Abstract
Growth factors, such as colony-stimulating factors (CSFs), epidermal growth factors (EGFs), and fibroblast growth factors (FGFs) are signaling proteins that control a wide range of cellular functions. Although growth factor networks are critical for intercellular communication and tissue homeostasis, their abnormal production or regulation occurs in various pathologies. Clinical strategies that target growth factors or their receptors are used to treat a variety of conditions, but have yet to be adopted for cardiovascular disease. In this review, we focus on M-CSF, GM-CSF, IL-3, EGFR, and FGF21. We first discuss the efficacy of targeting these growth factors in other disease contexts (i.e. inflammatory/autoimmune diseases, cancer, or metabolic disorders) and then consider arguments for or against targeting them to treat cardiovascular disease.
Keywords: atherosclerosis, inflammation, growth factors, immunotherapy, myeloid cells
Introduction
Ischemic heart disease, the leading cause of non-communicable death in the U.S. and abroad, typically stems from atherosclerosis.1, 2 Aging, lifestyle factors, and genetic susceptibility converge to promote the accumulation of lipid-laden atherosclerotic plaques in the intimal walls of arterial vessels.3–5 Over decades, these plaques contribute to many life-threatening complications, including myocardial infarction (MI) or stroke.6 Atherosclerotic plaque buildup was once thought to be a slow, passive process driven by cholesterol accrual (i.e. a lipid storage disorder). However, the identification of inflammatory and immune biomarkers (e.g. C-reactive protein (CRP), interleukin (IL)-6, anti-oxidized low-density lipoprotein (oxLDL) antibodies) and evidence that innate and adaptive immune cells enter plaques and contribute to their formation has led to atherosclerosis’ re-classification as a lipid-driven chronic inflammatory disease.7–9 It is now clear that leukocytes not only play important roles in driving inflammation in atherosclerosis and other forms of cardiovascular disease (CVD) but also contribute to cardiac development and heart function.10, 11
Despite the considerable advances in our understanding of inflammation and leukocyte involvement in CVD, treatment options for targeting inflammatory mediators in atherothrombotic disorders remain limited. The currently preferred drugs for primary and secondary prevention of MI, stroke, and death from other CVD complications are statins, a class of cholesterol biosynthesis inhibitors.12, 13 Statins are well-known to reduce hyperlipidemia, a major CVD risk factor, by lowering low-density lipoprotein (LDL) levels.14, 15 In addition, statins perform many pleiotropic, immunomodulatory, functions, such as the ability to suppress T cell activation, inhibit MHCII and adhesion molecule expression on vascular cells, and lower CRP, an inflammatory biomarker and independent predictor of major adverse cardiovascular events (MACE)12–14, 16–18. Because of the pleiotropic effects of statins, however, it has remained unclear whether targeting inflammatory mediators could prove beneficial for treating CVD independently of lipid-lowering therapies.
Recently, the Canakinumab Anti-inflammatory Thrombosis Outcome Study (CANTOS) trial was designed to test the inflammatory hypothesis of atherothrombosis by targeting IL-1β, an active inflammatory mediator in atherosclerosis. The trial was a success: IL-1β neutralization lowered recurrent cardiovascular events, providing support for the inflammatory hypothesis and rationale for developing focused therapies that target active inflammatory cascades.19–21 Importantly, blocking IL-1β, which is active in the acute inflammatory response, reduced CRP and IL-6 without affecting lipid levels.22, 23 There is no question that lowering lipids with statins is an effective approach for mitigating cardiovascular risk24, but CANTOS has opened up another dimension concerning treatment strategy that should be considered in more depth, namely, the development of focused immunotherapeutic approaches to treat CVD.
Growth Factors and Their Receptors as Potential Immunotherapy Targets to Treat Cardiovascular Inflammation
Growth factors are signaling proteins that mediate diverse functions related to cellular differentiation and proliferation. Though important to homeostasis, their abnormal production or regulation can promote disease. Immunotherapeutic approaches, including those that target growth factors and their receptors, have demonstrated clinical success against cancers and acute or chronic inflammatory/autoimmune disorders, but have not been widely adopted for CVD.24, 25
Though many different kinds of growth factors abound, we will limit our discussion to a handful for which there is both clinical evidence for treating cancer, inflammatory/autoimmune diseases, or metabolic disorders and pre-clinical evidence for treating cardiovascular disease (Table 1). First, the colony-stimulating factors (CSFs) are glycoproteins that regulate leukocyte survival, differentiation, and function.26, 27 In particular, the four CSFs – macrophage colony-stimulating factor (M-CSF; also called CSF-1), granulocyte-macrophage colony-stimulating factor (GM-CSF; also called CSF-2), granulocyte colony-stimulating factor (G-CSF; also called CSF-3), and interleukin-3 (IL-3; previously called multi-CSF) – differentially promote the proliferation, differentiation, mobilization, and survival of myeloid cells, which are derived from hematopoietic stem and progenitor cells (HSPCs) found in bone marrow niches and, in cases of extramedullary hematopoiesis, the spleen. These growth factors also regulate the mature myeloid cell functions in the steady state and under pathologic, inflammatory conditions.26, 27 Second, the epidermal growth factor receptor (EGFR), which binds multiple EGF ligands, and fibroblast growth factor 21 (FGF21) have multiple functions that modulate vascular smooth muscle cells, cardiomyocytes, cardiac fibroblasts, endothelial cells, adipocytes, and immune cells, with new functions arising.28, 29
Table 1.
Overview of Growth Factor Pathways in Cardiovascular Disease and Their Available Therapies
| Growth Factor Pathway | Clinically Available Therapies | Potential Role in CVD | Rationale for Targeting in CVD | Caveats for Targeting in CVD |
|---|---|---|---|---|
| M-CSF/CSF-1R | Monoclonal antibodies Small molecule inhibitors (TKIs) |
Pathogenica | • Clinically predicts atherosclerosis progression and adverse outcome [42, 43] • Induces monocyte recruitment, promotes macrophage differentiation and survival, drives aortic inflammation in atherosclerosis [48, 53, 56] • Drives lesional accumulation of monocyte-derived DCs in atherosclerosis [58] • Elevates monocytosis in the bone marrow due to poor sleep in atherosclerosis [56] • Alters expression of genes in macrophages to favor inflammation, cholesterol retention, and uptake [45, 54, 55] • Induces monocyte production and monocyte/macrophage accumulation in viral myocarditis [61] • Promotes vascular inflammation and macrophage accumulation in aortic aneurysm [62] |
• Role in myocardial ischemia is protective – mediates proper cardiac function, repair, and angiogenesis in models of MI [63–67] • Important roles in host defense and homeostastic functions that may be compromised if inhibited [68–72] • Reducing inflammatory macrophages with CSF-1R therapies in models remains in question [27] • Unclear how inhibiting M-CSF may affect the functions of IL-34, an alternate CSF-1R ligand [32, 73] |
| GM-CSF/GMRα:βc | Monoclonal antibodies Recombinant protein |
Pathogenic | • Promotes survival and/or proliferation of myeloid progenitors and progeny in atherosclerosis and MI [85–89] • Induces TH1 immunity via generation of DCs in the spleen in atherosclerosis [90] • Regulates the accumulation and proliferation of myeloid cells, DCs, and T cells in atherosclerotic lesions [91,92] • Promotes macrophage apoptosis and oxidative stress in advanced lesions via IL-23 in atherosclerosis [93] • Promotes cardiac dysfunction after MI [89] • Drives production of inflammatory cytokines, myeloid cell recruitment, and/or proliferation in KD, EAM, aortic aneurysm, and MI [89, 100, 102, 104] • Promotes DC infiltration into the myocardium in EAM and after MI [98, 99] • Induces pathogenic TH17 cells and by signaling on DCs to sustain inflammation in EAM [105,107] |
• Autoantibodies against GM-CSF interfere with the priming of neutrophil antimicrobial functions in patients with pulmonary alveolar proteinosis [110] • Autoantibodies against GM-CSF are associated with inflammation in IBD [111] • Promotes intestinal barrier integrity [112] |
| IL-3/IL-3Rα:βc | Monoclonal antibodies | Pathogenic | • Promotes survival and proliferation of myeloid progenitors and progeny in atherosclerosis [85–87] • Drives monocyte recruitment and local T cell proliferation in EAM [109] • Stimulates monocyte adhesion and activation in vitro [108] • Stimulates SMC migration and proliferation in vitro [108] • Stimulates EC proliferation, adhesion molecule expression, and cytokine production in vitro [108] |
• Role in atherosclerosis, MI, and other types of CVD has not yet been tested in vivo or requires further study |
| G-CSF/CSF-3R | Monoclonal antibodies Recombinant protein |
Protectiveb | • Reduces fibrotic areas after MI [121, 122] • Accelerates vessel formation after MI [119, 121, 122] • Improves cardiac function after MI [119–121] • Protects against cardiac remodeling after MI [119] • Protects against apoptosis including in cardiomyocytes after MI [119, 120] |
• Clinical trials utilizing G-CSF for post-MI repair yielded inconclusive effects [118] • Conflicting reports on the role of G-CSF infusion in models of atherosclerosis and modestly protective overall [125] • Mechanisms surrounding the effects of cells mobilized by G-CSF on CVD are still unclear/inconclusive [118, 123, 124] |
| EGFs/EGFR | Monoclonal antibodies Small molecule inhibitors (TKIs) |
Pathogenic | • Promotes macrophage accumulation, oxLDL uptake, and inflammatory cytokine production in atherosclerosis [136, 138] • Induces T cell activation, proliferation, and cytokine production in peripheral lymphoid organs and in atherosclerotic plaques [137] • Stimulates monocyte migration in vitro [134] • Amphiregulin, an EGFR ligand, produced by macrophages promotes cardiac dysfunction and fibrosis after MI [140] • Promotes cardiac and vascular hypertrophy and dysfunction via effects on both SMCs and ECs (e.g. proliferation, migration, activation, etc.) in vascular diseases including hypertension and AAA [131, 133] |
• Role in atherosclerosis, MI, and other types of CVD has not yet been tested in vivo or requires further study |
| FGF21/FGFR:KLB | Receptor agonists Engineered analogs |
Protective | • Attenuates plaque formation and macrophage accumulation in part by inhibiting liver cholesterol biosynthesis in atherosclerosis [153] • Inhibits smooth muscle cell proliferation in atherosclerosis [153] • Prevents foam cell formation by increasesing cholesterol efflux and reducing expression of inflammatory mediators in vitro [159–161] • Counteracts AngII-induced hypertension and vascular inflammation by inducing ACE2 in adipocytes and renal cells [158] • Attenuates cardiac remodeling and promotes repair by promoting cardiomyocyte survival after Mi [154, 157] • Ameliorates cardiac fibrosis, hypertrophy, oxidative stress, and inflammation in part via signaling in cardiomyocytes in diabetic cardiomyopathy [156] |
• Role in atherosclerosis, MI, and other types of CVD requires further study, especially with respect to FGF21 effects on leukocytes and the activities of specific FGFRs |
Protective in settings of myocardial ischemia.
Overall appears to be protective, but conflicting data between animal models and clinical trials for G-CSF infusion post-MI. Conflicting data and only modest effects surrounding studies in models of atherosclerosis.
Abbreviations: AAA, abdominal aortic aneursym; ACE2, Angiotensin converting enzyme 2; AngII, Angiotensin II; CVD, cardiovascular disease; EAM, Experimental autoimmune myocarditis; EC, Endothelial cell; FGFR, Fibroblast growth factor receptor; GMRα, GM-CSFRα; IBD, Inflammatory bowel disease; IL-34, Interleukin-34; KD, Kawasaki disease; KLB, β-klotho co-receptor; MI, myocardial infarction; oxLDL, oxidized low-density lipoprotein; SMC, Smooth muscle cell; TH, T helper; TKIs, tyrosine kinase inhibitors.
Macrophage Colony-Stimulating Factor
Among CSFs, M-CSF is a key growth factor relevant to the production, proliferation, survival, and function of many mononuclear phagocytes. As such, M-CSF is readily detectable in the circulation and is produced locally in tissues by different cell types, including endothelial cells (ECs), epithelial cells, neurons, and osteoblasts. M-CSF’s receptor, CSF-1R (CD115), is expressed on most mononuclear phagocytes and their progenitors.27, 30–32 In Csf1r−/− and Csf1op/op mice (Csf1−/−) (the latter have a null mutation in the Csf1 locus), many tissue macrophages and osteoclasts are severely compromised.32–37 Moreover, dendritic cells (DCs), including the skin-resident Langerhans cells, depend on the M-CSF:CSF-1R axis for their development since all DCs augment CSF-1R during their differentiation and are highly reduced in Csf1−/− mice.38, 39 Although blood monocyte development from bone marrow precursors does not entirely rely on M-CSF, it can boost monocyte production and modulate their functional capacity to express cytokines and cell surface receptors.37, 39
To date, M-CSF and CSF-1R have been targeted for treating rheumatoid arthritis (RA) and cancer with monoclonal antibodies (mAbs) and oral kinase antagonists that inhibit the CSF-1R’s receptor tyrosine kinase (RTK) activity. Clinical trials for RA have reported minimal efficacy, failed to disclose information, or remain ongoing.27 In cancer, these strategies are still being tested for solid tumors as monotherapies and in combination with immunotherapies, and have yielded some benefit in phase I trials.40 The M-CSF:CSF-1R axis has not been targeted for cardiovascular disease despite some intriguing data. First, M-CSF is a causal inflammatory biomarker in coronary artery disease and strongly predicts atherosclerotic plaque progression and adverse outcome.41–43 Second, M-CSF is augmented in experimental atherosclerotic lesions, can also be detected in human plaques, and its expression increases in endothelial cells and macrophages exposed to oxLDL.44–47 Third, M-CSF is atherogenic in mouse models as both Csf1−/− and Csf1+/− mice on the Apoe−/− and Ldlr−/− backgrounds are protected from atherosclerosis in spite of elevated circulating cholesterol levels, which are curiously increased in fully-deficient animals.48–51 Likewise, Apoe−/− mice treated with anti-CSF-1R mAb or a CSF-1R kinase inhibitor develop smaller atherosclerotic lesions.52, 53 Fourth, work performed in vitro suggests that M-CSF signaling in macrophages promotes atherogenesis by altering metabolic pathways to favor cholesterol retention, inducing monocyte chemoattraction, and enhancing scavenger receptor activity, which aids LDL uptake.45, 53–55 Fifth, observations in vivo have revealed that signaling downstream of CSF-1R favors plaque progression by promoting aortic inflammatory gene expression and macrophage survival and by inducing monocyte production, differentiation, and recruitment from bone marrow precursors (Figure 1).48–50, 52, 53, 56 For example, we have recently discovered that poor sleep, a lifestyle risk factor for CVD, aggravates atherosclerosis through heightened monocyte production incited by M-CSF derived from pre-neutrophils in the bone marrow.4, 57 Sixth, M-CSF may act on monocytes in the aorta to drive the differentiation and accumulation of CD11c+ CD11b+ F4/80+ monocyte-derived DCs, which are highly reduced in Csf1−/− aortas and, in contrast to classical DC subsets, operate independently of Flt3:Flt3L signaling.58, 59 Although the role of M-CSF-dependent myeloid-derived DCs in atherosclerosis is still unclear, this DC subset becomes dramatically elevated in the aortas of atherosclerotic mice along with CD11c− macrophages and may perpetuate chronic inflammation by sustaining T cell activation, proliferation, and inflammatory cytokine production (Figure 1).58–60 In acute models of inflammation in CVD, M-CSF also exerts pathologic activities. During myocarditis, M-CSF rises in the heart, where it influences the accumulation of inflammatory monocytes and macrophages, and it also drives bone marrow monocyte production, possibly by reaching the circulation.61 Following aortic aneurysm, M-CSF increases in the vasculature and promotes inflammation and macrophage accumulation, though whether M-CSF recruits monocytes to the vasculature during aortic aneurysm is unknown.62
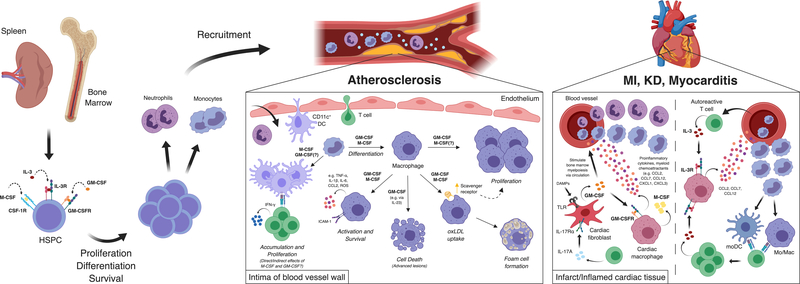
Figure 1.
Pathologic effects of colony-stimulating factors in cardiovascular disease. M-CSF, GM-CSF, and IL-3 promote hematopoiesis in the bone marrow and spleen by triggering hematopoietic stem and progenitor cell proliferation, survival, and differentiation into myeloid cell subsets (i.e. monocytes and neutrophils), especially in the setting of hypercholesterolemia. Myeloid cells are recruited from the blood into cardiovascular organs, including the arterial vessels and myocardial tissues, via chemokine gradients established in part by CSFs acting on cells in the tissue sites (e.g. cardiac macrophages). In the intima of arterial vessels, GM-CSF and M-CSF promote the differentiation of monocytes into macrophages and monocyte-derived DCs, macrophage proliferation, activation, and production of inflammatory mediators and chemokines, foam cell formation via scavenger receptor upregulation, and cell death in advanced lesions, all of which contribute to inflammation and the accrual of leukocytes. Although GM-CSF also promotes the accumulation of CD11c+ dendritic cells and T cells in plaques, it is unclear whether this occurs directly or indirectly. Within cardiac tissues in settings of myocardial inflammation (e.g. MI, KD, myocarditis), GM-CSF produced by fibroblasts can stimulate myeloid cell recruitment by signaling on cardiac macrophages’ production of chemoattractants as well as stimulate bone marrow hematopoiesis distally via the circulation. M-CSF secreted by cardiac macrophages can also promote distal bone marrow myelopoiesis in myocarditis. CD4+ T cells promote cardiac fibroblasts’ production of GM-CSF in part via the production of IL-17A. In myocarditis, IL-3 produced by autoreactive T cells stimulates cardiac macrophages to produce monocyte chemoattractants, which guides monocyte recruitment and differentiation into monocyte-derived APCs. In turn, monocyte-derived APCs stimulate local T cell proliferation which thereby fuels leukocyte accumulation and cardiac inflammation.
While the above observations suggest that inhibiting the M-CSF:CSF-1R axis may be beneficial in chronic and acute cardiovascular inflammation by dampening monocyte infiltration and the development of inflammatory macrophages and myeloid-derived DCs, challenges remain. Specifically targeting M-CSF during bouts of myocardial ischemia, such as in MI, may instead prove detrimental to host physiology based on multiple animal studies. For example, introducing recombinant M-CSF into animals after experimental MI improves their heart function, promotes cardiomyocyte survival, and stimulates infarct repair and angiogenesis.63–65 Such positive effects of M-CSF appear to rely, in part, on the mobilization of bone marrow CXCR4+ cells and monocytes, which differentiate into macrophages and proliferate in healing infarcts, as well as the anti-inflammatory functions of cardiac-resident CSF-1R+ macrophages, which participate in reparative processes after an MI.64, 66, 67 Thus, hampering monocyte infiltration and macrophage differentiation and function by blocking M-CSF may interfere with proper myocardial tissue repair.
Apart from the Janus-like roles of M-CSF in cardiovascular inflammation and ischemia, other potential hurdles that may arise downstream of M-CSF inhibition also require consideration. First, suppressing M-CSF or its receptor may compromise important monocyte and tissue macrophage host defense and homeostatic functions, such as thermogenesis, neuron pruning, iron recycling, and gut motility.68–72 Secondly, preclinical disease models show the actual efficiency of reducing inflammatory macrophages with CSF-1R-directed therapies requires further clarification.27 Complicating things further, IL-34, a recently identified CSF-1R ligand that competes with M-CSF for binding, regulates mononuclear phagocytes with somewhat overlapping yet distinct functions and may independently contribute to inflammatory disorders.32, 73 Overall, it remains unclear how targeting the M-CSF:CSF-1R axis would fare as a treatment strategy for CVD. Rather, it may be more effective to block or deplete M-CSF’s cellular sources, such as ECs and myeloid cells, with carefully directed therapies for atherothrombotic disorders in which M-CSF is believed to stimulate pathologic, inflammatory myeloid cell activity.44, 57, 61
Granulocyte-Macrophage Colony-Stimulating Factor and Interleukin-3
Although GM-CSF and IL-3 are classified as CSFs, they are also, like IL-5, β common (βc) cytokines that rely on the βc receptor (CD131; also CSF-2RB or IL-3Rβ) for signaling. To elicit signaling, the βc subunit forms a heterodimeric surface receptor with cytokine-specific receptor α subunits, such as GM-CSFRα, IL-3Rα, and IL-5Rα.74 Though produced in the steady state, particularly in the lung, GM-CSF production can be augmented during injury or infection by many cell types, including T cells, B cells, macrophages, and fibroblasts, among others, while GM-CSF’s cellular targets are primarily myeloid cells, hematopoietic progenitors, and some non-myeloid populations (e.g. neurons, epithelium, endothelium). Consequently, GM-CSF has been linked to several inflammatory and autoimmune disorders.75–77 In clinical trials, directly targeting the GM-CSF:GM-CSFRα axis with mAbs in RA has been successful up to phase II, whereas phase I/II trials for multiple sclerosis, psoriasis, and asthma are either ongoing or deem the therapy tolerable for patients.27, 76 In the clinic, GM-CSF’s immunostimulatory effects can overcome immune suppression in sepsis, boost myeloid cell recovery after chemotherapy, and induce hematopoietic regeneration after bone marrow transplantation.26, 78 Inflammatory stimuli such as infection and injury also augment IL-3, which appears to be more T cell-restricted. However, human mast cells and innate response activator (IRA) B cells, which are B1-derived cells that induce myelopoiesis by secreting growth factor cytokines, can also produce IL-3.74, 79, 80 IL-3 has numerous targets since myeloid cells, including the granulocytic subtypes, plasmacytoid dendritic cells, hematopoietic progenitors, and ECs, can be regulated by IL-3.27, 74, 81–83 In contrast to GM-CSF, the IL-3:IL-3Rα axis has not been clinically targeted to inhibit inflammation, though IL-3Rα-specific mAbs have been used to eliminate leukemic cells, which highly augment IL-3Rα in hematologic malignancies.84
Preclinical reports suggest that GM-CSF and IL-3 drive cardiovascular inflammation by promoting leukocyte accumulation in cardiovascular organs (Figure 1). In experimental atherosclerosis, plaque myeloid cell accumulation is influenced by the distal expansion of βc receptor+ HSPCs, which are found in the bone marrow and spleen. Work using Apoe−/− mice and Abca1−/− Abcg1−/− mice with defective cholesterol efflux showed that hypercholesterolemia elevates the βc receptor on HSPCs. In turn, HSPC βc receptor signaling via STAT5 induces their proliferation and survival, leading to monocytosis, neutrophilia, and, subsequently, leukocyte accumulation in plaques.85–88 Similarly, βc receptor signaling on bone marrow HSPCs gives rise to inflammatory monocytes and neutrophils, which mediate left ventricular rupture in the infarcted myocardium in experimental MI.89 Though the potential sources and individual roles of GM-CSF and IL-3 have not been thoroughly addressed in atherosclerosis, experimental evidence suggests that both growth factors can promote HSPC proliferation and that IRA B cells can peripherally produce GM-CSF and IL-3.85–88, 90 GM-CSF derived from IRA B cells can also boost adaptive TH1 immunity in atherosclerosis via inflammatory DC generation in peripheral organs.90 In MI, however, bone marrow HSPC proliferation appears to rely distinctly on circulating GM-CSF derived from cardiac fibroblasts rather than from a peripheral cellular source.89
Apart from its roles in driving myelopoiesis in the bone marrow and spleen in atherosclerosis and MI, GM-CSF also seems to control leukocyte accumulation and cardiovascular inflammation by acting on locally situated cells in plaques and cardiac tissue (Figure 1). For example, in nascent lesions, GM-CSF regulates the proliferation and accrual of intimal leukocytes, including CD11c+ dendritic cells (DCs), CD11b+ myeloid cells, and T cells, whereas in established lesions, it triggers inflammatory cytokine and reactive oxygen species (ROS) production and promotes apoptosis and plaque necrosis.91–93 It remains unclear whether GM-CSF’s local actions are specific to the Ldlr−/− background because of the divergent effects of GM-CSF deficiency and administration in Apoe−/− models.94, 95 Nevertheless, in models of MI, Kawasaki disease (KD), myocarditis, and aortic aneurysm, GM-CSF promotes myeloid cell and DC infiltration, proliferation, and pro-inflammatory cytokine secretion, a result that aligns with GM-CSF’s pathogenic activities in atherosclerosis.89, 96–103 Although more work is needed to define how GM-CSF locally induces myeloid cell recruitment and instigates myocardial inflammation, cardiac fibroblasts produce GM-CSF, which stimulates cardiac macrophages to express monocyte and neutrophil chemoattractants (e.g. CCL2, CCL7, CCL12, CXCL1, CXCL3), leading to myeloid cell accumulation in KD and MI.89, 102 Moreover, models of MI and myocarditis show that GM-CSF secretion by cardiac fibroblasts depends upon the hallmark T helper 17 (TH17) cytokine IL-17A.103, 104 Because GM-CSF also promotes pathogenic TH17 cell differentiation by stimulating DCs to produce IL-23 and IL-6, it is possible that cardiac fibroblasts, DCs, and TH17 cells form a collaborative loop via GM-CSF to both initiate and sustain myocardial inflammation and possibly trigger the development of heart failure.105–107
IL-3 may locally aggravate atherogenesis by stimulating monocyte activation and adhesion, EC activation, and smooth muscle cell (SMC) proliferation in plaques,108 but these observations have yet to be confirmed in vivo. Yet IL-3 does critically amplify myocardial inflammation.109 Specifically, self-reactive, cardiac-infiltrating CD4+ T cells produce IL-3 that provokes cardiac inflammation, leukocyte accumulation, and myocardial dysfunction in autoimmune myocarditis. By locally stimulating IL-3R+ cardiac macrophages to express monocyte chemoattractants, IL-3 mediates Ly-6Chigh monocyte recruitment and differentiation into monocyte-derived antigen-presenting cells (APCs), which subsequently amplify local T cell proliferation in cardiac tissue (Figure 1).109 Ultimately, whether IL-3 directly affects atherosclerosis, MI, and other CVD complications warrants further study.
Taken together, observations on GM-CSF and IL-3 in CVD suggest that inhibiting either growth factor might dampen inflammatory myeloid cell production in peripheral lymphoid organs and prevent inflammatory leukocyte recruitment and accumulation in myocardial tissues. At this point in time, however, more evidence is needed to establish IL-3 as a decisive target in CVD treatment. In addition, caution should be exercised regarding GM-CSF blockade as a therapeutic strategy. Because GM-CSF is important for priming myeloid cell antimicrobial functions and maintaining mucosal barrier homeostasis,110–113 blocking GM-CSF might predispose individuals to infection or inflammation at barrier sites. Therefore, depleting the relevant cellular sources of GM-CSF or defining a safe therapeutic dosage to inhibit GM-CSF may be more optimal for CVD treatment.
Granulocyte Colony-Stimulating Factor
G-CSF is produced by stromal-lineage cells such as ECs, epithelial cells, and fibroblasts as well as macrophages and other hematopoietic cells. Like GM-CSF and IL-3, G-CSF is typically induced by inflammation in response to host infection and injury. Because neutrophils express the most CSF-3R, the G-CSF receptor, its myeloid lineage regulation capacity is somewhat limited to controlling neutrophil production and function.27, 114 Accordingly, G-CSF primarily induces granulopoiesis by modulating the proliferation and mobilization of HSPCs, which can then give rise to mature neutrophils, and G-CSF also regulates neutrophil release from the bone marrow.114, 115 Thus, Csf3−/− mice harbor fewer bone marrow granulocyte precursors and exhibit neutropenia.116, 117
In the clinic, G-CSF is administered following chemotherapy for several types of cancer. G-CSF reduces the severity and duration of chemotherapy-induced neutropenia and protects against febrile neutropenia, infection-related mortality, and early mortality from all causes, with minor side-effects.26, 114 Although it is pathogenic in preclinical models of inflammatory/autoimmune diseases, such as arthritis, experimental autoimmune uveitis, and granulomatous disease, in which neutrophils and other myeloid cells drive harmful inflammatory responses, G-CSF is not yet a clinical target in such conditions. Additionally, G-CSF may perform potentially protective functions in various nervous system disease models.27 Since G-CSF’s role in inflammation appears disease dependent, the effect(s) of G-CSF depletion or administration could be either beneficial or detrimental depending on the inflammatory condition.
G-CSF’s functional role in CVD remains in question because of inconsistencies among clinical trials and preclinical disease models. For example, due to its known ability to mobilize stem and progenitor cells, G-CSF was used to treat acute MI alongside stem cell-based regimens intended to improve cardiac regeneration and function in damaged myocardial tissue.118 G-CSF was considered an attractive therapeutic candidate because it had been shown to protect against cardiac remodeling, fibrosis, and cardiomyocyte apoptosis while promoting angiogenesis and re-endothelialization in experimental MI animal models.119–122 Unfortunately, however, clinical trials utilizing G-CSF for post-MI repair were inconclusive, possibly due to differences in timing, administration route, and G-CSF dosage among the studies.118 At present, the mechanisms governing G-CSF’s effects after MI, including how G-CSF influences hematopoietic cell function during post-MI recovery, require further exploration.123, 124
In preclinical models of atherosclerosis, conflicting reports demonstrate that G-CSF infusion either promotes or prevents plaque progression. While a pooled study analysis suggests that G-CSF likely inhibits atherogenesis by mobilizing progenitor cells,125 whether those cells replace dysfunctional ECs and stimulate angiogenesis or give rise to neutrophils and monocytes that alter plaque evolution needs further mechanistic study.125–128 Ultimately, differing animal models, dosages, administration methods, and lesion measurement time points may have caused inconsistent results among the atherosclerosis G-CSF studies.125 Given its modest and inconsistent clinical effects, G-CSF infusion is unlikely to become a treatment strategy for cardiovascular inflammation. Additionally, since G-CSF’s function is potentially protective in CVD, blocking or altering its production or availability may not yield any substantial benefit.
The Epidermal Growth Factor Receptor Axis
EGFR is a cell surface receptor with downstream RTK activity that guides tissue development and maintains tissue homeostasis, principally in epithelial lineages. In response to seven EGF family ligands, which have distinct tissue-specific expression patterns, EGFR expands and sustains pools of undifferentiated, stem cell-derived progenitors by controlling their proliferation, migration, and survival.129 Due to its pathological role in the outgrowth of several carcinomas and brain tumors, EGFR has become a critical therapeutic target in cancer. Typically, oncogenic mutations, in-frame deletions, and genetic rearrangements in the EGFR locus trigger EGFR over-expression or over-activation.130 Consequently, uncontrolled EGFR signaling can promote tumor angiogenesis, proliferation, and metastasis, as well as inhibit apoptosis in malignant cells. Multiple blocking antibodies and small molecule inhibitors have been developed and safely utilized to counteract EGFR’s pro-tumorigenic activity.28, 129, 130
While EGFR signaling appears to be important for cardiac development,131 EGFR and EGF family members have gained more attention as detrimental players in vascular disorders. Within the vascular system, EGFR and its ligands are expressed by SMCs and ECs and can regulate their proliferation, migration, survival, and production of angiogenic factors and ROS (Figure 2).131–133 Most work on the EGF:EGFR axis in CVD has therefore focused on the role of EGFR stimulation or transactivation in non-immune cells. Although EGFR and EGFs are expressed in atherosclerotic plaques, more progress has been made in understanding how EGFR and EGF signaling promotes abdominal aortic aneurysm formation, cardiac remodeling, endothelial dysfunction, fibrosis, hypertension, and neointimal hyperplasia.131–133 In vascular disease models, mAb therapies and small molecule inhibitors have successfully antagonized the pathologic vascular activities of SMCs and ECs.133
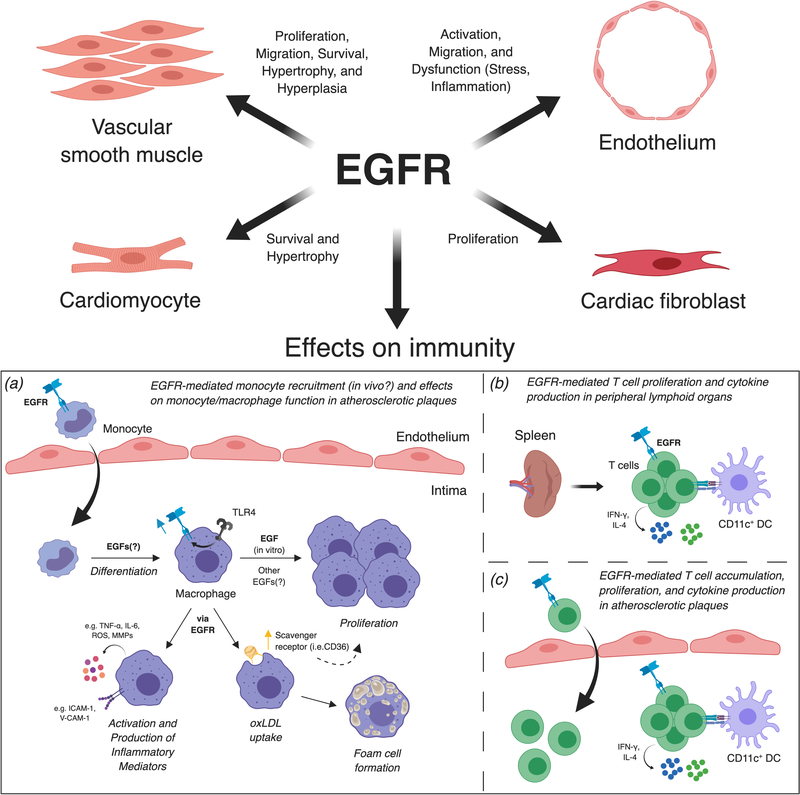
Figure 2.
Pathologic effects of epidermal growth factor receptor in cardiovascular disease. Most work surrounding the pathologic effects of EGFR on vascular inflammation has focused on smooth muscle cell proliferation, migration, and survival, and endothelial cell activation and dysfunction, though more recent data has suggested EGFR also affects cardiomyocytes and fibroblasts. Regarding the immune effects of EGFR in CVD: (a) EGFR promotes macrophage accumulation, activation, foam cell formation, and macrophage production of inflammatory mediators in atherosclerotic vessels. Though EGF has been reported to mediate monocyte adhesion and chemotaxis as well as macrophage proliferation in vitro, it remains unknown whether EGF:EGFR interactions (including which EGFs) drive monocyte recruitment, differentiation, and macrophage proliferation in vivo. (b, c) EGFR promotes CD4+ T cell proliferation and cytokine production as well as T cell accumulation in peripheral lymphoid organs and atherosclerotic plaques. It is unknown whether EGFR signaling affects CD4+ T helper cell differentiation in atherosclerosis, drives T cell recruitment into atherosclerotic plaques, or affects local T cell proliferation and cytokine production in plaques.
Recent work on EGFR expression in certain leukocyte subsets, including cardiac macrophages and activated T cells, has indicated that blocking the receptor may be a worthwhile strategy since EGFR may drive detrimental leukocyte activity in CVD.134–137 Mechanistic approaches suggest that EFGR signaling in macrophages provokes plaque progression in animal models of atherosclerosis (Figure 2). Ldlr−/− mice reconstituted in vitro with LysMCre/+ Egfrfl/fl bone marrow showed that myeloid-specific EGFR deficiency decreases macrophage accumulation in plaques, reduces macrophage oxLDL uptake by lowering CD36, and limits macrophages’ production of TNF-α and IL-6.136 Similarly, inhibiting EGFR signaling in mice abates macrophage accumulation, reduces expression of pro-inflammatory mediators and adhesion molecules, and attenuates development of oxidative stress in aortic plaques, supporting the idea of targeting EGFR in atherosclerosis.138 Moreover, oxLDL may promote foam cell formation in part by inducing EGFR signaling downstream of TLR4 activation.138, 139 EGFR may also control CD4+ T cell function and, by extension, promote atherosclerotic plaque development (Figure 2).137 More specifically, EGFR co-localizes with CD4+ T cells in atherosclerotic plaques and modulates these cells’ capacity to undergo activation, proliferate, and produce cytokines, including IFN-γ. Importantly, blocking EGFR signaling with an EGFR-specific mAb or conditionally deleting EGFR expression in CD4+ T cells limited atherosclerosis by suppressing effector CD4+ T cell activation, infiltration, proliferation, and cytokine production.137
Despite these recent discoveries, many questions regarding how EGFR and EGF ligands affect immunity and inflammation in CVD remain unanswered. Although monocytes express EGFR and EGF purportedly acts as a monocyte chemoattractant, it is unknown whether EGFR signaling guides monocytes into atherosclerotic plaques or affects their phenotype and function in plaques in vivo.134 While there is evidence that amphiregulin, an EGFR ligand, secreted by cardiac macrophages enhances cardiac fibrosis and myocardial dysfunction in experimental MI, it is unclear if EGFR signaling affects macrophage functions during MI.140 Additionally, whether EGFR and its ligands are produced by or signal in other cells in CVD remains unexplored. Overall, emerging work suggests that blocking EGFR might curtail adaptive and innate immune-driven cardiovascular inflammation and foam cell formation in atherosclerosis. How EGFR signaling affects other forms of CVD awaits exploration.
Fibroblast Growth Factor 21
Counteracting the metabolic syndrome is an urgent clinical need because its components (i.e. central obesity, insulin resistance, elevated fasting glucose, dyslipidemia, and systemic hypertension) are major risk factors for CVD, type 2 diabetes (T2D), and all-cause mortality.141, 142 In recent years, studies have shown that the FGF endocrine hormone FGF21 has beneficial effects on metabolism, thus making it an attractive therapeutic target to offset cardiometabolic risk. FGF21 is broadly expressed in many tissues, including the liver, adipose tissue, and pancreas, and acts on multiple cell types in a paracrine or endocrine manner by signaling via several FGF receptors (FGFRs) and its co-receptor β-klotho (KLB).143 In diabetes and obesity models, FGF21 improves insulin sensitivity, lowers fasting glucose and triglycerides, induces weight loss, and promotes both energy expenditure and white adipose tissue browning by uncoupling protein (UCP)1-dependent and -independent mechanisms (Figure 3).144–149 Moreover, human studies and early clinical trials in obese patients with T2D indicate that FGF21 and its analogs protect against obesity-related disorders through similar mechanisms, potentially by regulating brown adipose tissue activity and energy expenditure.29, 150, 151 Based on FGF21’s protective metabolic effects in humans and animal models, multiple FGF21 analogs and FGFR agonists are currently under development and used for treating obesity-related diseases.29, 143
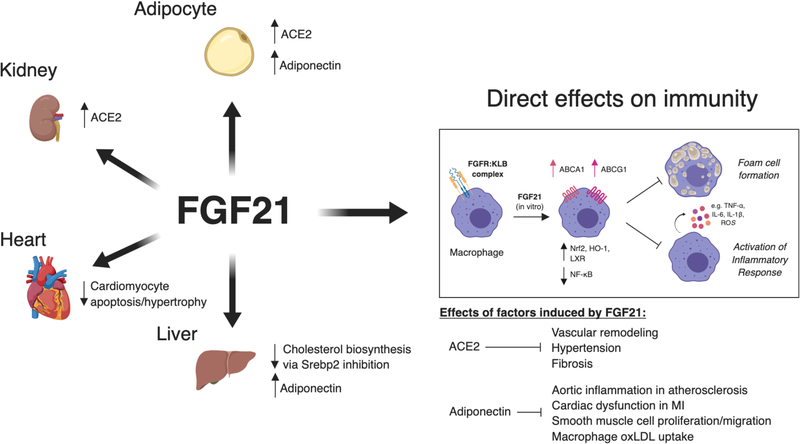
Figure 3.
Protective effects of fibroblast growth factor 21 in cardiovascular disease. Most work on the protective effects of FGF21 in CVD has focused on FGF21 signaling primarily in tissues, such as the liver, kidney, adipose tissue, and heart (i.e. effects on cardiomyocytes). The induction of angiotensin converting enzyme 2 (ACE2) in adipocytes and renal cells by FGF21 attenuates pathologic vascular remodeling, fibrosis, and inflammation in hypertension. The induction of adiponectin in adipocytes and the liver by FGF21 limits vascular inflammation in atherosclerosis and cardiac dysfunction in MI and suppresses smooth muscle cell proliferation and macrophage oxLDL uptake directly. In atherosclerosis, FGF21 directly inhibits cholesterol biosynthesis by signaling on hepatocytes. The immune effects of FGF21 have primarily been studied in vitro (see box). FGF21 suppresses macrophage pro-inflammatory cytokine production and foam cell formation by inducing the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway and upregulation of cholesterol efflux machinery (e.g. ABCA1, ABCG1) in vitro. It is unknown whether FGF21 impacts myeloid cell and other leukocyte functions in cardiovascular disease in vivo.
Since FGF21’s identification as a protective hormone against cardiometabolic risk factors and a potential biomarker for atherosclerosis, multiple studies have focused on evaluating the FGF21’s effects in CVD models.152 FGF21 appears to suppress atherosclerotic plaque accumulation by reducing hypercholesterolemia, oxidative stress, and SMC proliferation via adiponectin-dependent and -independent mechanisms.152, 153 In addition, FGF21 directly inhibits cardiomyocyte apoptosis, attenuates pathological cardiac remodeling, and protects against cardiac hypertrophy, thus preventing myocardial dysfunction and injury in ischemic heart tissue and diabetic cardiomyopathy (Figure 3).154–157 More recently, FGF21 was shown to inhibit hypertension and vascular inflammation by inducing angiotensin-converting enzyme 2 in adipocytes and renal cells.158 However, FGF21’s impact on leukocytes in cardiovascular organs and metabolically relevant sites, such as the liver and adipose tissue, in the setting of CVD is largely unexplored. Since it prevents macrophage accumulation, inflammation, and fibrosis in atherosclerosis and hypertension models, FGF21 may prove to regulate monocyte and macrophage recruitment, proliferation, and inflammatory functions in vessels and myocardial tissues.153, 158 Indeed, preliminary in vitro evidence suggests that FGF21 regulates cholesterol efflux, oxLDL uptake, and foam cell formation in THP1 macrophages and inhibits macrophages’ inflammatory capacity through the Nrf2 pathway (Figure 3).159–161 Moreover, recent data from diet-induced obesity and adaptive thermogenesis models indicate that FGF21 promotes anti-inflammatory macrophage polarization in adipose depots and white adipose tissue browning, which could effectively prevent adipose tissue from adopting pro-inflammatory profiles.162–164 In context of cardiovascular health and disease, agonizing FGF21 pathways might be advantageous because the adipose tissue is an active endocrine organ that can favor cardiovascular disease progression by inducing chronic, low-grade inflammation via hormones and pro-inflammatory mediators (i.e. “adipokines”).142, 165 Likewise, leukocytes that infiltrate adipose tissue in obesity can further release an inflammatory milieu that impacts CVD and metabolic disease outcomes.165 In sum, FGF21 may act directly on macrophages or other cell types in vivo and thereby suppress inflammation by altering macrophage polarization states, potentially at sites local and distal to cardiovascular organs. Although FGF21’s functional role on leukocytes in obesity and CVD remains unclear, preliminary evidence suggests administering either FGF21 or agonists that stimulate the FGF21 signaling pathway may help combat obesity-associated inflammation and thus offset cardiometabolic risk factors that promote cardiovascular inflammation and its downstream complications, including ischemic heart disease and stroke.
Conclusions
As the availability of immunotherapies and other “precision medicine” biologics continues to grow, physicians and scientists alike should evaluate novel targeted therapies in experimental models of CVD to provide a proof-of-concept for eventual adoption in cardiovascular medicine. Mounting evidence from preclinical models of atherosclerosis, myocardial infarction, and other cardiovascular disorders calls for a closer look at immunomodulatory growth factors, including the CSFs, EGF ligands, and FGF21, as potential immunotherapy targets for the treatment of CVD. Inhibiting the signaling of hematopoietic growth factors, such as M-CSF, GM-CSF, and IL-3, and the EGFR blunts the expansion and inflammatory functions of innate and adaptive immune cells in cardiovascular organs and at sites distal to the heart and coronary vasculature, such as the bone marrow and spleen. On the other hand, agonizing the FGF21 pathway induces a favorable cardiometabolic profile, which may protect against the development of CVD. Utilizing clinically available growth factor-targeted therapies to block or alter the bioavailability of candidate growth factors may ultimately help prevent adverse cardiac events in people with CVD risk factors and aid individuals who have already suffered from the complications of cardiovascular inflammation.
Supplementary Material
Highlights section.
The CANTOS trial provides rationale for designing focused immunotherapeutic strategies that target immunomodulatory mediators in cardiovascular disease.
Growth factors are immunomodulatory agents that control cellular functions, including cell proliferation, differentiation, survival, and mobilization, among others.
Growth factors have been successfully targeted in the clinic for a variety of inflammatory/autoimmune diseases, cancer, or metabolic disorders, yet they have not been adopted for cardiovascular disease treatment.
This review highlights recent and newly emerging immmunomodulatory effects of growth factors in cardiovascular inflammation, particularly for the colony-stimulating factors (M-CSF, GM-CSF, G-CSF, IL-3), the epidermal growth factor axis, and fibroblast growth factor 21.
Acknowledgements
The authors acknowledge Cameron S. McAlpine for helpful comments and Kaley Joyes for editing the manuscript. Figure illustrations were created with BioRender (https://biorender.io) under a Standard Academic License.
Sources of Funding
This work was supported by an NIH Ruth L. Kirschstein National Research Service Award (NRSA) Individual Predoctoral Fellowship F31HL147364 from the NHLBI (to J.E.M.) and NIH grants R35HL135752, R01HL128264, P01HL131478, an American Heart Association Established Investigator Award, and the Patricia and Scott Eston MGH Research Scholar Award (to F.K.S.).
Nonstandard Abbreviations
- CSF-1R
colony stimulating factor-1 receptor
- CVD
cardiovascular disease
- DC
dendritic cell
- EC
endothelial cell
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- FGF
fibroblast growth factor
- HSPC
hematopoietic stem and progenitor cell
- IRA
innate response activator
- MI
myocardial infarction
- oxLDL
oxidized low-density lipoprotein
- SMC
smooth muscle cell
Footnotes
Disclosures
None.
References
- 1.Mokdad AH, Ballestros K, Echko M, et al. The state of us health, 1990–2016: Burden of diseases, injuries, and risk factors among us states. JAMA. 2018;319:1444–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roth GA, Abate D, Aba SM, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the global burden of disease study 2017. Lancet. 2018;392:1736–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kathiresan S, Srivastava D. Genetics of human cardiovascular disease. Cell. 2012;148:1242–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nahrendorf M, Swirski FK. Lifestyle effects on hematopoiesis and atherosclerosis. Circ Res. 2015;116:884–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Head T, Daunert S, Goldschmidt-Clermont PJ. The aging risk and atherosclerosis: A fresh look at arterial homeostasis. Front Genet. 2017;8:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Libby P Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013;368:2004–2013 [DOI] [PubMed] [Google Scholar]
- 7.Ross R Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126 [DOI] [PubMed] [Google Scholar]
- 8.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843 [DOI] [PubMed] [Google Scholar]
- 9.Libby P, Lichtman AH, Hansson GK. Immune effector mechanisms implicated in atherosclerosis: From mice to humans. Immunity. 2013;38:1092–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339:161–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swirski FK, Nahrendorf M. Cardioimmunology: The immune system in cardiac homeostasis and disease. Nat Rev Immunol. 2018 [DOI] [PubMed] [Google Scholar]
- 12.Wang CY, Liu PY, Liao JK. Pleiotropic effects of statin therapy: Molecular mechanisms and clinical results. Trends Mol Med. 2008;14:37–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Q, Liao JK. Pleiotropic effects of statins. - basic research and clinical perspectives. Circ J. 2010;74:818–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nissen SE, Tuzcu EM, Schoenhagen P, Crowe T, Sasiela WJ, Tsai J, Orazem J, Magorien RD, O’Shaughnessy C, Ganz P, Reversal of Atherosclerosis with Aggressive Lipid Lowering I. Statin therapy, ldl cholesterol, c-reactive protein, and coronary artery disease. N Engl J Med. 2005;352:29–38 [DOI] [PubMed] [Google Scholar]
- 15.Nelson RH. Hyperlipidemia as a risk factor for cardiovascular disease. Prim Care. 2013;40:195–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ridker PM, Rifai N, Pfeffer MA, Sacks FM, Moye LA, Goldman S, Flaker GC, Braunwald E. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and recurrent events (care) investigators. Circulation. 1998;98:839–844 [DOI] [PubMed] [Google Scholar]
- 17.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr., Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ, Group JS. Rosuvastatin to prevent vascular events in men and women with elevated c-reactive protein. N Engl J Med. 2008;359:2195–2207 [DOI] [PubMed] [Google Scholar]
- 18.Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: An individual participant meta-analysis. Lancet. 2010;375:132–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Libby P Interleukin-1 beta as a target for atherosclerosis therapy: Biological basis of cantos and beyond. J Am Coll Cardiol. 2017;70:2278–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131 [DOI] [PubMed] [Google Scholar]
- 21.Ridker PM. Anticytokine agents: Targeting interleukin signaling pathways for the treatment of atherothrombosis. Circ Res. 2019;124:437–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ridker PM, Libby P, MacFadyen JG, Thuren T, Ballantyne C, Fonseca F, Koenig W, Shimokawa H, Everett BM, Glynn RJ. Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: Analyses from the canakinumab anti-inflammatory thrombosis outcomes study (cantos). Eur Heart J. 2018;39:3499–3507 [DOI] [PubMed] [Google Scholar]
- 23.Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ, Group CT. Relationship of c-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: A secondary analysis from the cantos randomised controlled trial. Lancet. 2018;391:319–328 [DOI] [PubMed] [Google Scholar]
- 24.Caspi RR. Immunotherapy of autoimmunity and cancer: The penalty for success. Nat Rev Immunol. 2008;8:970–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zupancic E, Fayad ZA, Mulder WJM. Cardiovascular immunotherapy and the role of imaging. Arterioscler Thromb Vasc Biol. 2017;37:e167–e171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Metcalf D The colony-stimulating factors and cancer. Nat Rev Cancer. 2010;10:425–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamilton JA, Cook AD, Tak PP. Anti-colony-stimulating factor therapies for inflammatory and autoimmune diseases. Nat Rev Drug Discov. 2016;16:53–70 [DOI] [PubMed] [Google Scholar]
- 28.Yarden Y, Pines G. The erbb network: At last, cancer therapy meets systems biology. Nat Rev Cancer. 2012;12:553–563 [DOI] [PubMed] [Google Scholar]
- 29.Sonoda J, Chen MZ, Baruch A. Fgf21-receptor agonists: An emerging therapeutic class for obesity-related diseases. Horm Mol Biol Clin Investig. 2017;30. [DOI] [PubMed] [Google Scholar]
- 30.Pixley FJ, Stanley ER. Csf-1 regulation of the wandering macrophage: Complexity in action. Trends Cell Biol. 2004;14:628–638 [DOI] [PubMed] [Google Scholar]
- 31.Mossadegh-Keller N, Sarrazin S, Kandalla PK, Espinosa L, Stanley ER, Nutt SL, Moore J, Sieweke MH. M-csf instructs myeloid lineage fate in single haematopoietic stem cells. Nature. 2013;497:239–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chitu V, Stanley ER. Regulation of embryonic and postnatal development by the csf-1 receptor. Curr Top Dev Biol. 2017;123:229–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cecchini MG, Dominguez MG, Mocci S, Wetterwald A, Felix R, Fleisch H, Chisholm O, Hofstetter W, Pollard JW, Stanley ER. Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development. 1994;120:1357–1372 [DOI] [PubMed] [Google Scholar]
- 34.Ryan GR, Dai XM, Dominguez MG, Tong W, Chuan F, Chisholm O, Russell RG, Pollard JW, Stanley ER. Rescue of the colony-stimulating factor 1 (csf-1)-nullizygous mouse (csf1(op)/csf1(op)) phenotype with a csf-1 transgene and identification of sites of local csf-1 synthesis. Blood. 2001;98:74–84 [DOI] [PubMed] [Google Scholar]
- 35.Dai XM, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, Kapp S, Sylvestre V, Stanley ER. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002;99:111–120 [DOI] [PubMed] [Google Scholar]
- 36.Sasmono RT, Oceandy D, Pollard JW, Tong W, Pavli P, Wainwright BJ, Ostrowski MC, Himes SR, Hume DA. A macrophage colony-stimulating factor receptor-green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood. 2003;101:1155–1163 [DOI] [PubMed] [Google Scholar]
- 37.Dai XM, Zong XH, Sylvestre V, Stanley ER. Incomplete restoration of colony-stimulating factor 1 (csf-1) function in csf-1-deficient csf1op/csf1op mice by transgenic expression of cell surface csf-1. Blood. 2004;103:1114–1123 [DOI] [PubMed] [Google Scholar]
- 38.MacDonald KP, Rowe V, Bofinger HM, Thomas R, Sasmono T, Hume DA, Hill GR. The colony-stimulating factor 1 receptor is expressed on dendritic cells during differentiation and regulates their expansion. J Immunol. 2005;175:1399–1405 [DOI] [PubMed] [Google Scholar]
- 39.Chitu V, Stanley ER. Colony-stimulating factor-1 in immunity and inflammation. Curr Opin Immunol. 2006;18:39–48 [DOI] [PubMed] [Google Scholar]
- 40.Cannarile MA, Weisser M, Jacob W, Jegg AM, Ries CH, Ruttinger D. Colony-stimulating factor 1 receptor (csf1r) inhibitors in cancer therapy. J Immunother Cancer. 2017;5:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saitoh T, Kishida H, Tsukada Y, Fukuma Y, Sano J, Yasutake M, Fukuma N, Kusama Y, Hayakawa H. Clinical significance of increased plasma concentration of macrophage colony-stimulating factor in patients with angina pectoris. J Am Coll Cardiol. 2000;35:655–665 [DOI] [PubMed] [Google Scholar]
- 42.Ikonomidis I, Lekakis J, Revela I, Andreotti F, Nihoyannopoulos P. Increased circulating c-reactive protein and macrophage-colony stimulating factor are complementary predictors of long-term outcome in patients with chronic coronary artery disease. Eur Heart J. 2005;26:1618–1624 [DOI] [PubMed] [Google Scholar]
- 43.Sjaarda J, Gerstein H, Chong M, Yusuf S, Meyre D, Anand SS, Hess S, Pare G. Blood csf1 and cxcl12 as causal mediators of coronary artery disease. J Am Coll Cardiol. 2018;72:300–310 [DOI] [PubMed] [Google Scholar]
- 44.Rajavashisth TB, Andalibi A, Territo MC, Berliner JA, Navab M, Fogelman AM, Lusis AJ. Induction of endothelial cell expression of granulocyte and macrophage colony-stimulating factors by modified low-density lipoproteins. Nature. 1990;344:254–257 [DOI] [PubMed] [Google Scholar]
- 45.Clinton SK, Underwood R, Hayes L, Sherman ML, Kufe DW, Libby P. Macrophage colony-stimulating factor gene expression in vascular cells and in experimental and human atherosclerosis. Am J Pathol. 1992;140:301–316 [PMC free article] [PubMed] [Google Scholar]
- 46.Rosenfeld ME, Yla-Herttuala S, Lipton BA, Ord VA, Witztum JL, Steinberg D. Macrophage colony-stimulating factor mrna and protein in atherosclerotic lesions of rabbits and humans. Am J Pathol. 1992;140:291–300 [PMC free article] [PubMed] [Google Scholar]
- 47.Rajavashisth TB, Yamada H, Mishra NK. Transcriptional activation of the macrophage-colony stimulating factor gene by minimally modified ldl. Involvement of nuclear factor-kappa b. Arterioscler Thromb Vasc Biol. 1995;15:1591–1598 [DOI] [PubMed] [Google Scholar]
- 48.Smith JD, Trogan E, Ginsberg M, Grigaux C, Tian J, Miyata M. Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein e. Proc Natl Acad Sci U S A. 1995;92:8264–8268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qiao JH, Tripathi J, Mishra NK, Cai Y, Tripathi S, Wang XP, Imes S, Fishbein MC, Clinton SK, Libby P, Lusis AJ, Rajavashisth TB. Role of macrophage colony-stimulating factor in atherosclerosis: Studies of osteopetrotic mice. Am J Pathol. 1997;150:1687–1699 [PMC free article] [PubMed] [Google Scholar]
- 50.Rajavashisth T, Qiao JH, Tripathi S, Tripathi J, Mishra N, Hua M, Wang XP, Loussararian A, Clinton S, Libby P, Lusis A. Heterozygous osteopetrotic (op) mutation reduces atherosclerosis in ldl receptor- deficient mice. J Clin Invest. 1998;101:2702–2710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Villiers WJ, Smith JD, Miyata M, Dansky HM, Darley E, Gordon S. Macrophage phenotype in mice deficient in both macrophage-colony-stimulating factor (op) and apolipoprotein e. Arterioscler Thromb Vasc Biol. 1998;18:631–640 [DOI] [PubMed] [Google Scholar]
- 52.Murayama T, Yokode M, Kataoka H, Imabayashi T, Yoshida H, Sano H, Nishikawa S, Nishikawa S, Kita T. Intraperitoneal administration of anti-c-fms monoclonal antibody prevents initial events of atherogenesis but does not reduce the size of advanced lesions in apolipoprotein e-deficient mice. Circulation. 1999;99:1740–1746 [DOI] [PubMed] [Google Scholar]
- 53.Shaposhnik Z, Wang X, Lusis AJ. Arterial colony stimulating factor-1 influences atherosclerotic lesions by regulating monocyte migration and apoptosis. J Lipid Res. 2010;51:1962–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Villiers WJ, Fraser IP, Hughes DA, Doyle AG, Gordon S. Macrophage-colony-stimulating factor selectively enhances macrophage scavenger receptor expression and function. J Exp Med. 1994;180:705–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Irvine KM, Andrews MR, Fernandez-Rojo MA, Schroder K, Burns CJ, Su S, Wilks AF, Parton RG, Hume DA, Sweet MJ. Colony-stimulating factor-1 (csf-1) delivers a proatherogenic signal to human macrophages. J Leukoc Biol. 2009;85:278–288 [DOI] [PubMed] [Google Scholar]
- 56.Zhang M, Zhu H, Ding Y, Liu Z, Cai Z, Zou MH. Amp-activated protein kinase alpha1 promotes atherogenesis by increasing monocyte-to-macrophage differentiation. J Biol Chem. 2017;292:7888–7903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McAlpine CS, Kiss MG, Rattik S, et al. Sleep modulates haematopoiesis and protects against atherosclerosis. Nature. 2019;566:383–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Choi JH, Cheong C, Dandamudi DB, Park CG, Rodriguez A, Mehandru S, Velinzon K, Jung IH, Yoo JY, Oh GT, Steinman RM. Flt3 signaling-dependent dendritic cells protect against atherosclerosis. Immunity. 2011;35:819–831 [DOI] [PubMed] [Google Scholar]
- 59.Busch M, Westhofen TC, Koch M, Lutz MB, Zernecke A. Dendritic cell subset distributions in the aorta in healthy and atherosclerotic mice. PLoS One. 2014;9:e88452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koltsova EK, Garcia Z, Chodaczek G, Landau M, McArdle S, Scott SR, von Vietinghoff S, Galkina E, Miller YI, Acton ST, Ley K. Dynamic t cell-apc interactions sustain chronic inflammation in atherosclerosis. J Clin Invest. 2012;122:3114–3126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meyer IS, Goetzke CC, Kespohl M, Sauter M, Heuser A, Eckstein V, Vornlocher HP, Anderson DG, Haas J, Meder B, Katus HA, Klingel K, Beling A, Leuschner F. Silencing the csf-1 axis using nanoparticle encapsulated sirna mitigates viral and autoimmune myocarditis. Front Immunol. 2018;9:2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang X, Liu S, Weng X, Wu T, Yu L, Xu Y, Guo J. Brg1 trans-activates endothelium-derived colony stimulating factor to promote calcium chloride induced abdominal aortic aneurysm in mice. J Mol Cell Cardiol. 2018;125:6–17 [DOI] [PubMed] [Google Scholar]
- 63.Yano T, Miura T, Whittaker P, Miki T, Sakamoto J, Nakamura Y, Ichikawa Y, Ikeda Y, Kobayashi H, Ohori K, Shimamoto K. Macrophage colony-stimulating factor treatment after myocardial infarction attenuates left ventricular dysfunction by accelerating infarct repair. J Am Coll Cardiol. 2006;47:626–634 [DOI] [PubMed] [Google Scholar]
- 64.Morimoto H, Takahashi M, Shiba Y, Izawa A, Ise H, Hongo M, Hatake K, Motoyoshi K, Ikeda U. Bone marrow-derived cxcr4+ cells mobilized by macrophage colony-stimulating factor participate in the reduction of infarct area and improvement of cardiac remodeling after myocardial infarction in mice. Am J Pathol. 2007;171:755–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Okazaki T, Ebihara S, Asada M, Yamanda S, Saijo Y, Shiraishi Y, Ebihara T, Niu K, Mei H, Arai H, Yambe T. Macrophage colony-stimulating factor improves cardiac function after ischemic injury by inducing vascular endothelial growth factor production and survival of cardiomyocytes. Am J Pathol. 2007;171:1093–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Frangogiannis NG, Mendoza LH, Ren G, Akrivakis S, Jackson PL, Michael LH, Smith CW, Entman ML. Mcsf expression is induced in healing myocardial infarcts and may regulate monocyte and endothelial cell phenotype. Am J Physiol Heart Circ Physiol. 2003;285:H483–492 [DOI] [PubMed] [Google Scholar]
- 67.Leblond AL, Klinkert K, Martin K, Turner EC, Kumar AH, Browne T, Caplice NM. Systemic and cardiac depletion of m2 macrophage through csf-1r signaling inhibition alters cardiac function post myocardial infarction. PLoS One. 2015;10:e0137515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haldar M, Kohyama M, So AY, et al. Heme-mediated spi-c induction promotes monocyte differentiation into iron-recycling macrophages. Cell. 2014;156:1223–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Muller PA, Koscso B, Rajani GM, et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158:300–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Theurl I, Hilgendorf I, Nairz M, et al. On-demand erythrocyte disposal and iron recycling requires transient macrophages in the liver. Nat Med. 2016;22:945–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baghdadi M, Umeyama Y, Hama N, Kobayashi T, Han N, Wada H, Seino KI. Interleukin-34, a comprehensive review. J Leukoc Biol. 2018;104:931–951 [DOI] [PubMed] [Google Scholar]
- 74.Hercus TR, Kan WLT, Broughton SE, et al. Role of the beta common (βc) family of cytokines in health and disease. Cold Spring Harb Perspect Biol. 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Becher B, Tugues S, Greter M. Gm-csf: From growth factor to central mediator of tissue inflammation. Immunity. 2016;45:963–973 [DOI] [PubMed] [Google Scholar]
- 76.Shiomi A, Usui T, Mimori T. Gm-csf as a therapeutic target in autoimmune diseases. Inflamm Regen. 2016;36:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wicks IP, Roberts AW. Targeting gm-csf in inflammatory diseases. Nat Rev Rheumatol. 2016;12:37–48 [DOI] [PubMed] [Google Scholar]
- 78.Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: A novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13:260–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kritas SK, Saggini A, Cerulli G, Caraffa A, Antinolfi P, Pantalone A, Saggini R, Frydas S, Rosati M, Tei M, Speziali A, Pandolfi F, Conti P. Interrelationship between il-3 and mast cells. J Biol Regul Homeost Agents. 2014;28:17–21 [PubMed] [Google Scholar]
- 80.Weber GF, Chousterman BG, He S, et al. Interleukin-3 amplifies acute inflammation and is a potential therapeutic target in sepsis. Science. 2015;347:1260–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lantz CS, Boesiger J, Song CH, Mach N, Kobayashi T, Mulligan RC, Nawa Y, Dranoff G, Galli SJ. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature. 1998;392:90–93 [DOI] [PubMed] [Google Scholar]
- 82.Brizzi MF, Formato L, Dentelli P, Rosso A, Pavan M, Garbarino G, Pegoraro M, Camussi G, Pegoraro L. Interleukin-3 stimulates migration and proliferation of vascular smooth muscle cells: A potential role in atherogenesis. Circulation. 2001;103:549–554 [DOI] [PubMed] [Google Scholar]
- 83.McKenna K, Beignon AS, Bhardwaj N. Plasmacytoid dendritic cells: Linking innate and adaptive immunity. J Virol. 2005;79:17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Testa U, Pelosi E, Frankel A. Cd 123 is a membrane biomarker and a therapeutic target in hematologic malignancies. Biomark Res. 2014;2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, Tall AR. Atp-binding cassette transporters and hdl suppress hematopoietic stem cell proliferation. Science. 2010;328:1689–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Murphy AJ, Akhtari M, Tolani S, Pagler T, Bijl N, Kuo CL, Wang M, Sanson M, Abramowicz S, Welch C, Bochem AE, Kuivenhoven JA, Yvan-Charvet L, Tall AR. Apoe regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J Clin Invest. 2011;121:4138–4149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Robbins CS, Chudnovskiy A, Rauch PJ, et al. Extramedullary hematopoiesis generates ly-6c(high) monocytes that infiltrate atherosclerotic lesions. Circulation. 2012;125:364–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang M, Subramanian M, Abramowicz S, Murphy AJ, Gonen A, Witztum J, Welch C, Tabas I, Westerterp M, Tall AR. Interleukin-3/granulocyte macrophage colony-stimulating factor receptor promotes stem cell expansion, monocytosis, and atheroma macrophage burden in mice with hematopoietic apoe deficiency. Arterioscler Thromb Vasc Biol. 2014;34:976–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Anzai A, Choi JL, He S, et al. The infarcted myocardium solicits gm-csf for the detrimental oversupply of inflammatory leukocytes. J Exp Med. 2017;214:3293–3310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hilgendorf I, Theurl I, Gerhardt LM, et al. Innate response activator b cells aggravate atherosclerosis by stimulating t helper-1 adaptive immunity. Circulation. 2014;129:1677–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shaposhnik Z, Wang X, Weinstein M, Bennett BJ, Lusis AJ. Granulocyte macrophage colony-stimulating factor regulates dendritic cell content of atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2007;27:621–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu SN, Chen M, Jongstra-Bilen J, Cybulsky MI. Gm-csf regulates intimal cell proliferation in nascent atherosclerotic lesions. J Exp Med. 2009;206:2141–2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Subramanian M, Thorp E, Tabas I. Identification of a non-growth factor role for gm-csf in advanced atherosclerosis: Promotion of macrophage apoptosis and plaque necrosis through il-23 signaling. Circ Res. 2015;116:e13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ditiatkovski M, Toh BH, Bobik A. Gm-csf deficiency reduces macrophage ppar-gamma expression and aggravates atherosclerosis in apoe-deficient mice. Arterioscler Thromb Vasc Biol. 2006;26:2337–2344 [DOI] [PubMed] [Google Scholar]
- 95.Haghighat A, Weiss D, Whalin MK, Cowan DP, Taylor WR. Granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor exacerbate atherosclerosis in apolipoprotein e-deficient mice. Circulation. 2007;115:2049–2054 [DOI] [PubMed] [Google Scholar]
- 96.Maekawa Y, Anzai T, Yoshikawa T, Sugano Y, Mahara K, Kohno T, Takahashi T, Ogawa S. Effect of granulocyte-macrophage colony-stimulating factor inducer on left ventricular remodeling after acute myocardial infarction. J Am Coll Cardiol. 2004;44:1510–1520 [DOI] [PubMed] [Google Scholar]
- 97.Sho E, Sho M, Hoshina K, Kimura H, Nakahashi TK, Dalman RL. Hemodynamic forces regulate mural macrophage infiltration in experimental aortic aneurysms. Exp Mol Pathol. 2004;76:108–116 [DOI] [PubMed] [Google Scholar]
- 98.Naito K, Anzai T, Sugano Y, Maekawa Y, Kohno T, Yoshikawa T, Matsuno K, Ogawa S. Differential effects of gm-csf and g-csf on infiltration of dendritic cells during early left ventricular remodeling after myocardial infarction. J Immunol. 2008;181:5691–5701 [DOI] [PubMed] [Google Scholar]
- 99.Blyszczuk P, Behnke S, Luscher TF, Eriksson U, Kania G. Gm-csf promotes inflammatory dendritic cell formation but does not contribute to disease progression in experimental autoimmune myocarditis. Biochim Biophys Acta. 2013;1833:934–944 [DOI] [PubMed] [Google Scholar]
- 100.Ye P, Chen W, Wu J, Huang X, Li J, Wang S, Liu Z, Wang G, Yang X, Zhang P, Lv Q, Xia J. Gm-csf contributes to aortic aneurysms resulting from smad3 deficiency. J Clin Invest. 2013;123:2317–2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Son BK, Sawaki D, Tomida S, Fujita D, Aizawa K, Aoki H, Akishita M, Manabe I, Komuro I, Friedman SL, Nagai R, Suzuki T. Granulocyte macrophage colony-stimulating factor is required for aortic dissection/intramural haematoma. Nat Commun. 2015;6:6994. [DOI] [PubMed] [Google Scholar]
- 102.Stock AT, Hansen JA, Sleeman MA, McKenzie BS, Wicks IP. Gm-csf primes cardiac inflammation in a mouse model of kawasaki disease. J Exp Med. 2016;213:1983–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen G, Bracamonte-Baran W, Diny NL, Hou X, Talor MV, Fu K, Liu Y, Davogustto G, Vasquez H, Taegtmeyer H, Frazier OH, Waisman A, Conway SJ, Wan F, Cihakova D. Sca-1(+) cardiac fibroblasts promote development of heart failure. Eur J Immunol. 2018;48:1522–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wu L, Ong S, Talor MV, Barin JG, Baldeviano GC, Kass DA, Bedja D, Zhang H, Sheikh A, Margolick JB, Iwakura Y, Rose NR, Cihakova D. Cardiac fibroblasts mediate il-17a-driven inflammatory dilated cardiomyopathy. J Exp Med. 2014;211:1449–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sonderegger I, Iezzi G, Maier R, Schmitz N, Kurrer M, Kopf M. Gm-csf mediates autoimmunity by enhancing il-6-dependent th17 cell development and survival. J Exp Med. 2008;205:2281–2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Myers JM, Cooper LT, Kem DC, Stavrakis S, Kosanke SD, Shevach EM, Fairweather D, Stoner JA, Cox CJ, Cunningham MW. Cardiac myosin-th17 responses promote heart failure in human myocarditis. JCI Insight. 2016;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wu L, Diny NL, Ong S, Barin JG, Hou X, Rose NR, Talor MV, Cihakova D. Pathogenic il-23 signaling is required to initiate gm-csf-driven autoimmune myocarditis in mice. Eur J Immunol. 2016;46:582–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.von der Thusen JH, Kuiper J, van Berkel TJ, Biessen EA. Interleukins in atherosclerosis: Molecular pathways and therapeutic potential. Pharmacol Rev. 2003;55:133–166 [DOI] [PubMed] [Google Scholar]
- 109.Anzai A, Mindur JE, Halle L, et al. Self-reactive cd4(+) il-3(+) t cells amplify autoimmune inflammation in myocarditis by inciting monocyte chemotaxis. J Exp Med. 2019;216:369–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Uchida K, Beck DC, Yamamoto T, Berclaz PY, Abe S, Staudt MK, Carey BC, Filippi MD, Wert SE, Denson LA, Puchalski JT, Hauck DM, Trapnell BC. Gm-csf autoantibodies and neutrophil dysfunction in pulmonary alveolar proteinosis. N Engl J Med. 2007;356:567–579 [DOI] [PubMed] [Google Scholar]
- 111.Han X, Uchida K, Jurickova I, Koch D, Willson T, Samson C, Bonkowski E, Trauernicht A, Kim MO, Tomer G, Dubinsky M, Plevy S, Kugathsan S, Trapnell BC, Denson LA. Granulocyte-macrophage colony-stimulating factor autoantibodies in murine ileitis and progressive ileal crohn’s disease. Gastroenterology. 2009;136:1261–1271, e1261–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Egea L, Hirata Y, Kagnoff MF. Gm-csf: A role in immune and inflammatory reactions in the intestine. Expert Rev Gastroenterol Hepatol. 2010;4:723–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Brown RL, Sequeira RP, Clarke TB. The microbiota protects against respiratory infection via gm-csf signaling. Nat Commun. 2017;8:1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bendall LJ, Bradstock KF. G-csf: From granulopoietic stimulant to bone marrow stem cell mobilizing agent. Cytokine Growth Factor Rev. 2014;25:355–367 [DOI] [PubMed] [Google Scholar]
- 115.Eyles JL, Hickey MJ, Norman MU, Croker BA, Roberts AW, Drake SF, James WG, Metcalf D, Campbell IK, Wicks IP. A key role for g-csf-induced neutrophil production and trafficking during inflammatory arthritis. Blood. 2008;112:5193–5201 [DOI] [PubMed] [Google Scholar]
- 116.Molineux G, Pojda Z, Dexter TM. A comparison of hematopoiesis in normal and splenectomized mice treated with granulocyte colony-stimulating factor. Blood. 1990;75:563–569 [PubMed] [Google Scholar]
- 117.Lieschke GJ, Grail D, Hodgson G, Metcalf D, Stanley E, Cheers C, Fowler KJ, Basu S, Zhan YF, Dunn AR. Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood. 1994;84:1737–1746 [PubMed] [Google Scholar]
- 118.Shim W, Mehta A, Lim SY, Zhang G, Lim CH, Chua T, Wong P. G-csf for stem cell therapy in acute myocardial infarction: Friend or foe? Cardiovasc Res. 2011;89:20–30 [DOI] [PubMed] [Google Scholar]
- 119.Ohtsuka M, Takano H, Zou Y, Toko H, Akazawa H, Qin Y, Suzuki M, Hasegawa H, Nakaya H, Komuro I. Cytokine therapy prevents left ventricular remodeling and dysfunction after myocardial infarction through neovascularization. FASEB J. 2004;18:851–853 [DOI] [PubMed] [Google Scholar]
- 120.Harada M, Qin Y, Takano H, et al. G-csf prevents cardiac remodeling after myocardial infarction by activating the jak-stat pathway in cardiomyocytes. Nat Med. 2005;11:305–311 [DOI] [PubMed] [Google Scholar]
- 121.Deindl E, Zaruba MM, Brunner S, Huber B, Mehl U, Assmann G, Hoefer IE, Mueller-Hoecker J, Franz WM. G-csf administration after myocardial infarction in mice attenuates late ischemic cardiomyopathy by enhanced arteriogenesis. FASEB J. 2006;20:956–958 [DOI] [PubMed] [Google Scholar]
- 122.Sato T, Suzuki H, Kusuyama T, Omori Y, Soda T, Tsunoda F, Shoji M, Iso Y, Koba S, Geshi E, Katagiri T, Kawachi K, Wakabayashi K, Takeyama Y. G-csf after myocardial infarction accelerates angiogenesis and reduces fibrosis in swine. Int J Cardiol. 2008;127:166–173 [DOI] [PubMed] [Google Scholar]
- 123.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673 [DOI] [PubMed] [Google Scholar]
- 124.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668 [DOI] [PubMed] [Google Scholar]
- 125.Liu M, Liu K, Chen D, Chen H, Sun K, Ju X, Lan J, Zhou Y, Wang W, Pang L. The effect of granulocyte colony-stimulating factor on the progression of atherosclerosis in animal models: A meta-analysis. Biomed Res Int. 2017;2017:6705363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Matsumoto T, Watanabe H, Ueno T, Tsunemi A, Hatano B, Kusumi Y, Mitsumata M, Fukuda N, Matsumoto K, Saito S, Mugishima H. Appropriate doses of granulocyte-colony stimulating factor reduced atherosclerotic plaque formation and increased plaque stability in cholesterol-fed rabbits. J Atheroscler Thromb. 2010;17:84–96 [DOI] [PubMed] [Google Scholar]
- 127.Tousoulis D, Briasoulis A, Vogiatzi G, Valatsou A, Kourkouti P, Pantopoulou A, Papageorgiou N, Perrea D, Stefanadis C. Effects of direct infusion of bone marrow-derived progenitor cells and indirect mobilization of hematopoietic progenitor cells on atherosclerotic plaque and inflammatory process in atherosclerosis. Int J Cardiol. 2013;168:4769–4774 [DOI] [PubMed] [Google Scholar]
- 128.Sinha SK, Mishra V, Nagwani S, Rajavashisth TB. Effects of g-csf on serum cholesterol and development of atherosclerotic plaque in apolipoprotein e-deficient mice. Int J Clin Exp Med. 2014;7:1979–1989 [PMC free article] [PubMed] [Google Scholar]
- 129.Schneider MR, Yarden Y. The egfr-her2 module: A stem cell approach to understanding a prime target and driver of solid tumors. Oncogene. 2016;35:2949–2960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sigismund S, Avanzato D, Lanzetti L. Emerging functions of the egfr in cancer. Mol Oncol. 2018;12:3–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Makki N, Thiel KW, Miller FJ, Jr. The epidermal growth factor receptor and its ligands in cardiovascular disease. Int J Mol Sci. 2013;14:20597–20613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dreux AC, Lamb DJ, Modjtahedi H, Ferns GA. The epidermal growth factor receptors and their family of ligands: Their putative role in atherogenesis. Atherosclerosis. 2006;186:38–53 [DOI] [PubMed] [Google Scholar]
- 133.Forrester SJ, Kawai T, O’Brien S, Thomas W, Harris RC, Eguchi S. Epidermal growth factor receptor transactivation: Mechanisms, pathophysiology, and potential therapies in the cardiovascular system. Annu Rev Pharmacol Toxicol. 2016;56:627–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lamb DJ, Modjtahedi H, Plant NJ, Ferns GA. Egf mediates monocyte chemotaxis and macrophage proliferation and egf receptor is expressed in atherosclerotic plaques. Atherosclerosis. 2004;176:21–26 [DOI] [PubMed] [Google Scholar]
- 135.Walter W, Alonso-Herranz L, Trappetti V, Crespo I, Ibberson M, Cedenilla M, Karaszewska A, Nunez V, Xenarios I, Arroyo AG, Sanchez-Cabo F, Ricote M. Deciphering the dynamic transcriptional and post-transcriptional networks of macrophages in the healthy heart and after myocardial injury. Cell Rep. 2018;23:622–636 [DOI] [PubMed] [Google Scholar]
- 136.Zeboudj L, Giraud A, Guyonnet L, Zhang Y, Laurans L, Esposito B, Vilar J, Chipont A, Papac-Milicevic N, Binder CJ, Tedgui A, Mallat Z, Tharaux PL, Ait-Oufella H. Selective egfr (epidermal growth factor receptor) deletion in myeloid cells limits atherosclerosis-brief report. Arterioscler Thromb Vasc Biol. 2018;38:114–119 [DOI] [PubMed] [Google Scholar]
- 137.Zeboudj L, Maitre M, Guyonnet L, et al. Selective egf-receptor inhibition in cd4(+) t cells induces anergy and limits atherosclerosis. J Am Coll Cardiol. 2018;71:160–172 [DOI] [PubMed] [Google Scholar]
- 138.Wang L, Huang Z, Huang W, Chen X, Shan P, Zhong P, Khan Z, Wang J, Fang Q, Liang G, Wang Y. Inhibition of epidermal growth factor receptor attenuates atherosclerosis via decreasing inflammation and oxidative stress. Sci Rep. 2017;8:45917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Howell KW, Meng X, Fullerton DA, Jin C, Reece TB, Cleveland JC, Jr. Toll-like receptor 4 mediates oxidized ldl-induced macrophage differentiation to foam cells. J Surg Res. 2011;171:e27–31 [DOI] [PubMed] [Google Scholar]
- 140.Liu L, Jin X, Hu CF, Zhang YP, Zhou Z, Li R, Shen CX. Amphiregulin enhances cardiac fibrosis and aggravates cardiac dysfunction in mice with experimental myocardial infarction partly through activating egfr-dependent pathway. Basic Res Cardiol. 2018;113:12. [DOI] [PubMed] [Google Scholar]
- 141.Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, Rinfret S, Schiffrin EL, Eisenberg MJ. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113–1132 [DOI] [PubMed] [Google Scholar]
- 142.Han TS, Lean ME. A clinical perspective of obesity, metabolic syndrome and cardiovascular disease. JRSM Cardiovasc Dis. 2016;5:2048004016633371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kliewer SA, Mangelsdorf DJ. A dozen years of discovery: Insights into the physiology and pharmacology of fgf21. Cell Metab. 2019;29:246–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kharitonenkov A, Shiyanova TL, Koester A, et al. Fgf-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Moller DE, Kharitonenkov A. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149:6018–6027 [DOI] [PubMed] [Google Scholar]
- 146.Xu J, Lloyd DJ, Hale C, et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58:250–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chau MD, Gao J, Yang Q, Wu Z, Gromada J. Fibroblast growth factor 21 regulates energy metabolism by activating the ampk-sirt1-pgc-1alpha pathway. Proc Natl Acad Sci U S A. 2010;107:12553–12558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Samms RJ, Smith DP, Cheng CC, Antonellis PP, Perfield JW, 2nd, Kharitonenkov A, Gimeno RE, Adams AC. Discrete aspects of fgf21 in vivo pharmacology do not require ucp1. Cell Rep. 2015;11:991–999 [DOI] [PubMed] [Google Scholar]
- 149.Jimenez V, Jambrina C, Casana E, et al. Fgf21 gene therapy as treatment for obesity and insulin resistance. EMBO Mol Med. 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hanssen MJ, Broeders E, Samms RJ, Vosselman MJ, van der Lans AA, Cheng CC, Adams AC, van Marken Lichtenbelt WD, Schrauwen P. Serum fgf21 levels are associated with brown adipose tissue activity in humans. Sci Rep. 2015;5:10275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Vinales KL, Begaye B, Bogardus C, Walter M, Krakoff J, Piaggi P. Fgf21 is a hormonal mediator of the human “thrifty” metabolic phenotype. Diabetes. 2019;68:318–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kokkinos J, Tang S, Rye KA, Ong KL. The role of fibroblast growth factor 21 in atherosclerosis. Atherosclerosis. 2017;257:259–265 [DOI] [PubMed] [Google Scholar]
- 153.Lin Z, Pan X, Wu F, Ye D, Zhang Y, Wang Y, Jin L, Lian Q, Huang Y, Ding H, Triggle C, Wang K, Li X, Xu A. Fibroblast growth factor 21 prevents atherosclerosis by suppression of hepatic sterol regulatory element-binding protein-2 and induction of adiponectin in mice. Circulation. 2015;131:1861–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu SQ, Roberts D, Kharitonenkov A, Zhang B, Hanson SM, Li YC, Zhang LQ, Wu YH. Endocrine protection of ischemic myocardium by fgf21 from the liver and adipose tissue. Sci Rep. 2013;3:2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Planavila A, Redondo-Angulo I, Villarroya F. Fgf21 and cardiac physiopathology. Front Endocrinol (Lausanne). 2015;6:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wu F, Wang B, Zhang S, Shi L, Wang Y, Xiong R, Pan X, Gong F, Li X, Lin Z. Fgf21 ameliorates diabetic cardiomyopathy by activating the ampk-paraoxonase 1 signaling axis in mice. Clin Sci (Lond). 2017;131:1877–1893 [DOI] [PubMed] [Google Scholar]
- 157.Tang TT, Li YY, Li JJ, et al. Liver-heart crosstalk controls il-22 activity in cardiac protection after myocardial infarction. Theranostics. 2018;8:4552–4562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pan X, Shao Y, Wu F, et al. Fgf21 prevents angiotensin ii-induced hypertension and vascular dysfunction by activation of ace2/angiotensin-(1–7) axis in mice. Cell Metab. 2018;27:1323–1337 e1325 [DOI] [PubMed] [Google Scholar]
- 159.Lin XL, He XL, Zeng JF, Zhang H, Zhao Y, Tan JK, Wang Z. Fgf21 increases cholesterol efflux by upregulating abca1 through the erk½-ppargamma-lxralpha pathway in thp1 macrophage-derived foam cells. DNA Cell Biol. 2014;33:514–521 [DOI] [PubMed] [Google Scholar]
- 160.Yu Y, He J, Li S, Song L, Guo X, Yao W, Zou D, Gao X, Liu Y, Bai F, Ren G, Li D. Fibroblast growth factor 21 (fgf21) inhibits macrophage-mediated inflammation by activating nrf2 and suppressing the nf-kappab signaling pathway. Int Immunopharmacol. 2016;38:144–152 [DOI] [PubMed] [Google Scholar]
- 161.Wang N, Li JY, Li S, Guo XC, Wu T, Wang WF, Li DS. Fibroblast growth factor 21 regulates foam cells formation and inflammatory response in ox-ldl-induced thp-1 macrophages. Biomed Pharmacother. 2018;108:1825–1834 [DOI] [PubMed] [Google Scholar]
- 162.Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, Spiegelman BM. Fgf21 regulates pgc-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Huang Z, Zhong L, Lee JTH, Zhang J, Wu D, Geng L, Wang Y, Wong CM, Xu A. The fgf21-ccl11 axis mediates beiging of white adipose tissues by coupling sympathetic nervous system to type 2 immunity. Cell Metab. 2017;26:493–508 e494 [DOI] [PubMed] [Google Scholar]
- 164.Li H, Wu G, Fang Q, Zhang M, Hui X, Sheng B, Wu L, Bao Y, Li P, Xu A, Jia W. Fibroblast growth factor 21 increases insulin sensitivity through specific expansion of subcutaneous fat. Nat Commun. 2018;9:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Oikonomou EK, Antoniades C. The role of adipose tissue in cardiovascular health and disease. Nat Rev Cardiol. 2019;16:83–99 [DOI] [PubMed] [Google Scholar]
- 166. Reiner Z Statins in the primary prevention of cardiovascular disease. Nat Rev Cardiol. 2013;10:453–464 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.