Abstract
Anaplasma phagocytophilum, the agent of human anaplasmosis, is an obligate intracellular bacterium that uses multiple survival strategies to persist in Ixodes scapularis ticks. Our previous study showed that A. phagocytophilum efficiently induced the tyrosine phosphorylation of several Ixodes proteins that includes extended phosphorylation of actin at tyrosine residue Y178. In order to identify the tyrosine kinase responsible for the A. phagocytophilum induced tyrosine phosphorylation of proteins, we combed the I. scapularis genome and identified a non-receptor Src tyrosine kinase ortholog. I. scapularis Src kinase showed high degree of amino acid sequence conservation with Dsrc from Drosophila melanogaster. We noted that at different developmental stages of I. scapularis ticks, larvae expressed significantly higher levels of src transcripts in comparison to the other stages. We found that A. phagocytophilum significantly reduced Src levels in unfed nymphs and in nymphs while blood feeding (48 h during feeding) in comparison to the levels noted to relative uninfected controls. However, A. phagocytophilum increased Src levels in fully engorged larvae and nymphs (48 h post feeding) and in vitro tick cells in comparison to the relative uninfected controls. Inhibition of Src kinase expression and activity by treatment with src-dsRNA or Src-inhibitor, respectively, significantly reduced A. phagocytophilum loads in ticks and tick cells. Overall, our study provides evidence for the important role of I. scapularis Src kinase in facilitating A. phagocytophilum colonization and survival in the arthropod vector.
Keywords: Anaplasma phagocytophilum, Ixodes scapularis, Src kinase, ticks, Src inhibitor, blood feeding
INTRODUCTION
Extracellular molecules transduce signals across the plasma membrane through transmembrane receptors that allow amplification of intracellular signaling to control many of the cellular activities. One type is the receptor protein tyrosine kinase (RPTK) family that has intrinsic protein tyrosine kinase activity (Hunter, 2002; van der Geer, 2002). The other type is the non-receptor protein tyrosine kinases (nRPTKs), the cytoplasmic enzymes that regulate many cellular functions by turning- on or off several other proteins inside a cell (Hanke et al., 1996; Hunter, 2002; van der Geer, 2002). Both types of PTKs are regulated by tyrosine phosphorylation. Signal cascade initiation is mainly dependent on RPTKs, but not on nRPTKs due to the lack of receptor-like features such as an extracellular ligand-binding domain and transmembrane-spanning regions (Boonyaratanakornkit and Edwards, 2007; Hunter, 2002; van der Geer, 2002). Although, most of the nRPTKs are cytosolic enzymes, some of them are anchored to the cell membrane through the modification of the amino-terminal ends (Hunter, 2002; van der Geer, 2002). The most important nRPTKs of the Src family contain the typical myristoylated terminus, which is a region of positively charged residues (Hanke et al., 1996; Roskoski Jr., 2005, 2004; and Brickell, 1992; Superti‐Furga and Courtneidge, 1995). Src family of nRPTKs also contains a short region with low sequence identity, Src homology domains, SH3 and SH2, for the negative regulation of Src and a tyrosine kinase domain with enzymatic activity to add phosphate group on target substrates and a short carboxyl-terminal domain (Roskoski Jr., 2005, 2004; Brickell, 1992). Disruption in phospho-tyrosine binding, due to mutations in the SH2 and SH3 domains leads to increased Src kinase activity (Roskoski Jr., 2005, 2004; Brickell, 1992). In Src, the kinase activity is dependent on two important regulatory tyrosine phosphorylation sites (Roskoski Jr., 2005). The C-terminal tail of Src contains the most important Tyr (Tyrosine)-527 residue that represses the kinase activity (Roskoski Jr., 2005). Another autoregulatory phosphorylation site Tyr-416 in the activation loop of Src, can suppress the transformation ability of the activating Tyr-527 to Phe mutation (Roskoski Jr., 2005). It has been shown that Rous sarcoma virus (RSV) has a form of viral v-Src, an oncogenic variant of Src that has shown the significance of the Tyr-527 phosphorylation site. Due to the C-terminal truncation of the v-Src and absence of this Tyr-527 site, the oncogenic enzyme is maintained to be constitutively active in RSV infected cells (Roskoski Jr., 2004; Liu et al., 2015; Roskoski Jr., 2015). Very little is known about the role of Src tyrosine kinase in arthropods. Recently, silkworm Bombyx mori Src ortholog with highly conserved sequence and protein tyrosine kinase activity was reported (Meng et al., 2015; Song et al., 2016). The Src kinase in silkworms promoted the antimicrobial peptides (AMPs) possibly through activation of p38 MAPK and Akt signaling cascades (Meng et al., 2015; Song et al., 2016). Also, several bacterial pathogens have shown impressive collection of molecular mechanisms for their survival in diverse niches of the host. Listeria monocytogenes entry using the Internalin A (InlA)-invasion pathways is dependent on the Src tyrosine kinase phosphorylation of the E-cadherin cytoplasmic tail (Alto and Orth, 2012; Pizarro-Cerdá et al., 2016). Another study from rickettsial pathogen (Rickettsia montanensis) interactions with Dermacentor variabilis ticks has suggested protein tyrosine kinases in general (including Src kinase) to be important arthropod protein(s) for rickettsial invasion (Petchampai et al., 2015). Treatment with Src family protein tyrosine kinase inhibitor PP2 at concentrations of 250 μM showed significant reduction in percentage of relative invasion in comparison to the 1% DMSO control. Although, no changes were found in 2.5 and 25 μM treated groups (Petchampai et al., 2015).
Anaplasma phagocytophilum is an obligate intracellular pathogen and a causative agent of human anaplasmosis, one of the most common arthropod-borne diseases in the United States, Europe and Asia (Bakken and Dumler, 2015; Dumler et al., 2005). Several studies have shown that A. phagocytophilum primarily infects and persists within human neutrophils, delays apoptosis, inhibits NADPH oxidase activity, subvert phagolysosome biogenesis and modulates cell signaling in its vector (Cabezas-Cruz et al., 2016; Carlyon and Fikrig, 2006, 2003; Carlyon et al., 2004; de la Fuente et al., 2016; Khanal et al., 2017; Neelakanta et al., 2010; Rikihisa, 2011, 2010; Sukumaran et al., 2006; Sultana et al., 2010; Taank et al., 2017; Thomas and Fikrig, 2007). Additionally, the effector proteins from type-IV secretion system of Anaplasma modulate mammalian host cell signaling (Carlyon and Fikrig, 2006; Nelson et al., 2008; Rikihisa, 2010). The blacklegged, Ixodes scapularis, ticks serve as the primary vector host for this pathogen in Northeastern and Upper Midwest part of the United States (Sonenshine, 2005). Anaplasma phagocytophilum is transstadially transmitted to different arthropod stages such as larvae, nymphs, and adults during development (Anderson and Magnarelli,L.A., 2008). In human neutrophils, A. phagocytophilum induces the tyrosine phosphorylation of Rho-Kinase (ROCK1) (Thomas and Fikrig, 2007). Our previous study has shown that A. phagocytophilum selectively modulates arthropod signaling by altering actin phosphorylation to regulate the salp16 gene transcription in association with RNA polymerase II and TATA box-binding protein (Sultana et al., 2010). The mechanisms used by A. phagocytophilum to influence the tyrosine protein phosphorylation, other than serine/threonine PI3K-PAK1 signaling in its arthropod vector, are not known (Sultana et al., 2010). To reveal important insights on A. phagocytophilum survival strategies in arthropod host, we have addressed the role of I. scapularis Src tyrosine kinase. The importance of Src tyrosine kinase in tick-rickettsial pathogen Anaplasma phagocytophilum interactions has not been explored. In this study, we explore whether A. phagocytophilum modulates Src tyrosine kinase signaling in the arthropod host. We provide substantial evidence to show that this tick-borne pathogen regulates the highly conserved Src tyrosine kinase for its survival in the medically important vector.
MATERIALS AND METHODS
Ticks, tick cells, Mice and infection.
The laboratory-reared I. scapularis ticks were used throughout this study. Ticks used in this study are larvae, nymphs and adult (male and female) were obtained from a continuously maintained tick colony at the BEI/Center for disease Control (CDC). The I. scapularis tick cell line ISE6 and HGE1-GFP A. phagocytophilum strain was a generous gift from Dr. Ulrike Munderloh at the University of Minnesota (St Paul, MN) and was maintained as described (Felsheim et al., 2006; Kurtti et al., 2008; Munderloh et al., 2003, 1996). Anaplasma phagocytophilum isolate NCH-1 (obtained from BEI resources, USA) was used in in vivo studies that involved work with mice and tick blood feeding. Anaplasma phagocytophilum isolates (HGE1-GFP or HZ) strains were used in the in vitro cell line experiments. HGE1-GFP is indicated as A. phagocytophilum-GFP strain and HZ strain is indicated as A. phagocytophilum-HZ strain. C3H/HeN mice (4–6 weeks old female, Charles River Laboratories, USA) were used throughout this study. To generate uninfected or A. phagocytophilum–infected unfed nymphs, larvae were fed on either uninfected or A. phagocytophilum–infected C3H/HeN mice and allowed to molt into nymphs. Tick rearing was conducted in an incubator at 23 ± 2°C with 95% relative humidity and a 14/10 hour light/dark photoperiod regiment as described in our previous studies. In acquisition experiments, nymphs were fed on either uninfected or A. phagocytophilum–infected C3H/HeN mice. The dsRNA- or mock-treated uninfected nymphs were fed on A. phagocytophilum-infected mice and upon repletion; ticks were processed further for RNA and DNA extractions.
Ethical statement.
All animal work was carried out in strict accordance with the regulations of the Care and Use of Laboratory Animals of the National Institute of Health. The Institutional Animal Care and Use Committee (IACUC; Animal Welfare Assurance Number: A3172–01) approved the protocol (permit number: 16–017) used in this study. Under the association with Assessment and Accreditation of Laboratory Animal Care (AALAC) Program, animal husbandry was provided at the current institution. Prior to handling animals, acepromazine tranquilizer was administered to minimize anxiety and/or discomfort and all efforts were made to minimize mice suffering.
RNA or DNA extractions and Quantitative Real-time PCR (QRT-PCR) Analysis.
Using the Aurum total RNA mini kit (Bio-Rad, USA) and following the manufacturer’s instructions, we isolated total RNA from different developmental stages of ticks (larvae, nymphs and adult females or males) or unfed/fed uninfected or A. phagocytophilum- infected ticks or dsRNA/inhibitor- or mock -treated ticks or tick cells, respectively. The generated RNA was converted to cDNA using Bio-RAD iScript cDNA synthesis kit (Bio-RAD, USA) and used as template for quantifying Src kinase transcripts with the forward and reverse oligonucleotides 5’ CGCGCACGGACGAGGA 3’ and 5’ GTTCTGCCTCGATGGACTTCAGT 3’, respectively. QRT-PCR was performed as described (Sultana et al., 2016) using CFX96 QPCR machine (Bio-RAD, USA) and iQ-SYBR Green Supermix (Bio-RAD, USA). To quantify bacterial burden in ticks, genomic DNA from A. phagocytophilum–infected unfed or fed nymphs or dsRNA/inhibitor- or mock-treated ticks or tick cells was extracted using DNeasy kit (QIAGEN) and processed for PCR with primers specific for the A. phagocytophilum p44 gene as reported in (Khanal et al., 2018; Taank et al., 2017). In QRT-PCR reactions, the standard curve was generated using 10-fold serial dilutions starting from 2 ng to 0.00002 ng (for tick actin and P44 to detect A. phagocytophilum) or 0.67 to 0.0000067 ng (for Src) of known quantities of respective fragments. As an internal control and to normalize the amount of template, I. scapularis beta-actin or 16S was quantified using oligonucleotides as described in (Taank et al., 2018, 2017).
Immunoblotting analysis.
Immunoblotting was performed as described in (Sultana et al., 2010; Zhou et al., 2018). Total lysates from unfed or fed ticks or ISE6 tick cells were prepared in modified RIPA buffer (BioExpress/VWR, USA) supplemented with EDTA-free protease inhibitor cocktail (Sigma, USA). Protein concentrations were determined by Bradford (BCA) protein assay kit (Pierce/ThermoScientific, USA). Primary (Catalog number sc-5266) and secondary (Catalog number sc-2005) antibodies were obtained from Santa Cruz Biotechnology Inc. (USA) and used as per company recommendations. Total lysates from each group (uninfected or infected), 30 μg (for tick cells and unfed ticks) or 10 μg (for fed ticks) were loaded onto 12% reducing SDS-PAGE gels for immunoblotting. Ponceau S-stained or Coomassie stained gel images served as controls. Antibody binding was detected with WesternBright ECL kit (Advansta, BioExpress). Blots were imaged using Chemidoc MP imaging system and processed using Image Lab (4.1) software obtained from the manufacturer (Bio-RAD, USA).
dsRNA synthesis, tick microinjections and transfections.
The dsRNA synthesis was performed as described (Khanal et al., 2018; Neelakanta et al., 2010; Sultana et al., 2010; Taank et al., 2017). Briefly, the src gene fragment (334 bp) was generated by PCR (using gene specific primers containing BglII (forward primer) and KpnI (reverse primer) restriction enzyme sites using forward and reverse oligonucleotides 5’ CCAGATCTCGAGCTCCAAGAACACCAAGA 3’ and 5’ CGGGTACCGCGCGAACCACCAGT 3’, respectively. Since Src fragment (334 bp) had a KpnI site at 188 bp, the resulting digestion product yielded a product of 187 bp that was purified and cloned into BglII-KpnI sites of the L4440 double T7 Script II vector. The Escherichia coli DH5alpha strain was used as a host for generating the clone with Src kinase fragment in L4440 plasmid. The dsRNA complementary to src gene sequences were synthesized using the MEGAscript RNAi Kit (Ambion Inc., USA) and following manufacturer’s instructions. Microinjections of src-dsRNA and mock (buffer alone) were performed as described (Khanal et al., 2018; Neelakanta et al., 2010; Sultana et al., 2010; Taank et al., 2017). To prepare dsRNA, nearly, 1 μg of L4440 vector containing src sequences was used as a template and was eluted in 50 μl of elution buffer (Ambion Inc., USA). Using Eppendorf and Zeiss Microinjection system, we injected src-dsRNA (~4.2 nl/tick, 1 × 1012 molecules/μl) into the bodies of uninfected unfed nymphs. Microinjected ticks were incubated at room temperature for ~4 hours for acclimatization in a desiccator and fed on A. phagocytophilum–infected mice. Fully engorged ticks were then analyzed for src kinase expression after 48 h post repletion to determine silencing efficiency by QRT-PCR. To achieve silencing of src kinase in ISE6 cells, Lipofectamine transfection (3 μl/1 ml culture) reagent (Invitrogen/ThermoFisher Scientific Inc., USA) was used. Briefly, 1 × 105 tick cells were seeded in L-15B300 medium on to 12 well plates and incubated for overnight. After cell attachment and spreading for overnight, 500 ng of dsRNA mixed with Lipofectamine reagent was added. The data shown in Supplementary Figure 5 was generated with 300 ng of dsRNA. We included at least six replicates to perform this experiment. After 6 hours, 2X L15-B300 medium was added and plates were further incubated for another 18 h. Cell-free A. phagocytophilum (isolated from infected HL-60 cells as described in (Sultana et al., 2010; Thomas and Fikrig, 2007)) was added after 24 h post transfection and cells were incubated for additional 24 h and processed for RNA or DNA extractions. Human promyelocytic cell line (HL-60, obtained from American Type Culture Collection, USA) was used to maintain A. phagocytophilum NCH1 or A. phagocytophilum-GFP strains, and cell-free bacteria isolated from these cells were used for in vitro infection studies as described (Thomas and Fikrig, 2007). Silencing efficiency and the bacterial burden was determined as mentioned in other sections.
Inhibitor studies.
The Src kinase inhibitor purchased from Cayman Chemicals (USA) is a potent competitive inhibitor of both Src and Lck (IC50 = 44 and 88 nm, respectively) as well as Csk and Yes kinases. Main stocks (5 mM) of Src kinase inhibitor were made in DMSO solutions. A 1:10 dilution of the stocks was prepared in DMSO to a final concentration of 1 mM and used for all experiments. Mock solution used was DMSO alone. Tick cells (1× 10 5) were seeded onto 12 well plates and incubated for 16–20 hours. Following overnight incubation, tick cells were treated with 5-μM of Src inhibitor for either 4 or 24 hours followed by A. phagocytophilum infection. Equal volumes of mock DMSO solution (corresponding to 5-μM volume) were added to control cells. Cells were collected at respective time points and processed further for RNA or DNA extractions to measure src transcripts and A. phagocytophilum loads. Inhibitor studies were performed in two independent experiments. One experiment retrieved the 24-hour inhibition data and the second experiment retrieved the 4-hour inhibition data.
Statistics.
Using GraphPad Prism6 software and Microsoft Excel 2010, we analyzed the statistical significance in the data sets. For data to compare two means, the non-paired, two-tailed Student’s t-test was performed. P values of <0.05 were considered significant in all analysis. Wherever necessary, statistical test and P values used are reported.
Supporting Information:
Additional supplementary file includes 6 supplementary figures and figure legends.
RESULTS
Comparison of Src tyrosine kinase from I. scapularis ticks to other insects and human.
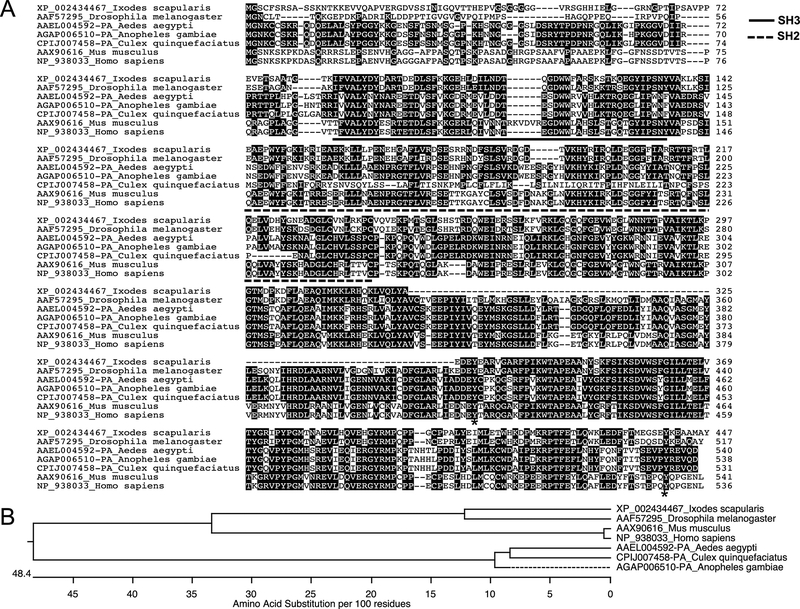
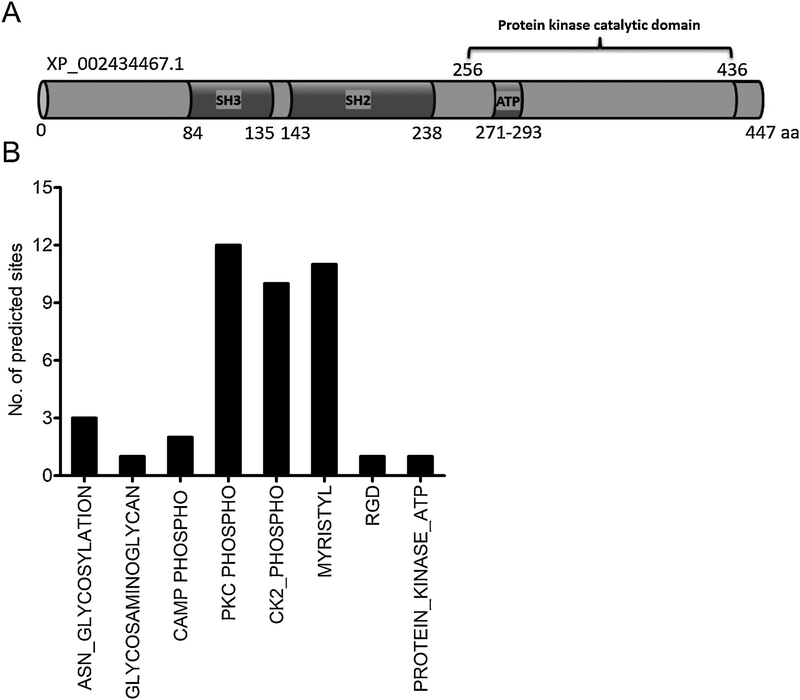
The information on Src tyrosine kinase in vector-pathogen interactions and bacterial survival is needed to recognize the fundamental processes of protein tyrosine phosphorylation in arthropods. We amplified and detected a single band, as I. scapularis encoded one gene transcript for the Src tyrosine kinase in ticks (GenBank acc. no. XP_002434467.1) (Supplementary Fig. 1A). Quantitative Real-Time PCR (QRT-PCR) amplified a product of 159 bp from cDNA prepared from total RNA of I. scapularis- unfed nymphs, 48 h post fed (PF) ticks, and ISE6 tick cells (Supplementary Fig. 1A). Using human Src tyrosine kinase primary amino acid sequence (GenBank acc. no. NP_938033) as a query sequence in the VectorBase, we recognized the Src homology-domains (SH2 and SH3) containing tick ortholog from I. scapularis (XP_002434467) (Fig. 1A). ClustalW alignment of I. scapularis Src tyrosine kinase amino acid sequence revealed 79.3% identity with Drosophila melanogaster Src (GenBank acc. no. AAF57295) (Fig. 1A and Supplementary Fig. 1B). In addition, Aedes aegypti (GenBank acc. no. AAEL004592-PA), Anopheles gambiae (GenBank acc. no. AGAP006510-PA) and Culex quinquefasciatus (GenBank acc. no. CPIJ007458) orthologs showed 45.1%, 45% and 38.6% identity, respectively with I. scapularis Src kinase (GenBank acc. no. XP_002434467) (Fig. 1A and Supplementary Fig. 1B). Both mouse (GenBank acc. no. AAX90616) and human (GenBank acc. no. NP_938033), orthologs showed 55.9% and 55.6% identity to the I. scapularis Src kinase, respectively (Fig. 1A and Supplementary Fig. 1B). Furthermore, the phylogenetic analysis revealed that I. scapularis Src kinase belongs within the same clade with D. melanogaster Dsrc (Fig. 1B). Both human and mouse Src kinase counterparts form a sub-clade within the main clade, suggesting higher degree of identity (Fig. 1B). The mosquito Src kinase orthologs from A. aegypti, A. gambiae and C. quinquefasciatus belonged to a same clade suggesting less divergence of this protein sequence in mosquitoes. Domain analysis of I. scapularis Src primary amino acid sequences revealed presence of Src homology SH2 domain (aa 143–238), Src homology SH3 domain (aa 84–135), Protein kinases ATP-binding region signature (aa 271–293), and a protein kinase catalytic domain (aa 256–436) and the C-terminal region (Fig. 2A). Also, the protein feature prediction analysis revealed presence of three Asparagine (ASN)-glycosylation sites of 3 residues length (aa 111–113, 285–287, 348–350), one glycosaminoglycan site of 4 residues length (aa 44–48), two cAMP phosphorylation sites of 4 residues length (aa 210–214, 418–422), twelve PKC phosphorylation sites of 3 residues length (aa 10–13, 14–17, 100–103, 124–127, 178–181, 186–189, 192–195, 213–216, 253–256, 264–267, 294–297, 353–356), ten CK2 phosphorylation sites of 4 residues length (aa 14–18, 37–41, 95–99, 113–117, 125–129, 186–190, 216–220, 253–257, 342–346, 380–384), eleven myristoylation sites of 6 residues length (aa 2–8, 34–40, 43–49, 45–51, 48–54, 230–236, 249–255, 282–288, 362–368, 378–384, 399–405), one RGD site with 3 residues (aa 25–27) and one protein kinase ATP binding site of 23 residues length (aa 271–293) (Fig. 2B). The presence of multiple myristoylation sites at region aa 43–54 suggests increased posttranslational modification at this site.
Figure 1: Alignment and phylogenetic analysis of I. scapularis Src tyrosine kinase.
A) The deduced I. scapularis Src tyrosine kinase amino acid sequence alignment (with other orthologs) using ClustalW program in DNASTAR Lasergene is shown. Residues that match are shaded in black color. GenBank accession numbers for D. melanogaster, A. aegypti, A. gambiae, C. quinquefasciatus, M. musculus, and H. sapiens Src sequences are shown. VectorBase accession numbers for I. scapularis, A. aegypti, A. gambiae and C. quinquefasciatus Src are provided. The NCBI accession numbers used for D. melanogaster, M. musculus and H. sapiens are also provided. Total length of the amino acid sequence is provided at one end (on the right) of each sequence. Asterisk in the sequence indicates possible tyrosine phosphorylation sites involved in Src activation and deactivation. B) Phylogenetic analysis was performed in DNASTAR by ClustalW slow/accurate alignment method using Gonnet as default value for protein weight matrix. Dotted line indicates variation in the A. gambiae forming a sub-clade; in comparison to the A. aegypti and C. quinquefasciatus Src. Scale at the bottom denotes amino acid substitutions per 100 amino acid residues.
Figure 2: Domain analysis and prediction of modification sites in I. scapularis Src kinase.
A) Domain analysis of I. scapularis Src kinase primary amino acid sequence at PROSITE (ExPASy) and NCBI protein database is shown. Structurally, I. scapularis Src consist of the SH3 domain, a SH3-SH2 connector, a SH2 domain, a SH2-kinase linker, a kinase domain, Protein kinases ATP-binding region signature and a C-terminal tail regulatory region. The phosphorylation of tyrosine site (Tyr416 in the catalytic domain and C-terminal region) conserved in the I. scapularis Src. B) Bioinformatic analysis showing I. scapularis Src kinase modification sites and relevant amino acids. I. scapularis Src kinase, contain three Asparagine (ASN)-glycosylation sites, one glycosaminoglycan site, two cAMP phosphorylation sites, twelve PKC phosphorylation sites, ten CK2 phosphorylation sites, eleven myristoylation sites, one RGD site and one protein kinase ATP binding site.
Expression of Src tyrosine kinase is developmentally regulated in I. scapularis ticks.
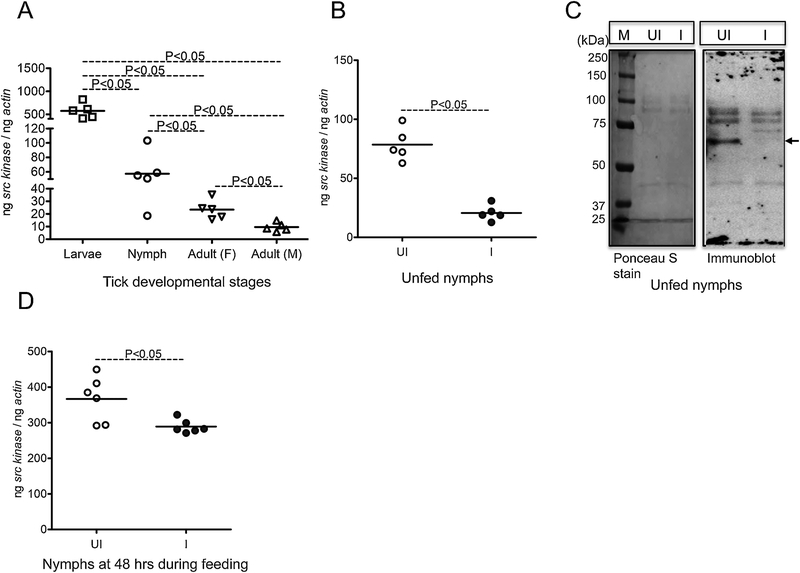
I. scapularis ticks have four stages in their life cycle: eggs, larvae, nymphs, and adult. To understand the importance of Src tyrosine kinase in tick life cycle, we analyzed src expression in tick developmental stages. QRT-PCR analysis showed that Src kinase is expressed in all tick developmental stages. The src mRNA levels were significantly (P<0.05) higher in larvae when compared to the levels noted in other developmental stages (Fig. 3A). Also, significant (P<0.05) difference in the src mRNA levels was noted between nymphs and adult female or male ticks (Fig. 3A). Comparison of src expression between adult females and male ticks revealed significant (P<0.05) differences, where adult female ticks had increased src expression in comparison to the adult male ticks (Fig. 3A). The significant increase in src expression in larval ticks when compared to the other stages suggests its important role in the early tick development.
Figure 3. Src tyrosine kinase levels in unfed I. scapularis nymphal ticks are reduced upon A. phagocytophilum infection.
A) QRT-PCR analysis showing expression of I. scapularis Src kinase at different tick developmental stages in uninfected unfed-larvae, nymphs, adult female and adult male ticks. Each open square, open circle, and open inverted triangle or triangle represents one individual tick. B) Src kinase expression between unfed uninfected nymphs and unfed A. phagocytophilum-infected nymphs is shown. C) Immunoblot showing reduced potential Src kinase expression at the protein level in unfed uninfected ticks and, upon A. phagocytophilum-infection. The arrow denotes a prominent intensity band between 50–60 kDa (potential phosphorylated Src protein band). The Ponceau S stained image serve as control. M indicates marker, and the marker sizes are shown in kDa on the left. D) Src kinase expression between uninfected nymphs and A. phagocytophilum-infected nymphs collected at 48 h during feeding is shown. Open circles represents uninfected (UI) and closed circles represent infected (I) ticks in both (B) and (D). Each circle represents one tick. The mRNA levels of src are normalized to tick beta-actin mRNA levels. P value from non-paired Student’s t-test is shown.
Anaplasma phagocytophilum down-regulates Src tyrosine kinase expression in unfed nymphs and in nymphs during feeding.
I. scapularis ticks transmit various pathogens to humans, including rickettsial pathogen A. phagocytophilum. The role for Src in tick-pathogen interactions has not been studied. We therefore generated A. phagocytophilum- infected unfed nymphs by feeding uninfected larvae on infected mice. The larvae were then molted to A. phagocytophilum-infected unfed nymphs. QRT-PCR analysis revealed that src levels were significantly (P<0.05) down-regulated in A. phagocytophilum-infected unfed nymphs in comparison to uninfected controls (Fig. 3B). Based on the amino acid sequence length (GenBank Acc. No. XP_002434467), Src protein size would be around 49–50 kDa. Immunoblotting showed a dramatic reduction in the levels of a prominent intensity band between 50–60 kDa (potential phosphorylated Src protein band) in A. phagocytophilum-infected unfed nymphs in comparison to the uninfected controls (Fig. 3C). The Ponceau S stained image indicates loading controls for protein amounts used in immunoblotting analysis (Fig. 3C). We then analyzed src levels in nymphs during feeding on A. phagocytophilum-infected mice. QRT-PCR analysis revealed significant (P<0.05) down regulation in src levels in nymphs during feeding on A. phagocytophilum-infected mice in comparison to the levels noted in nymphs during feeding on uninfected mice (Fig. 3D). We also noted that src levels were 4–5 fold increased in nymphs during feeding in comparison to the levels noted in unfed nymphs for both (A. phagocytophilum- infected and uninfected) groups of ticks (Fig. 3B & D).
Anaplasma phagocytophilum upregulates Src tyrosine kinase expression in fed ticks.
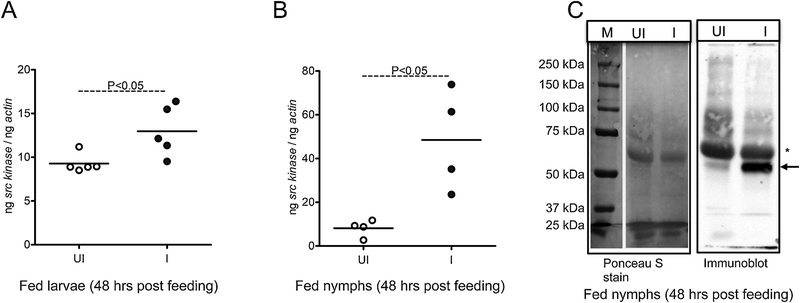
We then analyzed src levels in ticks that are fully engorged and dropped off from the host. To generate fed ticks, uninfected unfed larvae or nymphal ticks were fed on A. phagocytophilum-infected or uninfected mice and collected after repletion. Significant (P<0.05) increase in the src levels was noted in A. phagocytophilum-infected fed larvae (Fig. 4A) and nymphs (Fig. 4B) in comparison to relative uninfected controls (Fig. 4A & B). Immunoblotting showed dramatic increased level of a prominent intensity band at approximately 50 kDa (potential phosphorylated Src protein band) in A. phagocytophilum-infected fed nymphal ticks in comparison to uninfected control (Fig. 4C). Detection of possible host IgG heavy chain band was also noted in the both fed tick samples (Fig. 4C). The Ponceau S stained image indicates loading controls for protein amounts used in immunoblotting analysis (Fig. 4C). Furthermore, the observation of increased src levels in uninfected nymphs during feeding (Fig. 3D) and dramatic reduction in fully engorged uninfected nymphs (Fig. 4B) suggests differential role for Src in tick feeding.
Figure 4. Src tyrosine kinase is upregulated in post-fed A. phagocytophilum-infected I. scapularis ticks.
Expression of src kinase in ticks upon A. phagocytophilum acquisition (48 h post repletion) is shown from uninfected (UI) and infected (I) post-fed nymphs (A) or larvae (B). In panel A each circle indicates one individual tick. In panel B, each circle represents data obtained from 5–7 pooled ticks. The mRNA levels of src are normalized to tick beta-actin mRNA levels. Open circle represents uninfected (UI) and closed circles represent infected (I) ticks in (A) and (B). P value from non-paired Student’s t-test is shown. C) Immunoblotting analysis showing expression of potential Src kinase in fed uninfected (UI) or A. phagocytophilum-infected (I) nymphs analyzed after 48 h post-feeding. Arrow to the right indicates prominent intensity band at approximately 50 kDa (potential phosphorylated Src protein band). The Ponceau S stained image serve as control. Asterisk indicates possible host IgG heavy chain. M indicates marker, and sizes are shown in kDa.
Anaplasma phagocytophilum up-regulates src expression in tick cells.
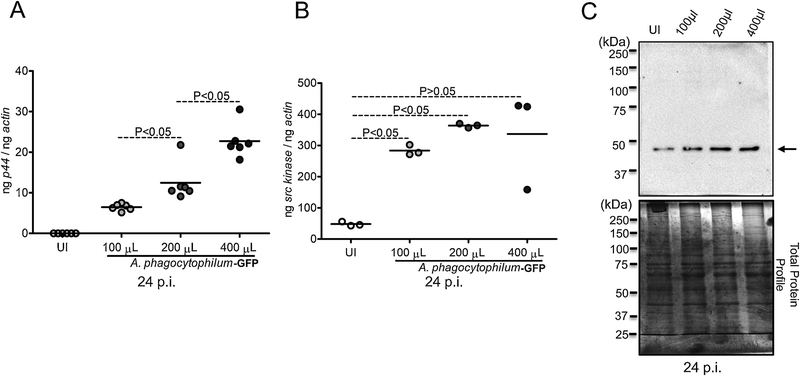
Next, we determined, if A. phagocytophilum affects Src levels in tick cells. Using I. scapularis in vitro tick cell line ISE6, we analyzed Src levels upon A. phagocytophilum-GFP strain infection with different doses. Tick cells were infected with 100 μl, 200 μl or 400 μl suspensions containing A. phagocytophilum-GFP strain. As expected, A. phagocytophilum-GFP strain burden in tick cells increased with an increase in bacterial dose (Fig. 5A). We found that the src transcript levels were significantly (P<0.05) increased in A. phagocytophilum-GFP infected tick cells at 24 post infection (p.i.) at all tested doses in comparison to the uninfected controls (Fig. 5B). In addition, immunoblotting analysis showed similar increase in the Src protein levels in A. phagocytophilum-GFP infected tick cells in comparison to the uninfected controls at all tested doses (Fig. 5C). Coomassie blue-stained gel image indicates total protein profile and serves as loading control for the amount of protein used in immunoblotting analysis (Fig. 5C). Furthermore, to test if the up-regulation of arthropod Src is a common response to all A. phagocytophilum strains, we infected tick cells with A. phagocytophilum-HZ strain at different doses and analyzed src levels at 24 p.i. QRT-PCR analysis revealed that the src transcript levels were significantly (P<0.05) increased in A. phagocytophilum-HZ strain infected tick cells at all tested doses in comparison to the uninfected controls (Supplementary Fig. 2). These data not only support in vivo observation in ticks but also suggests that upregulation of src in tick cells could be a general response to all A. phagocytophilum strains.
Figure 5. A. phagocytophilum upregulates Src kinase levels in tick cells.
A) QRT-PCR analysis showing A. phagocytophilum-GFP strain burden upon infection of tick cells with different bacterial doses (100, 200 and 400 μl of suspension contaning A. phagocytophilum-GFP strain prepared from infected HL-60 cells) at 24 h post infection. The uninfected (UI) cells serve as control. B) The upregulated src transcript levels are shown in the same samples treated with different doses of A. phagocytophilum-GFP strain in tick cells at 24 p.i.. Each circle represents data from one independent well of the culture plate. The mRNA levels of src and P44 (A. phagocytophilum gene) are normalized to tick beta-actin levels. P value from non-paired Student’s t-test is shown. C) Immunoblotting analysis with anti-Src antibody showing levels of Src kinase protein at approximately 49–50 kDa position in tick cells infected with A. phagocytophilum at different doses at 24 p.i. Total profile of proteins observed on Coomassie stained gel serves as a loading control.
Silencing and inhibition of Src tyrosine kinase reduces A. phagocytophilum loads in ticks and tick cells.
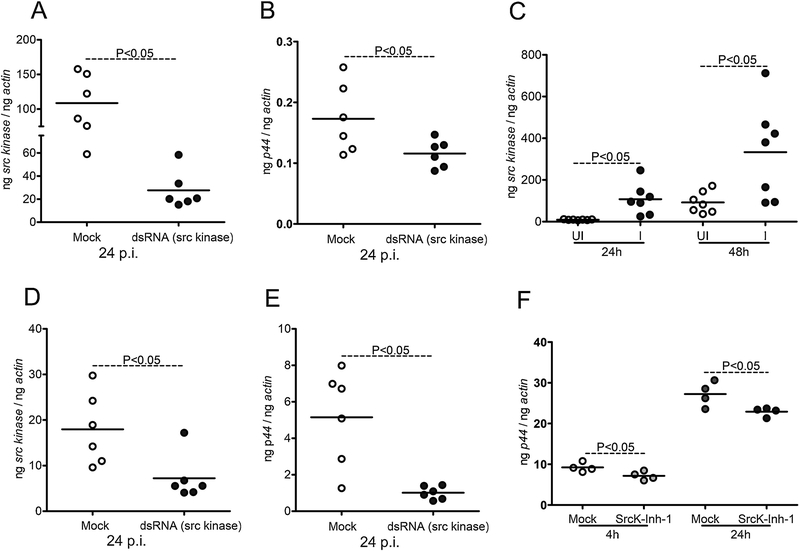
The observation of A. phagocytophilum-associated upregulation of Src levels in tick cells suggests an important role for this kinase in the bacterial survival. To determine if knockdown of src expression affects A. phagocytophilum loads in ticks, we amplified the Src product (187 bp fragment) and cloned into L4440 vector (Supplementary Figure 3A). All cloned sequences were analyzed and the correct clone was considered for further studies (Supplementary Fig. 3B). The src-dsRNA was synthesized from the cloned L4440 vector. The src-dsRNA or mock control were microinjected into the uninfected unfed nymphal body. After four hours recovery, these ticks were allowed to feed on A. phagocytophilum-infected mice. Fully engorged ticks that were dropped off from the host were collected and processed for QRT-PCR analysis. Significant (P<0.05) reduction in the src levels were noted in src-dsRNA injected ticks in comparison to mock-injected controls (Fig. 6A). In addition, significant (P<0.05) reduction in the A. phagocytophilum loads was also noted in src-dsRNA injected ticks in comparison to mock-injected ticks (Fig. 6B). These data show that silencing of src affects A. phagocytophilum acquisition from the murine host to ticks and provide important evidence for the role of Src in tick-A. phagocytophilum interactions. Next, we determined the levels of src in ISE6 tick cells upon A. phagocytophilum-HZ strain infection at different days p.i. We noted that A. phagocytophilum-HZ strain replicated actively in tick cells with significant (P<0.05) increase in the bacterial burden at day 3 in comparison to early points (Supplementary Fig. 4A). Significant upregulation of src transcripts levels were noted in A. phagocytophilum-HZ strain infected tick cells at 24 and 48 h p.i. in comparison to uninfected controls (Fig. 6C). No differences in the src transcript levels were observed at other tested time points (days 3, 5, 7 and 10 p.i.) between A. phagocytophilum-HZ strain infected and uninfected controls (Supplementary Fig. 4B). These data suggest a role for Src in the early phases of A. phagocytophilum-tick interactions. Furthermore, silencing of src expression in tick cells revealed similar results as observed in ticks. We tested two mock controls (mock elution buffer and mock-dsRNA that was synthesized from multiple cloning site of L4440 vector) to determine src-silencing efficiency in tick cells. QRT-PCR analysis revealed a significant knockdown of src expression in tick cells transfected with src-dsRNA in comparison to tick cells treated with mock controls (Fig. 6D and Supplementary Fig. 5). No significant differences in the src levels were observed between mock-elution buffer-treated cells and mock-dsRNA-treated cells (Supplementary Fig. 5). In addition, we did not observe any morphological differences upon treatment of tick cells with mock control or src-dsRNA followed by A. phagocytophilum-infection (Supplementary Fig. 6). A significant (P<0.05) reduction in A. phagocytophilum-HZ loads was noted in src-dsRNA-treated cells in comparison to the mock-treated control cells (Fig. 6E). To further support the role of Src in tick-A. phagocytophilum-interactions, we treated tick cells with Src inhibitor at 5 μM concentration followed by infection with A. phagocytophilum-HZ strain. At both 4 and 24 h p.i. significant (P<0.05) reduction in A. phagocytophilum-HZ loads was noted in Src-inhibitor-treated tick cells in comparison to the mock-treated controls (Fig. 6F). Collectively, these data not only suggests that Src kinase is critical for A. phagocytophilum survival in ticks and tick cells but also provides a model to understand rickettsial pathogen-tick interactions.
Figure 6: RNAi-mediated silencing of src in ticks and tick cells affects A. phagocytophilum growth and survival.
A) QRT-PCR analysis showing silencing efficiency of src expression (A) or bacterial burden (B) upon A. phagocytophilum infection in 48 h post-fed mock or src-dsRNA treated ticks. In panel A and B, Open circle represents A. phagocytophilum-infected mock-treated and closed circles represent infected src-dsRNA treated ticks. Each circle represents one tick. C) Levels of src transcripts in tick cells upon A. phagocytophilum infection at 24 and 48 h p.i. is shown. Open circle represents uninfected (UI) and closed circles represent infected (I) tick cells. QRT-PCR analysis showing reduced src transcript levels (D) or bacterial burden (E) upon treatment with mock or src-dsRNA in A. phagocytophilum-infected tick cells is shown. F) A. phagocytophilum burden in mock (DMSO) or 5 μM of Src inhibitor-treated tick cells at 4 or 24 h post infection is shown. The data shown in D-F is from A. phagocytophilum-infected ISE6 tick cells -treated with mock or src-dsRNA or inhibitor. Open circle represents A. phagocytophilum-infected mock treated and closed circles represent infected-src-dsRNA or inhibitor treated tick cells in panels D-F. Each circle represents data from one independent culture plate well. The mRNA levels of src and P44 DNA levels are normalized to tick beta-actin levels. P value from non-paired Student’s t-test is shown.
DISCUSSION
Among the post-translational modification of proteins during signal transduction, phosphorylation of proteins is the most widespread and extensively studied event in both vertebrate and invertebrate systems. Generally, proteins phosphorylate and dephosphorylate continuously as a feedback mechanism. Only a small amount of the phosphorylated protein from a total pool is sufficient to trigger the downstream changes and the relay of signal transduction. In our previous study (Sultana et al., 2010), we have shown that only a small portion of proteins out of total pool from I. scapularis ticks are phosphorylated at their tyrosine residue(s) as detected by immunoblotting with phospho- tyrosine antibodies. Several studies have also shown that A. phagocytophilum modulates the tyrosine phosphorylation of proteins to a greater extent (Carlyon and Fikrig, 2003; de la Fuente et al., 2016; Khanal et al., 2017; Sultana et al., 2010; Thomas and Fikrig, 2007). In order to identify the tyrosine kinase responsible for A. phagocytophilum-induced phosphorylation of proteins, we combed the I. scapularis genome and found an annotated sequence of putative Src tyrosine kinase with 447 aa sequence.
ClustalW alignment of I. scapularis Src amino acid sequence revealed different percentage of identity with other arthropods such as Ae. aegypti (45.1%), An. gambiae (45%) and C. quinquefasciatus (38.6%) suggesting an early emergence of I. scapularis Src when compared to the mosquito orthologs. The high degree of conservation with D. melanogaster (79.3%) Src, but not with mosquitoes, suggests conservation in structure and function of I. scapularis Src. In Drosophila, there are two src (Dsrc) homologs, Dsrc64 and Dsrc41. Dsrc64 was initially considered as a unique ortholog of the vertebrate c-src, but more recently, Dsrc41 was shown to be the closest relative of vertebrate c-src (Hoffman-Falk et al., 1983; Simon et al., 1985, 1983; Wadsworth, 1990; Wadsworth et al., 1990). Dsrc41 has been shown to be involved in cytoskeleton regulation, disorganization of the actin fibers and adherent junctions between cells. Comparison of the amino acids that may be essential for the distinct functions of the c-src gene products from Drosophila and vertebrate sequences places the gene duplication event prior to the Chordate-Arthropod divergence (Wadsworth, 1990; Wadsworth et al., 1990). High degree of identity and sharing of the same clade, suggests I. scapularis Src to be closely related in activation and regulation of the signaling cascades like its ortholog Drsc41.
The non-receptor Src family of tyrosine kinases shares a common architecture that underlies a combined regulatory mechanism (Hanke et al., 1996; Roskoski Jr., 2004; Superti-Furga and Courtneidge, 1995). There are at least nine members of this family that includes c-Src, Lck, Yes, Yrk, Blk, Fyn, Fgr, Lyn, Hck and Lck (Hanke et al., 1996; Roskoski Jr., 2004; Superti-Furga and Courtneidge, 1995). The c-Src is the widely expressed family member that performs variety of cellular functions (Roskoski Jr., 2005, 2004, 2015; Superti-Furga and Courtneidge, 1995; Wadsworth, 1990). The N-terminal region consists of 9–12 aa, that is essential for the membrane attachment and function of Src (Roskoski Jr., 2004, 2015). The SH2 domain from I. scapularis and D. melanogaster shares 90% identity (with 86 aa match out of 96 aa), while the SH3 domain shows an identity of 94% (49 match out of 52 aa). Tyrosine phosphorylation of c-Src at Tyr527, normally keeps it in inactive state, which is stabilized by binding of this residue to the SH2 domain or by binding of the SH2-kinase linker to the SH3 domain (Roskoski Jr., 2005, 2004, 2015; and Brickell, 1992). In I. scapularis Src, Tyr527 residue is at 440 aa position that is a highly conserved tyrosine residue. We assume that in I. scapularis, Try440 may be important in regulating activation of tick Src kinase. The presence of several protein translational modification sites in I. scapularis Src further suggests that this tyrosine kinase is an important regulator of various signaling pathways in ticks.
Tick-borne pathogens persist for longer periods and survive at different developmental stages, particularly in starving unfed nymphal stages. Obligate intracellular microbes including A. phagocytophilum use the vector molecular repertoire or signal transduction components and events to survive in those persistent times and/or to facilitate transmission to the vertebrate host. The highest expression of Src kinase in larvae followed by nymphs in comparison to the adult stages suggests an important role for this kinase and tyrosine phosphorylation in tick growth and development. The high expression of Src kinase in adult females in comparison to the adult male suggests a possible role in reproduction and/or oviposition. The observation of reduced levels of Src kinase in A. phagocytophilum unfed nymphal ticks and increased levels during pathogen entry into ticks (upon feeding) suggests a critical maintenance of protein tyrosine phosphorylation events in the presence of nutrients and energy. The observation of lower loads of Src kinase in unfed nymphs where, A. phagocytophilum is transstadially maintained, further suggest that it is perhaps a strategy from the microbe to keep its host alive longer for its own survival. The induced Src levels in ticks upon pathogen acquisition suggest a critical role for this kinase in tick-A. phagocytophilum interactions. The A. phagocytophilum induced Src tyrosine kinase levels in post fed larvae and nymphs perhaps suggest an immediate requirement of tyrosine phosphorylation of signaling proteins that is essential for the pathogen replication and transmigration from the midgut to the salivary gland tissues. Our previous research (Sultana et al., 2010) has clearly indicated that tyrosine phosphorylation of total proteins is induced in A. phagocytophilum-infected whole fed ticks (72 h post-fed), salivary glands and gut tissues, in comparison to the uninfected ticks. Also, the tyrosine phosphorylation of total proteins in unfed ticks was less in comparison to the fed ticks. The findings from the current study and our previous study (Sultana et al., 2010) suggests that Src is an important kinase in the induction of tyrosine phosphorylation of proteins upon A. phagocytophilum infection.
The upregulation of Src at both transcript and protein levels suggests an increase in the intracellular levels of tyrosine phosphorylation of proteins that might be essential for A. phagocytophilum replication and colonization. Our previous study (Sultana et al., 2010)showing increased tyrosine phosphorylation of total proteins upon A. phagocytophilum-infection in tick cells directly correlated with the increased Src kinase levels. Furthermore, silencing of Src kinase in both ticks (in vivo, 48 h post fed) and in tick cells (in vitro) suggests a direct relation between A. phagocytophilum replication and requirement of Src kinase in facilitating bacterial survival. Treatment with Src kinase inhibitor also showed reduced A. phagocytophilum loads, suggesting a need for this important kinase in A. phagocytophilum colonization and survival in its vector host. Our previous studies suggested that A. phagocytophilum could act as a facultative beneficial microbe for tick survival at cold temperatures (Khanal et al., 2018; Neelakanta et al., 2010) and modulates extended actin phosphorylation to regulate arthropod gene expression (Sultana et al., 2010). This study further strengthens this information on the modulation of the interplay between Src kinase and the induced tyrosine phosphorylation of host proteins for A. phagocytophilum colonization and survival in the vector host.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to acknowledge Dr. Ulrike Munderloh for tick cells and HGE1-GFP A. phagocytophilum strain. Also, we would like to acknowledge the BEI resources. “The following reagent was obtained through BEI Resources, NIAID, NIH: Anaplasma phagocytophilum, Strain NCH-1, NR-48807.” This study was supported by independent start-up funds from Old Dominion University to GN and HS and in part from NIH R03 AI092156–01 award to HS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
Authors declare no conflicts of interests.
REFERENCES
- Alto NM, Orth K, 2012. Subversion of cell signaling by pathogens. Cold Spring Harb. Perspect. Biol 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JF, and Magnarelli LA, 2008. Biology of ticks. Infect.Dis.Clin.North Am 22, 195–215. [DOI] [PubMed] [Google Scholar]
- Bakken JS, Dumler JS, 2015. Human Granulocytic Anaplasmosis. Infect. Dis. Clin. North Am 29, 341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonyaratanakornkit V, Edwards DP, 2007. Receptor mechanisms mediating non-genomic actions of sex steroids. Semin. Reprod. Med 25, 139–153. [DOI] [PubMed] [Google Scholar]
- Brickell PM, 1992. The p60c-src family of protein-tyrosine kinases: structure, regulation, and function. Crit. Rev. Oncog 3, 401–446. [PubMed] [Google Scholar]
- Cabezas-Cruz A, Alberdi P, Ayllón N, Valdés JJ, Pierce R, Villar M, de la Fuente J, 2016. Anaplasma phagocytophilum increases the levels of histone modifying enzymes to inhibit cell apoptosis and facilitate pathogen infection in the tick vector Ixodes scapularis. Epigenetics 11, 303–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlyon JA, Fikrig E, 2006. Mechanisms of evasion of neutrophil killing by Anaplasma phagocytophilum. Curr. Opin. Hematol 13, 28–33. [DOI] [PubMed] [Google Scholar]
- Carlyon JA, Fikrig E, 2003. Invasion and survival strategies of Anaplasma phagocytophilum. Cell. Microbiol 5, 743–754. [DOI] [PubMed] [Google Scholar]
- Carlyon JA, Latif DA, Pypaert M, Lacy P, Fikrig E, 2004. Anaplasma phagocytophilum utilizes multiple host evasion mechanisms to thwart NADPH oxidase-mediated killing during neutrophil infection. Infect. Immun 72, 4772–4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Fuente J, Estrada-Peña A, Cabezas-Cruz A, Kocan KM, 2016. Anaplasma phagocytophilum Uses Common Strategies for Infection of Ticks and Vertebrate Hosts. Trends Microbiol 24, 173–180. [DOI] [PubMed] [Google Scholar]
- Dumler JS, Choi KS, Garcia-Garcia JC, Barat NS, Scorpio DG, Garyu JW, Grab DJ, Bakken JS, 2005. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg. Infect. Dis 11, 1828–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsheim RF, Herron MJ, Nelson CM, Burkhardt NY, Barbet AF, Kurtti TJ, Munderloh UG, 2006. Transformation of Anaplasma phagocytophilum. BMC Biotechnol. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA, 1996. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor: Study of Lck- and FynT-dependent T cell activation. J. Biol. Chem 271, 695–701. [DOI] [PubMed] [Google Scholar]
- Hoffman-Falk H, Einat P, Shilo BZ, Hoffmann FM, 1983. Drosophila melanogaster DNA clones homologous to vertebrate oncogenes: Evidence for a common ancestor to the src and abl cellular genes. Cell 32, 589–598. [DOI] [PubMed] [Google Scholar]
- Hunter T, 2002. Protein-Tryosine Kinases. Annu. Rev. Biochem 54, 897–930. [DOI] [PubMed] [Google Scholar]
- Khanal S, Sultana H, Catravas JD, Carlyon JA, Neelakanta G, 2017. Anaplasma phagocytophilum infection modulates expression of megakaryocyte cell cycle genes through phosphatidylinositol-3-kinase signaling. PLoS One 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanal S, Taank V, Anderson JF, Sultana H, Neelakanta G, 2018. Arthropod transcriptional activator protein-1 (AP-1) aids tick-rickettsial pathogen survival in the cold. Sci. Rep 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtti TJ, Mattila JT, Herron MJ, Felsheim RF, Baldridge GD, Burkhardt NY, Blazar BR, Hackett PB, Meyer JM, Munderloh UG, 2008. Transgene expression and silencing in a tick cell line: A model system for functional tick genomics. Insect Biochem. Mol. Biol 38, 963–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Kovacevic Z, Peng Z, Jin R, Wang P, Yue F, Zheng M, Huang ML-H, Jansson PJ, Richardson V, Kalinowski DS, Lane DJR, Merlot AM, Sahni S, Richardson DR, 2015. The molecular effect of metastasis suppressors on Src signaling and tumorigenesis: new therapeutic targets. Oncotarget 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng M, Liu C, Peng J, Qian W, Qian H, Tian L, Li J, Dai D, Xu A, Li S, Xia Q, Cheng D, 2015. Homeodomain protein Scr regulates the transcription of genes involved in juvenile hormone biosynthesis in the silkworm. Int. J. Mol. Sci 16, 26166–26185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munderloh UG, Blouin EF, Kocan KM, Ge NL, Edwards WL, Kurtti TJ, 1996. Establishment of the Tick (Acari: Ixodidae)-Borne Cattle Pathogen Anaplasma marginale (Rickettsiales: Anaplasmataceae) in Tick Cell Culture. J. Med. Entomol 33, 656–664. [DOI] [PubMed] [Google Scholar]
- Munderloh UG, Tate CM, Lynch MJ, Howerth EW, Kurtti TJ, Davidson WR, 2003. Isolation of an Anaplasma sp. organism from white-tailed deer by tick cell culture. J. Clin. Microbiol 41, 4328–4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelakanta G, Sultana H, Fish D, Anderson JF, Fikrig E, 2010. Anaplasma phagocytophilum induces Ixodes scapularis ticks to express an antifreeze glycoprotein gene that enhances their survival in the cold. J. Clin. Invest 120, 3179–3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CM, Herron MJ, Felsheim RF, Schloeder BR, Grindle SM, Chavez AO, Kurtti TJ, Munderloh UG, 2008. Whole genome transcription profiling of Anaplasma phagocytophilum in human and tick host cells by tiling array analysis. BMC Genomics 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petchampai N, Sunyakumthorn P, Banajee KH, Verhoeve VI, Kearney MT, Macaluso KR, 2015. Identification of host proteins involved in rickettsial invasion of tick cells. Infect. Immun 83, 1048–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizarro-Cerdá J, Charbit A, Enninga J, Lafont F, Cossart P, 2016. Manipulation of host membranes by the bacterial pathogens Listeria, Francisella, Shigella and Yersinia. Semin. Cell Dev. Biol 60, 155–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y, 2011. Mechanisms of obligatory intracellular infection with Anaplasma phagocytophilum. Clin. Microbiol. Rev 24, 469–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y, 2010. Anaplasma phagocytophilum and Ehrlichia chaffeensis: Subversive manipulators of host cells. Nat. Rev. Microbiol 8, 328–339 [DOI] [PubMed] [Google Scholar]
- Roskoski R Jr., 2015. Src protein-tyrosine kinase structure, mechanism, and small molecule inhibitors. Pharmacol. Res 94, 9–25. [DOI] [PubMed] [Google Scholar]
- Roskoski R Jr., 2005. Src kinase regulation by phosphorylation and dephosphorylation. Biochem. Biophys. Res. Commun 331, 1–14. [DOI] [PubMed] [Google Scholar]
- Roskoski R Jr., 2004. Src protein-tyrosine kinase structure and regulation. Biochem. Biophys. Res. Commun 324, 1155–1164. [DOI] [PubMed] [Google Scholar]
- Simon MA, Drees B, Kornberg T, Bishop JM, 1985. The nucleotide sequence and the tissue-specific expression of Drosophila c-src. Cell 42, 831–840. [DOI] [PubMed] [Google Scholar]
- Simon MA, Kornberg TB, Bishop JM, 1983. Three loci related to the src oncogene and tyrosine-specific protein kinase activity in Drosophila. Nature 302, 837–839. [DOI] [PubMed] [Google Scholar]
- Sonenshine DE, 2005. The Biology of Tick Vectors of Human Disease. Tick-Borne Dis. Human, D.T.D Goodman Jesse L., Sonenshine Daniel E., Ed. Am. Soc. Microbiol Washingt. D.C. 12–35. [Google Scholar]
- Song L, Li X, Xia Q, Hua X, Wang F, 2016. Identification of Tyrosine Kinase Src Responsible for Antimicrobial Peptides Production in Bombyx mori. Protein Pept. Lett 24, 174–180. [DOI] [PubMed] [Google Scholar]
- Sukumaran B, Narasimhan S, Anderson JF, DePonte K, Marcantonio N, Krishnan MN, Fish D, Telford SR, Kantor FS, Fikrig E, 2006. An Ixodes scapularis protein required for survival of Anaplasma phagocytophilum in tick salivary glands. J. Exp. Med 203, 1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana H, Neelakanta G, Kantor FS, Malawista SE, Fish D, Montgomery RR, Fikrig E, 2010. Anaplasma phagocytophilum induces actin phosphorylation to selectively regulate gene transcription in Ixodes scapularis ticks. J. Exp. Med 207, 1727–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana H, Patel U, Toliver M, Maggi RG, Neelakanta G, 2016. Molecular identification and bioinformatics analysis of a potential anti-vector vaccine candidate, 15-kDa salivary gland protein (Salp15), from Ixodes affinis ticks. Ticks Tick. Borne. Dis 7, 46–53. [DOI] [PubMed] [Google Scholar]
- Superti-Furga G, Courtneidge SA, 1995. Structure- function relationships in Src family and related protein tyrosine kinases. BioEssays 17, 321–330. [DOI] [PubMed] [Google Scholar]
- Taank V, Dutta S, Dasgupta A, Steeves TK, Fish D, Anderson JF, Sultana H, Neelakanta G, 2017. Human rickettsial pathogen modulates arthropod organic anion transporting polypeptide and tryptophan pathway for its survival in ticks. Sci. Rep 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taank V, Zhou W, Zhuang X, Anderson JF, Pal U, Sultana H, Neelakanta G, 2018. Characterization of tick organic anion transporting polypeptides (OATPs) upon bacterial and viral infections. Parasites and Vectors 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas V, Fikrig E, 2007. Anaplasma phagocytophilum specifically induces tyrosine phosphorylation of ROCK1 during infection. Cell. Microbiol 9, 1730–1737. [DOI] [PubMed] [Google Scholar]
- Van der Geer P, 2002. Receptor Protein-Tyrosine Kinases and Their Signal Transduction Pathways. Annu. Rev. Cell Dev. Biol 10, 251–337. [DOI] [PubMed] [Google Scholar]
- Wadsworth SC, 1990. Drosophila src family proteins. Comp. Biochem. Physiol. -- Part B Biochem 97, 403–406. [DOI] [PubMed] [Google Scholar]
- Wadsworth SC, Muckenthaler FA, Vincent WS, 1990. Differential expression of alternate forms of a Drosophila src protein during embryonic and larval tissue differentiation. Dev. Biol 138, 296–312. [DOI] [PubMed] [Google Scholar]
- Zhou W, Woodson M, Neupane B, Bai F, Sherman MB, Choi KH, Neelakanta G, Sultana H, 2018. Exosomes serve as novel modes of tick-borne flavivirus transmission from arthropod to human cells and facilitates dissemination of viral RNA and proteins to the vertebrate neuronal cells. PLoS Pathog 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.