Abstract
Several large epidemiological and animal studies demonstrate a direct correlation between dietary heme iron intake and/or systemic iron levels and cancer risk in several cancers including colorectal cancer (CRC). However, the precise mechanisms for how heme iron contributes to CRC and how cancer cells respond to heme iron-induced stress are still unclear. Previously we have shown that one of the stress-inducible proteins, Sestrin2 (SESN2), is a novel tumor suppressor in colon by limiting endoplasmic reticulum stress and mammalian target of rapamycin complex 1 (mTORC1) signaling and tumor growth. But the relationship between heme iron and SESN2, especially in the context of colon carcinogenesis, was not investigated previously. Here, we found that hemin dose-dependently increased SESN2 expression in an oxidative stress and nuclear factor (erythroid-derived 2)-like 2 (NFE2L2, NRF2)-dependent manner. Since SESN2 overexpression reduced hemin-induced oxidative stress, SESN2 could be an important target of NRF2 exerting antioxidant function. Indeed, expression of several oxidative stress responsive proteins such as NRF2 and its target genes was reduced by SESN2. Although we formerly reported that SESN2 expression was reduced after p53 mutation in colon tumors, mouse colon tumors, which have intact p53 and NRF2, induced SESN2 expression in response to iron stimulus. Although SESN2 overexpression decreased murine colon tumor cell growth both in vitro and in vivo, it rendered colon cancer cells more resistant to hemin-induced apoptosis and therefore promoted tumor growth during hemin treatment. Taken together, although SESN2 generally suppresses tumorigenesis, it can produce tumor-promoting role in iron-rich environment by suppressing oxidative stress-associated cancer cell death.
Keywords: Sestrin2, Hemin, Oxidative stress, Colorectal cancer, Nuclear factor (erythroid-derived 2)-like 2
1. Introduction
Colorectal cancer (CRC) is the second most prevalent cancer and the second leading cause of cancer-related deaths when men and women are combined in the United States (Bray et al., 2018). Understanding the mechanisms of growth and progression of CRC is essential to improve treatment. Consumption of red meat, which is enriched with heme, is associated with significant risk of developing CRC (Sandhu et al., 2001; Larsson and Wolk, 2006). Heme may explain the relationship between colon cancer and red meat (eg. beef, pork, lamb) intake since no link between white meat (e.g. fish, chicken) intake and CRC has been found in either men or women (Sesink et al., 1999; Willett et al., 1990; Giovannucci et al., 1994). Furthermore, heme iron is sufficient to promote colon tumorigenesis (Bastide et al., 2015). Dietary heme is predominantly from hemoglobin and myoglobin in meats and can be directly absorbed in the small intestine (Oates and West, 2006; West and Oates, 2008). Heme carrier protein-1 (HCP1) was proposed to be responsible for the intestinal heme absorption, though later studies indicate that it might not be a physiologically relevant heme transporter (West and Oates, 2008). At the cellular level, endocytosis of hemoproteins linked with heme-responsive gene-1 (HRG1) imports extracellular free heme into colonic epithelia (Rajagopal et al., 2008; Gulec et al., 2014; Shayeghi et al., 2005; White et al., 2013).
Heme (ferroprotoporphyrin, Fe2+) is a tetrapyrrole ring containing an atom of ferrous iron in its center, whereas hemin (ferriprotoporphyrin, ferric heme, Fe3+ ) contains a ferric iron, which can be further oxidized yielding ferryl species (Kagan et al., 2001; Itoh et al., 2001). Administration of hemin to macrophages leads to intracellular accumulation of ferrous heme (Mullebner et al., 2017). Heme-containing enzymes are critical for many biological processes such as mitochondrial respiration (e.g. cytochrome C), oxygen transport and storage (e.g. hemoglobin and myoglobin), detoxification (e.g. cytochrome P450), antioxidant defenses (e.g. catalase, peroxidase), and signal transduction processes (e.g. nitric oxide synthase) (Nagababu and Rifkind, 2004; Ryter and Tyrrell, 2000; Shimizu, 2012). Hemin is genotoxic and cytotoxic to human colon cells (Glei et al., 2006; Gemelli et al., 2014).
Our previous study has also shown that the major intestinal iron uptake transporter divalent metal transporter (DMT) 1 is highly increased in colon tumors by hypoxia inducible factor-2α (Xue et al., 2012). Moreover, pharmacological inhibition or genetic disruption of colonic DMT1 decreases colon tumorigenesis via iron/cyclin-dependent kinase 1/Janus kinas 1/signal transducer and activator of transcription 3 signaling pathway (Xue et al., 2016). Mitochondrial iron accumulation, oxidative stress and colon tumorigenesis are increased by intestine-specific overexpression of six-transmembrane epithelial antigen of prostate 4 (STEAP4), a ferrireductase which is highly increased in CRC. In contrast, STEAP4-enhanced colon tumorigenesis is reduced when deferiprone was used to chelate mitochondrial iron in mice (Xue et al., 2017). These studies suggest that iron homeostasis is critical for colon carcinogenesis. However, the response of cancer cells to heme iron stress is not fully understood.
A recent study showed that heme and iron induced a cellular protein aggregation in response to oxidative stress in macrophage (Vasconcellos et al., 2016). Cellular stress response, which is a defensive reaction of cells to environmental damage, plays a critical role in redox sensing and regulation, DNA damage repair and cell cycle control (Kultz, 2005). Sestrins (SESNs) are a family of proteins that are highly conserved and are upregulated in cells under oxidative stress and metabolic restriction (Seo et al., 2015; Hwang et al., 2018; Byun et al., 2017). SESN proteins consist of three distinct family members, SESN1, SESN2, and SESN3 (Pasha et al., 2017). SESN1 (also named as p53 activated gene 26, PA26) was first identified as a novel target gene of p53 induced by genotoxic stress and has the property of the growth arrest DNA damage (GADD) gene family (Velasco-Miguel et al., 1999). SESN2 (also named as hypoxia induced gene #95, Hi95) was discovered to be induced by prolonged hypoxia, oxidative stress or DNA damage (Budanov et al., 2002). In contrast, SESN3 is regulated by RAS GTPase-activating protein and forkhead box protein O but not p53 to modulate intracellular reactive oxygen species (ROS) levels (Kopnin et al., 2007; Nogueira et al., 2008). During oxidative stress, SESNs suppress oxidative stress through various mechanisms (Ho et al., 2016), including direct oxidoreductase activity (Kim et al., 2015), autophagic degradation of kelch-like ECH-associated protein1 (KEAP1) and activation of NRF2 (Bae et al., 2013; Rhee and Bae, 2015; Ro et al., 2014) and dysfunctional mitochondria (Lee et al., 2010). Previously, we and others have shown that loss of SESN2 promotes colon tumor growth through increased endoplasmic reticulum stress and p53-mediated control over mTORC1 signaling (Ro et al., 2016; Budanov and Karin, 2008; Peng et al., 2014). Here, we tested the hypothesize that hemin can activate SESN2 by increasing ROS and NRF2 in CRC. Also, the interaction of hemin and SESN2 on colon tumor development was assessed in vitro and in vivo.
2. Materials and methods
2.1. Cell culture
Human HCT116, RKO, and murine MC38 CRC cells were maintained at 37 °C in 5% CO2 and cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin (VWR, Radnor, PA).
2.2. Animals
All mice were maintained in a standard cage in light and temperature-controlled room and were allowed standard chow and water excepted as indicated. Animal studies were performed in accordance of the Institute of Laboratory Animal Resources guidelines and approved by the Institutional Animal Care and Use Committee (IACUC) at the University of New Mexico Health Sciences Center (Protocol# HSC-18–200699) and followed the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978). A colon-specific tumor model driven by the tamoxifen-inducible caudal type homeobox 2 (CDX2) ERT2-Cre promoter and monoallelic flox of the frequently mutated adenomatous polyposis coli (APC) gene in CRC patients (CDX2ERT2ApcF/+ ), were utilized and treated as previously described (Xue et al., 2016). To be noted, the p53 gene is wildtype in this model. Briefly, CDX2ERT2ApcF/+ mice (n = 18) were treated with 100 mg/kg tamoxifen for 3 consecutive days, and then one day later were given AIN93G diet containing 1000 mg/kg of iron (1000Fe, high-iron diet) or iron sufficient AIN93G diet containing 35 mg/kg of iron (35Fe, control-iron diet, Dyets, Bethlehem, PA). Two days after the initiation of iron diet treatment, these mice were treated with 1.5% dextran sulfate sodium (DSS) for 7 days (inflammatory phase). Thereafter mice were placed on regular drinking water for 14 days (recovery phase), and one more inflammatory and recovery cycles were performed to establish the CRC model. CDX2 ApcF/+ mice (n = 14), in which colon tumors are developed spontaneously with aging, were sacrificed at 3 months old. For subcutaneous xenograft study, to test the role of SESN2 under immuneintact condition, parental MC38 and MC38 syngeneic cells with SESN2 overexpression were treated with or without 100 μM hemin (Sigma, St Louis, MO) overnight, trypsinized and resuspended in sterile 1× phosphate-buffered saline (PBS). Cells were counted and diluted with 1× PBS to a concentration 1 × 107cells/mL, and then 100 μl containing 1 × 106 total cells were injected into the flanks of C57BL/6 mice (n = 13). Two weeks later, mice were sacrificed and tumors were collected. Both male and female animals were used in this study and no influence of sex on the results of the study was observed.
2.3. Western blot analysis
Cells or tumor tissues were lysed with radioimmunoprecipitation assay buffer and incubated on ice for 30 min. After incubation, cell extracts were centrifuged for 10 min at 4 °C. The supernatant was collected for Bradford assay to quantify protein concentration. Equal amounts (30 μg-50 μg) of protein were loaded for sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), along with molecular weight marker. The gels were run for 1 h 30 min at 120 V. The proteins inside the gels were transferred onto nitrocellulose membrane for 1 h at 18 V using semi-dry transfer method. The membranes were blocked with 3% milk for 1 h. Primary antibodies against SESN2, Flag, ferritin heavy chain (FTH1), nuclear factor-like 2 (NRF2), cleaved caspase 3 (cCasp3), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Cell Signaling Technology, Danvers, MA), green fluorescence protein (GFP), NAD(P)H Quinone Dehydrogenase 1 (NQO1), heme oxygenase-1 (HO-1), proliferating cell nuclear antigen (PCNA) and cyclin D1 (CCND1) (Santa Cruz Biotechnology, Dallas, TX) were used. Secondary antibodies against Rabbit (Cell signaling Technology, Danvers, MA) and Mouse IgG (Cell signaling Technology, Danvers, MA) were used.
2.4. 2′,7′-Dichlorofluorescein diacetate (DCFDA) staining
Cells (2 × 105 cells/well) were plated into 24 well plates. After 24 h, drugs were added at indicated concentrations. After 24 h, the cells were washed one time with DMEM. Pre-warmed DMEM containing 10uM DCFDA (Cayman Chemical Company, Ann Arbor, MI) was added and the cells were incubated at 37 °C for 30 min. The cells were washed once with DMEM and twice with 1× PBS. Representative images were taken using the GFP channel of an Invitrogen™ EVOS™ FL Auto Imaging System (Thermo Fisher Scientific). Fluorescence intensity was quantified using a SpectraMax M2 Microplate Reader (Molecular Devices, Radnor, PA) at excitation 485 nm and emission 530 nm. The intensity was normalized with protein concentration.
2.5. Transfection
For transient overexpression, pLU-CMV-Flag-hSESN2 WT or empty plasmids were transfected into HCT116 and RKO using polyethylenimine (PEI). Experiments were carried out 24 hours post-transfection. For stable overexpression, MC38 cells were transfected with pLC242-GFP-SESN2 plasmid (#100519, Addgene) and selected with 1 μg/ml blasticidin.
2.6. Quantitative polymerase chain reaction (qPCR) analysis
Total RNA was extracted using IBI Isolate DNA/RNA Reagent Kit (IB47602, IBI Scientific, Dubuque, IA). qPCR was performed using a LightCycler 480 instrument (Roche Diagnostics, Indianapolis, IN). The following pre-designed primers were used: mouse Sesn2 forward TCC GAGTGCCATTCCGAGAT, mouse Sesn2 reverse TCCGGGTGTAGACCC ATCAC, 18S forward GTAACCCGTTGAACCCCATT and 18S reverse CCATCCAATCGGTAGTAGCG.
2.7. Thiazolyl blue tetrazolium bromide (MTT) assay
Cells were plated at a concentration of 5 × 104 cells/mL in a 24 well plates. 125 μL 5 mg/mL MTT (Sigma, MO) was added to each plate and incubated for 30 min. Dimethyl sulfoxide (DMSO) was added and absorbance was measured at 570 nm using a SpectraMax M2 Microplate Reader (Molecular Devices, Molecular Devices, Radnor, PA).
2.8. Statistical analysis
Data were expressed as mean ± SD. p values were calculated by independent t-test, one-way and two-way analysis of variance (ANOVA). p < .05 was considered significant.
3. Results
3.1. SESN2 is dose-dependently increased by hemin in CRC cells
To test the effect of hemin on SESN2 expression, HCT116 and RKO CRC cells were treated with different physiologically relevant concentrations of hemin (0–100 μM) (Goldstein et al., 2003) (Fig. 1). The initial reason we chose these two cell lines is that both of these cell lines express wild-type p53 and therefore the basal level of SESN2 expression is detectable (Ro et al., 2016). The doses chosen for hemin are based on a recent report that male 4-week-old F344 rats fed with a 1% hemoglobin diet for 100 days resulted in 200–300 μM heme in rat fecal water (Bastide et al., 2015). Hemin can be catabolized into equimolar amounts of iron, carbon monoxide and biliverdin by the enzyme HO-1 (Abraham and Kappas, 2008; Gozzelino et al., 2010). Carbon monoxide and biliverdin are cytoprotective, but iron can be toxic due to generation of ROS through Fenton reaction. Thus, the ferroxidase and iron storage protein FTH1 is induced to bind and oxidize iron to avoid the detrimental effect. As expected, FTH1 was increased by hemin in both HCT116 and RKO cell lines (Fig. 1A and C). Interestingly, SESN2 was also dose-dependently increased by hemin (Fig. 1A and B). SESN2 is induced in ulcerative colitis but is downregulated in CRC in a p53-dependent manner (Ro et al., 2016). However, hemin can lead to the nuclear export and cytosolic degradation of p53 (Shen et al., 2014). Since hemin can suppress p53 activity, SESN2 induction in HCT116 and RKO cell lines should be through a mechanism that is independent of p53.
Fig. 1.
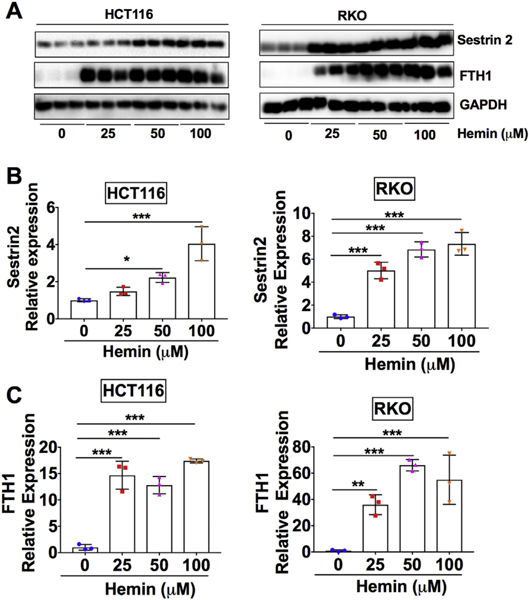
SESN2 is dose-dependently increased by hemin in CRC cells.
(A) Western blot analysis and (B and C) quantification in HCT116 and RKO CRC cells. Cells were treated with different doses of hemin as indicated for overnight. *p < .05, **p < .01 and ***p < .001.
3.2. Hemin-induced SESN2 is dependent on oxidative stress and NRF2 in CRC cells
We searched for a possible mechanism of how SESN2 is induced upon hemin challenge. Previous reports showed that SESN2 was highly upregulated by H2O2 in different cell lines (Liu et al., 2017; Ishihara et al., 2013). Consistently, we found here that SESN2 was dose-dependently upregulated by H2O2 in both HCT116 and RKO CRC cells (Fig. S1A and S1B). Heme is known to induce ROS through the activation of nicotinamide adenine dinucleotide phosphate oxidase or via the mitochondria (Barcellosde-Souza et al., 2013; Hasan and Schafer, 2008; Dutra et al., 2014). To assess whether hemin can indeed increase ROS generation, DCFDA staining was performed. DCFDA is a cell-permeable fluorogenic probe to quantify intracellular ROS (Owusu-Ansah et al., 2008). It is rapidly oxidized by ROS species such as nitric oxide and H2O2 in cells to form fluorescent 2′,7′-dichlorofluorescein (Gabriel et al., 1997). We found that hemin dose-dependently increased green fluorescence indicating increased oxidative stress levels in both cell lines (Fig. S2A and S2B). To further determine whether hemin-induced SESN2 is dependent on oxidative stress, N-Acetyl Cysteine (NAC) was utilized. NAC is a widely used thiol-containing antioxidant and generates reduced glutathione (Aldini et al., 2018). It interacts with OH of H2O2, thus decreasing ROS levels. Previously, NAC inhibited hemin-induced ROS surge and cellular stress response in vascular smooth muscle cells (Hasan and Schafer, 2008). To confirm that hemin-induced ROS levels were decreased by NAC, DCFDA staining was performed. NAC and hemin co-treatment generated less green fluorescence intensity compared to hemin-only treatment (Fig. 2A and B). At the same time, we found that NAC significantly reduced hemin-increased SESN2 expression in both HCT116 and RKO cells (Fig. 2C and D).
Fig. 2.
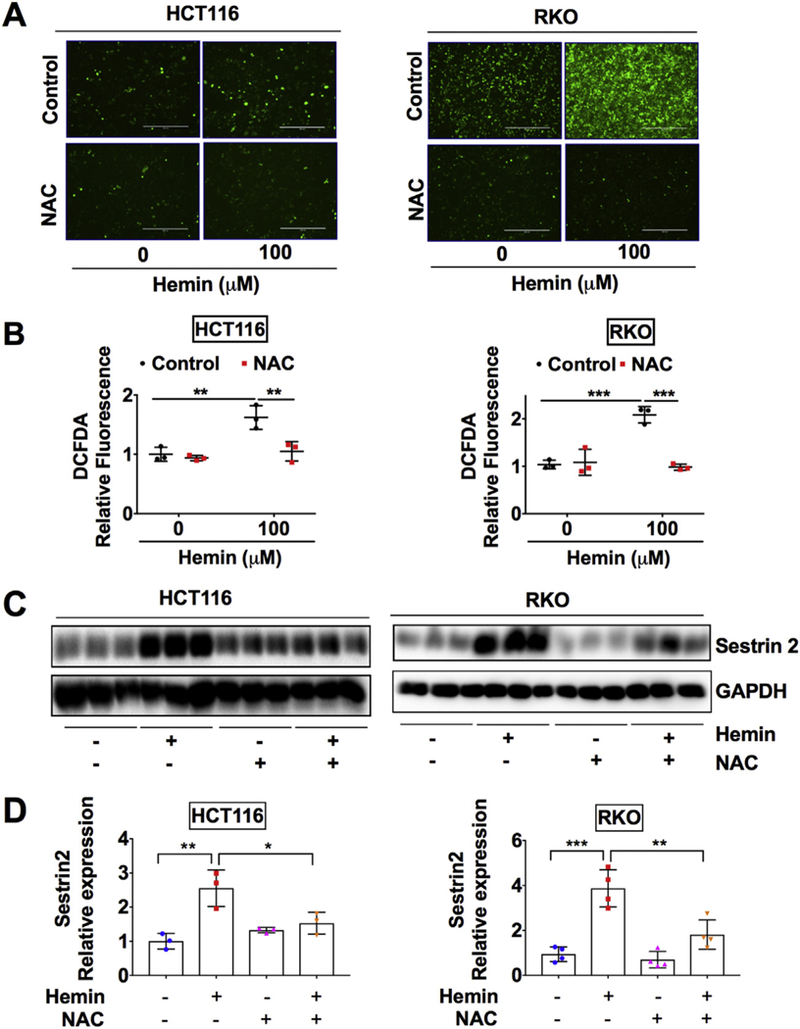
Hemin induced oxidative stress and SESN2 can be reduced by NAC in CRC cells.
(A) Representative green fluorescence images, (B) quantification of DCFDA staining, (C) Western blot analysis and (D) quantification in HCT116 and RKO CRC cells. Cells were treated with 100 μM hemin or 1 mM NAC as indicated for overnight. *p < .05, **p < .01 and ***p < .001.
It is well established that the increase of ROS production is accompanied with the induction of a large number of cellular antioxidant genes via transcriptional activation of the antioxidant response elements (ARE) in their promoters (Nguyen et al., 2009). NRF2 is a master transcriptional regulator that binds with ARE and activates antioxidant gene expression (Pall and Levine, 2015). Previously, it has been shown that NRF2-ARE pathway induces SESN2 (Shin et al., 2012). Here we found that ML-385, a NRF2 specific inhibitor that binds to the NRF2 DNA binding domain and interferes its transcriptional activity (Singh et al., 2016), abolished hemin-induced SESN2 expression (Fig. 3A and B). These results indicate that hemin induces SESN2 through inducing oxidative stress and subsequent activation of NRF2 transcription factor.
Fig. 3.
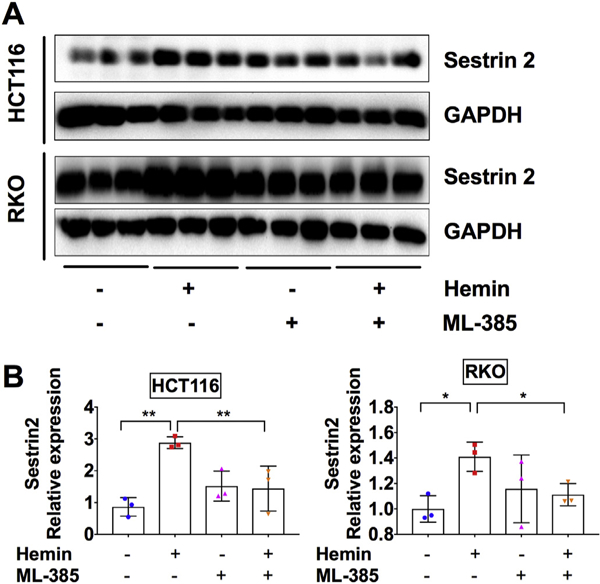
SESN2 induction by hemin is dependent on NRF2 activity in CRC cells.
(A) Western blot analysis and (B) quantification in HCT116 and RKO CRC cells. Cells were treated with 100 μM hemin or 5 μM NRF2 inhibitor ML385 as indicated for overnight. *p < .05 and **p < .01.
3.3. SESN2 overexpression reduces hemin-induced oxidative stress and the expression of oxidative stress responsive proteins
To understand the role of SESN2 induction during hemin-induced oxidative stress, SESN2 was overexpressed in both HCT116 and RKO cells. Given that SESN2 has antioxidant properties (Byun et al., 2017) and overexpression of SESN2 inhibited glucose deprivation-induced ROS production (Seo et al., 2015), DCFDA staining was also applied. Consistently, hemin increased the DCFDA fluorescence intensity in RKO cells, which was dramatically decreased by SESN2 overexpression in RKO cells (Fig. 4A and B). Thus, SESN2 can reduce hemin-induced oxidative stress. As expected, the expression of SESN2 was dramatically increased by the SESN2 plasmid (Fig. 4C). Consistent with a previous report (Vasconcellos et al., 2016), we found that NRF2 was increased by hemin. However, hemin-induced NRF2 expression was repressed by SESN2 overexpression (Fig. 4C and Fig. S3A). Similarly, the expression of two NRF2 targets and antioxidant proteins, NQO1 and HO-1, were induced by hemin and decreased by SESN2 overexpression (Fig. 4C, Fig. S3B and S3C). This is consistent with known function of SESN2 in downregulating oxidative stress. Together, SESN2 can reduce hemin-induced oxidative stress response.
Fig. 4.
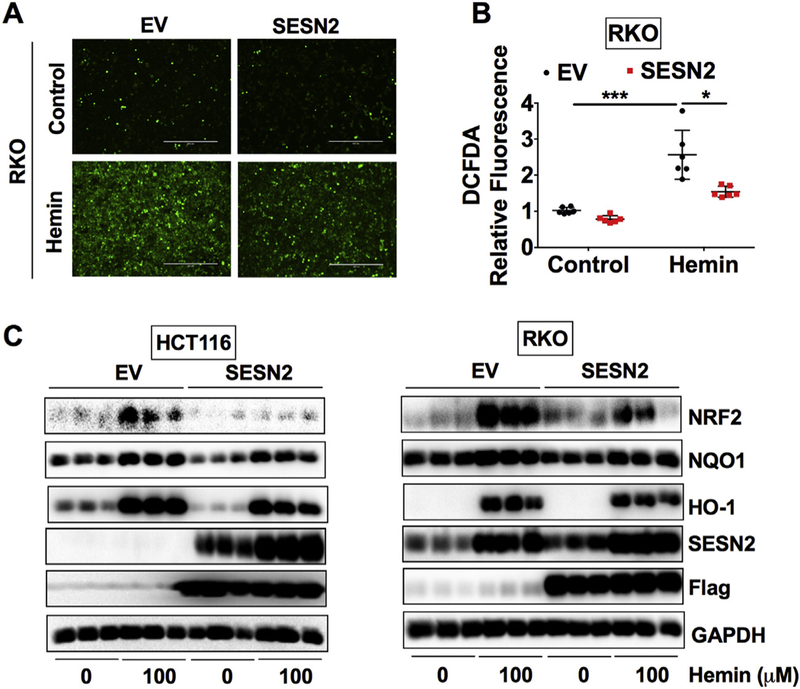
SESN 2 overexpression reduces the expression of hemin-enhanced oxidative stress responsive proteins in CRC cells.
(A) Representative green fluorescence images, (B) quantification of DCFDA staining and (C) Western blot analysis in HCT116 or RKO cells. Cells were transfected with human SESN2 plasmid or empty vector (EV) for 24 h and then these cells were treated with or without 100 μM hemin for overnight. *p < .05 and ***p < .001.
3.4. Sesn2 expression is increased in mouse colon tumors
To test the expression of SESN2 in vivo, normal and tumor colon tissues were collected from the colitis-associated and iron-driven CDX2ERT2ApcF/+ CRC mouse model as described previously (Xue et al., 2016). Interestingly, in the control-iron diet group (35 Fe), the mRNA expression of Sesn2 was significantly increased in the tumor colon tissues compared to normal colon tissues, which was further enhanced by high-iron diet (1000 Fe) treatment (Fig. 5A). To tease out the effect of DSS-induced inflammation on the expression of Sesn2, the sporadic CRC model of CDX2 ApcF/+ mice were also tested. Similarly, the mRNA expression of Sesn2 was increased in the tumor colon tissues compared to normal colon tissues (Fig. 5B). Together, the mRNA expression of Sesn2 is increased in mouse colon tumors and increased by heme iron.
Fig. 5.
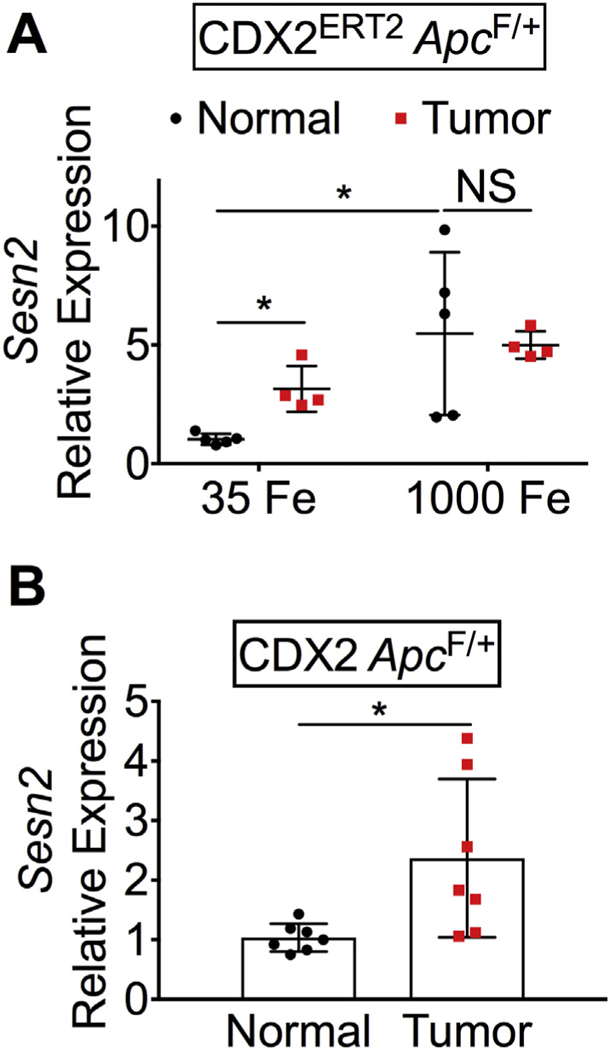
SESN2 expression is increased in mouse colon tumors.
qPCR analysis in (A) CDX2ERT2ApcF/+ mice and (B) CDX2 ApcF/+ mice. *p < .05; NS, not significant.
3.5. SESN2 overexpression and hemin modulates colon tumor cell growth
In addition to its pro-oxidative and cytotoxic effects, heme also activates innate immune responses and inflammation (Dutra et al., 2014). Additionally, SESN2 has been shown to suppress proinflammatory signaling in macrophages (Yang et al., 2015). Thus, we tested the roles of hemin and SESN2 in colon tumor growth using the syngeneic murine MC38 CRC cells, which can grow into tumors under an immune-sufficient environment in vivo. To test the direct role of SESN2 on colon tumor cell growth, MTT assay was first conducted in MC38 cells with SESN2 overexpression. SESN2 overexpression in MC38 cells was confirmed by Western blot analysis (Fig. 6A). SESN2 overexpression significantly reduced the cell survival rate compared to the empty vector control group (Fig. 6B). Next, MC38 cells were injected into C57BL/6 mice to test the effect of SESN2 overexpression in vivo. After subcutaneously injection, MC38 cells developed into large tumor (> 1 cm) within 2 weeks (Fig. 6C). The developed tumor weight was significantly lower in the SESN2 overexpression group compared to the control group (Fig. 6D).
Fig. 6.
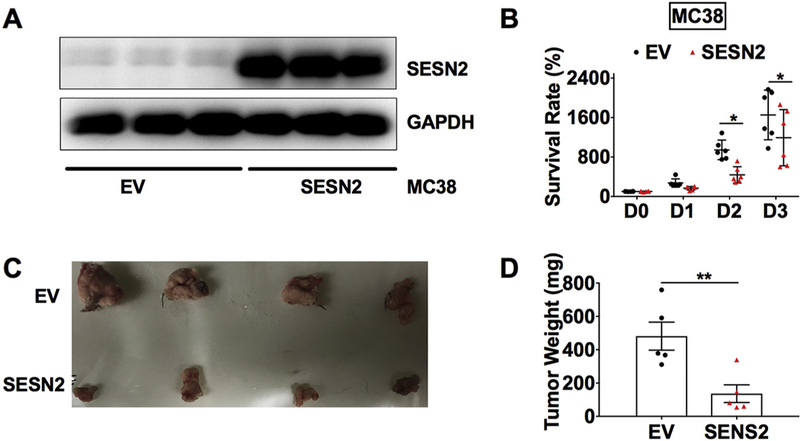
SESN2 overexpression reduces tumor cell growth and tumor size.
(A) Western blot analysis and (B) cell survival rate determined by MTT assay for MC38 cells stably expressed SESN2 or EV. (C) Representative images of MC38 tumors and (D) tumor weight at 14 days after subcutaneously injection of MC38 cells. *p < .05 and **p < .01.
To test the role of hemin on colon tumor growth, we treated MC38 cells with hemin overnight before the subcutaneous injection (Fig. 7). Interestingly, hemin treatment significantly increased the resulting tumor size of SESN2 overexpressing MC38 cells (Fig. 7A). Hemin treatment didn’t affect the tumor weight in the control MC38 cells, whereas SESN2 overexpression consistently reduced tumor weight (Fig. 7B). Western blot analysis showed that the expression of cCasp3, a marker of apoptosis, was not changed by hemin treatment in the tumor formed by control MC38 cells or by SESN2 overexpression. However, hemin treatment of SESN2 overexpression cells significantly reduced the expression of cCasp3 in the formed tumor compared to non-treated control cells (Fig. 7C and D). In contrast, the cell proliferation marker PCNA and the cell cycle related gene CCND1 were not changed either by hemin or SESN2 overexpression in the tumor formed by MC38 cells (Fig. 7C, E and F). Together, these data demonstrate that SESN2 has a dual role on tumor cell growth.
Fig. 7.
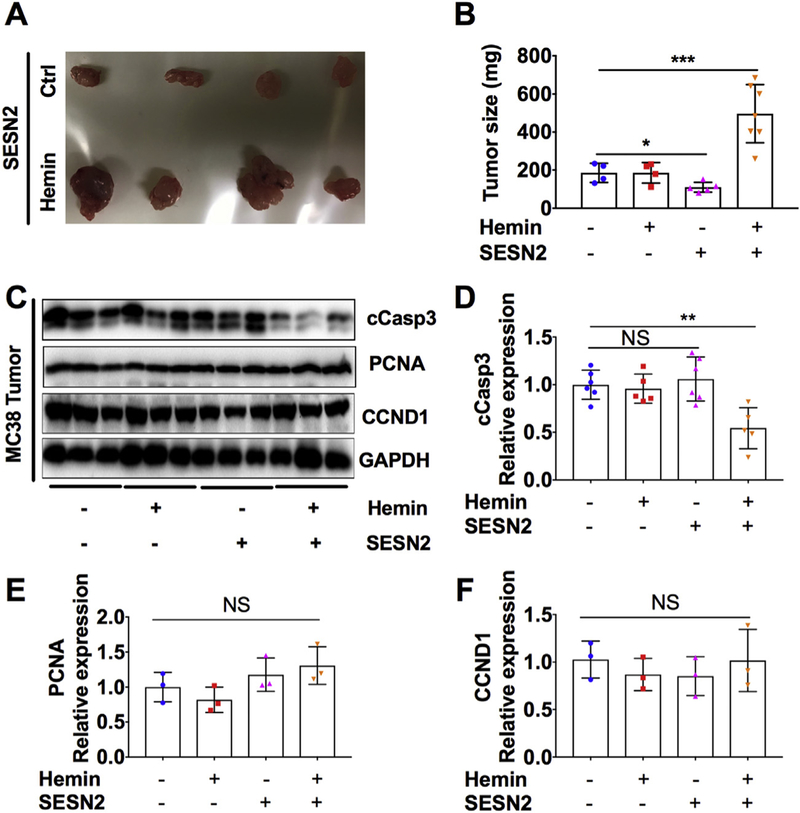
SESN2 overexpression increases tumor growth in the presence of hemin.
(A) Representative images of MC38 tumors and (B) tumor weight at 10 days after subcutaneously injection of MC38 cells treated with or without 100 μM hemin for overnight before injection. (C–F) Western blot analysis and quantification in MC38 tumors. **p < .01 and ***p < .001. NS, not significant.
4. Discussion
Our data herein shows that hemin can activate SESN2 via inducing ROS and NRF2, whereas oxidative stress can be reduced by SESN2 overexpression. Also, we have demonstrated that SESN2 was highly upregulated in colon tumors from both colitis-associated CRC model CDX2ERT2ApcF/+ mice and sporadic CRC model CDX2 ApcF/+ mice. Furthermore, we found SESN2 overexpression can decrease tumor growth under normal iron conditions. However, surprisingly, SESN2 overexpression together with hemin treatment leads to an increase in tumor growth, indicating a complicated scenario under the high iron condition.
Previously, hemin has been reported to reduce the expression of p53 (Shen et al., 2014), which is known to regulate the expression of SESN2 (Ro et al., 2016). Interestingly, here we found that SESN2 was increased by hemin in colon epithelial cells. It has been reported that the induction of SESN2 by DNA damaging treatments occurs in a p53-dependent manner, whereas its induction in response to prolonged hypoxia or by oxidative stress is p53-independent (Budanov et al., 2002). Akt signaling mediated SESN2 activation is also independent of p53 (Ben-Sahra et al., 2013). Also, in contrast to reduced expression of SESN2 in human colon tumors (Ro et al., 2016), here we found an increased expression of SESN2 in mouse colon tumors. This may be due to the fact that there is increased hypoxia signaling (Xue et al., 2016) and a lack of p53 mutation in these animal models. It would be interesting to see whether chronic hemin treatment will eventually lead to decreased expression of p53 and SESN2.
A previous report showed that SESN2 activates NRF2 through p62 dependent autophagic degradation of KEAP1 triggered by the acute lipogenic stimulus in the liver (Bae et al., 2013). Under normal conditions, KEAP1-Cullin3-E3 Ubiquitin ligase complex mediates the poly-ubiquitination and proteasomal degradation of NRF2 (Ma, 2013). Here we found that SESN2 overexpression repressed the expression of NRF2 and its targets NQO1 and HO-1 (Loboda et al., 2016). These results indicate that SESN2 may modulate NRF2 expression through KEAP1 independent mechanism (Bryan et al., 2013).
The effect of SESN2 on cell viability is complicated. Previous reports have shown that SESN2 overexpression leads to inhibited cell growth and proliferation in many cultured cells, but it also protects against apoptosis caused by hypoxia or H2O2 in cancer cells and oxidized low-density lipoprotein in macrophages (Budanov et al., 2002, 2004; Hu et al., 2015). Recently it has been reported that SESN2 induction is essential for cancer cell survival upon glutamine depletion (Byun et al., 2017) or under glycolysis inhibition induced energetic stress (Ben-Sahra et al., 2013). In our study, we found that SESN2 reduced hemin-induced ROS in CRC cells and suppressed colon tumor growth in the absence of hemin, but the colon tumor growth was enhanced in the presence of both hemin and high levels of SESN2. To be noted, we acutely treated MC38 cells with hemin overnight before the subcutaneously xenograft. Aqueous solutions of hemin can be very unstable, with a half-life of a few hours (Cannon et al., 1995). However, addition of antioxidants can reduce radicals, and led to slower degradation process and increased stabilization of hemin. This may partly explain why the increased tumor weight and reduced cCasp3 was only observed in the presence of both hemin and a high level of SESN2. It has been recently reported that dietary heme iron but not systemic heme promoted colon tumorigenesis (Constante et al., 2017), therefore we may need to put our mice on heme iron rich diet and then perform orthotropic transplantation of SESN2 overexpression MC38 cells into the cecum of mice. This may be more accurate in assessing the combinational effect of hemin and SESN2 on colon tumor growth under a more physiological condition.
A previous study has shown that hemin counteracts the cell death effects of imatinib via repressing NRF2 in leukemia cells (Bonovolias and Tsiftsoglou, 2009). NRF2 knockdown suppresses hypoxia activated angiogenesis and colon tumor growth in the HT29 and HCT116 xenograft mouse models (Kim et al., 2011). However, Nrf2 knockout mice crossed with Apcmin/+ mice or treated with azoxymethane and DSS decreases the antioxidative enzymes including NQO1, potentiates inflammation and enhances intestinal carcinogenesis (Cheung et al., 2014; Khor et al., 2008). Thus, it would be interesting to see the colon tumor growth rate after SESN2 depletion or NRF2 induction under a high hemin condition.
In summary, we have found that hemin induces SESN2 via ROS, which is a protective mechanism for suppressing oxidative stress and colon tumor growth, while high amount of SESN2 also protects hemin-induced cell death and leads to increased tumor growth (Fig. 8). Thus, similar to NRF2 (Tong et al., 2015; Gonzalez-Donquiles et al., 2017; Sebens et al., 2011), SESN2 may have dual roles in tumor suppression as well as tumor promotion depending on the context.
Fig. 8.
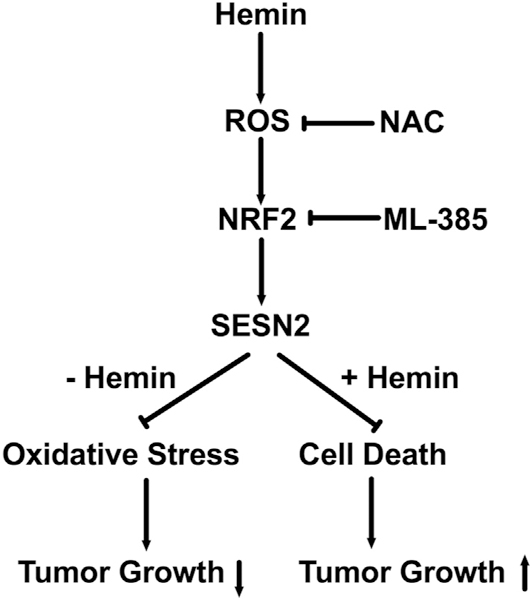
Proposed working model.
Hemin treatment induces ROS and activates NRF2, which lead to the enhanced expression of SESN2. The induction of SESN2 by hemin can be inhibited by the antioxidant NAC and NRF2 inhibitor ML385. In the absence of hemin, SESN2 overexpression leads to reduced oxidative stress and reduced tumor growth, whereas in the presence of hemin, SESN2 overexpression protects cell death and promotes tumor growth.
Supplementary Material
Acknowledgments
We thank Dr. Weiping Zou at University of Michigan for generously providing MC38 cells, Dr. Jun Hee Lee at University of Michigan for generously providing pLU-CMV-Flag-hSESN2 WT plasmid and editing the manuscript. This work was supported in part by the National Institutes of Health (K01DK114390), a Research Scholar Grant from the American Cancer Society (RSG-18–050-01-NEC), a Research Pilot Project Grant from University of New Mexico Environmental Health Signature Program and Superfund (P42 ES025589), and the Dedicated Health Research Funds from the University of New Mexico School of Medicine. Support for in vivo experiments in this paper was provided by the University of New Mexico Cancer Center Animal Models Shared Resource and a Shared Resources Pilot Project Award, funded by NCI 2P30 CA118100 (PI Willman, C.) “UNM Cancer Center Support Grant”. D.M.F was supported by the Academic Science Education and Research Training program at the University of New Mexico Health Sciences Center(NIGMS Institutional Research and Academic Career Development Award; K12-GM088021). The authors have no financial conflicts of interest.
Abbreviations:
- ARE
antioxidant response elements
- cCasp3
cleaved caspase 3
- CCND1
cyclin D1
- CRC
colorectal cancer
- DCFDA
2′,7′-Dichlorofluorescein diacetate
- DMSO
dimethyl sulfoxide
- DMT
divalent metal transporter
- DSS
dextran sulfate sodium
- EV
empty vector
- FTH1
ferritin heavy chain
- GADD
growth arrest DNA damage
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GFP
green fluorescence protein
- HCP1
Heme carrier protein-1
- HO-1
heme oxygenase-1
- HRG1
heme-responsive gene-1
- H2O2
hydrogen peroxide
- KEAP1
kelch-like ECH-associated protein1
- mTORC1
mammalian target of rapamycin complex 1
- NAC
N-Acetyl Cysteine
- NFE2L2 or NRF2
nuclear factor (erythroid-derived 2)-like 2
- NQO1
NAD(P)H Quinone Dehydrogenase 1
- PCNA
proliferating cell nuclear antigen
- ROS
reactive oxygen species
- SESN2
Sestrin2
- STEAP4
six-transmembrane epithelial antigen of prostate 4.
Footnotes
Conflict of interest statement
The authors have no financial conflicts of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.taap.2019.04.025.
References
- Abraham NG, Kappas A, 2008. Pharmacological and clinical aspects of heme oxygenase. Pharmacol. Rev. 60 (1), 79–127. [DOI] [PubMed] [Google Scholar]
- Aldini G, et al. , 2018. N-Acetylcysteine as an antioxidant and disulphide breaking agent: the reasons why. Free Radic. Res. 52 (7), 751–762. [DOI] [PubMed] [Google Scholar]
- Bae SH, et al. , 2013. Sestrins activate Nrf2 by promoting p62-dependent autophagic degradation of Keap1 and prevent oxidative liver damage. Cell Metab. 17 (1), 73–84. [DOI] [PubMed] [Google Scholar]
- Barcellos-de-Souza P, et al. , 2013. Heme modulates intestinal epithelial cell activation: involvement of NADPHox-derived ROS signaling. Am. J. Phys. Cell Physiol. 304 (2), C170–C179. [DOI] [PubMed] [Google Scholar]
- Bastide NM, et al. , 2015. A central role for heme iron in colon carcinogenesis associated with red meat intake. Cancer Res. 75 (5), 870–879. [DOI] [PubMed] [Google Scholar]
- Ben-Sahra I, et al. , 2013. Sestrin2 integrates Akt and mTOR signaling to protect cells against energetic stress-induced death. Cell Death Differ. 20 (4), 611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonovolias ID, Tsiftsoglou AS, 2009. Hemin counteracts the repression of Bcl-2 and NrF2 genes and the cell killing induced by imatinib in human Bcr-Abl(+) CML cells. Oncol. Res. 17 (11–12), 535–547. [DOI] [PubMed] [Google Scholar]
- Bray F, et al. , 2018. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68 (6), 394–424. [DOI] [PubMed] [Google Scholar]
- Bryan HK, et al. , 2013. The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem. Pharmacol. 85 (6), 705–717. [DOI] [PubMed] [Google Scholar]
- Budanov AV, Karin M, 2008. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell 134 (3), 451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budanov AV, et al. , 2002. Identification of a novel stress-responsive gene Hi95 involved in regulation of cell viability. Oncogene 21 (39), 6017–6031. [DOI] [PubMed] [Google Scholar]
- Budanov AV, et al. , 2004. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science 304 (5670), 596–600. [DOI] [PubMed] [Google Scholar]
- Byun JK, et al. , 2017. A positive feedback loop between Sestrin2 and mTORC2 is required for the survival of glutamine-depleted lung Cancer cells. Cell Rep. 20 (3), 586–599. [DOI] [PubMed] [Google Scholar]
- Cannon JB, Yunker MH, Luoma N, 1995. The effect of aggregation inhibitors and antioxidants on the stability of hemin solutions. PDA J. Pharm. Sci. Technol. 49 (2), 77–82. [PubMed] [Google Scholar]
- Cheung KL, et al. , 2014. Nrf2 knockout enhances intestinal tumorigenesis in Apc(min/ +) mice due to attenuation of anti-oxidative stress pathway while potentiates inflammation. Mol. Carcinog. 53 (1), 77–84. [DOI] [PubMed] [Google Scholar]
- Constante M, et al. , 2017. Dietary Heme induces gut Dysbiosis, aggravates colitis, and potentiates the development of adenomas in mice. Front. Microbiol. 8, 1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra FF, et al. , 2014. Hemolysis-induced lethality involves inflammasome activation by heme. Proc. Natl. Acad. Sci. U. S. A. 111 (39), E4110–E4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel C, et al. , 1997. Determination of nitric oxide generation in mammalian neurons using dichlorofluorescin diacetate and flow cytometry. J. Pharmacol. Toxicol. Methods 38 (2), 93–98. [DOI] [PubMed] [Google Scholar]
- Gemelli C, et al. , 2014. Cytotoxic effect of hemin in colonic epithelial cell line: involvement of 18 kDa translocator protein (TSPO). Life Sci. 107 (1–2), 14–20. [DOI] [PubMed] [Google Scholar]
- Giovannucci E, et al. , 1994. Intake of fat, meat, and fiber in relation to risk of colon cancer in men. Cancer Res. 54 (9), 2390–2397. [PubMed] [Google Scholar]
- Glei M, et al. , 2006. Hemoglobin and hemin induce DNA damage in human colon tumor cells HT29 clone 19A and in primary human colonocytes. Mutat. Res. 594 (1–2), 162–171. [DOI] [PubMed] [Google Scholar]
- Goldstein L, et al. , 2003. Hemin induces an iron-dependent, oxidative injury to human neuron-like cells. J. Neurosci. Res. 73 (1), 113–121. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Donquiles C, et al. , 2017. The NRF2 transcription factor plays a dual role in colorectal cancer: a systematic review. PLoS One 12 (5), e0177549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozzelino R, Jeney V, Soares MP, 2010. Mechanisms of cell protection by heme oxygenase-1. Annu. Rev. Pharmacol. Toxicol. 50, 323–354. [DOI] [PubMed] [Google Scholar]
- Gulec S, Anderson GJ, Collins JF, 2014. Mechanistic and regulatory aspects of intestinal iron absorption. Am. J. Physiol. Gastrointest. Liver Physiol. 307 (4), G397–G409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan RN, Schafer AI, 2008. Hemin upregulates Egr-1 expression in vascular smooth muscle cells via reactive oxygen species ERK-½-Elk-1 and NF-kappa B. Circ. Res. 102 (1), 42–50. [DOI] [PubMed] [Google Scholar]
- Ho A, et al. , 2016. Biochemical basis of Sestrin physiological activities. Trends Biochem. Sci. 41 (7), 621–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu HJ, et al. , 2015. Upregulation of Sestrin2 expression protects against macrophage apoptosis induced by oxidized low-density lipoprotein. DNA Cell Biol. 34 (4), 296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang HJ, et al. , 2018. Knockdown of Sestrin2 increases lipopolysaccharide-induced oxidative stress, apoptosis, and fibrotic reactions in H9c2 cells and heart tissues of mice via an AMPK-dependent mechanism. Mediat. Inflamm. 2018, 6209140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara M, et al. , 2013. Sestrin-2 and BNIP3 regulate autophagy and mitophagy in renal tubular cells in acute kidney injury. Am. J. Physiol. Ren. Physiol. 305 (4), F495–F509. [DOI] [PubMed] [Google Scholar]
- Itoh T, et al. , 2001. Hemin (Fe(3 + ))— and heme (Fe(2 + ))—smectite conjugates as a model of hemoprotein based on spectrophotometry. Bioconjug. Chem. 12 (1), 3–6. [DOI] [PubMed] [Google Scholar]
- Kagan VE, et al. , 2001. Antioxidant mechanisms of nitric oxide against iron-catalyzed oxidative stress in cells. Antioxid. Redox Signal. 3 (2), 189–202. [DOI] [PubMed] [Google Scholar]
- Khor TO, et al. , 2008. Increased susceptibility of Nrf2 knockout mice to colitis-associated colorectal cancer. Cancer Prev. Res. (Phila.) 1 (3), 187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, et al. , 2011. NRF2 blockade suppresses colon tumor angiogenesis by inhibiting hypoxia-induced activation of HIF-1alpha. Cancer Res. 71 (6), 2260–2275. [DOI] [PubMed] [Google Scholar]
- Kim H, et al. , 2015. Janus-faced Sestrin2 controls ROS and mTOR signalling through two separate functional domains. Nat. Commun. 6, 10025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopnin PB, et al. , 2007. Repression of sestrin family genes contributes to oncogenic Ras-induced reactive oxygen species up-regulation and genetic instability. Cancer Res. 67 (10), 4671–4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kultz D, 2005. Molecular and evolutionary basis of the cellular stress response. Annu. Rev. Physiol. 67, 225–257. [DOI] [PubMed] [Google Scholar]
- Larsson SC, Wolk A, 2006. Meat consumption and risk of colorectal cancer: a meta-analysis of prospective studies. Int. J. Cancer 119 (11), 2657–2664. [DOI] [PubMed] [Google Scholar]
- Lee JH, et al. , 2010. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science 327 (5970), 1223–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, et al. , 2017. Role of sestrin2 in H2O2-induced PC12 apoptosis. Neurosci. Lett. 646, 1–7. [DOI] [PubMed] [Google Scholar]
- Loboda A, et al. , 2016. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell. Mol. Life Sci. 73 (17), 3221–3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, 2013. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 53, 401–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullebner A, et al. , 2017. Interaction between mitochondrial reactive oxygen species, Heme Oxygenase, and nitric oxide synthase stimulates phagocytosis in macrophages. Front. Med. (Lausanne) 4, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagababu E, Rifkind JM, 2004. Heme degradation by reactive oxygen species. Antioxid. Redox Signal. 6 (6), 967–978. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Nioi P, Pickett CB, 2009. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 284 (20), 13291–13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira V, et al. , 2008. Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell 14 (6), 458–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oates PS, West AR, 2006. Heme in intestinal epithelial cell turnover, differentiation, detoxification, inflammation, carcinogenesis, absorption and motility. World J. Gastroenterol. 12 (27), 4281–4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owusu-Ansah E,Yavari A, and Banerjee U, A protocol for _in vivo_ detection of reactive oxygen species. 2008. [Google Scholar]
- Pall ML, Levine S, 2015. Nrf2, a master regulator of detoxification and also anti-oxidant, anti-inflammatory and other cytoprotective mechanisms, is raised by health promoting factors. Sheng Li Xue Bao 67 (1), 1–18. [PubMed] [Google Scholar]
- Pasha M, et al. , 2017. Sestrin2 as a novel biomarker and therapeutic target for various diseases. Oxidative Med. Cell. Longev. 2017, 3296294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng M, Yin N, Li MO, 2014. Sestrins function as guanine nucleotide dissociation inhibitors for rag GTPases to control mTORC1 signaling. Cell 159 (1), 122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal A, et al. , 2008. Haem homeostasis is regulated by the conserved and concerted functions of HRG-1 proteins. Nature 453 (7198), 1127–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SG, Bae SH, 2015. The antioxidant function of sestrins is mediated by promotion of autophagic degradation of Keap1 and Nrf2 activation and by inhibition of mTORC1. Free Radic. Biol. Med. 88 (Pt B), 205–211. [DOI] [PubMed] [Google Scholar]
- Ro SH, et al. , 2014. Sestrin2 promotes Unc-51-like kinase 1 mediated phosphorylation of p62/sequestosome-1. FEBS J. 281 (17), 3816–3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro SH, et al. , 2016. Tumor suppressive role of sestrin2 during colitis and colon carcinogenesis. Elife 5, e12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter SW, Tyrrell RM, 2000. The heme synthesis and degradation pathways: role in oxidant sensitivity. Heme oxygenase has both pro-and antioxidant properties. Free Radic. Biol. Med. 28 (2), 289–309. [DOI] [PubMed] [Google Scholar]
- Sandhu MS, White IR, McPherson K, 2001. Systematic review of the prospective cohort studies on meat consumption and colorectal cancer risk: a meta-analytical approach. Cancer Epidemiol. Biomark. Prev. 10 (5), 439–446. [PubMed] [Google Scholar]
- Sebens S, et al. , 2011. Inflammatory macrophages induce Nrf2 transcription factor-dependent proteasome activity in colonic NCM460 cells and thereby confer anti-apoptotic protection. J. Biol. Chem. 286 (47), 40911–40921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo K, Ki SH, Shin SM, 2015. Sestrin2-AMPK activation protects mitochondrial function against glucose deprivation-induced cytotoxicity. Cell. Signal. 27 (7), 1533–1543. [DOI] [PubMed] [Google Scholar]
- Sesink AL, et al. , 1999. Red meat and colon cancer: the cytotoxic and hyper proliferative effects of dietary heme. Cancer Res. 59 (22), 5704–5709. [PubMed] [Google Scholar]
- Shayeghi M, et al. , 2005. Identification of an intestinal heme transporter. Cell 122 (5), 789–801. [DOI] [PubMed] [Google Scholar]
- Shen J, et al. , 2014. Iron metabolism regulates p53 signaling through direct heme-p53 interaction and modulation of p53 localization, stability, and function. Cell Rep. 7 (1), 180–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, 2012. Binding of cysteine thiolate to the Fe(III) heme complex is critical for the function of heme sensor proteins. J. Inorg. Biochem. 108, 171–177. [DOI] [PubMed] [Google Scholar]
- Shin BY, et al. , 2012. Nrf2-ARE pathway regulates induction of Sestrin-2 expression. Free Radic. Biol. Med. 53 (4), 834–841. [DOI] [PubMed] [Google Scholar]
- Singh A, et al. , 2016. Small molecule inhibitor of NRF2 selectively intervenes therapeutic resistance in KEAP1-deficient NSCLC Tumors. ACS Chem. Biol. 11 (11), 3214–3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong YH, et al. , 2015. Keap1-Nrf2 pathway: a promising target towards lung cancer prevention and therapeutics. Chronic Dis. Transl. Med. 1 (3), 175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcellos LR, et al. , 2016. Protein aggregation as a cellular response to oxidative stress induced by heme and iron. Proc. Natl. Acad. Sci. U. S. A. 113 (47), E7474–E7482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco-Miguel S, et al. , 1999. PA26, a novel target of the p53 tumor suppressor and member of the GADD family of DNA damage and growth arrest inducible genes. Oncogene 18 (1), 127–137. [DOI] [PubMed] [Google Scholar]
- West AR, Oates PS, 2008. Mechanisms of heme iron absorption: current questions and controversies. World J. Gastroenterol. 14 (26), 4101–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White C, et al. , 2013. HRG1 is essential for heme transport from the phagolysosome of macrophages during erythrophagocytosis. Cell Metab. 17 (2), 261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett WC, et al. , 1990. Relation of meat, fat, and fiber intake to the risk of colon cancer in a prospective study among women. N. Engl. J. Med. 323 (24), 1664–1672. [DOI] [PubMed] [Google Scholar]
- Xue X, et al. , 2012. Hypoxia-inducible factor-2alpha activation promotes colorectal cancer progression by dysregulating iron homeostasis. Cancer Res. 72 (9), 2285–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue X, et al. , 2016. Iron uptake via DMT1 integrates cell cycle with JAK-STAT3 Signaling to promote colorectal tumorigenesis. Cell Metab. 24 (3), 447–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue X, et al. , 2017. Quantitative proteomics identifies STEAP4 as a critical regulator of mitochondrial dysfunction linking inflammation and colon cancer. Proc. Natl. Acad. Sci. U. S. A. 114 (45), E9608–E9617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JH, et al. , 2015. Role of sestrin2 in the regulation of proinflammatory signaling in macrophages. Free Radic. Biol. Med. 78, 156–167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


