Abstract
Objective
Our goal is to analyze the trend of colorectal cancer (CRC) regarding the death, incidence and prevalence rates over time, and to provide epidemiological knowledge basis for health policy revision by comparing data about fatal outcomes of CRC in 2017 to those data in 1990, which was extracted from the Global Burden of Disease (GBD).
Methods
The time trend and changes of CRC burden from 1990 to 2017 were measured by using the methods and results from the Institute for Health Metrics and Evaluation (IHME) GBD 2017, based on the rates of death, incidence and prevalence.
Results
The death rate of CRC is 13.24/100,000, accounting for 1.79% of total deaths in China in 2017. In 1990, CRC ranked 21st in all causes of death in China compared to its 11th ranking in 2017. The death, incidence and prevalence rate of CRC were standardized by the age scale of the global population in 2010, the change of standardized death rate of CRC was not significant, from 9.33/100,000 in 1990 to 10.10/100,000 in 2017. The standardized incidence rate of CRC significantly increased from 12.18/100,000 in 1990 to 22.42/100,000 in 2017. The standardized prevalence rate of CRC significantly increased from 44.55/100,000 in 1990 to 118.40/100,000 in 2017. The trend of the prevalence rate in both genders grow higher in 2017 compared to the 1990, resulting in 141.6%, 209.8% and 189.0% for the studied three age groups (15−49, 50−69 and 70+ years old), respectively. The death rate increased in the age groups of 50−69 and 70+ years in both genders (8.6% and 31.0% respectively), in contrast to a decrease of death rate in the age group of 15−49 years old (−10.8%).
Conclusions
China experienced a stunning increase in terms of incidence and prevalence rate of CRC from 1990 to 2017. To decrease the burden of CRC, prevention and management of known risk factors should be promoted through national polices.
Keywords: Colorectal cancer, death, incidence, prevalence, epidemiology
Introduction
Colorectal cancer (CRC) is a common malignant gastrointestinal cancer in China. It was shown in the latest data released by the National Cancer Center in 2017 that CRC ranked the fourth in terms of incidence of malignant tumors and the fifth in terms of death rate (1). In 2018, nearly two million newly diagnosed CRC cases and over 800,000 CRC related deaths are expected worldwide (2). CRC incidence and death rates have been stabilizing or decreasing in highly developed countries through the enormous efforts over the last decades (3). However, a rapid growth of CRC has been seen in many low-income and middle-income countries (4).
As one of the largest developing countries, China has been experiencing a rapid economic growth and health care reform. The diet and other factors related to CRC risk are also changing rapidly in China. One of the major public health issues is the rapid growth in CRC incidence and the accompanying increase in disease burden (5,6). According to the data from the National Central Cancer Registry (NCCR) of China, it was estimated that there were 274,841 new CRC cases (157,355 males and 117,486 females) diagnosed in 2010, which accounted for nearly one tenth of the global CRC burden in that year (7). Therefore, it is urgent to understand the epidemiological trends of CRC in China, so that related health policies accommodated to the trend of CRC prevalence, including prevention and management of cancer, can be established as soon as possible to improve public health.
Data were obtained from the Institute for Health Metrics and Evaluation (IHME) Global Burden of Disease (GBD) (https://vizhub.healthdata.org). The sample size of the data about the CRC related death, incidence and prevalence was large and provided a solid base for the analysis. By analyzing the data, we can see what is the ranking and distribution of CRC deaths of China in the world. The results obtained from our study should be useful for understanding CRC better, evaluating current prevention strategies, planning to manage disease burden national wide, and improving health management system to meet future challenges.
Materials and methods
Data sources
Death, incidence and prevalence data were extracted from the official website of GBD 2017 Study led by IHME for free. The GBD is hierarchically organized by geographic units or locations, with 7 super-regions, 21 regions nested within these super-regions, and 195 countries or territories within 21 regions. In the GBD Study, 95% uncertainty intervals (95% UIs) were determined based on the 25th and 975th ranked values across all 1,000 draws (8). Uncertainty analysis is the estimation of range or distribution of uncertainty in estimates based on an assessment of the uncertainty or confidence intervals for all data and parameter inputs. UIs should ideally include all sources of uncertainty, including those arising from systematic biases and measurement error. In contrast, generally reported confidence intervals are based solely on the variation observed in sample data (9).
In the database, we chose “China” for the location, “colon and rectum cancer” for cause, and “death”, “incidence” and “prevalence” for measures. The cause of death attributed each death to a single underlying cause that began the series of events leading to death, in accordance with International Classification of Diseases (ICD) 10 principles. The GBD study organized causes of death in a hierarchical list containing four levels, and the cause of colon and rectum cancer was in level 3 which represented the finest level of detail by cause, such as CRC, stomach cancer or stroke.
In the GBD study, the causes of death database of IHEM includes vital registration (VR), verbal autopsy (VA), registry, survey, police and surveillance data (10). Non-fatal estimation began with the compilation of data sources from a diverse set of possible sources, including 21 possible Global Health Data Exchange (GHDx) data types such as scientific literature, survey data and epidemiological surveillance data (11).
Statistical analysis
Descriptive analysis was conducted on CRC death, incidence and prevalence data by gender, age and year. Age grouping was based on the GBD 2017 published data. Three rough age groups (15−49 years old, 50−69 years old and 70+ years old) were used to analyze the time trend of CRC. Cases were age-grouped by every 5 years in the detailed descriptions of death, incidence and prevalence of CRC in 2017. Since the death, incidence and prevalence rates of CRC were zero for those patients younger than 15 years old, the groups of 0−4 years old, 5−9 years old and 10−14 years old were combined to a single age group. The changes of each parameter were calculated by comparing the data of year 2017 to the data of year 1990.
Results
Trend of CRC death, incidence and prevalence over time
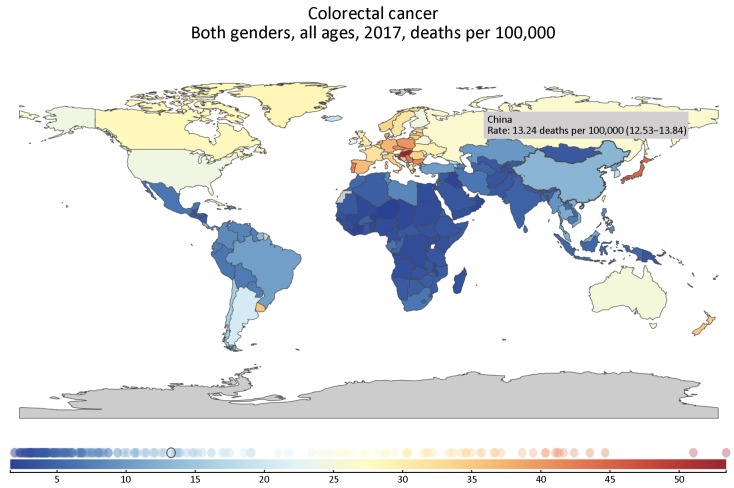
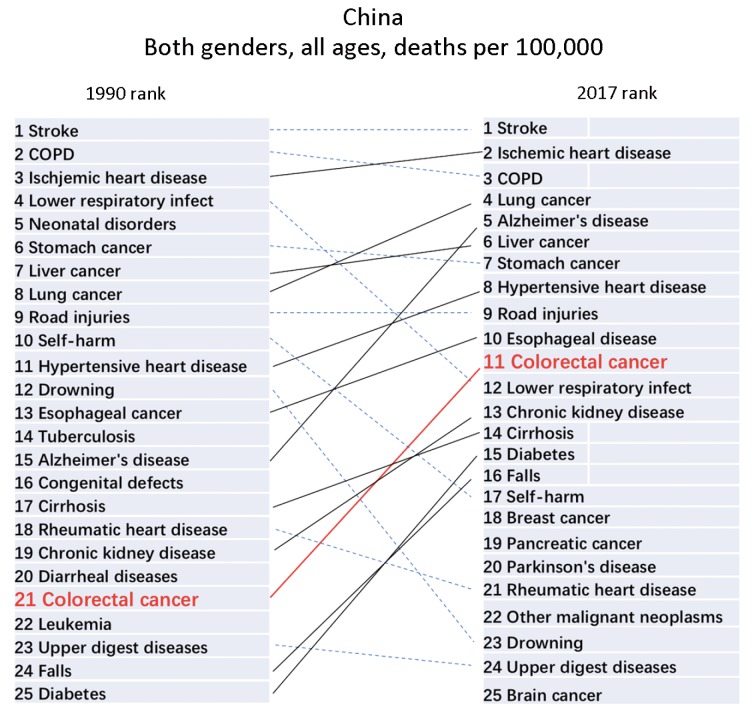
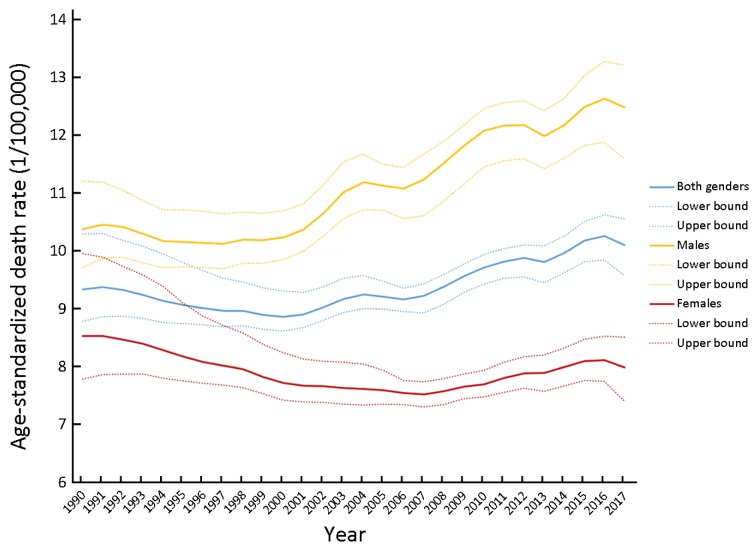
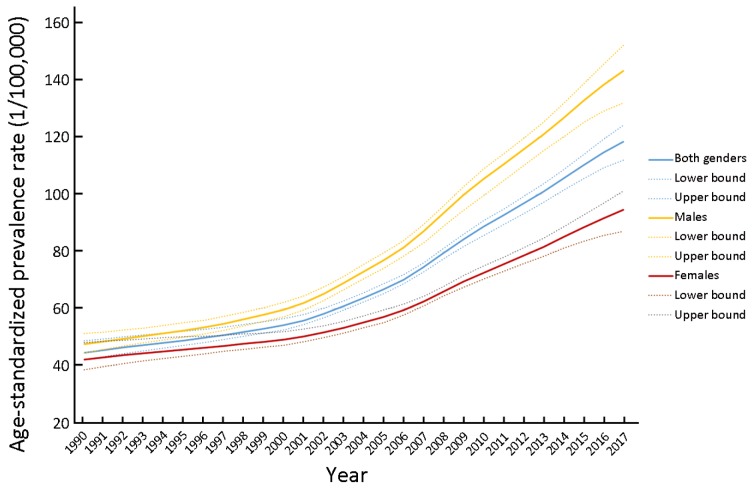
The death rate of CRC was 13.24/100,000 (95% UI: 12.53, 13.84) in China in 2017 (Figure 1, Supplementary Table S1), we present the death rate, incidence and prevalence rate of CRC in China by gender and age in 2017 (Table 1). From the geographical distribution of CRC deaths in Figure 1, we can see that China’s CRC deaths are obviously higher than those of South Asia and Africa, but significantly lower than those of developed countries such as Europe and the United States. CRC of China ranked 21st among all causes of death in 1990, while ranked 11th in 2017 (Figure 2). The death, incidence and prevalence rates of CRC were standardized by age scale of global population in 2010. The standardized death rate of CRC increased from 9.33/100,000 in 1990 (8.78−10.29) to 10.10/100,000 in 2017 (9.57−10.55) (Figure 3), which was not statistically significant. The standardized death rate of CRC was slightly higher in male than in female in 1990 (10.37/100,000 vs. 8.53/100,000). In contrast, the standardized death rate of CRC in male obviously increased as compared to that in female in 2017 (12.48/100,000 vs. 7.98/100,000). The standardized incidence rate of CRC increased from 12.18/100,000 (11.45−13.41) in 1990 to 22.42/100,000 (95% UI: 21.20, 23.49) in 2017. The standardized incidence rate of CRC was similar in male and in female in 1990 (13.35/100,000vs. 11.23/100,000). In contrast, the standardized incidence rate of CRC in male obviously increased as compared to that in female in 2017 (27.67/100,000 vs. 17.56/100,000) (Table 2). The standardized prevalence rate of CRC significantly increased from 44.55/100,000 (95% UI: 41.98, 48.69) in 1990 to 118.40/100,000 (95% UI: 111.92, 124.17) in 2017 for both genders, and it was slightly higher in male than in female in 1990 (47.47/100,000 vs. 42.10/100,000). Similar to incidence rate, the standardized prevalence rate of CRC was obviously higher than in male than in female in 2017 (143.20/100,000 vs. 94.53/100,000) (Figure 4).
1.
Geographical distribution of death rate per 100,000 colorectal cancer cases worldwide in 2017.
S1.
Death rate of CRC in China, 1990−2017 (per 100,000)
| Year | Both genders (95% UI) | Male (95% UI) | Female (95% UI) |
| CRC, colorectal cancer; 95% UI, 95% uncertainty interval. | |||
| 1990 | 6.34 (5.96, 6.98) | 6.45 (6.05, 6.97) | 6.22 (5.66, 7.25) |
| 1991 | 6.48 (6.12, 7.10) | 6.62 (6.25, 7.10) | 6.32 (5.81, 7.33) |
| 1992 | 6.58 (6.24, 7.18) | 6.75 (6.41, 7.19) | 6.40 (5.93, 7.34) |
| 1993 | 6.65 (6.35, 7.24) | 6.83 (6.50, 7.23) | 6.47 (6.04, 7.38) |
| 1994 | 6.71 (6.43, 7.30) | 6.89 (6.58, 7.26) | 6.51 (6.11, 7.36) |
| 1995 | 6.80 (6.56, 7.34) | 7.03 (6.73, 7.40) | 6.56 (6.21, 7.30) |
| 1996 | 6.91 (6.68, 7.40) | 7.17 (6.87, 7.55) | 6.63 (6.32, 7.28) |
| 1997 | 7.02 (6.81, 7.44) | 7.31 (7.00, 7.67) | 6.72 (6.42, 7.29) |
| 1998 | 7.16 (6.95, 7.54) | 7.49 (7.19, 7.87) | 6.80 (6.52, 7.34) |
| 1999 | 7.27 (7.06, 7.65) | 7.66 (7.35, 8.03) | 6.85 (6.58, 7.34) |
| 2000 | 7.40 (7.18, 7.78) | 7.88 (7.58, 8.26) | 6.88 (6.60, 7.37) |
| 2001 | 7.57 (7.37, 7.91) | 8.13 (7.82, 8.49) | 6.99 (6.72, 7.41) |
| 2002 | 7.84 (7.64, 8.16) | 8.50 (8.18, 8.90) | 7.14 (6.87, 7.54) |
| 2003 | 8.12 (7.91, 8.45) | 8.94 (8.57, 9.38) | 7.26 (6.98, 7.69) |
| 2004 | 8.39 (8.16, 8.71) | 9.31 (8.93, 9.72) | 7.42 (7.14, 7.84) |
| 2005 | 8.57 (8.36, 8.82) | 9.51 (9.16, 9.83) | 7.57 (7.32, 7.92) |
| 2006 | 8.75 (8.56, 8.95) | 9.73 (9.32, 10.03) | 7.73 (7.51, 7.97) |
| 2007 | 9.06 (8.79, 9.27) | 10.15 (9.61, 10.52) | 7.92 (7.70, 8.14) |
| 2008 | 9.47 (9.17, 9.68) | 10.69 (10.12, 11.04) | 8.19 (7.94, 8.43) |
| 2009 | 9.92 (9.62, 10.13) | 11.27 (10.65, 11.61) | 8.49 (8.25, 8.74) |
| 2010 | 10.33 (10.02, 10.58) | 11.83 (11.20, 12.20) | 8.76 (8.51, 9.04) |
| 2011 | 10.76 (10.44, 11.01) | 12.32 (11.74, 12.72) | 9.13 (8.84, 9.44) |
| 2012 | 11.17 (10.80, 11.44) | 12.77 (12.17, 13.22) | 9.49 (9.18, 9.85) |
| 2013 | 11.44 (11.02, 11.76) | 13.02 (12.40, 13.52) | 9.78 (9.37, 10.16) |
| 2014 | 12.01 (11.62, 12.37) | 13.74 (13.07, 14.29) | 10.21 (9.80, 10.63) |
| 2015 | 12.67 (12.21, 13.08) | 14.60 (13.83, 15.26) | 10.65 (10.21, 11.15) |
| 2016 | 13.14 (12.60, 13.62) | 15.21 (14.30, 16.01) | 10.99 (10.49, 11.54) |
| 2017 | 13.24 (12.53, 13.84) | 15.30 (14.18, 16.19) | 11.10 (10.30, 11.85) |
1.
Death rate, incidence and prevalence rate of CRC in China by gender and age in 2017 (per 100,000)
| Age (year) | Death rate (95% UI) | Prevalence rate (95% UI) | Incidence rate (95% UI) | ||||||||
| Both genders | Male | Female | Both genders | Male | Female | Both genders | Male | Female | |||
| CRC, colorectal cancer; 95% UI, 95% uncertainty interval. | |||||||||||
| 0−14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 15−19 | 0.11
(0.10, 0.12) |
0.13
(0.12, 0.16) |
0.08
(0.07, 0.10) |
2.27
(2.02, 2.53) |
2.70
(2.33, 3.16) |
1.78
(1.47, 2.09) |
0.36
(0.32, 0.40) |
0.43
(0.37, 0.50) |
0.28
(0.23, 0.33) |
||
| 20−24 | 0.23
(0.20, 0.26) |
0.26
(0.22, 0.30) |
0.20
(0.16, 0.24) |
5.83
(5.07, 6.51) |
6.44
(5.54,7.53) |
5.19
(4.02, 6.15) |
0.89
(0.77, 1.00) |
0.99
(0.85, 1.15) |
0.79
(0.61, 0.94) |
||
| 25−29 | 0.69
(0.63, 0.74) |
0.80
(0.71, 0.88) |
0.58
(0.52, 0.65) |
20.93
(19.30, 22.49) |
23.75
(20.93, 26.23) |
18.06
(15.84, 20.11) |
3.16
(2.91, 3.39) |
3.60
(3.17, 3.96) |
2.71
(2.38, 3.02) |
||
| 30−34 | 1.30
(1.19, 1.40) |
1.48
(1.33, 1.63) |
1.11
(0.98, 1.23) |
35.79
(32.81, 38.60) |
40.26
(35.51, 44.44) |
31.20
(27.44, 34.79) |
5.46
(5.01, 5.89) |
6.16
(5.42, 6.80) |
4.74
(4.16, 5.28) |
||
| 35−39 | 1.76
(1.61, 1.90) |
2.00
(1.79, 2.21) |
1.52
(1.32, 1.69) |
44.19
(40.11, 47.93) |
49.44
(44.00, 54.75) |
38.75
(33.82, 43.29) |
6.70
(6.08, 7.26) |
7.52
(6.69, 8.34) |
5.84
(5.09, 6.53) |
||
| 40−44 | 3.06
(2.81, 3.26) |
3.52
(3.16, 3.83) |
2.57
(2.29, 2.83) |
70.34
(64.52, 75.43) |
81.12
(72.23, 88.85) |
59.15
(52.38, 65.55) |
10.61
(9.73, 11.38) |
12.26
(10.95, 13.43) |
8.90
(7.87, 9.87) |
||
| 45−49 | 6.45
(6.03, 6.85) |
7.75
(7.05, 8.43) |
5.09
(4.63, 5.54) |
128.31
(119.79, 136.45) |
153.75
(139.60, 167.80) |
101.91
(92.08, 110.54) |
19.91
(18.57, 21.18) |
23.95
(21.76, 26.19) |
15.71
(14.17, 17.06) |
||
| 50−54 | 10.92
(10.22, 11.60) |
13.44
(12.23, 14.61) |
8.37
(7.75, 9.02) |
216.31
(202.25, 230.80) |
261.00
(236.53, 285.36) |
171.26
(157.20, 186.05) |
33.66
(31.45, 35.88) |
40.98
(37.08, 44.69) |
26.27
(24.11, 28.58) |
||
| 55−59 | 13.90
(13.08, 14.71) |
17.39
(15.95, 18.75) |
10.35
(9.53, 11.13) |
267.02
(250.57, 283.31) |
329.86
(299.60, 358.68) |
202.84
(185.62, 220.03) |
41.81
(39.19, 44.37) |
52.09
(47.32, 56.60) |
31.31
(28.65, 33.96) |
||
| 60−64 | 28.31
(26.70, 30.02) |
35.45
(32.70, 38.16) |
21.12
(19.38, 22.80) |
486.13
(457.09, 516.26) |
596.47
(545.65, 642.89) |
375.04
(341.25, 408.01) |
79.02
(74.26, 83.86) |
98.20
(89.94, 105.90) |
59.71
(54.28, 64.99) |
||
| 65−69 | 47.22
(44.31, 49.76) |
59.46
(54.35, 63.79) |
35.35
(32.50, 38.14) |
708.73
(663.22, 750.23) |
872.01
(794.80, 943.63) |
550.31
(502.82, 597.25) |
121.78
(113.77, 128.92) |
152.58
(138.88, 165.04) |
91.90
(83.88, 99.66) |
||
| 70−74 | 62.41
(58.42, 65.73) |
76.26
(70.32, 81.73) |
49.14
(44.79, 53.11) |
749.72
(700.57, 792.70) |
894.73
(821.10, 964.81) |
610.75
(555.73, 663.97) |
140.89
(131.45, 148.85) |
171.89
(157.60, 185.40) |
111.18
(101.09, 121.02) |
||
| 75−79 | 93.72
(88.21, 98.56) |
115.30
(106.27, 123.41) |
73.65
(67.40, 79.15) |
791.87
(745.14, 834.94) |
958.23
(885.52, 1,026.92) |
637.15
(584.29, 685.99) |
175.43
(164.93, 185.04) |
217.01
(200.25, 233.01) |
136.75
(124.84, 147.38) |
||
| 80−84 | 132.85
(123.72, 139.09) |
162.73
(149.34, 173.48) |
108.52
(99.56, 116.11) |
529.65
(494.24, 555.36) |
647.18
(592.05, 691.72) |
433.93
(398.04, 464.65) |
175.41
(163.40, 184.23) |
220.32
(202.05, 235.80) |
138.84
(126.71, 149.24) |
||
| 85−89 | 161.51
(153.23, 169.23) |
197.76
(182.54, 210.44) |
136.98
(127.84, 146.13) |
568.44
(537.52, 596.89) |
692.81
(631.64, 738.64) |
484.32
(452.79, 515.08) |
213.76
(202.34, 225.01) |
269.37
(246.42, 288.27) |
176.14
(163.90, 187.88) |
||
| 90−94 | 173.06
(164.47, 181.03) |
228.98
(210.83, 246.58) |
144.66
(135.60, 153.60) |
515.11
(491.05, 539.24) |
673.09
(623.37, 722.50) |
434.90
(408.26, 461.39) |
229.39
(218.15, 240.59) |
312.39
(288.06, 336.49) |
187.25
(175.03, 199.39) |
||
| 95+ | 171.57
(161.84, 181.63) |
235.50
(214.35, 255.39) |
148.34
(137.61, 160.24) |
190.06
(180.23, 200.38) |
257.15
(237.76, 276.24) |
165.69
(155.17, 177.00) |
226.80
(212.98, 240.77) |
321.98
(292.06, 352.57) |
192.21
(178.02, 208.09) |
||
2.
Ranking of death rates for death-related causes in China. Both genders and all age groups were included. Top 25 causes of death and their corresponding ranking order in 1990 and in 2017 were shown.
3.
Age-standardized death rate of colorectal cancer (CRC) in both genders, male and female only. Results were obtained from the data of Institute for Health Metrics and Evaluation (IHME) Global Burden of Disease (GBD) from 1990 to 2017 (per 100,000).
2.
Age-standardized incidence rate of CRC in China, 1990−2017 (per 100,000)
| Year | Both genders (95% UI) | Male (95% UI) | Female (95% UI) |
| CRC, colorectal cancer; 95% UI, 95% uncertainty interval. | |||
| 1990 | 12.18 (11.45, 13.41) | 13.35 (12.48, 14.43) | 11.23 (10.24, 13.04) |
| 1991 | 12.36 (11.68, 13.53) | 13.58 (12.81, 14.55) | 11.36 (10.46, 13.07) |
| 1992 | 12.45 (11.83, 13.59) | 13.71 (12.99, 14.60) | 11.41 (10.59, 13.02) |
| 1993 | 12.49 (11.93, 13.57) | 13.74 (13.03, 14.57) | 11.45 (10.70, 12.98) |
| 1994 | 12.49 (11.97, 13.53) | 13.74 (13.08, 14.48) | 11.43 (10.73, 12.84) |
| 1995 | 12.57 (12.13, 13.52) | 13.90 (13.27, 14.65) | 11.44 (10.84, 12.68) |
| 1996 | 12.70 (12.29, 13.57) | 14.09 (13.46, 14.78) | 11.51 (10.98, 12.59) |
| 1997 | 12.81 (12.42, 13.56) | 14.25 (13.61, 14.92) | 11.58 (11.08, 12.53) |
| 1998 | 12.95 (12.58, 13.63) | 14.49 (13.86, 15.21) | 11.63 (11.14, 12.49) |
| 1999 | 13.01 (12.64, 13.65) | 14.66 (14.07, 15.35) | 11.59 (11.12, 12.35) |
| 2000 | 13.12 (12.75, 13.77) | 14.94 (14.34, 15.63) | 11.53 (11.08, 12.25) |
| 2001 | 13.37 (13.02, 13.93) | 15.35 (14.75, 16.05) | 11.64 (11.20, 12.27) |
| 2002 | 13.81 (13.45, 14.33) | 16.05 (15.42, 16.78) | 11.85 (11.39, 12.47) |
| 2003 | 14.30 (13.91, 14.83) | 16.90 (16.17, 17.71) | 12.02 (11.55, 12.67) |
| 2004 | 14.76 (14.37, 15.26) | 17.60 (16.83, 18.37) | 12.25 (11.79, 12.91) |
| 2005 | 15.03 (14.68, 15.48) | 17.94 (17.25, 18.56) | 12.46 (12.04, 12.98) |
| 2006 | 15.33 (14.95, 15.69) | 18.31 (17.45, 18.90) | 12.70 (12.34, 13.09) |
| 2007 | 15.89 (15.38, 16.28) | 19.14 (18.05, 19.86) | 13.03 (12.66, 13.41) |
| 2008 | 16.69 (16.11, 17.08) | 20.25 (19.06, 20.94) | 13.53 (13.11, 13.93) |
| 2009 | 17.51 (16.93, 17.91) | 21.38 (20.13, 22.05) | 14.06 (13.65, 14.48) |
| 2010 | 18.27 (17.68, 18.71) | 22.48 (21.15, 23.23) | 14.52 (14.06, 14.98) |
| 2011 | 18.95 (18.34, 19.41) | 23.28 (22.01, 24.08) | 15.05 (14.56, 15.60) |
| 2012 | 19.61 (18.95, 20.12) | 24.03 (22.86, 24.90) | 15.59 (15.01, 16.19) |
| 2013 | 20.10 (19.33, 20.69) | 24.50 (23.24, 25.44) | 16.06 (15.35, 16.72) |
| 2014 | 21.02 (20.29, 21.66) | 25.68 (24.33, 26.77) | 16.69 (15.99, 17.44) |
| 2015 | 21.97 (21.19, 22.73) | 27.00 (25.54, 28.21) | 17.28 (16.52, 18.12) |
| 2016 | 22.54 (21.62, 23.41) | 27.82 (26.06, 29.27) | 17.63 (16.74, 18.56) |
| 2017 | 22.42 (21.20, 23.49) | 27.67 (25.57, 29.33) | 17.56 (16.21, 18.72) |
4.
Age-standardized prevalence rate of colorectal cancer (CRC) in both genders, male and female only. Results were obtained from the data of Institute for Health Metrics and Evaluation (IHME) Global Burden of Disease (GBD) from 1990 to 2017 (per 100,000).
Gender differences of CRC death and prevalence in different age groups
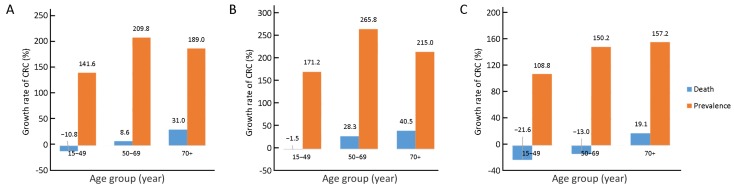
The death and prevalence data of CRC in 1990 were compared to those data in 2017 in age groups of 15−49 years old, 50−69 years old and 70+ years old (Figure 5). The results revealed a higher prevalence rate of CRC in both genders in all three age groups in 2017 (141.6%, 209.8% and 189.0%, respectively), while the death rate of CRC increased in age groups older than 50 years in both genders (8.6% in 50−69 year-old group and 31.0% in 70+ year-old group), but decreased in 15−49 year-old group (−10.8%).
5.
Comparison of age-related death rate and prevalence rate of colorectal cancer (CRC) between 1990 and 2017. Data were presented in both genders (A), male only (B) and female only (C).
We then analyzed the data based on gender of patients. The result showed that the prevalence rate of CRC in male grew higher in 2017 compared to that in 1990 in all three age groups (171.2%, 265.8% and 215.0%, respectively), the death rate of CRC increased in age groups older than 50 years (28.3% in 50−69 year-old group and 40.5% in 70+ year-old group), but decreased in age group of 15−49 years old (−1.5%). Similarly, the prevalence rate of CRC was higher in all three age groups in 2017 compared to those in 1990 (108.8%, 150.2% and 157.2%, respectively). Interestingly, the death rate of CRC in female significantly decreased in age group of 15−49 and 50−69 years old (−21.6% and −13.0%, respectively), but increased in age group of 70+ years old (19.1%).
Age characteristics in CRC death, incidence and prevalence
The apparent discordancy between younger and older patients with respect to CRC-related death, incidence and prevalence rates prompted us to an age-stratified analysis of trends. According to the data in 2017 at the official website of the IHME, patient data were grouped based on every 5 years of age. From the data discussed above, it was concluded that the incidence of CRC was less than 20/100,000, the prevalence of CRC was less than 100/100,000 and the death rate of CRC was less than 5/100,000 in both male and female in groups younger than 45 years old. However, once the age of patients reached 60 years or older, the incidence of CRC surged to about 100/100,000, the prevalence of CRC surged to about 500/100,000 and the death rate increased to about 30/100,000. Similarly, the prevalence rate of CRC peaked at the age of 75−79 years old with a rate of 791.87/100,000. However, the incidence and death rate increased with age, reaching 229.39/100,000 and 173.06/100,000 in the 90−94 year-old age group (Table 1).
Discussion
Many changes in society are expected to impact the incidence, prevalence and death of CRC, especially the increasing use of screening methods for CRC and the rapid development of the country. At present, there are little data reported on whether these developments have affected CRC-associated incidence, prevalence and death. The data network platform of IHME is currently the most representative and authoritative continuous data system on the incidence, prevalence and death of CRC all over the world, and data from each involved country are very detailed, including China. The published data set can reflect trends on incidence, prevalence and death of various diseases and present characteristics on different genders, ages and territories. The present study documented these changes and revealed a striking increasing trend of CRC-related incidence, prevalence and death in different age groups. Meanwhile, the prevalence rate of CRC, which is common in Western countries, has been increasing rapidly in Asian countries, including China and South Korea, in recent decades (4,12-14). Our data call for programs aimed at reducing the burden of CRC in China.
A secondary analysis was involved in the current study based on data of the official website of IHME between 1990 and 2017.
According to data from IHME, CRC ranked 21st in all causes of death in 1990, while it ranked 11th in 2017. The standardized death rate of CRC increased insignificantly from 9.33/100,000 in 1990 to 10.10/100,000 in 2017. The standardized incidence rate of CRC increased from 12.18/100,000 in 1990 (95% UI: 11.45, 13.41) to 22.42/100,000 in 2017 (95% UI: 21.20, 23.49). CRC is one of the most diagnosed cancers in the United States, the American Cancer Society estimated that there were 140,250 newly diagnosed CRC patients and 50,630 death caused by CRC in 2018 (15). Similarly, CRC is the first tumor by incidence in European Region (ER) according to the World Health Organization (WHO), with 471,000 new cases each year and a mean death rate of 28.2 per 100,000 population (16). Besides, CRC incidence and death rate in low- and middle-income countries are increasing (4,17). It is the fifth most common cancer in sub-Saharan Africa (18). CRC incidence and death rate in a population are also related to the prevalence of some modifiable risk factors. For CRC, those modifiable risk factors, such as physical activity, smoking, alcohol drinking and diet, have drawn attention (19). One of the debatable risk factors is fat-intake. Previous study has shown that CRC risk was higher in high fat-intake groups than that in low fat-intake groups (20). However, a recent systematic review and meta-analysis reported that dietary fats and fatty acids had no effects on the risk of CRC based on a systematic analysis of publications found on PubMed, Web of Science, and the Cochrane library for articles related to dietary fat and the risk of CRC (21). Independent of exercise and obesity, prolonged sedentary TV watching time, a surrogate for a more inactive lifestyle, was found associated with increased risk of young-onset CRC (yCRC), particularly of rectal cancer (22). In addition to the above discussed factors, the death rate of CRC is also related to race. Alshareef et al. investigated the correlation between race and death among CRC patients in the US during 2007−2014 based on a retrospective cohort study using data from the Surveillance, Epidemiology, and End Results (SEER) Program. They observed a significant increased risk of death in black and American Indian/Alaska Native patients with CRC compared to white patients (23). This study showed that although the incidence of CRC has increased significantly, the death rate of CRC did not change significantly. The reason was closely related to the progress of a series of diagnostic and therapeutic measures, such as colonoscopy screening, standardized surgical methods, neoadjuvant radiotherapy and chemotherapy, targeted therapy, and so on (24,25).
We compared the death and prevalence data of CRC in 2017 to those data in 1990 in three age groups including 15−49 years old, 50−69 years old and 70+ years old. The result revealed a trend of higher prevalence rate of CRC in both genders in all three age groups in 2017, compared to that in 1990, and that the death rate of CRC in patients older than 50 years old increased in both genders. Over the past 20 years, the prevalence of CRC has increased significantly in both males and females in all age groups. According to the incidence data in 2017, the incidence of CRC was less than 20/100,000 in both male and female in groups younger than 45 years old. However, once the age of patients reached 60 or older, the incidence of CRC surged to about 100/100,000, and peaked at the age of 90−94 years old with a rate of 229.39/100,000. CRC is the third leading cause of cancer-related death in the USA (15), a measurable increase of CRC incidence among individuals younger than age 50, namely yCRC, has been observed, which can also be traced back to 1990 (26-28). Besides, an increase of yCRC incidence was also reported. Those patients with yCRC are typically under the age for a routine CRC screening (29). Routine screening of adults over 50 years old has contributed to a decline in the incidence of CRC in this population (30). On the other hand, some registries reported a rising incidence of CRC even among young adults at 20−39 years of age, although the absolute incidence in this age group remains far lower than the incidence in adults over 50 years old (31,32). Randomized controlled trials have shown that screening is associated with a reduction in death rate (33). Furthermore, CRC screening based on stool testing and flexible sigmoidoscopy has reduced CRC-related death rate by 16% and 22%−31%, respectively (34,35). The WHO ER examined the association factors in CRC surveillance epidemiology and screening, and found that available resources (as measured by gross national income) appear to be the major factor in the Colorectal Cancer Surveillance Epidemiology and Screening (36). According to results from studies focused on cost-effectiveness, CRC screening was cost-effective compared with no screening (37). Recently, the American Center Society has adjusted its guidelines and proposed lowering the age for average risk CRC screening from 50 years old to 45 years old (38). Further studies are needed to evaluate testing strategies based on age and risk for individuals. It is hoped that by trying to reduce the screening age of colonoscopy to deal with precancerous lesions of CRC as early as possible and to change people’s lifestyle, the incidence of CRC can be further reduced through the above two means.
Limitations exist in terms of comprehensiveness and timeliness of information in our research. For instance, we were only able to analyze data on the national prevalence, incidence and death of CRC, other parameters such as geographical characteristics, risk factors of morbidity, the course of disease and other information on CRC were still lacking. In addition, since the data set was a summary of cases reported by different Chinese researchers, the phenomenon of report omission was inevitable, indicating that our research data are underestimated. Further special survey can be carried out focusing on risk factors of CRC to improve the relevant information.
Conclusions
Our study presented evidence for a steady increase in CRC incidence and prevalence in China over the past two decades. The prevention and management of known risk factors, such as smoking, alcohol drinking and diet, should be improved through effective national policies. Direct measures and strategies aimed at lowering risk factors and improving treatment should be proposed to stop the growth trend of CRC incidence, and help to decrease the burden of CRC.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 81541050), National Key Technologies R&D Program (No. 2015BAI13B09), the Beijing Natural Science Foundation of China (No. 7154191, 7184198), the Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (Code: ZYLX201504), and the Beijing Municipal Administration of Hospitals’ Youth Program (No. QML20160105). Support Project of High-level Teachers in Beijing Municipal Universities in the Period of 13th Five-year Plan (No. IDHT20170516). National Key Technologies R&D Program of China (No. 2017YFC0110904). Basic-Clinical Cooperation Program from Capital Medical University (No. 17JL18).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Contributor Information
Xin Gao, Email: gaoxin@ncncd.Chinacdc.cn.
Zhongtao Zhang, Email: zhangzht@medmail.com.cn.
References
- 1.Chen W, Zheng R, Zhang S, et al Cancer incidence and mortality in China in 2013: an analysis based on urbanization level. Chin J Cancer Res. 2017;29:1–10. doi: 10.21147/j.issn.1000-9604.2017.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, et al Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–24. doi: 10.3322/caac21492. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Fedewa SA, et al Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177–93. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 4.Arnold M, Sierra MS, Laversanne M, et al Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–91. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 5.Chen W, Zheng R, Baade PD, et al Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 6.Zheng R, Zeng H, Zhang S, et al National estimates of cancer prevalence in China, 2011. Cancer Lett. 2016;370:33–8. doi: 10.1016/j.canlet.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Zheng ZX, Zheng RS, Zhang SW, et al Colorectal cancer incidence and mortality in China, 2010. Asian Pac J Cancer Prev. 2014;15:8455–60. doi: 10.7314/APJCP.2014.15.19.8455. [DOI] [PubMed] [Google Scholar]
- 8.GBD 2017 Mortality Collaborators Global, regional, and national age-sex-specific mortality and life expectancy, 1950−2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1684–735. doi: 10.1016/S0140-6736(18)31891-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alan DL, Colin DM, Majid E, et al. Global Burden of Disease and Risk Factors. New York: Oxford University Press, 2006.
- 10.GBD 2017 Causes of Death Collaborators Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980−2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–88. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990−2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu W, Jiang J, Xie L, et al Mortality Trends in Colorectal Cancer in China During 2000−2015: A Joinpoint Regression and Age-Period-Cohort Analysis. Prev Chronic Dis. 2018;13; 15:E156. doi: 10.5888/pcd15.180329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng RS, Sun KX, Zhang SW, et al Report of cancer epidemiology in China, 2015. Zhonghua Zhong Liu Za Zhi. 2019;41:19–28. doi: 10.3760/cma.j.issn.0253-3766.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Sung JJ, Lau JY, Goh KL, et al Increasing incidence of colorectal cancer in Asia: implications for screening. Lancet Oncol. 2005;6:871–6. doi: 10.1016/S1470-2045(05)70422-8. [DOI] [PubMed] [Google Scholar]
- 15.American Cancer Society. Cancer Facts & Figures 2018. Atlanta: American Cancer Society, 2018. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2018/cancer-facts-and-figures-2018.pdf
- 16.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence And Mortality Worldwide: IARC Cancer Base No. 11. Lyon: IARC Scientific Publication, 2014.
- 17.Shah D, Makharia GK, Ghoshal UC, et al Burden of gastrointestinal and liver diseases in India, 1990-2016. Indian J Gastroenterol. 2018;37:439–45. doi: 10.1007/s12664-018-0892-3. [DOI] [PubMed] [Google Scholar]
- 18.Bray F, Colombet M, Mery L, et al. Cancer Incidence in Five Continents, Vol XI. Lyon: IARC Scientific Publication, 2017.
- 19.American Cancer Society. Colorectal Cancer Risk Factors. Available online: https://www.cancer.org/cancer/colon-rectal-cancer/causes-risks-prevention/risk-factors.html
- 20.Chun YJ, Sohn SK, Song HK, et al Associations of colorectal cancer incidence with nutrient and food group intakes in Korean adults: a case-control study. Clin Nutr Res. 2015;4:110–23. doi: 10.7762/cnr.2015.4.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim M, Park K Dietary fat intake and risk of colorectal cancer: a systematic review and meta-analysis of prospective studies. Nutrients. 2018;10:E1963. doi: 10.3390/nu10121963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen LH, Liu PH, Zheng X, et al Sedentary Behaviors, TV Viewing Time, and Risk of Young-Onset Colorectal Cancer. JNCI Cancer Spectr. 2018;(2):pky073. doi: 10.1093/jncics/pky073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alshareef SH, Alsobaie NA, Aldeheshi SA, et al Association between race and cancer-related mortality among patients with colorectal cancer in the United States: A Retrospective Cohort Study. Int J Environ Res Public Health. 2019;16:E240. doi: 10.3390/ijerph16020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu Z, Cheng P, Yang F, et al Long-term outcomes in patients with ypT0 rectal cancer after neoadjuvant chemoradiotherapy and curative resection. Chin J Cancer Res. 2018;30:272–81. doi: 10.21147/j.issn.1000-9604.2018.02.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H, Guo J, Gao S, et al Prognostic factors for transarterial chemoembolization combined with sustained oxaliplatin-based hepatic arterial infusion chemotherapy of colorectal cancer liver metastasis. Chin J Cancer Res. 2017;29:36–44. doi: 10.21147/j.issn.1000-9604.2017.01.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Cancer Society. Colorectal Cancer Facts & Figures 2017-2019. Atlanta: American Cancer Society, 2017. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/colorectal-cancer-facts-and-figures/colorectal-cancer-facts-and-figures-2017-2019.pdf
- 27.Murphy CC, Singal AG, Baron JA, et al Decrease in incidence of young-onset colorectal cancer before recent increase. Gastroenterology. 2018;155:1716–9.e4. doi: 10.1053/j.gastro.2018.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Venugopal A, Stoffel EM Colorectal Cancer in Young Adults. Curr Treat Options Gastroenterol. 2019;17:89–98. doi: 10.1007/s11938-019-00219-4. [DOI] [PubMed] [Google Scholar]
- 29.Bailey CE, Hu CY, You YN, et al Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg. 2015;150:17–22. doi: 10.1001/jamasurg.2014.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy CC, Sandler RS, Sanoff HK, et al Decrease in incidence of colorectal cancer among individuals 50 years or older after recommendations for population-based screening. Clin Gastroenterol Hepatol. 2017;15:903–9.e6. doi: 10.1016/j.cgh.2016.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh KE, Taylor TH, Pan CG, et al Colorectal cancer incidence among young adults in California. J Adolesc Young Adult Oncol. 2014;3:176–84. doi: 10.1089/jayao.2014.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tawadros PS, Paquette IM, Hanly AM, et al Adenocarcinoma of the rectum in patients under age 40 is increasing: impact of signet-ring cell histology. Dis Colon Rectum. 2015;58:474–8. doi: 10.1097/DCR.0000000000000318. [DOI] [PubMed] [Google Scholar]
- 33.Fitzpatrick-Lewis D, Ali MU, Warren R, et al Screening for colorectal cancer: A systematic review and meta-analysis. Clin Colorectal Cancer. 2016;15:298–313. doi: 10.1016/j.clcc.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Heresbach D, Manfredi S, D’halluin PN, et al Review in depth and meta-analysis of controlled trials on colorectal cancer screening by faecal occult blood test. Eur J Gastroenterol Hepatol. 2006;18:427–33. doi: 10.1097/00042737-200604000-00018. [DOI] [PubMed] [Google Scholar]
- 35.Schoen RE, Pinsky PF, Weissfeld JL, et al Colorectal cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med. 2012;366:2345–57. doi: 10.1056/NEJMoa1114635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altobelli E, Rapacchietta L, Marziliano C, et al Differences in colorectal cancer surveillance epidemiology and screening in the WHO European Region. Oncol Lett. 2019;17:2531–42. doi: 10.3892/ol.2018.9851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirst Y, Kerrison R, Kobayashi LC, et al Text Reminders in Colorectal Cancer Screening (TRICCS): Protocol for a randomised controlled trial. BMC Public Health. 2016;16:74. doi: 10.1186/s12889-016-2733-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolf AMD, Fontham ETH, Church TR, et al Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;68:250–81. doi: 10.3322/caac.21457. [DOI] [PubMed] [Google Scholar]