Abstract
Background:
Blood pressure visit-to-visit variability (BPV) is a novel risk factor for deleterious long-term cardiac and renal outcomes in the general population. We hypothesized that patients with systemic lupus erythematosus (SLE) have greater BPV than control subjects and that BPV is associated with a higher comorbidity burden.
Methods:
We studied 899 patients with SLE and 4,172 matched controls using de-identified electronic health records from an academic medical center. We compared BPV measures in patients with SLE and control subjects and examined the association between BPV and patients’ characteristics.
Results:
Patients with SLE had higher systolic BPV 9.7% [7.8%-11.8%] than the control group 9.2% [7.4%-11.2%], p<0.001 by coefficient of variation. Additional measures of systolic BPV (i.e., standard deviation, average real variation, successive variation, and maximum measure-to-measure change) were also significantly higher in patients with SLE than in control subjects. In patients with SLE, BPV correlated significantly with age, creatinine, C-reactive protein, triglyceride concentrations, and the Charlson comorbidity score (all p<0.05). Hydroxychloroquine use was associated with reduced BPV (p<0.001), whereas use of anti-hypertensives, cyclophosphamide, mycophenolate mofetil, and corticosteroids was associated with increased BPV (p<0.05).
Conclusion:
Patients with SLE had higher BPV than controls, and this increased BPV was associated with greater Charlson comorbidity scores, several clinical characteristics, and immunosuppressant medications. In particular, hydroxychloroquine prescription was associated with lower BPV.
Blood pressure (BP) routinely fluctuates. Elevated blood pressure variability intensifies the stress on blood vessels and leads to endothelial dysfunction, which promotes early target organ damage.1 In the general population, the extent of BP fluctuations over time provide additional, independent prognostic information compared with both isolated office readings and average ambulatory blood pressure measures.2
Greater systolic blood pressure visit-to-visit variability (BPV), regardless of BP, is related to worse long-term outcomes, including increased risk for all-cause mortality, coronary heart disease, cerebrovascular events, heart failure, and chronic kidney disease.3-7 Studies from the US Veterans8 and the Valsartan Antihypertensive Long-term Use Evaluation trial (VALUE)9 further support these findings.
Systemic lupus erythematosus (SLE) is associated with both a 3-fold increase in mortality compared to the general population10 and a higher comorbidity burden. A recent study indicated that systolic blood pressure varies during follow-up in this patient population; 46% of SLE patients fluctuated between being classified as normotensive and hypertensive over a year period.11 This study further suggested that both lupus disease activity and the use of corticosteroids were associated with increased systolic blood pressure (SBP).11 However, there is no published research on the relationship between standardized measures of BPV and SLE and whether BPV contributes to a higher comorbidity burden in patients with SLE.
The aims for this study were (1) to compare BPV between a group of patients with SLE and a group of frequency-matched controls; and (2) to examine the relationship between BPV and both the markers of inflammation and the burden of comorbidity in patients with SLE.
Methods:
SLE and Control Groups
We used the de-identified electronic health record database (EHR) at Vanderbilt University Medical Center in Nashville, TN to assemble a cohort of patients with SLE and frequency-matched control patients.12 The EHR includes 2.8 million patient records, with approximately 1 million patient records containing longitudinal data. We identified SLE patients using a previously validated algorithm with a positive predictive value (PPV) of 94%.13 This algorithm includes patients with an ICD-9 code for SLE (710.0) on four or more occasions and an antinuclear antibody (ANA) titer greater than or equal to 1:160, while excluding patients with ICD-9 codes for either systemic sclerosis (710.1) or dermatomyositis (710.3). After assembling the group of patients with SLE, we used frequency-matching based on age (within 5 years), race, and gender to generate a control group in a 5:1 ratio. Using ICD-9 codes, we excluded patients with autoimmune disease (see supplementary Table 1). For both the SLE patients and controls, we excluded individuals who were younger than 18 years old, had fewer than three outpatient visits over five years, or had outpatient BP measures on fewer than three unique dates to ensure sufficient data for analysis of BPV.
Follow-up:
In the SLE group, the beginning of the study period was defined as the date of the first ICD-9 code for SLE (710.0); in the control group, the study period began at the date of the first ICD-9 code for any diagnosis. The observation period continued until the first of the following: (1) date of the last ICD-9 code in the medical record, (2) end of the study (June 2017), or (3) death.
Blood Pressure Variability
We extracted outpatient BP measures for both the SLE patients and controls from the EHR. We limited our analysis to outpatient measures to minimize variability due to acute illnesses. Values were identified as inpatient measures if they coincided with an inpatient CPT code (see supplementary Table 2); these measures were then excluded. When multiple outpatient BP values were available from the same date, we used the median as a single value in our primary analysis.
For quality control, we excluded any measure with SBP lower than 30 or greater than 300 as well as measures with a diastolic blood pressure (DBP) lower than 10 or greater than 120. The primary outcome was the SBP coefficient of variation (SBP CV). Secondary outcomes included standard deviation, average real variation, and successive variation for both SBP and DBP, along with CV for DBP.14, 15
Maximum Measure-to-Measure Change in Systolic Blood Pressure
We also examined a measure of variability that is immediately accessible in the clinic: an individual’s largest change in systolic blood pressure from one visit or measurement to the next. For this analysis, we used all outpatient SBP values; we did not limit the analysis to the median on dates that had multiple SBP values. We examined whether these maximum changes were associated with clinical characteristics.
Charlson Scores
We retrieved ICD-9 codes for the multiple categories of comorbidities that constitute the Charlson comorbidity score from the EHR (see supplementary Table 2).16 We only included codes that appeared on or followed the date when the patient met our inclusion criteria. An individual was considered positive for a category if a single representative code was present. We compared the frequency of each comorbidity category between groups using Pearson’s chi-squared tests.
Other Covariates:
We extracted demographic and comorbid variables, including age, race, ethnicity, sex, height, weight, immunologic markers (i.e., antinuclear antibody positivity and anti-double stranded DNA), complement levels (i.e., C3 and C4), creatinine, inflammatory markers (i.e., erythrocyte sedimentation rate and C-reactive protein), cholesterol and triglyceride concentrations, use of medications (i.e., antihypertensives, antimalarials, cyclophosphamide, mycophenolate mofetil, azathioprine, corticosteroids, and other immunosuppressants/immunomodulators), and estimated glomerular filtration rate (eGFR) using computer programming in Structured Query Language.17
Statistical Analysis
We used Wilcoxon rank-sum tests to assess the significance of differences in the outcome measures between the groups. In patients with SLE, we utilized similar methods to compare BPV by gender and race. C3, C4, C-reactive protein (CRP), BMI, creatinine, Charlson comorbidity index, and age were analyzed for potential relationships with SBP CV as well as the maximum change in SBP using Spearman correlations. To further explore the association between the Charlson comorbidity score and BPV, we built proportional odds logistic regression models for both univariate and multivariate (adjusted for age, race, and sex) models. Additionally, we used univariate and multivariate linear regression to analyze whether use of cyclophosphamide, mycophenolate mofetil, azathioprine, corticosteroids, and other immunosuppressants/immunomodulators impacted the significance of our findings. All analyses were conducted using STATA (Version 15.1, College Station, TX).
Institutional Review Board
This study was approved by the Vanderbilt University IRB, which waived the need for informed consent (IRB# 170421).
Results:
Patient Characteristics:
We identified 899 patients with SLE and 4,172 control subjects. Characteristics of each group are displayed in Table 1. We found no significant differences in age, gender, or race/ethnicity, as predicted by our matching for these characteristics. There were statistically significant but small differences in other characteristics, including BMI, renal function, and cholesterol. More SLE patients than controls used antihypertensive agents (see supplementary Table 3 for a complete listing). Overall, the median follow-up was 7.3 person-years; controls generally had more person years of follow-up than patients with SLE (7.6 versus 6.5, respectively).
Table 1.
Characteristics for Patients with SLE and Controls
| Patients with SLE (N=899) |
Matched Controls (N=4,172) |
p-value | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 42 [30-53] | 41 [30-52] | 0.77 |
| Female (%) | 807 (90) | 3811 (91) | 0.13 |
| Race | 0.30 | ||
| White (%) | 635 (71) | 3018 (72) | |
| Black (%) | 214 (24) | 969 (23) | |
| Other or Unknown (%) | 50 (6) | 185 (4) | |
| Ethnicity | 0.12 | ||
| Hispanic (%) | 25 (3) | 132 (3) | |
| BMI (kg/m2) | 26.7 [23.1-32.2] | 27.8 [23.5-33.3] | <0.001 |
| Person years of observation time | 6.5 [3.4-10.6] | 7.6 [3.7-12.2] | <0.001 |
| Blood Pressure Observations | |||
| Number of readings per person | 21 [9-44] | 17 [8-35] | <0.001 |
| Number of dates with | 19 [9-38] | 15 [7-29] | <0.001 |
| observations per person | |||
| Renal Function | |||
| Creatinine (mg/d) | 0.82 [0.70-1.00] | 0.80 [0.70-0.90] | <0.001 |
| eGFR (ml/min/1.73m2) | 85 [65-106] | 90 [75-109] | <0.001 |
| Cardiovascular Measures | |||
| Total Cholesterol (mg/dL) | 180 [152-208] | 189 [161-218] | <0.001 |
| HDL-C (mg/dL) | 52 [39-64] | 54 [44-67] | <0.001 |
| LDL-C (mg/dL) | 99 [79-124] | 107 [85-131] | <0.001 |
| Triglycerides (mg/dL) | 123 [86-180] | 101 [70-155] | <0.001 |
| Hypertension (%) | 479 (53) | 1837 (44) | <0.001 |
| Antihypertensive agents | |||
| ACE inhibitors | 354 (39) | 1209 (29) | <0.001 |
| ARBs | 253 (28) | 743 (18) | <0.001 |
| Beta-blockers | 439 (49) | 1608 (39) | <0.001 |
| Calcium channel blockers | 359 (40) | 1094 (26) | <0.001 |
| Others | 518 (58) | 1888 (45) | <0.001 |
Continuous values are presented as median [interquartile range] and categorical variables are presented as N (%).
N=5071 except as noted for the following variables: BMI (N=4709), Creatinine (N=4737), EGFR (N=4764), Cholesterol (N=2648), HDL-C (N=2569), LDL-C (N=2487), and Triglycerides (N=2623).
Blood Pressure Variability in Patients with SLE and Control Subjects
SBP CV was significantly higher in patients with SLE compared to controls, with a median of 9.7% (7.9%-11.8%) in the SLE group compared to 9.2% (7.4%-11.2%) in the control group (p<0.001). All measures of SBP variability were significantly elevated in the SLE group compared to controls (Table 2). All measures of DBP variability were also elevated in patients with SLE compared to controls, and two of the four were statistically significant (Table 2). Additionally, the median maximum change in SBP from measure to measure was higher in the SLE group compared to controls [36 (26-47) and 33 (24-44) mmHg, p<0.001, respectively]. In determining these measures, the SLE group had significantly more BP readings and dates of observation compared to the control group. SLE patients had a median of 21 BP readings across 19 dates, whereas control patients had a median of 17 BP readings across 15 dates. We completed multivariate linear regression to determine if the differences in the numbers of measures or dates impacted our results; the differences in SBP CV between SLE patients and controls remained significant (β=0.49, 95% CI: 0.26-0.73, p<0.001).
Table 2.
BPV Measures for Patients with SLE and Controls
| Patients with SLE (N=899) |
Matched Controls (N=4172) |
p-value | |
|---|---|---|---|
| Systolic Blood Pressure | |||
| Coefficient of variation (%) | 9.7 [7.9-11.8] | 9.2 [7.4-11.2] | <0.001 |
| Standard deviation (mm Hg) | 11.9 [9.7-15.1] | 11.2 [8.9-14.1] | <0.001 |
| Average real variation (mm Hg) | 12.1 [9.7-15.5] | 11.8 [9.2-14.7] | 0.004 |
| Successive variation (mm Hg) | 15.0 [12.0-19.3] | 14.5 [11.4-18.3] | <0.001 |
| Diastolic Blood Pressure | |||
| Coefficient of variation (%) | 11.1 [9.1-13.3] | 10.8 [8.8-13.0] | 0.008 |
| Standard deviation (mm Hg) | 8.3 [6.7-10.1] | 8.0 [6.5-9.7] | 0.009 |
| Average real variation (mm Hg) | 8.6 [6.8-10.4] | 8.4 [6.7-10.2] | 0.14 |
| Successive variation (mm Hg) | 10.6 [8.6-12.9] | 10.4 [8.3-12.6] | 0.055 |
| Maximum Measure-to-Measure SBP Change (mm Hg) | 36.0 [26.0-47.0] | 33.0 [24.0-44.0] | <0.001 |
Values are presented as median [interquartile range].
Association of Blood Pressure Variability with Clinical Characteristics and Medication Use in SLE Patients
Age, creatinine, C-reactive protein, triglycerides, LDL cholesterol, and a higher Charlson comorbidity score were associated with increased BPV, whereas BMI, C3, C4, and HDL cholesterol were not (Table 3). Similarly, creatinine, C-reactive protein, triglycerides, HDL cholesterol, and a higher Charlson comorbidity score were all associated with increased DBP CV, whereas BMI, C3, C4, and LDL cholesterol were not.
Table 3.
Factors Associated with SBP CV in SLE Patients
| Spearman Coefficient | p-value | |
|---|---|---|
| Age (years) | 0.15 | <0.001 |
| Body mass index (kg/m2) | −0.01 | 0.84 |
| Creatinine (mg/dL) | 0.14 | <0.001 |
| C-reactive protein (mg/L) | 0.12 | 0.002 |
| HDL cholesterol (mg/dL) | −0.09 | 0.07 |
| LDL cholesterol (mg/dL) | 0.10 | 0.03 |
| Triglycerides (mg/dL) | 0.23 | <0.001 |
| C3 (mg/dL) | −0.05 | 0.13 |
| C4 (mg/dL) | 0.003 | 0.94 |
| Charlson comorbidity score | 0.18 | <0.001 |
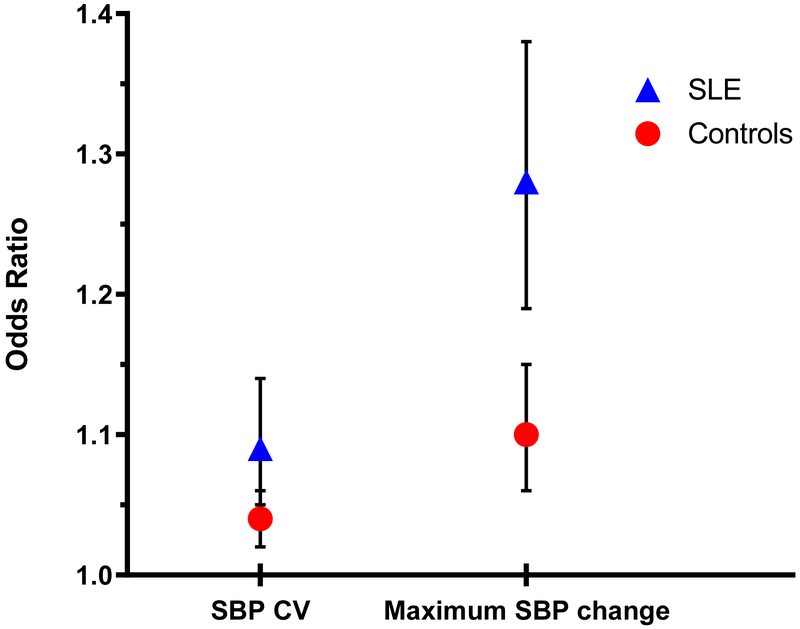
In additional analysis, the association between BPV and the Charlson comorbidity score was independent of age, sex, and race (Figure 1). Of the 899 patients in the SLE group, 845 (94.0%) had been prescribed hydroxychloroquine and 54 (6.0%) had not. The median SBP CV was 9.63% (7.76-11.74) in patients prescribed hydroxychloroquine and 11.04% (9.24-14.42) in patients that were not (p<0.001). With immunosuppressant and immunomodulator use, SLE patients had a higher median SBP CV; this difference was significant with cyclophosphamide, mycophenolate mofetil, and corticosteroids, but not with azathioprine or other immunosuppressants/immunomodulators (see supplementary Table 4 for details). SLE patients who used anti-hypertensive medications also had a significantly higher median SBP CV (supplementary Table 5). We completed multivariate linear regression to confirm that the differences in SBP CV between the SLE patients and controls subjects remained significant when adjusted for anti-hypertensive use as well as sex, age, race, the Charlson comorbidity score, number of total BP readings (β=0.34, 95% CI: 0.11-0.57, p=0.003).
Figure 1. Relationship Between BPV and Comorbidity Index by Charlson Comorbidity Score in Patients with SLE and Control Subjects.
Odds ratios with 95% CI calculated using proportional odds logistic regression, adjusted for age, sex, and race. Results for SBP change are based are expressed by 10 mmHg change
Association of Maximum Measure-to-Measure Change in Systolic Blood Pressure with Clinical Characteristics in SLE Patients
Age, BMI, creatinine, C-reactive protein, triglycerides, and higher Charlson comorbidity score were associated with a larger maximum change in SBP from measure to measure; HDL, LDL, C3, and C4 were not (Table 4).
Table 4.
Factors Associated with the Maximum Measure-to-Measure SBP Change in SLE Patients
| Spearman Coefficient | p-value | |
|---|---|---|
| Age (years) | 0.23 | <0.001 |
| Body mass index (kg/m2) | 0.11 | 0.002 |
| Creatinine (mg/dL) | 0.13 | <0.001 |
| C-reactive protein (mg/L) | 0.17 | <0.001 |
| HDL cholesterol (mg/dL) | −0.03 | 0.56 |
| LDL cholesterol (mg/dL) | −0.01 | 0.81 |
| Triglycerides (mg/dL) | 0.18 | <0.001 |
| C3 (mg/dL) | 0.03 | 0.44 |
| C4 (mg/dL) | 0.07 | 0.06 |
| Charlson comorbidity score | 0.26 | <0.001 |
Discussion:
To the best of our knowledge, this is the first study to demonstrate the following: 1) patients with SLE have higher rates of visit-to-visit blood pressure variability when compared to control subjects; 2) higher variability is associated with C-reactive protein, age, and creatinine in SLE patients; and 3) increased BPV and a higher comorbidity burden are independently associated in SLE patients.
Given that different publications have referred different measures of BP variability, we prespecified the coefficient of variation as our primary outcome but also aimed to quantify standard deviation, average real variation, and successive variation. All measures, with the exception of DBP average real variation and DBP successive variation, were significantly greater in patients with SLE supporting the notion—previously observed in the general population—that these different measures yield similar results.15 Additionally, we explored a simpler assessment of SBP changes to identify risk related to variations in BP amidst the clinical setting. Our secondary analysis examined individuals’ largest change in SBP from one measure to the next. These results indicate that large changes in SBP are also associated with a higher comorbidity burden and may offer clinicians a more accessible means of determining risk-related variability. In our study, the magnitude of difference in the SBP CV was 0.5% (9.7% in the SLE group compared to 9.2% in the control group). Most studies have examined quartiles or quintiles of BPV in the general population and compared outcomes between these groups. A recent metaanalysis demonstrated an increased association between systolic BPV and all-cause mortality, cardiovascular mortality, and cardiovascular events.18 Still, defining a clinically meaningful degree of variability has remained elusive. One study demonstrated a 0.8% absolute difference in SBP CV in association with ischemic strokes compared to a control group.19 Our study suggests an even smaller difference may be clinically meaningful.
We examined the relationships between SBP CV and disease characteristics in patients with SLE. Age, creatinine, and C-reactive protein were significantly associated with SBP CV, whereas complement levels were not. The associations we found between BPV with age and renal function are consistent with previous findings reported in the general population.4, 20 Higher creatinine levels in SLE may reflect either chronic kidney damage or may be evidence of active lupus nephritis. Furthermore, our study suggests an association between a higher comorbidity index and BPV, and the association with CRP indicates a link between underlying inflammation and BPV.
Immunosuppression has previously been shown to predict lower BPV in SLE patients.11 In our study there was a significant reduction in SBP CV in patients who had been prescribed hydroxychloroquine. It is currently recommended that all patients with SLE receive hydroxychloroquine unless there is a contraindication.21 In contrast, there was increased SBP CV in patients who received cyclophosphamide and mycophenolate mofetil. It is possible this is a result of higher disease burden, as these agents are recommended for treatment of more severe lupus manifestations.22 Azathioprine did not have a relationship with SBP CV in our study.
There has been evidence in the general population that calcium channel blockers and diuretics reduce BPV whereas other agents such as beta-blockers and angiotensin-converting enzyme inhibitors or angiotensin II receptor blocker agents may increase BPV.23, 24 Our study indicates that the use of any antihypertensive agent was associated with higher BPV. This may seem counterintuitive, but it is likely explained by the relationship between hypertension and BPV. With increasing absolute blood pressure values we see increasing variability in blood pressure measures. Also, we cannot conclude whether any agent was better than other in this regard because we did not assess the temporal association between variability.
Our results should be interpreted in light of some limitations. First, we could not account for medication compliance or seasonal variability, which are both factors suggested to influence BPV.3 Because we used a de-identified EHR, which only included data collected through routine clinical care at a single tertiary care center, we did not have data from clinical encounters outside of this location. Despite these limitations, the association between SLE and BPV appears robust, and further research is needed to examine whether interventions to reduce BPV will have an impact on comorbidity burden and long-term outcomes in patients with SLE.
This study has a few potential implications for clinical practice and future research. First, because BPV correlates with hypertensive end-organ damage, our results indicating that BPV is higher in patients with SLE and associated with a higher comorbidity burden is of interest. 3-7 Controlling BPV could potentially be a target for future interventions. Further, previously used methods to ascertain BPV rely on relatively complicated mathematical equations. Thus, we used a simpler measure of BPV—the maximum change in SBP between ambulatory visits—and had similar findings.
Supplementary Material
Supplementary Table 1. Inpatient CPT Codes
Supplementary Table 2. ICD-9 Codes for Charlson Comorbidity Score
Supplementary Table 3. Anti-hypertensive Classes and Generic Medications
Supplementary Table 4. SBP CV and Use of Immunosuppressants and Immunomodulators in Patients with SLE
Supplementary Table 5. SBP CV and Use of Anti-hypertensives in Patients with SLE
Supplementary Table 6. Factors Associated with DBP CV in Patients with SLE
Acknowledgments
Financial /Support: TR, JSG, and CPC received funding from the Rheumatology Research Foundation, MMS received funding from NIH-NIDDK grant DK108444. The datasets used for the analyses described were obtained from the Vanderbilt University Medical Center Synthetic Derivative which is supported by institutional funding and by the CTSA grant ULTR000445 from NCATS/NIH. AB received funding from NIH/NICHD 5K12HD043483-12 and NIH/NIAMS 1 K08AR072757-01. This project was supported by the Rheumatology Research Foundation, Lupus Research Alliance, and a K-23 award from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (K23AR064768). CPC is also supported by grant GM126535 from NIGMS.
Footnotes
Conflicts of Interest: The authors have no COIs to disclose.
References
- 1.Kim MK, Han K, Park YM, et al. Associations of Variability in Blood Pressure, Glucose and Cholesterol Concentrations, and Body Mass Index With Mortality and Cardiovascular Outcomes in the General Population. Circulation 2018; 138: 2627–2637. DOI: 10.1161/CIRCULATIONAHA.118.034978. [DOI] [PubMed] [Google Scholar]
- 2.Parati G, Ochoa JE, Salvi P, et al. Prognostic value of blood pressure variability and average blood pressure levels in patients with hypertension and diabetes. Diabetes Care 2013; 36 Suppl 2: S312–324. DOI: 10.2337/dcS13-2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parati G, Ochoa JE, Lombardi C, et al. Assessment and management of blood-pressure variability. Nature Reviews Cardiology 2013; 10: 143. [DOI] [PubMed] [Google Scholar]
- 4.Muntner P, Shimbo D, Tonelli M, et al. The relationship between visit-to-visit variability in systolic blood pressure and all-cause mortality in the general population: findings from NHANES III, 1988 to 1994. Hypertension 2011; 57: 160–166. [DOI] [PubMed] [Google Scholar]
- 5.Muntner P, Whittle J, Lynch AI, et al. Visit-to-visit variability of blood pressure and coronary heart disease, stroke, heart failure, and mortality: a cohort study. Annals of Internal Medicine 2015; 163: 329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whittle J, Lynch AI, Tanner RM, et al. Visit-to-Visit Variability of BP and CKD Outcomes: Results from the ALLHAT. Clinical Journal of the American Society of Nephrology 2016: CJN. 04660415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kronish IM, Lynch Al, Oparil S, et al. The association between antihypertensive medication nonadherence and visit-to-visit variability of blood pressure: findings from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Hypertension 2016; 68: 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gosmanova EO, Mikkelsen MK, Molnar MZ, et al. Association of systolic blood pressure variability with mortality, coronary heart disease, stroke, and renal disease. Journal of the American College of Cardiology 2016; 68: 1375–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehlum MH, Liestøl K, Kjeldsen SE, et al. Blood pressure variability and risk of cardiovascular events and death in patients with hypertension and different baseline risks. European Heart Journal 2018; 39: 2243–2251. [DOI] [PubMed] [Google Scholar]
- 10.Yurkovich M, Vostretsova K, Chen W, et al. Overall and cause-specific mortality in patients with systemic lupus erythematosus: a meta-analysis of observational studies. Arthritis Care and Research 2014; 66: 608–616. [DOI] [PubMed] [Google Scholar]
- 11.Nikpour M, Gladman DD, Ibanez D, et al. Variability over time and correlates of cholesterol and blood pressure in systemic lupus erythematosus: a longitudinal cohort study. Arthritis Research and Therapy 2010; 12: R125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roden DM, Pulley JM, Basford MA, et al. Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clinical Pharmacology & Therapeutics 2008; 84: 362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnado A, Casey C, Carroll RJ, et al. Developing electronic health record algorithms that accurately identify patients with systemic lupus erythematosus. Arthritis Care and Research 2017; 69: 687–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levitan EB, Kaciroti N, Oparil S, et al. Blood pressure measurement device, number and timing of visits, and intra-individual visit-to-visit variability of blood pressure. The Journal of Clinical Hypertension 2012; 14: 744–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levitan E, Kaciroti N, Oparil S, et al. Relationships between metrics of visit-to-visit variability of blood pressure. Journal of Human Hypertension 2013; 27: 589. [DOI] [PubMed] [Google Scholar]
- 16.Deyo RA, Cherkin DC and Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of Clinical Epidemiology 1992; 45: 613–619. [DOI] [PubMed] [Google Scholar]
- 17.Xu H, Stenner SP, Doan S, et al. MedEx: a medication information extraction system for clinical narratives. J Am Med Inform Assoc 2010; 17: 19–24. DOI: 10.1197/jamia.M3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevens SL, Wood S, Koshiaris C, et al. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. 2016; 354: ¡4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hata Y, Kimura Y, Muratani H, et al. Office blood pressure variability as a predictor of brain infarction in elderly hypertensive patients. 2000; 23: 553–560. [DOI] [PubMed] [Google Scholar]
- 20.Hussein WF and Chang TI. Visit-to-visit variability of systolic blood pressure and cardiovascular disease. Current Hypertension Reports 2015; 17:14. [DOI] [PubMed] [Google Scholar]
- 21.Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, et al. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. 2010; 69: 20–28. [DOI] [PubMed] [Google Scholar]
- 22.Hahn BH, Mcmahon MA, Wilkinson A, et al. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. 2012; 64: 797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muntner P, Levitan EB, Lynch AI, et al. Effect of Chlorthalidone, Amlodipine, and Lisinopril on Visit-to-Visit Variability of Blood Pressure: Results From the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. The Journal of Clinical Hypertension 2014; 16: 323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Webb AJ, Fischer U, Mehta Z, et al. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis. The Lancet 2010; 375: 906–915. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Inpatient CPT Codes
Supplementary Table 2. ICD-9 Codes for Charlson Comorbidity Score
Supplementary Table 3. Anti-hypertensive Classes and Generic Medications
Supplementary Table 4. SBP CV and Use of Immunosuppressants and Immunomodulators in Patients with SLE
Supplementary Table 5. SBP CV and Use of Anti-hypertensives in Patients with SLE
Supplementary Table 6. Factors Associated with DBP CV in Patients with SLE