Abstract
The anterior cerebral artery (ACA) contains anatomical variants that are closely related to its embryology and development. In this study, the authors reviewed the most commonly encountered variants of the ACA and anterior communicating artery. They also reviewed the embryological origins of these variants as well as a variety of associated pathologies. Several variants are described and highlighted with illustrations including: (1) the aberrant origin of the ACA from the internal carotid artery and its developmental association with the ophthalmic artery; (2) the persistent olfactory artery; (3) the azygous ACA; (4) the triplicated ACA; and (5) multiple anterior communicating arteries. The formation of aneurysms is associated with such variants, thus their knowledge and the embryology behind their development are crucial to prevent injury to the patient.
Keywords: Aneurysm, anomalies, anterior cerebral artery, variant, vascular malformation
INTRODUCTION
The anterior cerebral artery (ACA) and its branches contain both clinically significant and incidental variations. The ACA is the primary blood supply to the developing telencephalon in both amphibians and mammals and to the frontal lobes in humans. With key anastomoses to the ophthalmic artery, anterior choroidal artery (AchA), and external carotid artery branches, knowledge of ACA anatomy is crucial for surgeons and angiographers in diagnosing and treating pathological lesions while preventing complications. This review highlights the main anatomical variants of the ACA and several pathological entities involved with such variations.
SEGMENTS/COURSE
There are two main classification schemes for the segments of the ACA. The first, described by Fischer in 1938, delineates five segments: (1) Precommunicating (A1); (2) below the genu of the corpus callosum (A2); (3) around the genu of the corpus callosum (A3); (4) the terminal branch of the A4; and (5) the terminal branch of the A5 [1]. Rhoton [2] supported this classification system with a few modifications, including dividing the ACA into a proximal (precommunicating) and distal (postcommunicating) segment, as well as further distinguishing the post-communicating ACA into infracallosal (A2), precallosal (A3), supracallosal (A4), and posterocallosal (A5) segments. The supracallosal segments transition into posterocallosal segments where the ACA crosses the coronal suture. Osborn [3] acknowledges several classification schemes for the ACA segments, but utilizes the second, simpler classification scheme with three segments: (1) the horizontal/precommunicating artery (A1) which runs over the ipsilateral optic nerve and chiasm; (2) the vertical/postcommunicating artery (A2) which enters into the interhemispheric fissure anterior to the lamina terminalis; and (3) the distal ACA and cortical branches (A3). The ACA terminates in the choroid plexus in the roof of the third ventricle after passing around the splenium of the corpus callosum [4].
VASCULAR TERRITORY
Cadaveric examination shows that the area perfused by the ACA has the greatest variability compared to the middle cerebral and posterior cerebral arteries [5]. The vascular territory of the ACA includes vessels to the anterior perforating substance via the medial striate arteries and recurrent arteries (caudate, septal nuclei), and provides blood to the rostrum, genu and body of the corpus callosum, as well as the anterior two thirds of the cingulate gyrus, internal frontal, paracentral, and parietal gyri. Through the recurrent artery of Heubner, anterior portions of the pallidum and caudate nuclei, as well as the anteroinferior portion of the internal capsule, rely on blood supply from the ACA [6]. The perforators of the anterior communicating artery (A-comm) also supply blood to portions of the optic chiasm, fornix, hypothalamus, and hypophysis. The extent of supply from the ACA to the medial cerebral hemispheres varies with shared supply from the posterior cerebral artery. A choroidal supply to the tela choroidea of the third ventricle is observed only in aneurysmal malformations due to arrested development of vein of Galen. In these cases, the ACA and AchA share a vascular supply to the limbic structures known as the limbic arterial arch [1]. A meningeal branch to the anterior portion of the falx may arise from the ACA at the precallosal segment [1].
EMBRYOLOGY
Through evolution from fish to mammals, the developing telencephalon is supplied by the internal carotid artery (ICA), which comprises a rostral and caudal trunk [1]. The rostral trunk, from which the ACA eventually develops, has both medial and lateral divisions that are referred to as the medial and lateral olfactory arteries, respectively. The medial olfactory artery eventually becomes the ACA with branches of the lateral olfactory artery gradually giving rise to the middle cerebral artery, the recurrent artery of Heubner, the AchA, and lateral striate. The choroidal blood supply connects with branches from the caudal trunk of the ICA, which later becomes the posterior circulatory system. Variations during these evolutionary developments set the stage for several anatomical variants involving the ACA.
The embryology of the ophthalmic artery is also closely related to the development of the ACA. Lasjaunias described the embryology of the ophthalmic artery as a joining of two separate arteries during development. A ventral ophthalmic artery (VOA), which arises from the ACA, joins with the dorsal ophthalmic artery (DOA) arising from the cavernous segment of the ICA, forming the proper ophthalmic artery which passes through the optic canal to supply the orbit. This anastomosis, termed the primitive ophthalmic artery, becomes definitive when the DOA and VOA regress during development [1]. The olfactory artery, if persistent in adult mammals, is a remnant of the medial olfactory artery in lower order animals. Its usual course is along the olfactory tracts, eventually turning back on itself at the olfactory bulb to join the ACA proper distally [7]. The “origin” of the olfactory artery lies in the location of the A1-A2 junction.
The embryology of the A-comm can be traced back to reptiles where there is a midline fusion of the paired medial olfactory arteries, which is a branch of the rostral trunk of the ICA. If only one medial olfactory artery exists, as is seen in an azygous configuration of the ACA, no true A-comm is present due to the absence of this fusion.
VARIANT ANATOMY/PATHOLOGY
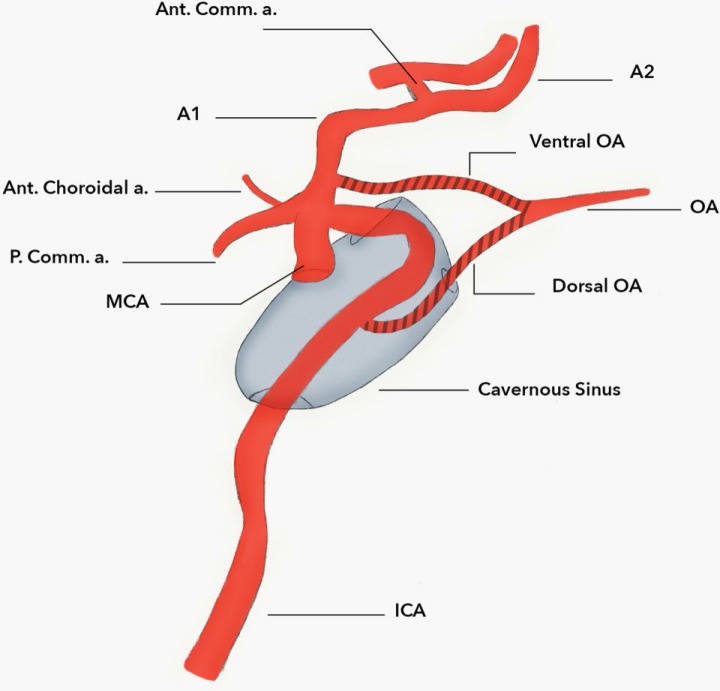
An aberrant ACA originating from the proximal intradural ICA with close relationships to the development of the ophthalmic artery has been described [1]. In 1976, Nutik and Dilenge [8] highlighted this anomaly. A patient presented with subarachnoid hemorrhage and an MRI revealed a large left-sided cavernous ICA vessel coursing medially and dorsally that anastomosed with the contralateral ACA [8]. There was an associated saccular aneurysm on this vessel where a normal A-comm would have been [8]. The course of the vessel described by Nutik is likely the DOA. Lasjaunias et al.[1] described how several anatomical variants may arise from the development of the ophthalmic artery with the ACA. A VOA which arises from the ACA forms an anastomosis with the DOA from the cavernous ICA in the optic canal to supply the orbit. This anastomosis, termed the primitive ophthalmic artery, becomes definitive when the DOA and VOA regress during development as mentioned earlier [1] (Figure 1).
Figure 1. An artist’s depiction of VOA and DOA variants, shown giving rise to a definitive OA.
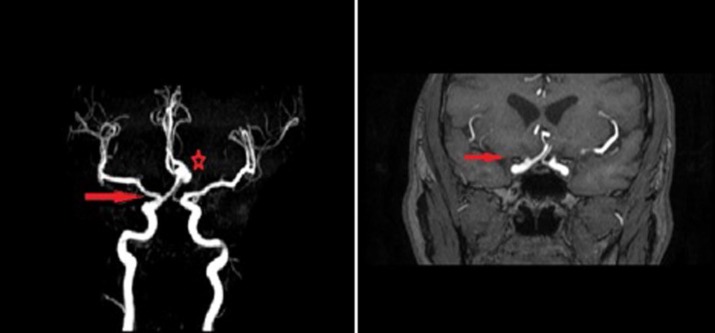
Any failure of regression may lead to either the ACA arising from the DOA or, in other words, from the ICA which provides the so-called infraoptic ACA (Figure 2), or the definitive ophthalmic artery arising from the ACA (also termed the VOA) variant (Figure 3).
Figure 2. MRA demonstrating a right-sided DOA variant where the right-sided ACA arises from the cavernous segment of the ICA (red arrow) (left). There is also a large anterior communicating artery aneurysm present (red star). MRA demonstrating the right ACA coursing below the optic tract (red arrow) on the right side representing the dorsal ophthalmic arterial variant configuration (right).
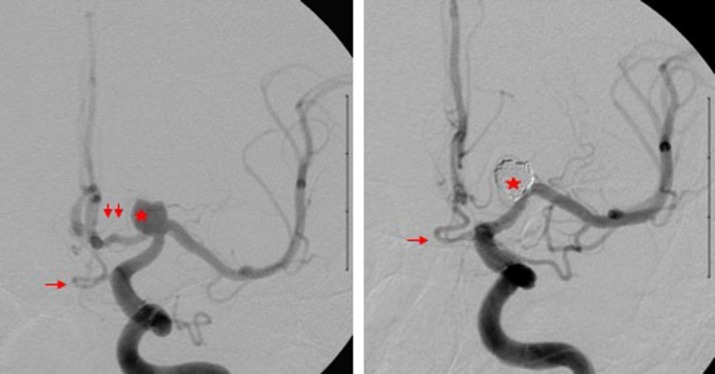
Figure 3. Digital subtraction angiography AP view before (left) and after (right) stent-assisted coiling of carotid terminus aneurysm (red star) demonstrating the dual supply to the A2 segment of the left A2 ACA via the A1 segment (double red arrow) and persistent VOA variant (red arrow). After coiling (right), the A2 segment is only supplied by the infraoptic persistent VOA as the A1 segment is occluded by the coils at the neck of the aneurysm.
Incomplete regression may also lead to infundibular or full aneurysm formation as noted at both DOA and VOA sites [9, 10]. Occasionally, both origins of the ophthalmic artery can persist and serve as a collateral pathway.
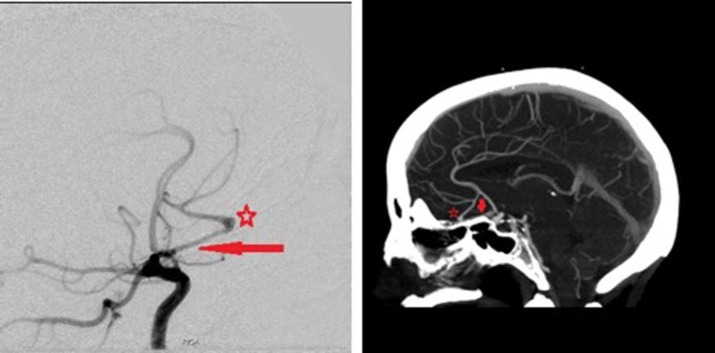
The persistent primitive olfactory artery (PPOA) is another precommunicating anatomical variant. In 2012, Horie et al. [11] described the embryological origin of the ACA and its association with the PPOA, explaining how the terminal ICA during development is termed the primitive olfactory artery and from this parent vessel the ACA arises and courses between the developing hemispheres. If the primitive olfactory artery fails to regress, it becomes the PPOA which may arise from either the ICA or ACA to supply distal ACA territory or terminate in the nasal cavity as the ethmoidal artery. The reported incidence of a PPOA is 0.14% in a published case series [12]. If the PPOA remains intradural, it typically runs along the olfactory tract toward the crista galli and forms a hairpin turn posterior to the olfactory bulb. Distally, this vessel may supply portions of the ACA territory or may enter the nasal cavity as an ethmoidal artery [13]. The PPOA is often associated with aneurysm formation and has also been implicated in anosmia due to aberrations in the blood supply to the olfactory tract [14] (Figure 4).
Figure 4. Digital subtraction angiography demonstrating a right-sided PPOA (red arrow) with an associated aneurysm (red star) (left). The distal PPOA supplies the frontal lobe. Computed tomography angiography demonstrating a PPOA (red arrow) with an associated aneurysm (right). The vessel runs along the olfactory tract of the gyrus rectus and connects with the distal ACA.
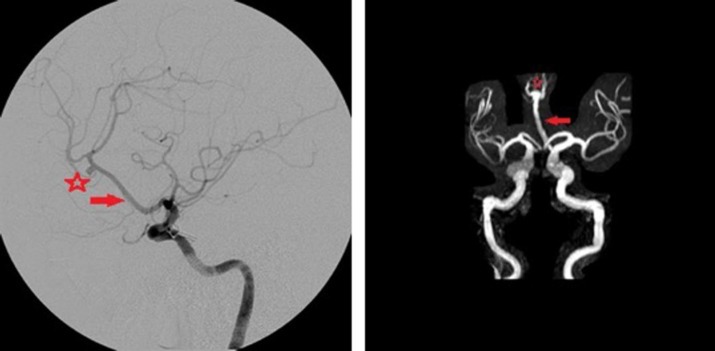
The azygous ACA variant demonstrates a single midline vessel originating from the joining of bilateral A1 segments near the vicinity of the normal A-comm. In most instances, a true A-comm is hypoplastic or absent [15]. The midline vessel lying in the inter-hemispheric fissure serves as the common blood supply to the medial hemispheres. Lasjaunias et al. [1] report an azygous ACA incidence of 0.3%. A true azygous ACA does not branch along its course over the corpus callosum, but a “fused” ACA trunk which has been differentiated from the azygous ACA anatomy does divide into separate distal ACA vessels at or distal to the genu of the corpus callosum [1]. At this dividing point, saccular aneurysms are common, ranging from 13% to 71% in the reported literature [16] (Figure 5).
Figure 5. Digital subtraction angiography demonstrating an azygous variant of the ACA (red arrow) with a single midline ACA vessel distal to the anterior communicating artery (left). An aneurysm (red star) is arising proximal to the origin of the pericallosal artery. MRA demonstrating the Azygous ACA variant (red arrow) (right). An aneurysm is seen at the origin of the pericallosal arteries (red star).
Other clinically significant pathologies include reports of azygous ACA occlusion leading to bi-hemispheric and corpus callosum infarcts [8]. The azygous ACA anatomy has often been associated with other midline facial and brain abnormalities including holoprosencephaly, dysgenesis of the corpus callosum, and porencephalic cysts [12]. A relatively similar anatomic variant to the azygous ACA is the bihemispheric ACA, in which the ACA is supplied from a single A1 vessel and the contralateral A1 is hypoplastic and primarily supplies orbitofrontal and frontopolar branches. In this variant, a unilateral ACA supplies the medial portions of both hemispheres distal to the A2 segment. If bilateral ACA vessels are present distal to the A2 segment, one vessel supplies only the callosomarginal territory unilaterally whereas the bihemispheric ACA supplies both pericallosal territories along with its unilateral callosomarginal territory [1].
As described previously, the azygous ACA variant has been reported in association with bi-hemispheric infarcts, and in 2005, Burbank and Morris [17] described the rare origin of a left ACA from the supraclinoid right ICA. This origin had only been described once previously in the literature and was discovered in the setting of ischemia of the left hemisphere. The origin of the left ACA was adjacent to but separate from the origin of the right ophthalmic artery. In this rare case, occlusion of the right ICA would lead to bi-hemispheric infarcts in the ACA territories.
Closely associated with the azygous ACA is another variant where three ACA vessels exist distal to the A-comm called a triplicated ACA. As Lemay and Gooding described in 1966, during fetal life, one to three ACA vessels exist with variations in adult life persisting as either involution of two of the three vessels or failure of involution of any of the three vessels [14]. In 2006, Uchino et al. [7] reported 27 instances of triplicated ACA morphology detected on magnetic resonance angiography (MRA) with associated pathologies including an A-comm aneurysm and two unilateral A1 aplasias. In this variant, the third vessel may supply a variable amount of normal ACA territory including the chiasmatic/paraolfactory area, the corpus callosum as far distally as the splenium, or the medial cortex [1].
The triplicated ACA must also be differentiated from the median artery of the corpus callosum (MACC), which is a midline vessel originating from or near the A-comm that supplies varying amounts of blood to the bilateral corpus callosum. When present, if its course ends at the rostrum it is often named the subcallosal artery, whereas a more extensive supply to the corpus callosum including the genu and portions of the body is properly named the MACC [18]. Lasjaunias et al. [1] report this vessel as a normal variant representing a fused pericallosal artery rather than persistence of an embryological entity. The reported incidence is as high as 13% during microsurgical examination [19]. Occlusion of this vessel may result in bilateral corpus callosum infarcts at or near the level of the genu.
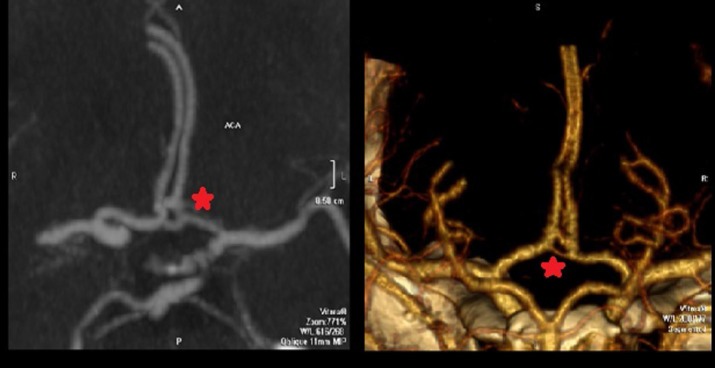
The A-comm artery is often referred to as a “complex” due to high variability in the number of bridging vessels between right and left ACAs. One to three A-comm vessels may be present at different locations with a “supreme A-comm” cited as distally as the genu of the corpus callosum [20, 21] (Figure 6).
Figure 6. CT angiography (CTA) demonstrating the presence of two anterior communicating arteries (A-comm) (red star) (left). CTA 3-D reconstruction demonstrating two separate A-comm vessels (red star) (right). In both images, asymmetry of the A-comm vessels is observed which is common when multiple (vessels) are present.
In fact, a single A-comm may only be present in 60% of cases with three bridging vessels present in as often as 10% of specimens [20]. Fenestrations in the A-comm or at the A1-A2 junction are also common with a higher association with aneurysm formation in these patients [22]. Asymmetry of an A1 was also associated with a higher incidence of ipsilateral aneurysm formation at the A-comm [23].
CONCLUSION
The ACA and its variations carry a rich blood supply to structurally eloquent areas, including the frontal lobe, orbit, corpus callosum, and basal ganglia, making it a clinically significant vascular structure. Pathologies involving the ACA and its branches, particularly in the setting of anatomic variations, are extensive and clinicians must be aware of these variations when treating aneurysms, embolizing tumors, or performing complex surgical procedures including corpus callosotomies or intraventricular lesions.
Acknowledgements
None.
REFERENCES
- Lasjaunias P, et al. Surgical neuro-angiography. Vol. 1. Berlin: Springer; 2006. [Google Scholar]
- Rhoton AL. Cranial anatomy and surgical approaches. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- Osborn AG. Diagnostic cerebral angiography. Philadelphia, PA: Lippincott Williams & Wilkins; 1999. [Google Scholar]
- Gibo H, et al. Carter LP, et al. Neurovascular surgery. USA: McGraw Hill; 1994. Cranial vascular anatomy of the anterior circulation ; pp. 11–22. [Google Scholar]
- van der Zwan A, et al. Variability of the territories of the major cerebral arteries. J Neurosurg. 1992;77(6):927–940. doi: 10.3171/jns.1992.77.6.0927. [DOI] [PubMed] [Google Scholar]
- Tatu L, et al. Arterial territories of the human brain cerebral hemispheres. Neurology. 1998;50(6):1699–1708. doi: 10.1212/WNL.50.6.1699. [DOI] [PubMed] [Google Scholar]
- Uchino A, et al. Anterior cerebral artery variations detected by MR angiography. Neuroradiology. 2006;48(9):647–652. doi: 10.1007/s00234-006-0110-3. [DOI] [PubMed] [Google Scholar]
- Nutik S, Dilenge D. Carotid-anterior cerebral artery anastomosis: case report. J Neurosurg. 1976;44(3):378–382. doi: 10.3171/jns.1976.44.3.0378. [DOI] [PubMed] [Google Scholar]
- Baltsavias G, et al. Persistent ventral ophthalmic artery associated with supraclinoid internal carotid artery aneurysm: case report and review of the literature. J Neuroradiol. 2012;39(3):186–189. doi: 10.1016/j.neurad.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Tanaka M. Persistent primitive dorsal ophthalmic artery associated with paraclinoid internal carotid artery aneurysm. J Neuroendovasc Ther. 2009;3:41. doi: 10.5797/jnet.3.39. [DOI] [Google Scholar]
- Horie N, et al. New variant of persistent primitive olfactory artery associated with a ruptured aneurysm: Case report. J Neurosurg. 2012;117(1):26–28. doi: 10.3171/2012.3.JNS111932. [DOI] [PubMed] [Google Scholar]
- Uchino A, et al. Persistent primitive olfactory artery: MR angiographic diagnosis. Surg Radiologic Anat. 2011;33(3):197–201. doi: 10.1007/s00276-010-0743-0. [DOI] [PubMed] [Google Scholar]
- Nozaki K, et al. Cerebral aneurysm associated with persistent primitive olfactory artery aneurysm. Acta Neurochir (Wien) 1998;140(4):397–402. doi: 10.1007/s007010050114. [DOI] [PubMed] [Google Scholar]
- Kim MS, Lee GJ. Diagnosis of persistent primitive olfactory artery using computed tomography angiography. J Korean Neurosurg Soc. 2011;49(5):290–291. doi: 10.3340/jkns.2011.49.5.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemay M, Gooding CA. The clinical significance of the azygos anterior cerebral artery (ACA) Am J Roentgenol. 1966;98(3):602–610. doi: 10.2214/ajr.98.3.602. [DOI] [PubMed] [Google Scholar]
- Huh JS, et al. Saccular aneurysm of the azygos anterior cerebral artery: three case reports. J Korean Neurosurg Soc. 2007;42(4):342–345. doi: 10.3340/jkns.2007.42.4.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbank NS, Morris PP. Unique anomalous origin of the left anterior cerebral artery. Am J Neuroradiol. 2005;26(10):2533–2535. [PMC free article] [PubMed] [Google Scholar]
- Bradac GB. Applied cerebral angiography. Berlin: Springer; 2017. [Google Scholar]
- Ogawa A, et al. Vascular anomalies associated with aneurysms of the anterior communicating artery: microsurgical observations. J Neurosurg. 1990;72(5):706–709. doi: 10.3171/jns.1990.72.5.0706. [DOI] [PubMed] [Google Scholar]
- Perlmutter D, Rhoton AL., Jr Microsurgical anatomy of the distal anterior cerebral artery. J Neurosurg. 1978;49(2):204–228. doi: 10.3171/jns.1978.49.2.0204. [DOI] [PubMed] [Google Scholar]
- Laitinen L, Snellman A. Aneurysms of the pericallosal artery: a study of 14 cases verified angiographically and treated mainly by direct surgical attack. J Neurosurg. 1960;17:447–458. doi: 10.3171/jns.1960.17.3.0447. [DOI] [PubMed] [Google Scholar]
- Kwak R, et al. Anterior communicating artery aneurysms with associated anomalies. J Neurosurg. 1980;52(2):162–164. doi: 10.3171/jns.1980.52.2.0162. [DOI] [PubMed] [Google Scholar]
- Kayembe KN, et al. Cerebral aneurysms and variations in the circle of Willis. Stroke. 1984;15(5):846–850. doi: 10.1161/01.STR.15.5.846. [DOI] [PubMed] [Google Scholar]