Abstract
Background
Lung protective ventilation has not been evaluated in patients with brain injury. It is unclear whether applying positive end-expiratory pressure (PEEP) adversely affects intracranial pressure (ICP) and cerebral perfusion pressure (CPP). We aimed to evaluate the effect of PEEP on ICP and CPP in a large population of patients with acute brain injury and varying categories of acute lung injury, defined by PaO2/FiO2.
Method
Retrospective data were collected from 341 patients with severe acute brain injury admitted to the ICU between 2008 and 2015. These patients experienced a total of 28,644 paired PEEP and ICP observations. Demographic, hemodynamic, physiologic, and ventilator data at the time of the paired PEEP and ICP observations were recorded.
Results
In the adjusted analysis, a statistically significant relationship between PEEP and ICP and PEEP and CPP was found only among observations occurring during periods of severe lung injury. For every centimeter H2O increase in PEEP, there was a 0.31 mmHg increase in ICP (p = 0.04; 95 % CI [0.07, 0.54]) and a 0.85 mmHg decrease in CPP (p = 0.02; 95 % CI [−1.48, −0.22]).
Conclusion
Our results suggest that PEEP can be applied safely in patients with acute brain injury as it does not have a clinically significant effect on ICP or CPP. Further prospective studies are required to assess the safety of applying a lung protective ventilation strategy in brain-injured patients with lung injury.
Keywords: Acute brain injury, Acute respiratory distress syndrome, Cerebral perfusion pressure, Intracranial pressure, Intracranial hypertension, Mechanical ventilation
Introduction
Acute respiratory distress syndrome (ARDS) develops frequently in patients suffering from severe, acute brain injury [1]. Despite the high incidence of ARDS in this population [2, 3], the optimal ventilation strategy to be utilized has yet to be elucidated. While lung protective ventilation for patients with ARDS has been extensively studied and shown to reduce both morbidity and mortality [4, 5], it is unclear whether these strategies benefit patients with acute brain injury. Historically, such patients have been excluded from these trials given concerns that a lung protective strategy, which utilizes a combination of positive end-expiratory pressure (PEEP) and low tidal volumes, may have an adverse effect on intracranial pressure (ICP) and cerebral perfusion pressure (CPP). In the setting of limited information to guide management, clinicians often institute a ventilation strategy that utilizes larger tidal volumes and decreased levels of PEEP relative to patients without neurologic injury [6].
While specific ventilator strategies for patients with ARDS and severe brain injury have not yet been evaluated, the relationship between applied PEEP and ICP has been a subject of clinical investigation for decades. Initial studies from the 1970s examined the effect of PEEP on ICP and yielded conflicting results [7, 8]. Frost demonstrated that applying PEEP between 5 and 12 cm H2O (and even transiently up to 40 cm H2O) improved arterial oxygenation without raising ICP [7], whereas Shapiro reported a >10 mmHg increase in ICP in more than 50 % of their cohort when PEEP was applied in the 4–8 cm H2O range [8]. It is difficult to draw conclusions from these early studies given their small sample sizes (≤12 patients) and the lack of patient demographic and clinical data. More contemporary work has demonstrated that PEEP has a variable impact on ICP when applied to patients with a variety of neurologic injuries, but overall this was considered modest [9–13].
Taken together, the complex interaction between mechanical ventilation and cerebral hemodynamics appear to be influenced by multiple patient-specific factors. Given the significant proportion of patients with severe, acute brain injury who develop hypoxic respiratory failure, we believe that improving our understanding of how these physiologic variables influence each other could suggest an optimal strategy of mechanical ventilation. In this hypothesis-generating study, we sought to evaluate the effect of mechanical ventilation on ICP and CPP in a large population of patients with acute brain injury. Specifically, we hypothesized that PEEP could be applied safely to patients with severe brain injury without causing intracranial hypertension or dangerous reductions in CPP.
Methods
Assembly of the Cohort
Patients 18 years of age or older admitted to the surgical or neuroscience intensive care units at Beth Israel Deaconess Medical Center, Boston, MA, from 2008 to 2015 with acute severe brain injury (Glasgow Coma Score [GCS] < 9) who were mechanically ventilated and had ICP monitoring were eligible for inclusion. Patients receiving chronic, intermittent mechanical ventilation for a preexisting condition at the time of admission or those with a lumbar drain were excluded. The institutional review board approved the study with a waiver of informed consent (2014P000410). Metavision (iMDsoft version 5.45.64), an electronic patient medical record system, was used to identify eligible patients and collect ventilation, physiologic, laboratory, medication, and demographic data.
Data Collection
The primary exposure of interest was total PEEP. Details of ventilation were recorded including PEEP, peak and plateau pressures, static lung compliance, respiratory rate, tidal volume, and fraction of inspired oxygen (FiO2). We defined severity of lung injury by the ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) as used by the Berlin criteria for ARDS [14]. Accordingly, the absence of lung injury was classified by a PaO2/FiO2 > 300, mild lung injury as PaO2/FiO2 > 200 and ≤300, moderate as PaO2/FiO2 > 100 and ≤200, and severe as PaO2/FiO2 < 100.
ICP was recorded and validated at regular intervals, about once per h. For analysis, data were paired so that the closest total PEEP value prior to the validated ICP value was used, which comprised a single observation in our data set. CPP was electronically calculated by subtracting intracranial pressure from mean arterial pressure.
Statistical Analyses
Categorical variables are presented as frequencies or proportions. Continuous covariates including hemodynamic variables, ICP, CPP, and PEEP are presented as mean (±standard deviation) or median (interquartile range) depending on the distribution of the data. Normality was assessed using the Shapiro–Wilk test. Given the exploratory nature of this study, we did not perform an a priori sample size calculation.
Locally weighted scatterplot smoothing (LOESS) was employed to test whether the relationship between ICP and PEEP followed a linear distribution. Once confirmed, generalized estimating equations (GEE) with robust variance were used to fit linear models, accounting for repeated measures per subject. Using a GEE model, we first tested for an interaction between PEEP and severity of lung injury as determined by PaO2/FiO2. After assessing the interaction (which was found to be significant), we stratified the data by severity of lung injury and assessed the relationship between PEEP observations and both ICP and CPP, fitting univariate and multivariate models that account for clustering within the data. Variable selection in the multivariate model was assessed using forward model selection. SAS 9.3 (SAS Institute, Cary, NC) was used for all analyses. All tests were two sided and p values <0.05 were considered statistically significant.
Results
A total of 341 patients met the inclusion criteria for this study (Fig. 1). The study population was predominantly Caucasian (58.1 %) and included a slightly larger proportion of men (56.3 %) with median age of 56.3 years old. On admission to the ICU, the median GCS score was 6 (IQR 3–7). Subarachnoid hemorrhage was the most common diagnosis (37.5 %) followed by intracerebral hemorrhage (23.5 %) and traumatic brain injury (20.5 %). Overall, patients were ventilated for a median of 3.2 days (IQR 1.2–8.9) and experienced a median ICU and hospital length of stay of 11 and 16 days, respectively (Table 1).
Fig. 1.
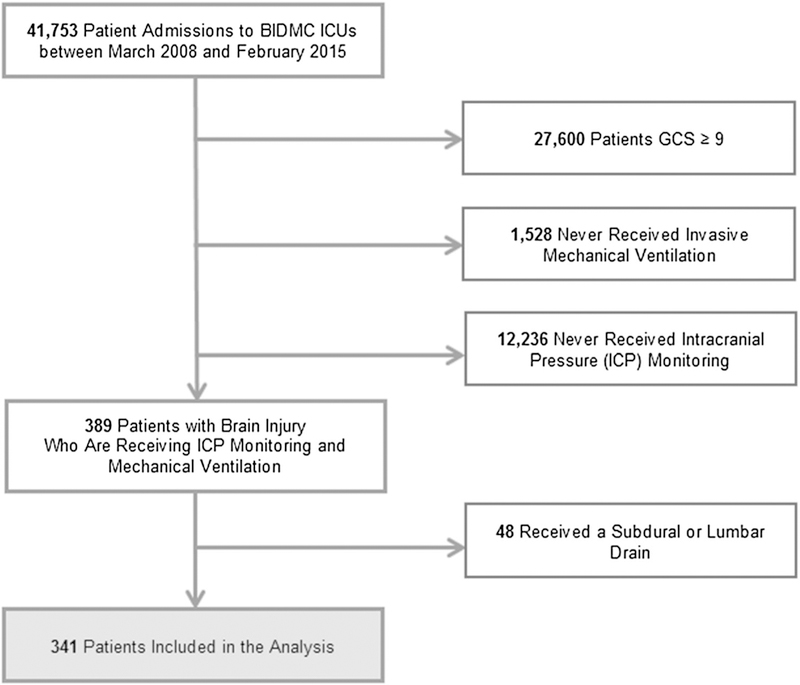
Assembly of the study cohort
Table 1.
Patient characteristics
| Variablesa | Entire cohort (n = 341) |
|---|---|
| Male gender | 192 (56.30) |
| Age, years | 56.30 (42.53–68.30) |
| White race | 198 (58.06) |
| Glasgow Coma Score on admission | 6 (3–7) |
| Category/cause of brain injury | |
| Subarachnoid hemorrhage | 128 (37.54) |
| Intraparenchymal hemorrhage | 80 (23.46) |
| Traumatic brain injury | 70 (20.53) |
| Brain tumor | 23 (6.74) |
| Liver failure | 10 (2.93) |
| Intraventricular hemorrhage | 10 (2.93) |
| Otherb | 9 (2.64) |
| Cerebrovascular accident | 8 (2.35) |
| Subdural hemorrhage | 5 (1.47) |
| ICP monitor type* | |
| Bolt | 76 (22.29) |
| Ventricular | 266 (78.01) |
| Neurovent | 1 (0.29) |
| Invasive ventilation time, days | 3.23 (1.24–8.91) |
| ICU length of stay, days | 11.19 (6.23–17.05) |
| Hospital LOS, days | 16.00 (9.00–24.00) |
| In-hospital mortality | 103 (30.21) |
| Number of recorded ICP measurements (per patient) | 87 (48–120) |
ICP intracranial pressure, LOS length of stay
Two patients had both an intraparenchymal hemorrhage and subarachnoid hemorrhage during their admission. An additional two patients were documented as switching between ventricular and bolt monitors during their ICU stay
Variables are presented as N (%) or median (IQR) depending on the type
Other includes anoxic brain injury post cardiac arrest, chronic small vessel infarct, meningitis, posterior reversible encephalopathy syndrome related to methylprednisolone-induced hypertension, septic thromboemboli, and hydrocephalus
Table 2 describes the clinical parameters of the cohort with respect to mechanical ventilation and hemodynamic data closest to each ICP observation, stratified according to the severity of lung injury. Overall, of 28,644 observations (validated paired PEEP and ICP data points), 71.3 % were recorded at times classified as without concurrent lung injury, whereas 15.2 % were recorded at times classified as mild lung injury, 11.6 % as moderate, and 1.9 % as severe.
Table 2.
Clinical parameters
| Variables | All observationsa (n = 28,644) |
No lung injury (n = 20,430) |
Mild lung injury (n = 4346) |
Moderate lung injury (n = 3329) |
Severe lung injury (n = 539) |
|---|---|---|---|---|---|
| Ventilation parameters | |||||
| Positive end-expiratory pressure (PEEP) | 5 (5–5) | 5 (5–5) | 5 (5–10) | 10 (5–10) | 10 (5–12) |
| Tidal volume | 500 (450–550) | 500 (450–500) | 500 (450–550) | 500 (450–550) | 450 (400–500) |
| Fraction of inspired oxygen (FiO2) | 40 (40–50) | 40 (40–50) | 40 (40–50) | 55 (50–60) | 80 (80–100) |
| Minute volume | 9.70 (7.90–11.60) | 9.10 (7.60–10.90) | 10.10 (8.40–12.10) | 10.90 (9.20–13.00) | 11.10 (8.70–13.60) |
| Respiratory rate | 16 (14–20) | 16 (14–20) | 18 (16–22) | 20 (16–24) | 22 (16–28) |
| Peak inspiratory pressure | 18 (12–23) | 17 (11–21) | 20 (15–24) | 22 (16–28) | 25 (18–32) |
| Plateau pressure | 17 (15–20) | 16 (14–19) | 19 (16–21) | 21 (18–24) | 24 (20–30) |
| Mean airway pressure | 9 (7–11) | 8 (7–10) | 10 (8–13) | 12 (9–15) | 13 (9–19) |
| Oxygen saturation (SpO2) | 98 (96–100) | 99 (97–100) | 98 (97–100) | 97 (95–99) | 96 (93–99) |
| Oxygenation index (OI) | 3.31 (2.07–5.63) | 2.09 (1.62–2.78) | 3.91 (3.09–5.14) | 7.78 (5.70–10.82) | 15.79 (10.77–23.90) |
| Static lung compliance > 40 | 23,595 (82.37) | 17,892 (87.58) | 3174 (73.03) | 2233 (67.08) | 296 (54.92) |
| Hemodynamic parameters | |||||
| Mean arterial blood pressure (ABP) | 90 (80–101) | 92 (82–103) | 87 (78–98) | 86 (79–97) | 82 (75–92) |
| Central venous pressure (CVP) | 11 (8–14) | 10 (7–13) | 11 (8–14) | 12 (10–16) | 14 (10–17) |
| Laboratory parameters | |||||
| PaO2 | 127 (96–168) | 167 (143–196) | 113 (100–130) | 86 (75–97) | 69 (62–79) |
| PaCO2 | 36 (33–40) | 36 (33–40) | 36 (32–41) | 37 (33–41) | 38 (33–42) |
| pH | 7.44 (7.40–7.47) | 7.44 (7.40–7.47) | 7.44 (7.41–7.47) | 7.44 (7.39–7.47) | 7.39 (7.34–7.47) |
| PaO2/FiO2 | 283 (196–388) | 398 (343–458) | 250 (226–277) | 158 (134–180) | 86 (74–93) |
Variables are presented as N (%) or median (IQR) depending on the type
Acute lung injury is defined as PaO2/FiO2 > 300; mild lung injury is defined as PaO2/FiO2 = 201–300 mmHG; moderate lung injury is defined as PaO2/FiO2 = 101–200 mmHG; severe lung injury is defined as PaO2/FiO2 < 100
Each observation refers to a paired ICP and PEEP data point, not an individual patient
Observations occurring during periods of severe lung injury experienced higher median PEEP values (10 cm H2O) than those with no or mild lung injury (5 cm H2O in both groups), as well as increased variability in PEEP measurements as compared to other groups. Tidal volumes were lower among severe lung injury observations as compared to those with less advanced forms of lung injury (median 450 vs. 500 mL); and FiO2 appeared to be higher, with interquartile ranges from 0.8 to 1.0 as compared to 0.4–0.6 in the other groups. As expected, there appeared to be a smaller proportion of observations with normal lung compliance (> 40 ml/cm H2O) in this subgroup (54.9 %) compared to observations with no lung injury (87.6 %), mild lung injury (73.0 %), and moderate lung injury (67.1 %). Oxygenation, defined by both the median PaO2 and oxygenation index (OI), was lower for observations with severe lung injury compared to those without lung injury (PaO2: 69 vs. 167 mmHg; OI: 15.8 vs. 2.1).
Mean arterial blood pressure was found to be lower among observations with severe lung injury (median 82, IQR 75–92 mmHg) than in the other lung injury strata (IQR 78–103 for all observations; Table 2).
When assessing intracranial hemodynamics among the varying categories of lung injury, the median ICP was rather similar across all observations, while the cerebral perfusion pressure was lower in the severe lung injury group (median 70 mmHg) than the values obtained in all other groups (74, 75, and 81 mmHg for moderate, mild, and no lung injury, respectively; Table 3). With regard to therapy provided to patients, a greater percentage of data points with severe lung injury received hyperotonic saline therapy (15.0 %) and vasopressors (57.0 %) as compared to their moderate (12.9 and 38.5 %), mild (12.6 and 35.3 %), and no lung injury (6.3 and 17.3 %) counterparts. Similar percentages of observations in each stratum of the cohort were administered mannitol (Table 3).
Table 3.
Clinical parameters
| Variables | All observationsa (n = 28,644) |
No lung injury (n = 20,430) |
Mild lung injury (n = 4346) |
Moderate lung injury (n = 3329) |
Severe lung injury (n = 539) |
|---|---|---|---|---|---|
| Cerebral parameters | |||||
| Intracranial pressure (ICP) | 10 (7–14) | 10 (7–13) | 11 (8–15) | 11 (8–16) | 11 (7–15) |
| Cerebral perfusion pressure (CPP) | 79 (68–90) | 81 (70–92) | 75 (66–86) | 74 (65–85) | 70 (61–80) |
| Interventions | |||||
| Received vasopressors | 6660 (23.25) | 3537 (17.31) | 1534 (35.30) | 1282 (38.51) | 307 (56.96) |
| Received mannitol | 1617 (5.65) | 1127 (5.52) | 263 (6.05) | 194 (5.83) | 33 (6.12) |
| Received hypertonic saline | 2339 (8.17) | 1281 (6.27) | 549 (12.63) | 428 (12.86) | 81 (15.03) |
Variables are presented as N (%) or median (IQR) depending on the type
Acute lung injury is defined as PaO2/FiO2 > 300; mild lung injury is defined as PaO2/FiO2 = 201–300 mmHG; moderate lung injury is defined as PaO2/FiO2 = 101–200 mmHG; severe lung injury is defined as PaO2/FiO2 < 100
Each observation refers to a paired ICP and PEEP data point, not an individual patient
Figure 2 displays the crude, unadjusted analysis of the relationship between PEEP and ICP, stratified by severity of lung injury. Results from the unadjusted analysis are given in Table 4, accounting for the correlation between an individual patient’s repeated observation. Univariate analyses indicated that for every centimeter H2O increase in PEEP, patients with no acute lung injury experienced an increase of 0.14 mmHg in ICP (p = 0.05), which compares to 0.08 mmHg (p = 0.26) in patients with mild lung injury, 0.12 mmHg (p = 0.04) in moderate lung injury, and 0.33 mmHg(p = 0.01) in severe lung injury. After adjusting for tidal volume, min volume, respiratory rate, peak inspiratory pressure, mean arterial blood pressure, PaCO2, and administration of vasopressors or hyperosmolar therapy, a significant relationship between PEEP and ICP persisted only in the severe lung injury group (p = 0.04). In this patient cohort, for every centimeter H2O increase in PEEP, there was a statistically significant increase in ICP of 0.31 mmHg (95 % CI [0.07, 0.54]).
Fig. 2.
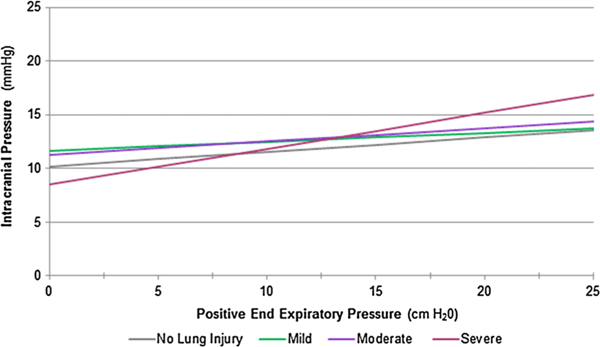
Univariate analysis of the relationship between ICP and PEEP at varying strata of lung injury. Depicted above are the results from the univariate GEE model of the relationship between PEEP and ICP, accounting for repeated measures per subject. Results are stratified by severity of lung injury defined by PaO2/FiO2
Table 4.
Unadjusted and adjusted analyses of ICP stratified by severity of lung injury
| Unadjusted PEEP beta estimate (95 % confidence interval) |
p value | Adjusteda PEEP beta estimate (95 % confidence interval) |
p value | |
|---|---|---|---|---|
| No acute lung injury | 0.14 (0.01, 0.26) | 0.05 | −0.16 (−0.38, 0.05) | 0.12 |
| Mild lung injury | 0.08 (−0.06, 0.23) | 0.26 | −0.03 (−0.20, 0.14) | 0.70 |
| Moderate lung injury | 0.12 (0.01, 0.23) | 0.04 | 0.08 (−0.03, 0.20) | 0.21 |
| Severe lung injury | 0.33 (0.10, 0.57) | 0.01 | 0.31 (0.07, 0.54) | 0.04 |
Each beta estimate represents the associated change in ICP with a one-point increase in PEEP, taking into account the correlation associated with repeated measures per patient
No acute lung injury is defined as PaO2/FiO2 > 300; mild lung injury is defined as PaO2/FiO2 = 201–300 mmHG; moderate lung injury is defined as PaO2/FiO2 = 101–200 mmHG; severe lung injury is defined as PaO2/FiO2 < 100
Models are adjusted for tidal volume, min volume, respiratory rate, peak inspiratory pressure, mean arterial blood pressure, PaCO2 and receiving vasopressors, mannitol or hypertonic saline
A similar observation was made when looking at the relationship between PEEP and CPP (Fig. 3; Table 5). Results from both the univariate and multivariate models indicate a significant relationship in the severe lung injury group (p ≤ 0.02); however, no association was found in the moderate, mild, or no lung injury strata. After adjusting for relevant confounders (pH, tidal volume, min volume, respiratory rate, peak inspiratory pressure, and administration of vasopressors or hyperosmolar therapy), application of one centimeter of H2O of PEEP resulted in a 0.85 mmHg (95 % CI [−1.48, −0.22]) decrease in CPP.
Fig. 3.

Univariate analysis of the relationship between CPP and PEEP and varying strata of lung injury. Depicted above are results from the univariate GEE model of the relationship between PEEP and CPP, accounting for repeated measures per subject. Results are stratified by severity of lung injury defined by PaO2/FiO2
Table 5.
Unadjusted and adjusted analyses of CPP stratified by severity of lung injury
| Unadjusted PEEP beta estimate (95 % confidence interval) |
p value | Adjusteda PEEP beta estimate (95 % confidence interval) |
p value | |
|---|---|---|---|---|
| No acute lung injury | −0.28 (−0.68, 0.11) | 0.17 | 0.41 (−0.02, 0.84) | 0.07 |
| Mild lung injury | −0.19 (−0.56, 0.18) | 0.33 | 0.06 (−0.38, 0.50) | 0.79 |
| Moderate lung injury | −0.08 (−0.41, 0.25) | 0.63 | 0.17 (−0.21, 0.56) | 0.38 |
| Severe lung injury | −1.22 (−1.81, −0.63) | <0.0001 | −0.85 (−1.48, −0.22) | 0.02 |
Each beta estimate represents the associated change in CPP with a one-point increase in PEEP, taking into account the correlation associated with repeated measures per patient
No acute lung injury is defined as PaO2/FiO2 > 300; mild lung injury is defined as PaO2/FiO2 = 201–300 mmHG; moderate lung injury is defined as PaO2/FiO2 = 101–200 mmHG; severe lung injury is defined as PaO2/FiO2 < 100
Models are adjusted for pH, tidal volume, min volume, respiratory rate, peak inspiratory pressure, and receiving vasopressors, mannitol or hypertonic saline
Discussion
We present the largest analysis of the relationship between PEEP and ICP in the literature. Our results show that in patients with severe, acute brain injury, the application of PEEP had no effect on either ICP or CPP for those without severe lung injury. A statistically significant relationship was found between PEEP and both ICP and CPP in the severe lung injury group only. Despite this, the increase in ICP was modest over the range of applied PEEP values, suggesting a statistically but not clinically meaningful increase. That is, holding all other covariates constant, a 5 cm H2O increase in PEEP would potentially increase ICP by 1.6 mmHg with a related 4.3 mmHg decrease in CPP. Upon retrospective review, these findings would suggest that PEEP can potentially be safely applied to most mechanically ventilated patients with severe brain injury.
Our study is consistent with previous findings that PEEP has a clinically insignificant effect on ICP across a wide range of patients with acute brain injury. Georgiadis et al. [13] found that PEEP had no effect on ICP in patients with acute stroke. Others have shown this to be the case for patients with traumatic brain injury and subarachnoid hemorrhage [12, 15]. Frost’s study demonstrated that PEEP had no influence on ICP, even at supranormal levels of PEEP (up to 40 cm H2O) [7]. However, our findings conflict with others, such as Shapiro’s study that reported a clinically significant rise in ICP when PEEP was applied in the range of 4–8 cm H2O [8]. It is difficult to compare our findings with those from Shapiro’s study as only limited data regarding demographics or mechanical ventilation was reported.
Investigators have postulated several mechanisms to explain the interdependent relationship between PEEP and ICP [16]. Caricato and colleagues demonstrated that cerebral hemodynamics and ICP were not influenced by the application of PEEP in patients with low lung compliance (<45 mL/cm H2O) [17]. Effectively, these patients were thought to be “protected” from further ICP increases when higher levels of PEEP were applied because less compliant lungs did not effectively transmit the increased pressure to the entire intrathoracic space. A smaller study reported conflicting data, showing that the ICP increased to a greater extent when PEEP was applied to patients with low lung compliance [9]. Interestingly, in our adjusted analysis, the presence of normal lung compliance was not predictive of ICP or CPP. Our conflicting results may reflect the heterogeneity of parenchymal lung injury and changes in regional compliance seen in ARDS; and, therefore, the relationship between lung compliance, PEEP, and ICP may depend on factors that we were unable to measure in this study.
What constitutes an optimal CPP range remains to be determined and is likely patient specific. While there is no definitive evidence to suggest a safe lower limit, most guidelines recommend maintaining CPP >60 mmHg to reduce the risk of exacerbating secondary brain injury [18]. In addition, it is well established that cerebral autoregulation (CA) plays an important role in maintaining constant cerebral blood flow over a wide range of CPP, though this mechanism is often unpredictably impaired in patients with severe brain injury. When CA is impaired, cerebral blood flow becomes passively dependent on perfusion. Unfortunately, assessing the integrity of CA is challenging and not routinely measured in most intensive care units. Without knowing whether CA was impaired in our population, it is difficult to understand the clinical relevance of changes in CPP. While our results suggest that the influence of PEEP on CPP likely depends on the degree of lung injury, the observed changes were relatively modest, unless lung injury is most severe.
In evaluating our practice of mechanical ventilation in this cohort of patients with acute, severe brain injury, we appear to base PEEP and FiO2 decisions on the severity of lung injury. This is evidenced by our use of lower tidal volumes and higher PEEP settings among data points in which the patients experienced severe lung injury (Table 2). However, the use of a “lung protective” strategy applied to this patient population needs to be studied further to understand whether utilizing this strategy translates into a similar mortality benefit that has been shown in ARDS patients without brain injury [5].
This study has several limitations. Given the study’s retrospective design, any results from our data should be interpreted with caution when determining their clinical application. In addition, decision making and reasons for choices in ICP and mechanical ventilation management could not be elucidated. Residual confounding may also complicate the results of our study given its retrospective nature. Specifically, for patients with external ventricular drains, we were unable to account for cerebrospinal fluid drainage, an effective strategy for reducing ICP. It is, therefore, possible that changes in these covariates could affect our conclusions in a particular subset of patients. In addition, while we looked broadly at the impact of PEEP on CPP, we were not able to measure other important parameters of cerebral hemodynamics such as changes to cerebral autoregulation, cerebral compliance, or cerebral blood flow velocities. Our cohort included patients with a broad range of neurologic diagnoses as we wanted to broadly study the impact of PEEP on ICP and CPP. In creating a heterogenous cohort, it may be difficult to generalize our results to patients with a particular neurologic diagnosis. Lastly, our data were obtained from a single tertiary care medical center; and therefore our results may not be generalizable to other ICUs. Our surgical and neuroscience ICUs employ standardized, tiered algorithmic strategies to manage intracranial hypertension, which is likely similar to other medical centers; however, it is still possible that our clinical management may differ in this patient population.
Despite these limitations, our study has significant strengths. To date, we have performed the largest human study that assesses the interaction between PEEP and intracranial physiology in the acute care setting. In addition, our database contained a robust amount of physiologic information, in particular with regard to hemodynamics, mechanical ventilation, and severity of lung injury. This allowed us to test a model that adjusted for clinically relevant covariates in assessing the impact of PEEP.
Conclusions
In conclusion, the application of PEEP for patients with varying degrees of acute lung injury and concomitant severe, acute brain injury does not appear to have a clinically significant effect on ICP or CPP. However, our findings should be applied with caution as further prospective studies are needed to assess the safety and clinical outcomes of applying a lung protective ventilation strategy to patients with both lung and brain injuries.
Acknowledgments
Funding information This study was not funded by any grant.
Footnotes
Compliance with Ethical Standards
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Aisiku IP, Yamal JM, Doshi P, Rubin ML, Benoit JS, Hannay HJ, Tilley BC, Gopinath S, Robertson CS. The incidence of ARDS and associated mortality in severe TBI utilizing the Berlin definition. J Trauma Acute Care Surg. 2015;80:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rincon F, Ghosh S, Dey S, Maltenfort M, Vibbert M, Urtecho J, McBride W, Moussouttas M, Bell R, Ratliff JK. Impact of acute lung injury and acute respiratory distress syndrome after traumatic brain injury in the United States. Neurosurgery. 2012;71: 795–803. [DOI] [PubMed] [Google Scholar]
- 3.Oddo M, Nduom E, Frangos S, MacKenzie L, Chen I, Maloney-Wilensky E, Kofke WA, Levine JM, LeRoux PD. Acute lung injury is an independent risk factor for brain hypoxia after severe traumatic brain injury. Neurosurgery. 2010;67:338–44. [DOI] [PubMed] [Google Scholar]
- 4.Briel M, Meade M, Mercat A, Brower RG, Talmor D, Walter SD, Slutsky AS, Pullenayegum E, Zhou Q, Cook D. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA. 2010;303:865–73. [DOI] [PubMed] [Google Scholar]
- 5.Laffey JG, Kavanagh BP. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury. N Engl J Med. 2000;343:812. [PubMed] [Google Scholar]
- 6.Pelosi P, Ferguson ND, Frutos-Vivar F, Anzueto A, Putensen C, Raymondos K, Apezteguia C, Desmery P, Hurtado J, Abroug F. Management and outcome of mechanically ventilated neurologic patients. Crit Care Med. 2011;39:1482–92. [DOI] [PubMed] [Google Scholar]
- 7.Frost EA. Effects of positive end-expiratory pressure on intracranial pressure and compliance in brain-injured patients. J Neurosurg. 1977;47:195–200. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro HM, Marshall LF. Intracranial pressure responses to PEEP in head-injured patients. J Trauma Acute Care Surg. 1978; 18:254–6. [DOI] [PubMed] [Google Scholar]
- 9.Cooper KR, Boswell PA, Choi SC. Safe use of PEEP in patients with severe head injury. J Neurosurg. 1985;63:552–5. [DOI] [PubMed] [Google Scholar]
- 10.McGuire G, Crossley D, Richards J, Wong D. Effects of varying levels of positive end-expiratory pressure on intracranial pressure and cerebral perfusion pressure. Crit Care Med. 1997;25: 1059–62. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X-y, Yang Z-j, Wang Q-x, Fan H-r. Impact of positive end-expiratory pressure on cerebral injury patients with hypoxemia. Am J Emerg Med. 2011;29:699–703. [DOI] [PubMed] [Google Scholar]
- 12.Muench E, Bauhuf C, Roth H, Horn P, Phillips M, Marquetant N, Quintel M, Vajkoczy P. Effects of positive end-expiratory pressure on regional cerebral blood flow, intracranial pressure, and brain tissue oxygenation. Crit Care Med. 2005;33:2367–72. [DOI] [PubMed] [Google Scholar]
- 13.Georgiadis D, Schwarz S, Baumgartner R, Veltkamp R, Schwab S. Influence of positive end-expiratory pressure on intracranial pressure and cerebral perfusion pressure in patients with acute stroke. Stroke. 2001;32:2088–92. [DOI] [PubMed] [Google Scholar]
- 14.Force ADT. Acute respiratory distress syndrome. Jama. 2012; 307:2526–33. [DOI] [PubMed] [Google Scholar]
- 15.Huynh T, Messer M, Sing RF, Miles W, Jacobs DG, Thomason MH. Positive end-expiratory pressure alters intracranial and cerebral perfusion pressure in severe traumatic brain injury. J Trauma Acute Care Surg. 2002;53:488–93. [DOI] [PubMed] [Google Scholar]
- 16.Stevens RD, Lazaridis C, Chalela JA. The role of mechanical ventilation in acute brain injury. Neurol Clin. 2008;26:543–63. [DOI] [PubMed] [Google Scholar]
- 17.Caricato A, Conti G, Della Corte F, Mancino A, Santilli F, Sandroni C, Proietti R, Antonelli M. Effects of PEEP on the intracranial system of patients with head injury and subarachnoid hemorrhage: the role of respiratory system compliance. J Trauma Acute Care Surg. 2005;58:571–6. [DOI] [PubMed] [Google Scholar]
- 18.Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons. Guidelines for the management of severe traumatic brain injury. J Neurotrauma 2007;24(Suppl 1):S1–106. [DOI] [PubMed] [Google Scholar]


