Abstract
Objective
Crizotinib has demonstrated promising efficacy in patients with anaplastic lymphoma kinase (ALK)-positive non-small-cell lung cancer (NSCLC) in clinical trials. We conducted this retrospective multicenter study to assess the outcomes of crizotinib therapy in, to our knowledge, a large sample cohort of patients with ALK-positive advanced NSCLC.
Methods
We reviewed the medical records of 484 unselected ALK-positive NSCLC patients treated with crizotinib at 5 cancer centers in China from January 2013 to November 2017. Clinical data were collected from the initiation of crizotinib therapy to Response Evaluation Criteria in Solid Tumors (RECIST)-defined progressive disease (PD).
Results
A total of 428 eligible ALK-positive NSCLC patients were enrolled, 273 (63.8%) of whom received crizotinib as first-line treatment. The median progression-free survival (PFS) and overall survival (OS) from the initiation of crizotinib treatment were 14.4 [95% confidence interval (95% CI), 12.4−16.4] months and 53.4 (95% CI, 33.7−73.1) months, respectively. In subgroup analyses, patients who received crizotinib as first-line treatment showed a higher disease control rate (DCR) and a longer median OS compared with second-/later-line crizotinib treatment (94.8% and OS not reachedvs. 89.0% and 40.5 months, respectively). For 261 patients with RECIST-defined PD, multivariate Cox analysis revealed that in patients who received first-line crizotinib therapy, continued crizotinib beyond progressive disease (CBPD) and next-generation ALK inhibitors after crizotinib failure were associated with improved survival.
Conclusions
This study has demonstrated the clinically meaningful benefit of crizotinib treatment in a large cohort of Chinese ALK-positive NSCLC patients. CBPD and next-generation ALK inhibitor treatment may provide improved survival after RECIST-defined progression on crizotinib.
Keywords: Crizotinib, anaplastic lymphoma kinase, non-small-cell lung cancer, real-world study
Introduction
Anaplastic lymphoma kinase (ALK) gene rearrangements are found in approximately 3%−7% of non-small-cell lung cancer (NSCLC) patients (1), with a higher incidence (9.7%) in the Chinese population (2). Crizotinib is a first-generation inhibitor of ALK-kinase activity and it has demonstrated superiority over conventional chemotherapy in advanced ALK-positive NSCLC in a series of PROFILE clinical trials (3-7). Several small, single-center retrospective studies also have shown the great efficacy of crizotinib in real-world clinical settings (8-10). However, large-scale data on the clinical application and patient outcomes associated with crizotinib in China are still limited.
The phase III PROFILE 1014 study had established the role of first-line crizotinib therapy for newly diagnosed ALK-positive NSCLC patients, and its updated survival analysis reported a survival probability at 4 years of 56.6% (11). The long-term survival was based on the significant effect of crizotinib on progression-free survival (PFS), and the impact of highly effective post-progression therapy on the outcome could not be ignored. Continuing crizotinib beyond progressive disease (CBPD) may be potentially beneficial and was recommended in patients with asymptomatic or isolated lesion progression (12). The newer generation ALK inhibitors (e.g. ceritinib, alectinib, brigatinib and lorlatinib) have been found to be effective for patients who experience progression on crizotinib in early phase clinical trials (13-16) and they may provide better survival benefit in comparison with other systemic treatment options. In addition, the efficacy of conventional chemotherapy after the failure of crizotinib is still debatable (17). So real-world investigations on the survival outcomes of different sequencing therapies are still needed to inform the optimal treatment after progressive disease (PD) on crizotinib.
We therefore carried out a large, multicenter, real-world study to evaluate the treatment patterns and outcomes with crizotinib therapy in an unselected population of ALK-positive NSCLC patients. We also explored the effects of different post-progression systemic treatments on survival to provide evidence for treatment options in clinical practice.
Materials and methods
Study population and procedures
We retrospectively collected data on consecutive ALK-positive patients who were treated from January 2013 to November 2017 at 5 comprehensive cancer centers in China (all of which had enrolled at least 10 eligible patients). All the patients who met the following criteria were retrospectively included: 1) histologically- or cytologically-diagnosed locally advanced, recurrent or metastatic NSCLC; 2) positive for an ALK rearrangement; 3) aged 18 years old or older; and 4) received at least 21 d of crizotinib treatment. In this study, crizotinib was administered orally at a dose of 250 mg twice daily, and proper dose adjustment or drug discontinuation was given due to adverse reactions. The data were collected from the time of the primary NSCLC diagnosis until the patients’ death or the end of the study period. Clinical data were extracted from medical records, and survival information was obtained from telephone follow-up by investigators at each center. The study was approved by the Ethics Committee of Cancer Hospital, Chinese Academy of Medical Sciences (Approval No. 18-082/1660).
Histology and molecular testing
Tumor histology was classified by the pathologists in the Department of Pathology at each center using the standard World Health Organization criteria. A positive ALK status was detected by one of the following testing methods: 1) a fluorescence in situ hybridization (FISH) assay using the Vysis ALK Break Apart FISH probe kit (Abbott Molecular, IL, USA); 2) Ventana immunohistochemistry (IHC) anti-ALK (D5F3); 3) quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) technology; and 4) next-generation sequencing (NGS) methods. Epidermal growth factor receptor (EGFR) mutations were also analyzed by using a direct DNA sequencing method or NGS technology.
Evaluation criteria
All patients were evaluated by computed tomography (CT) of the thorax and abdomen, enhanced magnetic resonance imaging (MRI) of the brain, and a whole body bone scan before the initiation of therapy. In addition, routine hematology tests, biochemistry analyses, and electrocardiograms (ECGs) were performed. Treatment responses were evaluated one month after crizotinib initiation and then approximately every two months during crizotinib treatment until drug withdrawal. Adverse events were classified and graded according to the Common Terminology Criteria for Adverse Events, version 4.0.
Definitions and study endpoints
Based on the treatment history retrospectively obtained from the medical records, all patients were divided into a first-line crizotinib therapy group and a second-/later-line crizotinib therapy group. Patients who continued crizotinib following Response Evaluation Criteria in Solid Tumors (RECIST)-defined PD were defined as the CBPD group. The subsequent drugs administered following crizotinib failure and the responses to them were monitored. The study endpoint was PFS (from crizotinib initiation to the first RECIST-defined PD or death from any cause) and overall survival (OS) (from crizotinib initiation to death or the last follow-up).
Statistical analysis
Two-sided Fisher’s exact tests were used for analyzing patients’ basic characteristics and comparing response rates between the different groups. The Kaplan-Meier method was applied to estimate PFS and OS endpoints, and Cox proportional hazard models were used to assess the impacts of various factors on survival outcomes. A P-value of less than 0.05 was considered to indicate statistical significance. Statistical analysis was performed at the last study follow-up date (Nov 30, 2017) using IBM SPSS Statistics (Version 19.0; IBM Corp., New York, USA).
Results
Patient characteristics and treatments
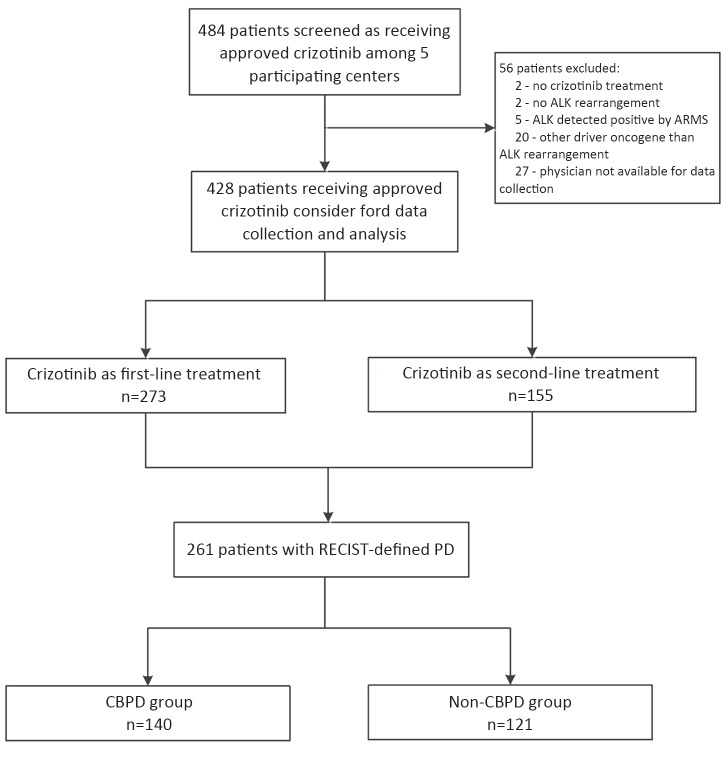
A total of 484 patients were enrolled for data collection, and 428 with advanced ALK-positive NSCLC met the inclusion criteria (Supplementary Figure S1). The baseline demographic and clinical characteristics of the patients are presented in Table 1.
S1.
Patient flow in the study. ALK, anaplastic lymphoma kinase; ARMS, amplification refractory mutation system; RECIST, Response Evaluation Criteria in Solid Tumors; PD, progressive disease; CBPD, crizotinib beyond progressive disease.
1.
Baseline characteristics of population at the time of crizotinib initiation (N=428)
| Characteristics | n | % |
| ECOG PS, Eastern Cooperative Oncology Group performance status. | ||
| Age (year) | ||
| Median (range) | 51 (18−82) | |
| <65 | 377 | 88.1 |
| ≥65 | 51 | 11.9 |
| Sex | ||
| Male | 207 | 48.4 |
| Female | 221 | 51.6 |
| Smoking status | ||
| Former-smoker | 125 | 29.2 |
| Never-smoker | 303 | 70.8 |
| Histology | ||
| Adenocarcinoma | 413 | 96.5 |
| Squamous carcinoma | 4 | 0.9 |
| Large cell | 3 | 0.7 |
| Other | 8 | 1.9 |
| ECOG PS | ||
| 0 | 251 | 58.6 |
| 1 | 148 | 34.6 |
| 2 | 21 | 4.9 |
| 3 | 8 | 1.9 |
| Stage | ||
| IIIA−IIIB | 27 | 6.3 |
| IV | 401 | 93.7 |
| Brain metastasis | ||
| Yes | 95 | 22.2 |
| No | 333 | 77.8 |
| Line of therapy before crizotinib | ||
| 0 | 273 | 63.8 |
| 1 | 88 | 20.6 |
| ≥2 | 67 | 15.6 |
| Metastatic lesions except brain metastasis | ||
| Lung | 190 | 44.4 |
| Pleural | 157 | 36.7 |
| Liver | 69 | 16.1 |
| Bone | 144 | 33.6 |
| Adrenal gland | 28 | 6.5 |
| Lymph node | 281 | 65.7 |
| Others | 36 | 8.4 |
The patients’ median age was 51 (range: 18−82) years and 93.7% (401/428) of patients had stage IV disease. In total, 399 patients (93.2%) had an Eastern Cooperative Oncology Group (ECOG) performance score (PS) of 0−1 and 95 patients (22.2%) had brain metastasis at baseline. A total of 273 patients (63.8%) received crizotinib as a first-line regimen, 88 (20.6%) as second-line treatment, and 67 (15.7%) as third or further-line treatment. Other first-line therapies included chemotherapy (n=140; a pemetrexed-based regimen accounted for 66.4%) and EGFR-tyrosine kinase inhibitors (EGFR-TKIs) (n=15; administration of EGFR-TKIs included 2 patients with simultaneousEGFR exon 21 mutations and 13 patients who received EGFR-TKIs treatment for over 21 d before the final genetic test results were available or for economic reasons). The median number of days to initiation of crizotinib treatment after the diagnosis of metastatic NSCLC was 50 d. At the time of analysis, 220 patients were still receiving crizotinib. The main reason for drug withdrawal in 200 patients was disease progression (189/200, 94.5%).
Efficacy of crizotinib treatment
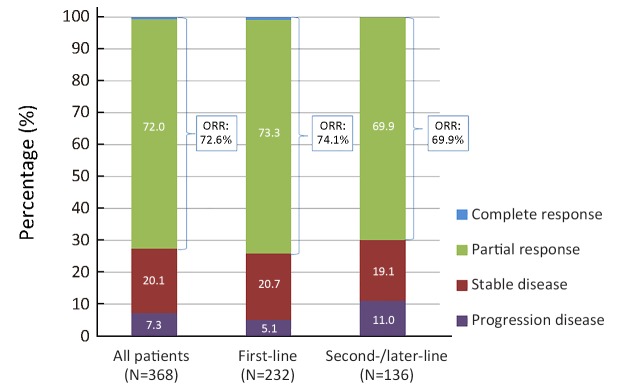
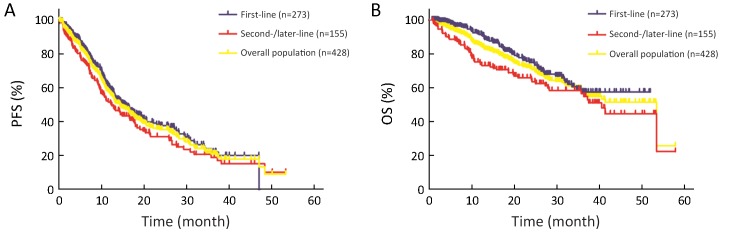
Of the 368 patients with evaluable lesions at baseline, complete response (CR) was achieved in 2 cases, partial response (PR) in 265 cases, stable disease (SD) in 74 cases, and PD in 27 patients; thus, the objective response rate (ORR) was 72.6% (267/368), and the disease control rate (DCR) was 92.7% (341/368). The ORR of the 232 patients with evaluable lesions who received first-line crizotinib was higher than that of patients of the second-/later-line group (74.1% vs. 69.9%, P=0.791), and the DCR was also significantly superior in the first-line therapy group (94.8% vs. 89.0%, P=0.038) (Figure 1). During a median follow-up duration of 18.7 months [range, 0.72−57.9; interquartile range (IQR), 10.2−28.5], 263 (61.4%) patients had disease progression. The median PFS with initial crizotinib therapy was 14.4 [95% confidence interval (95% CI), 12.350−16.371] months, and the median treatment duration of crizotinib therapy was 13.6 months. The median PFS of the 273 patients who received crizotinib as first-line therapy was longer than that of the second-/later-line therapy group, but the difference was not statistically significant [15.5 (95% CI, 12.354−18.728) monthsvs. 12.8 (95% CI, 9.722−15.786) months; hazard ratio (HR)=0.810; P=0.093) (Supplementary Figure S2A).
1.
Best clinical responses to crizotinib treatment in patients with evaluable lesions (n=368); the objective response rate (ORR) and disease control rate (DCR) for first-line vs. second-/later-line crizotinib were 74.1% vs. 69.9% (P=0.791) and 94.8% vs. 89.0% (P=0.038), respectively.
S2.
Kaplan-Meier curves of progression-free survival (PFS) (A) and overall survival (OS) (B) for 428 patients. Patients who received first-line treatment showed longer PFS and OS than patients in the second-/later-line therapy group [PFS: 15.5 (95% CI, 12.354−18.728) monthsvs. 12.8 (95% CI, 9.722−15.786) months; HR=0.810; P=0.093); OS: not reached (95% CI not estimated)vs. 40.5 months (95% CI, 34.650−46.333); HR=0.664; P=0.023]. 95% CI, 95% confidence interval; HR, hazard ratio.
OS analysis
Eleven patients were lost to follow-up and 125 patients had died by the time of the last follow-up. The median OS from the initiation of crizotinib was 53.4 (95% CI, 33.699−73.055) months (Supplementary Figure S2B). The 1-, 2- and 3-year survival rates were 83%, 70% and 57%, respectively. In the subgroup analysis, the median OS for the first-line therapy group had not been reached but was significantly longer in comparison with the second-/later-line therapy group [not reached (95% CI not estimated) vs. 40.5 months (95% CI, 34.650−46.333); HR=0.664; P=0.023] (Supplementary Figure S2B). The 1-, 2- and 4-year OS rate for the patients who used crizotinib as first-line therapy was 92%, 75% and 59.0%, respectively.
We further analyzed the effects of several possible factors that may influence OS. The multivariable Cox regression analysis showed that patients with an ECOG PS score of 0−1 had significantly longer survival than those with an ECOG PS score of ≥2 (median OS, 53.4 monthsvs. 10.3 months, HR=0.267; 95% CI, 0.161−0.444; P<0.001). The baseline brain metastasis status (P=0.843) and prior lines of crizotinib therapy (P=0.069) had no significant impact on survival.
Effect on OS of systemic treatments following RECIST-defined progression on crizotinib
Since there were 2 patients lost to follow-up after disease progression, 261 patients with documented RECIST-defined progression on crizotinib were analyzed, with a median OS from the time of crizotinib progression of 15.3 (95% CI, 11.376−19.181) months. Multivariate Cox analysis revealed that patients who received first-line crizotinib therapy, with ≥ median PFS, continued CBPD and received next-generationALK inhibitors after crizotinib failure were associated with improved survival, both from the time of crizotinib progression and from the initiation of crizotinib treatment (Table 2).
2.
Cox multivariate analysis of OS from time of disease progression on crizotinib therapy and from time of initial crizotinib treatment in patients with documented progressive disease (N=261)
| Characteristics | OS from crizotinib progression | OS from crizotinib initiation | |||||||
| Univariable analysis | Multivariable analysis | Univariable analysis | Multivariable analysis | ||||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | ||
| OS, overall survival; ECOG PS, Eastern Cooperative Oncology Group performance status; PFS, progression-free survival; NA, not available; CBPD, crizotinib beyond progressive disease; ALK, anaplastic lymphoma kinase; HR, hazard ratio; 95% CI, 95% confidence interval. | |||||||||
| ECOG PS | |||||||||
| 0−1vs. 2−3 | 0.385 (0.236−0.630) | <0.001 | 0.909 (0.525−1.574) | 0.732 | 0.300 (0.184−0.490) | <0.001 | 0.635 (0.366−1.102) | 0.107 | |
| Brain metastasis | |||||||||
| Yes vs. No | 0.881 (0.578−1.344) | 0.557 | 0.867 (0.555−1.355) | 0.530 | 0.917 (0.598−1.405) | 0.690 | 0.870 (0.556−1.361) | 0.543 | |
| No. of treatment lines before crizotinib | |||||||||
| 0 vs. ≥1 | 0.723 (0.515−1.040) | 0.082 | 0.646 (0.439−0.951) | 0.027 | 0.730 (0.512−1.040) | 0.081 | 0.610 (0.413−0.901) | 0.013 | |
| PFS with crizotinib | |||||||||
| ≥median vs.
<median |
0.478 (0.320−0.716) | <0.001 | 0.534 (0.353−0.809) | 0.003 | 0.236 (0.156−0.359) | <0.001 | 0.188 (0.121−0.291) | <0.001 | |
| Progression pattern | |||||||||
| Extracranial
progress vs. intracranial progress |
1.606 (1.031−2.502) | 0.036 | 1.449 (0.883−2.379) | 0.142 | 1.748 (1.120−2.730) | 0.014 | 1.415 (0.869−2.303) | 0.163 | |
| Comprehensive
progress vs. intracranial progress |
9.314 (5.543−15.649) | <0.001 | 7.322 (3.964−13.526) | <0.001 | 6.229 (3.760−10.321) | <0.001 | 5.408 (2.955−9.895) | <0.001 | |
| NA vs.
intracranial progress |
1.626 (0.678−3.900) | 0.277 | 1.330 (0.519−3.411) | 0.553 | 2.356 (0.980−5.665) | 0.056 | 1.569 (0.613−4.019) | 0.348 | |
| CBPD | |||||||||
| Yes vs. No | 0.467 (0.326−0.669) | <0.001 | 0.580 (0.372−0.905) | 0.016 | 0.407 (0.284−0.585) | <0.001 | 0.556 (0.354−0.874) | 0.011 | |
| Next-generation ALK inbibitors after progression on crizotinib | |||||||||
| Yes vs. No | 0.459 (0.307−0.686) | <0.001 | 0.310 (0.198−0.483) | <0.001 | 0.550 (0.368−0.824) | 0.004 | 0.309 (0.195−0.489) | <0.001 | |
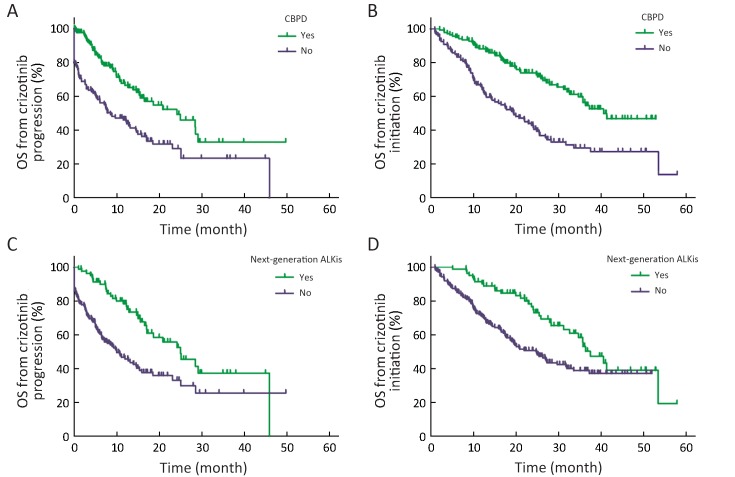
CBPD ± local treatment was documented in 140 patients, while 121 patients were classified as non-CBPDs. The baseline and post-progression characteristics of CBPD and non-CBPD patients were shown in Supplementary Table S1. Patients who never smoked, had an ECOG PS score of 0−1, had only intracranial progression and <median PFS were more frequently in the CBPD population. The median OS from the time of progression on crizotinib was significantly longer in CBPD patients than in non-CBPD patients [median OS 24.1 (95% CI, 14.801−33.396) monthsvs. 8.5 (95% CI, 4.248−12.801) months; HR=0.467; P<0.001] (Supplementary Figure S3A). Similarly, the median OS from the start of initial crizotinib treatment was also longer in the CBPD group than the non-CBPD group [median OS 40.5 (95% CI not estimated) months vs. 19.5 (95% CI, 13.992−24.959) months; HR=0.407; P<0.001] (Supplementary Figure S3B).
S1.
Baseline and post-progression characteristics of patients who continued CBPD and those who did not (N=261)
| Characteristics | Total | Continued CBPD | χ2 | P | |
| No | Yes | ||||
| CBPD, crizotinib beyond progressive disease; ECOG PS, Eastern Cooperative Oncology Group performance status; NA, missing data; PFS, progression-free survival; ALK, anaplastic lymphoma kinase. | |||||
| Age (year) | 1.018 | 0.313 | |||
| <65 | 228 | 103 | 125 | ||
| ≥65 | 33 | 18 | 15 | ||
| Sex | 0.601 | 0.438 | |||
| Male | 127 | 62 | 65 | ||
| Female | 134 | 59 | 75 | ||
| Smoking status | 3.933 | 0.047 | |||
| Former-smoker | 75 | 42 | 33 | ||
| Never-smoker | 186 | 79 | 107 | ||
| Histology | 0.067 | 0.796 | |||
| Adenocarcinoma | 249 | 115 | 134 | ||
| Non-adenocarcinoma | 12 | 6 | 6 | ||
| ECOG PS score | 10.322 | 0.001 | |||
| 0−1 | 238 | 103 | 135 | ||
| 2−3 | 23 | 18 | 5 | ||
| Stage | 0.343 | 0.558 | |||
| IIIA−IIIB | 13 | 5 | 8 | ||
| IV | 248 | 116 | 132 | ||
| Brain metastasis | 1.668 | 0.196 | |||
| Yes | 59 | 23 | 36 | ||
| No | 202 | 98 | 104 | ||
| Line of therapy before crizotinib | 0.010 | 0.921 | |||
| 0 | 154 | 71 | 83 | ||
| ≥1 | 107 | 50 | 57 | ||
| Progression pattern | 67.695 | <0.001 | |||
| Intracranial progress | 94 | 15 | 79 | ||
| Extracranial progress | 113 | 61 | 52 | ||
| Comprehensive progress | 42 | 36 | 6 | ||
| NA | 12 | 9 | 3 | ||
| Initial PFS with crizotinib | 9.959 | 0.002 | |||
| ≥median | 100 | 34 | 66 | ||
| <median | 161 | 87 | 74 | ||
| Next-generation ALK inhibitors after progression on crizotinib | 9.430 | 0.002 | |||
| Yes | 83 | 50 | 33 | ||
| No | 178 | 71 | 107 | ||
S3.
Kaplan-Meier curves for overall survival from the time of progressive disease and from the time of initial crizotinib treatment. (A,B) Crizotinib beyond progressive disease (CBPD) vs. non-CBPD: the median overall survival (OS) from the time of crizotinib progression was significantly longer in the CBPD group than in the non-CBPD group (P<0.001) and the median OS from the start of initial crizotinib treatment was also longer in the CBPD group than in the non-CBPD group (P<0.001); (C,D) Receipt of next-generationALK inhibitors vs. no receipt of next-generation ALK inhibitors: the median OS from the time of crizotinib progression was significantly longer in patients who received next-generation ALK inhibitors (P<0.001), and so was the median OS from the start of initial crizotinib treatment (P=0.004).
A possible reason for the survival advantage seen among CBPD group was that 33 patients received next-generation ALK inhibitors as second-/later-line therapy following crizotinib progression. To address this, we evaluated the impact of receiving next-generation ALK inhibitors after crizotinib failure and found that patients who received next-generation ALK inhibitors following progression on crizotinib had better survival outcomes both from the time of disease progression than those who didn’t [median OS 24.9 (95% CI, 17.166−32.670) monthsvs. 10.7 (95% CI, 6.764−14.547) months; HR=0.459; P<0.001] and from the initiation of crizotinib therapy [median OS 37.4 (95% CI, 31.812−43.073) monthsvs. 24.8 (95% CI, 18.817−30.757) months; HR=0.550; P=0.004] (Supplementary Figures S3C,D).
We further conducted subgroup survival analyses and found that patients who received crizotinib [n=140; median OS, 40.5 months (95% CI not estimated)] or next generation ALK inhibitors [n=42; median OS, 53.4 months (95% CI not estimated)] as the first-line therapy following crizotinib progression both showed better survival outcomes than patients who received chemotherapy [n=33; median OS, 19.5 (95% CI, 12.688−26.263) months] and best supportive care [n=46; median OS, 9.4 (95% CI, 6.381−12.373) months].
Safety and adverse events
The most common toxicity recorded with crizotinib was elevated transaminases (45.5% of patients), with grade 3−4 elevations occurring in 5.4%. Other common adverse events (occurring in >20%) were diarrhea (29.8%), nausea (25.6%), vomiting (21.9%), leukopenia (23.8%), vision disorder (21.2%), and edema (20.7%), which were mainly grade 1 or 2 events. Fifty-five patients had dosage reductions or temporary drug withdrawal during crizotinib treatment, and the main reasons were adverse events (including 9 for a prolonged QTc, 14 for elevated transaminases, and 10 for gastrointestinal reactions). Four patients permanently stopped crizotinib treatment because of gastrointestinal intolerance reactions or grade 3 elevated transaminases.
Discussion
To our knowledge, this study is a large sample multicenter, retrospective study conducted thus far to evaluate efficacy and survival with crizotinib treatment in patients with ALK-positive NSCLC. We demonstrated the clinically meaningful benefit of crizotinib treatment, with a median OS of 53.4 months for the total population, and found that, CBPD and next-generation ALK inhibitor treatment may provide survival improvements after RECIST-defined progression on crizotinib in real-world clinical settings. The OS data of our study was highly consistent with that of PROFILE 1014, in which the survival probability at 4 years with crizotinib was 56.6%.
CBPD might have favorably impact on survival outcomes. Ou et al. (12) reported that median OS values from the time of crizotinib initiation were 29.6 months in the CBPD group and 10.8 months in non-CBPD group, and a similar survival benefit was observed in a French multicenter study (18). In our study, patients in the CBPD group also had an improved OS compared with the non-CBPD group. The persistence of this benefit following disease progression on crizotinib confirmed the validity of the CBPD treatment pattern. A possible reason for the survival advantage seen in the CBPD group was the higher frequency of patients with better ECOG PS scores and only intracranial progression, which can be controlled by local therapy. Moreover, 23.6% of the patients in the CBPD group received next-generation ALK inhibitors following re-progression on crizotinib, which may prolong the survival.
Next-generation ALK inhibitors were effective and well-tolerated drugs for treatment strategies following crizotinib failure, and some of these drugs have even shown superior efficacy to crizotinib in the primary treatment of ALK-positive NSCLC (19). Retrospective studies have reported prolonged OS in patients who received initial therapy with crizotinib followed by ceritinib or alectinib (18,20-22). The updated survival results from the PROFILE 1014 study mentioned above also showed that patients who received crizotinib followed by another ALK inhibitor had the longest OS, while patients randomized to chemotherapy followed by no ALK inhibitor or other treatment had the worst OS (11). This survival benefit was also confirmed by the French retrospective study (18). In our study, we found that next generation ALK inhibitors were associated with a potential survival benefit in comparison with other systemic treatment options, but how to optimally arrange the order of different generation ALK inhibitors is still an unanswered question.
Previous randomized controlled trials (RCTs) have reported a median PFS of 7.7−11.1 months for crizotinib, which was longer than that for standard first-line or second-line chemotherapy (5-7). The median PFS in our study was 14.4 months for the overall population and 15.5 months for first-line crizotinib therapy, and both were longer than results achieved in RCTs. As the baseline characteristics of our patients were similar to those of patients in previous studies (23,24), the longer PFS achieved in our real-world study may be partly due to the higher proportional discontinuance of crizotinib treatment for adverse events in the RCTs. Other limitations in RCTs come from the restrictive inclusion criteria and unalterable treatment patterns, which means that any benefit observed in a selected population might not reflect that in daily practice. So, the large samples from the real-world study act as a supplement to the RCT study, and it can comprehensively evaluate how ALK-positive NSCLC patients benefit from crizotinib.
Several limitations of this retrospective analysis cannot be ignored. Firstly, the characteristics of the groups are partially imbalanced due to bias in patient selection. Secondly, since next-generation ALK inhibitors have not been approved in China, only a small proportion of patients received these treatments by participating in clinical trials or travelling to other countries. Although we provided a general sequencing of treatment after crizotinib failure, we were not able to compare the efficacy of different next-generation ALK inhibitors, and further analyses are therefore needed. Thirdly, as OS was determined in only 30% of patients by the last follow-up, the median OS in the first-line crizotinib therapy group wasn’t reached and it will be necessary to extend the follow-up period to obtain long-term survival data. Fourthly, the adverse event information of some patients was obtained by telephone follow-up, and some subjective adverse events, such as, vision disorder, gastrointestinal reactions and rash, could not be recorded completely. This might be the reason for the relatively lower incidence of adverse events than those recorded in RCTs.
Conclusions
Our study provides the largest real-world evidence on the survival benefit of crizotinib in ALK-positive NSCLC patients, and is highly consistent with the OS result of the PROFILE 1014 study. Further studies comparing the survival benefit of different ALK inhibitors are needed to inform optimal treatment for clinical practice.
Acknowledgements
This work was supported by Pfizer investigator funding.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Soda M, Choi YL, Enomoto M, et al Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–6. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 2.Wang Z, Zhang X, Bai H, et al EML4-ALK rearrangement and its clinical significance in Chinese patients with advanced non-small cell lung cancer. Oncology. 2012;83:248–56. doi: 10.1159/000341381. [DOI] [PubMed] [Google Scholar]
- 3.Camidge DR, Bang YJ, Kwak EL, et al Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13:1011–9. doi: 10.1016/S1470-2045(12)70344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blackhall F, Ross Camidge D, Shaw AT, et al Final results of the large-scale multinational trial PROFILE 1005: efficacy and safety of crizotinib in previously treated patients with advanced/metastatic ALK-positive non-small-cell lung cancer. ESMO Open. 2017;2:e000219. doi: 10.1136/esmoopen-2017-000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw AT, Kim DW, Nakagawa K, et al Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–94. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 6.Solomon BJ, Mok T, Kim DW, et al First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–77. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 7.Wu YL, Lu S, Lu Y, et al Results of PROFILE 1029, a Phase III Comparison of First-Line Crizotinib versus Chemotherapy in East Asian Patients with ALK-Positive Advanced Non-Small Cell Lung Cancer. J Thorac Oncol. 2018;13:1539–48. doi: 10.1016/j.jtho.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 8.Cui S, Zhao Y, Dong L, et al Is there a progression-free survival benefit of first-line crizotinib versus standard chemotherapy and second-line crizotinib in ALK-positive advanced lung adenocarcinoma? A retrospective study of Chinese patients. Cancer Med. 2016;5:1013–21. doi: 10.1002/cam4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen G, Chen X, Zhang Y, et al A large, single-center, real-world study of clinicopathological characteristics and treatment in advanced ALK-positive non-small-cell lung cancer. Cancer Med. 2017;6:953–61. doi: 10.1002/cam4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou J, Zheng J, Zhang X, et al Crizotinib in patients with anaplastic lymphoma kinase-positive advanced non-small cell lung cancer versus chemotherapy as a first-line treatment. BMC Cancer. 2018;18:10. doi: 10.1186/s12885-017-3720-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solomon BJ, Kim DW, Wu YL, et al Final overall survival analysis from a study comparing first-line crizotinib versus chemotherapy in ALK-mutation-positive non-small-cell lung cancer. J Clin Oncol. 2018;36:2251–8. doi: 10.1200/JCO.2017.77.4794. [DOI] [PubMed] [Google Scholar]
- 12.Ou SH, Jänne PA, Bartlett CH, et al Clinical benefit of continuing ALK inhibition with crizotinib beyond initial disease progression in patients with advanced ALK-positive NSCLC. Ann Oncol. 2014;25:415–22. doi: 10.1093/annonc/mdt572. [DOI] [PubMed] [Google Scholar]
- 13.Kim DW, Mehra R, Tan DSW, et al Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol. 2016;17:452–63. doi: 10.1016/S1470-2045(15)00614-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw AT, Gandhi L, Gadgeel S, et al Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol. 2016;17:234–42. doi: 10.1016/S1470-2045(15)00488-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ou SH, Ahn JS, De Petris L, et al Alectinib in crizotinib-refractory ALK-rearranged non-small-cell lung cancer: A phase II global study. J Clin Oncol. 2016;34:661–8. doi: 10.1200/jco.2016.34.4_suppl.661. [DOI] [PubMed] [Google Scholar]
- 16.Shaw AT, Felip E, Bauer TM, et al Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol. 2017;18:1590–9. doi: 10.1016/S1470-2045(18)30649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berge EM, Lu X, Maxson D, et al Clinical benefit from pemetrexed before and after crizotinib exposure and from crizotinib before and after pemetrexed exposure in patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer. Clin Lung Cancer. 2013;14:636–43. doi: 10.1016/j.cllc.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duruisseaux M, Besse B, Cadranel J, et al Overall survival with crizotinib and next-generation ALK inhibitors in ALK-positive non-small-cell lung cancer (IFCT-1302 CLINALK): a French nationwide cohort retrospective study. Oncotarget. 2017;8:21903–17. doi: 10.18632/oncotarget.15746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peters S, Camidge DR, Shaw AT, et al Alectinib versus Crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377:829–38. doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 20.Chiari R, Metro G, Iacono D, et al Clinical impact of sequential treatment with ALK-TKIs in patients with advanced ALK-positive non-small cell lung cancer: Results of a multicenter analysis. Lung Cancer. 2015;90:255–60. doi: 10.1016/j.lungcan.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Gainor JF, Tan DS, De Pas T, et al Progression-free and overall survival in ALK-positive nsclc patients treated with sequential crizotinib and ceritinib. Clin Cancer Res. 2015;21:2745–52. doi: 10.1158/1078-0432.CCR-14-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito K, Hataji O, Kobayashi H, et al Sequential therapy with crizotinib and alectinib in ALK-rearranged non-small cell lung cancer-A multicenter retrospective study. J Thorac Oncol. 2017;12:390–6. doi: 10.1016/j.jtho.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 23.Shaw AT, Yeap BY, Mino-Kenudson M, et al Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27:4247–53. doi: 10.1200/JCO.2009.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gainor JF, Varghese AM, Ou SH, et al ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: an analysis of 1,683 patients with non-small cell lung cancer. Clin Cancer Res. 2013;19:4273–81. doi: 10.1158/1078-0432.CCR-13-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]