Abstract
Purpose
Chemotherapy-induced peripheral neuropathy (CIPN) with associated chronic pain is a common and disabling condition. Current treatments for neuropathic pain in CIPN are largely ineffective, with unfavorable side-effects. The capsaicin 8% patch (capsaicin 179 mg patch) is approved for the treatment of neuropathic pain: a single topical cutaneous application can produce effective pain relief for up to 12 weeks. We assessed the therapeutic potential of capsaicin 8% patch in patients with painful CIPN, and its mechanism of action.
Patients and methods
16 patients with chronic painful CIPN (mean duration 2.5 years), in remission for cancer and not receiving chemotherapy, were treated with 30 min application of capsaicin 8% patch to the feet. Symptoms were monitored using the 11-point numerical pain rating scale (NPRS), and questionnaires. Investigations were performed at baseline and three months after patch application, including skin biopsies with a range of markers, and quantitative sensory testing (QST).
Results
Patients reported significant reduction in spontaneous pain (mean NPRS: −1.27; 95% CI 0.2409 to 2.301; p=0.02), touch-evoked pain (−1.823; p=0.03) and cold-evoked pain (−1.456; p=0.03). Short-Form McGill questionnaire showed a reduction in neuropathic (p=0.0007), continuous (p=0.01) and overall pain (p=0.004); Patient Global Impression of Change showed improvement (p=0.001). Baseline skin biopsies showed loss of intra-epidermal nerve fibers (IENF), and also of sub-epidermal nerve fibers quantified by image analysis. Post-patch application skin biopsies showed a significant increase towards normalization of intra-epidermal and sub-epidermal nerve fibers (for IENF: structural marker PGP9.5, p=0.009; heat receptor TRPV1, p=0.027; regenerating nerve marker GAP43, p=0.04). Epidermal levels of Nerve Growth Factor (NGF), Neurotrophin-3 (NT-3), and Langerhans cells were also normalized. QST remained unchanged and there were no systemic side-effects, as in previous studies.
Conclusion
Capsaicin 8% patch provides significant pain relief in CIPN, and may lead to regeneration and restoration of sensory nerve fibers ie, disease modification.
Keywords: capsaicin, neuropathic pain, chemotherapy, skin biopsy
Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) is a common and often disabling adverse effect of common cancer treatments,1–3 which may persist for years, and affect the quality of life.4–7 CIPN is a cause of dose-reduction or discontinuation of chemotherapy treatment, with consequences for prognosis.8–10 Despite the prevalence and impact of CIPN, there is no treatment for its prevention or cure.
More than 30% of patients treated with neurotoxic chemotherapy agents develop peripheral neuropathy.11 The risk is higher with cisplatin, paclitaxel, docetaxel, vincristine, oxaliplatin, and bortezomib.12,13 The platinum-based14 and other drugs15–18 may lead to the “coasting” phenomenon ie, an increase in the severity of symptoms after cessation of chemotherapy treatment.
Chemotherapy agents exert effects on peripheral nerve fibers,19 with reduced amplitude of the sensory action potentials, and the involvement of small sensory fibers leading to the development of pain.20 The diverse underlying cellular and molecular mechanisms include loss of intra-epidermal nerve fibers (IENF),21 mitochondrial changes,22 neuronal viability,23 sodium channels,24 potassium channels,25 transient receptor potential vanilloid receptors (TRPV),26 Langerhans cells,27 oxidative stress,28 mitogen activated protein kinase (MAPK),29 N-methyl-d-aspartate (NMDA) receptors,30 neuropeptide Y,31 nitric oxide,32 5-HT2A,33 protein kinase C,34 calpains and caspases,35 and phosphoglycerate dehydrogenase (3PGDH).36 The involvement of small sensory fibers in CIPN has been well documented.37–41
Randomized clinical trials have been conducted with drugs currently used for neuropathic pain in CIPN, such as Gabapentin,42 Lamotrigine,43 Nortriptyline,44 Amitriptyline,45 and Duloxetine.46,47 Two studies have evaluated the effectiveness of topical agents incorporating Amitriptyline, Ketamine, and Baclofen.48,49 One study was conducted to determine the effect of an oro-mucosal cannabis-based spray.50 The results of these trials have been largely disappointing,51 and only Duloxetine showed a small statistical effect on pain outcome measures.
Topical capsaicin formulations are widely used to manage pain. Low-concentration creams for daily skin application over weeks have been available for decades. Application of the high-dose capsaicin 8% patch (also known as capsaicin 179 mg patch, Qutenza) reduces neuropathic pain after a single 30 to 60 min application,52–62 with pain relief maintained up to 12 weeks.63–65 Capsaicin is an agonist which acts on TRPV1, the heat and capsaicin receptor, and recent advances in the understanding of its mechanism and site of action have been reviewed.66 Our recent study showed its effect and mechanisms in amputation stump pain and phantom limb syndrome.67
Our aim in this study was to assess the effect of capsaicin 8% patch in patients with painful CIPN in relation to its underlying mechanism of action, using a range of pathophysiological markers in skin biopsies, and neurophysiological tests. The skin biopsy markers included nociceptor subsets, and their target-derived neurotrophic factors, which maintain nociceptors, regulate their phenotype, and induce nerve regeneration. The neurophysiological tests included standard neurophysiological techniques and quantitative sensory testing (QST).
Our hypothesis was that the capsaicin 8% patch may relieve pain but also induce nerve regeneration and/or restoration of the nerve fiber phenotype, by “pruning” the abnormal nerve fibers. The capsaicin 8% patch causes a reversible “cutaneous nerve terminal axotomy” ie, nerve terminal degeneration followed by regeneration. As the patients were in remission and now not receiving chemotherapy, which adversely affects both their nerves and target skin cells, hence their nerve fibers may now regenerate more “normally” following capsaicin 8% patch treatment.
Materials and methods
Study design
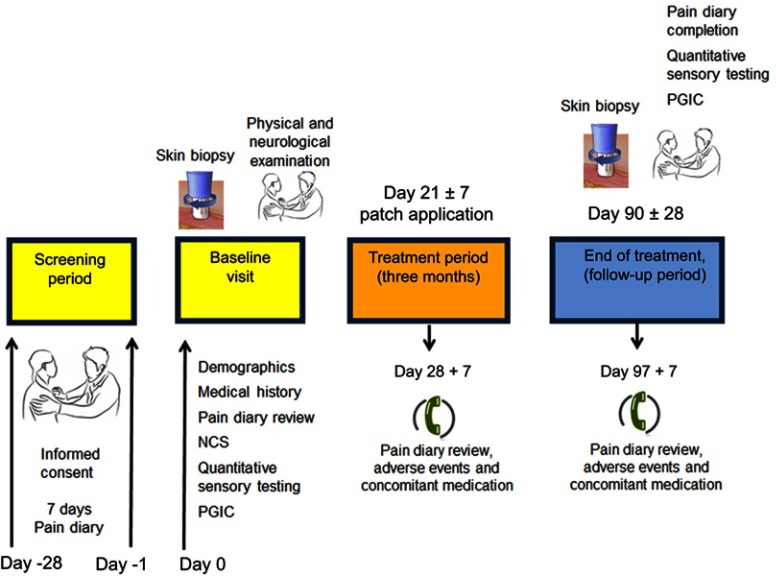
This was a single center, open-label, longitudinal study with capsaicin 8% patch treatment as licensed, conducted in patients who attended the Peripheral Neuropathy Unit, Imperial College London, based at Hammersmith Hospital, Imperial College Healthcare NHS Trust. The study was approved by the London Fulham Research Ethics Committee (Ethics reference number: 12/LO/0895). The study involved hospital visits and telephone calls, as shown in Figure 1. Patients attended the hospital unit for study visits 1 to 3, and the application of the capsaicin 8% patches was carried out in the hospital as previously described in detail, with illustrations.67 The capsaicin 8% patches covered the feet and distal calf, including the region of the pretreatment baseline skin biopsies, after they had fully healed. All patients had a total number of 4 patches, 2 for each foot.
Figure 1.
Study flow diagram.
Abbreviations: NCS, nerve conduction study, PGIC, patient global impression of change.
Participants
All participants provided written informed consent, and this study was conducted in accordance with the Declaration of Helsinki.
Patients with painful CIPN for at least 3 months prior to enrollment, and aged between 18–80 years, were eligible for inclusion in the study. 16 patients with different types of cancer, who had received chemotherapy (mainly platinum, taxane, and proteasome inhibitor compounds) and developed symptoms of CIPN, were enrolled. Patients Demographic and characteristics are outlined in Table 1. All patients fulfilled the criteria for neuropathy outlined by the National Cancer Institute of Canada Common Toxicity Criteria (NCIC-CTC), including pain and hypersensitivity. Patients were considered suitable for the study if their symptoms had been stable on their prescribed medical treatment for 8 weeks prior to enrollment. Patients described symptoms in their lower limbs, most commonly numbness, pins, and needles, tingling and burning pain or discomfort. All patients reported pain in their feet; most were taking treatment for pain at the start of the study (gabapentin, pregabalin, amitriptyline, duloxetine, tramadol, oxycodone, or a combination of these).
Table 1.
Patients’ demographics and clinical characteristics
| Patients demographics | |
|---|---|
| Age (years, range) | 64 (45–79) |
| Number of patients | 16 |
| Number of male patients (%) | 8 (50) |
| Ethnic origin, % | |
| Asian or Asian British | 25.0 |
| Caucasian Other |
68.0 7.0 |
| Clinical characteristics | |
| Duration of CIPN (years, range) | 2.5 (5 month-8 years) |
| Pain level at baseline (NPRS, mean [SEM]) | 6.6 (0.43) |
| Number of patients taking pain medications at baseline | 12 (75%) |
| Acetaminophen (Paracetamol) Gabapentinoids (Pregabalin and Gabapentin) |
2 4 |
| Tricyclic anti-depressants/SNRIs Opioids Other analgesic combinations |
1 4 1 |
| Patient cancer types: Colon cancer Multiple myeloma Lung cancer Ovary cancer |
7 6 1 2 |
| Patient chemotherapy types: Bortezomib Platinum/taxane or both |
6 10 |
Abbreviations: CIPN, chemotherapy-induced peripheral neuropathy; SEM, standard error of the mean; SD, standard deviation; NPRS, numerical pain rating scale; SNRIs, serotonin and norepinephrine reuptake inhibitors.
Clinical symptoms and pain assessment scales
Patients were given a study diary to complete starting on the day of screening and continuing for the next 7 days. The diary collected numerical pain rating scores (NPRS) twice daily. An 11-point numerical rating scale (NRPS), with the 0 point being “no pain” and the 10 point being “pain as bad as you can imagine,” was used to describe “pain on average in the last 24 hrs” for spontaneous and evoked pain. After 7 days of completing this diary, a member of the study team contacted the patients by telephone and averaged the result of their NPRS to determine their eligibility for the study. Only patients with average pain intensity equal or greater than 4/10 on the NPRS for spontaneous pain were eligible to participate further in the study and were advised to continue with the study diary until the end-of-study follow up visit. Symptoms were also assessed using the Short Form McGill Pain Questionnaire (SF-MPQ-2).68 The standard Patient Global Impression of Change (PGIC) was recorded.
Clinical examination and assessment of neuropathy
Clinical examination and tests were performed to confirm that patients had a predominantly sensory, length-dependent neuropathy. The Neuropathy Impairment Score Lower Limbs (NIS-LL)69 was recorded.
Nerve conduction studies were performed once at the start of the study for all patients. Nerve conduction studies of the common peroneal (including F wave studies) and sural nerves in the right leg were performed in a standardized manner by the same examiner on a Medtronic Keypoint electromyogram (Medtronic, Minneapolis, MN, USA). Sural antidromic sensory action potentials of <5 µV amplitude and 40 m/s conduction velocity were considered abnormal, and common peroneal nerve (compound muscle action potential from extensor digitorum brevis) values <3 mV amplitude, and 40 m/s conduction velocity were considered abnormal.70 An F-wave latency >60 ms was considered abnormal.
Most patients (66%) had at least one abnormality on the nerve conduction study, 11% had both motor and sensory abnormality. F-waves were absent in 1 patient. The mean ± SEM (range) for the peroneal motor action potential was 3.8±0.5 (1.2–6.3) µV and for the peroneal conduction velocity was 46±1.4 (40–56.8) m/s. The mean ± SEM (range) for the sural sensory action potential was 6.8±1.4 (0.0–16) µV, and for the sural nerve velocity was 44.1±6.3 (0.0–67) m/s.
Quantitative sensory testing (QST)
For quantitative sensory testing (QST), thresholds for light touch were measured using Semmes–Weinstein hairs (made by A. Ainsworth, University College London, UK), No. 1 (0.0174 g) to No 20 (263.0 g). The number of the hair with the lowest force reliably detected by the patient on the dorsum of the toe was recorded. Values > No. 3 monofilament (0.0479 g) were considered abnormal.70 Vibration perception thresholds were measured using a biothesiometer (Biomedical Instrument Company, Newbury, OH, USA) placed on the metatarsophalangeal joint of the big toe. Three ascending and three descending trials were carried out, and the mean value obtained. Values >12 V were considered abnormal.71
Thermal perception thresholds were performed as described in previous publications72,73 using the TSA II–NeuroSensory Analyzer (Medoc, Ramat Yishai, Israel). A 30 mm x 30 mm thermode was used and thermal thresholds determined in the soles of the feet (under the instep), right lateral calf and palms of the hands (thenar eminence) for warm perception, cool perception, heat pain and cold pain from a baseline temperature of 32 °C, with a change in temperature of 1°C/s. The mean of three consecutive tests for each modality was recorded. Values >6.4 °C for warm sensation, >2.3 °C for cool sensation and >10.4 °C for heat pain, were considered abnormal.70,72,73
Calf skin biopsy and immunohistochemistry
Two 3.5-mm diameter skin punch biopsies were collected under local anesthesia from the distal lateral calf of 16 patients with CIPN on visit 1 before capsaicin 8% patch application, and repeated 3 months after patch application. Skin biopsies collected from age and gender-matched 12 healthy volunteers were analyzed alongside the CIPN patient biopsies, as controls. The immunohistochemical methods and antibodies used here had been reported previously.74–76 One of the two skin biopsies was snap frozen and stored at −70 °C, and the other immersed in fixative (modified Zamboni’s fluid – 2% formalin; 0.01 M phosphate buffer; 15% saturated picric acid (pH 7.2), then washed in phosphate buffered saline (PBS; 0.1 M phosphate; 0.9% w/v saline; pH 7.3) containing 15% w/v sucrose for an hour, before snap freezing in optimum cutting tissue embedding medium (Tissue-Tek OCT, RA Lamb Ltd, Eastbourne, U.K.). Frozen sections (15µm thickness) were collected onto poly-L-lysine (Sigma, Poole, UK) coated glass slides and post-fixed in freshly prepared, 4% w/v paraformaldehyde in 0.15M phosphate buffered saline (PBS) for 30 min. Sections of pre-fixed tissue were collected in the same way and allowed to air dry for markers. Endogenous peroxidase was blocked by incubation in methanol containing 0.3% w/v hydrogen peroxide for 30 mins for both post- and pre- (Zamboni) fixed sections. After rehydration, appropriately processed sections were incubated overnight with primary antibodies (n=16 biopsies, unless stated otherwise, as tissue was not enough to study all markers in some biopsies). The antibodies were to the structural nerve marker PGP 9.5 (Rabbit, RA95/06, 1:40,000; Ultraclone, Isle of Wight, UK), the heat and capsaicin receptor transient receptor potential vanilloid 1 TRPV1 (Rabbit, C22, 1:10,000; GlaxoSmithKline, Harlow, UK), the human sensory neuron-specific receptor SNSR, marker of IB-4 nociceptor subset (Rabbit, 1:15,000; gift from Astra Zeneca, Montreal, Canada) nerve regeneration marker, growth associated protein GAP-43 (G9264, Mouse, 7B10, 1:80,000; Sigma, Poole, UK), recombinant human Nerve Growth Factor (Genentech Inc, San Francisco, USA, Rabbit, 12,756/71, 1:2000), NT3 (Rabbit, C/845 No 883, 1:50,000, Amgen, Thousand Oaks, USA) epidermal Langerhans cells marker S-100 (Rabbit, Z311, 1:40,000, Dakocytomation, Dako UK, Ltd, Cambridge, UK). Sites of primary antibody attachment were revealed using nickel-enhanced, avidin-biotin peroxidase (ABC - Vector Laboratories, Peterborough, UK) as previously described.74–76 Sections were counterstained for nuclei in 0.1% w/v aqueous neutral red, air dried and mounted in xylene-based mountant (DPX; BDH/Merck, Poole, UK), prior to analysis. Negative controls included omission of primary antibodies or their replacement with pre-immune serum.
Nerve fibers were counted along the length of four nonconsecutive sections. The length of epithelium in each counted section was measured using computerized microscopy software (Olympus ANALYSIS 5.0 Soft, Olympus UK, Southend, Essex, UK) and results expressed as fibers/mm length of the section. Sub-epidermal nerve immune-reactivity obtained as a percentage (% area) measured by image analysis where digital photomicrographs were captured via video link to an Olympus BX50 microscope. The grey-shade detection threshold was set at a constant level to allow detection of positive immuno-staining and the area of highlighted immuno-reactivity was expressed as a percentage (% area) of the field scanned. Images were captured (x40 objective magnification) along the entire length, and the mean values were used for statistical analysis. Quantification was performed by two independent blinded observers, and there was no significant difference between observers. Validation of these methods, including vs PGP9.5 IENF in 50µm thickness sections, have been published previously.
Statistical analysis
Data were analyzed using GraphPad Prism version 5.0 for Windows (GraphPad Prism Software, San Diego, CA, USA). The statistical test used was the paired two-tailed Mann-Whitney test. Values were compared before and after the treatment with capsaicin 8% patch. For all statistical tests, p-values <0.05 were considered significant.
Results
Neuropathy Impairment Score Lower Limbs (NIS-LL) showed a significant improvement after treatment with the capsaicin 8% patch (p=0.01), with a reduction of the mean score ± SEM of 1.875±0.40 (Table 2).
Table 2.
Results before and after capsaicin 8% patch: spontaneous pain (NPRS), short form McGill pain questionnaire, patient global impression of change, quantitative sensory testing, and contact heat evoked potentials
| Numerical Pain Rating Scale (NPRS), mean ± SEM | |||
|---|---|---|---|
| Pre patch application | Post patch application | p-value | |
| Spontaneous pain Light touch evoked pain Cold evoked pain |
6.6±0.4 4.8±0.8 4.0±0.8 |
5.3±0.5 2.6±0.7 2.6±0.8 |
0.01 0.02 0.03 |
| Short Form McGill pain Questionnaire (SFMPQ), mean ± SEM | |||
| Pre patch application | Post patch application | p-value | |
| Continuous pain Intermittent pain Affective pain Neuropathic pain Overall pain |
27.9±3.6 21.2±3.8 9.5±2.8 30.5±3.3 83.6±12.3 |
14.9±2.9 14.0±3.1 9.4±2.4 19.5±2.6 53.5±8.7 |
0.001 ns ns 0.0007 0.003 |
| Patient Global Impression of Change (PGIC), mean ± SEM | |||
| Pre patch application | Post patch application | p-value | |
| PGIC score | 4.2±0.2 | 2.8±0.3 | 0.003 |
| Quantitative Sensory Testing (QST), mean ± SEM | |||
| Pre patch application | Post patch application | p-value | |
| Cool Threshold (°C) Warm Threshold (°C) Cold Pain Threshold (°C) Heat Pain Threshold (°C) Vibration Threshold (V) Monofilament Threshold (gm) |
20.4±2.1 44.3±1.2 9.6±2.1 47.9±0.7 33.6±3.4 34.9±22.2 |
20.7±1.7 43.9±0.9 10.4±2.4 48.2±0.6 28.7±3.0 2.3±1.4 |
ns ns ns ns ns ns |
| Neuropathy Impairment Score Lower Limbs (NIS-LL), mean ± SEM | |||
| Pre patch application | Post patch application | p-value | |
| NIS-LL Score | 10.5±1.2 | 8.6±0.8 | 0.01 |
Abbreviations: SEM, standard error of the mean; NPRS, numerical pain rating scale; ns, not significant; PGIC, patient global impression of change; QST, quantitative sensory testing; NIS-LL, neuropathy impairment score lower limbs; °C, Celsius degree; V, Volt; gm, gram.
Pain scores and questionnaires
There was a significant reduction in the average (±SEM) daily NPRS for spontaneous pain, −1.271 (±0.077), p=0.02, three months after capsaicin 8% patch application (baseline week vs week 12 after patch application). There was also a significant reduction in scores for pain evoked by touch −1.823 (±0.07), p=0.03, and cold −1.456 (±0.06), p=0.03 (Table 2).
Short-Form McGill Pain Questionnaire (SF-MPQ-2) showed a significant reduction in the continuous (−13.0±0.66, p=0.001) and neuropathic (−11.7±0·72, p=0.0007) pain scores. There was no significant difference in the intermittent and affective pain scores.
Patient Global Impression of Change (PGIC) showed significant improvement, p=0.0029 (Table 2).
Quantitative sensory testing
All patients showed abnormalities on QST pre-treatment compared to normal values reported previously,77 in accord with other laboratories. There was no significant change after treatment (p>0.05, Table 2).
Immunohistochemistry
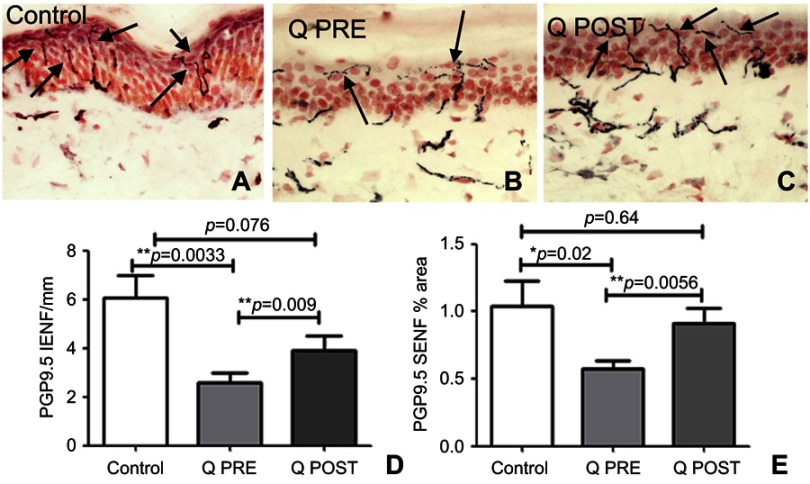
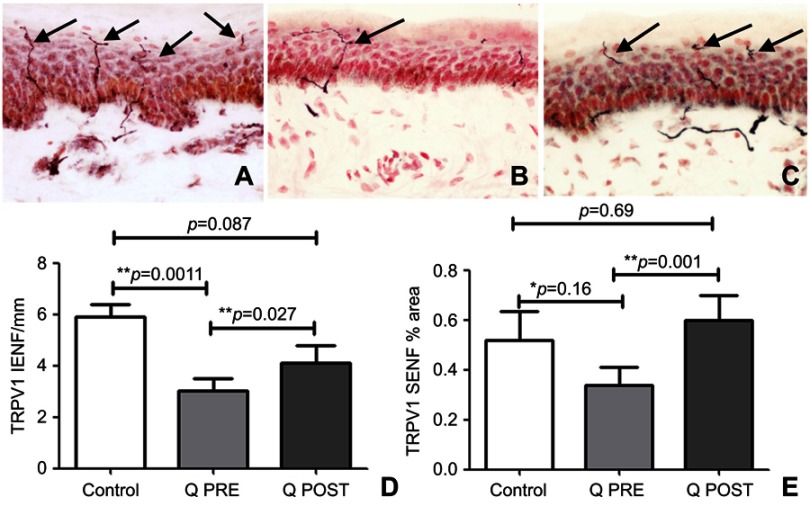
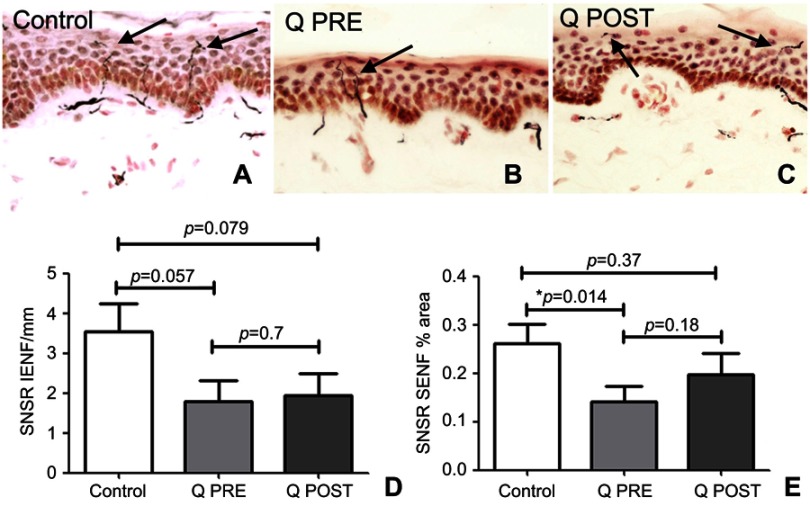
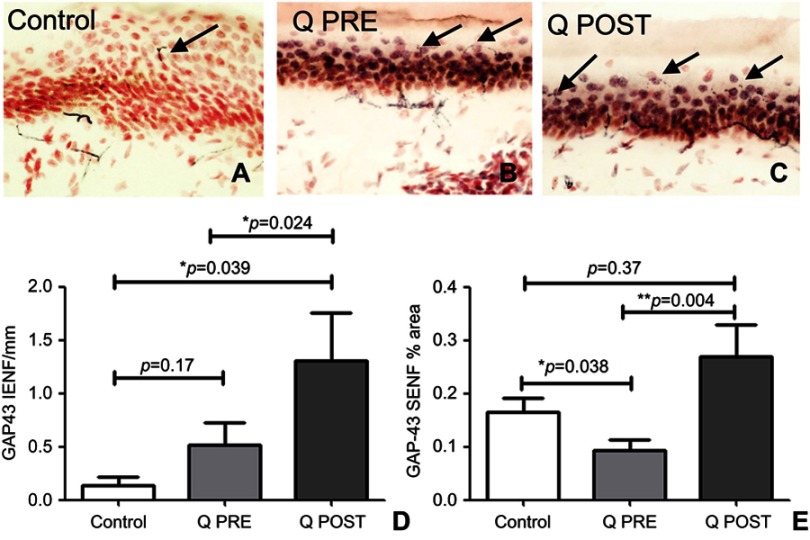
Skin biopsies at baseline showed fewer PGP9.5-immunoreactive intra-epidermal nerve fibers (IENF) counts than controls; the latter were in accord with our previously published normative data, for all the skin biopsy markers used in this study.70,76–78,84 There was a significant increase in PGP9.5 IENF fibers after capsaicin 8% patch treatment patients (p=0.009, Figure 2), and in subepithelial nerve fibers (SENF), (p=0.0056). There was also a significant increase in TRPV1 IENF fibers (p=0.027, Figure 3), and in TRPV1 SENF (p=0.001). There were no significant differences between Sensory Neuron-specific Receptors (SNSR) IENF and SNSR SENF (p=0.7 and 0.18 respectively; Figure 4). GAP-43 immunoreactive IENF were significantly more abundant after capsaicin 8% patch treatment p=0.04, and also GAP-43 SENF (p=0.004), Figure 5).
Figure 2.
Immunohistochemistry in skin biopsies for PGP9.5, before and after capsaicin 8% patch treatment. Intra-epidermal nerve fibers (arrowed) and sub-epidermal nerve fibers from (A) control subjects, at the baseline visit (B, Q PRE) and, after capsaicin 8% patch treatment (C, Q POST), magnification x40. (D) Bar chart of intra-epidermal nerve fibers for PGP 9.5 counts, (E) bar chart of sub-epidermal (SENF) analysis (% area).
Notes: *Significant; **very significant.
Figure 3.
Immunohistochemistry in skin biopsies for TRPV1, before and after capsaicin 8% patch treatment. Intra-epidermal nerve fibers (arrowed) and sub-epidermal nerve fibers from (A) control subjects, at the baseline visit (B, Q PRE) and, after capsaicin 8% patch treatment (C, Q POST), magnification x40. (D) Bar chart of intra-epidermal nerve fibers for TRPV1 (IENF) counts; (E) bar chart of sub-epidermal (SENF) analysis (% area) for TRPV1.
Notes: *Significant; **very significant.
Figure 4.
Immunohistochemistry in skin biopsies for SNSR, before and after capsaicin 8% patch treatment. Intra-epidermal nerve fibers (arrowed) and sub-epidermal nerve fibers from (A) control subjects, at the baseline visit (B, Q PRE) and, after capsaicin 8% patch treatment (C, Q POST), magnification x40. (D) Bar chart of intra-epidermal nerve fibers for SNSR (IENF) counts; (E) bar chart of sub-epidermal (SENF) analysis (% area) for SNSR.
Note: *Significant.
Figure 5.
Immunohistochemistry in skin biopsies for GAP43, before and after capsaicin 8% patch treatment. Representative image of intra-epidermal nerve fibers (arrowed) and sub-epidermal nerve fibers from (A) control subjects, at the baseline visit (B, Q PRE) and, after capsaicin 8% patch treatment (C, Q POST), magnification x40. (D) Bar chart of intra-epidermal nerve fibers for GAP43 (IENF) counts; (E) bar charts of sub-epidermal (SENF) analysis (% area) for GAP43.
Notes: *Significant; **very significant.
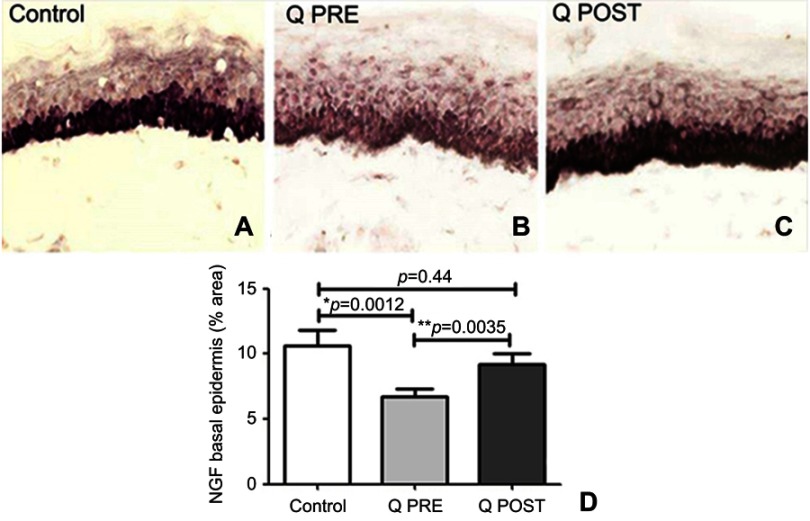
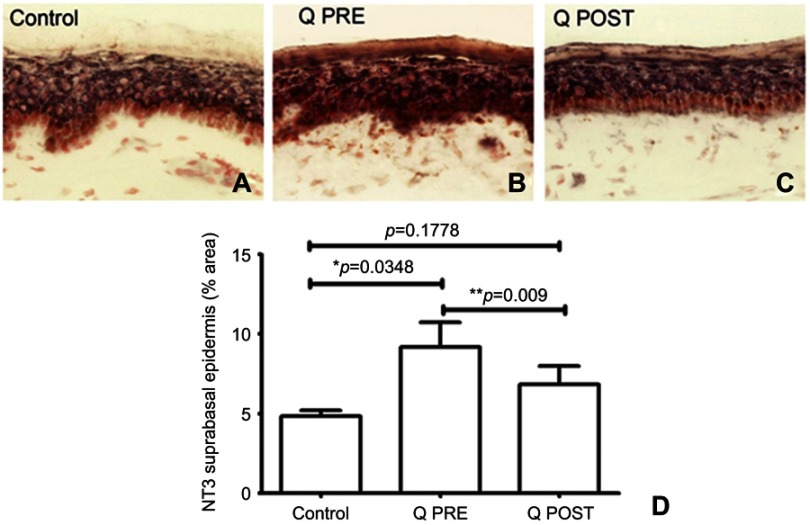
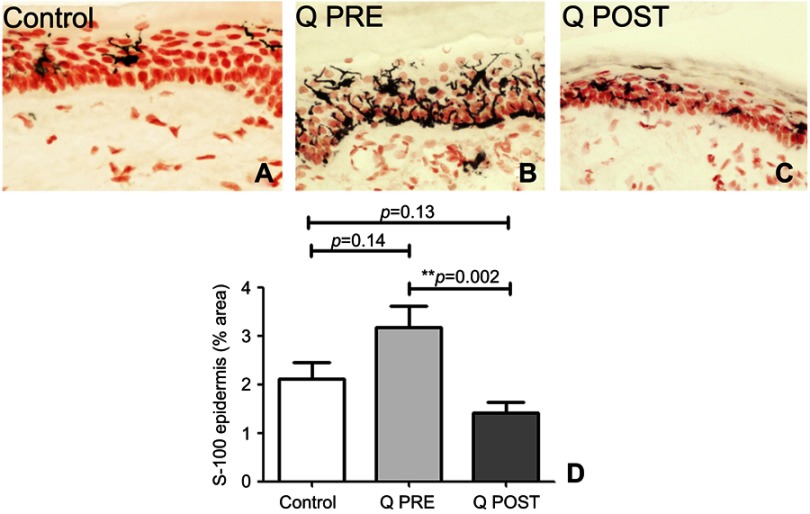
NGF antibodies labeled basal keratinocytes which express NGF which normally helps maintain the IENF73,78–80 (Figure 6). In this study, there was a decrease of NGF in basal keratinocytes compared to controls at baseline (p=0.012), but an increase towards normal values after treatment with capsaicin 8% patch (p=0.0035, Figure 6); further, this reversal appeared to restore levels towards normal values compared to controls (p=0.44, Figure 6). In both control and CIPN subjects, Neurotrophin 3 (NT3) antibodies labeled suprabasal keratinocytes (Figure 7). There was a significant increase in NT3 levels in CIPN patients before treatment compared to control subjects (p=0.0348 (Figure 7), which was abolished after treatment with capsaicin 8% patch (p=0.1778, Figure 7); this decrease of NT3 was significant (p=0.009, Figure 7). S100 antibody labeled Langerhans cells (LCs) (Figure 8). These were decreased, towards normal levels, after capsaicin 8% patch treatment (p=0.002, Figure 8).
Figure 6.
Immunohistochemistry in skin biopsies for NGF, before and after capsaicin 8% patch treatment. NGF immunostaining of basal epidermis in calf skin obtained from (A) control subjects, and CIPN patients before (B, Q PRE) and after capsaicin 8% patch treatment (C, Q POST), magnification x40. (D) Bar chart showing the basal cell NGF image analysis (% area).
Notes: *Significant; **very significant.
Figure 7.
Immunohistochemistry in skin biopsies for NT3, before and after capsaicin 8% patch treatment. NT3 immunostaining from (A) control subjects, and CIPN patients before (B, Q PRE) and after capsaicin 8% patch treatment (C, Q POST). (D) Bar chart showing NT3 suprabasal image analysis (% area).
Notes: *Significant; **very significant.
Figure 8.
Immunohistochemistry in skin biopsies for Langerhans cells (LCs), before and after capsaicin 8% patch treatment. LCs immunostaining in the epidermis of calf skin from (A) control subjects, and CIPN patients before (B, Q PRE) and after capsaicin 8% patch treatment (C, Q POST), magnification x40. (D) Bar chart showing LCs image analysis (% area).
Note: **Very significant.
Discussion
Chemotherapy-induced peripheral neuropathy with associated chronic pain has a major impact on the quality of life of cancer patients, including those in remission from cancer. Current symptomatic treatments used for neuropathic pain have limited efficacy with significant side-effects, and there are no preventive measures for development of CIPN, or amelioration of established painful CIPN.
In this study, CIPN patients reported significant pain reduction following a single 30 min treatment with the capsaicin 8% patch - in spontaneous pain, touch-evoked pain and cold-evoked pain. Their Short-Form McGill questionnaire showed a reduction in neuropathic, continuous and overall pain scores; Patient Global Impression of Change also showed improvement. The effect-size on pain relief by capsaicin 8% patch was similar to that for chronic neuropathic pain caused by other conditions, and as reported recently in two open label treatment studies for painful CIPN.81,82 QST remained unchanged, and there were no systemic side-effects, as in previous clinical trials.
The novel findings in the study were the changes observed in skin biopsy markers. The baseline skin biopsies showed loss of intra-epidermal nerve fibers (IENF), as in painful small fiber neuropathy caused by several other conditions. Post-patch application skin biopsies showed a significant increase towards normalization of intra-epidermal and sub-epidermal nerve fibers for the pain-neuronal structural marker PGP9.5, capsaicin and heat receptor TRPV1, and regenerating nerve fibers with the selective marker GAP43. Epidermal Nerve Growth Factor (NGF), Neurotrophin-3 (NT-3), and Langerhans cells were also changed towards normalization post-patch application.
Capsaicin 8% patch is a topical formulation for the treatment of peripheral neuropathic pain; we have reviewed its mechanism of action in detail.66 In brief, capsaicin is the pungent “hot” ingredient in chili peppers, a natural selective agonist of the vanilloid receptor TRPV1. It is released rapidly from the capsaicin 8% patch and leads to an overstimulation of skin nociceptors - they are “defunctionalized” acutely, and are no longer able to respond as previously to the range of stimuli that may cause pain in patients with peripheral neuropathy.66 The defunctionalization occurs in nociceptor cutaneous terminals, as the patch has an effect on their mitochondrial function to the dermis, with a concentration gradient. A single application of capsaicin 8% patch can provide pain relief for up to 3 months or more – however, the effect of the patch is reversible, and nerve fiber terminals usually regenerate, hence some patients require 3 monthly patch applications for pain relief.
For this study, our hypothesis was that a single application of capsaicin 8% patch, by “pruning” the abnormal nerve fibers, may induce nerve regeneration and restoration of the nerve fiber phenotype in skin biopsies, as the patients were now not receiving chemotherapy which adversely affects both nerves and target skin cells. The milieu in the absence of chemotherapy agents may enable more healthy interactions between the regenerating sensory nerve fibers and their target organs, leading to restoration of nociceptor phenotype and expression of neurotrophic factors by the target organ.66,73,79 The sensory neuropeptides eg, calcitonin gene-related peptide CGRP when released by nerve terminals enhances keratinocyte proliferation and their expression of NGF.73,79 The results of this study are in accord with our hypothesis. Thus, the capsaicin 8% patch provides significant pain relief in CIPN, and may also lead to regeneration and restoration of sensory nerve fibers ie, disease modification.
The potential roles of the key epidermal neurotrophins in painful peripheral neuropathies, and the inverse correlation between NGF and NT-3 levels observed before and after treatment with capsaicin 8% patch, is in agreement with our previous publications and reviews.73,79,83,84 Decreased epidermal expression and levels of NGF, eg, induced by cancer chemotherapy which is toxic to epidermal keratinocytes expressing NGF, may lead to reduced IENF. The increased level of NT-3 observed at baseline in this study has been reported previously in association with epidermal denervation in small fiber painful peripheral neuropathy, and attributed to a possible compensatory mechanism.84 The persistence of these neurotrophic factor changes and their dependent innervation following cessation of chemotherapy observed at baseline in our study deserve further investigation, particularly long-term epigenetic mechanisms.
The potential role of Langerhans cells in painful CIPN has been described;27 their changes following treatment with capsaicin 8% patch towards normalization in our study suggests a contribution in pain relief, or a secondary effect. The interaction between neuro-inflammatory, neuro-degenerative and neuro-regenerative mechanisms at different stages of painful neuropathies are complex, and also deserve further studies in CIPN.
Future studies should include randomized clinical trials in a greater numbers of participants, in comparison with placebo and also other treatments for neuropathic pain. These will address some of the limitations of this initial study, including spontaneous improvements in pain and skin biopsy markers – we regard the latter as unlikely, as the patients all had persistent neuropathic pain over months to years, and on assessments showed features of chronic CIPN. To our knowledge, repeat skin biopsies have not been analyzed previously in patients with CIPN, without or following treatments. We have reported a decline of nerve fiber markers in skin biopsies repeated after 6 months in a natural history study of patients with chronic diabetic sensory polyneuropathy.77
Conclusion
Capsaicin 8% patch provides significant pain relief in CIPN, and may lead to nerve regeneration and restoration of sensory nerve fibers ie, disease modification. These mechanistic changes following capsaicin 8% patch treatment in painful CIPN are promising, and deserve further study for pain relief and disease modification.
Acknowledgments
We thank Paul Facer, formerly Research Assistant, Imperial College London, for help with the tissue studies. This work was supported by Astellas Pharma Europe Ltd. The study was sponsored by Imperial College London.
Author contributions
The study was conceived by PA, clinical assessments were conducted by EE, RP, KN, PM and PA, tissue studies and review were performed by YY, PD and PA, statistical analysis was by EE, YY and RP, the patients were recruited with HG, HW, LK, and AR, who reviewed clinical data. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
PA has received speaker fees for symposia and meetings organized by Astellas UK, but no remuneration for this investigator-led study. PA also reports grants from Astellas, during the conduct of the study. HG reports is jointly employed by Imperial College and Astra Zeneca and received personal fees from Sensorkinesis. HG is also the Chief Scientific Officer and patent holder for Papyrus Therapeutics, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
- 1.Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C. Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. Eur J Cancer. 2008;44(11):1507–1515. doi: 10.1016/j.ejca.2008.04.018 [DOI] [PubMed] [Google Scholar]
- 2.Cioroiu C, Weimer LH. Update on chemotherapy-induced peripheral neuropathy. Curr Neurol Neurosci Rep. 2017;17(6):47. doi: 10.1007/s11910-017-0757-7 [DOI] [PubMed] [Google Scholar]
- 3.Chaudhry V, Chaudhry M, Crawford TO, Simmons-O’Brien E, Griffin JW. Toxic neuropathy in patients with pre-existing neuropathy. Neurology. 2003;60(2):337–340. doi: 10.1212/01.wnl.0000043691.53710.53 [DOI] [PubMed] [Google Scholar]
- 4.Kandula T, Park SB, Cohn RJ, Krishnan AV, Farrar MA. Pediatric chemotherapy induced peripheral neuropathy: A systematic review of current knowledge. Cancer Treat Rev. 2016;50:118–128. doi: 10.1016/j.ctrv.2016.09.005 [DOI] [PubMed] [Google Scholar]
- 5.Tofthagen C. Patient perceptions associated with chemotherapy-induced peripheral neuropathy. Clin J Oncol Nurs. 2010;14(3):E22–E28. doi: 10.1188/10.CJON.E22-E28 [DOI] [PubMed] [Google Scholar]
- 6.Bakitas MA. Background noise: the experience of chemotherapy-induced peripheral neuropathy. Nurs Res. 2007;56(5):323–331. doi: 10.1097/01.NNR.0000289503.22414.79 [DOI] [PubMed] [Google Scholar]
- 7.Winters-Stone KM, Horak F, Jacobs PG, et al. Falls, functioning, and disability among women with persistent symptoms of chemotherapy-induced peripheral neuropathy. J Clin Oncol. 2017:JCO2016713552. doi: 10.1200/JCO.2016.71.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richardson PG, Sonneveld P, Schuster MW, et al. Reversibility of symptomatic peripheral neuropathy with bortezomib in the phase III APEX trial in relapsed multiple myeloma: impact of a dose-modification guideline. Br J Haematol. 2009;144(6):895–903. doi: 10.1111/j.1365-2141.2008.07573.x [DOI] [PubMed] [Google Scholar]
- 9.Bhatnagar B, Gilmore S, Goloubeva O, et al. Chemotherapy dose reduction due to chemotherapy induced peripheral neuropathy in breast cancer patients receiving chemotherapy in the neoadjuvant or adjuvant settings: a single-center experience. Springerplus. 2014;3:366. doi: 10.1186/2193-1801-3-366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanabe Y, Hashimoto K, Shimizu C, et al. Paclitaxel-induced peripheral neuropathy in patients receiving adjuvant chemotherapy for breast cancer. Int J Clin Oncol. 2013;18(1):132–138. doi: 10.1007/s10147-011-0352-x [DOI] [PubMed] [Google Scholar]
- 11.Seretny M, Currie GL, Sena ES, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain. 2014;155(12):2461–2470. doi: 10.1016/j.pain.2014.09.020 [DOI] [PubMed] [Google Scholar]
- 12.Velasco R, Bruna J. [Chemotherapy-induced peripheral neuropathy: an unresolved issue]. Neurologia. 2010;25(2):116–131. [PubMed] [Google Scholar]
- 13.Cavaletti G, Marmiroli P. Chemotherapy-induced peripheral neurotoxicity. Nat Rev Neurol. 2010;6(12):657–666. doi: 10.1038/nrneurol.2010.160 [DOI] [PubMed] [Google Scholar]
- 14.Siegal T, Haim N. Cisplatin-induced peripheral neuropathy. Frequent off-therapy deterioration, demyelinating syndromes, and muscle cramps. Cancer. 1990;66(6):1117–1123. [DOI] [PubMed] [Google Scholar]
- 15.Haim N, Epelbaum R, Ben‐Shahar M, Yarnitsky D, Simri W, Robinson E. Full dose vincristine (without 2‐mg dose limit) in the treatment of lymphomas. Cancer. 1994;73(10):2515–2519. doi: [DOI] [PubMed] [Google Scholar]
- 16.Grisold A, Ackerl M, Surböck B, Giometto B, Grisold W. Multifocal neuropathy in vinorelbine treatment for breast cancer (P6.186). Neurology. 2017. Available from: http://www.neurology.org/content/88/16_Supplement/P6.186.short [Google Scholar]
- 17.Hilkens PH, Verweij J, Vecht CJ, Stoter G, van den Bent MJ. Clinical characteristics of severe peripheral neuropathy induced by docetaxel (Taxotere). Ann Oncol. 1997;8(2):187–190. doi: 10.1023/a:1008245400251 [DOI] [PubMed] [Google Scholar]
- 18.Verstappen CC, Koeppen S, Heimans JJ, et al. Dose-related vincristine-induced peripheral neuropathy with unexpected off-therapy worsening. Neurology. 2005;64(6):1076–1077. doi: 10.1212/01.WNL.0000154642.45474.28 [DOI] [PubMed] [Google Scholar]
- 19.Kerckhove N, Collin A, Condé S, Chaleteix C, Pezet D, Balayssac D. Risk factors of chemotherapy-induced peripheral neuropathies: a comprehensive literature review. Front Pharmacol. 2017;8:86. doi: 10.3389/fphar.2017.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaggi AS, Singh N. Mechanisms in cancer-chemotherapeutic drugs-induced peripheral neuropathy. Toxicology. 2012;291(1–3):1–9. doi: 10.1016/j.tox.2011.10.019 [DOI] [PubMed] [Google Scholar]
- 21.Siau C, Bennett GJ. Dysregulation of cellular calcium homeostasis in chemotherapy-evoked painful peripheral neuropathy. Anesth Analg. 2006;102(5):1485–1490. doi: 10.1213/01.ane.0000204318.35194.ed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouillot S, Martin-Negrier ML, Vital A, et al. Peripheral neuropathy associated with mitochondrial disorders: 8 cases and review of the literature. J Peripher Nerv Syst. 2002;7(4):213–220. [DOI] [PubMed] [Google Scholar]
- 23.Sun X, Windebank AJ. Calcium in suramin-induced rat sensory neuron toxicity in vitro. Brain Res. 1996;742(1–2):149–156. doi: 10.1016/s0006-8993(96)01003-7 [DOI] [PubMed] [Google Scholar]
- 24.Ling B, Authier N, Balayssac D, Eschalier A, Coudore F. Behavioral and pharmacological description of oxaliplatin-induced painful neuropathy in rat. Pain. 2007;128(3):225–234. doi: 10.1016/j.pain.2006.09.016 [DOI] [PubMed] [Google Scholar]
- 25.Kagiava A, Tsingotjidou A, Emmanouilides C, Theophilidis G. The effects of oxaliplatin, an anticancer drug, on potassium channels of the peripheral myelinated nerve fibers of the adult rat. Neurotoxicology. 2008;29(6):1100–1106. doi: 10.1016/j.neuro.2008.09.005 [DOI] [PubMed] [Google Scholar]
- 26.Ta LE, Bieber AJ, Carlton SM, Loprinzi CL, Low PA, Windebank AJ. Transient receptor potential vanilloid 1 is essential for cisplatin-induced heat hyperalgesia in mice. Mol Pain. 2010;6:15. doi: 10.1186/1744-8069-6-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siau C, Xiao W, Bennett GJ. Paclitaxel- and vincristine-evoked painful peripheral neuropathies: loss of epidermal innervation and activation of Langerhans cells. Exp Neurol. 2006;201(2):507–514. doi: 10.1016/j.expneurol.2006.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joseph EK, Chen X, Bogen O, Levine JD. Oxaliplatin acts on IB4-positive nociceptors to induce an oxidative stress-dependent acute painful peripheral neuropathy. J Pain. 2008;9(5):463–472. doi: 10.1016/j.jpain.2008.01.335 [DOI] [PubMed] [Google Scholar]
- 29.Scuteri A, Galimberti A, Maggioni D, et al. Role of MAPKs in platinum-induced neuronal apoptosis. Neurotoxicology. 2009;30(2):312–319. doi: 10.1016/j.neuro.2009.01.003 [DOI] [PubMed] [Google Scholar]
- 30.Flatters SJ, Bennett GJ. Ethosuximide reverses paclitaxel- and vincristine-induced painful peripheral neuropathy. Pain. 2004;109(1–2):150–161. doi: 10.1016/j.pain.2004.01.029 [DOI] [PubMed] [Google Scholar]
- 31.Jamieson SM, Liu JJ, Connor B, Dragunow M, McKeage MJ. Nucleolar enlargement, nuclear eccentricity and altered cell body immunostaining characteristics of large-sized sensory neurons following treatment of rats with paclitaxel. Neurotoxicology. 2007;28(6):1092–1098. doi: 10.1016/j.neuro.2007.04.009 [DOI] [PubMed] [Google Scholar]
- 32.Mihara Y, Egashira N, Sada H, et al. Involvement of spinal NR2B-containing NMDA receptors in oxaliplatin-induced mechanical allodynia in rats. Mol Pain. 2011;7:8. doi: 10.1186/1744-8069-7-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hansen N, Uçeyler N, Palm F, et al. Serotonin transporter deficiency protects mice from mechanical allodynia and heat hyperalgesia in vincristine neuropathy. Neurosci Lett. 2011;495(2):93–97. doi: 10.1016/j.neulet.2011.03.035 [DOI] [PubMed] [Google Scholar]
- 34.Galeotti N, Vivoli E, Bilia AR, Vincieri FF, Ghelardini C. St. John’s Wort reduces neuropathic pain through a hypericin-mediated inhibition of the protein kinase Cgamma and epsilon activity. Biochem Pharmacol. 2010;79(9):1327–1336. doi: 10.1016/j.bcp.2009.12.016 [DOI] [PubMed] [Google Scholar]
- 35.Joseph EK, Levine JD. Caspase signaling in neuropathic and inflammatory pain in the rat. Eur J Neurosci. 2004;20(11):2896–2902. doi: 10.1111/j.1460-9568.2004.03750.x [DOI] [PubMed] [Google Scholar]
- 36.Kiya T, Kawamata T, Namiki A, Yamakage M. Role of satellite cell-derived L-serine in the dorsal root ganglion in paclitaxel-induced painful peripheral neuropathy. Neuroscience. 2011;174:190–199. doi: 10.1016/j.neuroscience.2010.11.046 [DOI] [PubMed] [Google Scholar]
- 37.Koskinen MJ, Kautio A-L, Haanpää ML, et al. Intraepidermal nerve fibre density in cancer patients receiving adjuvant chemotherapy. Anticancer Res. 2011;31(12):4413–4416. [PubMed] [Google Scholar]
- 38.Krøigård T, Schrøder HD, Qvortrup C, et al. Characterization and diagnostic evaluation of chronic polyneuropathies induced by oxaliplatin and docetaxel comparing skin biopsy to quantitative sensory testing and nerve conduction studies. Eur J Neurol. 2014;21(4):623–629. doi: 10.1111/ene.12353 [DOI] [PubMed] [Google Scholar]
- 39.Boyette-Davis JA, Cata JP, Driver LC, et al. Persistent chemoneuropathy in patients receiving the plant alkaloids paclitaxel and vincristine. Cancer Chemother Pharmacol. 2013;71(3):619–626. doi: 10.1007/s00280-012-2047-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaudhry V, Cornblath DR, Polydefkis M, Ferguson A, Borrello I. Characteristics of bortezomib- and thalidomide-induced peripheral neuropathy. J Peripher Nerv Syst. 2008;13(4):275–282. doi: 10.1111/j.1529-8027.2008.00193.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richardson PG, Xie W, Mitsiades C, et al. Single-agent bortezomib in previously untreated multiple myeloma: efficacy, characterization of peripheral neuropathy, and molecular correlations with response and neuropathy. J Clin Oncol. 2009;27(21):3518–3525. doi: 10.1200/JCO.2008.18.3087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rao RD, Michalak JC, Sloan JA, et al. Efficacy of gabapentin in the management of chemotherapy-induced peripheral neuropathy: a phase 3 randomized, double-blind, placebo-controlled, crossover trial (N00C3). Cancer. 2007;110(9):2110–2118. doi: 10.1002/cncr.23008 [DOI] [PubMed] [Google Scholar]
- 43.Rao RD, Flynn PJ, Sloan JA, et al. Efficacy of lamotrigine in the management of chemotherapy-induced peripheral neuropathy: a phase 3 randomized, double-blind, placebo-controlled trial, N01C3. Cancer. 2008;112(12):2802–2808. doi: 10.1002/cncr.23482 [DOI] [PubMed] [Google Scholar]
- 44.Hammack JE, Michalak JC, Loprinzi CL, et al. Phase III evaluation of nortriptyline for alleviation of symptoms of cis-platinum-induced peripheral neuropathy. Pain. 2002;98(1–2):195–203. [DOI] [PubMed] [Google Scholar]
- 45.Kautio AL, Haanpää M, Saarto T, Kalso E. Amitriptyline in the treatment of chemotherapy-induced neuropathic symptoms. J Pain Symptom Manage. 2008;35(1):31–39. doi: 10.1016/j.jpainsymman.2007.02.043 [DOI] [PubMed] [Google Scholar]
- 46.Smith EML, Pang H, Cirrincione C, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy a randomized clinical trial. JAMA. 2013;309(13):1359–1367. doi: 10.1001/jama.2013.2813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirayama Y, Ishitani K, Sato Y, et al. Effect of duloxetine in Japanese patients with chemotherapy-induced peripheral neuropathy: a pilot randomized trial. Int J Clin Oncol. 2015;20(5):866–871. doi: 10.1007/s10147-015-0810-y [DOI] [PubMed] [Google Scholar]
- 48.Gewandter JS, Mohile SG, Heckler CE, et al. A phase III randomized, placebo-controlled study of topical amitriptyline and ketamine for chemotherapy-induced peripheral neuropathy (CIPN): a University of Rochester CCOP study of 462 cancer survivors. Support Care Cancer. 2014;22(7):1807–1814. doi: 10.1007/s00520-014-2158-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barton DL, Wos EJ, Qin R, et al. A double-blind, placebo-controlled trial of a topical treatment for chemotherapy-induced peripheral neuropathy: NCCTG trial N06CA. Support Care Cancer. 2011;19(6):833–841. doi: 10.1007/s00520-010-0911-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lynch ME, Cesar-Rittenberg P, Hohmann AG, Double-Blind A. Placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain. J Pain Symptom Manage. 2014;47(1):166–173. doi: 10.1016/j.jpainsymman.2013.02.018 [DOI] [PubMed] [Google Scholar]
- 51.Gewandter JS, Dworkin RH, Finnerup NB, Mohile NA. Painful chemotherapy-induced peripheral neuropathy: lack of treatment efficacy or the wrong clinical trial methodology? Pain. 2017;158(1):30–33. doi: 10.1097/j.pain.0000000000000653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Webster LR, Nunez M, Tark MD, et al. Tolerability of NGX-4010, a capsaicin 8% dermal patch, following pretreatment with lidocaine 2.5%/prilocaine 2.5% cream in patients with post-herpetic neuralgia. BMC Anesthesiol. 2011;11:25. doi: 10.1186/1471-2253-11-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Webster LR, Peppin JF, Murphy FT, Tobias JK, Vanhove GF. Tolerability of NGX-4010, a capsaicin 8% patch, in conjunction with three topical anesthetic formulations for the treatment of neuropathic pain. J Pain Res. 2012;5:7–13. doi: 10.2147/JPR.S25272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Webster LR, Peppin JF, Murphy FT, Lu B, Tobias JK, Vanhove GF. Efficacy, safety, and tolerability of NGX-4010, capsaicin 8% patch, in an open-label study of patients with peripheral neuropathic pain. Diabetes Res Clin Pract. 2011;93(2):187–197. doi: 10.1016/j.diabres.2011.04.010 [DOI] [PubMed] [Google Scholar]
- 55.Webster LR, Malan TP, Tuchman MM, Mollen MD, Tobias JK, Vanhove GF. A multicenter, randomized, double-blind, controlled dose finding study of NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia. J Pain. 2010;11(10):972–982. doi: 10.1016/j.jpain.2010.01.270 [DOI] [PubMed] [Google Scholar]
- 56.Simpson DM, Brown S, Tobias J, NGX-4010 C107 Study Group. Controlled trial of high-concentration capsaicin patch for treatment of painful HIV neuropathy. Neurology. 2008;70(24):2305–2313. doi: 10.1212/01.wnl.0000314647.35825.9c [DOI] [PubMed] [Google Scholar]
- 57.Brown S, Simpson DM, Moyle G, et al. NGX-4010, a capsaicin 8% patch, for the treatment of painful HIV-associated distal sensory polyneuropathy: integrated analysis of two phase III, randomized, controlled trials. AIDS Res Ther. 2013;10(1):5. doi: 10.1186/1742-6405-10-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Irving GA, Backonja MM, Dunteman E, et al. A multicenter, randomized, double-blind, controlled study of NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia. Pain Med. 2011;12(1):99–109. doi: 10.1111/j.1526-4637.2010.01004.x [DOI] [PubMed] [Google Scholar]
- 59.Clifford DB, Simpson DM, Brown S, et al. A randomized, double-blind, controlled study of NGX-4010, a capsaicin 8% dermal patch, for the treatment of painful HIV-associated distal sensory polyneuropathy. J Acquir Immune Defic Syndr. 2012;59(2):126–133. doi: 10.1097/QAI.0b013e31823e31f7 [DOI] [PubMed] [Google Scholar]
- 60.Wagner T, Poole C, Roth-Daniek A. The capsaicin 8% patch for neuropathic pain in clinical practice: a retrospective analysis. Pain Med. 2013;14(8):1202–1211. doi: 10.1111/pme.12143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Backonja MM, Malan TP, Vanhove GF, Tobias JK, C102/106 Study Group. NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia: a randomized, double-blind, controlled study with an open-label extension. Pain Med. 2010;11(4):600–608. doi: 10.1111/j.1526-4637.2009.00793.x [DOI] [PubMed] [Google Scholar]
- 62.Simpson DM, Gazda S, Brown S, et al. Long-term safety of NGX-4010, a high-concentration capsaicin patch, in patients with peripheral neuropathic pain. J Pain Symptom Manage. 2010;39(6):1053–1064. doi: 10.1016/j.jpainsymman.2009.11.316 [DOI] [PubMed] [Google Scholar]
- 63.Maihofner C, Heskamp M-L. Prospective, non-interventional study on the tolerability and analgesic effectiveness over 12 weeks after a single application of capsaicin 8% cutaneous patch in 1044 patients with peripheral neuropathic pain: first results of the QUEPP study. Curr Med Res Opin. 2013;29(6):673–683. doi: 10.1185/03007995.2013.792246 [DOI] [PubMed] [Google Scholar]
- 64.Maihöfner CG, Heskamp M-LS. Treatment of peripheral neuropathic pain by topical capsaicin: impact of pre-existing pain in the QUEPP-study. Eur J Pain. 2014;18(5):671–679. doi: 10.1002/j.1532-2149.2013.00415.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martini C, Yassen A, Olofsen E, Passier P, Stoker M, Dahan A. Pharmacodynamic analysis of the analgesic effect of capsaicin 8% patch (QutenzaTM) in diabetic neuropathic pain patients: detection of distinct response groups. J Pain Res. 2012;5:51–59. doi: 10.2147/JPR.S30406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anand P, Bley K. Topical capsaicin for pain management: therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br J Anaesth. 2011;107(4):490–502. doi: 10.1093/bja/aer260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Privitera R, Sinisi M, Mihaylov D, Leech R, Anand P. Capsaicin 8% patch treatment for amputation stump and phantom limb pain: a clinical and functional MRI study. J Pain Res. 2017;10:1623–1634. doi: 10.2147/JPR.S140925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30(2):191–197. [DOI] [PubMed] [Google Scholar]
- 69.Bril V. NIS-LL: the primary measurement scale for clinical trial endpoints in diabetic peripheral neuropathy. Eur Neurol. 1999;41(Suppl 1):8–13. doi: 10.1159/000052074 [DOI] [PubMed] [Google Scholar]
- 70.Atherton DD, Facer P, Roberts KM, et al. Use of the novel Contact Heat Evoked Potential Stimulator (CHEPS) for the assessment of small fiber neuropathy: correlations with skin flare responses and intra-epidermal nerve fiber counts. BMC Neurol. 2007;7:21. doi: 10.1186/1471-2377-7-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Coppini DV, Wellmer A, Weng C, Young PJ, Anand P, Sonksen PH. The natural history of diabetic peripheral neuropathy determined by a 12-year prospective study using vibration perception thresholds. J Clin Neurosci. 2001;8(6):520–524. doi: 10.1054/jocn.2001.0893 [DOI] [PubMed] [Google Scholar]
- 72.Wellmer A, Misra VP, Sharief MK, Kopelman PG, Anand P. A double-blind placebo-controlled clinical trial of recombinant human brain-derived neurotrophic factor (rhBDNF) in diabetic polyneuropathy. J Peripher Nerv Syst. 2001;6(4):204–210. [DOI] [PubMed] [Google Scholar]
- 73.Anand P, Terenghi G, Warner G, Kopelman P, Williams-Chestnut RE, Sinicropi DV. The role of endogenous nerve growth factor in human diabetic neuropathy. Nat Med. 1996;2(6):703–707. doi: 10.1038/nm0696-703 [DOI] [PubMed] [Google Scholar]
- 74.Ragé M, Van Acker N, Facer P, et al. The time course of CO2 laser-evoked responses and of skin nerve fiber markers after topical capsaicin in human volunteers. Clin Neurophysiol. 2010;121(8):1256–1266. doi: 10.1016/j.clinph.2010.02.159 [DOI] [PubMed] [Google Scholar]
- 75.Gopinath P, Wan E, Holdcroft A, et al. Increased capsaicin receptor TRPV1 in skin nerve fibers and related vanilloid receptors TRPV3 and TRPV4 in keratinocytes in human breast pain. BMC Womens Health. 2005;5(1):2. doi: 10.1186/1472-6874-5-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Facer P, Mathur R, Pandya SS, Ladiwala U, Singhal BS, Anand P. Correlation of quantitative tests of nerve and target organ dysfunction with skin immunohistology in leprosy. Brain. 1998;121(Pt 12):2239–2247. doi: 10.1093/brain/121.12.2239 [DOI] [PubMed] [Google Scholar]
- 77.Narayanaswamy H, Facer P, Misra VP, et al. A longitudinal study of sensory biomarkers of progression in patients with diabetic peripheral neuropathy using skin biopsies. J Clin Neurosci. 2012;19(11):1490–1496. doi: 10.1016/j.jocn.2011.12.026 [DOI] [PubMed] [Google Scholar]
- 78.Anand P, Terenghi G, Birch R, et al. Endogenous NGF and CNTF levels in human peripheral nerve injury. Neuroreport. 1997;8(8):1935–1938. doi: 10.1097/00001756-199705260-00028 [DOI] [PubMed] [Google Scholar]
- 79.Anand P. Neurotrophic factors and their receptors in human sensory neuropathies. Prog Brain Res. 2004;146:477–492. doi: 10.1016/S0079-6123(03)46030-5 [DOI] [PubMed] [Google Scholar]
- 80.Yiangou Y, Facer P, Sinicropi DV, et al. Molecular forms of NGF in human and rat neuropathic tissues: decreased NGF precursor-like immunoreactivity in human diabetic skin. J Peripher Nerv Syst. 2002;7(3):190–197. [DOI] [PubMed] [Google Scholar]
- 81.Filipczak-Bryniarska I, Krzyzewski RM, Kucharz J, et al. High-dose 8% capsaicin patch in treatment of chemotherapy-induced peripheral neuropathy: single-center experience. Med Oncol. 2017;34(9):162. doi: 10.1007/s12032-017-1015-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Le Marec C, Berard J, Queneuille I, et al. Improvement of chemotherapy induced neuropathy (CIN) in cancer patients using capsaicin 8% patch. J Clin Oncol. 2017;34(15_suppl). doi: 10.1200/JCO.2016.34.15_suppl.e14031 [DOI] [Google Scholar]
- 83.Anand P. Nerve growth factor regulates nociception in human health and disease. Br J Anaesth. 1995;75(2):201–208. doi: 10.1093/bja/75.2.201 [DOI] [PubMed] [Google Scholar]
- 84.Kennedy AJ, Wellmer A, Facer P, et al. Neurotrophin-3 is increased in skin in human diabetic neuropathy. J Neurol Neurosurg Psychiatry. 1998;65:393–395. doi: 10.1136/jnnp.65.3.393 [DOI] [PMC free article] [PubMed] [Google Scholar]