The crystal structure of protein kinase CK2a1 in complex with 5-iodotubercidin, an ATP-analogue inhibitor, was determined at 1.78 Å resolution. This structure should facilitate the discovery of selective CK2a1 inhibitors.
Keywords: protein kinase CK2, ATP-analogue inhibitor, selectivity, off-target kinases, structural comparison
Abstract
Protein kinase CK2a1 is a serine/threonine kinase that plays a crucial role in the growth, proliferation and survival of cells and is a well known target for tumour and glomerulonephritis therapies. Here, the crystal structure of the kinase domain of CK2a1 complexed with 5-iodotubercidin (5IOD), an ATP-mimetic inhibitor, was determined at 1.78 Å resolution. The structure shows distinct structural features and, in combination with a comparison of the crystal structures of five off-target kinases complexed with 5IOD, provides valuable information for the development of highly selective inhibitors.
1. Introduction
Protein kinase CK2a1 is a serine/threonine protein kinase that regulates the growth, proliferation and survival of a variety of cells. CK2a1 is an important target protein in serious diseases such as cancer and glomerulonephritis (Duncan & Litchfield, 2008 ▸; Yamada et al., 2005 ▸). Structural studies of CK2a1 have contributed to the production of potent inhibitors, including CX-4945, the first clinical CK2a1 inhibitor (Siddiqui-Jain et al., 2010 ▸).
In the development of antikinase drugs, researchers are faced with selectivity issues against off-target kinases. Crystal structures of target kinases serve to increase the potency of inhibitors, but are insufficient for obtaining high selectivity. Occasionally, comparative structural dissections lead to a drastic enhancement in the inhibitory selectivity between the target kinase and off-target kinases. Furthermore, common and/or disparate insights into the binding mode of a particular compound to protein kinases may confer clues for the development of highly selective inhibitors. Crystal structures of Src-family kinases with staurosporine, a well known broad-spectrum kinase inhibitor, provided structural insights that have enhanced the understanding of inhibitory selectivity in the Src family (Kinoshita et al., 2006 ▸; Miyano et al., 2009 ▸).
Here, we report the crystal structure of CK2a1 in complex with 5-iodotubercidin (5IOD) [Fig. 1 ▸(c)], an ATP-mimetic inhibitor, at 1.78 Å resolution. Comparative structural analyses of the complexes of 5IOD with CK2a1, CK1g2 (unpublished, but available in the Protein Data Bank), ERK1 (Kinoshita et al., 2008 ▸), ERK2 (Kinoshita et al., 2016 ▸), haspin (Eswaran et al., 2009 ▸) and CLK1 (Heroven et al., 2018 ▸) provided valuable structural insights for structure-based drug discovery of novel CK2a1-selective inhibitors.
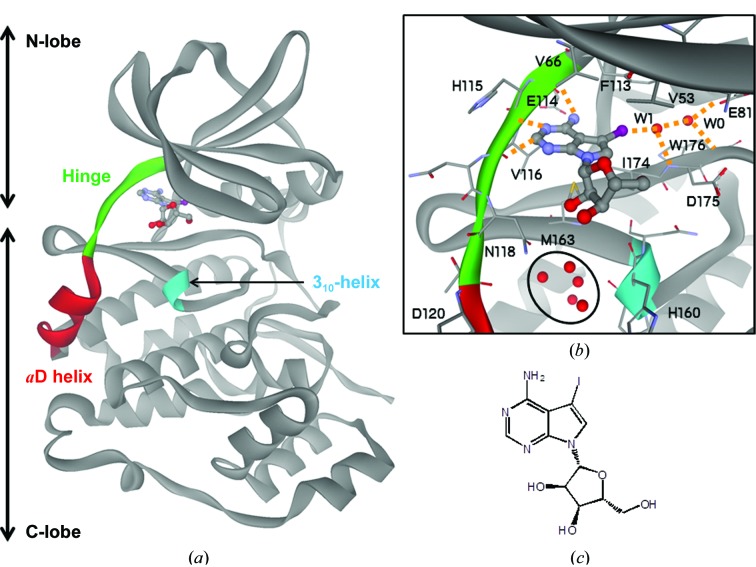
Figure 1.
Crystal structure of 5-iodotubercidin (5IOD) bound to the ATP-binding site of CK2a1. (a) Overall structure of the 5IOD–CK2a1 complex. (b) Mode of interaction of 5IOD with CK2a1. Hydrogen bonds are shown as orange dotted lines. The clustered water molecules in the αD pocket are shown as red spheres and are circled. (c) Chemical structure of 5IOD.
2. Materials and methods
2.1. Macromolecule production
Recombinant human CK2a1 was prepared according to a previously reported protocol (Kinoshita et al., 2013 ▸). The gene coding for human CK2a1 (1–335) was cloned into the pGEX-6P-1 vector (GE Healthcare) at BamHI and EcoRI restriction sites. Escherichia coli strain HMS174 (DE3) cells (Novagen) were transformed with the cloned plasmid and cultured in LB medium supplemented with 100 µg ml−1 ampicillin. Protein expression was induced with 0.2 mM isopropyl β-d-1-thiogalactopyranoside and the cells were incubated at 298 K for a further 15 h. The cells were harvested, resuspended in a buffer consisting of 150 mM NaCl, 25 mM Tris–HCl pH 7.4 and sonicated. After removing the cellular debris by centrifugation, the supernatant was loaded onto glutathione Sepharose 4B resin (GE Healthcare) and the CK2a1 protein was digested with 80 U ml−1 PreScission protease (GE Healthcare). The eluted CK2a1 was further purified by anion-exchange chromatography on a Mono Q column (GE Healthcare) using a linear gradient of 0–0.5 M NaCl in a buffer consisting of 25 mM Tris–HCl pH 8.0, 5 mM dithiothreitol.
2.2. Crystallization
The purified CK2a1 protein was concentrated to 5 mg ml−1 in anion-exchange elution buffer and used for crystallization. Prism-shaped crystals of apo CK2a1 were obtained by the sitting-drop vapour-diffusion method. Crystallization drops were prepared by mixing 2 µl protein solution with an equal volume of reservoir solution composed of 20–25% ethylene glycol as a precipitant and were equilibrated against 500 µl reservoir solution at 277 K. This condition was the same as the previous result (Sekiguchi et al., 2009 ▸).
2.3. Data collection and processing
A piece of powdered 5-iodotubercidin (5IOD) with dimensions of approximately 0.1 mm (MedChemExpress) was placed into the crystallization drop. A large fraction of 5IOD remained in the solid state, and the subtly dissolved compound probably soaked into the apo CK2a1 crystals on storage overnight. The ethylene glycol used as a precipitant acted as a cryoprotectant. Diffraction data were collected using synchrotron radiation on beamline BL44XU at SPring-8 using an MX-300HE (Rayonix) detector. The X-ray diffraction set was processed and scaled using HKL-2000 (Otwinowski & Minor, 1997 ▸). Data-collection parameters and statistics are shown in Table 1 ▸.
Table 1. Data collection and processing.
Values in parentheses are for the outer shell.
| Diffraction source | BL44XU, SPring-8 |
| Wavelength (Å) | 0.9 |
| Temperature (K) | 95 |
| Detector | MX-300HE |
| Crystal-to-detector distance (mm) | 250 |
| Rotation range per image (°) | 0.5 |
| Total rotation range (°) | 0–180 |
| Exposure time per image (s) | 0.5 |
| Space group | P212121 |
| a, b, c (Å) | 48.21, 78.94, 82.84 |
| Mosaicity (°) | 0.8 |
| Resolution range (Å) | 50.0–1.78 (1.81–1.78) |
| Total No. of reflections | 224679 |
| No. of unique reflections | 30925 |
| Completeness (%) | 100 (100) |
| Multiplicity | 7.3 (7.2) |
| 〈I/σ(I)〉 | 23.3 (2.5) |
| R r.i.m. † | 0.103 (0.966) |
The redundancy-independent merging R factor R r.i.m. was estimated by multiplying the conventional R merge value by the factor [N/(N − 1)]1/2, where N is the data multiplicity.
2.4. Structure solution and refinement
Initial phasing was performed by the molecular-replacement method with MOLREP (Vagin & Teplyakov, 2010 ▸) using a previously reported structure of CK2a1 (PDB entry 3war; Kinoshita et al., 2013 ▸) as the search model. Refinement, electron-density map calculations and model building were performed using REFMAC5 (Murshudov et al., 2011 ▸), the PHENIX package (Adams et al., 2010 ▸) and Coot (Emsley et al., 2010 ▸). The refinement statistics are shown in Table 2 ▸. The final set of coordinates was deposited in the Protein Data Bank as entry 6jwa.
Table 2. Structure solution and refinement.
Values in parentheses are for the outer shell.
| Resolution range (Å) | 50.00–1.78 (1.83–1.78) |
| Completeness (%) | 100.0 (96.7) |
| No. of reflections, working set | 28870 (2782) |
| No. of reflections, test set | 1997 (189) |
| Final R cryst (%) | 18.5 (26.0) |
| Final R free (%) | 22.5 (32.1) |
| No. of non-H atoms | |
| Protein | 2762 |
| Ligand | 44 |
| Water | 157 |
| Total | 2963 |
| R.m.s. deviations | |
| Bonds (Å) | 0.007 |
| Angles (°) | 1.047 |
| Average B factors (Å2) | |
| Protein | 25.5 |
| Ligand | 26.0 |
| Water | 33.1 |
| Ramachandran plot | |
| Most favoured (%) | 96.92 |
| Allowed (%) | 2.46 |
3. Results and discussion
3.1. Binding mode of 5-iodotubercidin to CK2a1
The crystal structure of CK2a1 adopts the conserved protein kinase fold, with the N-terminal and C-terminal lobes connected by the hinge region [Fig. 1 ▸(a)]. 5-Iodotubercidin (5IOD) binds to CK2a1 at the ATP-binding site, which is a hydrophobic groove located between the lobes [Fig. 1 ▸(a)]. The 4-aminopyrrolopyrimidine moiety of 5IOD is anchored by three hydrogen bonds to the carbonyl O atoms of Glu114 and Val116 and the NH group of Val116 in the hinge region, and the I atom forms a hydrogen bond to a water molecule (W1) ligated by the carbonyl O atom of Asp175 [Fig. 1 ▸(b)]. W1 also forms a hydrogen bond to a water molecule (W0) that is highly conserved among CK2a1 crystal structures and is ligated by the carbonyl O atom of Trp176 and the carboxyl group of Glu81. The pyrrolopyrimidine plane is bracketed by Val53, Val66, Met163 and Ile174 via hydrophobic interactions [Fig. 1 ▸(b)], resulting in the formation of a hydrophobic spine (C-spine) that is conserved among all kinases (Taylor & Kornev, 2011 ▸). The ribose moiety of 5IOD forms no hydrogen bonds to CK2a1, even though the ability of this moiety to form hydrogen bonds is high. Five clustered water molecules are bound to a small hydrophobic pocket, referred to as the αD pocket, located between the αD and 310-helices near the ribose-binding region [Fig. 1 ▸(b)]. This cluster of water molecules is likely to act as a damper for movement of the αD helix, which is a critical switch in CK2a1 to use either ATP or GTP as a phosphate source (Niefind et al., 1999 ▸). Asp120 is likely to function as an N-cap residue (Richardson & Richardson, 1988 ▸) of the αD helix, which stabilizes the positive dipole moment of the α-helix structure.
3.2. Comparison of the binding modes of 5IOD between CK2a1 and other protein kinases
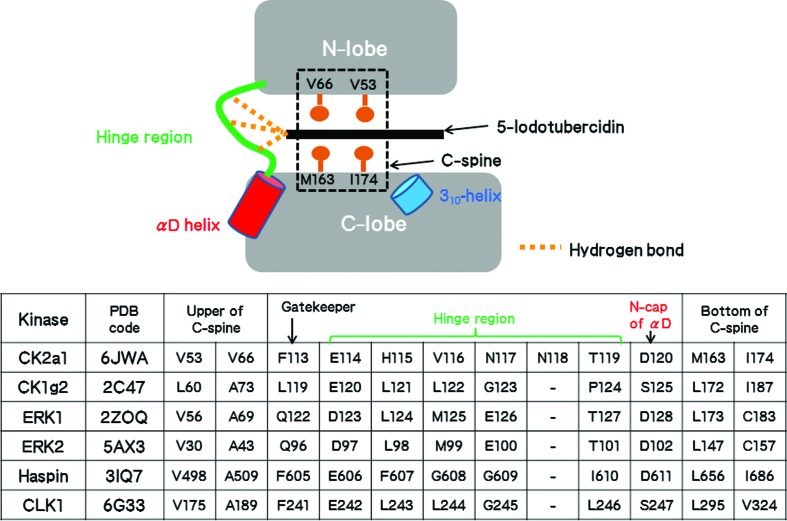
Besides the structure of CK2a1, five crystal structures of 5IOD–protein kinase complexes are available from the Protein Data Bank (Fig. 2 ▸). The interaction of 5IOD with the hinge region is essentially conserved in the structures of CK2a1, CK1g2, ERK1, ERK2, haspin and CLK1. The 4-aminopyrrolopyrimidine moiety of 5IOD binds at a similar position in all structures via three hydrogen bonds to the hinge region (Table 3 ▸). These interactions use main-chain atoms and are thus independent of differences in the amino-acid sequences (Fig. 2 ▸). The more bulky hydrophobic residues that form the C-spine narrow the ATP-binding site of CK2a1 when compared with those of other kinases (Fig. 2 ▸). Therefore, planarity of inhibitors is likely to be a structural requirement for CK2a1 selectivity.
Figure 2.
Amino-acid residues of the protein kinases involved in the interaction with the 4-aminopyrrolopyrimidine moiety of 5-iodotubercidin.
Table 3. Inhibitory activities and/or binding dissociation constants of 5-iodotubercidin against the kinases and the number of electrostatic interactions.
| Kinase | IC50 (µM) | K d † (µM) | 4-Aminopyrrolopyrimidine | Ribose | I atom |
|---|---|---|---|---|---|
| CK2a1 | 10.9† | 3 hydrogen bonds | None | 1 hydrogen bond | |
| CK1g2 | 0.4† | 3 hydrogen bonds | 3 hydrogen bonds | 1 hydrogen bond | |
| ERK1 | 1.2‡ | 3 hydrogen bonds | 3 hydrogen bonds | 1 hydrogen bond | |
| ERK2 | 0.9‡ | 3 hydrogen bonds | 3 hydrogen bonds | 1 hydrogen bond | |
| Haspin | 0.009§ | 0.006¶ | 3 hydrogen bonds | 3 hydrogen bonds | 1 hydrogen bond, 1 halogen–π interaction |
| CLK1 | 0.007¶ | 3 hydrogen bonds | 3 hydrogen bonds | 1 hydrogen bond, 1 halogen–π interaction |
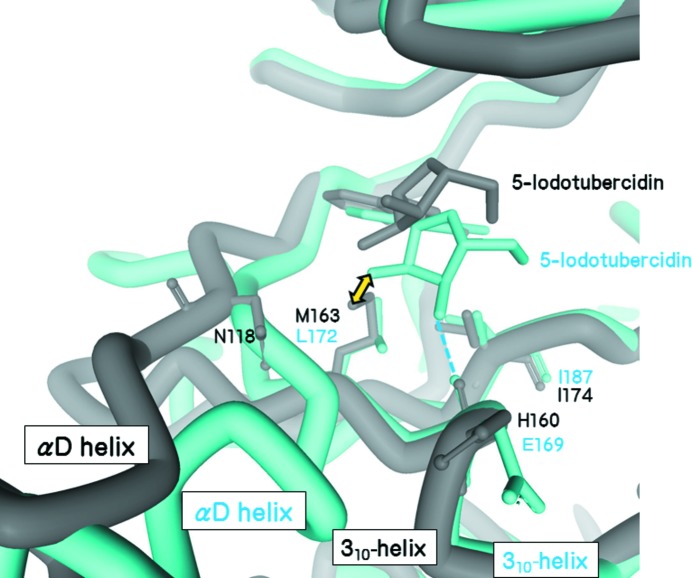
The ribose moiety of 5IOD binds tightly to all kinases except CK2a1 (Table 3 ▸); the carbonyl group in the 310-helix and the side chains in the αD helix act as hydrogen-bond acceptors or donors. The αD helix of CK2a1 is displaced when compared with those of the other kinases and it cannot participate in hydrogen bonding to the ribose moiety (Fig. 3 ▸). In constrast, the configuration of the carbonyl group in the 310-helix is well conserved among these kinases (Fig. 3 ▸). However, Met163 of CK2a1 disturbs hydrogen bonding to the ribose moiety owing to steric hindrance (Fig. 3 ▸). Furthermore, Met163 is immobilized by the side-chain atoms of Asn118, which is a unique insertion residue in the hinge region. The structure revealed that Ile174, Met163 and Asn118 form a wider hydrophobic planar structure in the ribose-binding region of CK2a1 (Fig. 3 ▸).
Figure 3.
Superimposition of the 5-iodotubercidin complexes of CK2a1 (grey) and CK1g2 (blue). The ribose moiety in the CK2a1 complex is located in the upper position when compared with that of CK1g2. The position of the ribose moiety in the CK1g2 structure would cause steric clashes with Met163 of CK2a1 (yellow arrow).
The I atom of 5IOD faces the gatekeeper residue in all 5IOD complexes. The higher inhibitory activities of 5IOD for haspin and CLK1 are likely to be attributable to the halogen–π interaction between the gatekeeper phenylalanine residue and the I atom (Heroven et al., 2018 ▸). Although the gatekeeper residue of CK2a1 is Phe113, as found in haspin and CLK1, this residue is distal from the I atom. The reduced interaction of the ribose and I atom of 5IOD with CK2a1 is most likely to account for the lower inhibitory activity of CK2a1 (Table 3 ▸). The values for the inhibitory activity and/or the binding dissociation constant extracted from previous reports (Massillon et al., 1994 ▸; Kinoshita et al., 2008 ▸; Balzano et al., 2011 ▸; Heroven et al., 2018 ▸) are associated with the number of electrostatic interactions involving hydrogen bonds and halogen–aromatic π interactions (Table 3 ▸).
The iodine-ligated W1 in the CK2a1 complex is conserved in the 5IOD complexes of CK1g2, ERK1, CLK1 and haspin. The low-resolution ERK2 structure contains no water molecules, but has sufficient space to be bound by a water molecule. The water molecule W0 is conserved in haspin but not in the other four kinases. Thus, the W0 position is unique and could be available for increasing the selectivity of CK2a1 inhibitors.
The αD pocket was observed in the CK2a1 complex but not in the other five kinases. The single insertion residue in the hinge region of CK2a1 probably impedes the formation of the zipper-like interaction between the αD and 310-helices that is observed in other kinase structures such as that of CK1g2 (Fig. 3 ▸). Thus, the αD helix of CK2a1 is located in the outer region and distal from the 310-helix. The space afforded by the position of the αD helix is occupied by Asn118 and water molecules (Fig. 3 ▸). αD pocket binders have recently been discovered (Iegre et al., 2018 ▸) and are therefore suitable for producing highly selective CK2a1 inhibitors.
4. Conclusion
The 1.78 Å resolution structure of the 5IOD–CK2a1 complex and a comparative structural dissection of this structure and those of five other protein kinases provide structural insights for improving the inhibitory activity and selectivity of CK2a1 inhibitors. The hydrophobic regions of the ribose-binding site and the αD pocket are unique among protein kinases and are useful for structure-based drug discovery of CK2a1-selective inhibitors. Recently, Iegre and coworkers reported αD binders connected to an ATP-site inhibitor via a long flexible liker (Iegre et al., 2018 ▸). Likewise, 5IOD could be merged with αD binders at the hydroxyl groups in the ribose moiety.
Supplementary Material
PDB reference: CK2a1, complex with 5-iodotubercidin, 6jwa
Acknowledgments
Preliminary experiments and diffraction data collection were carried out on beamline BL17A at the Photon Factory (Proposal No. 2016G665) and on the Osaka University beamline BL44XU at SPring-8 (Proposal No. 2017B6717). The authors thank Edanz Group (http://www.edanzediting.com/ac) for editing a draft of this manuscript.
References
- Adams, P. D., Afonine, P. V., Bunkóczi, G., Chen, V. B., Davis, I. W., Echols, N., Headd, J. J., Hung, L.-W., Kapral, G. J., Grosse-Kunstleve, R. W., McCoy, A. J., Moriarty, N. W., Oeffner, R., Read, R. J., Richardson, D. C., Richardson, J. S., Terwilliger, T. C. & Zwart, P. H. (2010). Acta Cryst. D66, 213–221. [DOI] [PMC free article] [PubMed]
- Balzano, D., Santaguida, S., Musacchio, A. & Villa, F. (2011). Chem. Biol. 18, 966–975. [DOI] [PubMed]
- Duncan, J. S. & Litchfield, D. W. (2008). Biochim. Biophys. Acta, 1784, 33–47. [DOI] [PubMed]
- Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. (2010). Acta Cryst. D66, 486–501. [DOI] [PMC free article] [PubMed]
- Eswaran, J., Patnaik, D., Filippakopoulos, P., Wang, F., Stein, R. L., Murray, J. W., Higgins, J. M. G. & Knapp, S. (2009). Proc. Natl Acad. Sci. USA, 106, 20198–20203. [DOI] [PMC free article] [PubMed]
- Heroven, C., Georgi, V., Ganotra, G. K., Brennan, P., Wolfreys, F., Wade, R. C., Fernández-Montalván, A. E., Chaikuad, A. & Knapp, S. (2018). Angew. Chem. Int. Ed. 57, 7220–7224. [DOI] [PMC free article] [PubMed]
- Iegre, J., Brear, P., De Fusco, C., Yoshida, M., Mitchell, S. L., Rossmann, M., Carro, L., Sore, H. F., Hyvönen, M. & Spring, D. R. (2018). Chem. Sci. 9, 3041–3049. [DOI] [PMC free article] [PubMed]
- Kinoshita, T., Matsubara, M., Ishiguro, H., Okita, K. & Tada, T. (2006). Biochem. Biophys. Res. Commun. 346, 840–844. [DOI] [PubMed]
- Kinoshita, T., Nakaniwa, T., Sekiguchi, Y., Sogabe, Y., Sakurai, A., Nakamura, S. & Nakanishi, I. (2013). J. Synchrotron Rad. 20, 974–979. [DOI] [PMC free article] [PubMed]
- Kinoshita, T., Sugiyama, H., Mori, Y., Takahashi, N. & Tomonaga, A. (2016). Bioorg. Med. Chem. Lett. 26, 955–958. [DOI] [PubMed]
- Kinoshita, T., Yoshida, I., Nakae, S., Okita, K., Gouda, M., Matsubara, M., Yokota, K., Ishiguro, H. & Tada, T. (2008). Biochem. Biophys. Res. Commun. 377, 1123–1127. [DOI] [PubMed]
- Massillon, D., Stalmans, W., van de Werve, G. & Bollen, M. (1994). Biochem. J. 299, 123–128. [DOI] [PMC free article] [PubMed]
- Miyano, N., Kinoshita, T., Nakai, R., Kirii, Y., Yokota, K. & Tada, T. (2009). Bioorg. Med. Chem. Lett. 19, 6557–6560. [DOI] [PubMed]
- Murshudov, G. N., Skubák, P., Lebedev, A. A., Pannu, N. S., Steiner, R. A., Nicholls, R. A., Winn, M. D., Long, F. & Vagin, A. A. (2011). Acta Cryst. D67, 355–367. [DOI] [PMC free article] [PubMed]
- Niefind, K., Pütter, M., Guerra, B., Issinger, O. G. & Schomburg, D. (1999). Nature Struct. Biol. 6, 1100–1103. [DOI] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol. 276, 307–326. [DOI] [PubMed]
- Richardson, J. S. & Richardson, D. C. (1988). Science, 240, 1648–1652. [DOI] [PubMed]
- Sekiguchi, Y., Nakaniwa, T., Kinoshita, T., Nakanishi, I., Kitaura, K., Hirasawa, A., Tsujimoto, G. & Tada, T. (2009). Bioorg. Med. Chem. Lett. 19, 2920–2923. [DOI] [PubMed]
- Siddiqui-Jain, A., Drygin, D., Streiner, N., Chua, P., Pierre, F., O’Brien, S. E., Bliesath, J., Omori, M., Huser, N., Ho, C., Proffitt, C., Schwaebe, M. K., Ryckman, D. M., Rice, W. G. & Anderes, K. (2010). Cancer Res. 70, 10288–10298. [DOI] [PubMed]
- Taylor, S. S. & Kornev, A. P. (2011). Trends Biochem. Sci. 36, 65–77. [DOI] [PMC free article] [PubMed]
- Vagin, A. & Teplyakov, A. (2010). Acta Cryst. D66, 22–25. [DOI] [PubMed]
- Yamada, M., Katsuma, S., Adachi, T., Hirasawa, A., Shiojima, S., Kadowaki, T., Okuno, Y., Koshimizu, T. A., Fujii, S., Sekiya, Y., Miyamoto, Y., Tamura, M., Yumura, W., Nihei, H., Kobayashi, M. & Tsujimoto, G. (2005). Proc. Natl Acad. Sci. USA, 102, 7736–7741. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDB reference: CK2a1, complex with 5-iodotubercidin, 6jwa