Abstract
Objective
Tumor cells rely heavily on glycolysis regardless of oxygen tension, a phenomenon called the Warburg effect. Hexokinase II (HKII) catalyzes the first irreversible step of glycolysis and is often overexpressed in tumor cells. Mitochondrial HKII couples glycolysis and oxidative phosphorylation while maintaining mitochondrial membrane integrity. In this study, we investigated the role of HKII in promoting the Warburg effect in cancer cells.
Methods
HKII-mediated phosphorylation of the alpha subunit of pyruvate dehydrogenase (PDHA1) was tested in HEK293T cells and clear cell renal cell carcinoma (ccRCC) specimens using gene knockdown, western blotting, immunohistochemistry, and immunofluorescence.
Results
It was determined that HKII could not only transform glucose into glucose-6-phosphate, but also transfer the phosphate group of ATP onto PDHA1. In addition, it was found that HKII increased the phosphorylation of Ser293 on PDHA1, decreasing pyruvate dehydrogenase (PDH) complex activity and thus rerouting the metabolic pathway and promoting the Warburg effect. The overexpression of HKII correlated with the phosphorylation of PDHA1 and disease progression in ccRCC.
Conclusions
The data presented here suggest that HKII is an important biomarker in the evaluation and treatment of cancer.
Keywords: Hexokinase II, PDHA1, phosphorylation, Warburg effect
Introduction
Nearly all tumors share one significant phenomenon, the Warburg effect. The Warburg effect refers to tumor cells utilizing the anaerobic glycolysis pathway for energy production even when there is ample oxygen (1,2). The function of hexokinase (HK) is to catalyze the first irreversible step of glycolysis during which glucose is phosphorylated to glucose-6-phosphate (3-5). Although there are five HK isoforms (6,7), which are differentially expressed in various tissues, HKII (EC: 2.7.1.1) is the only isoform documented to be upregulated in tumors (8-11). HKII acts as a sensor for N-acetylglucosamine, and dissociation of HKII from voltage-dependent anion channel (VDAC) induces interleukin (IL)-1β and IL-18 production in an NLRP3-dependent manner (12). Phosphorylation of Thr473 of HKII by protein kinase B (AKT) increases HKII mitochondrial localization (13,14). Mito-HKII couples glycolysis and oxidative phosphorylation and maintains mitochondrial membrane integrity (11). Mito-HKII may prevent Bax-mediated apoptosis in proximal tubule cells (15). In addition, HKII is negatively regulated by Sirtuin-6 (SIRT6) and prevents autophagy-driven monocyte differentiation (16). HKII binds to and suppresses mTOR and positively regulates autophagy (17). HKII phosphorylates histone H2A in the absence of glucose (18). Therefore, it is possible that HKII might phosphorylate other proteins.
Pyruvate dehydrogenase (PDH) complex (PDC) links glycolysis and the tricarboxylic acid (TCA) cycle (19-21). PDC contains PDH (E1), dihydrolipoamide acetyltransferase (E2), and lipoamide dehydrogenase (E3) (22). The E1 enzyme is a heterotetramer of two alpha and two beta subunits. PDHA1 is the alpha subunit of PDH, which is phosphorylated by PDHK1, PDHK2, PDHK3, and PDHK4 and dephosphorylated by PDP1 and PDP2 (23-29). The phosphorylation of Ser293, Ser300, and Ser232 (23-29), the acetylation of Lys321 (30), and the desuccinylation of Lys83 and Lys244 on PDHA1 (31) decrease PDC activity and eventually promote tumor growth, confirming that PDC is an important regulation node for metabolism and signal transduction.
It was reported that von Hippel-Lindau (VHL) mutants in clear cell renal cell carcinoma (ccRCC) lead to activation of hypoxia-inducible factor (HIF)1α, increased glycolysis, and increased expression of HKII, GLUT1/3, pyruvate kinase 2 (PKM2), PDHK1, and lactate dehydrogenase A (LDHA) (32-34). Therefore, the relationship among downstream regulators of HIF1α pathway is elusive.
In this study, we investigated the role of HKII in promoting the Warburg effect in cancer cells. Our results suggest that HKII may be a significant biomarker in the evaluation and treatment of cancer.
Materials and methods
Cell culture, materials and antibodies
HEK293T cells (ATCC, CRL-11268) were cultured in Dulbecco’s modified eagle medium (DMEM) (Gibco, 12430-054) containing 10% fetal bovine serum (FBS) (Gibco, Thermo Fisher Scientific, MA, USA, 10099141) and HeLa cells (ATCC®, CCL-2TM) were cultured in minimum essential medium (MEM) (Gibco, 11095-080) containing 1% NEAA (Gibco, 11140-050) and 10% FBS (Gibco, 10099141). Cells were transfected with plasmids using Lipofectamine 2000 Transfection Reagent (Thermo Fisher Scientific, MA, USA, Cat. No. 12566014). Wild type HKII, HKII mutant, wild type PDHA1, PDHA1 S293A, and PDHA1 S293E were cloned into pcDNA3.1-Flag, pcDNA3.1-Myc, and pcDNA3.1-HA vectors, respectively. A HKII-specific siRNA (5’-TCGCATCTGCTTGCCTACTTCTTCA-3’) was used to knock down HKII gene, and a non-silencing siRNA oligonucleotide was used as a negative control. 3-Bromopyruvate (3BP, 16490-10G) and 2-deoxy-D-glucose (2-DG, D8375-10 mg) were purchased from Sigma-Aldrich, DAPI (D1306) from Invitrogen, anti-HKII antibody (Arg66209) from Arigobio, anti-PDHA1 (ab67592) and anti-phosphorylated PDHA1 (phosphor S293) (ab92696) antibodies from Abcam, and PDH enzyme microplate assay kit (ab109902) from Abcam.
Measurement of HKII activity
Recombinant Flag-HKII, His-HKII, and its mutants were affinity-purified from HEK293T cells overexpressing pcDNA3.1-Flag-HKII and from Escherichia coli BL21(DE3)plysS overexpressing pET22b-His-HKII, respectively. To assay the activity of purified HKII, 2 μg of purified protein was diluted in 20 μL of HK dilution buffer containing 20 mmol/L KH2PO4, 100 mmol/L KCl, 1 mmol/L MgCl2, 1 mmol/L ethylene diamine tetraacetic acid (EDTA), 1 mmol/L dithiothreitol (DTT), 60 g/L glycerol, and 1 g/L bovine serum albumin. Samples were loaded onto a microplate and mixed with 100 μL of reaction buffer containing 50 mmol/L HEPES pH 7.4, 100 mmol/L KCl, 8 mmol/L MgCl2, 5 mmol/L ATP, 0.5 mmol/L nicotinamide adenine dinucleotide phosphate (NADP), 1 U/mL glucose-6-phosphate dehydrogenase (G6PDH) (from Leuconostoc mesenteroides), 1 mmol/L DTT, 1 g/L bovine serum albumin, and 10 mmol/L D-(+)-glucose. Reactions were incubated at 37 °C for 1 h and stopped with 174 μL buffer C (0.46 mmol/L SDS, 300 mmol/L NaH2PO4, pH 8.0). Fluorescence of NADPH was measured at the excitation wavelength of 340 nm and the emission wavelength of 450 nm. Specific activity was determined using a NADPH standard.
Western blotting analysis
Standard procedures were followed for western blotting analysis. After cell harvest, the protein concentration was determined using Quantity One software (Bio-Rad, Hercules, CA, USA). Cell lysates were separated by 10 % SDS-PAGE, transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA, USA), and blocked with 5% (w/v) skim milk for 2 h at room temperature (RT). The membranes were incubated overnight at 4 °C with antibodies against PDHA1, S293-phosphorylated PDHA1, HKII, PDHK1, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), actin, and TOM40. The immune complexes were detected using a horseradish peroxidase-conjugated secondary antibody (1:3,000, Southern-Biotech) for 2 h at RT. Finally, the complexes were visualized using an enhanced chemiluminescence system (ECL; Pierce Company Woburn, MA, USA). Western blot signals were obtained by detecting chemiluminescence on a Typhoon FLA 9500 (GE Healthcare, Wisconsin, USA). ImageJ was used to analyze the signal intensities. Each blot shown in the figures is representative of at least three experiments.
Immunofluorescence analysis
Standard procedures were followed for immunofluorescence analysis. Cells seeded in 24-well plates were fixed with 4% paraformaldehyde followed by permeabilization with 1% triton. Cells were then incubated overnight at 4 °C with anti-PDHA1, anti-S293-phosphorylated PDHA1, and anti-HKII antibodies, and on the following day were detected with Alexa Fluor 555 goat anti-mouse IgG antibody. 4’,6-diamidino-2-phenylindole (DAPI) was used to stain the nuclei. Immunofluorescence images were observed on a fluorescence microscope (Leica, DMI4000B).
Measurement of PDH complex activity
The activity of PDH complex was measured using a PDH enzyme microplate assay kit (ab109902). This kit recognizes PDH in human, rat, mouse, and bovine cells, tissue extracts, and isolated mitochondria. Briefly, HKII was overexpressed or knocked down in HEK293T cells. Samples containing PDH complex were then prepared, loaded onto 96-well plates coated with anti-PDH monoclonal antibody, and incubated for 3 h at RT. A volume of 200 μL of assay solution was added to each well, optical density (OD450 nm) was measured in kinetic mode at RT for 30 min, and finally the relative activity of the PDH complex was calculated.
Measurement of growth curves
PDHA1 knock-down cells were transfected with HKII, wild type PDHA1, PDHA1 S293A, and PDHA1 S293E, respectively, and seeded into 96-well plates. Cell morphology was observed under an inverted microscope and the plates equilibrated to RT for approximately 30 min. The clear bottom of the plates was covered with white back seal and luminescence recorded with EnSpire (PerkinElmer).
Human samples and mutation screening
Human samples were obtained from Ruijing Hospital North, Shanghai Jiaotong University. Informed consents from the patients were obtained. The procedures related to human subjects were approved by Ethic Committees of Ruijing Hospital North, Shanghai Jiaotong University School of Medicine. The methods were carried out in accordance with the approved guidelines.
Immunohistochemistry (IHC)
Tissue sections were prepared from formalin-fixed paraffin embedded specimens. Antigen retrieval of renal cell carcinoma or breast cancer specimens was performed by incubating the slides in Tris-EDTA buffer (pH 8.4) at 99 °C for 60 min. Endogenous peroxidase activity was inactivated in methanol with 3% H2O2. Slides were incubated with primary antibody for 60 min, secondary antibody for 8 min, and DAB Chromagen staining for 8 min. All procedures were performed using stainer (BenchMark XT, Ventana) and the slides were scanned with a scanner (Ventana iScan Coreo). Quantification of IHC results was performed by an experienced pathologist. Signal intensity was calculated according to the number of positive areas and degree. Sections were stained with HKII (1:100), P-S293-PDHA1 (1:30), and Ki67 (1:100) antibodies using an Ultraview Detection Kit (Roche Diagnostics).
Statistical analysis
Significant differences between groups were determined using Student’s t-test. The significance level for statistical testing was set at P<0.05. IBM SPSS Statistics (Version 19.0; IBM Corp., New York, USA) was used for statistical analysis.
Results
HKII phosphorylates S293 of PDHA1
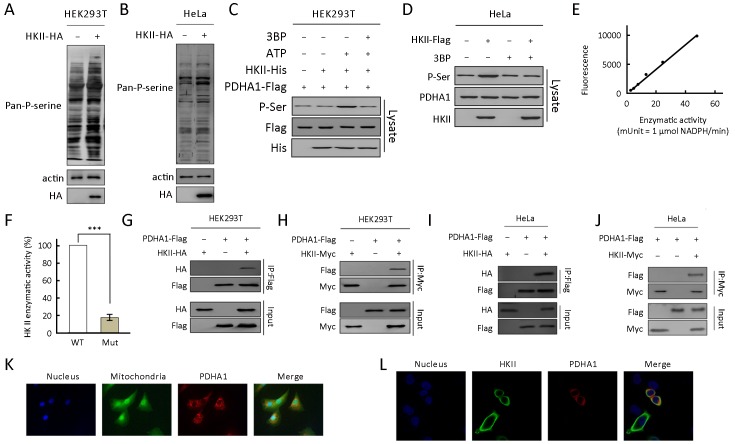
To identify the substrates of HKII, we used a quantitative phosphoproteomic approach (35,36). Our results show that HKII increased the overall serine phosphorylation levels of proteins in HEK293T and HeLa cell lysates (Figure 1A,B ). In addition, wild type HKII, but not its catalytically inactive mutant HKIImut (37), increased pan-phosphorylation of PDHA1 (Figure 1C,E,F ), and 3BP, an HKII inhibitor, decreased the pan-phosphorylation of PDHA1 (Figure 1D ).
1.
Hexokinase (HK) II interacts with and phosphorylates the alpha subunit of pyruvate dehydrogenase (PDHA1) in an ATP-dependent manner. (A) HA-tagged HKII was transfected into HEK293T cells and pan-phosphorylation level of serine (Pan-P-serine) was tested in HEK293T cell line; (B) HA-tagged HKII was transfected into HeLa cells and Pan-P-serine was tested in HeLa cell line; (C) Purified PDHA1 was treated with recombinant HKII in the presence and absence of ATP, and the levels of P-Ser of PDHA1 in the reaction mixture after treatment were determined; (D) Flag-tagged HKII was expressed in HeLa cells. Pan-P-serine levels of PDHA1 from cells cultured with and without 3BP (5 mmol/L) supplementation were determined; (E) Standard curve for HKII specific activity versus fluorescence (n=3); (F) Enzymatic activity of purified wild type HKII (WT) and HKIImut (Mut) were measured; (G) Flag-tagged PDHA1 and HA-tagged HKII were co-expressed in HEK293T cells. HKII co-purified with PDHA1 was detected by HA antibody; (H) Flag-tagged PDHA1 and Myc-tagged HKII were co-expressed in HEK293T cells. PDHA1 co-purified with HKII was detected by Flag antibody; (I) Flag-tagged PDHA1 and HA-tagged HKII were co-expressed in HeLa cells. HKII co-purified with PDHA1 was detected by HA antibody; (J) Flag-tagged PDHA1 and Myc-tagged HA were co-expressed in HeLa cells. PDHA1 co-purified with HKII was detected by Flag antibody; (K) PDHA1 was expressed in HeLa cells. PDHA1, mitochondria, and nucleus were marked with red, green, and blue fluorescence, respectively; (L) HKII and PDHA1 were co-expressed in HeLa cells. HKII, PDHA1, and nucleus were marked with green, red, and blue fluorescence, respectively. ***, P<0.001.
The phosphorylation levels of S293 of PDHA1 increased 100-fold after HKII treatment, suggesting that the PDH complex can be regulated by HKII. This hypothesis was confirmed through the co-expression of HKII and PDHA1 in HEK293T cells. HKII was co-purified with PDHA1 (Figure 1G ) and PDHA1 was co-purified with HKII (Figure 1H ), revealing an interaction between these proteins. In addition, HKII interacted with PDHA1 in HeLa cells (Figure 1I,J ). Moreover, incubation of purified PDHA1 with recombinant HKII resulted in an elevation of P-Ser levels of PDHA1 in an ATP-dependent manner (Figure 1A ), confirming that HKII phosphorylates PDHA1 directly. It was also found PDHA1 and HKII co-localized in the mitochondria (Figure 1K,L ), further supporting the phosphorylation of PDHA1 by HKII.
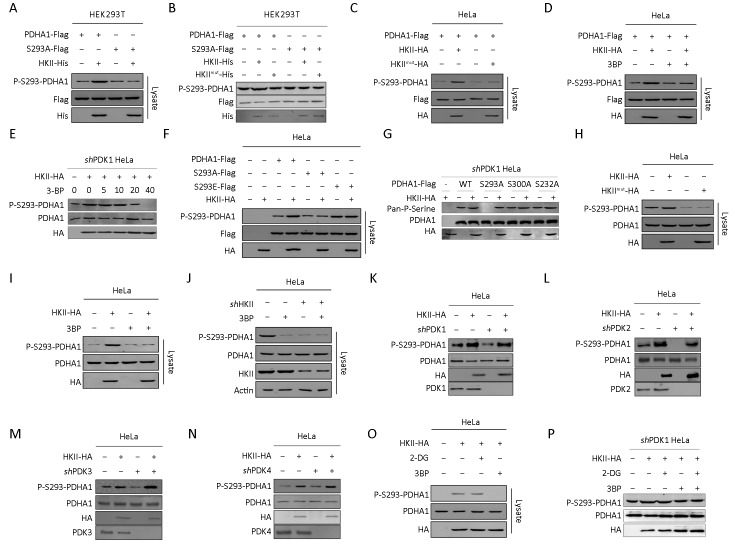
The P-S293 levels of purified PDHA1 increased after HKII, but not HKII mutant, treatment (Figure 2A−C ), confirming that S293 of PDHA1 may be the substrate of HKII. It was also found that 3BP decreased the phosphorylation levels of S293 on PDHA1 in a dose-dependent manner (Figure 2D,E ). Interestingly, HKII increased the phosphorylation of PDHA1 but not of its non-phosphorylation mimetic S293A and phosphorylation mimetic S293E mutants, indicating that S293 may be the substrate of HKII (Figure 2F,G ). To determine whether HKII phosphorylated endogenous PDHA1, the levels of P-S293 on endogenous PDHA1 were measured under HKII overexpression and HKII knockdown. Overexpression of wild type, but not mutant, HKII in HeLa cells increased the levels of endogenous P-S293 (Figure 2H ), and this event was inhibited by 3BP (Figure 2I ). Conversely, the reduction of HKII expression with short hairpin RNA (shRNA) decreased the levels of P-S293 on endogenous PDHA1 (Figure 2J ). It has been reported that S293, S300, and S232 of PDHA1 are phosphorylated by PDK1, PDK2, PDK3, and PDK4 (23-29). To exclude the effects of PDK1−4 on the phosphorylation levels of PDHA1, we knocked down PDK1−4. Our results show that HKII increased the phosphorylation levels of S293 of PDHA1 (Figure 2K−N ), whereas 3BP decreased the phosphorylation levels of S293 on PDHA1 in a dose-dependent manner in PDK1 knockdown cells (Figure 2E ). Interestingly, the glucose analogue 2-DG did not prevent HKII-mediated phosphorylation of PDHA1 in HeLa cells and in PDK1 knockdown HeLa cells (Figure 2O,P ). These results collectively support that HKII phosphorylates S293 of PDHA1.
2.
Hexokinase (HK) II phosphorylates Ser293 of the alpha subunit of pyruvate dehydrogenase (PDHA1). (A) Purified HKII was incubated with either wild type PDHA1 or PDHA1 mutant S293A. After incubation, PHDA1 was purified by Flag beads. Phosphorylation level of serine 293 of PDHA1 (P-S293-PDHA1) were determined and quantified; (B) Purified wide type PDHA1 and S293A mutant were treated with recombinant wide type HKII or its catalytic dead mutant HKIImut, and the levels of P-Ser of PDHA1 in the reaction mixture after treatment were determined; (C) Flag-tagged PDHA1 was co-expressed with either HA-tagged HKII or HA-tagged HKII mutant in HeLa cells. P-Ser levels of Flag bead-purified PDHA1 from each culture were determined and quantified; (D) Flag-tagged PDHA1 was co-expressed with HA-tagged HKII in HeLa cells. P-Ser levels of Flag bead-purified PDHA1 from each cell cultured with and without 3-bromopyruvate (3BP, 5 mmol/L) supplementation were determined and quantified; (E) PDK1 was knocked down in HeLa cells and HA-tagged HKII was transfected into PDK1 knockdown cells. Then the cells were treated with serial diluted 3BP and P-S293-PDHA1 was tested; (F) HA-tagged HKII was co-expressed with either Flag-tagged PDHA1, Flag-tagged PDHA1 mutants S293A or S293E in HeLa cells. The P-Ser levels of Flag bead-purified PDHA1 and mutants from each culture were determined and quantified; (G) PDK1 was knocked down in HeLa cells and HKII and PDHA1, S293A, S300A and S232A mutants were transfected into knockdown cells, then Pan-P-Serine was tested; (H) HA-tagged WT HKII or HA-tagged HKII mutant was overexpressed in HeLa cells. P-S293 levels of endogenous PDHA1 of each culture were determined; (I) HA-tagged HKII was overexpressed in HeLa cells. P-S293 levels of endogenous PDHA1 of each culture with and without 3BP (5 mmol/L) supplementation were determined; (J) P-S293 levels of endogenous PDHA1 in HeLa cells before and after HKII knockdown by independent shRNAs were compared; PDK1 (K); PDK2 (L); PDK3 (M) and PDK4 (N) were knocked down, respectively in HeLa cells and HKII was transfected into the cells, then P-S293-PHDA1 was tested.; (O) HA-tagged HKII overexpressed HeLa cells were treated with or without 2-DG (10 mmol/L) or 3BP (5 mmol/L) and P-S293 levels of endogenous PDHA1 were determined; (P) PDK1 was knocked down in HeLa cells and HA-tagged HKII was overexpressed in the cells. The endogenous P-S293 levels of PDHA1 of each culture with and without 3BP (5 mmol/L) or 2-DG (10 mmol/L) supplementation were determined.
HKII phosphorylates PDHA1 and regulates metabolic pathways
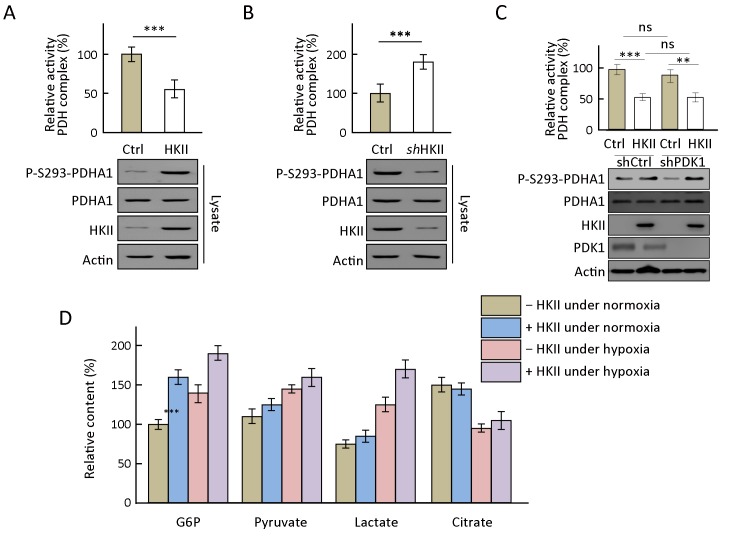
To confirm that whether HKII phosphorylates PDHA1 and reroutes its metabolic pathway, the activity of the PDH complex under HKII overexpression or knockdown was evaluated. The results show that overexpression of HKII increased the phosphorylation levels of PDHA1 but decreased the activity of the PDH complex (Figure 3A ). Conversely, knocking down HKII decreased PDHA1 phosphorylation levels and increased the activity of the PDH complex (Figure 3B ). To exclude the effects of PDK on the phosphorylation levels of PDHA1 and the activity of the PDH complex, PDK1 was knocked down in HeLa cells. The results show that, in these cells, HKII increased the phosphorylation levels of PDHA1 S293 but decreased the activity of the PDH complex significantly (Figure 3C ). Alterations in the levels of some metabolites through glycolysis and tricarboxylic acid cycle were further evaluated. The results show that, under hypoxic conditions, the overexpression of HKII re-routed metabolites into glycolysis, decreased citrate levels, and increased lactate levels (Figure 3D ), suggesting that hypoxia promotes HKII kinase activity.
3.
Hexokinase (HK) II decreases relative activity of pyruvate dehydrogenase (PDH) complex and regulates metabolic flux. (A) Relative activity of PDH complex and P-S293 levels of endogenous PDHA1 were determined and quantified in HKII-overexpressed cells; (B) Relative activity of PDH complex and P-S293 levels of endogenous PDHA1 were determined and quantified after HKII knockdown; (C) PDK1 was knocked down in HeLa cells and then HKII was transfected into the cells, the activity of PDH complex was measured by PDH enzyme microplate assay kit (ab109902); (D) HKII-overexpressing HeLa cells were incubated under normoxia or hypoxia and the amount of glucose 6-phosphate (G6P), pyruvate, lactate, and citrate were determined and quantified. **, P<0.01; ***, P<0.001.
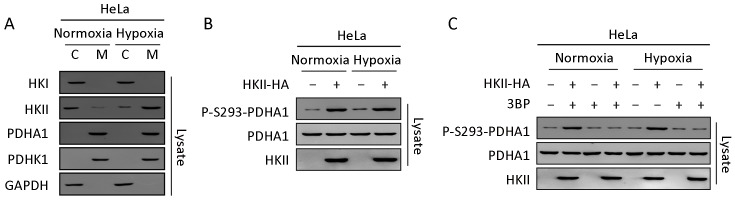
Although the expression of HKII is enhanced by the HIF signaling pathway (34), here we found that hypoxia promoted the translocation of HKII, but not HK I or the substrate PDHA1, from the cytoplasm into the mitochondria (Figure 4A ). Interestingly, the expression of PDHK1 in mitochondria was not increased by hypoxia. Consistent with previous data, hypoxia increased the phosphorylation of PDHA1 under overexpression of HKII (Figure 4B ). Similarly, the HKII inhibitor 3prevented the phosphorylation of HKII under hypoxia (Figure 4C ).
4.
Hypoxia promotes translocation of hexokinase (HK) II into mitochondria and increases phosphorylation of the alpha subunit of pyruvate dehydrogenase (PDHA1). (A) HeLa cells were incubated under normoxia or hypoxia conditions. Endogenous proteins of cytoplasm and mitochondria were isolated, respectively and endogenous HK I, HKII, PDHA1 and PDHK1 were determined and quantified; (B) P-S293 levels of endogenous PDHA1 of each cell cultured under normoxia or hypoxia were determined and quantified; (C) HA-tagged HKII was overexpressed in HeLa cells under normoxia or hypoxia. P-S293 levels of endogenous PDHA1 of cultures with or without 3-bromopyruvate (3BP; 5 mmol/L) were determined.
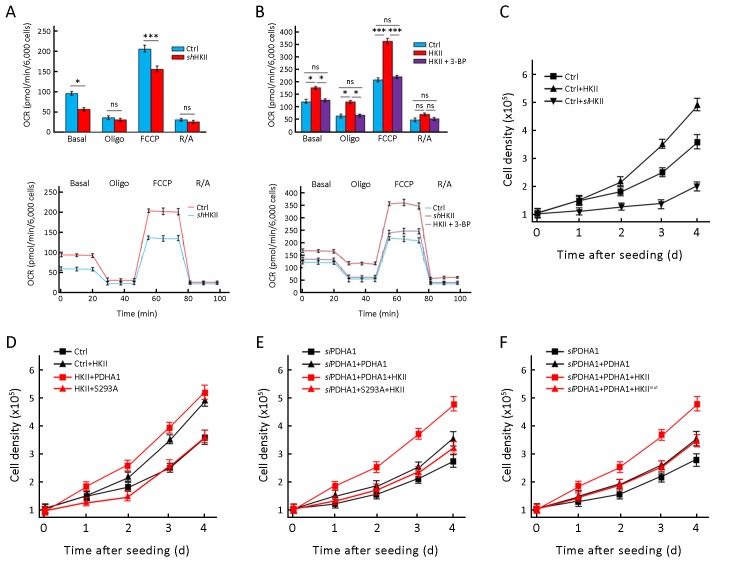
To test the effects of HKII on the oxygen consumption rate (OCR) of cells, HKII was knocked down in HeLa cells, which resulted in decreased OCRs (Figure 5A ). HKII knockdown slightly decreased basal respiration, but incubation with oligomycin prevented ATP production. Carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP) uncoupled oxidative phosphorylation but did not promote ATP synthesis ATP, indicating maximal respiration. Finally, when the cells were incubated with ntimycin A and retenone (respiratory chain inhibitors), mitochondrial oxygen consumption was completely blocked. As can be seen in Figure 5A , knocking down HKII decreased basal respiration and maximal respiration. Consistent with these results, overexpression of HKII increased the basal respiration and maximal respiration of HeLa cells, and 3BP prevented HKII-mediated increase in OCR (Figure 5B ).
5.
Hexokinase (HK) II increases oxygen consumption rate (OCR) of cells and promotes cell proliferation by phosphorylating S293 of the alpha subunit of pyruvate dehydrogenase (PDHA1). (A) OCRs were detected (bottom) for ctrl and HKII KD cells; (B) OCRs were detected (bottom) for ctrl and HKII overexpressed cells (with or without 3BP, 5 μmol/L). OCR under oligomycin, carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP), and antimycin A/rotenone (R/A) treatments, respectively; (C) Growth curves of control HEK293T cells, HKII overexpressing HEK293T cells and HKII knockdown HEK293T cells were determined; (D) Growth curves of control HEK293T cells, HKII overexpressing HEK293T cells, HKII & PHDA1 overexpressing HEK293T cells and HKII & S293A overexpressing HEK293T cells were determined; (E) PDHA1 was knocked down in HEK293T cells and then PDHA1 and S293A and HKII were re-introduced into the cells. Growth curves of siPDHA1 HEK293T cells, PDHA1 overexpressing siPDHA1 HEK293T cells, HKII & PHDA1 overexpressing siPDHA1 HEK293T cells and HKII & S293A overexpressing siPDHA1 HEK293T cells were determined; (F) PDHA1 was knocked down in HEK293T cells and then PDHA1 was transfected into cells and finally these cells were transfected with HKII or HKII mutation and growth curves were determined. *, P<0.05; **, P<0.01; ***, P<0.001.
Lastly, overexpression of HKII accelerated HEK293T proliferation, whereas knocking down HKII decreased HEK293T proliferation (Figure 5C ). When PDHA1 and HKII were overexpressed together in HEK293T cells, both PDHA1 and HKII increased cell proliferation. Interestingly, the growth-promoting effects of HKII were blocked by overexpression of mutant PDHA1 S293A (Figure 5D ). To determine the effects of endogenous PDHA1 on cell growth, PDHA1 was knocked down in HEK293T cells, and then HKII and wild type PDHA1 or mutant PDHA1 S293A were re-introduced. The results show that HEK293T cells transfected with HKII & PDHA1 grew faster than cells transfected with HKII & PDHA1 S293A ( Figure 5E ). In addition, wild type HKII, but not mutant HKII, increased cell proliferation in cells with re-introduced PDHA1 (Figure 5F ). These results support the hypothesis that phosphorylation of PDHA1 by HKII regulates cell growth.
HKII expression correlates with PDHA1 phosphorylation in disease progression in cancers
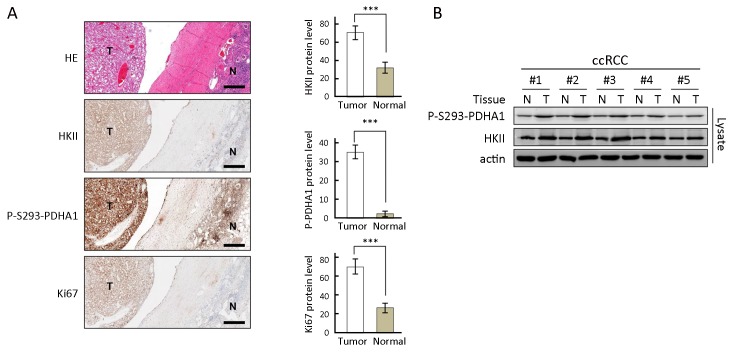
To evaluate a correlation between overexpression of HKII and phosphorylation of S293 of PDHA1 with tumor growth and disease progression, the levels of each protein were determined in ccRCC samples. IHC analysis was performed on 10 specimens that contained both normal and tumor tissues to illustrate differential expression of each marker in these tissues. In ccRCC, HKII was overexpressed with elevated phosphorylation of S293 of PDHA1 and increased expression of Ki67, an indicator of disease progression (Figure 6A ). The increased expression levels of HKII correlated with phosphorylation of P293 of PDHA1 in tumor tissues (Figure 6B ). These results indicate that HKII phosphorylates PDHA1 and plays a role in promoting RCC disease progression.
6.
Expression of hexokinase (HK) II is correlated with alpha subunit of pyruvate dehydrogenase (PDHA1) phosphorylation and clear cell renal cell carcinoma (ccRCC) progression. (A) HKII, P-S293-PDHA1, and Ki67 levels were determined in the same patient in ccRCC tumor tissues and adjacent normal tissues. Representative immunohistochemistry (left) and statistics (right, n=10) results are shown. Normal and tumor tissues are indicated by N and T, respectively. Pathologic results were confirmed by experienced pathologists. Scale bars correspond to 100 μm; (B) HKII and P-S293-PDHA1 levels in the same patient were also determined using western blotting. ***, P<0.001.
Discussion
HKII is overexpressed in most cancers (38-40), which leads to the detection of cancer by positron emission tomography (PET) imaging (41). A glucose analogue, 2-fluoro-2-deoxy glucose (18FDG), is phosphorylated by HKII at the 6th carbon using ATP to become 2-fluoro-2-deoxy glucose 6-phosphate (18FDG-6-P).
HKII (EC: 2.7.1.1) is the first irreversible enzyme of glycolysis and is typically overexpressed in tumor tissues. Mito-HKII couples glycolysis and oxidative phosphorylation, maintaining mitochondrial membrane integrity (11). 3BP was reported to inhibit the activity of HKII in a rabbit VX2 tumor model (42). HKII binds to and inhibits the mTOR complex 1 and potentiates glucose starvation and induces autophagy in cardiomyocyte and noncardiomyocyte cells. However, the protein kinase activity of HKII toward other enzymes was not reported.
PDC catalyzes the overall conversion of pyruvate to acetyl-CoA and CO2, and provides the primary link between glycolysis and the TCA cycle (19-21). PDHK1 phosphorylates Ser293 of PDHA1 and inhibits the activity of PDC (22-29). Y381 phosphorylation of PDP1 recruits ACAT1 to PDC, and K321 acetylation inhibits PDHA1 by recruiting PDK1, promoting glycolysis in cancer cells and consequently tumor growth (30). PDC was reported to translocate from the mitochondria to the nucleus and regulate the acetylation of specific lysine residues of histones, which is important for G1-S phase progression and for expression of S phase markers (43). It was reported that SIRT3 deacetylates and increases the activity of PDH complex (44), but SIRT5 desuccinylates PDHA1 and decreases the activity of the PDH complex (31). It was also reported that SIRT4 enzymatically hydrolyzes the lipoamide cofactors from the E2 component dihydrolipoyllysine acetyltransferase (DLAT), diminishing PDH activity (45). It was also reported that PDH is an important regulator of senescence induced by BRAFV600E, which is commonly mutated in melanoma and other cancers (46), further confirming that PDC is a regulatory node for metabolism and signal transduction.
It was recently reported that many metabolic enzymes have dual functions, metabolic activity and protein kinase activity. Although the protein kinase activity of PKM2 is controversial (47), PKM2 was reported to translocate into the nucleus and phosphorylate Thr11 of histone 3, Tyr705 of STAT3, Tyr207 of Bub3, Y118 of MLC2, and S202 & 203 of AKT1S1 ( 48-54). In addition, phosphoglycerate kinase 1 (PGK1) translocates from the cytoplasm into the mitochondria to phosphorylate T338 of PDHK1 and suppress the activity of PDH complex, promoting the Warburg effect (55). PGK1 was also reported to phosphorylate Beclin 1, promoting autophagy (56). ULK1/2 not only initiates autophagy, but also phosphorylates key glycolytic enzymes such as HK, phosphofructokinase 1 (PFK1), enolase 1 (ENO1), and the gluconeogenic enzyme fructose-1,6-bisphosphatase (FBP1) to regulate glucose metabolic fluxes (57).
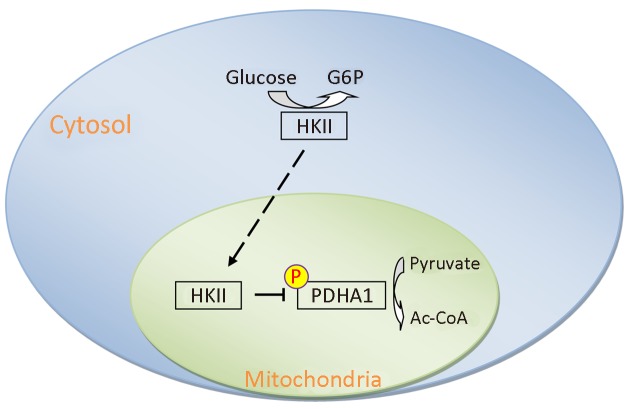
Here we determined that HKII not only transforms glucose into glucose-6-phosphate, but also transfers the phosphate group from ATP to PDHA1, inhibiting the activity of the PDH complex and promoting the Warburg effect (Figure 7 ). In addition, the overexpression of HKII correlated with the phosphorylation of PDHA1 and disease progression in ccRCC. Although many specific genes such as VHL, PBRM1, SETD2, BAP1, and KDM5C, are mutated in ccRCC (32,33), and HIF1α and HIF2α are targeted to inhibit the proliferation and growth of cancer cells (34,58), hypoxia induces the expression of HKII and stimulates the proliferation of human hepatoma cells (59). HIF1α also increased the expression of HK I, HKII, PKM2, PDHK1, and LDHA, thus increasing glycolysis and promoting the Warburg effect in ccRCC. The data presented here suggest that HKII may be a significant biomarker in the evaluation and treatment of cancer.
7.
Schematic diagram of hexokinase (HK) II-mediated alpha subunit of pyruvate dehydrogenase (PDHA1) phosphorylation leading to the Warburg effect. Overexpression of HKII leads to accumulation of glycolytic intermediates, and HKII-mediated inactivation of PDHA1 decreases metabolic flux through tricarboxylic acid (TCA) cycle. Both events increase the Warburg effect and promote growth and proliferation of tumor cells. G6P, glucose 6-phosphate.
Conclusions
It was found that HKII was a bifunctional enzyme, inhibited the activity of PDH complex and promoted Warburg effect through phosphorylating S293 of PDHA1. In addition, the overexpression of HKII correlated with the phosphorylation of PDHA1 and disease progression in renal clear cell carcinoma.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Contributor Information
Fei Yuan, Email: lfxiu2007@126.com.
Junli Zuo, Email: daphny2014@163.com.
References
- 1.Liberti MV, Locasale JW The Warburg Effect: How does it benefit cancer cells? Trends Biochem Sci. 2016;41:211–8. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vander Heiden MG, Cantley LC, Thompson CB Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao J, Liu J, Zhao W By blocking hexokinase-2 phosphorylation, limonin suppresses tumor glycolysis and induces cell apoptosis in hepatocellular carcinoma. Onco Targets Ther. 2018;11:3793–803. doi: 10.2147/ott.s165220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts DJ, Miyamoto S Hexokinase II integrates energy metabolism and cellular protection: Akting on mitochondria and TORCing to autophagy. Cell Death Differ. 2015;22:248–57. doi: 10.1038/cdd.2014.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Z, Zhang H, Lu W, et al Role of mitochondria-associated hexokinase II in cancer cell death induced by 3-bromopyruvate. Biochim Biophys Acta. 2009;1787:553–60. doi: 10.1016/j.bbabio.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo C, Ludvik AE, Arlotto ME, et al Coordinated regulatory variation associated with gestational hyperglycaemia regulates expression of the novel hexokinase HKDC1. Nat Commun. 2015;6:6069. doi: 10.1038/ncomms7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irwin DM, Tan H Molecular evolution of the vertebrate hexokinase gene family: Identification of a conserved fifth vertebrate hexokinase gene. Comp Biochem Physiol Part D Genomics Proteomics. 2008;3:96–107. doi: 10.1016/j.cbd.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Patra KC, Wang Q, Bhaskar PT, et al Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell. 2013;24:213–28. doi: 10.1016/j.ccr.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho N, Coomber BL Hexokinase II expression is correlated with colorectal cancer prognosis. Cancer Treat Commun. 2016;6:11–6. doi: 10.1016/j.ctrc.2016.02.008>. [DOI] [Google Scholar]
- 10.Ho N, Morrison J, Silva A, et al The effect of 3-bromopyruvate on human colorectal cancer cells is dependent on glucose concentration but not hexokinase II expression. Biosci Rep. 2016;36:e00299. doi: 10.1042/BSR20150267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathupala SP, Ko YH, Pedersen PL Hexokinase-2 bound to mitochondria: cancer’s stygian link to the " Warburg Effect” and a pivotal target for effective therapy. Semin Cancer Biol. 2009;19:17–24. doi: 10.1016/j.semcancer.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolf AJ, Reyes CN, Liang W, et al Hexokinase is an innate immune receptor for the detection of bacterial peptidoglycan. Cell. 2016;166:624–36. doi: 10.1016/j.cell.2016.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts DJ, Tan-Sah VP, Smith JM, et al Akt phosphorylates HK-II at Thr-473 and increases mitochondrial HK-II association to protect cardiomyocytes. J Biol Chem. 2013;288:23798–806. doi: 10.1074/jbc.M113.482026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Majewski N, Nogueira V, Bhaskar P, et al Hexokinase-mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol Cell. 2004;16:819–30. doi: 10.1016/j.molcel.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 15.Gall JM, Wong V, Pimental DR, et al Hexokinase regulates Bax-mediated mitochondrial membrane injury following ischemic stress. Kidney Int. 2011;79:1207–16. doi: 10.1038/ki.2010.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh A, Sen E Reciprocal role of SIRT6 and Hexokinase 2 in the regulation of autophagy driven monocyte differentiation. Exp Cell Res. 2017;360:365–74. doi: 10.1016/j.yexcr.2017.09.028. [DOI] [PubMed] [Google Scholar]
- 17.Roberts DJ, Tan-Sah VP, Ding EY, et al Hexokinase-II positively regulates glucose starvation-induced autophagy through TORC1 inhibition. Mol Cell. 2014;53:521–33. doi: 10.1016/j.molcel.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adams V, Griffin LD, Gelb BD, et al Protein kinase activity of rat brain hexokinase. Biochem Biophys Res Commun. 1991;177:1101–6. doi: 10.1016/0006-291x(91)90652-n. [DOI] [PubMed] [Google Scholar]
- 19.Patel MS, Roche TE Molecular biology and biochemistry of pyruvate dehydrogenase complexes. FASEB J. 1990;4:3224–33. doi: 10.1096/fasebj.4.14.2227213. [DOI] [PubMed] [Google Scholar]
- 20.Patel MS, Korotchkina LG Regulation of the pyruvate dehydrogenase complex. Biochem Soc Trans. 2006;34:217–22. doi: 10.1042/BST20060217. [DOI] [PubMed] [Google Scholar]
- 21.Roche TE, Hiromasa Y Pyruvate dehydrogenase kinase regulatory mechanisms and inhibition in treating diabetes, heart ischemia, and cancer. Cell Mol Life Sci. 2007;64:830–49. doi: 10.1007/s00018-007-6380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hiromasa Y, Fujisawa T, Aso Y, et al Organization of the cores of the mammalian pyruvate dehydrogenase complex formed by E2 and E2 plus the E3-binding protein and their capacities to bind the E1 and E3 components. J Biol Chem. 2004;279:6921–33. doi: 10.1074/jbc.M308172200. [DOI] [PubMed] [Google Scholar]
- 23.Lu CW, Lin SC, Chen KF, et al Induction of pyruvate dehydrogenase kinase-3 by hypoxia-inducible factor-1 promotes metabolic switch and drug resistance. J Biol Chem. 2008;283:28106–14. doi: 10.1074/jbc.M803508200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wynn RM, Kato M, Chuang JL, et al Pyruvate dehydrogenase kinase-4 structures reveal a metastable open conformation fostering robust core-free basal activity. J Biol Chem. 2008;283:25305–15. doi: 10.1074/jbc.M802249200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McFate T, Mohyeldin A, Lu H, et al Pyruvate dehydrogenase complex activity controls metabolic and malignant phenotype in cancer cells. J Biol Chem. 2008;283:22700–8. doi: 10.1074/jbc.M801765200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferriero R, Iannuzzi C, Manco G, et al Differential inhibition of PDKs by phenylbutyrate and enhancement of pyruvate dehydrogenase complex activity by combination with dichloroacetate. J Inherit Metab Dis. 2015;38:895–904. doi: 10.1007/s10545-014-9808-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kato M, Li J, Chuang JL, et al Distinct structural mechanisms for inhibition of pyruvate dehydrogenase kinase isoforms by AZD7545, dichloroacetate, and radicicol. Structure. 2007;15:992–1004. doi: 10.1016/j.str.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hitosugi T, Fan J, Chung TW, et al Tyrosine phosphorylation of mitochondrial pyruvate dehydrogenase kinase 1 is important for cancer metabolism. Mol Cell. 2011;44:864–77. doi: 10.1016/j.molcel.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shan C, Kang HB, Elf S, et al Tyr-94 phosphorylation inhibits pyruvate dehydrogenase phosphatase 1 and promotes tumor growth. J Biol Chem. 2014;289:21413–22. doi: 10.1074/jbc.M114.581124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan J, Shan C, Kang HB, et al Tyr phosphorylation of PDP1 toggles recruitment between ACAT1 and SIRT3 to regulate the pyruvate dehydrogenase complex. Mol Cell. 2014;53:534–48. doi: 10.1016/j.molcel.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park J, Chen Y, Tishkoff DX, et al SIRT5-mediated lysine desuccinylation impacts diverse metabolic pathways. Mol Cell. 2013;50:919–30. doi: 10.1016/j.molcel.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turajlic S, Larkin J, Swanton C SnapShot: renal cell carcinoma. Cell. 2015;163:1556–1556. doi: 10.1016/j.cell.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 33.Ricketts CJ, Crooks DR, Sourbier C, et al SnapShot: renal cell carcinoma. Cancer Cell. 2016;29:610–610. doi: 10.1016/j.ccell.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 34.Denko NC Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer. 2008;8:705–13. doi: 10.1038/nrc2468. [DOI] [PubMed] [Google Scholar]
- 35.Knight JD, Tian R, Lee RE, et al A novel whole-cell lysate kinase assay identifies substrates of the p38 MAPK in differentiating myoblasts. Skelet Muscle. 2012;2:5. doi: 10.1186/2044-5040-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang C, Ye M, Bian Y, et al Determination of CK2 specificity and substrates by proteome-derived peptide libraries. J Proteome Res. 2013;12:3813–21. doi: 10.1021/pr4002965. [DOI] [PubMed] [Google Scholar]
- 37.Tsai HJ, Wilson JE Functional organization of mammalian hexokinases: both N- and C-terminal halves of the rat type II isozyme possess catalytic sites. Arch Biochem Biophys. 1996;329:17–23. doi: 10.1006/abbi.1996.0186. [DOI] [PubMed] [Google Scholar]
- 38.Wilson JE Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J Exp Biol. 2003;206:2049–57. doi: 10.1242/jeb.00241. [DOI] [PubMed] [Google Scholar]
- 39.Mathupala SP, Ko YH, Pedersen PL Hexokinase II: cancer’s double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene. 2006;25:4777–86. doi: 10.1038/sj.onc.1209603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu JW, Sun P, Zhang DX, et al Hexokinase 2 regulates G1/S checkpoint through CDK2 in cancer-associated fibroblasts. Cell Signal. 2014;26:2210–6. doi: 10.1016/j.cellsig.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 41.Ko YH, Verhoeven HA, Lee MJ, et al A translational study " case report” on the small molecule " energy blocker” 3-bromopyruvate (3BP) as a potent anticancer agent: from bench side to bedside. J Bioenerg Biomembr. 2012;44:163–70. doi: 10.1007/s10863-012-9417-4. [DOI] [PubMed] [Google Scholar]
- 42.Ko YH, Pedersen PL, Geschwind JF Glucose catabolism in the rabbit VX2 tumor model for liver cancer: characterization and targeting hexokinase. Cancer Lett. 2001;173:83–91. doi: 10.1016/S0304-3835(01)00667-X. [DOI] [PubMed] [Google Scholar]
- 43.Sutendra G, Kinnaird A, Dromparis P, et al A nuclear pyruvate dehydrogenase complex is important for the generation of acetyl-CoA and histone acetylation. Cell. 2014;158:84–97. doi: 10.1016/j.cell.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 44.Ozden O, Park SH, Wagner BA, et al SIRT3 deacetylates and increases pyruvate dehydrogenase activity in cancer cells. Free Radic Biol Med. 2014;76:163–72. doi: 10.1016/j.freeradbiomed.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mathias RA, Greco TM, Oberstein A, et al Sirtuin 4 is a lipoamidase regulating pyruvate dehydrogenase complex activity. Cell. 2014;159:1615–25. doi: 10.1016/j.cell.2014.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaplon J, Zheng L, Meissl K, et al A key role for mitochondrial gatekeeper pyruvate dehydrogenase in oncogene-induced senescence. Nature. 2013;498:109–12. doi: 10.1038/nature12154. [DOI] [PubMed] [Google Scholar]
- 47.Hosios AM, Fiske BP, Gui DY, et al Lack of evidence for PKM2 protein kinase activity. Mol Cell. 2015;59:850–7. doi: 10.1016/j.molcel.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang W, Xia Y, Ji H, et al Nuclear PKM2 regulates β-catenin transactivation upon EGFR activation. Nature. 2011;480:118–22. doi: 10.1038/nature10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao X, Wang H, Yang JJ, et al Pyruvate kinase M2 regulates gene transcription by acting as a protein kinase. Mol Cell. 2012;45:598–609. doi: 10.1016/j.molcel.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang W, Xia Y, Hawke D, et al PKM2 phosphorylates histone H3 and promotes gene transcription and tumorigenesis. Cell. 2014;158:1210. doi: 10.1016/j.cell.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 51.Jiang Y, Li X, Yang W, et al PKM2 regulates chromosome segregation and mitosis progression of tumor cells. Mol Cell. 2014;53:75–87. doi: 10.1016/j.molcel.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang Y, Wang Y, Wang T, et al PKM2 phosphorylates MLC2 and regulates cytokinesis of tumour cells. Nat Commun. 2014;5:5566. doi: 10.1038/ncomms6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keller KE, Doctor ZM, Dwyer ZW, et al SAICAR induces protein kinase activity of PKM2 that is necessary for sustained proliferative signaling of cancer cells. Mol Cell. 2014;53:700–9. doi: 10.1016/j.molcel.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He CL, Bian YY, Xue Y, et al Pyruvate kinase M2 activates mTORC1 by phosphorylating AKT1S1. Sci Rep. 2016;6:21524. doi: 10.1038/srep21524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li X, Jiang Y, Meisenhelder J, et al Mitochondria-translocated PGK1 functions as a protein kinase to coordinate glycolysis and the TCA cycle in tumorigenesis. Mol Cell. 2016;61:705–19. doi: 10.1016/j.molcel.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qian X, Li X, Cai Q, et al Phosphoglycerate kinase 1 phosphorylates beclin1 to induce autophagy. Mol Cell. 2017;65:917–31. doi: 10.1016/j.molcel.2017.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li TY, Sun Y, Liang Y, et al ULK1/2 constitute a bifurcate node controlling glucose metabolic fluxes in addition to autophagy. Mol Cell. 2016;62:359–70. doi: 10.1016/j.molcel.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 58.Mathupala SP, Rempel A, Pedersen PL Glucose catabolism in cancer cells: identification and characterization of a marked activation response of the type II hexokinase gene to hypoxic conditions. J Biol Chem. 2001;276:43407–12. doi: 10.1074/jbc.M108181200. [DOI] [PubMed] [Google Scholar]
- 59.Gwak GY, Yoon JH, Kim KM, et al Hypoxia stimulates proliferation of human hepatoma cells through the induction of hexokinase II expression. J Hepatol. 2005;42:358–64. doi: 10.1016/j.jhep.2004.11.020. [DOI] [PubMed] [Google Scholar]