Abstract
Averaging behavioral data such as the nictitating membrane response (NMR) across subjects can conceal important individual and group differences. Analyses were conducted of NMR data from rabbits that were grouped based on the point during NMR conditioning when subjects produced 8 conditioned responses (CR) in a set of 10 trials. This resulted in five groups (Early Day 1, Late Day 1, Early Day 2, Late Day 2, Early Day 3) in which group differences in CR acquisition rates were found. Percent (%) CRs were not found to increase monotonically and between-session differences in % CR were found. Conditioning-specific reflex modification (CRM) of the NMR is a type of enhanced reflexive responding of the NMR that is detected when the unconditioned stimulus (US) is presented in the absence of the conditioned stimulus (CS) following paired classical conditioning. CRM occurred in some subjects in all five groups. Subjects from both the group that was fastest and the group that was slowest to reach the learning criterion had unconditioned response (UR) topographies following NMR conditioning that strongly resembled the CR-UR response sequence elicited during NMR conditioning. This finding was most pronounced when the US duration used to assess CRM was equivalent to that used during NMR conditioning, further evidence to support the hypothesis that CRM is a CR that has generalized from the CS to the US. While grouping data based on conditioning criteria did not facilitate identifying individuals more predisposed to exhibiting CRM, strong CRM only occurred in the groups that reached the conditioning criterion the fastest.
Keywords: Classical conditioning, Eyeblink, Reflex modification, NMR conditioning, Rabbit
1. Introduction
Schreurs, Oh, Hirashima, and Alkon (1995) reported that following robust nictitating membrane response (NMR) conditioning using a delay paradigm with a tone conditioned stimulus (CS) paired with an aversive electrodermal stimulation (ES) unconditioned stimulus (US) to the periorbital area, exaggerated responding to the US occurred when the US was later presented to the rabbit in the absence of the CS, a phenomenon termed conditioning-specific reflex modification (CRM). This exaggerated reflexive responding includes an increase in unconditioned response (UR) amplitude and area and a shift to later UR peak latencies than the rabbit exhibited prior to CS-US pairings. The basic CRM experiment is an ABA design where the baseline level of responding to USs of varying intensities and durations is assessed prior to (Pretest) and following (Posttest) NMR conditioning. CRM is associative in nature and has been found to be “conditioning-specific” because while it is observed in rabbits receiving CS-US pairings, it is not observed in sit control rabbits, nor in those given explicitly unpaired CS and US presentations (Schreurs et al., 1995). Gruart and Yeo (1995) and Wikgren and Korhonen (2001) have also reported enhanced reflexive responding to the US following classical conditioning of the NMR in rabbits.
Early observations of CRM noted the striking similarity between the topography of the UR and the CR following conditioning. More speci-fically, Gruart and Yeo (1995) and Schreurs et al. (1995) found that following CS-US pairings, the topography of the NMR during US-alone trials closely resembled topographies of the CR-UR response sequence elicited during acquisition, particularly at US intensities milder than the training intensity. From Pretest to Posttest, the UR developed from a uniphasic response to a multiphasic response with an increased amplitude and area. Additionally, on Posttest the UR peaks shifted later from US onset toward the point where the US would have occurred had the US-alone trial been a CS-US trial. For example, Schreurs, Smith-Bell, and Burhans (2011a) found that rabbits given CS-US pairings had significantly later peak latencies on Posttest at both 0.25-mA and 0.5-mA, with peaks occurring ~200 ms following ES-onset, than they exhibited on Pretest, with peaks occurring within ~100 ms of ES onset. These changes in the basic NM reflex suggest that the conditioning-specific changes in the UR observed on Posttest, particularly at intensities milder than the training intensity, may be due to the UR becoming a CR that has generalized from the CS to the US (Gruart & Yeo, 1995). In other words, the US may be triggering the generation of the response pattern that normally was elicited to the CS during CS-US pairings (Schreurs et al., 1995).
Additional evidence in support of the CR generalization hypothesis is that the strength of CRM was found to be a function of the strength of NMR conditioning. More specifically, manipulations that produced greater NMR conditioning levels or rates also increased the strength of CRM (Burhans, Smith-Bell, & Schreurs, 2008). For example, while one day of CS-US pairings resulted in a low level of NMR conditioning (17% CRs), both three and six days of CS-US pairings resulted in levels of conditioning in excess of 90% CRs (Schreurs et al., 1995). CRM was not observed in the one-day group, only a UR peak latency shift was observed on Posttest in the three-day group and the most robust CRM was observed in the group receiving six sessions of NMR conditioning. In another study, 1-mA, 2-mA and 4-mA periorbital ESs were found to support increasing rates of NMR conditioning while consequently increasing CRM strength (Seager, Smith-Bell, & Schreurs, 2003).
However, additional studies suggested that although CRM may share similar associative processes with the CR, the two can also be dissociated, indicating that CRM cannot be fully explained by the generalized CR hypothesis. For example, Schreurs, Shi, Pineda, and Buck (2000) found that CRs and CRM do not extinguish similarly. While CRs extinguished well in rabbits presented with six sessions of CS-alone extinction, CRM remained intact though somewhat reduced in amplitude and area. Alternatively, when CRM was successfully extinguished via US-alone presentations, CRs remained intact. Meanwhile, unpaired extinction, which involved presentations of both the CS and US, was observed to most successfully extinguish both CRs and CRM.
If CRM is a generalized CR, a US modality that supports a high level of NMR conditioning should also elicit strong CRM. However, it was found that although both a 2-mA ES and a moderately intense 4-PSI air puff (AP) supported similar terminal levels of NMR conditioning, in excess of 90% following six days of pairings, only conditioning with the 2-mA ES resulted in strong CRM (Buck, Seager, & Schreurs, 2001). However, robust CRM was observed when a more intense, and presumably, more aversive, 8-PSI AP was employed during NMR conditioning.
If CRM is a generalized CR, we could expect to see the strongest CRM in subjects that are most strongly conditioned. Our lab has found that 99% of research subjects become highly conditioned (> 80% CR) to the tone CS but high levels of NMR conditioning do not necessarily ensure strong CRM. In fact, only approximately 25% of our subjects show strong CRM with the remaining subjects showing moderate levels, low levels or even no CRM (Smith-Bell, Burhans, & Schreurs, 2012). When correlations were examined between CR dependent variables (e.g., frequency, onset latency, and area under the response curve) and level of CRM, the strongest predictors of CRM, as indexed by an increase in percent change in the magnitude of the area of the UR when examined following six sessions of NMR conditioning, were CR onset latency and CR area. Those rabbits whose CRs began more immediately after the onset of the CS and those rabbits with larger CR areas were more likely to exhibit strong CRM than other subjects.
Previous CRM experiments reported NMR conditioning data averaged across all rabbits receiving paired NMR conditioning (Burhans, Smith-Bell, & Schreurs, 2015; Schreurs et al., 1995, 2000). However, averaging group NMR conditioning data is known to mask behavioral phenomena and group averages may suggest that all subjects learn at the same rate in a monotonically increasing fashion (Gallistel, Fairhurst, & Balsam, 2004; Halverson, Hoffmann, Kim, Kish, & Mauk, 2016). By dividing subjects into groups based on the time point in a session when a specific learning criterion was met, Halverson et al. (2016) found systematic differences in the rate of conditioning and in CR amplitude.
Within-experiment variations in the levels of rabbit NMR conditioning may have an anatomical explanation. Using trace conditioning, Woodruff-Pak, Lehr, Li, and Liu-Chen (2010) reported higher levels of binding of αβ heteromeric nicotinic acetylcholine receptors in the hippocampus of both young and old rabbits who were designated “good learners” rather than “poor learners” of a difficult trace conditioning task. Van der Zee, Kronforst-Collins, Maizels, Hunzicker-Dunn, and Disterhoft (1997) found significant differences between the level of protein kinase C-γ immunoreactivity in the hippocampus of trace conditioned rabbits designated “good leaners” versus “slow learners.”
Anatomical differences in the cerebellum could influence variability in NMR acquisition during delay conditioning as well. Differences in learning-related synapse formation could play a factor. Kleim et al. (2002) reported that rats undergoing eyeblink conditioning had more excitatory synapses per interpositus nucleus neuron than unpaired or naïve controls. Age-related Purkinje cell loss and consequent decreases in cerebellar volume have been linked to compromised performance on delay eyeblink conditioning tasks in C57BL/6 mice aged 9–12 months (Vogel, Ewers, Ross, Gould, & Woodruff-Pak, 2002), and controlling for age-related hearing loss, Woodruff-Pak (2006) found a marginally significant inverse relationship between Purkinje cell counts and trials to criterion on a delay eyeblink conditioning task in C57BL/6 mice aged 4, 8 and 12 months. Schreurs, Gusev, Tomsic, Alkon, and Shi (1998) noted a strong relationship between cerebellar lobule HVI Purkinje cell dendritic excitability and % CR following one day of paired delay NMR conditioning in rabbits.
Taking into consideration that overall acquisition averages may be masking individual or group differences in CR and UR dependent variables, we separated subject data into five groups based on when they met a specific learning criterion (Halverson et al., 2016). Of particular interest was whether grouping subjects by CR data would consequently result in grouping subjects by strength of CRM. Because we have found that only a small subset of subjects show strong levels of CRM, we hoped to delineate aspects of NMR conditioning that could better clarify the relationship between NMR conditioning and CRM strength and to add to the debate of whether CRM is a CR that has generalized from the CS to the US.
2. Materials and methods
2.1. Subjects
Data were analyzed from 145 rabbits that were classically conditioned using our standard NMR delay conditioning paradigm. The data came from 34 rabbits in a published study (Burhans et al., 2015) and from 111 rabbits in four unpublished studies collected over a period of several years. Subjects were male, New Zealand White rabbits (Oryctolagus cuniculus), supplied by Harlan (Indianapolis, IN, USA) or Charles River (Saint Constant, Quebec, Canada) weighing 2.0–2.2 kg and aged 69–77 days upon arrival. Rabbits were housed in individual cages, given free access to food and water, and kept on a 12-h light/dark cycle. Upon arrival, rabbits were acclimated to housing conditions for one week prior to any behavioral manipulations and maintained in accordance with National Institutes of Health guidelines. The research was approved by the West Virginia University Animal Care and Use Committee.
2.2. Apparatus
The apparatus, data collection and analysis procedures for NMR conditioning have been described in detail previously (Schreurs, Smith-Bell, & Burhans, 2011b) and were modeled after those described by Gormezano (Coleman & Gormezano, 1971). During each behavioral session, each rabbit was placed in a natural sitting position in an adjustable Plexiglas box with ears restrained between layers of foam padding. The restrained rabbit was placed inside a sound-attenuating chamber (Coulbourn Instruments, Allentown, PA; Model E10–20) facing a stimulus panel containing a speaker and a houselight (10-W, 120 V), mounted at a 45° angle 15 cm anterior to and dorsal to the rabbit’s head. Each chamber ventilation fan created a continuous ambient noise level of 77 dB inside the chamber. The US ES was delivered by a programmable two-pole stimulator (Coulbourn Instruments, Model E13–35) via insulated wires connected to stainless steel Autoclip wound clips (Stoelting, Wood Dale, IL) placed 10 mm ventral and 10 mm posterior to the dorsal canthus of the right eye. Stimulus delivery, data collection, and analyses were accomplished using a custom programmed LabVIEW software system (National Instruments, Austin, TX).
The NMRs were transduced by a potentiometer (Novotechnik US Inc., Southborough, MA; Model P2201) connected at one end, via a freely moving ball and socket joint, to an L-shaped lever containing a hook that attached to a 6–0 nylon loop sutured into but not through the NM of the rabbit’s right eye. At the other end, the potentiometer was connected to a 12-bit analog-to-digital converter (5-ms sampling rate, 0.05-mm resolution), and individual A/D outputs were stored on a trial-by-trial basis for future analysis.
2.3. Procedure
All 145 rabbits received one behavioral session per day in the following order: one 30-min session of restraint habituation, one 80-min session of restraint and training chamber adaptation, one 80-trial session of US-alone testing (Pretest), six sessions of 80 paired delay CS–US presentations, and another 80-trial session of US-alone testing (Posttest). During the restraint habituation session, rabbits were placed in the Plexiglas restrainer with their ears restrained in the foam padding for 30 min. During the adaptation session, rabbits were restrained and prepared for US presentations and the recording of the NMR and adapted to the training chambers for the duration of subsequent sessions (80 min). On US-alone Pretest and Posttest sessions, rabbits received 80 US-alone trials presented on average every 60 s (50–70-s range). Each trial involved the presentation of one of 20 combinations of US intensity (0.1, 0.33, 0.5, 1.0, or 2.0-mA) and duration (10, 25, 50, or 100 ms). Four pseudorandomized sequences of the 20 combinations were presented during each US testing session, and the same intensity or duration did not occur on more than three consecutive trials. Each of the six NMR conditioning sessions consisted of 80 presentations of a 400-ms, 1-kHz, 82-dB tone CS co-terminating with a 100-ms, 2.0-mA US (300-ms ISI), delivered on average every 60 s (50–70-s range).
2.4. Data analysis
During the NMR conditioning phase of the experiments, movement of the NM was scored as a CR when the NM extension exceeded 0.5 mm and was initiated after CS onset but before US onset. The CR frequency was calculated as well as CR amplitude as the maximum NM extension in millimeters. CR onset latency was calculated as the latency from CS onset for a CR to reach 0.1-mm above baseline and CR criterion latency was calculated as the latency from CS onset for a response to reach a0.5-mm criterion. These dependent variables were measured in the time window from CS onset to US onset to avoid contamination by the subsequent presentation of the US. Area under the response curve (in arbitrary units) was calculated for the entire trial period that included the presentation of the US.
During the US-alone phase of the experiments (Pretest and Posttest), movement of the NM was scored as a UR when the NM extension exceeded 0.5 mm and was initiated within 300 ms of the onset of the US. During several studies when CRM was observed, a strong resemblance was noted between the UR topographies to lower intensity USs (0.25-mA and 0.5-mA) on Posttest and to CR–UR topographies during CS–US pairings, particularly in well-conditioned subjects (Buck et al., 2001; Burhans et al., 2008). UR frequency was calculated as well as UR amplitude as the maximum NM extension in millimeters. UR area was also calculated as the total area under the response curve in arbitrary units. To overcome the statistical limitations of empty data cells produced by subthreshold responses to the US, especially at low intensities, two additional UR measures were calculated, magnitude of the response amplitude and magnitude of the response area. These measures included the amplitude and area data from all NM responses above baseline (Garcia, Mauk, Weidemann, & Kehoe, 2003) even when the URs did not exceed 0.5 mm. Unless otherwise noted, reported UR data were for five US intensities (0.1, 0.33, 0.5, 1.0, and 2.0-mA) presented across the first 20 trials of Pretest and Posttest and averaged across four durations (10, 25, 50 and 100 ms).
Additional calculations include % Change from Pretest to Posttest in UR frequency, magnitude of UR amplitude and magnitude of UR area (Smith-Bell et al., 2012). Increases in dependent-variable measures from Pretest to Posttest were indicated by a positive % Change and decreases were indicated by a negative % Change. Trials with no response on Pretest but a response on Posttest were set as a 100% Change while trials with no response on Posttest but a response on Pretest were set as a −100% Change (Smith-Bell et al., 2012). To determine relationships between responding during NMR conditioning and CRM, we calculated correlation coefficients for CR frequency, CR onset latency, and CR area versus % Change in UR frequency, magnitude of UR amplitude, and magnitude of UR area.
It is lab convention to present Pretest and Posttest NMR topographies separated by intensity and averaged across groups and US durations (10, 25, 50 and 100 ms) just as it has been our convention to focus on the first set of 20 trials of US test data (Schreurs et al., 2000). The relatively mild US intensities of 0.33 and 0.5-mA generally elicit minimal responding on Pretest but following NMR conditioning elicit enhanced URs, and are thus an excellent intensity at which to measure CRM. To examine the shape and timing of NMRs during the first daily trials of NMR conditioning, response topographies were generated by averaging the first 500 ms of data, prior to the presentation of the US, across groups of rabbits. Individual NMR conditioning data are presented in topographical form as well.
The 145 subjects were divided into groups based on the point during the six sessions of NMR conditioning when they met the criterion of producing a CR 8 times within 10 consecutive trials (the 8/10 CR Criterion). The groups are referred to as Early Day 1 (meeting criterion within the first 40 trials of Session 1), Late Day 1 (meeting criterion within the final 40 trials of Session 1), Early Day 2 (meeting criterion within the first 40 trials of Session 2), Late Day 2 (meeting criterion within the final 40 trials of Session 2), and Early Day 3 (meeting criterion within the first 40 trials of Session 3). All subjects achieved one of these criteria.
2.5. Statistical analysis
Data were analyzed by repeated measures analysis of variance (ANOVA) using SPSS 18.0 (SPSS Inc., Chicago, IL, USA). Planned and follow up comparisons were Bonferroni corrected for the number of comparisons.
3. Results
3.1. Percentage CRs and CR amplitude
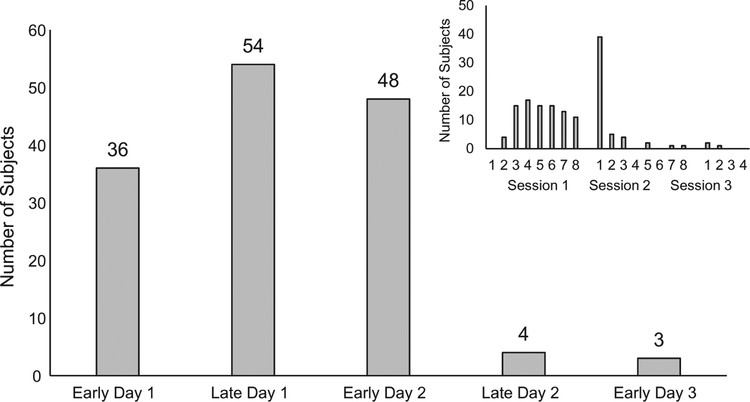
Fig. 1 shows the number of subjects reaching the 8/10 criterion in each of the consecutive half-sessions (larger plot) and in each block of ten trials (smaller plot). As can be seen in the larger plot, 25% (36) of the subjects reached criterion in Early Day 1, and 37% (54) reached criterion in Late Day 1. In the smaller plot, it can be seen that, after the first two blocks, these subjects were distributed fairly evenly throughout Day 1. Most of the remaining subjects (33%, 48) achieved criterion in Early Day 2, and, as shown in the smaller plot, most of those achieved criterion in the first block of Day 2.
Fig. 1.
The number of subjects assigned to each of five groups based on the point during six daily sessions of nictitating membrane response conditioning when they produced a conditioned response 8 times within 10 consecutive trials. All subjects achieved this criterion by Early Day 3. The groups are referred to as Early Day 1, Late Day 1, Early Day 2, Late Day 2 and Early Day3. The inset shows the specific 10-trial block during which subjects met criterion.
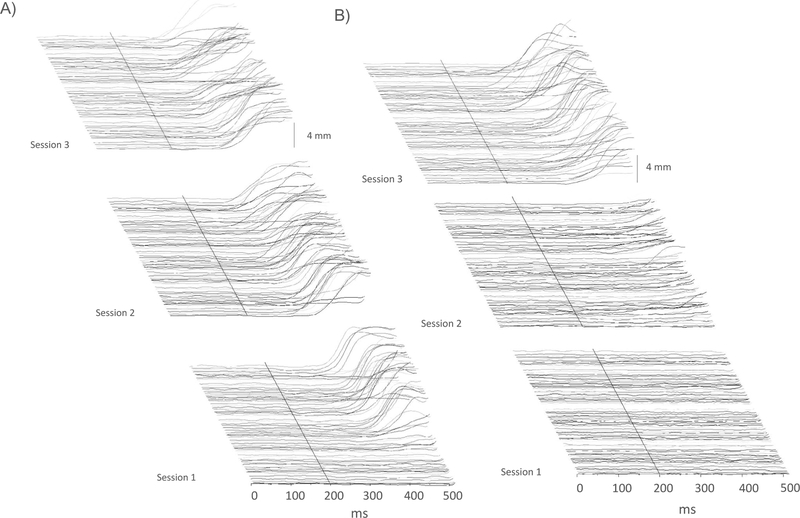
Waterfall plots in Fig. 2 show the NMR topographies during Sessions 1–3 of NMR conditioning for representative subjects from the Early Day 1 group (Panel A) and from the Early Day 3 group (Panel B). Panel A shows that CRs develop quite rapidly in the Early Day 1 subject and that once this subject begins producing CRs, they are sustained throughout the first three sessions of NMR conditioning. Panel B shows a subject with a slower and more sporadic emergence of CRs during Session 2, finally meeting criterion early in Session 3 and thereafter maintaining a high level of conditioned responding.
Fig. 2.
Waterfall plots of 80 trial-by-trial nictitating membrane response (NMR) topographies elicited during the first three sessions of NMR conditioning for a subject in the Early Day 1 Group that rapidly conditioned (Panel A) and for a subject in the Early Day 3 Group, the group that was the slowest to condition (Panel B). Session 1 Trial 1 is oriented at the bottom of the plots while Session 3 Trial 80 is oriented at the top of the plots. The x-axis scale is from 0 to 500 ms, with the tone conditioned stimulus presented at 200 ms where a solid black line has been placed. The plots end where the electrodermal stimulation unconditioned stimulus begins (500 ms); therefore, the unconditioned response is not shown.
Analysis of % CRs across all subjects yielded a significant main effect of sessions (F (5, 720) = 651.561, p < .001) with planned comparisons indicating a significant increase in % CRs from Session 1–2 (p < .001). Inspection of the raw % CR data revealed great variability in the individual % CR level during Session 2, with values ranging from 0 to 100% CRs, indicating that Session 2 is in fact, not an “aha” moment for all subjects.
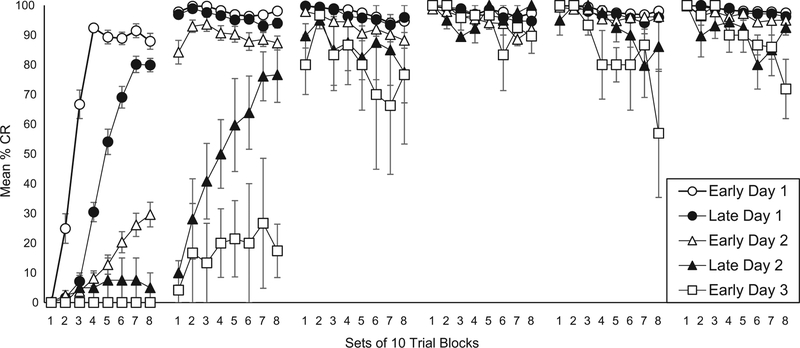
To examine possible between-session differences in % CR, data from each session were analyzed by eight sets of 10-trial blocks. Fig. 3 shows the mean % CR curves for each of the five derived groups. The Early Day 1 and Late Day 1 groups ended Session 1 with 87.9% CRs and 79.9% CRs, respectively, and both began Session 2 in excess of 95% CRs. Subsequently, the Early Day 2 group ended Session 1 at 29.6% CRs but began Session 2 at 84.3% CR, a dramatic between-session increase. The Early Day 3 group similarly exhibited a dramatic between-session increase from the end of Session 2 (17.4% CRs) to the beginning of Session 3 (80% CRs). Corroborating these between-session differences across groups, analysis of % CR yielded a significant interaction of Sessions X 10-trial Blocks X Groups (F (140,4900) = 17.047, p < .001).
Fig. 3.
Mean (± SEM) percentage conditioned responses (CRs) to the tone conditioned stimulus during eight 10-trial blocks during each of six daily sessions of nictitating membrane response (NMR) conditioning for five groups of rabbits. Subjects were assigned to one of five groups based on the point during NMR conditioning when they produced a CR 8 times in 10 consecutive trials. The group assignments are: Early Day 1 (white circles), Late Day 1 (black circles), Early Day 2 (white triangles), Late Day 2 (black triangles) and Early Day 3 (white squares).
To further test between-session improvements in % CR, a more focused analysis was conducted on the final 10 trials of Session 1 and the first 10 trials of Session 2. An analysis of % CR of these two blocks of 10 trials yielded a significant main effect of 10-trial Blocks (F(1140) = 25.755, p < .001) and a significant interaction of 10-trial Blocks X Groups (F (4140) = 28.530, p < .001). Planned comparisons yielded significant increases in % CR from the final 10 trials of Session 1 to the first 10 trials of Session 2 for the Early Day 1 group (p < .01), for the Late Day 1 group (p < .001) and for the Early Day 2 group (p < .001) but not for the Late Day 2 and Early Day 3 groups.
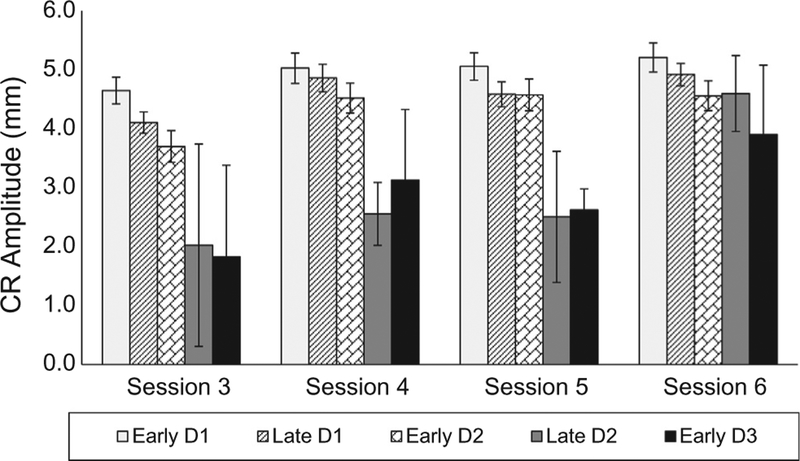
Fig. 4 depicts the averaged maximal CR amplitude for each of the five derived groups for Trial 1 of Sessions 3–6 because by Session 3, all rabbits had met the 80% CRs criterion and were reliably producing CRs. An analysis of CR amplitude yielded a significant main effect of Sessions (F (3408) = 9.397, p < .001) but no Sessions X Groups interaction. Follow-up comparisons yielded only a significant increase in CR amplitude from Session 3–4 (p < .001). While CR amplitude increased across sessions, group assignment did not appear significantly related to CR response size across sessions.
Fig. 4.
Mean (± SEM) nictitating membrane response (NMR) conditioned response (CR) peak amplitudes (measured in millimeters) during the CR period before unconditioned stimulus onset for each group for the first tone presentation (Trial 1) of Sessions 3, 4, 5, and 6 of NMR conditioning. Subjects were assigned to one of five groups based on the point during NMR conditioning when they produced a CR 8 times in 10 consecutive trials. The group assignments are: Early Day 1 (white bars), Late Day 1 (diagonal, black bars), Early Day 2 (diagonal brick bars), Late Day 2 (gray bars) and Early Day 3 (black bars).
3.2. Correlations between conditioned responding and CRM
Previously, our lab determined that approximately 25% of rabbits trained using our standard NMR conditioning paradigm would exhibit strong CRM (Smith-Bell et al., 2012). We reported that a subject’s susceptibility to expressing strong CRM, as evidenced by large positive changes from Pretest to Posttest in the frequency and magnitude of URs, was correlated with earlier CR onset latencies and larger CR areas during CS–US pairings. When the 145 animals in this study were combined, the data corroborate these findings for URs at the mild intensity of 0.33-mA. Specifically, larger area and earlier onset CRs are weakly correlated (r < 0.10) with increases in the percentage change from Pretest to Posttest in the UR frequency and in the magnitude amplitude and magnitude area of the UR. When these 0.33-mA data are separated by groups, small correlations (r = 0.10–0.29) are found between these measures of CRM and CR area and CR onset latency. As there are fewer significant correlations when the data are separated by groups than when all subjects are combined, there does not appear to be an advantage in separating the animals into groups based on CR performance to predict a group’s susceptibility to showing enhanced CRM.
A total of 61 rabbits (42.1%) from all 5 groups had at least one measure of CRM at 0.33, 0.5 and/or 1.0-mA that was one or more standard deviations above the mean % Change from Pretest to Posttest. These three particular US intensities were selected because CRM is most frequently observed at mild to moderate intensities (Schreurs et al., 1995). The percentage of subjects from the Early Day 1, Late Day 1, Early Day 2, Late Day 2 and Early Day 3 groups showing this level of CRM were 47%, 35%, 44%, 75%, and 33% respectively. Corroborating Smith-Bell et al. (2012) only 33 rabbits (23%) had at least one measure of CRM that was two standard deviations above the mean% change. The percentage of subjects from the derived groups showing this enhanced CRM were 28%, 19%, 27%, 0%, and 0% respectively. Grouping the data reveals that while CRM is demonstrated in all groups, the most enhanced CRM seems to be limited to the groups that meet the 8/10 CR Criterion the earliest.
3.3. UR amplitude
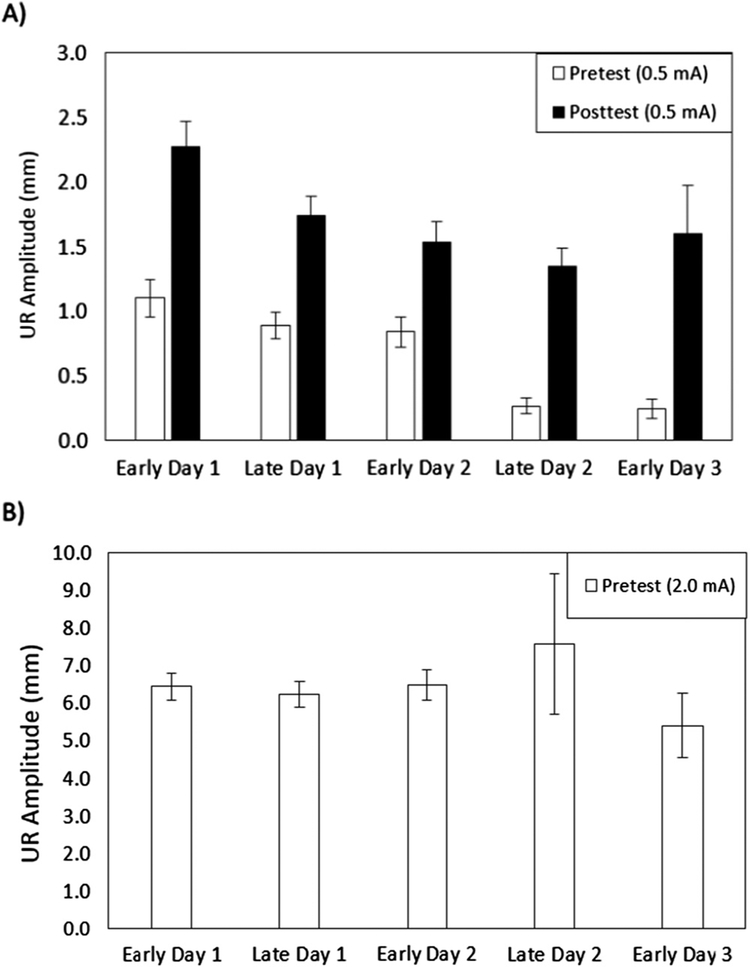
The Pretest and Posttest UR peak amplitude data at the 0.5-mA intensity are presented in Fig. 5, Panel A. The 0.5-mA trials were selected as they are the mid-point US intensity presented during Pretest and Posttest, and subjects tend to have more frequent above-baseline responding on Pretest at 0.5-mA than they do at 0.33-mA (with very little, if any, responding at 0.1-mA). Additionally, it can be difficult to assess CRM on the 1.0-mA and 2.0-mA trials as these intensities tend to produce very large Pretest responses that may be at or approaching the maximal NM response. Analysis yielded a significant main effect of Tests (F (1140) = 31.844, p < .001) indicating a Pretest to Posttest increase in UR amplitude which is indicative of CRM. However, there were no significant effects involving group. Note that the groups that were slowest to reach the 8/10 CR Criterion, the Late Day 2 and Early Day 3 groups, have the smallest Pretest UR amplitudes. While the initial reflexive responding to this mild 0.5-mA US is smaller than that of the other groups that go on to reach criterion more quickly, a one-way ANOVA conducted of the Pretest data found no significant between-group differences (F (4140) = 1.822, p = .128).
Fig. 5.
Mean (± SEM) maximum unconditioned response (UR) amplitude (measured in millimeters [mm]) to four presentations of a 0.5-mA unconditioned stimulus (US) during the Pretest (white bars) that occurred prior to a Posttest (black bars) which followed six daily sessions of nictitating membrane response (NMR) conditioning (Panel A). Mean (± SEM) maximum UR amplitude to the first presentation of a 100 ms, 2-mA US during Pretest (Panel B). This US was equivalent in duration and intensity to the US used during NMR conditioning. Subjects were assigned to one of five groups based on the point during NMR conditioning when they produced a conditioned response 8 times in 10 consecutive trials. The group assignments are: Early Day 1, Late Day 1, Early Day 2, Late Day 2 and Early Day 3.
Compared to the subjects that conditioned more quickly, the moderate 0.5-mA US during Pretest may have not been perceived as intensely by subjects in the two groups that were slowest to condition. In this case, the US would not reliably evoke climbing fibers to conduct the US signal to the Purkinje cells. We consequently assessed responding to the first presentation of the US utilized for conditioning (100 ms, 2-mA) during Pretest (Fig. 5, Panel B) and found that this US duration and intensity elicited URs of similar maximum amplitude among the five groups with no significant differences (F (4132) = 0.429, p = .788).
3.4. UR topographies and CRM
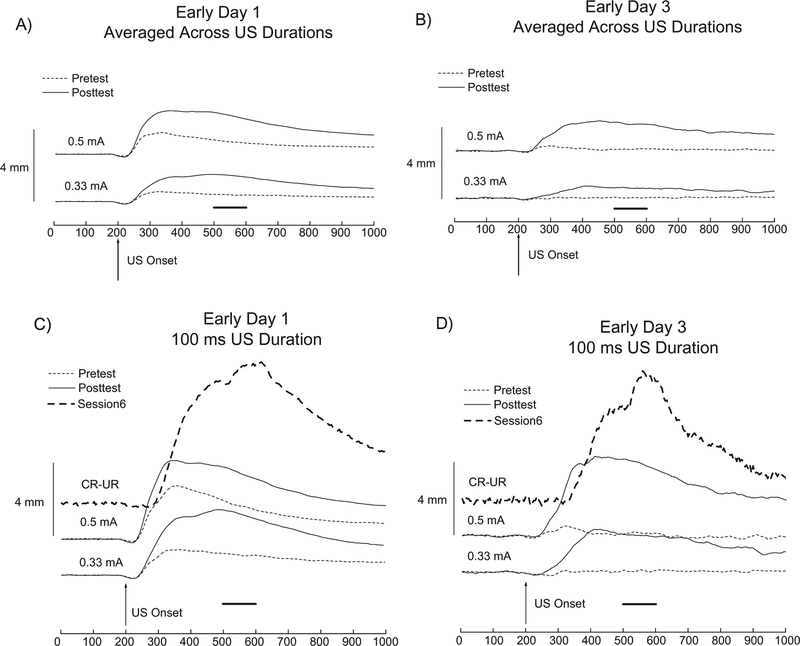
Fig. 6 shows NMR topographies for the 0.33 and 0.5-mA Pretest (dotted lines) and Posttest (solid lines) trials averaged across US durations during the first set of 20 trials for both the Early Day 1 Group (Panel A) and the Early Day 3 Group (Panel B). For both groups, CRM was evidenced by an increase in UR amplitude and area from Pretest to Posttest. An increase in peak latency on Posttest in response to the US was also observed (Buck et al., 2001), at times occurring near or when the US would have been presented had this been a paired CS-US trial (indicated by the solid black horizontal line between 500 and 600 ms).
Fig. 6.
Pretest (dotted line) vs Posttest (solid line) averaged group topographies of the unconditioned nictitating membrane response (NMR) averaged across four electrodermal unconditioned stimulus (US) durations for the Early Day 1 Group (n = 36) that rapidly conditioned (Panel A) and for the Early Day 3 Group (n = 3) that required two additional sessions to reach the 8/10 conditioned response (CR) criterion (Panel B). The top pair of traces of Panels A and B correspond to unconditioned responses (URs) averaged across four US durations (10, 25, 50 and 100 ms) at 0.5-mA during Pretest (dotted line) and Posttest (solid line) while the bottom pair of traces correspond to URs averaged across the same four durations at 0.33-mA during Pretest (dotted line) and Posttest (solid line). Data is similarly presented for the bottom two traces in Panels C and D, except that only the 100 ms US duration is shown. The top traces (dashed lines) of Panels C and D correspond to the averaged group CR-UR topography on Trial 1 of Session 6 of NMR conditioning. The Pretest was presented prior to and the Posttest was presented following six daily sessions of NMR conditioning. During Pretest and Posttest, the US was presented 200 ms after the start of the NMR trace recording (vertical arrows). Note the horizontal lines in the four panels correspond to the timing of the US presentation during NMR conditioning.
As the US employed during NMR conditioning was 100 ms in duration, this prompted us to focus on only the 100 ms duration 0.33 and 0.5-mA trials during the first set of 20 trials during Pretest and Posttest. Fig. 6 Panels C and D show remarkably robust CRM for the groups depicted in Panels A and B. A large increase in UR amplitude and area from Pretest to Posttest is visible for both the Early Day 1 Group (Panel C) and the Early Day 3 Group (Panel D) with the CRM elicited at the 100 ms duration being far more dramatic than when averaged across the four US durations. While the Pretest URs are uniphasic and smaller, the Posttest URs are more sprawling and multi-phasic in nature and are in some cases more than double the amplitude of the Pretest URs. Note that more than the Pretest topographies, the Posttest topographies resemble the shape and peak timing of the CR-UR from Trial 1 of Session 6 (top dashed traces).
While all subjects were trained to asymptote, CRM was observed in two groups that acquired CRs in very different manners. The Early Day 3 Group depicted in Fig. 6 Panels B and D met the 8/10 CR Criterion an entire two sessions later than the Early Day 1 Group depicted in Fig. 6 Panels A and C, with subjects giving over one hundred fewer CRs throughout the six sessions of NMR conditioning. Still, both groups similarly demonstrated an increase in UR area and amplitude from Pretest to Posttest with peaks of a more complicated nature occurring around the time that the US would have been presented had this been a paired CS-US trial.
4. Discussion
It is apparent from this study that averaging group data can mask important information about the conditioned rabbit NMR. Based on when subjects reached the criterion of producing 8 CRs in 10 consecutive trials during NMR conditioning (the “8/10 CR Criterion”), rabbits were divided into the Early Day 1, Late Day 1, Early Day 2, Late Day 2 and Early Day 3 groups. Analyzing CR data by 10-trial blocks revealed that % CR in the rabbit NM preparation does not always monotonically increase and revealed group and between-session differences in % CR. By analyzing CR and UR data by derived groups, we revealed that groups of rabbits do not respond uniformly to the CS or to the US.
This study also offers new information to support the hypothesis that CRM is a CR that has generalized from the CS to the US; however, this hypothesis still does not seem to completely explain CRM. It would have been ideal to find large correlations between group CR dependent variables and the incidence of CRM, as this may have improved our ability to predict a subject’s susceptibility to demonstrating CRM. Instead, the correlational and topographical data suggest CRM can be found in any group although the strongest CRM is limited to subjects that condition the fastest. It is worth noting that a full six sessions of NMR conditioning may have masked group differences in CRM. While some subjects did produce over one hundred fewer CRs than other subjects, all subjects were trained to asymptote and ultimately produced hundreds of CRs over the course of training.
4.1. Overnight incubation effects and consolidation of the CR
Our daily averaged data of 145 subjects suggest a between-session monotonic improvement in % CR for the rabbit NMR with the largest improvement in learning taking place between Session 1 and Session 2. The Early Day 1, the Late Day 1, and the Early Day 2 groups account for most of the subjects (138) in this study and do show dramatic increases in % CR from Session 1 to Session 2, with asymptotic or near-asymptotic performance during Session 2. The Late Day 2 group shows a similarly large increase in % CR from both Session 1 to Session 2 and from Session 2 to Session 3 while the Early Day 3 group shows only a dramatic improvement from Session 2 to Session 3. Additionally, the Late Day 2 and the Early Day 3 groups actually show between-session decrements in learning from Session 4 to Session 5. Clearly, between-sessions, the rabbit NM CR exhibits considerable individual and group variability (Gallistel et al., 2004).
Kehoe (2006) reported that for paired conditioning of the rabbit NMR, the CR acquisition rate was slowest within the first session and that the biggest and most reliable jump in % CR occurred between the first and second sessions of pairings. Post-training consolidation of the CS-US association quite likely plays a role in the session-to-session improvements in % CR with consolidation beginning immediately after learning, which has been shown to be dependent on the cerebellar cortex (Attwell, Cooke, & Yeo, 2002; Kellett, Fukunaga, Chen-Kubota, Dean, & Yeo, 2010; Scavio, Clift, & Wills, 1992).
Halverson et al. (2016) and Frey and Gavin (1975) have reported a benefit of overnight incubation of the partially conditioned eyeblink response. Frey and Gavin (1975) found that following paired training and during a recall test portion of the experiment, retention of the CR was found to be inferior when tested at intervals earlier than 24 h post-training (5 min, 4 h or 12 h) and was attributable to possible motor fatigue, decreased attention to the CS so soon after paired training, and to the CS-US association needing time to be rehearsed or consolidated. Interestingly, even the Early Day 1 and the Late Day 1 groups that were arguably well conditioned (≥80% CRs) at the end of Session 1, showed significant improvements following overnight incubation at the beginning of Session 2. It seems that the phenomenon of overnight incubation of the CR is not limited to only the partially conditioned rabbits but may also be demonstrated in well-conditioned subjects.
Between-subject variations in the efficiency of the neural pathways for NM conditioning may explain the individual differences in the rate of CR acquisition. Woodruff-Pak, Cronholm, and Sheffield (1990) found a significant negative correlation between Purkinje cell counts in the cerebellum and the number of trials to reach a learning criterion of 8 CRs in 9 consecutive trials in both young and middle-aged rabbits, indicating that the fewer Purkinje cells a subject had, the more trials needed to reach criterion. Additionally, they found a correlation between corrected cerebellar volume and performance on an eyeblink conditioning task in adult humans (Woodruff-Pak, Goldenberg, Downey-Lamb, Boyko, & Lemieux, 2000). In trace conditioning NM preparations that rely on the hippocampus, Woodruff-Pak et al. (2010) reported that rabbits that were “good learners” and “poor learners” had differing levels of hippocampal binding of αβ heteromeric nicotinic acetylcholine receptors and Van der Zee et al. (1997) reported significant differences in hippocampal levels of protein kinase C-γ immunoreactivity in “good leaners” versus “slow learners.”
4.2. Conditioning correlates of CRM
Schreurs et al. (1995) reported that the strength of CRM is dependent upon the strength of conditioning, finding more robust CRM in subjects given 3 or 6 daily sessions of classical conditioning than those given only one session of conditioning. While all the rabbits in this report reached the 8/10 CR Criterion by the early part of Session 3, some rabbits ultimately produced over 100 more CRs than other rabbits. Regardless of how many CRs a rabbit produced over the course of six sessions of NMR conditioning, some rabbits from all groups displayed CRM.
As previously reported (Burhans & Schreurs, 2008; Smith-Bell et al., 2012), approximately 25% of our subjects show robust CRM following paired NMR conditioning with a tone CS and a 2-mA electrodermal US. Examining the 145 subjects together, the current work corroborates these findings, specifically, that large CR areas and early CR onsets correlate with enhanced CRM though a more in-depth examination of the correlational data found that grouping rabbits based on the 8/10 CR Criterion does not further assist in predicting CRM.
While we found no significant group differences in Pretest UR amplitude at 0.5 or 2.0-mA or in CR peak amplitude during NMR conditioning, the Late Day 2 and the Early Day 3 groups clearly had smaller URs at 0.5-mA than did the other groups that reached the 8/10 CR Criterion more quickly. Regardless of the size of these responses, it is apparent that some rabbits in all groups show CRM as evidenced by an increase in UR amplitude from Pretest to Posttest.
4.3. CRM as a generalized CR
Chen, Bao, Lockard, Kim, and Thompson (1996), Schreurs, Oh, and Alkon (1996), Yang, Lei, Feng, and Sui (2015), and Freeman (2015) have described the neural circuitry required for delay eyeblink acquisition. Both the CS and US signals converge at the Purkinje cell from granule cells/parallel fibers and climbing fibers respectively. With stimulus pairings, long-term depression occurs at the parallel fiber to Purkinje cell synapses, the process underlying learning-related neural plasticity. The CS and US signals also converge at the deep cerebellar nuclei (DCN) via mossy fibers and climbing fiber collaterals respectively. This convergence and the consequent learning-related long-term potentiation ultimately lead to associative motor output. That both the CS and US signals converge at the DCN to produce the CR-UR suggests the possibility that the NMR produced on Posttest could be influenced by CS input.
As demonstrated in this study, the CR-UR sequence and the URs on Posttest following pairings share an undeniably similar topography that includes a latency shift in the UR peak amplitude. This finding was most pronounced when the US duration presented during Posttest was matched to the US duration presented during the six sessions of NMR conditioning. Recall that while CRM was demonstrated by some rabbits in each of the five groups, the strongest CRM was found only in the groups that were fastest to reach criterion.
Still, previous work in our lab has demonstrated a dichotomy between CRs and CRM. Schreurs et al. (2000) found that following CS-US pairings, extinction of the CR could best be achieved by CS-alone presentations that left CRM intact, though somewhat reduced in strength compared to animals who did not receive CS-alone trials. Additionally, CRM could be eliminated, but CRs preserved, by presenting US-alone trials following CS-US pairings.
The dichotomy between CRs and CRM extends to possible anatomical substrates. Using bilateral and unilateral lesions, Gruart and Yeo (1995) found that CRs were more sensitive to damage of the cerebellar cortex than were URs, confirming work in cats by Hesslow (1994) that CRs are much more likely than URs to be inhibited by stimulation of the c1 and c3 zones of the cerebellar cortex. Burhans and Schreurs (2008) also found that temporary inactivation of the central nucleus of the amygdala with muscimol impaired expression of CRM but not expression of CRs.
A CR versus CRM dichotomy was also found in a different system during an experiment exploring extinction of heart rate CRs and heart rate CRM in rabbits (Burhans, Smith-Bell, & Schreurs, 2010). Heart rate CRs could be extinguished via CS-alone OR unpaired CS/US presentations, but CRs remained stable when subjects received US-alone presentations or no further stimulus presentations. Meanwhile, heart rate CRM was found to diminish for subjects in all extinction groups except for one subset of rabbits in the unpaired CS/US presentation group.
4.4. Conclusion
Grouping NM data from rabbits based on when they met the 8/10 CR Criterion can reveal details of the CR and UR that are otherwise obscured by averaging session data across all subjects. Our results confirm work by others that the CR is not always a monotonically increasing response and that between-session incubation of the CR occurs.
Detailed examination of data from the groups that were earliest and latest to reach criterion revealed that, despite learning at very different rates, subjects from both groups exhibited CRM with UR topographies resembling the CR-UR sequence elicited during NMR conditioning, evidence to support the hypothesis that the UR following NMR conditioning is a CR that has generalized from the CS-US pairings to the US itself. Meanwhile, CR versus CRM correlational data lend support to the narrative that CRM cannot be fully explained as a generalized CR to the US. Grouping data did not enhance our ability to identify subjects that were more likely to show CRM but it did help delineate which subjects would not show enhanced CRM. Exploration of neuroanatomical differences could help to characterize which subjects are predisposed to learn the NMR conditioning task more slowly and consequently, which subjects are less likely to exhibit strong CRM. Also, examining data from subjects that have only just reached 80% CR, our standard criterion for inclusion in data analysis, and who have not received six entire sessions of NMR conditioning, may reveal more details about the relationship between CRs and CRM.
Acknowledgements
This work was supported in part by National Institute of Mental Health MH064715. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NIMH. Support was also provided by the West Virginia University Health Sciences Center bridge funding and the Blanchette Rockefeller Neurosciences Institute. We thank Lauren B. Burhans for assisting with data collection, for conducting statistical analyses and for providing invaluable comments on the manuscript.
References
- Attwell PJ, Cooke SF, & Yeo CH (2002). Cerebellar function in consolidation of a motor memory. Neuron, 34, 1011–1020. 10.1016/S0896-6273(02)00719-5. [DOI] [PubMed] [Google Scholar]
- Buck DL, Seager MA, & Schreurs BG (2001). Conditioning-specific reflex modification of the rabbit (Oryctolagus cuniculus) nictitating membrane response: Generality and nature of the phenomenon. Behavioral Neuroscience, 115(5), 1039–1047. 10.1037/0735-7044.115.5.1039. [DOI] [PubMed] [Google Scholar]
- Burhans LB, & Schreurs BG (2008). Inactivation of the central nucleus of the amygdala abolishes conditioning-specific reflex modification of the rabbit (Oryctolagus cuniculus) nictitating membrane response and delays classical conditioning. Behavioral Neuroscience, 122(1), 75–88. 10.1037/0735-7044.122.1.75. [DOI] [PubMed] [Google Scholar]
- Burhans LB, Smith-Bell CA, & Schreurs BG (2008). Conditioning-specific reflex modification of the rabbit’s nictitating membrane response and heart rate: Behavioral rules, neural substrates, and potential applications to posttraumatic stress disorder. Behavioral Neuroscience, 122(6), 1191–1206. 10.1037/a0013599. [DOI] [PubMed] [Google Scholar]
- Burhans LB, Smith-Bell CA, & Schreurs BG (2010). Effects of extinction on classical conditioning and conditioning-specific reflex modification of rabbit heart rate. Behavioural Brain Research, 206, 127–134. 10.1016/j.bbr.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burhans LB, Smith-Bell CA, & Schreurs BG (2015). Effects of extinction treatments on the reduction of conditioned responding and conditioned hyperarousal in a rabbit model of posttraumatic stress disorder (PTSD). Behavioral Neuroscience, 129(5), 611–620. 10.1037/bne0000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Bao S, Lockard JM, Kim JK, & Thompson RF (1996). Impaired classical eyeblink conditioning in cerebellar-lesioned and Purkinje cell degeneration (pcd) mutant mice. Journal of Neuroscience, 16(8), 2829–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman SR, & Gormezano I (1971). Classical conditioning of the rabbit’s (Oryctolagus cuniculus) nictitating membrane response under symmetrical CS-US interval shifts. Journal of Comparative and Physiological Psychology, 77, 447–455. 10.1037/h0031879. [DOI] [PubMed] [Google Scholar]
- Freeman JH (2015). Cerebellar learning mechanisms. Brain Research, 1621, 260–269. 10.1016/j.brainres.2014.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey PW, & Gavin W (1975). Overnight incubation of a partially conditioned eyeblink response in rabbits. Animal Learning & Behavior, 3(2), 114–118. 10.3758/BF03209111. [DOI] [Google Scholar]
- Gallistel CR, Fairhurst S, & Balsam P (2004). The learning curve: Implications of a quantitative analysis. Proceedings of the National Academy of Sciences of the United States of America, 101(36), 13124–13131. 10.1073/pnas.0404965101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia KS, Mauk MD, Weidemann G, & Kehoe EJ (2003). Covariation of alternative measures of responding in rabbit (Oryctolagus cuniculus) eyeblink conditioning during acquisition training and tone generalization. Behavioral Neuroscience, 117(2), 292–303. 10.1037/0735-7044.117.2.292. [DOI] [PubMed] [Google Scholar]
- Gruart A, & Yeo CH (1995). Cerebellar cortex and eyeblink conditioning: Bilateral regulation of conditioned responses. Experimental Brain Research, 104(3), 431–448. 10.1007/BF00231978. [DOI] [PubMed] [Google Scholar]
- Halverson HE, Hoffmann LC, Kim Y, Kish EA, & Mauk MD (2016). Systematic variation of acquisition rate in delay eyelid conditioning. Behavioral Neuroscience, 130(6), 553–562. 10.1037/bne0000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesslow G (1994). Inhibition of classically conditioned eyeblink responses by stimulation of the cerebellar cortex in the decerebrate cat. Journal of Physiology, 476(2), 245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe EJ (2006). Repeated acquisitions and extinctions in classical conditioning of the rabbit nictitating membrane response. Learning and Memory, 13(3), 366–375. 10.1101/lm.169306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellett DO, Fukunaga I, Chen-Kubota E, Dean P, & Yeo CH (2010). Memory consolidation in the cerebellar cortex. PLoS ONE, 5(7), e11737 10.1371/journal.pone.0011737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim JA, Freeman JH, Bruneau R, Nolan BC, Cooper NR, Zook A, & Walters D (2002). Synapse formation is associated with memory storage in the cerebellum. Proceedings of the National Academy of Sciences of the United States of America, 99(20), 13228–13231. 10.1073/pnas.202483399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scavio MJ, Clift PS, & Wills JC (1992). Posttraining effects of amphetamine, chlorpromazine, ketamine, and scopolamine on the acquisition and extinction of the rabbit’s conditioned nictitating membrane response. Behavioral Neuroscience, 106(6), 900–908. 10.1037/0735-7044.106.6.900S. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, Gusev PA, Tomsic D, Alkon DL, & Shi T (1998). Intracellular correlates of acquisition and long-term memory of classical conditioning in Purkinje cell dendrites in slices of rabbit cerebellar lobule HVI. Journal of Neuroscience, 18(14), 5498–5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs BG, Oh MM, & Alkon DL (1996). Pairing-specific long-term depression of Purkinje cell excitatory post synaptic potentials results from a classical conditioning procedure in the rabbit cerebellar slice. Journal of Neurophysiology, 74, 1051–1060. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, Oh MM, Hirashima C, & Alkon DL (1995). Conditioning-specific modification of the rabbit’s unconditioned nictitating membrane response. Behavioral Neuroscience, 109(1), 24–33. 10.1037/0735-7044.109.1.24. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, Shi T, Pineda IS, & Buck DL (2000). Conditioning the unconditioned response: Modification of the rabbit’s (Oryctolagus cuniculus) unconditioned nictitating membrane response. Journal of Experimental Psychology: Animal Behavior Processes, 26(2), 144–156. 10.1037/0097-7403.26.2.144. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, Smith-Bell CA, & Burhans LB (2011a). Incubation of conditioning-specific reflex modification: Implications for posttraumatic stress disorder. Journal of Psychiatric Research, 45(11), 1535–1541. 10.1016/j.jpsychires.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs BG, Smith-Bell CA, & Burhans LB (2011b). Unpaired extinction: Implications for treating post-traumatic stress disorder. Journal of Psychiatric Research, 45(5), 638–649. 10.1016/j.jpsychires.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seager MA, Smith-Bell CA, & Schreurs BG (2003). Conditioning-specific reflex modification of the rabbit (Oryctolagus cuniculus) nictitating membrane response: US intensity effects. Learning and Behavior, 31(3), 292–298. 10.3758/BF03195990. [DOI] [PubMed] [Google Scholar]
- Smith-Bell CA, Burhans LB, & Schreurs BG (2012). Predictors of susceptibility and resilience in an animal model of posttraumatic stress disorder. Behavioral Neuroscience, 126(6), 749–761. 10.1037/a0030713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Zee EA, Kronforst-Collins MA, Maizels ET, Hunzicker-Dunn M, &Disterhoft JF (1997). Γ-isoform-selective changes in PKC immunoreactivity after trace eyeblink conditioning in the rabbit hippocampus. Hippocampus, 7(3), 271–285. . [DOI] [PubMed] [Google Scholar]
- Vogel RW, Ewers M, Ross C, Gould TJ, & Woodruff-Pak DS (2002). Age-related impairment in the 250-millisecond delay eyeblink classical conditioning procedure in C57BL/6 mice. Learning & Memory, 9(5), 321–336. 10.1101/lm.50902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikgren J, & Korhonen T (2001). Interpositus nucleus inactivation reduces unconditioned response amplitude after paired but not explicitly unpaired treatment in rabbit eyeblink conditioning. Neuroscience Letters, 308(3), 181–184. 10.1016/S0304-3940(01)02000-6. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS (2006). Stereological estimation of Purkinje neuron number inC57BL/6 mice and its relation to associative learning. Neuroscience, 141(1), 233–243. 10.1016/j.neuroscience.2006.03.070. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Cronholm JF, & Sheffield JB (1990). Purkinje cell number related to rate of classical conditioning. NeuroReport, 1(2), 165–168. 10.1097/00001756-199010000-00020. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Goldenberg G, Downey-Lamb MM, Boyko OB, & Lemieux SK (2000). Cerebellar volume in humans related to magnitude of classical conditioning. NeuroReport, 14, 609–615. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Lehr MA, Li JG, & Liu-Chen LY (2010). Young and older good learners have higher levels of brain nicotinic receptor binding. Neurobiology of Aging, 31(6), 1032–1043. 10.1016/j.neurobiolaging.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Lei C, Feng H, & Sui J-F (2015). Review: The neural circuitry and molecular mechanisms underlying delay and trace eyeblink conditioning in mice. Behavioural Brain Research, 278, 307–314. 10.1016/j.bbr.2014.10.006. [DOI] [PubMed] [Google Scholar]