dodecyl sulphate (SDS), 1-μM aprotinin, 1-μM leupeptin, and 1-mM phenylmethane sulfonyl fluoride. The protein concentration was determined with a protein assay kit using bovine serum albumin standards according to the manufacturer's instructions (Bio-Rad Laboratories, Hercules, CA). Equal amounts of cell lysate were separated by SDS polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane (Hybond-C, Amersham Pharmacia Biotech Inc., Oakville, ON). Following blocking with Tris-buffered saline containing 5% w/v non-fat dry milk for 1 h, the membranes were incubated overnight at 4°C with anti-kisspeptin receptor (Neomarker, Fremont, CA), anti-phospho-ERK1/2 (Cell Signaling, Danvers, Massachusetts, USA), anti-ERK1/2 (Cell Signaling), anti-phospho-FAK (Cell Signaling), anti-FAK (Cell Signaling), anti-phospho-Src (Cell Signaling), anti-Src (Cell Signaling), anti-MMP-2 (Calbiochem), or anti-MMP-9 (Calbiochem) antibody diluted to 1:1000 in 5% skimmed milk followed by incubation with the horseradish peroxidase (HRP)-conjugated anti-mouse secondary antibody (1:5000; Santa Cruz Biotechnology, Inc. Dallas, Texas, USA) at room temperature for 1 h. The immunoreactive bands were detected with an enhanced chemiluminescence kit (Thermo Fisher Scientific, Waltham, Massachusetts, USA). The intensities of the bands were quantified by densitometric analysis using Scion Image software (Scion, Frederick, MD). The membrane was then stripped with stripping buffer (62.5-mM Tris, 10-mM Dithiothreitol (DTT), and 2% SDS, pH 6.7) at 50°C for 30 min and re-probed with β-actin antibody (Santa Cruz Biotechnology Inc.) as a loading control.
Immunohistochemistry
To demonstrate the expression of the kisspeptin and kisspeptin receptor protein in human decidual tissue, immunohistochemistry (IHC) was performed on sections of human decidual tissue using previously reported procedures (Chao et al., 2006). Four micrometer–thick formalin-fixed, paraffin-embedded tissue sections were deparaffinized in xylene and rehydrated through a graded series of ethanol solutions. The sections were then stained with anti-human kisspeptin or anti-human kisspeptin receptor polyclonal antibodies (Neomarker; 1:100) using an automated IHC stainer with the Ventana Basic 3,3′-diaminobenzidine Detection kit (Tucson, AZ) and diluting in antibody dilution buffer (Ventana Medical Systems, Inc.) overnight at 4°C, and rinsing in PBS three times (5 min per rinse). Primary antibody was applied and incubated using Amplifier Quanto (#TL-060-QHD; Thermo Fisher Scientific) and HRP Polymer Quanto (#TL-060-QHD) for 10 min according to the manufacturer’s protocol. Counterstaining was performed with hematoxylin. Sections were stained without the kisspeptin and kisspeptin receptor antibody as a negative control.
RT-PCR
Total RNA was extracted from cultured decidual stromal cells using TRIzol reagent (Invitrogen, Waltham, Massachusetts, USA). The total RNA concentration was assessed from spectrophotometric analysis at A260/280. Single-stranded complementary DNA (cDNA) was synthesized from 2 μg of total RNA by reverse transcription (RT) (Amersham Biosciences, Baie d’Urfe’, Quebec, Canada). We used the synthesized cDNA as a template for PCR amplification. To determine kisspeptin receptor expression, cDNA was amplified using the following primer pair: 5′GCTGGTACGTGACGGTGTTC3′ (sense) and 5′AGAGCCTACCCAGATGCTGAG3′ (anti-sense). PCR was performed using the following cycling conditions: 94°C for 5 min followed by 30 cycles of 94°C for 30 s, 60°C for 20 s, and 74°C for 70 s. The PCR products were electrophoresed on a 1.5% (w/v) agarose gel, visualized by ethidium bromide, and photographed under UV light. The RNA quality was tested by PCR amplification of human glyceraldehyde dehydrogenase (GAPDH) cDNA from the same RT reaction products that were used for PCR amplification of kisspeptin receptor using the following primer pair: 5′-ATGTTCGTCATGGGTGTGAACCA-3′ (sense) and 5′-TGGCAGGTTTTTCTAGACGGCAG-3′ (anti-sense) and the following cycling conditions: 94°C for 5 min followed by 21 cycles of 94°C for 60 s, 55°C for 35 s, and 72°C for 90 s.
The intensities of the bands were quantified by densitometric analysis using Scion Image software.
Small interfering RNA transfection
siGENOME ON-TARGETplus SMARTpool human kisspeptin receptor (GCUGGUACGUGACGGUGUU), FAK (GCGAUUAUAUGUUAGAGAU), Src (GCAGUUGUAUGCUGUGGUU), MMP-2 (ACAAGAACCAGAUCACAUA), MMP-9 (GCAUAAGGACGACGUGAAU) small interfering RNAs (siRNAs), and siCONTROL NON-TARGETINGpool siRNA were purchased from Dharmacon (Lafayette, CO, USA). The cells were transfected with siRNA (100 nM) using Lipofectamine RNAiMAX (Invitrogen). After a 24-h transfection, the medium was removed and changed to fresh serum-free medium. To examine the siRNA transfection, cells were transfected with 100-nM si-GLO (Dharmacon) for 24 h. The transfection efficiency was examined by fluorescent microscopy (Supplementary Fig. S1).
Invasion and migration assays
Invasion and migration assays were performed in Boyden chambers with minor modifications. Cell culture inserts (24-well, pore size 8 μm; BD Biosciences, Mississauga, ON) were seeded with 1 × 105 cells in 250 μL of DMEM medium with 0.1% (v/v) FBS. Inserts pre-coated with growth factor reduced Matrigel (40 μl, 1 mg/ml; BD Biosciences) were used for invasion assays, whereas un-coated inserts were used for migration assays. Medium with 10% (v/v) FBS (750 μl) was added to the lower chamber and served as a chemotactic agent. Non-invading/migrating cells were wiped from the upper side of the membrane, and cells on the lower side were fixed in cold methanol (−20°C) and air dried after 48-h (invasion) or 24-h (migration) incubation. To measure the invasion and migration of decidual stromal cells after 48 (invasion) and 24 (migration) hours of incubation, the kisspeptin analogues were applied in a dose-dependent manner at concentrations of 10 nM to 500 nM with a maximal effect at 500 nM. Each independent experiment was conducted with triplicate inserts. The cells that had not penetrated the filter were removed by wiping, and the cells that had invaded the lower surface of the filter were fixed with ice-cold methanol and stained with 0.5% crystal violet before air drying. Five microscopic fields were photographed under an optical microscope in each insert, and the cell number per field was calculated manually on the lower surface of the membrane.
Statistical analysis
The results are shown as mean ± SEM. Pair-wise comparisons were done with the t-test for paired data. Multiple comparisons were first analyzed by one-way analysis of variance, followed by Tukey’s multiple comparison tests. A significant difference was defined as P < 0.05.
Results
Expression of the kisspeptin receptor in decidual stromal cells
As shown in Fig. 1A, kisspeptin receptor (kisspeptin R) mRNA was identified in human decidual stromal cells purified from three distinct patients. The Ishikawa endometrial cancer cell line was used as a positive control. Knockdown of kisspeptin R in decidual stromal cells was achieved by 24-h treatment with 50-mM kisspeptin receptor siRNA. Immunohistochemical studies confirmed the presence of kisspeptin and kisspeptin R proteins in human decidual stromal cells (Fig. 1B).
Figure 1.
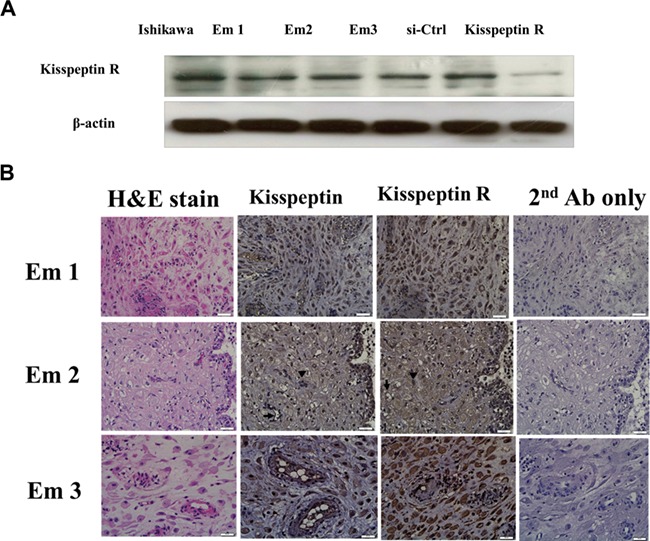
Kisspeptin receptor (kisspeptin R) protein expression in human decidual stromal cells. Results show cells from three different women (EM1, 2, and 3). (A) Immunoblots, Ishikawa endometrial cancer cells served as a positive control. In the final two columns, human decidual stromal cells were transfected with scrambled siRNA (si-Ctrl) or siRNA targeting human kisspeptin R. (B) Immunohistochemistry of decidual endometrium sections; column 1, hematoxylin and eosin staining; column 2, kisspeptin (brown staining) endothelial cells (arrow) and stromal cells (arrowhead) are indicated in row 2; column 3, kisspeptin receptor (brown staining), endothelial cells (arrow), and stromal cells (arrowhead) are indicated in row 2; column 4, second antibody only control. Micrographs were taken with the 400× objective lens. Scale bars represent 20 μm.
Kisspeptin regulates invasion and migration of decidual stromal cells
Kisspeptin agonist inhibited the invasion and migration of decidual stromal cells in a dose-dependent manner at concentrations from 100 nM to 500 nM. Maximal inhibition of invasion (37% inhibition) and migration (22% inhibition) was seen with 500 nM (Fig. 2).
Figure 2.
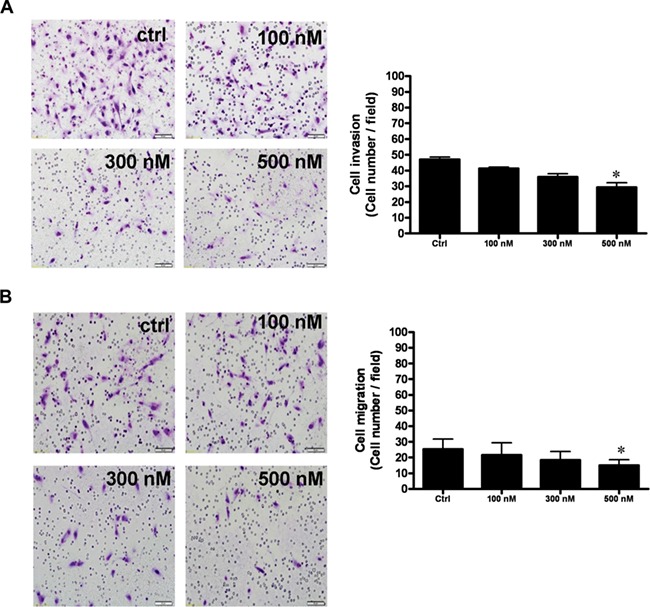
Kisspeptin agonist (K10) inhibits invasion and migration of decidual stromal cells. (A) Invasion, decidual stromal cells were seeded onto a Matrigel-precoated filter in Transwell chambers containing 0-, 100-, 300-, or 500-nM KP10. (B) Migration, decidual stromal cells were seeded on an uncoated porous filter in a transwell chamber containing 0-, 100-, 300-, or 500-nM KP10. KP10 inhibited the invasion and migration of decidual stromal cells in a dose-dependent manner with a maximal effect at 500 nM. After 48 (invasion, A) and 24 (migration, B) hours of incubation, cells on the upper side of the filter were removed and the invaded or migrated cells on the lower surface were fixed, stained with crystal violet, and counted. The photomicrographs show representative results. Bar charts show the mean number of invaded or migrated cells of four fields of triplicate wells from three independent experiments + SEM; *P < 0.05, versus control.
In contrast, kisspeptin antagonist stimulated the invasion and migration of decidual stromal cells, again in a dose-dependent manner at concentrations of 100 nM to 500 nM with a maximal effect at 500 nM (Fig. 3). For invasion, the maximum stimulation was 220%, and for migration, it was 285%.
Figure 3.
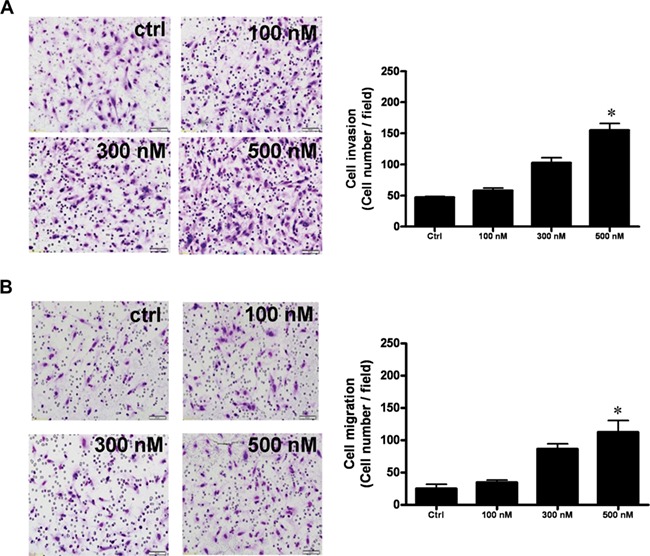
Kisspeptin antagonist (KP234) promotes invasion and migration of decidual stromal cells. (A) Invasion, decidual stromal cells were seeded onto a Matrigel-precoated filter in Transwell chambers containing 0-, 100-, 300-, or 500-nM KP234. (B) Migration, decidual stromal cells were seeded on an uncoated porous filter in a transwell chamber containing 0-, 100-, 300-, or 500-nM KP234. KP234 inhibited the invasion and migration of decidual stromal cells in a dose-dependent manner with a maximal effect at 500 nM. After 48 (invasion, A) and 24 (migration, B) hours of incubation, cells on the upper side of the filter were removed and the invaded or migrated cells on the lower surface were fixed, stained with crystal violet and counted. The photomicrographs show representative results. Bar charts show the mean number of invaded or migrated cells of four fields of triplicate wells from three independent experiments + SEM; *P < 0.05, versus control.
The kisspeptin-regulated cell motility is mediated by kisspeptin R in human decidual stromal cells
Treatment of human decidual stromal cells with 50-nM kisspeptin R siRNA decreased kisspeptin R m-RNA expression (Fig. 4B) and protein (Fig. 4A). Furthermore, knockdown of the endogenous kisspeptin receptor significantly rescued the kisspeptin agonist-induced inhibition of cell invasion and migration (Fig. 4C and D, upper column) and decreased kisspeptin antagonist-induced stimulation of cell invasion and migration (Fig. 4C and D, lower column). In brief, these results show that the kisspeptin-regulated cell invasion and migration in human decidual stromal cells requires kisspeptin receptors.
Figure 4.
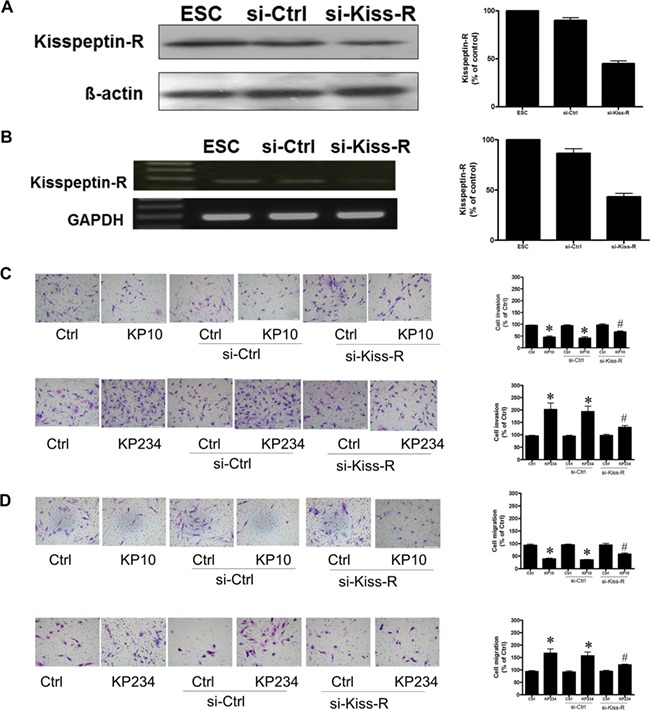
The kisspeptin regulation of cell motility is mediated by kisspeptin receptors (R) in human decidual stromal cells. Human decidual stromal cells (DSC) were transfected with human Kiss-R siRNA (si-Kiss-R) or scrambled siRNA (si-Ctrl). Kisspeptin receptor (kisspeptin-R, Kiss-R) levels were monitored by immunoblot assays. (A) The expression of kisspeptin-R mRNA in ESC was detected by electrophoresis of RT-PCR products and ethidium bromide staining (B). (C) The effects of si-Kiss-R transfection on the kisspeptin-regulated cell invasion. The cells were transfected with si-Kiss-R and treated with kisspeptin agonist (KP10) (500 nM) or kisspeptin antagonist (KP234) (500 nM) for 48 h. Invasion was measured by movement through a Matrigel-coated filter in a Transwell chamber. The results are expressed as the mean ± SEM of three independent experiments. (*P < 0.05, versus control; #P < 0.05, versus kisspeptin analogues). (D) The effects of si-Kiss-R transfection on kisspeptin-regulated cell migration. The cells were transfected with si-Kiss-R and treated with kisspeptin agonist (KP10) (500 nM) and kisspeptin antagonist (KP234) (500 nM) for 24 h. Migration was assessed by movement across an uncoated filter in a Transwell chamber. The results are expressed as the mean ± SEM of three independent experiments. (*P < 0.05, versus control; #P < 0.05, versus kisspeptin analogues). The photomicrographs depict representative fields of the lower surface of the membrane after cells on the upper surface had been removed and those on the lower surface fixed and stained with crystal violet.
The FAK–Src signaling of kisspeptin analogues in decidual stromal cells
To clarify the molecular mechanism of kisspeptin-regulated human decidual stromal cells motility, phosphorylation of FAK and Src was examined by immunoblot analysis. As shown in Fig. 5A, kisspeptin antagonist activated FAK and Src phosphorylation, while kisspeptin agonist suppressed it. Human decidual stromal cells were then treated with FAK siRNA or Src siRNA before adding kisspeptin antagonist. As shown in Fig. 5B, pretreatment of the cells with FAK siRNA nearly abolished the stimulation of cell invasion and migration by kisspeptin antagonist. In a similar way, pretreatment of the cells with Src siRNA markedly decreased the stimulation of cell invasion and migration by kisspeptin antagonist (Fig. 5C). These results indicate that kisspeptin antagonist stimulated motility of human decidual stromal cells through the activation of the FAK and Src signaling pathways.
Figure 5.
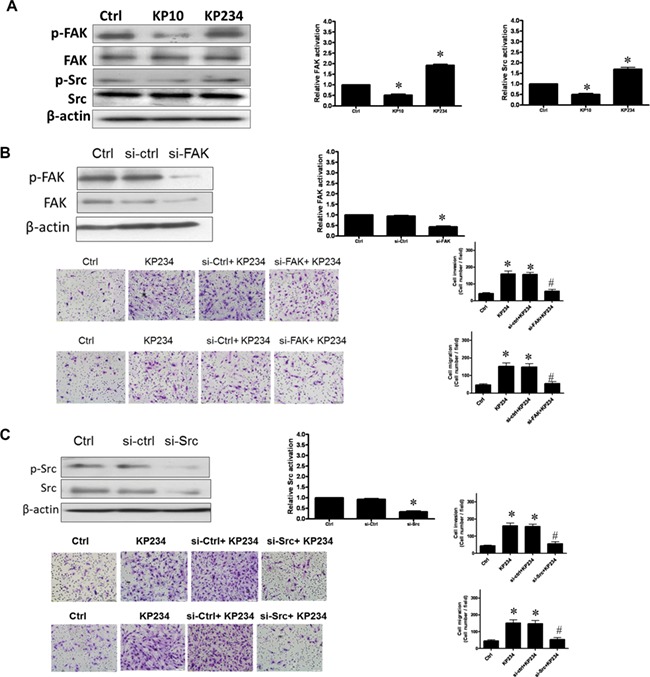
Signaling by kisspeptin analogues in decidual stromal cells involves FAK–Src. (A) The effects of kisspeptin analogues on FAK and Src phosphorylation. The cells were treated with kisspeptin agonist (KP10) (500 nM) and kisspeptin antagonist (KP234) (500 nM) for 2 h. Immunoblot analysis indicated increases in the phosphorylation of FAK and Src following 2 h of KP234 stimulation but decreases following 2 h of KP10 stimulation. (B) The effects of transfection with siRNA against human FAK (si-FAK) or scrambled siRNA (si-ctrl) on the KP234-stimulated activation of FAK. si-FAK but not si-ctrl produced significant decreases in the levels of FAK and FAK activation. Pretreatment with si-FAK attenuated the effects of KP234 on the induction of cell invasion and migration. The results are expressed as the mean ± SEM of three independent experiments. (*P < 0.05, versus control; #P < 0.05, versus KP234). (C) The effects of transfection with si-RNA against human Src (si-Src) or si-ctrl on the KP234-stimulated activation of Src. si-Src but not si-ctrl decreased expression of Src and Src activation. Pretreatment with si-Src attenuated the effects of KP234 on the induction of cell invasion and migration. The results are expressed as the mean ± SEM of three independent experiments. (*P < 0.05, versus control; #P < 0.05, versus KP234). The photomicrographs depict representative fields of the lower surface of the membrane after cells on the upper surface had been removed and those on the lower surface fixed and stained with crystal violet.
Kisspeptin-regulated cell motility is mediated by ERK1/2 signaling in human decidual stromal cells
Kisspeptin antagonist activated, while kisspeptin agonist decreased, ERK1/2 phosphorylation in a time-dependent manner (Fig. 6A). Kisspeptin antagonist-induced phosphorylation of ERK1/2 signaling was abolished by pre-treatment of cells with kisspeptin R siRNA but not with control siRNA (Fig. 6B). Pretreatment of cells with the ERK1/2 inhibitor (U0126) abolished kisspeptin antagonist-stimulated cell invasion and migration (Fig. 6C). These results suggest that kisspeptin regulates the motility of human decidual stromal cells through kisspeptin R and the activation of the ERK1/2 signaling pathways.
Figure 6.
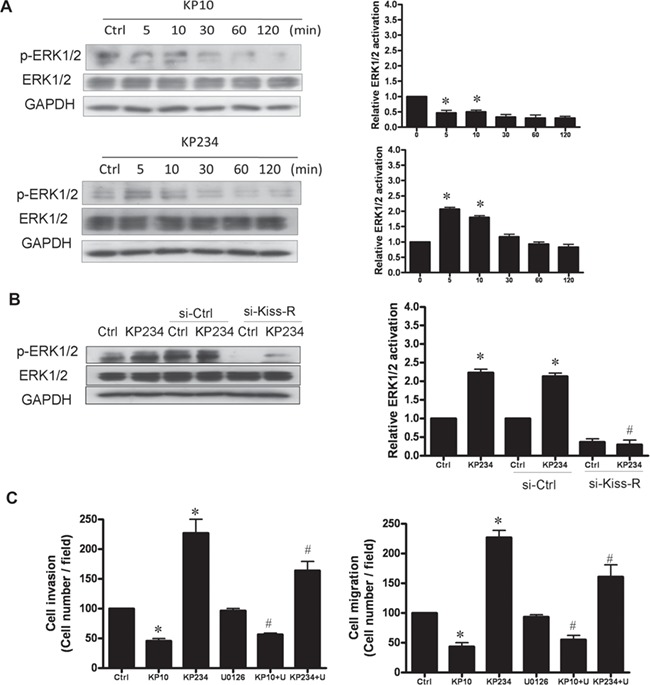
Kisspeptin regulation of cell motility in human decidual stromal cells involves ERK1/2. (A) The impact of kisspeptin analogues on ERK1/2 signaling activation. The cells were treated with kisspeptin agonist (KP10) (500 nM) and kisspeptin antagonist (KP234) (500 nM) for increasing times. Immunoblot analysis of phosphorylated ERK1/2 (p-ERK1/2) levels indicated increased p-ERK1/2 following 5 min of KP234 exposure, but a decrease following KP10 exposure. (B) Transfection with siRNA against human si-kisspeptin receptor (si-Kiss-R) decreased the level of p-ERK1/2 after KP234 treatment, whereas scrambled siRNA (si-ctrl) had no effect. (C) Pretreatment with the ERK1/2 inhibitor (U0126; 1 μM) for 30 min attenuated the positive effect of K234 and the negative effect of K10. Bars show the mean + SEM of three independent experiments in three different passages of the cell line; *P < 0.05, versus control; #P < 0.05, versus kisspeptin analogue).
Effects of kisspeptin-regulated MMP-2 and MMP-9 expression on the cell motility of decidual stromal cells
Treatment with kisspeptin agonist decreased expression of MMP-2 and MMP-9 by >50% (Fig. 7A), whereas treatment with kisspeptin antagonist stimulated MMP-2 and MMP-9 expression (Fig. 7B). In both cases, the action was abolished by the respective MMP siRNA. Pre-treatment of decidual stromal cells with either MMP-2 or MMP-9 siRNA abolished kisspeptin-regulated cell invasion and migration (Fig. 7C), demonstrating that MMP-2 and MMP-9 signaling were critical for the effects of kisspeptin on the motility of decidual stromal cells.
Figure 7.
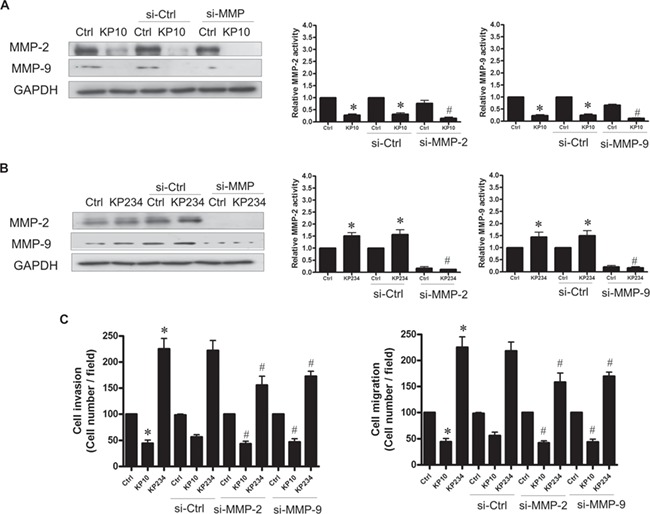
Kisspeptin analogues regulate MMP-2 and MMP-9 expression in decidual stromal cells. Immunoblot analysis demonstrates (A) that the kisspeptin agonist KP10 decreased MMP-2 and MMP-9 protein expression in decidual stromal cells. (B) The kisspeptin antagonist KP234 enhanced MMP-2 and MMP-9 protein expression in decidual stromal cells. (C) Pretreating the cells with siRNA against MMP-2 (si-MMP-2) or MMP-9 (si-MMP-9) decreased the stimulation of cell invasion and migration by KP234 but scrambled siRNA si-ctrl had no effect. The results are expressed as the mean ± SEM of three independent experiments. (*P < 0.05, versus control; #P < 0.05, versus kisspeptin analogue).
Discussion
Taken together, our data show that kisspeptin, via its receptor, regulates invasion and migration of human decidual stromal cells through FAK, Src, ERK1/2, and MMP signaling pathways (Fig. 8). Kisspeptin, first identified as a regulator of GnRH secretion in the hypothalamus, has also been shown to have local actions in peripheral reproductive tissues and processes (de Roux et al., 2003; Pinilla et al., 2012; Seminara et al., 2003), such as follicular maturation, suggesting that kisspeptin could be considered as a possible regulator in reproduction. It is thought that the embryo and decidual stroma control human embryonic trophoblast penetration (Grewal et al., 2008). The motility of human decidual stromal cells may mediate trophoblast invasion and subsequent implantation of the embryo. Therefore, the control of human decidual stromal cell movement could be pivotal during embryo implantation. The molecular mechanisms used by kisspeptin to control motility of human decidual stromal cells remain unclear. The kisspeptin receptor belongs to the GPCR family of transmembrane receptors. GPCRs have seven transmembrane domains and transmit their information via multiple G protein components, often inducing diverse mechanical signals (Novaira et al., 2009; Seminara et al., 2003). In the present study, we demonstrate that kisspeptin, through its receptor, can influence the invasive and migratory behavior of human decidual stromal cells through the FAK–Src–ERK1/2 and MMP signaling pathways.
Figure 8.
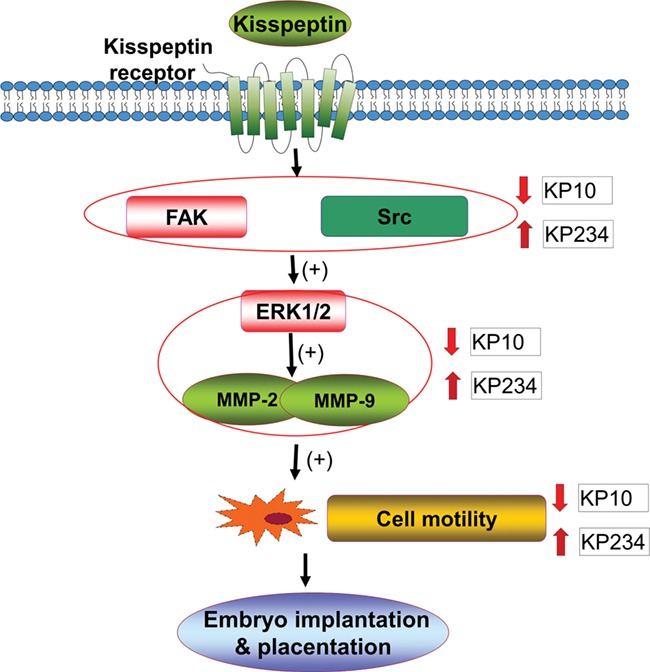
The proposed signaling pathways involved in kisspeptin-regulated motility of human decidual stromal cells. KP10 a kisspeptin agonist and KP234 a kisspeptin antagonist bind to kisspeptin receptors and regulate the invasion and migration of human decidual stromal cells by suppressing or activating, respectively, FAK and Src expression and consequently the activation of the ERK1/2 and MMPs pathways.
FAK and Src are considered to be a motility-promoting signaling complex by regulating adhesion through multiple signaling pathways (Schlaepfer et al., 2004; Webb et al., 2004). FAK siRNA and Src siRNA abolished kisspeptin-regulated cell invasion and migration of human decidual stromal cells, indicating that these effects of kisspeptin are dependent upon FAK and Src. The expression of FAK and Src was required for cell invasion and migration and is mediated via expression of MMPs (Wu et al., 2005). FAK-stimulated phosphorylation of ERK1/2 can also enhance transcription-associated increases in MMP expression (Hu et al., 2006). ERK1/2 signaling pathways regulate cellular responses to various extracellular impulses affecting cell function. Our results show that ERK1/2 signaling could play a pivotal role in kisspeptin-induced decidual stromal cell motility. In aggregate, manipulation of ERK1/2-mediated cell invasion and migration may elucidate the actions of kisspeptin on decidual stromal cells during embryo implantation.
Cell movement involves multiple processes including proteolysis, reduced cell adhesion, and enhanced cell migration. MMP-2 and MMP-9 play a critical role in cancer migration; their increased expression is correlated with increased invasion and migration of cancer cells (Davidson et al., 2002; Jedryka et al., 2012; Monge et al., 2007; Moser et al., 1994). MMP-2 and MMP-9 were essential for FAK–Src-stimulated cell invasion and migration. MMP-2 siRNA and MMP-9 siRNA abolished the ability of kisspeptin agonists/antagonists to regulate cell motility, indicating that the effects of kisspeptin in decidual stromal cells are mainly related to MMP-2 and MMP-9 activity.
In conclusion, our results demonstrate that kisspeptin can regulate the cell motility of decidual stromal cells, an important process in embryo implantation, through the binding of kisspeptin receptors, the activation of FAK and Src signaling, the phosphorylation of the ERK1/2 pathway, and the subsequent stimulation of the motility-related proteinases MMP-2 and MMP-9 activity. These data imply that the regulation of FAK–Src, ERK1/2, and MMPs expression by kisspeptin in the decidual endometrium is a possible target for increasing embryo implantation and brings a new concept concerning the mechanism of embryo implantation and decidual programming of human pregnancy.
Supplementary Material
Acknowledgements
The authors acknowledge W.J. Qiu and F.W. Chen at the Gynecological and Obstetrics Laboratory, Chang Gung Memorial Hospital, for their technical assistance in this study.
Authors’ roles
H.-M.W., H.-S.W. and P.C.K.L. performed the experiments, interpreted the results, and prepared the manuscript. H.-M.W., H.-S.W., H.-Y.H., Y.-K.S. and P.C.K.L. contributed to the scientific discussion and the manuscript editing. H.-S.W. and P.C.K.L. supervised in the design of the study and finalized the manuscript. All authors read and approved the final manuscript.
Funding
Ministry of Science and Technology, Taiwan (NSC-104-2314-B-182A-146-MY2, CMRPG3E0401, and CMRPG3E0402 to H.-M.W.); Canadian Institutes of Health Research (to P.C.K.L.). P.C.K.L. is the recipient of a Child & Family Research Institute Distinguished Investigator Award.
Conflict of interest
The authors declare that they have no competing interests.
References
- Baba T, Kang HS, Hosoe Y, Kharma B, Abiko K, Matsumura N, Hamanishi J, Yamaguchi K, Yoshioka Y, Koshiyama M et al. . Menstrual cyclic change of metastin/GPR54 in endometrium. Med Mol Morphol 2015;48:76–84. [DOI] [PubMed] [Google Scholar]
- Chao A, Wang TH, Lee YS, Hsueh S, Chao AS, Chang TC, Kung WH, Huang SL, Chao FY, Wei ML et al. . Molecular characterization of adenocarcinoma and squamous carcinoma of the uterine cervix using microarray analysis of gene expression. Int J Cancer 2006;119:91–98. [DOI] [PubMed] [Google Scholar]
- Chou CS, MacCalman CD, Leung PC. Differential effects of gonadotropin-releasing hormone I and II on the urokinase-type plasminogen activator/plasminogen activator inhibitor system in human decidual stromal cells in vitro. J Clin Endocrinol Metab 2003;88:3806–3815. [DOI] [PubMed] [Google Scholar]
- Davidson B, Goldberg I, Gotlieb WH, Kopolovic J, Ben-Baruch G, Nesland JM, Reich R. The prognostic value of metalloproteinases and angiogenic factors in ovarian carcinoma. Mol Cell Endocrinol 2002;187:39–45. [DOI] [PubMed] [Google Scholar]
- Davidson B, Konstantinovsky S, Kleinberg L, Nguyen MT, Bassarova A, Kvalheim G, Nesland JM, Reich R. The mitogen-activated protein kinases (MAPK) p38 and JNK are markers of tumor progression in breast carcinoma. Gynecol Oncol 2006;102:453–461. [DOI] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A 2003;100:10972–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellersen B, Reimann K, Samalecos A, Aupers S, Bamberger AM. Invasiveness of human endometrial stromal cells is promoted by decidualization and by trophoblast-derived signals. Hum Reprod 2010;25:862–873. [DOI] [PubMed] [Google Scholar]
- Grewal S, Carver JG, Ridley AJ, Mardon HJ. Implantation of the human embryo requires Rac1-dependent endometrial stromal cell migration. ProcNatl Acad Sci U S A 2008;105:16189–16194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol 2004;5:816–826. [DOI] [PubMed] [Google Scholar]
- Hu B, Jarzynka MJ, Guo P, Imanishi Y, Schlaepfer DD, Cheng SY. Angiopoietin 2 induces glioma cell invasion by stimulating matrix metalloprotease 2 expression through the alphavbeta1 integrin and focal adhesion kinase signaling pathway. Cancer Res 2006;66:775–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedryka M, Chrobak A, Chelmonska-Soyta A, Gawron D, Halbersztadt A, Wojnar A, Kornafel J. Matrix metalloproteinase (MMP)-2 and MMP-9 expression in tumor infiltrating CD3 lymphocytes from women with endometrial cancer. Int J Gynecol Cancer 2012;22:1303–1309. [DOI] [PubMed] [Google Scholar]
- Kim KY, Choi KC, Park SH, Auersperg N, Leung PC. Extracellular signal-regulated protein kinase, but not c-Jun N-terminal kinase, is activated by type II gonadotropin-releasing hormone involved in the inhibition of ovarian cancer cell proliferation. J Clin Endocrinol Metab 2005;90:1670–1677. [DOI] [PubMed] [Google Scholar]
- Kraus S, Naor Z, Seger R. Intracellular signaling pathways mediated by the gonadotropin-releasing hormone (GnRH) receptor. Arch Med Res 2001;32:499–509. [DOI] [PubMed] [Google Scholar]
- Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol 2006;18:516–523. [DOI] [PubMed] [Google Scholar]
- Monge M, Colas E, Doll A, Gonzalez M, Gil-Moreno A, Planaguma J, Quiles M, Arbos MA, Garcia A, Castellvi J et al. . ERM/ETV5 up-regulation plays a role during myometrial infiltration through matrix metalloproteinase-2 activation in endometrial cancer. Cancer Res 2007;67:6753–6759. [DOI] [PubMed] [Google Scholar]
- Moser TL, Young TN, Rodriguez GC, Pizzo SV, Bast RC Jr, Stack MS. Secretion of extracellular matrix-degrading proteinases is increased in epithelial ovarian carcinoma. Int J Cancer 1994;56:552–559. [DOI] [PubMed] [Google Scholar]
- Novaira HJ, Ng Y, Wolfe A, Radovick S. Kisspeptin increases GnRH mRNA expression and secretion in GnRH secreting neuronal cell lines. Mol Cell Endocrinol 2009;311:126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinilla L, Aguilar E, Dieguez C, Millar RP, Tena-Sempere M. Kisspeptins and reproduction: physiological roles and regulatory mechanisms. Physiol Rev 2012;92:1235–1316. [DOI] [PubMed] [Google Scholar]
- Salker M, Teklenburg G, Molokhia M, Lavery S, Trew G, Aojanepong T, Mardon HJ, Lokugamage AU, Rai R, Landles C et al. . Natural selection of human embryos: impaired decidualization of endometrium disables embryo-maternal interactions and causes recurrent pregnancy loss. PLoS One 2010;5: e10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer DD, Mitra SK, Ilic D. Control of motile and invasive cell phenotypes by focal adhesion kinase. Biochim Biophys Acta 2004;1692:77–102. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG et al. . The GPR54 gene as a regulator of puberty. N Engl J Med 2003;349:1614–1627. [DOI] [PubMed] [Google Scholar]
- Teklenburg G, Salker M, Molokhia M, Lavery S, Trew G, Aojanepong T, Mardon HJ, Lokugamage AU, Rai R, Landles C et al. . Natural selection of human embryos: decidualizing endometrial stromal cells serve as sensors of embryo quality upon implantation. PLoS One 2010;5: e10258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, Horwitz AF. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol 2004;6:154–161. [DOI] [PubMed] [Google Scholar]
- Wu HM, Cheng JC, Wang HS, Huang HY, MacCalman CD, Leung PC. Gonadotropin-releasing hormone type II induces apoptosis of human endometrial cancer cells by activating GADD45alpha. Cancer Res 2009;69:4202–4208. [DOI] [PubMed] [Google Scholar]
- Wu HM, Huang HY, Lee CL, Soong YK, Leung PC, Wang HS. Gonadotropin-releasing hormone type II (GnRH-II) agonist regulates the motility of human decidual endometrial stromal cells: possible effect on embryo implantation and pregnancy. Biol Reprod 2015;92:98. [DOI] [PubMed] [Google Scholar]
- Wu HM, Wang HS, Huang HY, Lai CH, Lee CL, Soong YK, Leung PC. Gonadotropin-releasing hormone type II (GnRH-II) agonist regulates the invasiveness of endometrial cancer cells through the GnRH-I receptor and mitogen-activated protein kinase (MAPK)-dependent activation of matrix metalloproteinase (MMP)-2. BMC Cancer 2013;13:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Gan B, Yoo Y, Guan JL. FAK-mediated src phosphorylation of endophilin A2 inhibits endocytosis of MT1-MMP and promotes ECM degradation. Dev Cell 2005;9:185–196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


