Abstract
The first case of invasive pulmonary infection due to the thermophilic fungus Thermoascus crustaceus in a kidney transplant recipient is described. For the identification of the fungal isolate, morphological aspects and molecular analysis have been used. The case report emphasizes this fungal species as an opportunistic human pathogen and underlines the importance of an accurate laboratory diagnosis for the correct management of the patient.
Keywords: Thermoascus crustaceus, thermophilic fungi, pulmonary infection, caspofungin, immunosuppression
Introduction
More than thirty distinct fungal species belonging to various taxonomical groups are able to thrive at higher temperatures and to exhibit an extremely active metabolic pattern including secretory enzymes like protease, lipase, amylase, laccase, trehalaze and others.1 Thermophilic fungi are uncommon isolates in clinical specimens despite their ubiquitous existence in the environment. They can affect immunocompromised hosts and some species can pose serious diagnostic problems due to their morphological resemblance with other related fungi.2 An accurate laboratory diagnostic is crucial for the subsequent management of the infection, particularly in patients at risk.
Case report
A 33-year-old Romanian woman, who had undergone kidney transplantation eight years before, from a living related donor, was admitted to our clinic with fever, rhinorrhea, cough and serous expectoration, four days after the onset of the symptoms (D4).
She had been diagnosed with nephrotic syndrome eleven years ago while working in Italy. One year later, after having returned to Romania, continuous ambulatory peritoneal dialysis (CAPD) was started because of severe impairment of the renal function (Chronic Kidney Disease – stage 5). After two years of CAPD, kidney transplantation from a living related donor (mother) was performed in Bucharest and follow-up was assured by the same clinic until last year. During this period, a chronic graft dysfunction (CGD) occurred, with a creatinine baseline of 2.5–2.8 mg/dL. Since January last year, the patient has been monitored in a clinic at the Renal Transplantation Center (Iași, Romania) and her history revealed four severe episodes of urinary tract infection and secondary serositis due to tacrolimus over dosage (amended after dose reduction). Anemia and arterial hypertension secondary to CGD were also diagnosed. The home treatment consisted of tacrolimus 0.5 mg twice a day, mofetil mycophenolate 1 g twice a day, prednisone 5 mg every two days, metoprolol 50 mg twice a day, moxonidine 0.4 mg twice a day, enalapril 5 mg daily, furosemide 0.5 mg/kg twice a day, and erythropoietin 5,000 U twice a week.
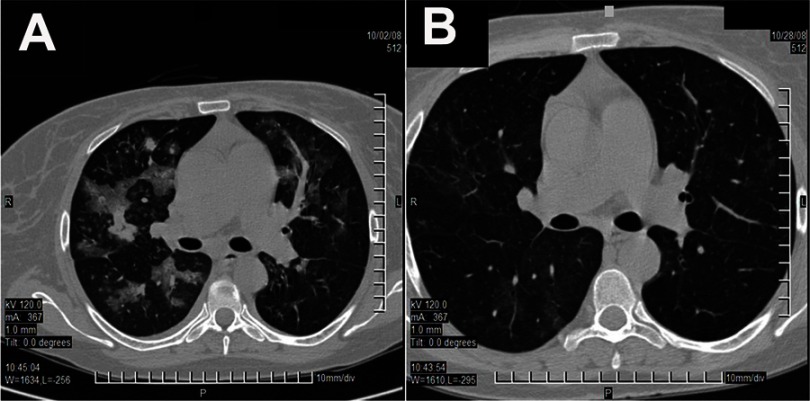
On D0, in the first day of the onset of the symptoms (fever, cough and rhinorrhea), the patient presented to the outpatient department of the county hospital where she was diagnosed with interstitial pneumonia and empirically treated with amoxicillin/clavulanic acid 1 g twice a day. Because of fever persistence under antibiotic therapy, the patient asked a consultation in our clinic four days later. On hospital admission, the patient was in mild stress, dehydrated and with low-grade fever. The vital signs and oxygen saturation were normal except a sinusal tachycardia (95 beats/minute) and crackles on the left lung base. Urine output was of 1,000 mL within the first 24 hrs. Laboratory tests revealed inflammatory syndrome (erythrocyte sedimentation rate: 42 mm/1 hr, C-Reactive Protein: 46 mg/dL, fibrinogen: 706 mg/dL), and renal dysfunction (creatinine − 4.8 mg/dL, urea − 200 mg/dL). The white blood cells count was normal (6,500/mm3). Although chest X-ray evidenced interstitial lung opacity, the microbiological examination of the sputum was negative. At that time, an interdisciplinary consultation team recommended moxifloxacin, pulsed-therapy with methylprednisolone, parenteral fluid therapy, and cancelation of diuretics and angiotensin-converting-enzyme inhibitors administration. This therapeutic approach improved the renal function with an important decrease of the nitrogen retention but had no effect against the respiratory symptoms. The fever persisted (38.0−38.7°C) while the cough and dyspnea were progressively exacerbating. Fiberoptic bronchoscopy revealed a normal aspect of the airways and the culture of bronchial aspirate exhibited Candida krusei in one sample. Most probably, it was an oral contamination because five other samples gave negative cultures. High-resolution computed tomography (CT) scan performed on D38 evidenced left basal condensation. In that context, the therapeutic protocol was modified with co-trimoxazole, plus meropenem, plus voriconazole, and valganciclovir being added to the previous schema to target the possible etiologies of infection in this immunocompromised patient. Under this therapy, the patient’s general status progressively deteriorated, the fever increased up to 40°C, dyspnea and cough got aggravated, hemoptysis and two episodes of acute pulmonary edema occurred. Crackles in both lower hemithorax were identified. Another CT scan performed on D51 revealed multiple condensation areas throughout both lungs (Figure 1A). There were identified non-systematized ground-glass diffuse areas around the bronchial vessels, disseminated in both lungs. The thickening of the alveolar septa and the lobar and segmental bronchial walls, an important increase in number and volume of previously described processes, and two 1.5 cm pulmonary condensation processes in the upper right lobe have also occurred. Air bronchogram was present.
Figure 1.
CT scan aspects of the pulmonary tissue: at D51, before starting the caspofungin treatment (A) and at D78, after the appropriate treatment was done (B).
No conclusive figures of Aspergillus infection (ie, “halo” sign or “air crescent” sign) were detected. The inflammatory syndrome became more severe (erythrocyte sedimentation rate: >150 mm/1 hr, C-Reactive Protein: 200 mg/dL, fibrinogen: 902 mg/dL). Another fiberoptic bronchoscopy showed mucopurulent and sanguinolent secretions. A bronchial aspirate and a transbronchial biopsy were performed and the samples were directed for microbiological investigations. Direct microscopic examination using calcofluor white revealed septate hyphal elements in both specimens. All cultures were negative for bacteria, but a filamentous fungus grew after 48 hrs of incubation at 37°C on all Sabouraud Dextrose Agar plates (Bio-Rad Laboratories, Hercules, USA) inoculated with both clinical samples. The fungus was presumptively identified as Paecilomyces sp. and the antifungal susceptibility testing was performed using Sensititre YO-10 plates (Trek Diagnostic Systems, East Grinstead, UK). Although no clinical breakpoints were available for this organism, low minimal inhibitory concentrations (MICs) were obtained for posaconazole (0.008 mg/L) and echinocandins (anidulafungin − 0.015 mg/L, caspofungin − 0.015 mg/L and micafungin − 0.008 mg/L) suggesting at least in vitro susceptibility.
At that point, the therapeutic protocol was modified with caspofungin being used as single antimicrobial agent for 28 days. Under this therapy, the patient improved continuously, the physical signs normalized, and the inflammatory syndrome disappeared (the last value of the C-Reactive Protein − 3.6 mg/dL). The last CT scan performed on D78 showed a marked reduction of condensation areas in both lungs (Figure 1B). In the anterior and posterior upper right lobe and apical-posterior and anterior upper left lobe segment, there was an increase of the peri- and intralobular pulmonary design, with a residual pneumonitis aspect associated with the sketching of fewer focal areas of residual ground-glass appearance at this level. Compared with the previous tomographic investigation, focal areas are much diminished in intensity and extension. The patient was discharged two weeks later, and no relapses occurred during a two-year follow-up.
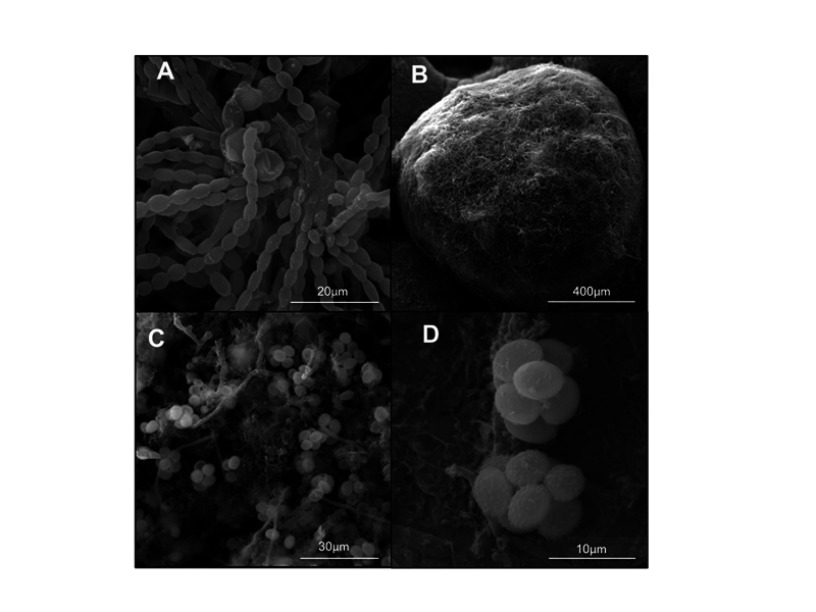
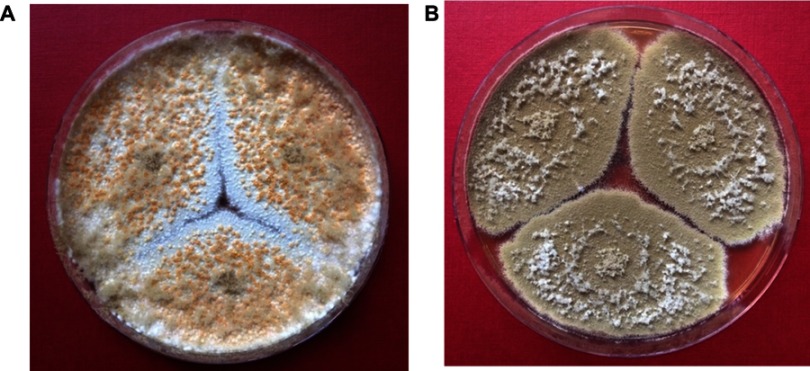
The isolate was subsequently processed for taxonomic purposes at Centraalbureau voor Schimmelcultures (now Westerdijk Fungal Biodiversity Institute) in Utrecht (The Netherlands) and “Petru Poni” Institute of Macromolecular Chemistry, and it was identified as Thermoascus crustaceus (anamorph Paecilomyces crustaceus) using morphological and molecular methods. The strain is available for access in the CBS Collection of Fungi (CBS 133,504). The main morphological aspects are shown in the Figure 2A–D. Colonies of our strain on malt-agar were spreading broadly, attaining a diameter of 8 cm within 7 days at 40°C. They were avellaneous at first, later switching to orange colors. Conidiophores arising from submerged hyphae or sometimes also as branches of the aerial hyphae, were up to 100 μm in length and 4–10 μm in diameter. Phialides were cylindrical with an abruptly tapering neck and measured 11–23×2.5–4.0 μm. Conidia varied from ellipsoidal to cylindrical, rounded at both ends, smooth-walled, hyaline to pale brown, 7–10×3–6 μm (Figure 2A). Chlamydospores were absent. Superficial ascomata were appearing in crusts, subspherical to hemispherical, non-ostiolate, yellowish-orange to reddish-orange, mostly 600–800 μm diameter, surrounded by a loose cover of aerial hyphae (Figure 2B). Peridium pseudoparenchymatous, asci ripening within 14 days on Oatmeal Agar, 8-spored, subspherical, irregularly arranged in the centrum (Figure 2C). Ascospores were pale yellow to pale brown, ovoidal to ellipsoidal, 7–8×4.5–6 μm, thick-walled, finely echinulate (Figure 2D). The isolate is thermophilic, growing faster between 37°C and 42°C than to lower temperatures. There was a clear difference between the colonies developed at 30°C and 37°C respectively, with the first exhibiting numerous ascomata on their surface (Figure 3A and B).
Figure 2.
Morphological aspects of the T. crustaceus isolate in scanning electron microscopy (ESEM Quanta 200-FEI, Hillsboro – USA): chains of conidia; (A) subspherical to hemispherical, non-ostiolate ascoma; (B) 8-spored asci; (C) thick-walled, finely echinulate ascospores (D).
Figure 3.
Macroscopic aspects of the T. crustaceus isolate after 5 days incubation at different temperatures on Potatoes Dextrose Agar: 30°C (A); 37°C (B).
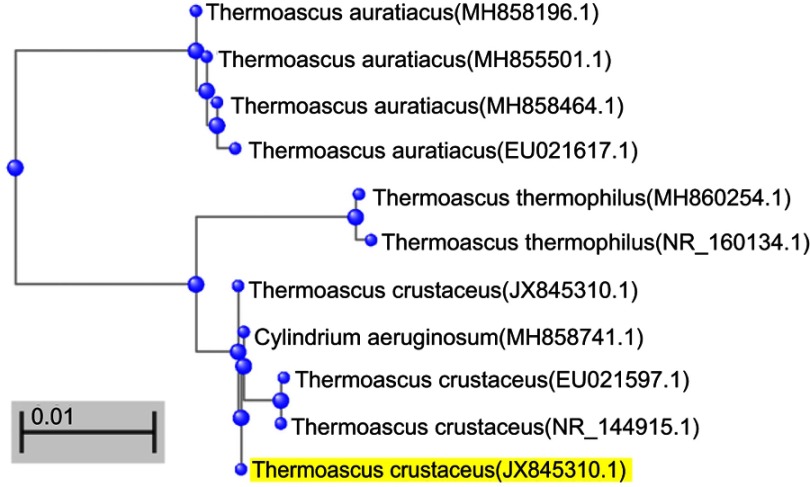
The phenotypic identification was further confirmed by molecular tests. DNA extraction was performed using the MoBio-UltraCleanTM Microbial DNA Isolation Kit according to the instructions of the manufacturer. Fragments containing the 26S ribosomal RNA gene, Large Subunit D1 and D2 region (LSU) were amplified using the primers LR0R (ACCCGCTGAACTTAAGC) and LR5 (TCCTGAGGGAAACTTCG).3 Fragments containing the Internal Transcribed Spacer 1 and 2, and the 5.8S gene (ITS) were amplified using the primers LS266 (GCATTCCCAAACAACTCGACTC) and V9G (TTACGTCCCTGCCCTTTGTA).4 The PCR fragments were sequenced with the ABI Prism® Big DyeTM Terminator v. 3.0 Ready Reaction Cycle sequencing kit. Samples were analyzed on an ABI PRISM 3700 Genetic Analyzer and contigs were assembled using the forward and reverse sequences with the SeqMan software from the LaserGene package. The sequences of our T. crustaceus isolate have been deposited in the GenBank database under the accession number JX845310. High percentages of similarity with other T. crustaceus strains were achieved for our isolate (Figure 4): 98.55% to T. crustaceus NRRL 1563 (GenBank accession No. EU021597.1), and 98.49% to T. crustaceus CBS181.67 (GenBank accession No. NR_144915.1).
Figure 4.
Phylogenetic tree of Thermoascus crustaceus based on confidently aligned internal transcribed spacer (ITS) sequences available in GenBank (constructed with BLASTN 2.9.0+).
Discussion
T. crustaceus is an ascomycetous thermophilic fungus ubiquitously distributed in compost, domestic wastes, soil, air, stored cereal grains, nesting materials of birds etc. from where it can be easily inhaled by humans.5 Also, it is more and more frequently isolated from pasteurized acid products being recognized as one of the most heat-resistant fungal species.6 Due to its capability to grow above the body temperature of mammals, this kind of fungi can act as potential human pathogens.2 Thermophilic fungi also have a pattern of protein expression that assures high levels of protein secretion outside the cells – especially thermotolerant enzymes (proteases, lipases).1,5 This may be responsible for the inflammatory syndrome occurred in our patient, even under glucocorticoid therapy.
The main risk factor for the development of an invasive pulmonary infection in our transplant patient was the long-term immunosuppression while the extension of the lesions was due to the lack of appropriate investigations and treatment in the first stages of the illness. Later, a proven invasive fungal infection was documented according to EORTC/MSG Consensus Group guidelines and an effective therapy was started.7
To our knowledge, this is the first report of an invasive fungal infection caused by T. crustaceus and it emphasizes the pathogenic abilities of the fungus to cause severe illness in humans, especially under immunosuppressive conditions. Only two cases of colonization with T. crustaceus of the peritoneal catheter were previously described in the literature.8,9 At 37°C, the colonies of T. crustaceus can mimic other related species (especially Paecilomyces variotii) due to their avellaneous color and powdery appearance. This could result in misidentification of the isolates, if additional investigations like thermotolerance and molecular tests are not performed.
Acknowledgments
The Ministry of Education and Research from Romania supported Mihai Mareș by CNCSIS-UEFISCDI PN II-RU 159/2010 grant. The authors are grateful to Martin Meijer and Professor Robert A. Samson from Westerdijk Fungal Biodiversity Institute (Utrecht, The Netherlands) for assistance with molecular identification and morphological description. Prior to initial manuscript submission, a written informed consent has been obtained from the patient stating that all case details and accompanying images can be published in a specialty journal. Also, the institutional approval for publication has been obtained from Renal Transplantation Center, C. I. Parhon Hospital in Iasi (Romania). The ethics committee of the same institution agreed on the publication of all case details.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Maheshwari R, Bharadwaj G, Bhat M. Thermophilic fungi: their physiology and enzymes. Microbiol Mol Biol Rev. 2000;64:461–488. doi: 10.1128/mmbr.64.3.461-488.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luangsa-ard JJ, Manoch L, Hywel-Jones N, Artjariyasripong S, Samson AR. Thermotolerant and thermoresistant paecilomyces and its teleomorphic states isolated from Thai forest and mountain soils. Kasetsart J. 2004;37:94–101. [Google Scholar]
- 3.Vilgalys R, Hester M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several cryptococcus species. J Bacteriol. 1990;172:4239–4246. doi: 10.1128/jb.172.8.4238-4246.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerrits van den Ende AHG, de Hoog GS. Variability and molecular diagnostics of the neurotropic species Cladophialophora bantiana. Stud Mycol. 1999;43:151–159. [Google Scholar]
- 5.Shaik YB. Inflammatory thermophilic fungi are used in biotechnology applications. Eur J Inflamm. 2006;4:147–155. doi: 10.1177/1721727X0600400303 [DOI] [Google Scholar]
- 6.Scaramuzza N, Berni E. Heat-resistance of hamigera avellanea and Thermoascus crustaceus isolated from pasteurized acid products. Int J Food Microbiol. 2014;168-169:63–68. doi: 10.1016/j.ijfoodmicro.2013.10.007 [DOI] [PubMed] [Google Scholar]
- 7.Pauw BD, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–1821. doi: 10.1086/588660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oz Y, Kiraz N, Ozkurt S, Soydan M. Colonization of peritoneal catheter with a thermophilic fungus, Thermoascus crustaceus: a case report. Med Mycol. 2010;48:1105–1107. doi: 10.3109/13693781003793838 [DOI] [PubMed] [Google Scholar]
- 9.Alvarez E, Castillo A, Iturrieta I. Fungal peritonitis by Thermoascus crustaceus in a peritoneal dialysis patient from Chile. Rev Iberoam Micol. 2017;34(4):225–228. doi: 10.1016/j.riam.2017.01.004 [DOI] [PubMed] [Google Scholar]