Abstract
The concept of a syndemic was proposed more than two decades ago to explain how large-scale social forces might give rise to co-occurring epidemics that synergistically interact to undermine health in vulnerable populations. This conceptual instrument has the potential to help policymakers and program implementers in their endeavors to improve population health. Accordingly, it has become an increasingly popular heuristic for advocacy, most notably in the field of HIV treatment and prevention. However, most empirical studies purporting to validate the theory of syndemics actually do no such thing. Tomori et al. (2018) provide a novel case study from India illustrating how the dominant empirical approach fails to promote deeper understanding about how hazardous alcohol use, illicit drug use, depression, childhood sexual abuse, and intimate partner violence interact to worsen HIV risk among men who have sex with men. In this commentary, I relate the theory of syndemics to other established social science and public health theories of disease distribution, identify possible sources of conceptual and empirical confusion, and provide concrete suggestions for how to validate the theory using a mixed-methods approach. The hope is that more evidence can be mobilized -- whether informed by the theory of syndemics or not -- to improve health and psychosocial wellbeing among vulnerable populations worldwide.
Keywords: HIV, mixed methods, multilevel analysis, population health, social determinants of health, stigma, syndemic, syndemics
Introduction
The theory of syndemics was first proposed by Singer (1996) to describe “synergistically related” (p.103) epidemics that cluster and arise from harmful social conditions. The structural aspect of his theory follows in the vein of what Krieger (2000) describes as “theories of disease distribution” (p.160), all of which have highlighted the role of large-scale forces in driving concentrated health disadvantage at the population level. This influential body of work includes, among others, ecosocial theory (Krieger, 1994), fundamental cause theory (Link & Phelan, 1995), and the theory of structural violence now prominently associated with Farmer (1996). Similarly, by the time Singer (1996) proposed his theory, the disease interaction concept had already become widely accepted among health care providers caring for patients with complex constellations of comorbidities, described in parallel literatures on multimorbidity (Diederichs, Berger, & Bartels, 2011; van den Akker, Buntinx, & Knottnerus, 1996) and dual diagnosis (Drake et al., 1991; Lehman, Myers, & Corty, 1989).
In the two decades since Singer (1996) first explicitly named the intertwined epidemics of Substance Abuse, Violence, and AIDS (“SAVA”) in Hartford, Conn., the menu of alphabet soup offerings has =expanded. We now have: VIDDA (Violence, Immigration and associated isolation, Depression, type 2 Diabetes, and Abuse) (Mendenhall, 2012), SUMIC (Substance Use, Mental Illness, and familial Conflict non-negotiation) (Robinson et al., 2016), SAVID (Substance use during condomless intercourse, Adolescent sexual abuse, Violence, Internalized Homonegativity, and Depression) (Adeboye et al., 2017), and, most recently, PHAMILIS (Physical Health problems, Abuse, Mental Illness, Loss, Instability, and Substance use) (Marcus, 2014; Marcus & Singer, 2017). These lexicalized acronyms do not feature the same stretch for meaning and association as those used, for example, in the field of cardiology (Berkwits, 2000; Orlowski & Christensen, 2002; Pottegård et al., 2014). However, this proliferation of acronyms appears to be consistent with the Procrustean efforts trending in other fields (Fallowfield & Jenkins, 2002; Pottegård et al., 2014).
While the theory of syndemics marries different aspects of well-known social science and medical theories to generate powerful predictions for program implementation and clinical intervention, empirical analysis has lagged theorizing in the field. On the one hand, social epidemiology has clearly established the role of social determinants in explaining differences in disease between and within populations (Berkman, Kawachi, & Glymour, 2014); and descriptive evidence for the phenomenon of disease clustering is fairly robust, at least at the level of the individual (Tsai & Venkataramani, 2016). On the other hand, however, with the exception of detailed anthropological studies (Mendenhall, 2012, 2015; Mendenhall et al., 2012), the field has so far failed to find convincing empirical evidence to support the third pillar of the theory, i.e., disease interaction. Systematic reviews have shown that most epidemiological studies in this literature -- all of which are based on individual-level data -- have attempted to document the existence of syndemics by using the “sum score” specification (Tsai & Burns, 2015; Tsai et al., 2017). This dominant modeling approach is so named because the omnibus exposure of interest is calculated as the sum total of health risks experienced by study participants, and this variable is then included in a regression model as either a continuous or a categorical covariate.
Epidemiological studies employing the sum score approach to analyze putative syndemics have proliferated in recent years, most notably in the field of HIV treatment and prevention. The classic article by Stall et al. (2003) was the first of many studies to adopt the sum score approach to explain HIV risk among men who have sex with men. It is the “patient zero” of this literature and has received more citations than either of the primary articles elaborating the theory’s conceptual basis (Singer, 1996, 2006). In this burgeoning literature, investigators’ claims of having identified synergistically interacting epidemics, based on regression analyses adopting the sum score specification, are often accompanied by appeals for complex, integrated and/or multicomponent interventions (Ferlatte et al., 2015; Li et al., 2016; Nehl et al., 2016; Wang et al., 2018).
But these appeals for multicomponent interventions, and the empirical basis for such appeals, are not consistent with each other (Fig. 1). It can be shown mathematically that the sum score specification conveys no information about the extent to which the candidate health risks interact (Tsai & Venkataramani, 2016). This proof was foreshadowed more than a decade earlier by Singer and Clair (2003), who reviewed disease count data from three New England cities while explicitly noting that “the data do not enable an assessment of disease interaction” (p.433). Further, one of the most commonly used specifications of the sum score essentially encodes an assumption that there exists a sufficient cause interaction (Tsai et al., 2017). Yet no program implementers or policy makers have drawn on the findings of this body of literature to argue for single component interventions to prevent or treat HIV. On the other hand, the conclusion that multicomponent interventions are absolutely essential for optimal outcomes could be drawn from a study documenting multiple sufficient pathways to disease (Eisenberg, Scott, & Porco, 2007; Wagner & Lanoix, 1958). Yet no program implementers or policy makers have used that literature to motivate the use of multicomponent interventions to prevent or treat HIV.
Fig 1.
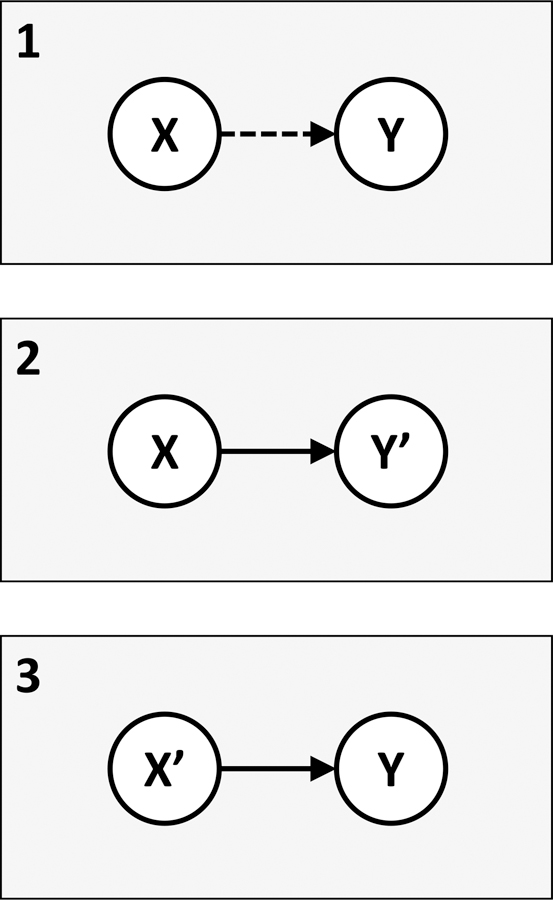
A simplified framework for understanding the relationship between findings based on the sum score approach vs. policy or programmatic recommendations for multicomponent interventions. Panel 1 depicts what is commonly observed in the literature on syndemics: investigators fit a regression model following the sum score approach (X) and then draw on the findings to advocate for complex, integrated, and/or multicomponent interventions (Y). The dashed line indicates that a conclusion of Y based on findings from X is incorrect, given the underlying math (Tsai & Venkataramani, 2016). Panel 2 recognizes that one of the most commonly used specifications of the sum score essentially encodes an assumption that there exists a sufficient cause interaction (Tsai et al., 2017). Such an assumption does not, however, militate for a multicomponent intervention. The findings of X would instead lead directly to the conclusion that a single component intervention (Y’) would be sufficient to prevent the outcome, because the mechanism of disease -- which the sufficient cause represents -- requires the presence of all health risks to operate. Panel 3 illustrates that if a multicomponent intervention is the desired policy or programmatic outcome, use of the sum score approach will not lead to this conclusion. One possibility, however, is to demonstrate that there are multiple sufficient pathways to disease (X’), as is often observed in the setting of enteric pathogens and diarrheal disease (Eisenberg, Scott, & Porco, 2007; Wagner & Lanoix, 1958). In none of these scenarios does X lead to Y.
How do epidemics “interact”?
Possibly the confusion over how to operationalize syndemics in the empirical literature stems from imprecision in how the theory of syndemics has been described (Fig. 2). One might anticipate the argument that the word “interaction” could be consistent with several different models of relating co-occurring epidemics to each other:
Mutually causal epidemics: Singer (1996) initially portrayed the SAVA syndemic diagrammatically as a triangle. The discussion in his initial treatise focuses on the manifold ways in which the three epidemics are thought to be mutually causal: substance abuse is a risk factor for violent victimization and/or HIV acquisition, victimization may lead to substance abuse and/or HIV acquisition, and people with HIV are at greater risk of victimization and substance abuse.
Synergistically interacting epidemics: In other elaborations of the theory of syndemics, the framing adopts the language of interaction or synergism. Synergy implies that the disease burden attributable to joint health risks exceeds the sum of the disease burden of the health risks in isolation (Rothman, 1974).
Serially causal epidemics: This category represents a somewhat heterogeneous group of models that are based on well-known theories about the potential adverse consequences of accumulating and/or serially causal health risks. There is no shortage of coined phrases here, including “insult accumulation” (Riley, 1989), “weathering” (Geronimus, 1992), “allostatic load” (McEwen & Stellar, 1993), “chains of risk” (Coie et al., 1993), and life course epidemiology (Kuh & Ben-Shlomo, 1997; Kuh et al., 2003).
Fig. 2.
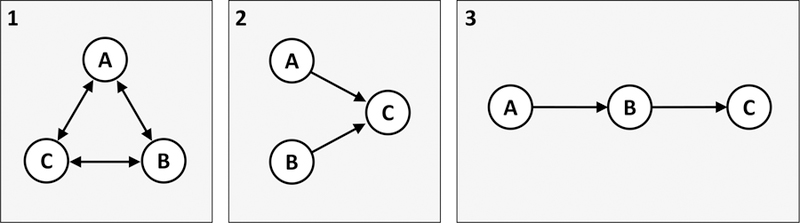
A simplified typology of three ways to operationalize how co-occurring epidemics relate to each other. A, B, and C can be thought of either as diseases at the individual level or as epidemics at the population level. Panel 1 depicts mutually causal epidemics, described by Singer (1996): A and B are mutually causal, B and C are mutually causal, and A and C are mutually causal. Panel 2 depicts synergistically interacting epidemics, highlighted in Singer and Clair (2003): A and B both cause C, and their total effect on C exceeds the sum of their individual effects alone. Panel 3 depicts serially causal epidemics: A causes B, which then causes C. This model is related to theories of accumulating health risks.
When a set of co-occurring epidemics is labeled a putative “syndemic,” which of these three models does the label imply?
In his initial formulation, Singer (1996) emphasized the interdependence and interrelatedness of substance abuse, violence, and HIV -- suggesting the model of mutually causal epidemics. However, the plain use of the word “interaction” instead suggests the model of synergistically interacting epidemics. Moreover, the exemplars provided in Singer and Clair (2003), the most highly cited précis of the theory, draw exclusively on the language of interaction and synergism. For example, persons with chronic hepatitis C virus infection who also engage in heavy alcohol use are at far greater risk of developing hepatocellular carcinoma compared with persons who only engage in heavy alcohol use or who only have chronic hepatitis C virus infection; persons with HIV who are co-infected with mycobacterium tuberculosis experience accelerated progression of HIV disease; and so forth. As for the model of serially causal epidemics, it is not explicitly described by Singer (1996, 2006) but is frequently cited by Stall and colleagues as being consistent with their conceptualization of syndemics (Herrick et al., 2013; Stall et al., 2015; Stall, Friedman, & Catania, 2007).
As elaborated further in Tsai et al. (2017), objection to the casual use of statistically distinct terms interchangeably cannot be dismissed as mere semantic quibbling. This practice contributes to ongoing confusion in the field because the three different models of co-occurring epidemics generate starkly different predictions about the potential effectiveness of treatment or prevention strategies. The model of mutually causal epidemics implies that addressing all epidemics or health risks may be necessary to effectively reduce the burden of disease (or that, in a single population with multiple epidemics, addressing all epidemics would be needed to achieve as much disease burden reduction as addressing the equivalent number of single epidemics present in separate populations). The various models of serially causal epidemics also imply different intervention strategies. In contrast, the model of synergistically interacting epidemics potentially implies that one epidemic or health risk can be addressed in isolation while leaving the other intact, and that doing so should be expected to result in a greater reduction in disease burden than otherwise would be expected if no interactions were present.
The Tomori et al. (2018) study
It is in this context that Tomori et al. (2018) offer a novel investigation into the epidemics of hazardous alcohol use, illicit drug use, depression, childhood sexual abuse, and intimate partner violence among men who have sex with men. Not all the studied health risks would be considered “epidemic,” as the prevalence of illicit drug use during the 6 months prior to interview was less than 3 percent. Nor did it appear that many of the health risks were co-occurring, as fewer than 15 percent of the sample reported two or more health risks. However, these health risks are among those most commonly investigated in empirical studies attempting to understand putative syndemics (Tsai & Burns, 2015; Tsai et al., 2017). The study participants, recruited at several sites in India through respondent-driven sampling, were interviewed as part of the baseline assessment for a cluster-randomized HIV prevention trial (Solomon et al., 2013; Solomon et al., 2015). The outcomes of interest in this analysis were condomless anal intercourse during the 6 months prior to interview and active syphilis infection determined by rapid plasma reagin and treponemal antibody testing.
Tomori et al. (2018) focused their analysis on estimating the associations between the exposures and outcomes. First, following most empirical studies in this literature, they followed the sum score approach by calculating an omnibus variable equal to the sum total of health risks (Tsai & Burns, 2015; Tsai et al., 2017). They found, as have most studies in this literature, a statistically significant association between the sum score and the outcome of condomless anal intercourse.
In describing those findings throughout the article, Tomori et al. (2018) adopt the regrettable terminology of their predecessors, referring to “additive associations” between the exposures and outcomes. In this literature, this phrase is often deployed -- on the basis of estimates derived from the sum score approach -- to emphasize multimorbidity and interrelatedness and, therefore, the need for complex, multicomponent interventions (Ferlatte et al., 2015; Li et al., 2016; Nehl et al., 2016; Wang et al., 2018). But the persisting use of the phrase in this context is regrettable because it actually does not carry substantive meaning. As emphasized in Tsai and Venkataramani (2016), the covariates in a linear regression model are assumed to have additive associations with the outcome. Compared with a conventional regression model in which the candidate health risks are entered as separate covariates, the sum score specification simply enforces the assumptions that the associations are not only additive but also equivalent -- while also increasing the probability of a statistically significant finding by reducing (from several to one) the number of parameters to be estimated.
In a novel departure from the literature, Tomori et al. (2018) included two- and three-way product terms in the regression models to test for the presence of interactions between the health risks. Out of 26 two- and three-way interactions tested for the two outcomes, they found evidence of synergy only between intimate partner violence and depression for the outcome of condomless anal intercourse, and between hazardous alcohol use and illicit drug use for the outcome of active syphilis. This pattern of largely null findings, which diverges from the estimates obtained in their analysis based on the sum score, provides a concrete, real-world illustration of what has been previously demonstrated mathematically (Tsai & Venkataramani, 2016): that fitting a regression model using the sum score specification can yield a statistically significant estimate consistent with a data-generating process characterized by no interactions between the exposures of interest.
Skeptical readers might examine these findings and, generalizing beyond these epidemics in this population, conclude -- while recalling the underlying math, which proves that most of the studies in this literature claim a finding that they do not (and cannot) demonstrate (Tsai & Venkataramani, 2016) -- that the empirical literature on syndemics is largely hokum. Tomori et al. (2018), on the other hand, suggest a “broader conceptualization of syndemics.” Essentially, they propose redefining syndemics so that regression models adopting the sum score approach can be reinterpreted as providing evidence consistent with the theory.
This proposal raises several concerns. First, retrospectively redefining the concept of a syndemic to be consistent with regression analyses that were motivated by a mistaken understanding of the theory would be a rather unconventional approach to validate the theory. Doing so is tantamount to moving the goal structure after the kick so a football that appears to be hooking wide ends up sailing through the uprights straight and true. Second, given the conceptual fuzziness described previously, the models of mutually causal, synergistically interacting, and serially causal epidemics could all potentially be consistent with the theory of syndemics. The foundational work by Singer (1996, 2006) is quite unclear on this matter. Irrespective of the model or models that emerge conceptually victorious from the scrum when the dust settles, this point is worth emphasizing: the sum score specification offers an appropriate test for none of these models. Put differently, the sum score specification conveys no information about the extent to which epidemics are mutually causal, synergistically interacting, or serially causal. Third, syndemics are theorized to be multilevel phenomena in which epidemics interact at both the level of populations and the level of individuals (Singer, 2006). Cumulative adversities, on the other hand, are largely conceptualized at the level of the individual (Cronholm et al., 2015). Redefining the concept of a syndemic strictly on the basis of individual-level analyses would not necessarily support expanding such a reconceptualization to the population level. Fourth, while the authors suggest that redefining syndemics could spur “greater integration of quantitative and qualitative methodologies,” such an outcome does not necessarily follow from the suggestion. More qualitative data can be brought to bear on the study of syndemics without accommodating the sum score approach (as is described in more detail below).
Need for new approaches in the study of syndemics
Tomori et al. (2018) advance the literature by providing an empirical example that starkly exposes the potential discrepancies that can emerge between analyses based on the sum score approach vs. those that test for synergistic interactions. But their analysis also highlights the need for new study designs that can help the field gain purchase on validating the theory of syndemics. Given the complexity of the large-scale social forces shaping how multiple epidemics converge to worsen the burden of disease, triangulation of data from multiple sources may be required (Patton, 1999). Below I discuss four approaches for potentially advancing the literature: extending analyses beyond the level of the individual; mapping the temporal cascade of health risks; using agent-based models to understand disease and intervention co-dynamics; and incorporating insights from anthropological field work. Each of these study designs is complementary to the others, and using them in tandem will enable investigators to minimize their limitations and reinforce their strengths.
Extending analyses beyond the level of the individual
To date, the field has focused exclusively on investigating how individual-level outcomes can be explained by individual-level covariates (Tsai & Burns, 2015; Tsai et al., 2017). This omission of population-level studies and contextual effects is conspicuous given that syndemics have been explicitly theorized as multilevel phenomena, e.g. as described by Singer (2006): “Recently, in our efforts to further delineate the concept of syndemic [sic], we have drawn attention to the fact that disease interaction occurs at both the population and individual levels” (p.39). Failure to explicitly model the colluding social forces responsible for syndemic health risks leads the field away from political, economic, and cultural explanations of concentrated health disadvantage and toward disembodied notions of comorbidity.
One would expect empirical studies of syndemics to be natural candidates for the application of multilevel models, which bridge analyses of ecological and individual-level data and permit simultaneous estimation of associations at different levels (Diez-Roux, 1998; Duncan, Jones, & Moon, 1998). Blakely and Woodward (2000) described three principal mechanisms through which ecological constructs can affect individual-level outcomes: cross-level effect modification (e.g. the association between men’s beliefs about gender roles and women’s experience of intimate partner violence is modified by laws disadvantaging women relative to men in their access to resources and factors of production), direct cross-level effects (e.g., laws disadvantaging women in access to resources and factors of production directly undermine women’s health by contributing to fear and stress), and indirect cross-level effects (e.g., laws disadvantaging women in access to resources and factors of production shape men’s beliefs about gender roles, which in turn elevate women’s risk for intimate partner violence). Ecological factors can also modify the effects of other ecological factors, consistent with ethnographically-informed theories about how the interplay between large-scale social forces shapes the HIV risk environment (Rhodes et al., 2005; Rhodes et al., 1999).
Mapping the temporal cascade of health risks
The models of serially causal epidemics used in life course epidemiology (Kuh et al., 2003) may or may not be consistent with the concept of a syndemic. If so, path analysis and social network analysis represent two methods that can be used to infer the causal relations between psychosocial health risks experienced by individuals. Path analysis is a form of structural equation modeling that can be used to examine both direct and indirect hypothesized associations between several different variables (Wright, 1920, 1921). At the level of the individual, serially causal health risks could manifest, for example, as child abuse leading to alcohol abuse, subsequently followed by alcohol dependence, major depressive disorder, and HIV transmission risk behavior; covariates at multiple levels of analysis could easily be accommodated. The estimation of direct and indirect effects has traditionally been undertaken by structural equation modelers, but counterfactual-based approaches have gained increasing popularity (VanderWeele, 2015).
The social network approach to explaining constellations of health risks might conceptualize them as mutually enhancing, reciprocally reinforcing nodes in an association network (Cramer et al., 2010). In contrast to path analysis, network analysis does not rely on an a priori specified model relating the nodes to each other; rather, the strength of the linkages (edges) and the centrality of certain nodes emerges from the analysis. Candidate causal structures can be explored using inference algorithms (Spirtes & Glymour, 1991). Such an analysis would also be able to identify situations in which low-prevalence health risks are highly central to the tangled web of comorbidity, or, conversely, situations in which health risks may be of high prevalence but nonetheless lack central importance.
Social network analysis has been increasingly used to illustrate psychiatric disorders as causal systems (McNally et al., 2015) or to clarify the role of symptom overlap in characterizing constellations of psychiatric disorders (Bekhuis et al., 2016; Bringmann et al., 2015). One recent analysis expanded this approach to include other psychosocial variables such as stigma, functioning, resilience, and service engagement (Galderisi et al., 2018). In the context of a longitudinal study, this type of analysis could be used to elucidate the processes through which the nodes interact with each other and exert potentially reinforcing effects. Nodes with high levels of correlations with other nodes (out-strength) could represent potential targets for intervention, as Tomori et al. (2018) usefully suggest, given that they have a greater likelihood of influencing other nodes in the network. McNally et al. (2015) have similarly speculated that targeting such nodes for intervention could trigger a “therapeutic cascade of downstream benefits” (p.845).
Using agent-based models to understand disease and intervention co-dynamics
The complex, multifactorial processes that outline the contours of syndemics may be well suited to applications of agent-based models. Agent-based models simulate heterogeneous, autonomous “agents” who are endowed with pre-programmed behavioral rules governing their interactions with each other and with the environment (Bonabeau, 2002). These interactions, when aggregated, generate system-wide phenomena that cannot be intuitively discerned from the behavioral rules alone (Coleman, 1990; Granovetter, 1978; Schelling, 1969, 1971). Importantly, the process of model construction would require analysts to make concrete their (presumably theory-based) assumptions about how they expect syndemics to operate. Analysts can assess the validity of their models by comparing the emergent descriptions to epidemiological data (Bearman, Moody, & Stovel, 2004; Hedström, 2005; Hedström & Bearman, 2009). Relevant examples of agent-based modeling of syndemics are scarce in the literature. Applications of other complex systems methods, such as system dynamics models, are similarly scarce (Batchelder et al., 2015). O’Neil and Sattenspiel (2010) employed an agent-based model to understand how the spread of influenza in North American aboriginal communities was influenced by seasonal settlement and mobility patterns (but their model did not incorporate interactions between influenza and other infectious diseases, e.g., tuberculosis).
One appealing feature of agent-based models is their ability to simulate the population-level effects of complex interventions. Agent-based models have been used, for example, to test the effectiveness of stigmatization and social network targeting strategies in addressing the obesity epidemic (Zhang et al., 2015), structural interventions to increase consumption of fresh produce among low-income households (Widener, Metcalf, & Bar-Yam, 2013), enhancing neighborhood collective efficacy to reduce racial and ethnic disparities in violent victimization (Cerda et al., 2014), and multicomponent interventions to improve diarrheal disease control (Mellor et al., 2012). The treatment effect estimates so obtained are causal, in the sense that counterfactual scenarios can be created ad infinitum (Marshall & Galea, 2015); but they are virtual, in the sense that the “real world” data against which the modeler attempts to compare the inferred counterfactual are actually not real -- only calibrated using real world data. Unfortunately, use of conventional methods to test models of synergistic disease interaction or the effectiveness of multicomponent interventions can encounter intractable difficulties due to the resource demands of full factorial study designs (Tsai & Burns, 2015; Tsai & Venkataramani, 2016). Thus, the disadvantages of using simulated data are potentially offset by the efficiency and promise of understanding how syndemics unfold and how they can be effectively addressed.
Incorporating insights from anthropological field work
Statistical and mathematical models must often be simplified to permit their estimation, but doing so can run the risk of over-simplifying disease co-dynamics (Rhodes et al., 2005). Anthropological approaches, on the other hand, can generate rich data that do not require reductive analyses. These range from ethnography to narrative interviews and mixed methods, and can provide unique insights into under-researched political, economic, and cultural mechanisms that influence disease clustering and amplify disease burden (Maher, 2002). The study of syndemics through an anthropological lens is perhaps best exemplified by the work of Mendenhall and colleagues (Mendenhall, 2012, 2015; Mendenhall et al., 2012).
Anthropological data can complement epidemiological and simulation studies by providing microfoundations for macro-level observations (Agar, 2005; Dean et al., 2000; Geller & Moss, 2008; Tubaro & Casilli, 2010; Yang & Gilbert, 2008). Moore et al. (2009), for example, describe a study of psychostimulant use and health outcomes among Australian youth in which ethnographic work informed some of the behavioral rules that were programmed into an agent-based model, and epidemiological data were used to validate the model’s emergent descriptions. More generally, anthropologists can develop concepts in the field, use the concepts to construct an agent-based model, and then test the consistency of their social theories by assessing the extent to which the emergent phenomena match their field observations. Some anthropologists are already employing similarly oriented workflows -- minus the agent-based modeling -- to good effect (Brown et al., 2009; Kohrt et al., 2009). Agent-based models can also suggest new questions for the fieldwork, thereby improving ongoing anthropological research.
Conclusion
The theory of syndemics has the potential to help policymakers and program implementers in their endeavors to improve population health. As theorized, syndemics are complex, multilevel phenomena, and there remain important opportunities to investigate how epidemics interact both at the level of populations and at the level of individuals and how they evolve across space and time. While the theory of syndemics has become an increasingly popular heuristic for advocacy, notably in the field of HIV treatment and prevention, most empirical studies purporting to validate the theory actually do no such thing. Their vacuousness is neatly illustrated in this novel contribution by Tomori et al. (2018). However, rather than broadening the concept of a syndemic, the field needs to significantly sharpen the theory’s empirical predictions so that investigators can have specific, falsifiable hypotheses to test using actual data. There is a danger that the haphazardly expanding concept of a syndemic will generate predictions so diffuse that the theory is rendered useless. I have suggested a few promising avenues for advancing the empirical literature on syndemics in the hope that evidence can be mobilized to improve the health and psychosocial wellbeing of vulnerable populations worldwide.
Acknowledgments:
I thank, without implicating, Brian T. Chan, John D. Kraemer, and Mark J. Siedner for their helpful comments on a previous draft of this manuscript.
BIBLIOGRAPHY
- Adeboye A, Ross MW, Wilkerson MJ, Springer A, Ahaneku H, Yusuf RA, et al. (2017). Syndemic production of HIV infection among Tanzanian MSM. Journal of Health Education Research & Development, 5(3), 1000231. [Google Scholar]
- Agar M (2005). Agents in living color: towards emic agent-based models. Journal of Artificial Societies and Social Simulation, 8(1). [Google Scholar]
- Batchelder AW, Gonzalez JS, Palma A, Schoenbaum E, & Lounsbury DW (2015). A social ecological model of syndemic risk affecting women with and at-risk for HIV in impoverished urban communities. American Journal of Community Psychology, 56(3–4), 229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearman PS, Moody J, & Stovel K (2004). Chains of affection: the structure of adolescent romantic and sexual networks. American Journal of Sociolology, 110(1), 44–91. [Google Scholar]
- Bekhuis E, Schoevers RA, van Borkulo CD, Rosmalen JG, & Boschloo L (2016). The network structure of major depressive disorder, generalized anxiety disorder and somatic symptomatology. Psychological Medicine, 46(14), 2989–2998. [DOI] [PubMed] [Google Scholar]
- Berkman LF, Kawachi I, & Glymour M (Eds.) (2014). Social epidemiology Oxford: Oxford University Press. [Google Scholar]
- Berkwits M (2000). CAPTURE! SHOCK! EXCITE! Clinical trial acronyms and the “branding” of clinical research. Annals of Internal Medicine, 133(9), 755–759. [DOI] [PubMed] [Google Scholar]
- Blakely TA, & Woodward AJ (2000). Ecological effects in multi-level studies. Journal of Epidemiology and Community Health, 54(5), 367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonabeau E (2002). Agent-based modeling: methods and techniques for simulating human systems. Proceedings of the National Academy of Sciences of the United States of America, 99 Suppl 3, 7280–7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringmann LF, Lemmens LH, Huibers MJ, Borsboom D, & Tuerlinckx F (2015). Revealing the dynamic network structure of the Beck Depression Inventory-II. Psychological Medicine, 45(4), 747–757. [DOI] [PubMed] [Google Scholar]
- Brown RA, Kuzara J, Copeland WE, Costello EJ, Angold A, & Worthman CM (2009). Moving from ethnography to epidemiology: lessons learned in Appalachia. Annals of Human Biology, 36(3), 248–260, 242 p following 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerda M, Tracy M, Ahern J, & Galea S (2014). Addressing population health and health inequalities: the role of fundamental causes. American Journal of Public Health, 104 Suppl 4, S609–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coie JD, Watt NF, West SG, Hawkins JD, Asarnow JR, Markman HJ, et al. (1993). The science of prevention. A conceptual framework and some directions for a national research program. American Psychologist, 48(10), 1013–1022. [DOI] [PubMed] [Google Scholar]
- Coleman JS (1990). Foundations of social theory Cambridge: Belknap Press. [Google Scholar]
- Cramer AO, Waldorp LJ, van der Maas HL, & Borsboom D (2010). Comorbidity: a network perspective. Behavioral and Brain Sciences, 33(2–3), 137–150; discussion 150–193. [DOI] [PubMed] [Google Scholar]
- Cronholm PF, Forke CM, Wade R, Bair-Merritt MH, Davis M, Harkins-Schwarz M, et al. (2015). Adverse childhood experiences: expanding the concept of adversity. American Journal of Preventive Medicine, 49(3), 354–361. [DOI] [PubMed] [Google Scholar]
- Dean JS, Gumerman GJ, Epstein JM, Axtell RL, Swedlund AC, Parker MT, et al. (2000). Understanding Anasazi culture change through agent-based modeling. In: Kohler TA & Gummerman GJ (Eds.), Dynamics in human and primate societies: agent-based modeling of social and spatial processes (pp. 179–205). Oxford: Oxford University Press. [Google Scholar]
- Diederichs C, Berger K, & Bartels DB (2011). The measurement of multiple chronic diseases--a systematic review on existing multimorbidity indices. Journal of Gerontology: Medical Sciences, 66(3), 301–311. [DOI] [PubMed] [Google Scholar]
- Diez-Roux AV (1998). Bringing context back into epidemiology: variables and fallacies in multilevel analysis. American Journal of Public Health, 88(2), 216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake RE, McLaughlin P, Pepper B, & Minkoff K (1991). Dual diagnosis of major mental illness and substance disorder: an overview. New Directions for Mental Health Services, (50), 3–12. [DOI] [PubMed] [Google Scholar]
- Duncan C, Jones K, & Moon G (1998). Context, composition and heterogeneity: using multilevel models in health research. Social Science and Medicine, 46(1), 97–117. [DOI] [PubMed] [Google Scholar]
- Eisenberg JN, Scott JC, & Porco T (2007). Integrating disease control strategies: balancing water sanitation and hygiene interventions to reduce diarrheal disease burden. American Journal of Public Health, 97(5), 846–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallowfield L, & Jenkins V (2002). Acronymic trials: the good, the bad, and the coercive. Lancet, 360(9346), 1622. [DOI] [PubMed] [Google Scholar]
- Farmer P (1996). Women, poverty, and AIDS. In: Farmer P, Connors M & Simmons J (Eds.), Women, poverty and AIDS: sex, drugs, and structural violence (pp. 3–38). Monroe: Common Courage Press. [Google Scholar]
- Ferlatte O, Dulai J, Hottes TS, Trussler T, & Marchand R (2015). Suicide related ideation and behavior among Canadian gay and bisexual men: a syndemic analysis. BMC Public Health, 15, 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galderisi S, Rucci P, Kirkpatrick B, Mucci A, Gibertoni D, Rocca P, et al. (2018). Interplay among psychopathologic variables, personal resources, context-related factors, and real-life functioning in individuals with schizophrenia: a network analysis. JAMA Psychiatry, in press. Epub 14 Feb 2018. doi: 10.1001/jamapsychiatry.2017.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller A, & Moss S (2008). Growing qawm: an evidence-driven declarative model of Afghan power structures. Advances in Complex Systems, 11(2), 321–335. [Google Scholar]
- Geronimus AT (1992). The weathering hypothesis and the health of African-American women and infants: evidence and speculations. Ethnicity and Disease, 2(3), 207–221. [PubMed] [Google Scholar]
- Granovetter M (1978). Threshold models of collective behavior. American Journal of Sociolology, 83(6), 1420–1443. [Google Scholar]
- Hedström P (2005). Dissecting the social: on the principles of analytical sociology Cambridge: Cambridge University Press. [Google Scholar]
- Hedström P, & Bearman P (Eds.) (2009). Oxford handbook of analytical sociology Oxford: Oxford University Press. [Google Scholar]
- Herrick AL, Lim SH, Plankey MW, Chmiel JS, Guadamuz TE, Kao U, et al. (2013). Adversity and syndemic production among men participating in the multicenter AIDS cohort study: a life-course approach. American Journal of Public Health, 103(1), 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohrt BA, Speckman RA, Kunz RD, Baldwin JL, Upadhaya N, Acharya NR, et al. (2009). Culture in psychiatric epidemiology: using ethnography and multiple mediator models to assess the relationship of caste with depression and anxiety in Nepal. Annals of Human Biology, 36(3), 261–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N (1994). Epidemiology and the web of causation: has anyone seen the spider? Social Science and Medicine, 39(7), 887–903. [DOI] [PubMed] [Google Scholar]
- Krieger N (2000). Epidemiology and social sciences: towards a critical reengagement in the 21st century. Epidemiologic Reviews, 22(1), 155–163. [DOI] [PubMed] [Google Scholar]
- Kuh D, & Ben-Shlomo Y (1997). A life course approach to chronic disease epidemiology: tracing the origins of ill-health from early to adult life Oxford: Oxford University Press. [Google Scholar]
- Kuh D, Ben-Shlomo Y, Lynch J, Hallqvist J, & Power C (2003). Life course epidemiology. Journal of Epidemiology and Community Health, 57(10), 778–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman AF, Myers CP, & Corty E (1989). Assessment and classification of patients with psychiatric and substance abuse syndromes. Hospital and Community Psychiatry, 40(10), 1019–1025. [DOI] [PubMed] [Google Scholar]
- Li R, Cai Y, Wang Y, Sun Z, Zhu C, Tian Y, et al. (2016). Psychosocial syndemic associated with increased suicidal ideation among men who have sex with men in Shanghai, China. Health Psychology, 35(2), 148–156. [DOI] [PubMed] [Google Scholar]
- Link BG, & Phelan J (1995). Social conditions as fundamental causes of disease. Journal of Health and Social Behavior, 35(Extra Issue), 80–94. [PubMed] [Google Scholar]
- Maher L (2002). Don’t leave us this way: ethnography and injecting drug use in the age of AIDS. International Journal of Drug Policy, 13(4), 311–325. [Google Scholar]
- Marcus R (2014). Women’s discourse on the homeless experience: it’s about love and loss (doctoral dissertation) Storrs: University of Connecticut. [Google Scholar]
- Marcus R, & Singer M (2017). The PHAMILIS stigma syndemic among homeless women. In: Lerman S, Ostrach B & Singer M (Eds.), Foundations of biosocial health: stigma and illness interactions (pp. 107–132). Lanham: Lexington Books. [Google Scholar]
- Marshall BD, & Galea S (2015). Formalizing the role of agent-based modeling in causal inference and epidemiology. American Journal of Epidemiology, 181(2), 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, & Stellar E (1993). Stress and the individual. Mechanisms leading to disease. Archives of Internal Medicine, 153(18), 2093–2101. [PubMed] [Google Scholar]
- McNally RJ, Robinaugh DJ, Wu GWY, Wang L, Deserno MK, & Borsboom D (2015). Mental disorders as causal systems: a network approach to posttraumatic stress disorder. Clinical Psychological Science, 3(6), 836–849. [Google Scholar]
- Mellor JE, Smith JA, Learmonth GP, Netshandama VO, & Dillingham RA (2012). Modeling the complexities of water, hygiene, and health in Limpopo Province, South Africa. Environmental Science and Technology, 46(24), 13512–13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendenhall E (2012). Syndemic suffering: social distress, depression, and diabetes among Mexican immigrant women Walnut Creek: Left Coast Press. [Google Scholar]
- Mendenhall E (2015). Syndemic suffering in Soweto: violence and inequality at the nexus of health transition in South Africa. Annals of Anthropological Practice, 38(2), 300–316. [Google Scholar]
- Mendenhall E, Shivashankar R, Tandon N, Ali MK, Narayan KM, & Prabhakaran D (2012). Stress and diabetes in socioeconomic context: a qualitative study of urban Indians. Social Science and Medicine, 75(12), 2522–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D, Dray A, Green R, Hudson SL, Jenkinson R, Siokou C, et al. (2009). Extending drug ethno-epidemiology using agent-based modelling. Addiction, 104(12), 1991–1997. [DOI] [PubMed] [Google Scholar]
- Nehl EJ, Klein H, Sterk CE, & Elifson KW (2016). Prediction of HIV sexual risk behaviors among disadvantaged African American adults Using a syndemic conceptual framework. AIDS and Behavior, 20(2), 449–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil CA, & Sattenspiel L (2010). Agent-based modeling of the spread of the 1918–1919 flu in three Canadian fur trading communities. American Journal of Human Biology, 22(6), 757–767. [DOI] [PubMed] [Google Scholar]
- Orlowski JP, & Christensen JA (2002). The potentially coercive nature of some clinical research trial acronyms. Chest, 121(6), 2023–2026. [DOI] [PubMed] [Google Scholar]
- Patton MQ (1999). Enhancing the quality and credibility of qualitative analysis. Health Services Research, 34(5 Pt 2), 1189–1208. [PMC free article] [PubMed] [Google Scholar]
- Pottegård A, Haastrup MB, Stage TB, Hansen MR, Larsen KS, Meegaard PM, et al. (2014). SearCh for humourIstic and Extravagant acroNyms and Thoroughly Inappropriate names For Important Clinical trials (SCIENTIFIC): qualitative and quantitative systematic study. BMJ, 349, g7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes T, Singer M, Bourgois P, Friedman SR, & Strathdee SA (2005). The social structural production of HIV risk among injecting drug users. Social Science and Medicine, 61(5), 1026–1044. [DOI] [PubMed] [Google Scholar]
- Rhodes T, Stimson GV, Crofts N, Ball A, Dehne K, & Khodakevich L (1999). Drug injecting, rapid HIV spread, and the ‘risk environment’: implications for assessment and response. AIDS, 13 Suppl A, S259–269. [PubMed] [Google Scholar]
- Riley JC (1989). Sickness, recovery and death: a history and forecast of ill-health Iowa City: University of Iowa Press. [Google Scholar]
- Robinson AC, Knowlton AR, Gielen AC, & Gallo JJ (2016). Substance use, mental illness, and familial conflict non-negotiation among HIV-positive African-Americans: latent class regression and a new syndemic framework. Journal of Behavioral Medicine, 39(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ (1974). Synergy and antagonism in cause-effect relationships. American Journal of Epidemiology, 99(6), 385–388. [DOI] [PubMed] [Google Scholar]
- Schelling TC (1969). Models of segregation. American Economic Review, 59(2), 488–493. [Google Scholar]
- Schelling TC (1971). Dynamic models of segregation. Journal of Mathematical Sociology, 1(2), 143–186. [Google Scholar]
- Singer M (1996). A dose of drugs, a touch of violence, a case of AIDS: conceptualizing the SAVA syndemic. Free Inquiry in Creative Sociology, 24(2), 99–110. [Google Scholar]
- Singer M (2006). A dose of drugs, a touch of violence, a case of AIDS, part 2: further conceptualizing the SAVA syndemic. Free Inquiry in Creative Sociology, 34(1), 39–53. [Google Scholar]
- Singer M, & Clair S (2003). Syndemics and public health: reconceptualizing disease in bio-social context. Medical Anthropology Quarterly, 17(4), 423–441. [DOI] [PubMed] [Google Scholar]
- Solomon SS, Lucas GM, Celentano DD, Sifakis F, & Mehta SH (2013). Beyond surveillance: a role for respondent-driven sampling in implementation science. American Journal of Epidemiology, 178(2), 260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon SS, Mehta SH, Srikrishnan AK, Vasudevan CK, McFall AM, Balakrishnan P, et al. (2015). High HIV prevalence and incidence among MSM across 12 cities in India. AIDS, 29(6), 723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spirtes P, & Glymour C (1991). An algorithm for fast recovery of sparse causal graphs. Social Science Computer Review, 9(1), 62–72. [Google Scholar]
- Stall R, Coulter RWS, Friedman MR, & Plankey MW (2015). Commentary on “Syndemics of psychosocial problems and HIV risk: A systematic review of empirical tests of the disease interaction concept” by A. Tsai and B. Burns. Social Science and Medicine, 145(1), 129–131. [DOI] [PubMed] [Google Scholar]
- Stall R, Friedman M, & Catania JA (2007). Interacting epidemics and gay mean’s health: a theory of syndemic production among urban gay men. In: Wolitski RJ, Stall R & Valdiserri RO (Eds.), Unequal opportunity: health disparities affecting gay and bisexual men in the United States (pp. 251–274). New York: Oxford University Press. [Google Scholar]
- Stall R, Mills TC, Williamson J, Hart T, Greenwood G, Paul J, et al. (2003). Association of co-occurring psychosocial health problems and increased vulnerability to HIV/AIDS among urban men who have sex with men. American Journal of Public Health, 93(6), 939–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomori C, McFall AM, Solomon SS, Aylur SK, Anand S, Balakrishnan K, et al. (2018). Is there synergy in syndemics? Psychosocial conditions and sexual risk among men who have sex with men in India. Social Science and Medicine, in press. Epub 23 March 2018. doi: 10.1016/j.socscimed.2018.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai AC, & Burns BFO (2015). Syndemics of psychosocial problems and HIV risk: a systematic review of empirical tests of the disease interaction concept. Social Science and Medicine, 139(1), 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai AC, Mendenhall E, Trostle JA, & Kawachi I (2017). Co-occurring epidemics, syndemics, and population health. Lancet, 389(10072), 978–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai AC, & Venkataramani AS (2016). Syndemics and health disparities: a methodological note. AIDS and Behavior, 20(2), 423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubaro P, & Casilli AA (2010). “An ethnographic seduction”: how qualitative research and agent-based models can benefit each other. Bulletin de Méthodologie Sociologique, 106, 59–74. [Google Scholar]
- van den Akker M, Buntinx F, & Knottnerus JA (1996). Comorbidity or multimorbidity; what’s in a name? A review of literature. European Journal of General Practice, 2(2), 65–70. [Google Scholar]
- VanderWeele TJ (2015). Explanation in causal inference: methods for mediation and interaction New York: Oxford University Press. [Google Scholar]
- Wagner EG, & Lanoix JN (1958). Excreta disposal for rural areas and small communities. WHO monograph series no. 39 Geneva: World Health Organization. [PubMed] [Google Scholar]
- Wang Z, Zhao X, Zhang Z, Luo M, Shen Q, Dong Y, et al. (2017). Co-occurring psychosocial problems and multiple sexual partners among men who have sex with men in Shanghai, China: a syndemic approach. Journal of Sex Research, in press. Epub 8 Dec 2017. doi: 10.1080/00224499.2017.1399333. [DOI] [PubMed] [Google Scholar]
- Widener MJ, Metcalf SS, & Bar-Yam Y (2013). Agent-based modeling of policies to improve urban food access for low-income populations. Applied Geography, 40(1), 1–10. [Google Scholar]
- Wright S (1920). The relative importance of heredity and environment in determining the piebald pattern of guinea-pigs. Proceedings of the National Academy of Sciences of the United States of America, 6(6), 320–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S (1921). Correlation and causation. Journal of Agricultural Research, 20(7), 557–585. [Google Scholar]
- Yang L, & Gilbert N (2008). Getting away from numbers: using qualitative observation for agent-based modeling. Advances in Complex Systems, 11(2), 175–185. [Google Scholar]
- Zhang J, Tong L, Lamberson PJ, Durazo-Arvizu RA, Luke A, & Shoham DA (2015). Leveraging social influence to address overweight and obesity using agent-based models: the role of adolescent social networks. Social Science and Medicine, 125, 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]


