Abstract
Purpose:
Monoclonal antibodies (mAbs) are commonly administered via subcutaneous (SC) route. However, bioavailability is often reduced after SC administration. In addition, the sequential transfer of mAbs through the SC tissue and lymphatic system is not completely understood. Therefore, major objectives of this study were a) To understand absorption of mAbs via the lymphatic system after SC administration using physiologically based pharmacokinetic (PBPK) modeling, and b) to demonstrate application of the model for prediction of SC pharmacokinetics (PK) of mAbs.
Methods:
A minimal PBPK model was constructed using various physiological parameters related to the SC injection site and lymphatic system. The remainder of the body organs were represented using a 2-compartment model (central and peripheral compartments), with parameters derived from available intravenous (IV) PK data. The IV and SC clinical PK data of a total of 10 mAbs were obtained from literature. The SC PK data were used to estimate the lymphatic trunk-lymph node (LN) clearance.
Results:
The mean estimated lymphatic trunk-LN clearance obtained from 37 SC PK profiles of mAbs was 0.00213 L/h (0.001332 to 0.002928, 95% confidence intervals). The estimated lymphatic trunk-LN clearance was greater for the mAbs with higher isoelectric point (pI). In addition, the estimated clearance increased with decrease in the bioavailability.
Conclusion:
The minimal PBPK model identified SC injection site lymph flow, afferent and efferent lymph flows, and volumes associated with the SC injection site, lymphatic capillaries and lymphatic trunk-LN as important physiological parameters governing the absorption of mAbs after SC administration. The model may be used to predict PK of mAbs using the relationship of lymphatic trunk-LN clearance and the pI. In addition, the model can be used as a bottom platform to incorporate SC and lymphatic in vitro clearance data for mAb PK prediction in the future.
INTRODUCTION
Monoclonal antibodies (mAbs) are an important class of therapeutic proteins (TPs) administered mainly via subcutaneous (SC) route due to shorter clinical visits for patients, the possibility of self-administration, and its less invasive nature. However, when compared to the intravenous (IV) route, SC injection has challenges associated with the incomplete bioavailability and pain-free administration of larger fluid volumes (1). The SC tissue and lymphatic system are important barriers for the absorption of mAbs. After SC administration, the mAbs travel through the lymphatic vessels and lymph nodes before reaching the systemic circulation. The SC bioavailability of mAbs is typically in the range of 52 to 80% (2, 3). Proteolysis within the lymphatic system and the SC injection site may be partially responsible for the reduced bioavailability of mAbs. The rate of neonatal Fc receptor (FcRn) binding and recycling exceeds the rate of lysosomal/endosomal trafficking of mAbs; therefore, endosomal proteolysis may contribute only minimally to low mAb bioavailability (3, 4). In addition, the target mediated drug disposition (TMDD) is often saturated due to limited receptors. Thus, mAbs are cleared slowly from the systemic circulation. The TPs like IgG1, IgG2 and IgG4 have a long half-life of around 23 days (3), which is substantially longer than other proteins of similar molecular weights. Further, the appearance rate of mAbs in the plasma is slow (Tmax generally 2–14 days) (3). The low bioavailability of mAbs may result from efficient pre-systemic clearance mechanisms or irreversible retention (and subsequent elimination) of significant dose at the injection site or in the surrounding tissues.
Charman et al. investigated the causes of reduced bioavailability of human growth hormone (hGH) protein using a sheep model. The SC injection site degradation was minimal for hGH, while the lymphatic proteolysis was mainly responsible for its reduced bioavailability after SC administration (5). We hypothesize that the interstitial proteolysis in the lymphatic system may be responsible for lower bioavailability of mAbs after SC administration. In this study, a minimal physiologically based pharmacokinetic (PBPK) model was used to understand the sequential transit of mAbs and to estimate lymphatic clearance using the SC pharmacokinetics (PK). The model was constructed using physiological parameters related to the SC injection site, lymphatic system and the reported clinical IV PK data. This study had the following major objectives: a) construction of the minimal PBPK model focusing on the lymphatic transit of mAbs after SC administration using physiologically relevant lymph flows and compartment volumes, b) estimation of the lymphatic clearance using literature SC PK data, and c) identification of the parameters governing the absorption of mAbs via the SC and lymphatic system.
Various PBPK models for TPs have been reported recently and as early as 1995 (Gill et al., Abbuqayyas and Balthasar, Garg and Balthasar, Baxter et al., Shah and Betts) (6–9). Some of the authors (Zhao et al., Chen et al., Elmeliegy et al., Li et al.) proposed the minimal PBPK approach to eliminate complexity associated with the models (3, 10–12). Although, these models may be useful for a mechanistic understanding of the clearance and absorption processes at the SC injection site and in the lymphatic system, use of PBPK modeling for TP PK prediction is limited (13). After SC injection, the mAb travels through the lymphatic capillary network, lymph nodes, lymphatic trunks and thoracic lymph duct before joining the systemic circulation. Hence, in this study, the lymphatic organs were incorporated in the minimal PBPK model, and the lymphatic trunk-lymph node (LN) clearance was estimated using the SC PK. Interestingly, it appears that the estimated lymphatic trunk-LN clearance was directly proportional to the isoelectric point (pI) of the mAb. In addition, the model must be combined in the future with in vitro proteolysis data obtained from the lymphatic system to predict SC PK and bioavailability of mAbs. Further, the variability in the PK due to different populations, disease conditions, formulations, novel delivery technologies, and biophysical properties of the TP may be addressed using the minimal PBPK model (14). Overall, the proposed minimal PBPK model can be used for mechanistic understanding of mAb absorption and prediction of PKPK after SC administration.
METHODS
Digitization of literature IV and SC PK data
The IV (35 profiles) and SC (37 profiles) PK of 10 mAbs were obtained from literature and digitized using WebPlotDigitizer (version 4.1) (15). This web-based tool has been extensively used in other reports (16). The IV PK profiles were used to estimate 2-compartment model parameters, while the SC PK profiles were used to estimate the lymphatic trunk-LN clearance.
Construction of the minimal PBPK model
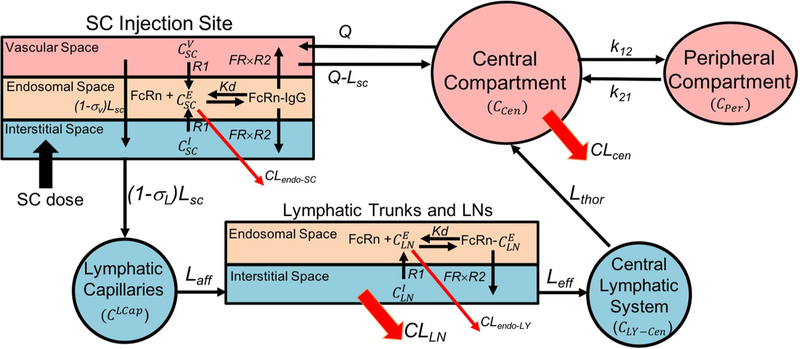
The minimal PBPK model was constructed using SimBiology (Matlab R2017a). The physiologically based model and simple compartmental model were combined to describe absorption of mAbs after SC administration (Figure 1). The model equations describing FcRn binding and transfer across interstitial, endosomal and vascular space were adopted from previously reported studies (3, 9, 17). However, additional physiological parameters related to the lymphatic system were either calculated or obtained from the literature (Table 1) and used to construct the model. The model parameters specific to mAbs are listed in Table 2. Furthermore, additional compartments were included to describe the sequential transfer of mAbs through the lymphatic system. It was assumed that the SC dose of mAb distributes equally in the SC interstitial space after the injection.
Figure 1.
Schematic of the minimal PBPK model for mAb absorption after SC injection
Table 1.
Human physiological parameters related to the SC injection site and lymphatic system
| Parameter | Value | Reference |
|---|---|---|
| (SC injection site interstitial volume) | 0.003115 L | (9) |
| (SC injection site endosomal volume) | 0.000025 L | (9) |
| (SC injection site vascular volume) | 0.00025 L | (9) |
| VLCap (Volume of the lymphatic capillaries) | 0.00033 L | Calculated (18–20) |
| (Combined interstitial volume of lymphatic trunk and LNs after SC injection in upper arm)a | 0.01408 L | Calculated (24–27) |
| (Combined interstitial volume of lymphatic trunk and LNs after SC injection in thigh) | 0.01349 L | Calculated (25–27, 31) |
| (Combined interstitial volume of lymphatic trunk and LNs after SC injection in abdomen)a | 0.01758 L | Calculated (25–27, 32) |
| (Combined endosomal volume of all LNs to which the mAb is exposed after SC injection in upper arm)a | 0.000014 mL | Calculated (24–29) |
| (Combined endosomal volume of all LNs to which the mAb is exposed after SC injection in thigh) | 0.0000126 mL | Calculated (28, 29, 31) |
| (Combined endosomal volume of all LNs to which the mAb is exposed after SC injection in abdomen) | 0.00001596 mL | Calculated (25–29, 32) |
| VLY-Cen (Volume of central lymphatic system) | 0.00888 L | Calculated (30) |
| VThor (Volume of thoracic lymph duct)b | 0.00884 L | Calculated (30) |
| VCC (Volume of cisterna chyli)b | 0.000039 L | Calculated (30) |
| LSC (Lymph flow at the SC injection site) | 0.0001356 L/h | (9) |
| LAff (Lymph flow afferent to LNs in human) | 0.00000564 L/h | Calculated (21, 22) |
| LEff (Lymph flow efferent to LNs in sheep) | 0.00387 L/h | (23) |
| LThor (Thoracic duct lymph flow rate) | 0.06 L/h | (33, 34) |
| QSC (Blood flow at the SC injection site) | 0.04992 L/h | (9) |
| CEndo (Concentration of endogenous mAb in endosomal compartment) | 10000 mg/L | (3) |
| Kd (Dissociation constant for antibody FcRn binding) | 45.36 mg/L | (35) |
| nPt (FcRn concentration in SC tissue or LNs) | 2880 mg/L | (36) |
Not used in the model (SC injection site was assumed as thigh)
Used to calculate total central lymphatic volume
Table 2.
mAb related parameters used in the minimal PBPK model
| Parameter | Value | Reference |
|---|---|---|
| σV (Vascular reflection coefficient) | 0.95 | (3, 17) |
| σL (Lymphatic reflection coefficient) | 0.2 | (3, 17) |
| R1 (Endosomal uptake rate of antibody) | 0.00000926 /h | (3,4) |
| R2 (Endosomal return rate of antibody) | 0.26 /h | (3,4) |
| FR (Recycling fraction of FcRn bound mAb) | 0.715 | (3) |
| CLEndo-SC (Endosomal clearance of mAb in SC injection site)a | 0.003675 L/h | (17) |
| CLEndo-LY (Endosomal clearance of antibody in LNs)b | 0.0001254 L/h | (17) |
Endosomal clearance in skin assumed to be similar to SC injection site
Endosomal clearance in spleen assumed to be similar to lymphatic trunk-LN
Physiological parameters related to the SC injection site and lymphatic system
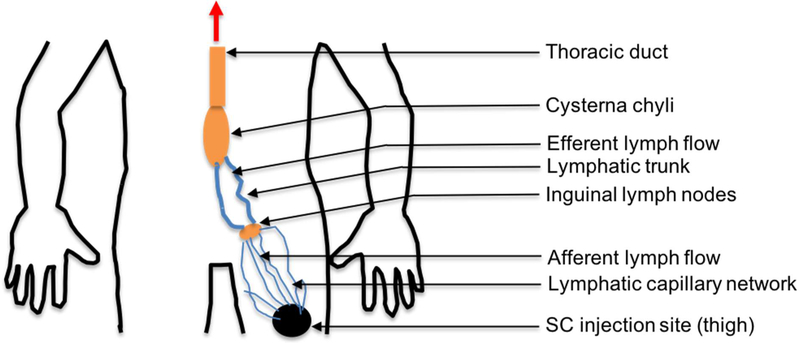
The physiological parameters related to the SC injection site and lymphatic system were either obtained or calculated from the literature (Table 1). The SC injection site volume was calculated by Gill et al. (9) using the diameter of the SC injection depot of radiolabeled IgG. While, the SC site lymph flow was measured using the rate of radiolabeled IgG loss from a SC administration site (9). Lymphatic capillary volume was calculated using the average distance between the injection site and sentinel LN (30 cm), the lymphatic network density per 1 cm annulus of arm skin (385 cm), and the average radius of lymphatic capillaries (0.0274 mm) (18–20). Afferent lymph flow was calculated using the reported lymphatic flow rate (40 mm/min) and radius (0.0274 mm) of the lymphatic capillaries in humans (21, 22). The efferent lymph flow rate in sheep was used in the model (23). The volume of the lymphatic trunk was calculated based on an approximate length of 30 cm and a radius of 1 mm (20). The total LN volume was calculated based on the number of LNs (45 to 50) to which which the mAb is exposed after SC injection in thigh (24–26). The average volume of cervical LNs (0.292 mL) reported in humans was used to calculate the total LN volume after SC injection in the upper arm, abdomen, and thigh (27). The SC injection of a TP in thigh would lead to its travel through the inguinal, iliac and lumbar LNs (Figure 2). Upon SC injection in the upper arm, the TP would travel through the cubital and axillary LNs followed by the subclavian trunk. After passing through the LNs and lymphatic trunks, TPs would join the central lymphatic system (thoracic duct and cysterna chyli), which are lymphatic vessels with greater diameters.
Figure 2.
Schematic representation of flow of mAbs or TPs after SC injection in thigh
The TP would join the systemic circulation via the thoracic duct if the SC injection site were left upper arm, whereas injections into the right upper arm, would enter either via the right thoracic duct or thoracic lymph duct (Figure 3). After abdominal SC injection, the TP would generally travel via inguinal, iliac, and lumbar LNs towards the cisterna chyli and thoracic duct, to enter the systemic blood circulation via the subclavian vein. The known anatomical structure of lymphatic system was utilized to define the sequential transfer of mAbs (24–27).
Figure 3.
Sequential transfer of mAbs towards the systemic circulation after SC injection in, A) Thigh, B) Abdomen, and C) Upper Arm.
The lymphatic trunk and LN volumes were combined to calculate the interstitial lymphatic trunk-LN volume. The total endosomal volume of LNs was calculated based on the endosomal volume in a peripheral mononuclear cell (28) and the number of lymphocytes in a LN (29). The endosomal LN volume and the combined lymphatic trunk-LN interstitial volume for various SC injection sites (thigh, abdomen, and upper arm) were approximately similar (Table 1).
The central lymphatic system’s volume was dependent on the site of SC injection. In the case of SC injection to the thigh, abdomen or left upper arm, the TP would travel through the thoracic lymph duct. In the case of thigh and abdominal injections, volume of the cisterna chyli should be included in the central lymphatic volume. For this model, it was assumed that the SC injection site was either thigh or abdomen, therefore, the volume of the thoracic duct and cisterna chyli were included in the central lymphatic volume. However, the volume of the central lymphatic system after injection in an upper arm or thigh was found to be similar, because the volume of the cisterna chyli was negligible as compared to the thoracic duct volume (Table 1). Volumes of the thoracic lymph duct and cisterna chyli were calculated based on literature values of length and diameter. In the case of the thoracic lymph duct, the length and diameter were 45 and 5 cm, respectively. While, in the case of cisterna chyli, the length and diameter were 2–5 mm and 1 cm, respectively (30).
Parameter estimation and sensitivity analysis
A nonlinear mixed-effects model with stochastic EM algorithm was used for estimation of the 2-compatmental IV PK parameters. The lymphatic trunk-LN clearance was estimated using either the nonlinear mixed-effects model with stochastic EM algorithm or a nonlinear least squares solver. Sensitivity analysis was performed on the physiological, mAb related and estimated parameters (Supplementary Figure 1 and 2). The parameters were altered by 0.1-, 0.3-, 0.5-, 0.7-, 1-, 3-, 5-, 7-, 10-, 50-and 100-fold of the original values as mentioned in Table 1. The lymphatic reflection coefficient was altered by 0.1-, 0.3-, 0.5-, 0.7-, 1-, 1.5-, 2-, 2.5-, 3-, 3.5-, 4-, 4.5-, 5-folds of the original parameter value and its impact on the PK of mAbs was determined.
Model validation
The model was validated by comparing observed and predicted PK profiles after SC administration. In addition, accuracy of the predictions was assessed by plotting ratios of Tmax-observed/Tmax-predicted, Cmax-observed/Cmax-predicted with the pI, lymphatic trunk-LN clearance, and bioavailability (Supplementary Figure 3, 4 and 5). The bioavailability and pI values for mAbs were obtained from the literature (37–50). In the case of anifrolumab, the pI value was estimated using the amino acid sequence (51) and ProtParam, a web-based tool (52).
RESULTS
Estimation of the 2-compartment IV parameters
The 2-compartment model was used to estimate the parameters using the literature PK data after IV bolus or infusion (Table 3). These parameters were different for each mAb, and they were fixed in the minimal PBPK model. The mAbs had average volume of 3.5571 L (standard deviation, ± 1.1081) for the central compartment and 1.8069 L (standard deviation, ± 1.0308) for the peripheral compartment. Mean values for CLcen, K12 and K21 were 0.01531 L/h, 0.0992 /h and 0.3448 /h, respectively.
Table 3.
The 2-compartmental model parameters for various mAbs after IV administration
| mAb | Vcen (L) | Vper (L) | CLcen (L/h) | K12 (/h) | K21 (/h) | Reference |
|---|---|---|---|---|---|---|
| Adalimumab | 3.2131 | 2.2382 | 0.01023 | 0.01162 | 0.01669 | (53) |
| Anifrolumab | 2.1732 | 3.7869 | 0.0111 | 0.02024 | 0.01161 | (54) |
| Belimumab | 3.0486 | 2.3877 | 0.009602 | 0.01635 | 0.0208 | (43, 44) |
| Canakinumab | 3.2897 | 2.3638 | 0.007541 | 0.009417 | 0.0131 | (40) |
| Daclizumab | 5.5255 | 1.762 | 0.01104 | 0.00257 | 0.00805 | (55) |
| Golimumab | 2.3293 | 2.3279 | 0.01467 | 0.01302 | 0.01302 | (42, 56) |
| Guselkumab | 4.9381 | 0.4131 | 0.0233 | 0.000871 | 0.0104 | (46) |
| Infliximab | 4.5782 | 1.2645 | 0.0169 | 0.8914 | 3.2276 | (57) |
| Tocilizumab | 3.5145 | 1.0064 | 0.03585 | 0.01084 | 0.0378 | (58) |
| Trastuzumab | 2.9608 | 0.5186 | 0.01291 | 0.0157 | 0.0896 | (59, 60) |
| Mean | 3.5571 | 1.8069 | 0.01531 | 0.0992 | 0.3448 | |
| Standard deviation |
Estimation of the lymphatic trunk-LN clearance
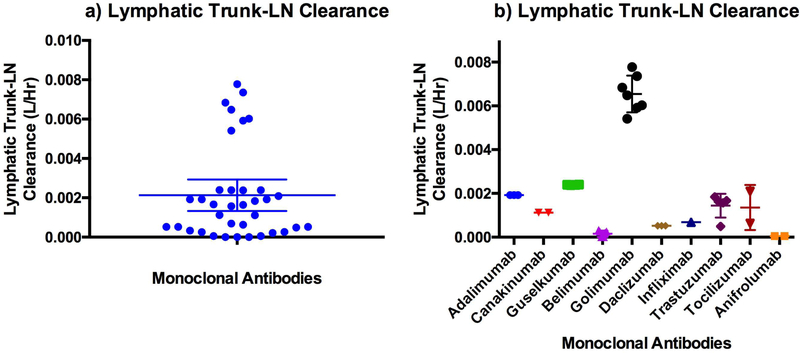
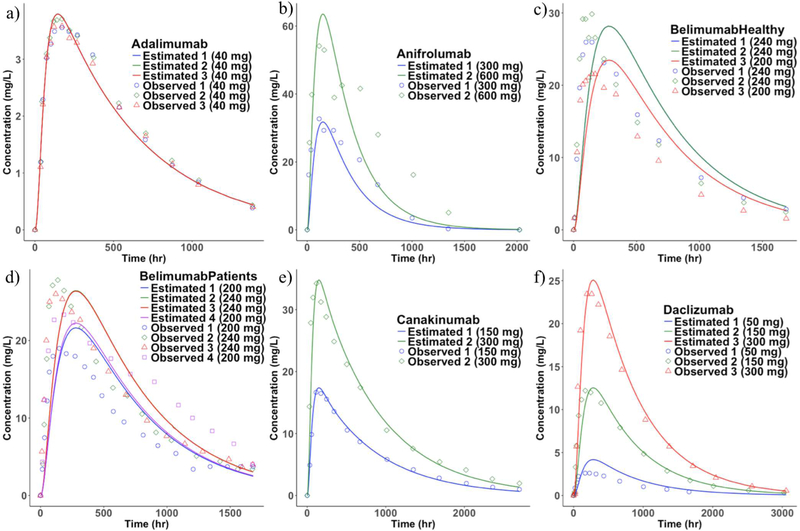
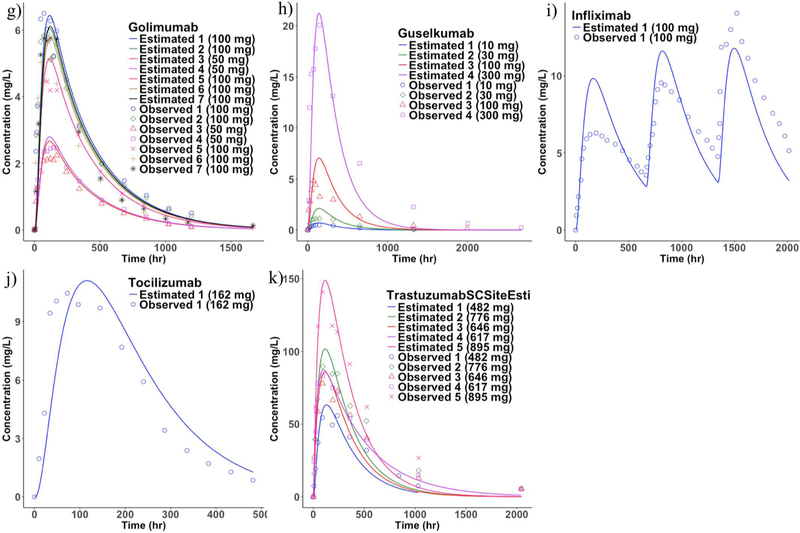
A single parameter (lymphatic trunk-LN clearance) was estimated and the rest of the model was fixed using literature values as described in the methods section. The clearance represents proteolysis of mAbs in the interstitial space of the lymphatic trunks and LNs (Figure 4). The model predicted a total of 37 SC PK profiles (10 mAbs with 26 different doses), which were compared with the mean observed published data (Figure 5 and 6). However, in the case of belimumab, the patient-PK profile was a geometric mean. The estimated lymphatic trunk-LN clearance was in the range of 0.0001495 to 0.007776 L/h with a mean of 0.00213 L/h (0.001332 to 0.002928, lower and upper 95% confidence intervals of the mean) for a total of 37 SC PK profiles. Average lymphatic trunk-LN clearance values for each mAb are shown in Table 4.
Figure 4.
Estimated lymphatic trunk-LN clearance for a) all mAbs used in this study, b) individual mAbs (Mean with 95% confidence interval)
Figure 5.
Observed and the model fitted SC PK profiles. a) adalimumab, b) anifrolumab, c) belimumab-Healthy, d) belimumab-patients, e) canakinumab, and f) daclizumab (Mean observed PK profiles were used for comparison with the model estimates. Observed belimumab-patient PK profile was geometric mean).
Figure 6.
Observed and the model fitted SC PK profiles. g) golimumab, h) guselkumab, i) infliximab, j) tocilizumab, and k) trastuzumab (Mean observed PK profiles were used for comparison with the model estimates)
Table 4.
Estimation of lymphatic trunk-LN clearance after SC administration of mAbs
| mAb (SC injection site) | SC Dose and (F) | Population | Ref. | Model estimated average CLln |
|---|---|---|---|---|
| Adalimumab (Lower abdomen) | 40 mg (64%) | Healthy volunteers | (39) | 0.00192 L/h |
| Anifrolumab (Abdomen) | 300, 600 mg (73%) | Healthy volunteers | (45) | 0.00005105 L/h |
| Belimumab* (Abdomen or thigh) | 200, 2*120, 240 mg (76%) | Lupus erythromatosus patients | (43) | 0.0002564 L/h |
| Belimumab (Abdomen or thigh) | 200, 2*120, 240 mg (76%) | Healthy volunteers | (44) | 0.00000024 L/h |
| Canakinumab (NA) | 150, 300 mg (70%) | Healthy volunteers | (40) | 0.001116 L/h |
| Daclizumab** (NA) Golimumab | 50, 150, 300 mg | Healthy volunteers | (55) | 0.0005207 L/h |
| (Abdomen/Thigh/Upper Arm) | 50, 100 mg (50 %) | Healthy volunteers | (41,42) | 0.00651 L/h |
| Guselkumab (NA) | 10, 30, 100, 300 mg (49%) | Healthy volunteers | (46, 47) | 0.002389 L/h |
| Infliximab (NA) | 100 mg (on day 0,28 and 56) (71%) | Rheumatoid arthritis patients | (61, 62) | 0.0006855 L/ha |
| Tocilizumab (Abdomen) | 162 mg (49%) | Healthy volunteers | (58) | 0.002085 L/ha |
| Tocilizumab (Thigh) | 162 mg (with hyaluronidase) | Healthy volunteers | (63) | 0.0006258 L/ha,b |
| Trastuzumab (Thigh) | 482, 645, and 776 mg (with hyaluronidase) | Healthy volunteers | (64) | 0.001621 L/ha,c |
| Trastuzumab (Thigh) | 895 mg (with hyaluronidase) | HER2-positive breast cancer patients | (64) | 0.0004889 L/ha,d |
| Trastuzumab (Thigh) | 600 mg (with hyaluronidase) | Healthy volunteers | (65) | 0.001839 L/ha,e |
Mean CLLN: 0.00213 L/h (Standard deviation: 0.002359, lower 95% confidence interval of the mean: 0.001332, upper 95% confidence interval of the mean: 0.002928 L/h)
Nonlinear mixed-effects model with stochastic EM algorithm used for estimation of the parameters unless indicated. All observed SC PK data were mean values unless indicated.
Nonlinear least squares solver
SC Site lymph flow (0.04474 L/h) and CLLN estimated simultaneously
SC Site lymph flow (0.002798 L/h) and CLLN estimated simultaneously
SC Site lymph flow (0.003112 L/h) and CLLN estimated simultaneously
SC Site lymph flow (0.01307 L/h) and CLLN estimated simultaneously
F: Bioavailability after SC administration
Geometric mean of the observed pharmacokinetic data
The model simulated median pharmacokinetic data
CLLN: Clearance of mAb in the lymphatic trunk-LN interstitial compartment
NA: Not available
Applications of the minimal PBPK model to evaluate impact of hyaluronidase in the mAb formulation
The mAbs for the SC administration are formulated as highly concentrated solutions in order to deliver higher doses (typically 500–900 mg). The injection volume cannot be increased more than 1–2 mL due to injection discomfort (66). However, hyaluronidase has been used in several studies to allow higher injection volumes by disrupting the complex network of the SC extracellular matrix formed by hyaluronic acid. In addition, the hyaluronidase enzyme can increase the rate of TP absorption leading to decreased Tmax, increased Cmax, increased area under the curve (AUC), and enhanced bioavailability of the TPs (66, 67). In this study, the SC PK data obtained after co-formulation of hyaluronidase, and tocilizumab and trastuzumab were used to estimate the lymphatic trunk-LN clearance (58, 63–65). Due to the co-formulation with hyaluronidase, Tmax was over-predicted for tocilizumab and trastuzumab. The Observed Tmax/Predicted Tmax ratio for tocilizumab and trastuzumab was 0.58 and 0.6, respectively. We hypothesized that the alteration of SC injection site by hyaluronidase may lead to altered SC injection site lymph flow. Therefore, lymphatic trunk-LN clearance and SC injection site lymph flow were estimated simultaneously (Table 4). After the simultaneous estimation of both the parameters, Tmax prediction was improved for tocilizumab (Observed Tmax/Predicted Tmax= 0.87). Similarly, trastuzumab Tmax prediction accuracy was also improved (Observed Tmax/Predicted Tmax= 0.82). The estimated SC injection site lymph flow for the co-formulation of the mAb and hyaluronidase was higher when compared to the original lymph flow used in the model. The estimated SC injection site lymph flow for tocilizumab was 0.04474 L/h, which was 330-fold higher than the minimal PBPK model’s value (Table 1). In the case of trastuzumab, the average SC injection site lymph flow was 0.002955 L/h (22-fold higher than the original value) for healthy volunteers and HER2-positive breast cancer patients (64) (observed and model estimated PK profiles shown in Figure 6). In the case of other SC PK profile (65) (data not shown) obtained from the healthy volunteers, SC site lymph flow was 0.01307 L/h (96-fold higher than the original value).
In addition to above analysis, the SC PK (tocilizumab) profiles obtained without co-formulation with hyaluronidase enzyme were also used for simultaneous estimation of the SC injection site lymph flow and lymphatic trunk-LN clearance using the minimal PBPK model. This was done to demonstrate that the change in the SC site lymph flow observed previously was in fact due to co-formulation of hyaluronidase and mAbs. In the case of tocilizumab without hyaluronidase, the SC site lymph flow was 0.0009557 L/h (only 7-fold higher than the original value). This proves that the SC injection site lymph flow was altered when hyaluronidase co-formulation strategy was used. For the SC profiles where this strategy was not used, the SC injection site lymph flow was relatively less affected.
Comparison of lymphatic trunk-LN clearance with pI and bioavailability of mAbs
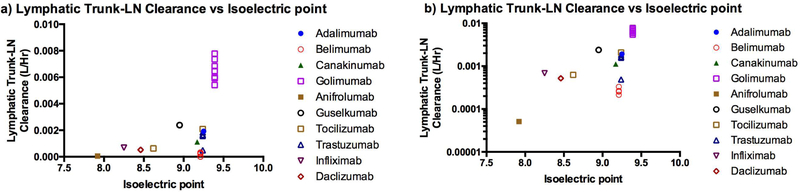
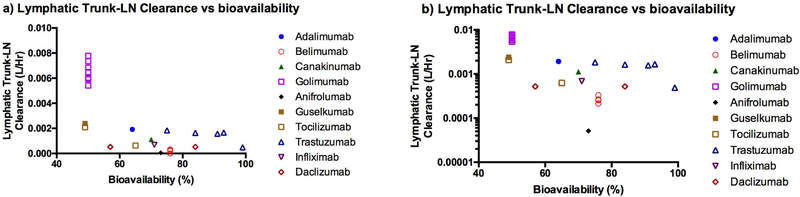
The estimated lymphatic trunk-LN clearance was compared with the pI (Figure 7) and bioavailability (Figure 8) of mAbs. The interstitial space has overall anionic charge due to cell surface of various immune cells in the LNs. The mAbs with higher pI had higher values of the estimated lymphatic trunk-LN clearance. The lymphatic trunk-LN clearance increased with decrease in bioavailability (obtained from literature) of the mAbs (Figure 8). This suggests that the model accounted for the lymphatic proteolysis of mAbs after SC administration. This may indicate that the lymphatic system was an important organ for clearance of mAbs.
Figure 7.
Correlation of the model estimated lymphatic trunk-LN clearance with the isoelectric point a) Linear scale, b) Logarithmic scale
Figure 8.
Correlation of the model estimated lymphatic trunk-LN clearance with the bioavailability a) Linear scale, b) Logarithmic scale (Trastuzumab: Co-formulated with hyaluronidase. Tocilizumab: Only one sample co-formulated with hyaluronidase.)
SENSITIVITY ANALYSIS
The sensitivity analysis showed that the SC injection site interstitial volume (), SC lymph flow (LSC), and lymphatic capillary volume (VLCap), and afferent lymph flow (LAff) were responsible for changes in Cmax and Tmax of mAbs (Supplementary Figure 1). In the case of lymphatic trunk-LN interstitial volume (), increases of the volume by 50-and 100-fold lead to alterations of mAb PK profiles, while changes by 0.1 to 10-fold of the original value did not alter Cmax or Tmax. The alteration of lymphatic trunk-LN clearance (CLLN) and efferent lymph flow (LEff) mainly lead to modification of the Cmax. In addition, changes in the thoracic duct lymph flow (LThor) and central lymphatic volume (VLY-Cen) did not impact PK of mAbs. This indicates that transit through the initial lymphatic system after SC injection is the rate determining step for mAb absorption instead of the thoracic duct. Therefore, SC injection site volume, SC injection site lymph flow, lymphatic capillary volume, afferent lymph flow, and efferent lymph flow are important physiological parameters for absorption of mAbs. Change in the thoracic duct lymph flow and central lymphatic volume did not alter the PK of mAbs (Supplementary Figures 1). Both the Cmax and Tmax were sensitive to change in the SC injection site volume, SC injection site lymph flow, lymphatic capillary volume, and afferent lymph flow (Supplementary Figures 3 to 7). However, changes in the efferent lymph flow had no impact on Tmax. In addition, when the lymphatic reflection coefficient was increased; Cmax decreased, while Tmax increased. There was no change in the PK profile after alteration of the vascular reflection coefficient (Supplementary Figure 2).
Model validation
Accuracy of the model prediction was evaluated by plotting observed and predicted values of Cmax and Tmax (Supplementary Figure 8). In addition, the ratio of observed and predicted Cmax and Tmax were plotted with the pI, lymphatic trunk-LN clearance, and bioavailability (Supplementary Figure 9, 10, and 11). In the case of Cmax, all predicted values were within 1.2-to 0.5-fold of the observed literature values. The predicted Tmax was within the range of 1.8 to 0.3-fold of the observed values.
DISCUSSION
In this manuscript, the sequential transfer of mAbs after SC administration via the lymphatic system is described using the minimal PBPK model. The SC injection site (interstitial, endosomal, vascular), lymphatic capillaries, lymphatic trunk-LNs (interstitial and endosomal), central lymphatic system (thoracic duct and cisterna chyli) compartments were used to describe the transit of mAbs after SC administration. The rest of the body was modeled with a 2-compartment model based on the literature IV PK data. The sequential transfer was based on the known anatomy of lymphatic system (24–27). However, detailed routes of TP transfer via different LNs remain to be investigated further. Also, some individuals may have alterations in the clearance patterns, for example the clearance in the arm, shoulder and thigh may be significantly changed after radical mastoidectomy. In addition, an injection site may clear into multiple adjacent lymph basins.
The clearance from the interstitial space of the SC injection site was not considered in the model, because a previously reported study indicated that protein (hGH) degradation was minimal at the SC injection site (5). In addition, simultaneous estimation of the clearance from the SC interstitial space and lymphatic trunk-LN interstitial compartment resulted in a minor contribution for the SC injection site proteolysis (data not shown). However, the endosomal proteolysis in the SC injection site and LNs was considered.
The lymphatic trunk-LN interstitial clearance was estimated using the minimal PBPK model and the literature SC PK data (Figure 4 and Table 4). The estimated clearance differed with dosing and population changes. This alteration of the estimated clearance may be due to disease condition, change in formulation, or the differences in posttranslational modifications (e.g. glycosylation). These differences were not considered in the model. The model demonstrated that the estimated lymphatic trunk-LN clearance of mAbs may correlate with the bioavailability (Figure 8). The estimated clearance increased when the bioavailability of the mAb decreased. This indicates efficiency of the model to account for the proteolysis of mAbs in the lymphatic system. According to the previously published reports, proteolysis was not observed after incubation of TPs with the freshly collected lymph, indicating absence of any protease enzymes in the lymph (5, 68). In this study, the lymphatic trunk volume (which mainly contains lymph fluid) and the LN (which mainly contains lymph node cells) volume were combined to represent the compartment responsible for proteolysis of the mAbs. The lymphatic trunk-LN compartment was mainly composed of the LN volume. The lymphatic trunk volume representing volume of the lymph fluid was very minor (< 7%).
Sensitivity analysis (Supplementary Figure 1) showed that the lymphatic trunk-LN interstitial space proteolysis was important to govern Cmax of the mAbs. The degradation of mAbs in the lymphatic system was primarily due to extracellular or interstitial proteolysis. The protease enzymes secreted by the LN and other immune cells in the interstitial space may play an important role in reducing the bioavailability of mAbs. Intracellular or endosomal proteolysis of mAbs was negligible due to FcRn binding-mediated protection. The intracellular proteolysis of mAbs in the lymphatic system may be dependent on their uptake by the lymphocytes. In this model, we used endosomal uptake rate (R1) from a literature PBPK model (estimated by fitting liver data) (3, 4). Alteration of the endosomal uptake rate by 0.1 to 100-fold of the original value did not change the PK of mAbs (data not shown). However, in vitro studies to calculate R1 may be useful for an accurate understanding of proteolytic processes in the SC injection site and lymphatic system. Disease conditions like inflammation may also increase proteolytic activity of the lymph. However, this remains to be further investigated. In addition, alteration of the recycling fraction of FcRn bound mAb, dissociation constant for, dissociation constant for antibody FcRn binding, and FcRn concentration did not change PK of mAbs (data not shown).
Wang et al. (68) confirmed proteolysis of erythropoietin in the presence of rat LN cell suspension. The protein completely disappeared after the number of LN cells in the incubation was increased. This indicates that LNs are responsible for proteolysis of TPs. In addition, after incubation of the protein with the SC tissue homogenate, 90–95% of the parent protein remained unaffected (68). Although the authors raised doubts about loss of proteolytic activity during preparation of the SC tissue homogenate, this finding corroborates our conclusion that the SC site degradation plays a minor role in governing the bioavailability. However, in another study, insulin was reported to degrade at the SC injection site (69). Detailed investigation of in vitro proteolysis of mAbs in various systems must be done to arrive at more definite conclusion about the cause of reduced bioavailability after SC administration.
The PK studies in humans indicate that variation in the injection site (abdomen and thigh) do not have any impact on Cmax and AUC0−∞ of belimumab (44). In another clinical study, golimumab’s median Tmax after SC injection in the thigh was 1.25-fold higher than SC injection in the abdomen and upper arm. The Cmax after thigh SC injection was 1.33-fold higher than SC injection in the upper arm, and the Cmax-abdomen was 1.24-fold higher than that of the upper arm (42). This suggests that the volumes of lymphatic compartments and lymph flows may not be significantly different for each of the SC injection sites. Therefore, lymphatic volumes and flows rates for one injection site may be applied to the other. The calculations used in the model showed that the interstitial volume of the lymphatic trunk-LN was similar for various SC injection sites (upper arm, abdomen, and thigh) (Table 1).
The prediction accuracy of the model was determined by plotting observed and predicted PK parameters (Supplementary Figure 8). There was no correlation of the prediction accuracy of Tmax or Cmax with the pI or bioavailability or the estimated lymphatic trunk-LN clearance of mAbs. This confirms that the pI of mAbs did not affect the uptake by lymphatic system. Similarly, lymphatic trunk-LN clearance and bioavailability did not govern the prediction accuracy of the model.
The model also showed that for the mAbs with higher pI, the estimated lymphatic trunk-LN clearance was greater. The cationic proteins with higher pI have a propensity to bind with the anionic cell surfaces and interstitial space (e.g. hyaluronic acid). Higher pI also leads to faster clearance of mAbs (70). Similar trend was observed for the mAbs investigated in this study (Figure 7). This correlation may be used to predict the lymphatic trunk-LN clearance based on the known pI of mAbs. The lymphatic clearance can be used in the minimal PBPK model to predict SC PK of the mAbs.
Sensitivity analysis of the model parameters indicate that the initial lymphatics are rate determining for absorption of mAbs via the SC route. Mainly, the SC injection site lymph flow, SC injection site volume, afferent lymph flow, efferent lymph flow, and lymphatic trunk-LN volume impacted Tmax, after their alteration by 0.1 to 100-fold of the original value. However, thoracic duct lymph flow had no effect on Tmax after 0.1 to 100-fold change in its value. Therefore, alteration of thoracic lymph duct flows due to disease condition may not alter overall PK of mAbs. However, the parameters associated with the initial lymphatic system are important to govern the absorption of mAbs and change in those parameters due to the disease conditions may significantly alter the SC PK of mAbs.
It was reported earlier that the PK of mAbs is prone to high inter-subject variability. Factors like body weight, age, sex, ethnicity, disease condition, immune status are responsible for variations in the PK (71). However, more research is needed to evaluate their influence on physiological parameters related to the SC injection site and lymphatic system. The minimal PBPK model may be used for prediction of bioavailability of mAbs using in vitro lymphatic proteolysis data and to evaluate the impact of changes in lymphatic flow rates on the PK. In addition, the model may be utilized to guide in vitro experiments for mechanistic prediction of the bioavailability. This study has explained various physiological parameters related to the SC injection site and lymphatic system responsible for regulating the PK of mAbs.
CONCLUSION
The lymphatic trunk-LN clearance was estimated using the minimal PBPK model. The physiological parameters related to the SC tissue and lymphatic system were used along with the 2-compartment IV parameters to construct the minimal PBPK model for prediction of SC PK of the mAbs. The model may serve as a platform to utilize the in vitro clearance data from the SC tissue and lymphatic system to predict SC PK of mAbs. The LNs were mainly responsible for proteolysis of mAbs leading to their reduced bioavailability. Therefore, LN cell suspension may be used to generate inputs for the PBPK model. However, the in vitro studies were beyond the scope of this manuscript. Further, this study identified SC injection site lymph flow, afferent lymph flow, efferent lymph flow, volumes associated with the SC injection site, and lymphatic trunk-LN clearance as important parameters responsible for absorption of mAbs. The lymphatic trunk-LN clearance increased with increase in the pI of mAbs. Therefore, the pI of mAbs can be used to calculate the lymphatic clearance. Overall, the model is useful to understand disposition of mAbs after SC administration.
Supplementary Material
ACKNOWLEDGEMENT
The authors were supported by a generous grant from NIH (R01CA173292). NV was partially supported by a Higuchi Fellowship and the Department of Pharmaceutical Chemistry, The University of Kansas. We are also grateful to the J.R. and Inez Jay funds, awarded to MLF by the Higuchi Biosciences Center at The University of Kansas.
GLOSSARY
Concentration of mAb in the vascular space of SC injection site
Concentration of mAb in the interstitial space of SC injection site
Concentration of mAb in the endosomal space of SC injection site
- CLCap
Concentration of mAb in the lymphatic capillary compartment
Concentration of mAb in the interstitial space of lymphatic trunk-LNs
Concentration of mAb in the endosomal space of lymphatic trunk-LNs
- CLY-Cen
Concentration of mAb in the central lymphatic system
- CCen
Concentration of mAb in the central compartment
- CPer
Concentration of mAb in the peripheral compartment
SC injection site interstitial volume
SC injection site vascular volume
SC injection site endosomal volume
- VLCap
Volume of the lymphatic capillaries
Combined interstitial volume of lymphatic trunk-LNs after SC injection in thigh
Combined endosomal volume of all LNs to which the mAb is exposed after SC injection in thigh
- VLY-Cen
Volume of central lymphatic system
- VCen
Volume of the central compartment
- VPer
Volume of the peripheral compartment
- LSC
Lymph flow at the SC injection site
- LAff
Lymph flow afferent to LNs
- LEff
Lymph flow efferent to LNs
- LThor
Thoracic duct lymph flow
- QSC
Blood flow at the SC injection site
- σL
Lymphatic reflection coefficient
- σV
Vascular reflection coefficient
- CLLN
Clearance of mAb in the lymphatic trunk-LN interstitial compartment
- CLEndo-SC
Endosomal clearance of mAb in the SC injection site
- CLEndo-LN
Endosomal clearance of mAb in LNs
- CLcen
Clearance of mAb from the central compartment
- K12
Transfer rate constant from the central compartment to the peripheral compartment
- K21
Transfer rate constant from the peripheral compartment to the central compartment
- fuSC
Unbound fraction of mAb in SC tissue
- fuLN
Unbound fraction of mAb in SC tissue
- CEndo
Concentration of endogenous mAb in endosomal compartment
- R1
Endosomal uptake rate of antibody
- R2
Endosomal return rate of antibody
- FR
Recycling fraction of FcRn bound mAb
- Kd
Dissociation constant for antibody FcRn binding
- nPt
FcRn concentration in SC tissue or LN
APPENDIX
In addition to the data figures in the Supplementary materials, all raw digitalized data and the SimBiology (Matlab 2017a) model file used in this study are archived and available free of charge at the University of Kansas Scholar Works Repository (https://kuscholarworks.ku.edu/handle/1808/26648)
Model equations
REFERENCES
- 1.Jackisch C, Müller V, Maintz C, Hell S, Ataseven B. Subcutaneous administration of monoclonal antibodies in oncology. Geburtshilfe Frauenheilkd. 2014;74(4):343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dirks NL, Meibohm B. Population pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49(10):633–659 [DOI] [PubMed] [Google Scholar]
- 3.Zhao L, Ji P, Li Z, Roy P, Sahajwalla CG. The antibody drug absorption following subcutaneous or intramuscular administration and its mathematical description by coupling physiologically based absorption process with the conventional compartment pharmacokinetic model. J Clin Pharmacol. 2013;53(3):314–325 [DOI] [PubMed] [Google Scholar]
- 4.Davda JP, Jain M, Batra SK, Gwilt PR, Robinson DH. A physiologically based pharmacokinetic (PBPK) model to characterize and predict the disposition of monoclonal antibody CC49 and its single chain Fv constructs. Int Immunopharmacol. 2008;8(3):401–413 [DOI] [PubMed] [Google Scholar]
- 5.Charman SA, Segrave AM, Edwards GA, Porter CJ. Systemic availability and lymphatic transport of human growth hormone administered by subcutaneous injection. J Pharm Sci. 2000;89(2):168–177 [DOI] [PubMed] [Google Scholar]
- 6.Abuqayyas L, Balthasar JP. Application of PBPK modeling to predict monoclonal antibody disposition in plasma and tissues in mouse models of human colorectal cancer. J Pharmacokinet Pharmacodyn. 2012;39(6):683–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baxter LT, Zhu H, Mackensen DG, Butler WF, Jain RK. Biodistribution of monoclonal antibodies: scale-up from mouse to human using a physiologically based pharmacokinetic model. Cancer Res. 1995;55(20):4611–4622 [PubMed] [Google Scholar]
- 8.Shah DK, Betts AM. Towards a platform PBPK model to characterize the plasma and tissue disposition of monoclonal antibodies in preclinical species and human. J Pharmacokinet Pharmacodyn. 2012;39(1):67–86 [DOI] [PubMed] [Google Scholar]
- 9.Gill KL, Gardner I, Li L, Jamei M. A bottom-up whole-body physiologically based pharmacokinetic model to mechanistically predict tissue distribution and the rate of subcutaneous absorption of therapeutic proteins. AAPS J. 2016;18(1):156–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X, DuBois DC, Almon RR, Jusko WJ. Interrelationships between Infliximab and rhTNF-α in Plasma using Minimal Physiologically-Based Pharmacokinetic (mPBPK) Models. Drug Metab Dispos. 2017;45(7):790–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elmeliegy M, Lowe P, Krzyzanski W. Simplification of complex physiologically based pharmacokinetic models of monoclonal antibodies. AAPS J. 2014;16(4):810–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li L, Gardner I, Dostalek M, Jamei M. Simulation of Monoclonal Antibody Pharmacokinetics in HumansUsing a Minimal Physiologically Based Model. AAPS J. 2014;16(5):1097–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sager JE, Yu J, Raguenau-Majlessi I, Isoherranen N. Physiologically based pharmacokinetic (PBPK) modeling and simulation approaches: a systematic review of published models, applications and model verification. Drug Metab Dispos. 2015;43:1823–1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong H, Chow TW. Physiologically based pharmacokinetic modeling of therapeutic proteins. J Pharm Sci. 2017;106(9):2270–2275 [DOI] [PubMed] [Google Scholar]
- 15.Rohatgi A WebPlotDigitizer, Web based tool to extract data from plots, images, and maps. 2018. Accessed on May 09, 2018 Available from: https://automeris.io/WebPlotDigitizer/.
- 16.Kuo I, Akpa BS. Validity of the Lipid Sink as a Mechanism for the Reversal of Local Anesthetic Systemic ToxicityA Physiologically Based Pharmacokinetic Model Study. Anesthesiology: The Journal of the American Society of Anesthesiologists. 2013;118(6):1350–1361 [DOI] [PubMed] [Google Scholar]
- 17.Garg A, Balthasar JP. Physiologically-based pharmacokinetic (PBPK) model to predict IgG tissue kinetics in wild-type and FcRn-knockout mice. J Pharmacokinet Pharmacodyn. 2007;34(5):687–709 [DOI] [PubMed] [Google Scholar]
- 18.Mellor R, Stanton A, Azarbod P, Sherman M, Levick J, Mortimer P. Enhanced cutaneous lymphatic network in the forearms of women with postmastectomy oedema. J Vasc Res. 2000;37(6):501–512 [DOI] [PubMed] [Google Scholar]
- 19.Nathanson SD, Nelson L, Karvelis KC. Rates of flow of technetium 99m-labeled human serum albumin from peripheral injection sites to sentinel lymph nodes. Ann Surg Oncol. 1996;3(4):329–335 [DOI] [PubMed] [Google Scholar]
- 20.Margaris K, Black RA. Modelling the lymphatic system: challenges and opportunities. J R Soc Interface. 2012;9(69):601–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischer M, Franzeck UK, Herrig I, Costanzo U, Wen S, Schiesser M, Hoffmann U, Bollinger A. Flow velocity of single lymphatic capillaries in human skin. Am J Physiol Heart Circ Physiol. 1996;270(1):H358–H363 [DOI] [PubMed] [Google Scholar]
- 22.Fujiwara M, Sawada M, Kasuya A, Matsushita Y, Yamada M, Fukamizu H, Magata Y, Tokura Y, Sakahara H. Measurement of cutaneous lymphatic flow rates in patients with skin cancer: area extraction method. J Dermatol. 2014;41(6):498–504 [DOI] [PubMed] [Google Scholar]
- 23.Quin J, Shannon A. The influence of the lymph node on the protein concentration of efferent lymph leaving the node. J Physiol. 1977;264(2):307–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lymphatics of the Upper Limb. Department of Neurobiology and Developmental Sciences, University of Arkansas for Medical Sciences; Accessed on April 14, 2018 Available from: http://anatomy.uams.edu/lymph_upperlimb.html. [Google Scholar]
- 25.Bontumasi N, Jacobson JA, Caoili E, Brandon C, Kim SM, Jamadar D. Inguinal lymph nodes: size, number, and other characteristics in asymptomatic patients by CT. Surg Radiol Anat. 2014;36(10):1051–1055 [DOI] [PubMed] [Google Scholar]
- 26.Usovich A, Borziak E. Variants in the number and size and the topography of the lumbar lymph nodes in the regional of the liver in the human adult. Arkh Anat Gistol Ehmbriol. 1982;83(7):29–33 [PubMed] [Google Scholar]
- 27.Ying M, Pang B. Three-dimensional ultrasound measurement of cervical lymph node volume. Br J Radiol. 2009;82(980):617–625 [DOI] [PubMed] [Google Scholar]
- 28.Corlier F, Rivals I, Lagarde J, Hamelin L, Corne H, Dauphinot L, Ando K, Cossec J, Fontaine G, Dorothée G. Modifications of the endosomal compartment in peripheral blood mononuclear cells and fibroblasts from Alzheimer’s disease patients. Transl Psychiatry. 2015;5(7):e595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sikorski E, Gerberick G, Ryan C, Miller C, Ridder G. Phenotypic analysis of lymphocyte subpopulations in lymph nodes draining the ear following exposure to contact allergens and irritants. Fundam Appl Toxicol. 1996;34(1):25–35 [DOI] [PubMed] [Google Scholar]
- 30.Skandalakis JE, Skandalakis LJ, Skandalakis PN. Anatomy of the lymphatics. Surg Oncol Clin N Am. 2007;16(1):1–16 [DOI] [PubMed] [Google Scholar]
- 31.Lymphatics of the Lower Limb. Department of Neurobiology and Developmental Sciences, University of Arkansas for Medical Sciences; Accessed on April 14, 2018 Available from: http://anatomy.uams.edu/lymph_lowerlimb.html. [Google Scholar]
- 32.Lymphatics of the Abdomen. Department of Neurobiology and Developmental Sciences, University of Arkansas for Medical Sciences; Accessed on April 14, 2018 Available from: http://anatomy.uams.edu/lymph_abdomen.html. [Google Scholar]
- 33.Giragossian C, Vage C, Li J, Pelletier K, Piché-Nicholas N, Rajadhyaksha M, Liras J, Logan A, Calle RA, Weng Y. Mechanistic Investigation of the Preclinical Pharmacokinetics and Interspecies Scaling of PF-05231023, a Fibroblast Growth Factor 21–Antibody Protein Conjugate. Drug Metab Dispos. 2015;43(6):803–811 [DOI] [PubMed] [Google Scholar]
- 34.Dumont AE, Mulholland JH. Flow rate and composition of thoracic-duct lymph in patients with cirrhosis. N Engl J Med. 1960;263(10):471–474 [DOI] [PubMed] [Google Scholar]
- 35.Zhou J, Johnson JE, Ghetie V, Ober RJ, Ward ES. Generation of mutated variants of the human form of the MHC class I-related receptor, FcRn, with increased affinity for mouse immunoglobulin G. J Mol Biol. 2003;332(4):901–913 [DOI] [PubMed] [Google Scholar]
- 36.Ferl GZ, Wu AM, DiStefano JJ. A predictive model of therapeutic monoclonal antibody dynamics and regulation by the neonatal Fc receptor (FcRn). Ann Biomed Eng. 2005;33(11):1640–1652 [DOI] [PubMed] [Google Scholar]
- 37.Center for drug evaluation and research, guselkumab application 761061Orig1s000 2018. Accessed on April 13, 2018 Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/761061Orig1s000OtherR.pdf.
- 38.Keith Doyle, Maria Halo, Harding Emma R, Lewis Peter, Mark Uden, Valeriu Damian, Xuan H. Antibody variants. In.; 2017.
- 39.Hillson J, Mant T, Rosano M, Huntenburg C, Alai-Safar M, Darne S, Palmer D, Pavlova BG, Doralt J, Reeve R. Pharmacokinetic equivalence, comparable safety, and immunogenicity of an adalimumab biosimilar product (M923) to Humira in healthy subjects. Pharmacol Res Perspect. 2018;6(1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chakraborty A, Tannenbaum S, Rordorf C, Lowe PJ, Floch D, Gram H, Roy S. Pharmacokinetic and pharmacodynamic properties of canakinumab, a human anti-interleukin-1β monoclonal antibody. Clin Pharmacokinet. 2012;51(6):e1–e18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ling J, Lyn S, Xu Z, Achira M, Bouman-Thio E, Shishido A, Ford J, Shankar G, Wagner C, Kim KT. Lack of racial differences in the pharmacokinetics of subcutaneous golimumab in healthy Japanese and Caucasian male subjects. J Clin Pharmacol. 2010;50(7):792–802 [DOI] [PubMed] [Google Scholar]
- 42.Xu Z, Wang Q, Zhuang Y, Frederick B, Yan H, Bouman-Thio E, Marini JC, Keen M, Snead D, Davis HM. Subcutaneous bioavailability of golimumab at 3 different injection sites in healthy subjects. J Clin Pharmacol. 2010;50(3):276–284 [DOI] [PubMed] [Google Scholar]
- 43.Yapa SWS, Roth D, Gordon D, Struemper H. Comparison of intravenous and subcutaneous exposure supporting dose selection of subcutaneous belimumab systemic lupus erythematosus Phase 3 program. Lupus. 2016;25(13):1448–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cai WW, Fiscella M, Chen C, Zhong ZJ, Freimuth WW, Subich DC. Bioavailability, pharmacokinetics, and safety of belimumab administered subcutaneously in healthy subjects. Clin Pharmacol Drug Dev. 2013;2(4):349–357 [DOI] [PubMed] [Google Scholar]
- 45.Tummala R, Rouse T, Berglind A, Santiago L. Safety, tolerability, and pharmacokinetics of subcutaneous and intravenous anifrolumab in healthy volunteers. In.ACR/ARHP Annual Meeting: BMJ Publishing Group Ltd; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhuang Y, Calderon C, Marciniak SJ, Bouman-Thio E, Szapary P, Yang T-Y, Schantz A, Davis HM, Zhou H, Xu Z. First-in-human study to assess guselkumab (anti-IL-23 mAb) pharmacokinetics/safety in healthy subjects and patients with moderate-to-severe psoriasis. Eur J Clin Pharmacol. 2016;72(11):1303–1310 [DOI] [PubMed] [Google Scholar]
- 47.Product monograph for Tremfya (guselkumab). Janssen Inc. Accessed on April 16, 2018 Available from: http://www.janssen.com/canada/sites/www_janssen_com_canada/files/prod_files/live/tremfya_cpm.pdf. [Google Scholar]
- 48.Infliximab DrugBank. 2018. Accessed on May 17, 2018 Available from: https://www.drugbank.ca/drugs/DB00065.
- 49.Daclizumab DrugBank. 2018. Accessed on May 17, 2018 Available from: https://www.drugbank.ca/drugs/DB00111.
- 50.Miao S, Fan L, Zhao L, Ding D, Liu X, Wang H, Tan W-S. Physicochemical and Biological Characterization of the Proposed Biosimilar Tocilizumab. Biomed Res Int. 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.International Nonproprietary Names for Pharmaceutical Substances (INN), WHO Drug Information, Vol. 28, No. 1, 2014 (INN List 71). Accessed on May 25, 2018 Available from: http://www.who.int/medicines/publications/druginformation/innlists/RL71.pdf.
- 52.Gasteiger E, Hoogland C, Gattiker A, Wilkins MR, Appel RD, Bairoch A. Protein identification and analysis tools on the ExPASy server In. The proteomics protocols handbook: Springer; 2005. p. 571–607. [Google Scholar]
- 53.Weisman MH, Moreland LW, Furst DE, Weinblatt ME, Keystone EC, Paulus HE, Teoh LS, Velagapudi RB, Noertersheuser PA, Granneman GR. Efficacy, pharmacokinetic, and safety assessment of adalimumab, a fully human anti-tumor necrosis factor-alpha monoclonal antibody, in adults with rheumatoid arthritis receiving concomitant methotrexate: a pilot study. Clin Ther. 2003;25(6):1700–1721 [DOI] [PubMed] [Google Scholar]
- 54.Goldberg A, Geppert T, Schiopu E, Frech T, Hsu V, Simms RW, Peng SL, Yao Y, Elgeioushi N, Chang L. Dose-escalation of human anti-interferon-α receptor monoclonal antibody MEDI-546 in subjects with systemic sclerosis: a phase 1, multicenter, open label study. Arthritis Res Ther. 2014;16(1):R57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Othman AA, Tran JQ, Tang MT, Dutta S. Population pharmacokinetics of daclizumab high-yield process in healthy volunteers: integrated analysis of intravenous and subcutaneous, single-and multiple-dose administration. Clin Pharmacokinet. 2014;53(10):907–918 [DOI] [PubMed] [Google Scholar]
- 56.Zhou H, Jang H, Fleischmann RM, Bouman-Thio E, Xu Z, Marini JC, Pendley C, Jiao Q, Shankar G, Marciniak SJ. Pharmacokinetics and safety of golimumab, a fully human anti-TNF-α monoclonal antibody, in subjects with rheumatoid arthritis. J Clin Pharmacol. 2007;47(3):383–396 [DOI] [PubMed] [Google Scholar]
- 57.Palaparthy R, Udata C, Hua SY, Yin D, Cai C-H, Salts S, Rehman MI, McClellan J, Meng X. A randomized study comparing the pharmacokinetics of the potential biosimilar PF-06438179/GP1111 with Remicade®(infliximab) in healthy subjects (REFLECTIONS B537–01). Expert Rev Clin Immunol. 2018;14(4):329–336 [DOI] [PubMed] [Google Scholar]
- 58.Zhang X, Georgy A, Rowell L. Pharmacokinetics and pharmacodynamics of tocilizumab, a humanized anti-interleukin-6 receptor monoclonal antibody, following single-dose administration by subcutaneous and intravenous routes to healthy subjects. Int J Clin Pharmacol Ther. 2013;51(6):443–455 [DOI] [PubMed] [Google Scholar]
- 59.Morita J, Tanaka M, Nomoto M, Matsuki S, Tsuru T, Matsuguma K, Shiramoto M. Pharmacokinetic bioequivalence, safety, and immunogenicity of DMB-3111, a trastuzumab biosimilar, and trastuzumab in healthy Japanese adult males: results of a randomized trial. BioDrugs. 2016;30(1):17–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pivot X, Deslypere JP, Park LS, Kim MJ, Lee W, Lee J. A Randomized Phase I Study Comparing the Pharmacokinetics of HD201, a Trastuzumab Biosimilar, With European Union–sourced Herceptin. Clin Ther. 2018;40(3):396–405.e394 [DOI] [PubMed] [Google Scholar]
- 61.Westhovens R, Houssiau F, Joly J, Everitt DE, Zhu Y, Sisco D, Van Hartingsveldt B, Mascelli MA, Graham MA, Durez P. A phase I study assessing the safety, clinical response, and pharmacokinetics of an experimental infliximab formulation for subcutaneous or intramuscular administration in patients with rheumatoid arthritis. J Rheumatol. 2006;33(5):847–853 [PubMed] [Google Scholar]
- 62.Zhu Y, Pendley C, Sisco D, Westhovens R, Durez P, Bouman-Thio E, Hartingsveldt B, Everitt D, Graham M. Pharmacokinetics and pharmacodynamics of infliximab, an anti-tumor necrosis factor-alpha monoclonal antibody, following single subcutaneous administrations in rheumatoid arthritis patients. Clin Pharmacol Ther. 2005;77(2) [Google Scholar]
- 63.Morcos PN, Zhang X, McIntyre C, Bittner B, Rowell L, Hussain Z. Pharmacokinetics and pharmacodynamics of single subcutaneous doses of tocilizumab administered with or without rHuPH20. Int J Clin Pharmacol Ther. 2013;51(7):537–548 [DOI] [PubMed] [Google Scholar]
- 64.Wynne C, Harvey V, Schwabe C, Waaka D, McIntyre C, Bittner B. Comparison of Subcutaneous and Intravenous Administration of Trastuzumab: A Phase I/Ib Trial in Healthy Male Volunteers and Patients With HER2- Positive Breast Cancer. J Clin Pharmacol. 2013;53(2):192–201 [DOI] [PubMed] [Google Scholar]
- 65.Wynne CJ, Ellis-Pegler RB, Waaka DS, Schwabe C, Lehle M, Heinzmann D, Mangat R, Li C, Dick AE, Cranshaw NA. Comparative pharmacokinetics of subcutaneous trastuzumab administered via handheld syringe or proprietary single-use injection device in healthy males. Cancer Chemother Pharmacol. 2013;72(5):1079–1087 [DOI] [PubMed] [Google Scholar]
- 66.Richter WF, Jacobsen B. Subcutaneous Absorption of Biotherapeutics: Knowns and Unknowns. Drug Metab Dispos. 2014;42(11):1881–1889 [DOI] [PubMed] [Google Scholar]
- 67.Liu S, Xie B, Wei W, Hui M, Su Z. Design and preparation of chimeric hyaluronidase as a chaperone for the subcutaneous administration of biopharmaceuticals. Biochem Eng J. 2016;112:32–41 [Google Scholar]
- 68.Wang W, Chen N, Shen X, Cunningham P, Fauty S, Michel K, Wang B, Hong X, Adreani C, Nunes CN. Lymphatic transport and catabolism of therapeutic proteins after subcutaneous administration to rats and dogs. Drug Metab Dispos. 2012;40(5):952–962 [DOI] [PubMed] [Google Scholar]
- 69.Okumura K, Komada F, Hori R. Fate of porcine and human insulin at the subcutaneous injection site. I. degradation and absorption of insulins in the rat. J Pharmacobiodyn. 1985;8(1):25–32 [DOI] [PubMed] [Google Scholar]
- 70.Bumbaca D, Boswell CA, Fielder PJ, Khawli LA. Physiochemical and biochemical factors influencing the pharmacokinetics of antibody therapeutics. AAPS J. 2012;14(3):554–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gill KL, Machavaram KK, Rose RH, Chetty M. Potential sources of inter-subject variability in monoclonal antibody pharmacokinetics. Clin Pharmacokinet. 2016;55(7):789–805 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.