Abstract
Background:
During a migraine attack, trigeminal activation results in the release of calcitonin gene-related peptide (CGRP), which stimulates the release of inflammatory cytokines playing an important role in migraine.
Objective:
We analyze the relation between CGRP and cytokines during attacks to explore the possible mechanism of migraine.
Materials and Methods:
Migraine patients and healthy control were recruited at the Department of Neurology, the Sixth People's Hospital of Fuyang City, between March 2018 and July 2018. The protein levels of interleukin (IL)-1β, IL-2, IL-6, IL-10, tumor necrosis factor-alpha (TNF-α), and CGRP were determined from the sera of patients with migraine and control subjects by enzyme-linked immunosorbent assay kits. Spearman's rank correlation coefficient was also determined to calculate the correlation between CGRP and inflammatory factors levels.
Results:
The level of IL-1β, IL-6, TNF-α, and CGRP in migraine group were significantly higher than normal group (P < 0.05). The level of CGRP was significantly correlated with IL-1 β (r = 0.30, P < 0.05) and IL-6 (r = 0.94, P < 0.05), but not significantly correlated with IL-2 (r =−0.047, P = 0.75), IL-10 (r = 0.12, P = 0.43), and TNF-α (r = 0.05, P = 0.72).
Conclusions:
In our study, we found migraine patients had a higher IL-6, IL-1β, and TNF level than healthy controls and the level of CGRP was related significantly with the level of IL-1β and IL-6. In conclusion, our results suggest that IL-1β and IL-6 may be involved in the pathogenesis of migraine attacks and CGRP related with the secretion of cytokines.
Keywords: Calcitonin gene-related peptide, cytokines, interleukin-6, migraine
INTRODUCTION
Migraine is a chronic neurovascular disorder, characterized by episodic headaches with autonomic symptoms and its pathogenesis remains unclear.[1,2] Many previous studies were in favor of the trigeminal neurovascular reflex theory which believed that the migraine attributes to neurogenic inflammation induced by vasoactive peptides released by the perivascular nerves.[3,4,5] During a migraine attack, trigeminal activation results in the release of calcitonin gene-related peptide (CGRP), which induces releasing of neurogenic inflammation and vasodilation in the leptomeningeal vessels giving rise to the typical migraine pain.[6,7]
CGRP is widely expressed in the nervous system and immunocytochemistry has shown that >50% of trigeminal neurons produce CGRP in the trigeminal system. It has been shown that the CGRP released after stimulation of the trigeminal ganglion in humans.[8,9] During acute severe migraine, CGRP is elevated in the cranial circulation in humans, and this release is reversed after successful treatment by sumatriptan.[10,11] It has been shown that CGRP stimulates the release of inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β, and IL-6 from human lymphocytes “in vitro.”[12] Therefore, it is possible that the pain of migraine attack is related to the release of CGRP and its ability to induce a secretion of cytokine from white cells, platelets, and endothelium.
Cytokines are small proteins produced by most cells in the body, which possess multiple biologic activities. Several evidence suggest that cytokines play an important role in several physiological and pathological settings such as inflammation and pain.[13,14,15] A few studies examined cytokine levels in migraine patients, but the results were controversial. Recently, pro-inflammatory cytokines such as IL-1β and TNF-α and anti-inflammatory cytokines such as IL-10 and IL-4 have been shown to play a significant role in the modulation of pain threshold, and they could contribute to trigeminal nerve fibers sensitization.[16] However, there was no study investigating possible correlation between CGRP and cytokines levels during attacks.
Because of different responses of cytokines, we investigated the levels of pro- and anti-inflammatory cytokines (TNF-α, IL-1β, IL-2, IL-6, and IL-10) in the serum of the migraine patients to understand the role of cytokines in migraine. Furthermore, we analyze the relation between CGRP and cytokines during attacks to explore the possible mechanism of migraine.
MATERIALS AND METHODS
Patients
Patients with migraine were recruited at the Department of Neurology, the Sixth People's Hospital of Fuyang City between March 2018 and July 2018. The migraine patients were diagnosed by two neurologists, according to the 2013 Headache Classification Committee of International Headache Society migraine diagnosis standard. All patients in the migraine group had not received any prophylactic migraine medication in the previous week and had not taken antibiotics within the previous 3 months. The healthy persons who had no personal or family history of migraine were recruited as controls. All participants underwent a detailed neurological examination and tests of head computed tomography angiography, head magnetic resonance imaging, electrocardiogram, white blood cell count, C-reactive protein (CRP), blood sugar, and lipids. Patients who had nonmigraine headache, inflammatory or autoimmune diseases, abnormal CRP levels, hypertension, diabetes mellitus, obesity, metabolic syndrome, or ischemic cerebrovascular disease were excluded from this study. The study was approved by the Ethical Committee of the Sixth People's Hospital of Fuyang City. Written informed consent was obtained from each participant.
Samples
Blood samples were obtained within 2 h from the onset of migraine headache from the jugular venous.[17] Blood samples were also obtained from healthy individuals. Inflammatory factor concentration in the serum was analyzed. Two 10 ml blood samples were drawn into glass tubes containing 35 μmol of dipotassium-EDTA and 1500 kallikrein inactivator units of trasylol. The tubes were kept in an ice bath and then centrifuged at 2000×g for 15 min at 4°C. The plasma was separated from the cells, stored at −80°, and analyzed through commercially available enzyme-linked immunosorbent assay (ELISA).
Enzyme-linked immunosorbent assay
The protein levels of IL-1 β, IL-2, IL-6, IL-10, TNF-α, and CGRP were determined from the sera of patients with migraine and control subjects. Commercial ELISA kits were used in accordance with the manufacturers' instructions. The assays were performed in duplicate in 96-well plates, and the results were presented as picograms per milliliter. The collected samples from each of the two groups were analyzed on the same day on one ELISA plate for each protein. The following kits were used: human IL-1β ELISA kit (ab100562; Abcam, Cambridge, MA, USA; detection threshold of 1.5 pg/ml; intra- and inter-assay coefficient of variation of <11%); human IL-2 ELISA kit (ab174444; Abcam, Cambridge, MA, USA; detection threshold of 9 pg/ml; intra- and inter-assay coefficient of variation of <6%); human IL-6 ELISA kit (ab46027; Abcam, Cambridge, MA, USA; detection threshold of 2 pg/ml; intra- and inter-assay coefficient of variation of <8.6%); human IL-10 ELISA kit (ab100549; Abcam, Cambridge, MA, USA; detection threshold of 1 pg/ml; intra- and inter-assay coefficient of variation of <10%); human TNF-α ELISA kit (ab46087; Abcam, Cambridge, MA, USA; detection threshold of 15 pg/ml; intra- and inter-assay coefficient of variation of <10.3%); human CGRP ELISA kit (CEA876Hu; USCN Life Science Inc., Hubei, China; detection threshold of 5.35 pg/ml; intra- and inter-assay coefficient of variation of <12%).
Statistical analysis
The inflammatory factor and CGRP concentration are presented as mean ± SEM Differences between groups were statistically analyzed using the t-test depending on different variables with SAS 8.0 Software (SAS Institute INC, USA). Spearman rank correlation coefficient was also determined to calculate the correlation between CGRP and inflammatory factors levels. P < 0.05 was considered statistically significant.
RESULTS
Ultimately, 47 patients with migraine (26 females and 21 males, mean age = 35.2 ± 9.3 years) and 38 healthy volunteers (22 females and 16 males, mean age = 32.4 ± 6.1 years) were included. Among the migraine patients, 20 were without aura (MO) (11 females and 9 males, mean age = 34.1 ± 5.3 years) and 27 were with aura (MA) (15 females and 12 males, mean age = 36.3 ± 10.4 years). There was no significant difference between the migraine and control group or MO and MA groups in age and gender (P > 0.05). There were 16 patients with episodic migraine (EM) (fewer than 15 headache days per month) and 31 patients with chronic migraine (CM) (15 or more headache days per month) [Table 1].
Table 1.
The characteristics of the migraine patients
| Items | Migraine (n=47) |
|---|---|
| Age (year) | 35.2±9.3 |
| Sex (female/male) | 26/21 |
| Aura | |
| With | 27 |
| Without | 20 |
| Types of frequency | |
| EM | 16 |
| CM | 31 |
EM=Episodic migraine, CM=Chronic migraine
Among all the inflammatory factors and CGRP we detected, the level of IL-1β, IL-6, TNF-α, and CGRP in migraine group were significantly higher than normal group (P < 0.05) [Table 2]. However, the levels of all inflammatory factors and CGRP were similar between MO and MA groups (P > 0.05) [Table 3].
Table 2.
Comparison of levels of inflammatory factor and calcitonin gene-related peptide between the migraine patients and the control group
| Items (pg/ml) | Migraine (n=47) | Control (n=38) | P |
|---|---|---|---|
| IL-1β | 24.6±12.6 | 3.4±1.4 | <0.05 |
| IL-2 | 452.1±110.1 | 430.7±44.4 | >0.05 |
| IL-6 | 206.6±111.3 | 2.8±0.56 | <0.05 |
| IL-10 | 6.9±1.9 | 7.2±1.3 | >0.05 |
| TNF-α | 105.3±45.6 | 30.7±13.8 | <0.05 |
| CGRP | 80.5±22.3 | 29.5±8.8 | <0.05 |
IL=Interleukin, TNF-α=Tumor necrosis factor-alpha, CGRP=Calcitonin gene-related peptide
Table 3.
Comparison of levels of inflammatory factor and calcitonin gene-related peptide between the migraine with or without aura
| Items (pg/ml) | Migraine with aura (n=27) | Migraine without aura (n=20) | P |
|---|---|---|---|
| IL-1β | 23.9±12.9 | 25.5±12.6 | >0.05 |
| IL-2 | 445.0±105.7 | 461.6±118.0 | >0.05 |
| IL-6 | 221.7±113.7 | 186.3±107.3 | >0.05 |
| IL-10 | 7.2±1.8 | 6.6±2.0 | >0.05 |
| TNF-α | 102.7±42.4 | 108.9±50.5 | >0.05 |
| CGRP | 84.8±22.2 | 74.7±21.6 | >0.05 |
IL=Interleukin, TNF-α=Tumor necrosis factor-alpha, CGRP=Calcitonin gene-related peptide
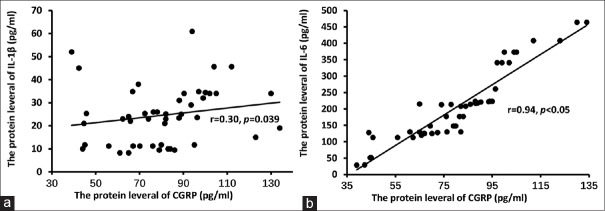
The level of CGRP was significantly correlated with IL-1β (r = 0.30, P < 0.05) and IL-6 (r = 0.94, P < 0.05) [Figure 1], but not correlated with IL-2 (r = −0.047, P = 0.75), IL-10 (r = 0.12, P = 0.43), or TNF-α (r = 0.05, P = 0.72).
Figure 1.
(a and b) Correlation between interleukin-1β, interleukin-6, and calcitonin gene-related peptide. Spearman rank correlation analysis showed that the level of calcitonin gene-related peptide was significantly correlated with interleukin-1β (r = 0.30, P < 0.05) and interleukin-6 (r = 0.94, P < 0.05)
DISCUSSION
Migraine is a primary headache disorder which seriously affects the daily life of patients.[1,18] As understanding of the neural pathways has advanced, CGRP-based mechanisms have arisen. CGRP is found in the trigeminovascular nociceptive system widely in the trigeminal ganglion and brain stem. CGRP may be involved in migraine at both a central and peripheral level. Previous studies showed that the stimulation of brainstem could activate the trigeminovascular system and cause releasing of CGRP.[19,20,21] Moreover, several studies showed the CGRP level in serum raised during migraine attack and fallen after effective treatment. The significance of CGRP in migraine pathophysiology has been supported by several lines of clinical evidence.[6,7] In our study, we found that the level of CGRP in migraine group was significantly higher than normal group, but it is similar between MO and MA groups. Similar results have been obtained measuring serum levels of CGRP in migraine patients during attacks. While the role of CGRP in migraine attack was still unclear, but several studies showed the CGRP may cause migraine by inducing secretion of cytokines.
Cytokines play an important role in several physiological courses, such as inflammation and pain. Pro-inflammatory cytokines, such as TNF-α, IL-1β, IL-6, and anti-inflammatory cytokines, such as IL-10 have been reported to play a significant role in the modulation of pain threshold and they could contribute to trigeminal nerve fibers sensitization.[22,23] Previous studies supported that cytokines might be considered in inflammation and hyperalgesia in migraine.[24,25] Similar to the earlier studies, we found migraine patients had a higher IL-6, IL-1β, and TNF level than healthy controls in our study, but we did not find a significant difference between migraine with aura and migraine without aura. The increasing IL-6, IL-1 β and TNF level support that cytokines may be related with the pathogenesis of migraine. The cause of elevated cytokine levels during attacks is unknown; however, CGRP may play a role in this event. It has been shown that CGRP stimulates the production and release of pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6 from human lymphocytes “in vitro” through the link to specific receptors on these cells.[10,26] In addition, the jugular plasma levels of CGRP are increased in the 1st h of migraine attacks, may cause neurogenic inflammation and vasodilation in animal models. Recent data have shown that CGRP possibly through stimulation of their selective receptors on T-cells, trigger the secretion of cytokines.[27,28] Therefore, it is possible that the pain of migraine attack is related to the release of CGRP and its ability to induce a secretion of cytokine from white cells, platelets, and endothelium. In our study, we found the level of CGRP was related significantly with the level of IL-1β (r = 0.30, P < 0.05) and IL-6 (r = 0.94, P < 0.05). In conclusion, our results suggest that IL-1β and IL-6 may be involved in the pathogenesis of migraine attacks and CGRP related with the secretion of cytokines. Following this hypothesis, possible future drugs that prevent cytokine production or block CGRP release from trigeminal vascular endings may be a useful strategy in the treatment of migraine.
In our study, the IL-6 level was related with CGRP level most significantly. However, the role of IL-6 to induce migraine has not yet been explored. Several studies showed that IL-6 can activate the mitogen-activated protein kinase (MAPK) signaling pathway in trigeminal ganglion neurons. Moreover, the induction and maintenance of various pain conditions were activated by the ERK1/2 MAPK pathway.[29,30] Recent work showed that the voltage-gated sodium channels, such as Nav1.3 and Nav1.8, can been regulated by MAPK pathway in DRG neurons and the voltage-gated sodium channels play important role in neuralgia.[31,32] Consequently, we believe the migraine may be caused by cytokines, such as IL-6 and IL-1β which induced by CGRP.
Our study had limitations certainly, such as we just collected the blood from jugular venous because a large number of studies had shown that the level of CGRP in jugular venous but not cubital venous was higher in migraine patients than healthy volunteers. In the present study, we did not compare the CGRP and cytokines between CM and EM patients as well as between in and out of an attack, we will explore these points in the next studies.
CONCLUSIONS
In our study, we found migraine patients had a higher IL-6, IL-1β, and TNF level than healthy controls and the level of CGRP was related significantly with the level of IL-1β and IL-6. In conclusion, our results suggest that IL-1β and IL-6 may be involved in the pathogenesis of migraine attacks and CGRP related with the secretion of cytokines.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Cutrer FM. Pathophysiology of migraine. Semin Neurol. 2010;30:120–30. doi: 10.1055/s-0030-1249222. [DOI] [PubMed] [Google Scholar]
- 2.Aurora SK, Kulthia A, Barrodale PM. Mechanism of chronic migraine. Curr Pain Headache Rep. 2011;15:57–63. doi: 10.1007/s11916-010-0165-z. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein DJ, Wang O, Saper JR, Stoltz R, Silberstein SD, Mathew NT, et al. Ineffectiveness of neurokinin-1 antagonist in acute migraine: A crossover study. Cephalalgia. 1997;17:785–90. doi: 10.1046/j.1468-2982.1997.1707785.x. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein DJ, Offen WW, Klein EG, Phebus LA, Hipskind P, Johnson KW, et al. Lanepitant, an NK-1 antagonist, in migraine prevention. Cephalalgia. 2001;21:102–6. doi: 10.1046/j.1468-2982.2001.00161.x. [DOI] [PubMed] [Google Scholar]
- 5.Guo S, Christensen AF, Liu ML, Janjooa BN, Olesen J, Ashina M, et al. Calcitonin gene-related peptide induced migraine attacks in patients with and without familial aggregation of migraine. Cephalalgia. 2017;37:114–24. doi: 10.1177/0333102416639512. [DOI] [PubMed] [Google Scholar]
- 6.Russo AF. Calcitonin gene-related peptide (CGRP): A new target for migraine. Annu Rev Pharmacol Toxicol. 2015;55:533–52. doi: 10.1146/annurev-pharmtox-010814-124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bigal ME, Walter S, Rapoport AM. Calcitonin gene-related peptide (CGRP) and migraine current understanding and state of development. Headache. 2013;53:1230–44. doi: 10.1111/head.12179. [DOI] [PubMed] [Google Scholar]
- 8.Messlinger K, Lennerz JK, Eberhardt M, Fischer MJ. CGRP and NO in the trigeminal system: Mechanisms and role in headache generation. Headache. 2012;52:1411–27. doi: 10.1111/j.1526-4610.2012.02212.x. [DOI] [PubMed] [Google Scholar]
- 9.Messlinger K, Fischer MJ, Lennerz JK. Neuropeptide effects on the trigeminal system: Pathophysiology and clinical significance for migraine. Schmerz. 2011;25:393. doi: 10.1007/s00482-011-1069-5. [DOI] [PubMed] [Google Scholar]
- 10.Karsan N, Goadsby PJ. CGRP mechanism antagonists and migraine management. Curr Neurol Neurosci Rep. 2015;15:25. doi: 10.1007/s11910-015-0547-z. [DOI] [PubMed] [Google Scholar]
- 11.Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28:183–7. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- 12.Holzmann B. Modulation of immune responses by the neuropeptide CGRP. Amino Acids. 2013;45:1–7. doi: 10.1007/s00726-011-1161-2. [DOI] [PubMed] [Google Scholar]
- 13.de Miguel M, Kraychete DC, Meyer Nascimento RJ. Chronic pain: Cytokines, lymphocytes and chemokines. Inflamm Allergy Drug Targets. 2014;13:339–49. doi: 10.2174/1871528114666150114170004. [DOI] [PubMed] [Google Scholar]
- 14.Zhao W, Wang Y, Fang Q, Wu J, Gao X, Liu H, et al. Changes in neurotrophic and inflammatory factors in the cerebrospinal fluid of patients with postherpetic neuralgia. Neurosci Lett. 2017;637:108–13. doi: 10.1016/j.neulet.2016.11.041. [DOI] [PubMed] [Google Scholar]
- 15.Wang K, Bao JP, Yang S, Hong X, Liu L, Xie XH, et al. A cohort study comparing the serum levels of pro – Or anti-inflammatory cytokines in patients with lumbar radicular pain and healthy subjects. Eur Spine J. 2016;25:1428–34. doi: 10.1007/s00586-015-4349-4. [DOI] [PubMed] [Google Scholar]
- 16.Kidd BL, Photiou A, Inglis JJ. The role of inflammatory mediators on nociception and pain in arthritis. Novartis Found Symp. 2004;260:122–33. [PubMed] [Google Scholar]
- 17.Uzar E, Evliyaoglu O, Yucel Y, Ugur Cevik M, Acar A, Guzel I, et al. Serum cytokine and pro-brain natriuretic peptide (BNP) levels in patients with migraine. Eur Rev Med Pharmacol Sci. 2011;15:1111–6. [PubMed] [Google Scholar]
- 18.Nye BL, Thadani VM. Migraine and epilepsy: Review of the literature. Headache. 2015;55:359–80. doi: 10.1111/head.12536. [DOI] [PubMed] [Google Scholar]
- 19.Walker CS, Hay DL. CGRP in the trigeminovascular system: A role for CGRP, adrenomedullin and amylin receptors? Br J Pharmacol. 2013;170:1293–307. doi: 10.1111/bph.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edvinsson L. The trigeminovascular pathway: Role of CGRP and CGRP receptors in migraine. Headache. 2017;57(Suppl 2):47–55. doi: 10.1111/head.13081. [DOI] [PubMed] [Google Scholar]
- 21.Aggarwal M, Puri V, Puri S. Serotonin and CGRP in migraine. Ann Neurosci. 2012;19:88–94. doi: 10.5214/ans.0972.7531.12190210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milligan ED, Penzkover KR, Soderquist RG, Mahoney MJ. Spinal interleukin-10 therapy to treat peripheral neuropathic pain. Neuromodulation. 2012;15:520–6. doi: 10.1111/j.1525-1403.2012.00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwilasz AJ, Grace PM, Serbedzija P, Maier SF, Watkins LR. The therapeutic potential of interleukin-10 in neuroimmune diseases. Neuropharmacology. 2015;96:55–69. doi: 10.1016/j.neuropharm.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rozen T, Swidan SZ. Elevation of CSF tumor necrosis factor alpha levels in new daily persistent headache and treatment refractory chronic migraine. Headache. 2007;47:1050–5. doi: 10.1111/j.1526-4610.2006.00722.x. [DOI] [PubMed] [Google Scholar]
- 25.Boćkowski L, Sobaniec W, Zelazowska-Rutkowska B. Proinflammatory plasma cytokines in children with migraine. Pediatr Neurol. 2009;41:17–21. doi: 10.1016/j.pediatrneurol.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Ramachandran R. Neurogenic inflammation and its role in migraine. Semin Immunopathol. 2018;40:301–14. doi: 10.1007/s00281-018-0676-y. [DOI] [PubMed] [Google Scholar]
- 27.Yamaguchi M, Kojima T, Kanekawa M, Aihara N, Nogimura A, Kasai K, et al. Neuropeptides stimulate production of interleukin-1 beta, interleukin-6, and tumor necrosis factor-alpha in human dental pulp cells. Inflamm Res. 2004;53:199–204. doi: 10.1007/s00011-003-1243-z. [DOI] [PubMed] [Google Scholar]
- 28.Sakuta H, Inaba K, Muramatsu S. Calcitonin gene-related peptide enhances cytokine-induced IL-6 production by fibroblasts. Cell Immunol. 1995;165:20–5. doi: 10.1006/cimm.1995.1182. [DOI] [PubMed] [Google Scholar]
- 29.Xia Y, Khoi PN, Yoon HJ, Lian S, Joo YE, Chay KO, et al. Piperine inhibits IL-1β-induced IL-6 expression by suppressing p38 MAPK and STAT3 activation in gastric cancer cells. Mol Cell Biochem. 2015;398:147–56. doi: 10.1007/s11010-014-2214-0. [DOI] [PubMed] [Google Scholar]
- 30.Ji M, Lu Y, Zhao C, Gao W, He F, Zhang J, et al. C5a induces the synthesis of IL-6 and TNF-α in rat glomerular mesangial cells through MAPK signaling pathways. PLoS One. 2016;11:e0161867. doi: 10.1371/journal.pone.0161867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zang Y, Xin WJ, Pang RP, Li YY, Liu XG. Upregulation of nav1.3 channel induced by rrTNF in cultured adult rat DRG neurons via p38 MAPK and JNK pathways. Chin J Physiol. 2011;54:241–6. doi: 10.4077/CJP.2011.AMM075. [DOI] [PubMed] [Google Scholar]
- 32.House CD, Wang BD, Ceniccola K, Williams R, Simaan M, Olender J, et al. Voltage-gated na+ channel activity increases colon cancer transcriptional activity and invasion via persistent MAPK signaling. Sci Rep. 2015;5:11541. doi: 10.1038/srep11541. [DOI] [PMC free article] [PubMed] [Google Scholar]